Abstract
Proteomics has emerged as an indispensable methodology for large-scale protein analysis in functional genomics. The Escherichia coli proteome has been extensively studied and is well defined in terms of biochemical, biological, and biotechnological data. Even before the entire E. coli proteome was fully elucidated, the largest available data set had been integrated to decipher regulatory circuits and metabolic pathways, providing valuable insights into global cellular physiology and the development of metabolic and cellular engineering strategies. With the recent advent of advanced proteomic technologies, the E. coli proteome has been used for the validation of new technologies and methodologies such as sample prefractionation, protein enrichment, two-dimensional gel electrophoresis, protein detection, mass spectrometry (MS), combinatorial assays with n-dimensional chromatographies and MS, and image analysis software. These important technologies will not only provide a great amount of additional information on the E. coli proteome but also synergistically contribute to other proteomic studies. Here, we review the past development and current status of E. coli proteome research in terms of its biological, biotechnological, and methodological significance and suggest future prospects.
INTRODUCTION
Escherichia coli, one of the best-characterized prokaryotes, has served as a model organism for countless biochemical, biological, and biotechnological studies. Since the completion of the E. coli genome-sequencing project (28), this organism has been characterized on the genome-wide scale in terms of its transcriptome, proteome, interactome, metabolome, and physiome by use of DNA microarray, two-dimensional (2-D) gel electrophoresis (2-DE) coupled with mass spectrometry (MS), liquid and gas chromatography coupled with MS, and bioinformatics (34, 176, 217, 226, 325). Recent advances in these functional genomics studies have facilitated understanding of global metabolic and regulatory alterations caused by genotypic and/or environmental changes. DNA microarray has proven to be a successful tool for monitoring whole-genome-wide expression profiles at the mRNA level (176). Similarly, proteomics can be employed to compare changes in the expression levels of many proteins under particular genetic and environmental conditions. Unlike transcriptomics, which focuses on gene expression, proteomics examines the levels of proteins and their changes in response to different genotypes and conditions. The studies on proteomes under well-defined conditions can provide a better understanding of complex biological processes and may allow inference of unknown protein functions. Most of all, proteomic approaches provide information about posttranslational modifications which cannot be obtained from mRNA expression profiles; these approaches have proven critical to our understanding of proper physiological protein function, translocation, and subcellular localization.
The most prominent developments within the field of proteomics to date are shown in Fig. 1. Although the first proteomic analyses were conducted 30 years ago, renewed interest in this field has been fueled by several recent advances, including the availability of public genome and protein databases, the development of database search engines capable of exploiting these databases, and the introduction of high-sensitivity, easy-to-use MS techniques. Other important recent advances include improved 2-DE, computer programs for analysis of the 2-D gel images, protocols for proteolytic digestion of proteins in excised gel pieces, and low-flow chromatography methods. Recently, in order to reduce complexity and detect low-abundance proteins, proteomics researchers have become increasingly aware of non-gel-based technologies combined with subcellular fractionation by n-dimensional chromatographies.
FIG. 1.
Major developments in the history of proteomics. Since the beginning of proteome studies in 1975, proteomics and the associated technologies have evolved dramatically, resulting in almost exponential increases in the number of resolved proteins and their identification and greatly enhancing our understanding of complex biological processes in a variety of organisms.
These advances in proteomics technologies led to the generation of unprecedentedly large amounts of proteome data, which are used in fundamental as well as applied research. Here, we review the technological and methodological advances in proteome research in terms of the E. coli proteome. Gel-based and non-gel-based approaches and predictive proteomics including 2-DE, MS, tandem mass spectrometry (MS/MS), and computational tools are reviewed. Applications of MS combined with pulldown methods to investigate the E. coli interactome are also reviewed. In addition, physiological responses to growth stage, temperature, pH, oxidative stress, and other environmental conditions revealed by proteome analysis are reviewed. Following the review on the applications of proteome studies in biotechnology, the future direction of proteomic studies is suggested. For those topics that are not covered in this paper, readers are recommended to refer to the following excellent review articles on E. coli: for phage or bacterial display, refer to reference 65; for protein microarray, refer to reference 21, and for information on the two-hybrid system, refer to reference 119.
