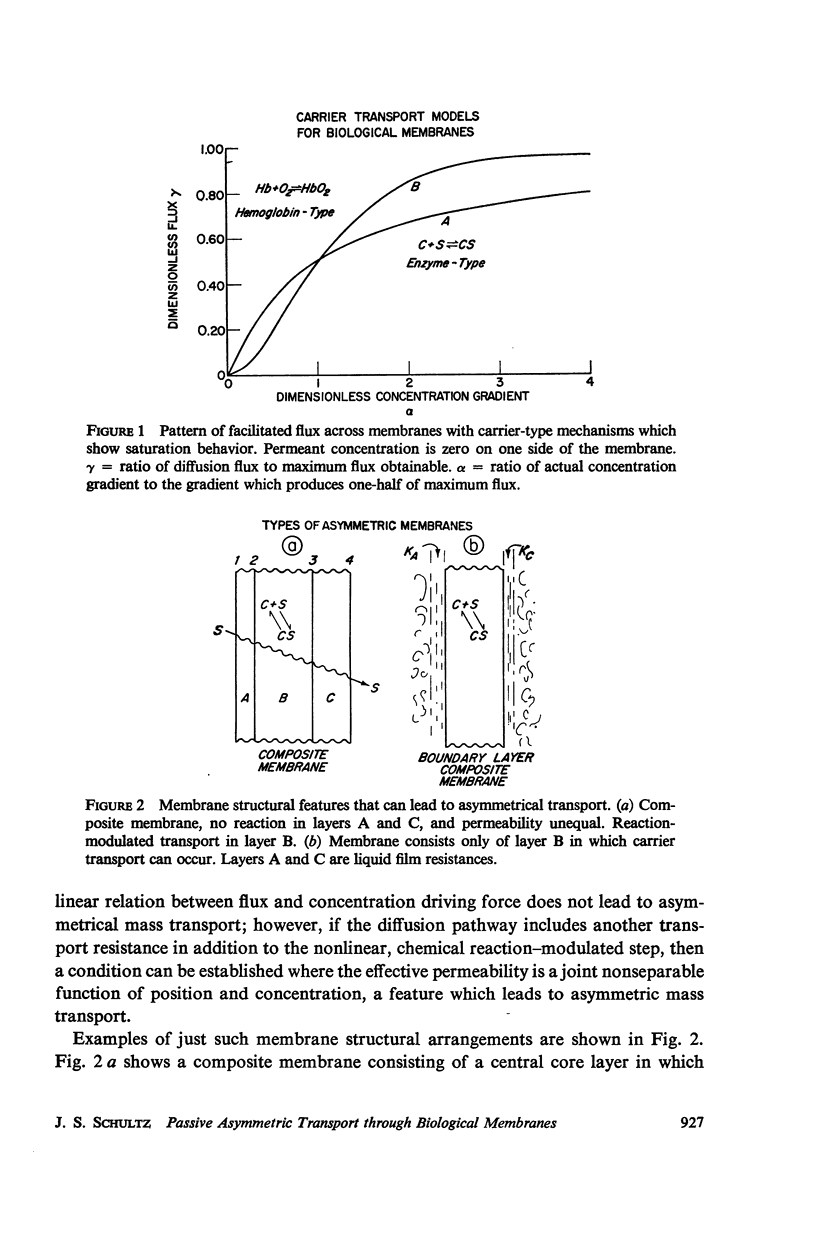
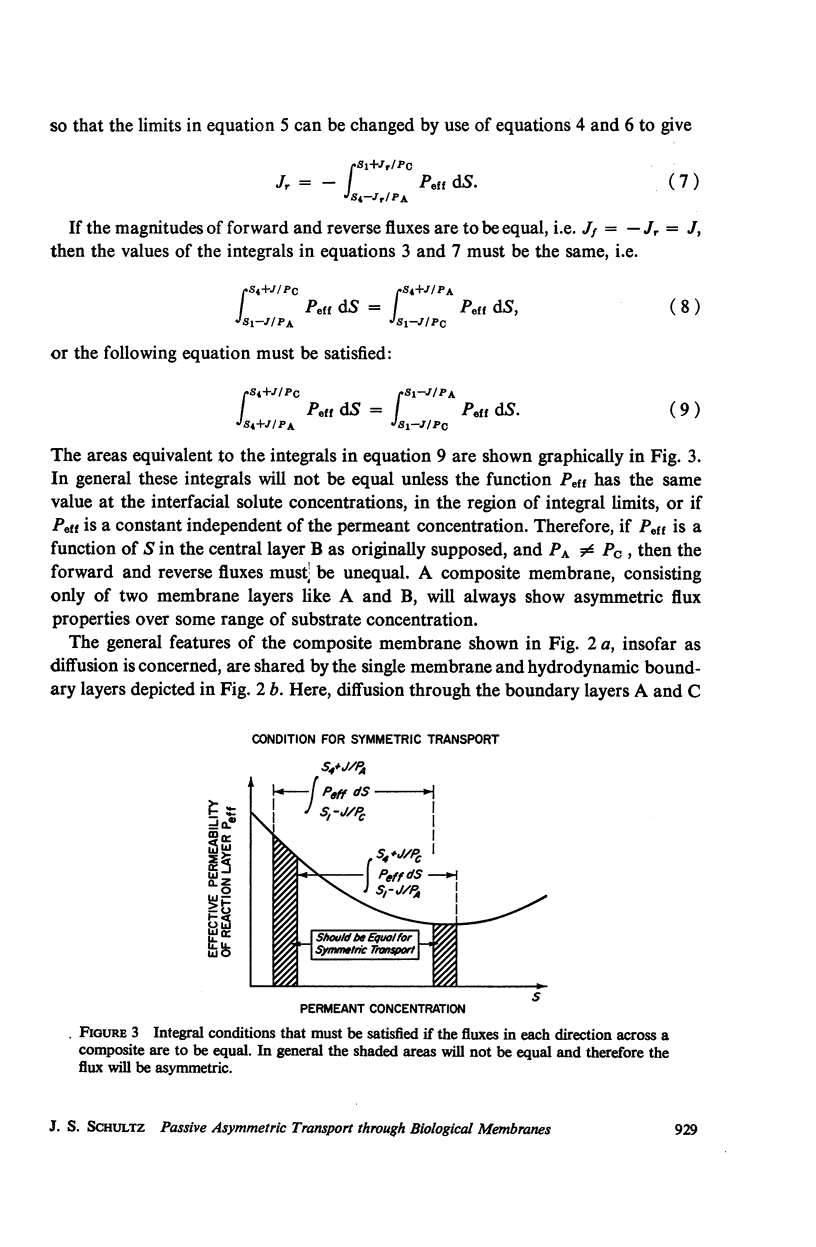
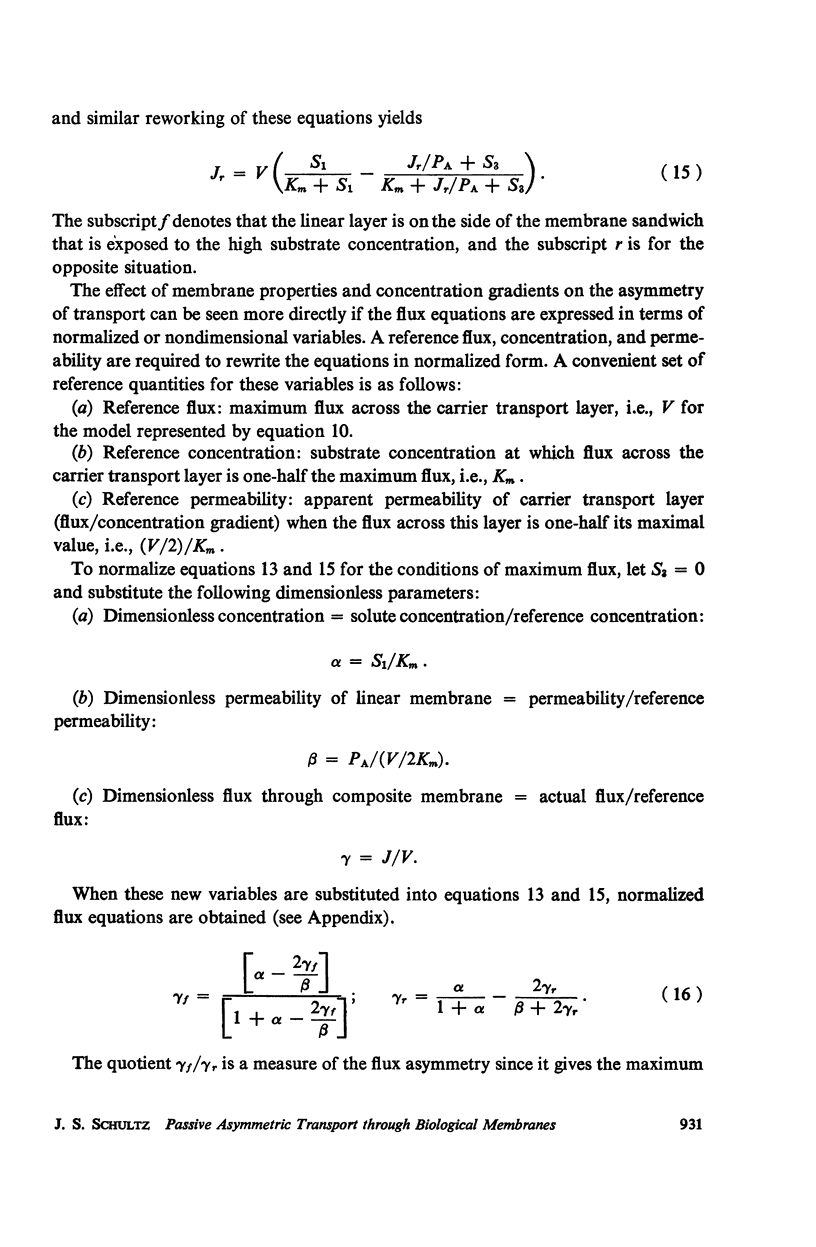
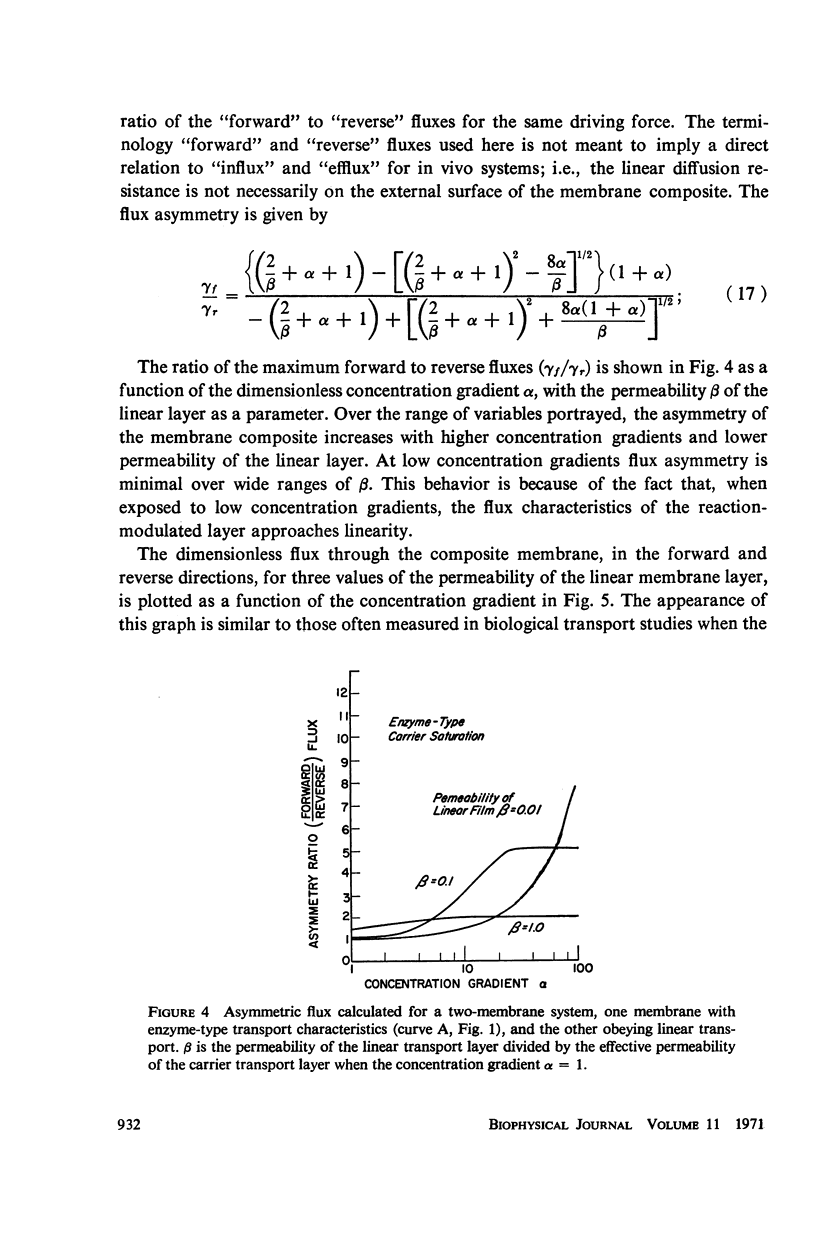
Abstract
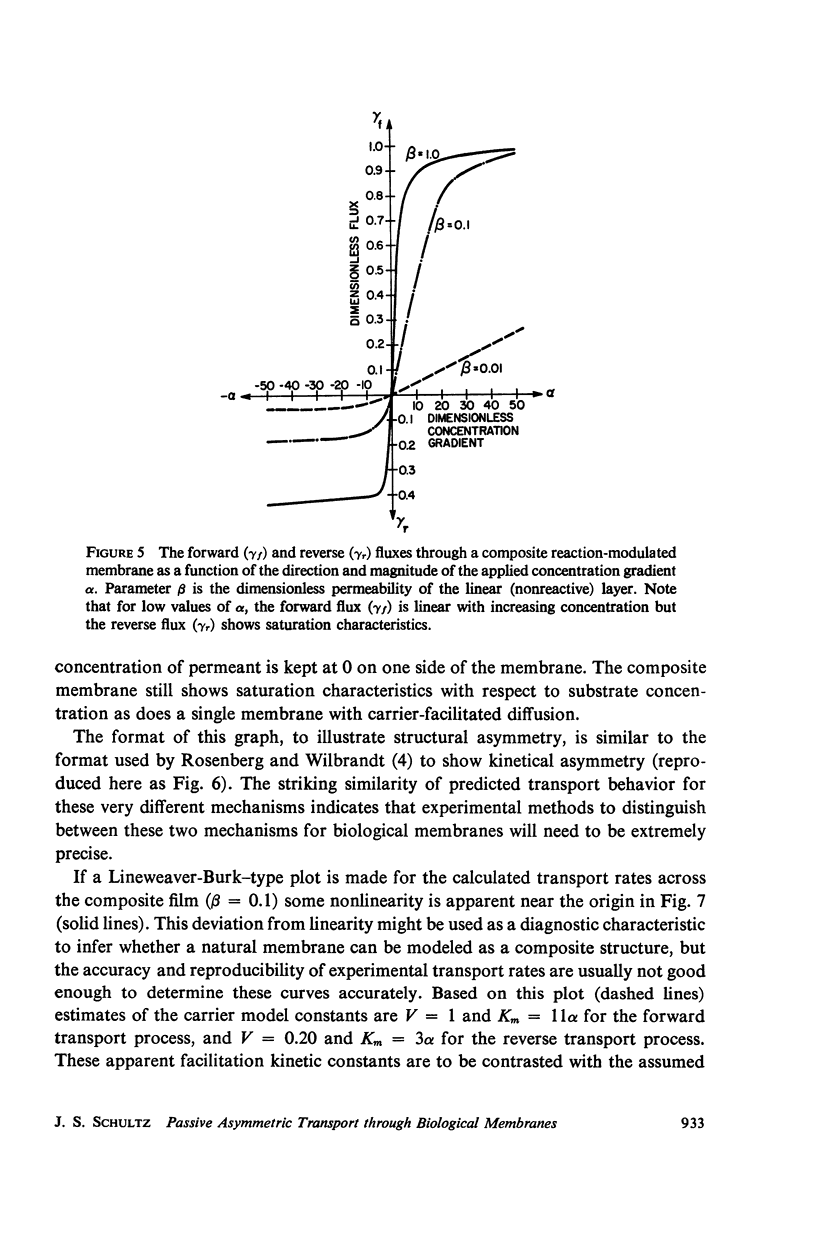
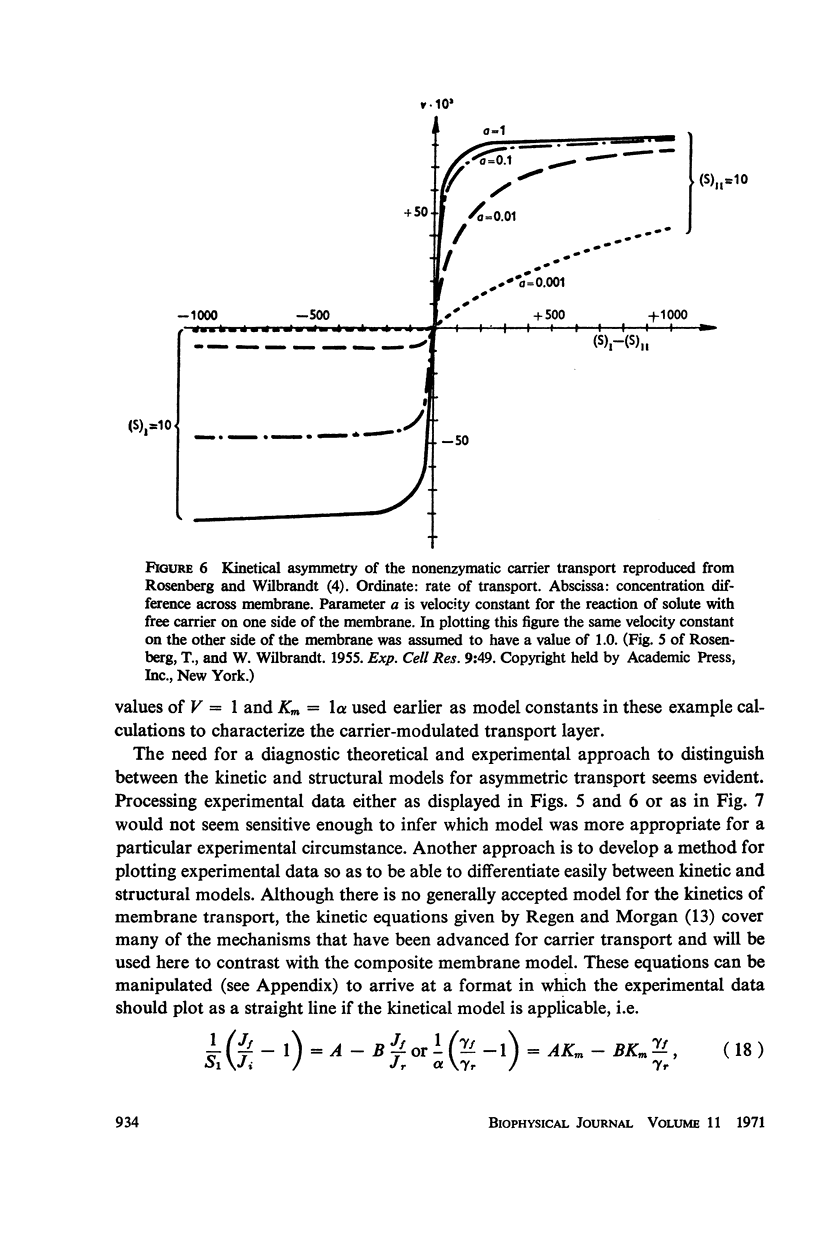
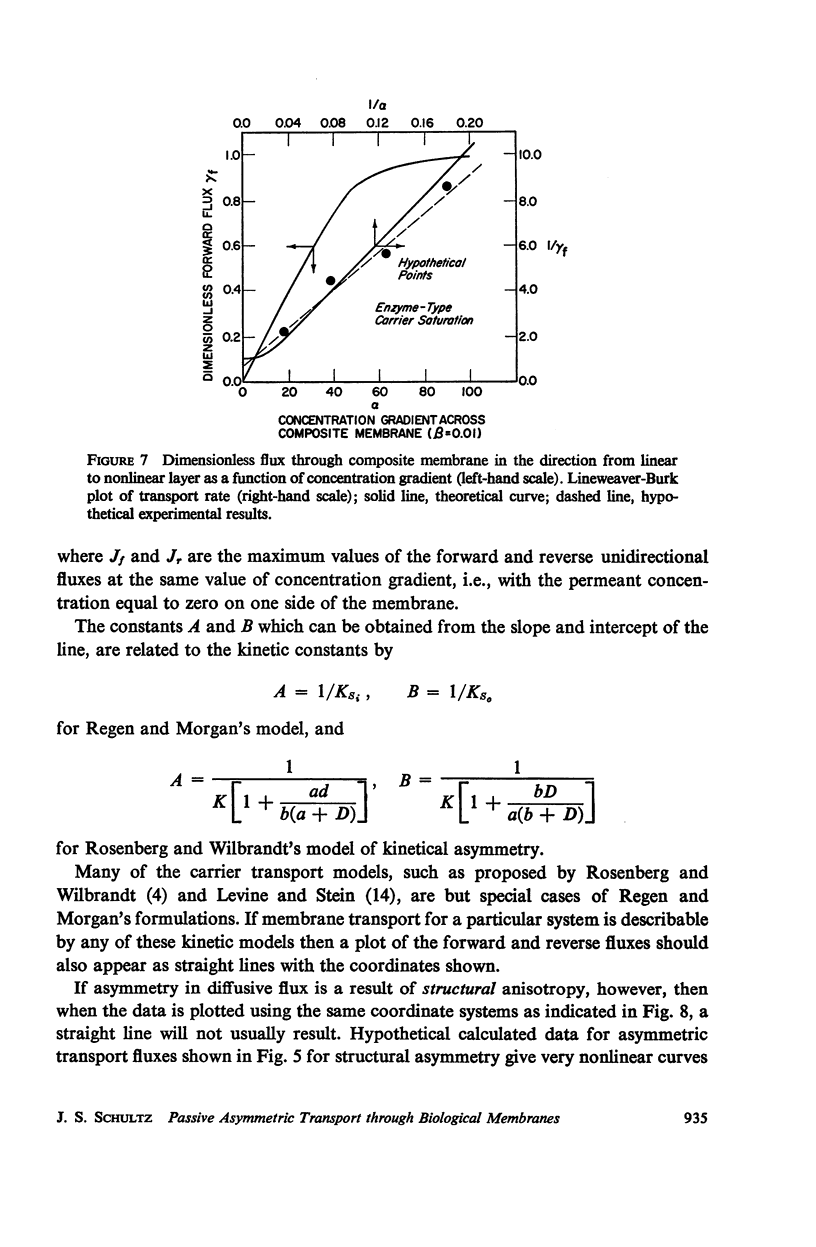
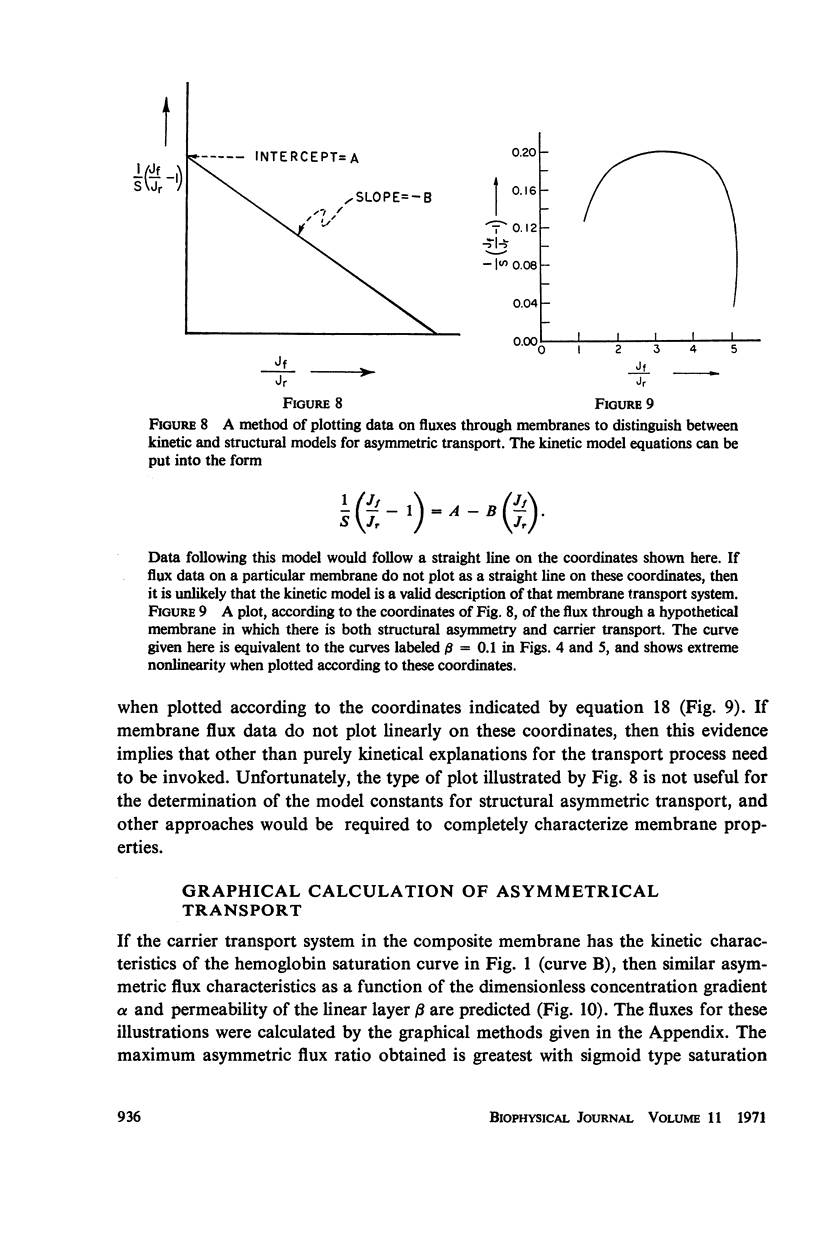
The magnitude of passive diffusional solute transfer through artificial membranes is usually considered to be independent of the