PROGRESS IN E. COLI PROTEOMIC TECHNOLOGY
The exploration of the E. coli proteome can be divided roughly into three phases: (i) the gel-based approaches, (ii) the non-gel-based approaches, and (iii) predictive proteomics (bioinformatics tools). The gel-based and non-gel-based approaches are defined as being based on separation of complex protein mixtures in gel and non-gel matrices, respectively, whereas predictive proteomics cover functional proteomic studies performed by computational tools in silico. These approaches overlap in time, and their evolutions have resulted in an almost exponential increase in the number and quality of resolved protein spots over the past 30 years (287) as increasingly complex separations have been developed to continue forward progress. In recent years, the E. coli proteome has been used as a standard for evaluating and validating new technologies and methodologies such as sample prefractionation, protein enrichment, 2-DE, protein detection, MS, combinatorial assays with n-dimensional chromatography and MS, and image analysis (Table 1) . In comparison to the proteomes of other organisms, the E. coli proteome provides an excellent model for various research needs based on the following advantages (161): (i) the availability of public databases such as SWISS-PROT (http://www.expasy.ch/ch2d/) and NCBI (http://www.ncbi.nlm.nih.gov/), which contain rich information on the proteins and corresponding genes; (ii) the existence of the E. coli SWISS-2DPAGE maps, which are based on a great deal of biochemical and biological data; and (iii) the fact that the E. coli proteome is less complex than those of other organisms such as humans and plants, boasting smaller open reading frame (ORF) products and less protein modification. Furthermore, as summarized in Fig. 2, the basic processes and strategies for an E. coli proteomic analysis have been well defined and optimized.
TABLE 1.
Summary of proteomic technologies used to study E. colia
| Analytical technique | Comment | Reference(s) |
|---|---|---|
| 2-D DIGE, MALDI-TOF-MS | Fluorescence 2-D DIGE was used for more-accurate quantitative proteome analysis using cyanine dyes such as Cy3 and Cy5 | 289, 321 |
| 2-DE | Three different commercially available instruments for isoelectric focusing (the Multiphor, the IPGphor, and the Protean IEF cell) were compared in terms of their performances with the following result: Protean IEF cell > Multiphor > IPGphor | 45 |
| 2-DE, N- and C-terminal sequence tags | Short N- and C-terminal sequence tags of 4 and 5 amino acid residues were applied for the identification of proteins separated on 2-D gels; to utilize this specificity of sequence tags of up to 6 amino acid residues for protein identification, the protein identification program TagIdent (http://www.expasy.org/tools/tagident.html) was created for prokaryotes; the TagIdent tool allows (i) the generation of a list of proteins close to a given pI and MW, (ii) the identification of proteins by matching a short sequence tag of up to 6 amino acids against proteins in the SWISS-PROT/TrEMBL databases close to a given pI and MW, and (iii) the identification of proteins by their mass, if this mass has been determined by mass spectrometric techniques | 312 |
| 2-D LC (AIX LC-HIC LC), 2-DE, MALDI-TOF-MS | Whole-cell lysates are fractionated over two dimensions of native-state liquid chromatography (2-D LC): a strong AIX, followed by a second separation on a HIC; the fractions were then digested with trypsin and identified by MALDI-TOF-MS; the first-dimension fractions were analyzed by 2-DE to validate the assignments of proteins obtained from 2-D LC coupled with MALDI-TOF-MS | 39 |
| 2-D LC SCX (SCX-RPLC or SEC-RPLC), ESI-MS, MALDI-TOF-MS | 2-D LC system was used for the separation of protein mixtures: or SEC followed by RPLC; interesting fractions were analyzed by MALDI-TOF-MS or ESI-MS | 224, 225, 301, 302 |
| 2-DE, MALDI-TOF-MS | An isolation method of outer membrane proteins using sequential extraction with sodium carbonate was introduced | 201 |
| Anion exchange chromatography, 2-DE, MALDI-TOF-MS | Nondenaturing anion exchange column chromatography was used to explore functional associations between individual proteins and to enrich less abundant proteins; successive fractions were analyzed using 2-D gels followed by MALDI-TOF-MS | 35 |
| Capillary LC, ESI-MS | Capillary columns packed with octadecyl-modified nonporous silica particles were used to separate proteins and peptides generated from enzymatic digests of proteins; this method could detect as little as 250 fmol of protein or 500 fmol of peptide on-column | 184 |
| Chromatography (covalent and/or metal affinity), RPLC-MS coupled with the use of isotope labeling | Covalent chromatography and immobilized metal affinity chromatography column loaded with copper were used to select peptides containing cysteine and histidine residues from tryptic digests of cell lysates, respectively; these peptides were labeled with succinic and deuterated succinic anhydride for two different samples, subsequently fractionated by RPLC, and then identified by MS and quantified by differential isotope labeling; this method reduces the complexity of protein digests and greatly simplifies database searches | 303, 305 |
| CIEF-ESI-MS | CIEF can provide high-resolution separations of complex protein mixtures and recently has been used primarily with conventional UV detection; CIEF was combined with ESI-MS to analyze complex protein mixtures on the intact protein level | 188 |
| CIEF-FTICR-MS coupled with the use of isotope labeling | CIEF-FTICR-MS was used to enhance sensitivity and accuracy of molecular mass measurements; the use of isotope labeling provides accurate quantitative proteomic analysis; this approach provides more-comprehensive and -precise measurements of differences in protein expression | 126, 127, 189, 300 |
| COFRADIC, LC-MS/MS | COFRADIC was applied to select and identify methionine peptides in a tryptic peptide mixture; in this strategy, the methionine oxidation reaction is carried out between two consecutive RPLC runs; after sorting methionine-containing peptides, 800 E. coli proteins were identified by LC-MS/MS | 78 |
| Column chromatography, 2-DE, MALDI-TOF-MS | Six similar reactive dyes (RB-4, RB-10, RB-72, RG-19, RR-120, and RY-3) were applied as reactive dye resin columns of affinity chromatography for fractionation of cell extracts prior to 2-DE to identify low-abundance proteins; distinctive protein profiles were obtained for the bound proteins recovered from the different reactive dye compounds and identified using MALDI-TOF-MS | 24 |
| Hydroxyapatite chromatography, 2-DE, MALDI-TOF-MS | Hydroxyapatite chromatography was used to enrich low-abundance proteins; enriched pools were analyzed by 2-DE and MALDI-TOF-MS. | 69 |
| ICAT-MS | ICAT-MS was used for a large-scale investigation to determine the degree of reproducibility and depth of proteome coverage of a typical ICAT-MS expt; however, the method was strongly biased to detect acidic proteins (pI, <7) and underrepresented small proteins (<10 kDa) and failed to show clear superiority over 2-DE methods in monitoring hydrophobic proteins from cell lysates | 202 |