direction of the concentration gradient driving force. It can be shown, however, that a composite membrane, having as one component a membrane with a chemical reaction-facilitated diffusion transport mechanism, can result in an asymmetrical flux. An asymmetric flux caused by this type of structural heterogeneity may be one mechanism contributing to the asymmetric properties of biological membranes. Similar vectorial fluxes can be generated in interfacial solute transfer through membranes if hydrodynamic boundary layers occur at the membrane interface and reversible chemical reactions with the permeant species are involved in either phase.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Koch A. L. Metabolic control through reflexive enzyme action. J Theor Biol. 1967 Apr;15(1):75–102. doi: 10.1016/0022-5193(67)90045-8. [DOI] [PubMed] [Google Scholar]
- Levine M., Stein W. D. The kinetic parameters of the monosaccharide transfer system of the human erythrocyte. Biochim Biophys Acta. 1966 Sep 26;127(1):179–193. doi: 10.1016/0304-4165(66)90488-0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein N. S., Leaf A. Evidence for a double series permeability barrier at the mucosal surface of the toad bladder. Ann N Y Acad Sci. 1966 Jul 14;137(2):556–565. doi: 10.1111/j.1749-6632.1966.tb50181.x. [DOI] [PubMed] [Google Scholar]
- Miller D. M. The kinetics of selective biological transport. 3. Erythrocyte-monosaccharide transport data. Biophys J. 1968 Nov;8(11):1329–1338. doi: 10.1016/s0006-3495(68)86559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REGEN D. M., MORGAN H. E. STUDIES OF THE GLUCOSE-TRANSPORT SYSTEM IN THE RABBIT ERYTHROCYTE. Biochim Biophys Acta. 1964 Jan 27;79:151–166. doi: 10.1016/0926-6577(64)90048-8. [DOI] [PubMed] [Google Scholar]
- ROSENBERG T., WILBRANDT W. The kinetics of membrane transports involving chemical reactions. Exp Cell Res. 1955 Aug;9(1):49–67. doi: 10.1016/0014-4827(55)90160-9. [DOI] [PubMed] [Google Scholar]
- SCHOLANDER P. F. TENSION GRADIENTS ACCOMPANYING ACCELERATED OXYGEN TRANSPORT IN A MEMBRANE. Science. 1965 Aug 20;149(3686):876–877. doi: 10.1126/science.149.3686.876. [DOI] [PubMed] [Google Scholar]
- Stannett V., Williams J. L., Gosnell A. B., Gervasi J. A. Membranes with anisotropic flow properties. J Polym Sci B. 1968 Mar;6(3):185–190. doi: 10.1002/pol.1968.110060306. [DOI] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]
- Wyman J. Facilitated diffusion and the possible role of myoglobin as a transport mechanism. J Biol Chem. 1966 Jan 10;241(1):115–121. [PubMed] [Google Scholar]
- de Bruijne A. W., van Steveninck J. Asymmetry of the yeast cell membrane with respect to influx and efflux of dimethylsulfoxide. Biochim Biophys Acta. 1970 Sep 15;211(3):555–564. doi: 10.1016/0005-2736(70)90261-0. [DOI] [PubMed] [Google Scholar]