| IEF gels, MALDI-TOF-MS | The proteins separated by IEF gels were identified by MALDI-TOF-MS; MS is substituted for the size-based separation of 2-D gels, resulting in the creation of a virtual 2-D gel; this approach provides advantages in mass resolution and accuracy over classical 2-D gels and can be readily automated | 178 |
| Immobilized trypsin columns | Immobilized trypsin columns were used for the digestion of cellular extracts that contained thousands of proteins; trypsin columns can be easily incorporated into multidimensional separation systems for automated proteomics. | 304 |
| LC, capillary sample concn and trypsin reaction, MALDI-TOF MS | The combined method of LC fractionation, nanoliter protein concn/digestion, and microspot MALDI-TOF-MS was used for low-mass proteome analysis | 142 |
| LC, ESI-MS | In-line LC-ESI-MS was used for identifying hydrophobic membrane proteins | 159, 310 |
| LC, ESI-MS | LC combined with MS/MS was used to obtain a protein profile of an E. coli strain; membrane proteins were analyzed after enrichment of membrane proteins; different E. coli proteins (1,147) were identified and compared with the transcription profile obtained on Affymetrix GeneChips | 49 |
| LC, MALDI-TOF-MS or ESI-MS | LC combined with MALDI-TOF-MS or ESI-MS was used to detect low-mass proteins (mass range, 2-19 kDa) | 54, 59 |
| LC, MALDI-TOF-MS or MS/MS coupled with the use of isotope labeling | A global isotope labeling (global internal standard technology) was used for quantitative proteomics; tryptic peptides labeled with by differential isotopic labeling were fractionated by RPLC and analyzed by MALDI-TOF-MS or MS/MS | 36 |
| MCE, 2-DE | Complex proteomes were prefractionated by MCE with isoelectric membranes prior to 2-DE to increase the load ability, resolution, and detection sensitivity of 2-D maps | 108 |
| MicroSol IEF, 2-DE | Complex proteomes were prefractionated with MicroSol IEF prior to 2-DE; this method increases protein loads, resolution, and dynamic detection range compared with unfractionated samples | 333 |
| Multiplexed protein quantitation, 2-D LC (SCX-RPLC), MALDI-TOF-MS or MS/MS | The multiplex strategy using amine-specific isobaric tags (iTRAQ reagents) and simultaneously compares multiple exptl conditions for up to four samples in parallel; all trypsin-digested mixtures were labeled with four isotopically labeled tags and then were mixed and subsequently analyzed by using 2-D LC and MS or MS/MS | 2 |
| Off-gel purification, 2-DE | A free-flow protein purification technique based on isoelectric electrophoresis prior to 2-DE was introduced to enhance separation efficiency | 248 |
| Organic solvent extractions, 2-DE, MALDI-TOF-MS | Hydrophobic membrane proteins were extracted with a mixture of chloroform and methanol prior to 2-DE | 200 |
| TAP, MALDI-TOF-MS or MS/MS. | The TAP procedure for isolating protein complexes was used for site-specific recombination to introduce a dual tagging cassette into chromosomal loci; the tagged bait proteins were expressed at endogenous levels and purified by affinity chromatography and then identified by using MALDI-TOF-MS or MS/MS | 34, 93 |
| Two-layer matrix/sample prepn method, MALDI-TOF-MS | Two-layer matrix/sample prepn method based on the deposition of a mixture of sample and matrix on top of a thin layer of matrix crystals was introduced to analyze protein and peptide samples containing sodium dodecyl sulfate up to approx 1% | 328 |
RPLC, reverse-phase liquid chromatography; SCX, strong cation exchange; SEC, size exclusion liquid chromatography; MicroSol, microscale solution; AIX, anion exchange column; HIC, hydrophobic interaction resin.
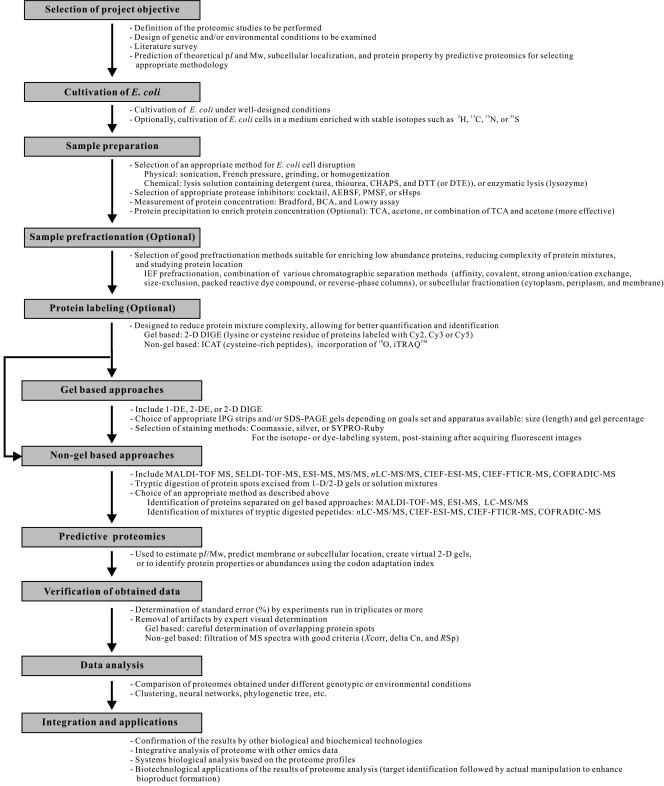
FIG. 2.
General steps for proteomic analysis and tips for success. Once the project objective is set, E. coli cells are cultured and sampled for proteome profiling. During this process, protein samples can be prefractionated or labeled differentially for better comparison of the results. Proteome profiles can be obtained by gel-based and/or non-gel-based approaches. Also, predictive proteomic studies can be performed to analyze a priori the characteristics of proteins in the proteome. Gel-based approaches and non-gel-based approaches are complementary and should be combined if possible to maximize the total number of proteins detected and identified. sHsps IbpA and IbpB were from E. coli and Hsp26 was from Saccharomyces cerevisiae (96). SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; AEBSF, aminoethyl benzylsufonyl fluoride or Pefabloc SC; BCA, bicinchoninic acid; delta Cn, correlation value (difference between the first hit and the second hit); DTE, dithioerythritol; DTT, dithiothreitol; iTRAQ, a multiplexed set of isobaric reagents that yield amine-derivatized peptides (iTRAQ reagents; Applied Biosystems, CA) (253); PMSF, phenylmethylsulfonyl fluoride; RSp, rank preliminary score; SELDI-TOF-MS, surface-enhanced laser desorption ionization-time of flight mass spectrometry; TCA, trichloroacetic acid; Xcorr, cross-correlation (measures how close the spectrum fits to the ideal spectrum).
Gel-Based Approaches
2-DE is currently the most widely used proteomic approach for analyzing the protein composition of cells, tissues, or biofluids and might even be called “classic” or “blue-collar” proteomics (316). 2-DE was first independently introduced by O'Farrell and Klose in 1975 (147, 220) and was first used for analyzing basic proteins (222). VanBogelen and colleagues (294) then pioneered the use of 2-DE for determining the protein composition of E. coli, and the technique has been intensively pursued by others since then (25, 83, 287). However, these initial studies of the E. coli proteome were limited by the fact that the complex protein mixtures were displayed only with respect to their positions on the 2-D gels and also by the lack of reproducibility among different laboratories. The later use of an immobilized pH gradient (IPG) gel instead of the carrier ampholyte method allowed researchers to apply 2-DE for easier and more-reproducible proteome analyses (25, 83). The current use of commercially available 18-cm IPG strips (pH, 3 to 10) along with high-sensitivity staining is generally able to resolve up to 1,000 to 1,500 protein spots in the case of the E. coli proteome (286). However, a large number of the protein spots are found in a 2-D gel of the E. coli proteome cluster at an isoelectric point of 4 to 7 and a molecular weight (MW) of 10 to 100 (294), representing a limitation of 2-D gel separation of unfractionated samples on IPG strips. Furthermore, despite the excellent sensitivity of MS, only the most abundant proteins from 2-D gels can be analyzed, leading to the exclusion of many low-abundance proteins.
One strategy for enhancing the capacities of 2-D gels involves parallel separation of replicate aliquots from unfractionated samples on a series of narrow-pH-range IPG gels (or zoom gels). The E. coli 3.5-10 SWISS-2DPAGE map shows 40% of the E. coli proteome (286), among which 231 proteins have been identified by techniques such as gel comparison, microsequencing, N-terminal sequencing, and amino acid composition analysis (Table 2) . In contrast, the use of narrow-range pH gradients (pH 4 to 5, 4.5 to 5.5, 5 to 6, 5.5 to 6.7, 6 to 9, and 6 to 11) was shown to potentially display proteins existing at low levels (up to a few protein molecules per cell), resulting in the discrimination of >70% of the entire E. coli proteome (Table 2; reference 287). The number of displayed proteins was higher than that identified by non-gel-based approaches, but not all of the proteins could be identified. The main benefit of using narrow-pH-range IPG strips is that the total number of protein spots per pH unit that can be separated increases due to higher spatial resolution. However, in practice this approach results in only a moderate increase in the number of proteins detected compared to that detected by use of a single broad-pH-range gel. Narrow-pH-range IPG gels show variable and unreliable separation of proteins, especially when unfractionated complex protein samples are analyzed, because proteins having pIs outside the pH range of the IPG strip usually cause massive precipitation and aggregation on the gel.
TABLE 2.
E. coli proteins identified on 2-D gelsa
| Protein nameb | Accession no.b | Descriptionb | pI/MW
|
Protein function and expressione | Reference(s) | ||
|---|---|---|---|---|---|---|---|
| Theoreticalc | Experimentald | ||||||
| AccA | P0ABD5 | Acetyl-CoA carboxylase carboxyl transferase subunit alpha | 5.76/35,110.35 | Acetyl-CoA carboxylase is a heterodecamer of four copies of biotin carboxyl carrier protein (AccB), two copies of biotin carboxylase (AccC), and two copies each of the two subunits of carboxyl transferase (AccA and AccD); AccC catalyzes the carboxylation of the carrier protein and then the transcarboxylase transfers the carboxyl group to form malonyl-CoA | 179; personal communication to J. E. Cronan | ||
| AccB | P0ABD8 | Biotin carboxyl carrier protein of acetyl-CoA carboxylase | 4.66/16,687.21 | 4.57/22,029 | See AccA; induced by high pH during anaerobic growth | 179, 286, 287, 323 | |
| 4.74/18,338 (4-5) | |||||||
| 5.01/18,459 (4-5) | |||||||
| 4.78/15,809 (4-5) | |||||||
| AccC | P24182 | Biotin carboxylase | 6.65/49,320.74 | See AccA | 179 | ||
| AccD | P0A9Q5 | Acetyl-CoA carboxylase carboxyl transferase subunit beta | 7.58/33,321.89 | See AccA | 179 | ||
| AceA | P0A9G6 | Isocitrate lyase | 5.16/47,521.57 | 5.19/44,130 (4.5-5.5) | Involved in glyoxylate bypass; activated by phosphorylation (on histidine) and inhibited by phosphoenolpyruvate (PEP), 3-phosphoglycerate, and succinate; induced by pH change; increases in the physiological short-term adaptation to glucose limitation | 27, 179, 287, 311 | |
| 5.12/44,130 (4.5-5.5) | |||||||
| 5.01/33,761 (4.5-5.5) | |||||||
| 4.99/33,624 (4.5-5.5) | |||||||
| 5.01/51,702 (5-6) | |||||||
| AceE | P0AFG8 | Pyruvate dehydrogenase E1 component | 5.46/99,537.29 | 5.40/99,289 | The pyruvate dehydrogenase complex contains multiple copies of three enzymatic components: pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2), and lipoamide dehydrogenase (E3); catalyzes the overall conversion of pyruvate to acetyl-CoA and CO2; increases during aerobic growth and after benzoic acid treatment | 179, 228, 270, 294, 321 | |
| 5.39/94,195 (DIGE 4.5-6.5) | |||||||
| AceF | P06959 | Dihydrolipoyllysine residue acetyltransferase component of pyruvate dehydrogenase complex | 5.09/65,964.87 | 5.01/77,450 | See AceE; increases during aerobic growth, during the low temp at 10°C, and after benzoic acid treatment | 133, 179, 228, 270, 294, 321 | |
| 5.09/78,105 (DIGE 4.5-6.5) | |||||||
| AckA | P0A6A3 | Acetate kinase | 5.85/43,290.45 | 5.84/44,001 | Involved in the activation of acetate to acetyl-CoA and the secretion of acetate; involved in the synthesis of most of the ATP formed catabolically during anaerobic growth; induced by low pH during anaerobic growth | 179, 228, 294, 287, 321, 323 | |
| 5.79/41,417 (DIGE 4.5-6.5) | |||||||
| 5.72/31,846 (5-6) | |||||||
| AcnA | P25516 | Aconitate hydratase 1 | 5.59/97,516.00 | Aconitate hydratases (AcnA and AcnB) serve as a protective buffer against the basal level of oxidative stress that accompanies aerobic growth by acting as a sink for reactive oxygen species and by modulating translation of the sodA transcript; AcnA enhances the stability of the sodA transcript, whereas AcnB lowers its stability; it is induced by iron and oxidative stress and may have an iron-responsive regulatory function | 151, 278 | ||
| AcnB | P36683 | Aconitate hydratase 2 | 5.24/93,498.11 | 5.30/95,000 (DIGE 4.5-6.5) | See AcnA | 151, 179, 278, 287, 321 | |
| 5.24/75,907 (4.5-5.5)
| |||||||
| AcpP | P0A6A8 | Acyl carrier protein | 3.98/8,508.33 | A carrier of the growing fatty acid chain in fatty acid biosynthesis; even though AcpP is an abundant protein (ca. 60,000 copies per cell), its small size (8.5 kDa) makes it difficult to detect by typical 2-DE; it can be detected after subcellular fractionation | 179 | ||
| AcrA | P0AE06 | Acriflavine resistance protein A | 6.08/39,723.72 | AcrAB is a drug efflux protein with broad substrate specificity | 179 | ||
| Add | P22333 | Adenosine deaminase | 5.36/36,397.46 | 179 | |||
| Ade (YicP) | P31441 | Adenine deaminase | 5.63/34,723.76 | 5.22/61,539 (DIGE 4.5-6.5) | Repressed by H-NS but activated by insertion of a variety of insertion elements into a region extending from −145 to +13 relative to the transcription start site; decreases after benzoic acid treatment | 321 | |
| AdhE | P0A9Q7 | Aldehyde-alcohol dehydrogenase | 6.33/95,996.05 | AdhE has three activities: alcohol dehydrogenase, acetaldehyde dehydrogenase, and PFL deactivase; PFL deactivase activity catalyzes the quenching of the pyruvate-formate-lyase catalyst in an iron-, NAD-, and CoA-dependent reaction; in aerobic conditions, it acts as a hydrogen peroxide scavenger; induced under anaerobic conditions in the absence of nitrate | 179 | ||
| Adk | P69441 | Adenylate kinase | 5.55/23,586.02 | 5.49/28,491 | Catalyzes the reversible transfer of the terminal phosphate group between ATP and AMP; essential for maintenance and cell growth; increases during phosphate limitation | 179, 228, 287, 293 | |
| 5.60/22,470 | |||||||
| 5.00/9,929 (4-5) | |||||||
| 4.99/9,991 (4.5-5.5) | |||||||
| 5.48/31,661 (5-6) | |||||||
| Agp | P19926 | Glucose-1-phosphatase | 5.38/43,560.36 | Absolutely required for the growth of E. coli in a high-phosphate medium containing glucose-1-phosphate as the sole carbon source; positively controlled by cAMP-CRP | 179 | ||
| AhpC | P0AE08 | Alkyl hydroperoxide reductase subunit C | 5.03/20,630.25 | 5.01/21,554 | Directly reduces organic hydroperoxides in its reduced dithiol form; may be directly degraded by ClpXP or modulated by a protease-dependent mechanism; induced by oxidative stress but repressed by sulfate or cysteine; increases during high-cell-density cultivation and acid or propionate condition during aerobic growth | 27, 179, 228, 266, 287, 294, 308, 321, 325 | |
| 5.09/23,123 (DIGE 4.5-6.5) | |||||||
| 5.08/21,705 (4.5-5.5) | |||||||
| 5.03/22,439 (4.5-5.5) | |||||||
| AhpF | P35340 | Alkyl hydroperoxide reductase subunit F | 5.47/56,177.11 | 5.44/53,485 (DIGE 4.5-6.5) | Serves to protect the cell against DNA damage by alkyl hydroperoxides; can use either NADH or NADPH as electron donor for direct reduction of redox dyes or of alkyl hydroperoxides when combined with the AhpC protein; induced by oxidative stress and after benzoic acid treatment | 179, 203, 266, 287, 321 | |
| 5.20/41,503 (5-6) | |||||||
| AlaS | P00957 | Alanyl-tRNA synthetase | 5.53/96,032.40 | 5.50/96,123 | 29, 179, 228, 294 | ||
| AldA | P25553 | Aldehyde dehydrogenase A | 5.07/52,141.60 | 5.11/52,363 (DIGE 4.5-6.5) | Acts on lactaldehyde as well as other aldehydes; induced by growth on fucose, rhamnose, arabinose, and amino acids such as glutamate; increases in the physiological short-term adaptation to glucose limitation | 179, 287, 308, 311, 321, 325 | |
| 5.29/42,547 (4.5-5.5) | |||||||
| 5.53/38,913 (4.5-5.5) | |||||||
| 5.43/50,034 (5-6) | |||||||
| Amn | P0AE12 | AMP nucleosidase | 5.90/53,994.91 | 6.38/47,693 | Involved in regulation of AMP concentrations; allosterically activated by Mg-ATP but inactivated by inorganic phosphate; induced by cAMP at limiting phosphate concentrations | 293 | |
| AmpC | P00811 | Beta-lactamase | 8.78/39,551.14 | 9.06/43,647 (6-11) | Serine beta-lactamase with substrate specificity for cephalosporins | 287 | |
| AnsA | P0A962 | l-Asparaginase I | 5.52/37,127.24 | AnsA converts l-asparagine into l-aspartate; there are two l-asparaginase isoenzymes: l-asparaginase I (AnsA), a low-affinity enzyme located in the cytoplasm, and l-asparaginase II (AnsB), a high-affinity secreted enzyme | 179 | ||
| AnsB | P00805 | l-Asparaginase II | 5.66/34,593.94 | See AnsA; induced by cAMP and anaerobiosis | 179 | ||
| AppA | P07102 | Periplasmic AppA protein | 6.11/44,689.86 | 5.42/46,694 | It is induced during entry into the stationary phase; its synthesis is triggered by carbon starvation, phosphate starvation, osmotic shift, or a shift from aerobic to anaerobic conditions; controlled by σS and AppY | 13, 228, 293 | |
| Apt | P69503 | Adenine phosphoribosyltransferase | 5.26/19,858.88 | 5.30/23,792 (DIGE 4.5-6.5) | Catalyzes a purine salvage reaction, resulting in the formation of AMP that is energically less costly than de novo synthesis | 179, 321 | |
| AraD | P08203 | l-Ribulose-5-phosphate 4-epimerase | 5.73/25,518.91 | 6.06/29,165 | Involved in l-arabinose catabolism | 294 | |
| AraF | P02924 | l-Arabinose-binding periplasmic protein | 5.61/33,210.05 | Involved in high-affinity l-arabinose membrane transport system; binds with high affinity to arabinose but can also bind d-galactose (approx 2-fold reduction) and d-fucose (approx 40-fold reduction) | 179 | ||
| ArcA | P0A9Q1 | Aerobic respiration control protein ArcA | 5.21/27,292.02 | 4.52/30,891 (DIGE 4.5-6.5) | ArcA/ArcB two-component regulatory system represses a wide variety of aerobic enzymes under anaerobic conditions, controls the resistance of E. coli to dyes, and may be involved in the osmoregulation of envelope proteins; when activated by arcB, it negatively regulates the expression of genes of aerobic function; it activates the transcription of the plfB operon by binding to its promoter | 125, 321 | |
| ArgD | P18335 | Acetylornithine/ succinyldiaminopimelate aminotransferase | 5.80/43,635.78 | 5.75/41,241 (DIGE 4.5-6.5) | Involved in both the arginine and lysine biosynthetic pathways | 287, 321 | |
| 5.19/33,559 (5-6) | |||||||
| ArgF | P06960 | Ornithine carbamoyltransferase chain F | 5.63/36,695.95 | 5.73/30,486 (5-6) | Ornithine carbamoyltransferase consists of ArgF and ArgI; involved in first step of arginine biosynthetic pathway | 287 | |
| 5.86/29,835 (5.5-6.7) | |||||||
| ArgG | P0A6E4 | Argininosuccinate synthase | 5.23/49,767.20 | 5.27/46,667 (DIGE 4.5-6.5) | Involved in seventh step of arginine biosynthetic pathway | 179, 287, 321 | |
| 5.28/46,209 (4.5-5.5) | |||||||
| 5.08/51,667 (5-6) | |||||||
| ArgI | P04391 | Ornithine carbamoyltransferase chain I | 5.46/36,775.92 | 5.43/39,776 | See ArgF; repressed during phosphate limitation | 228, 293 | |
| ArgS | P11875 | Arginyl-tRNA synthetase | 5.32/64,682.96 | 5.32/60,405 | Changes very little throughout the normal temp (23-37°C) and increases its level with increasing growth rate | 29, 109, 228, 230, 294 | |
| ArgT | P09551 | Lysine-arginine-ornithine-binding periplasmic protein | 5.22/25,784.99 | 5.15/26,055 (DIGE 4.5-6.5) | Involved in an arginine transport system; increases in the physiological short-term adaptation to glucose limitation; may be directly degraded by ClpAP and ClpXP, respectively, or be modulated by a protease-dependent mechanism | 179, 287, 308, 311, 321 | |
| 5.17/26,881 (4.5-5.5) | |||||||
| Theoreticalc | Experimentald | ||||||
| AroA | P0A6D3 | 3-Phosphoshikimate 1-carboxyvinyltransferase | 5.37/46,095.78 | 5.34/53,476 (5-6) | Involved in sixth step of chorismate biosynthesis from d-erythrose 4-phosphate and PEP | 287 | |
| 5.23/50,990 (5-6) | |||||||
| AroC | P12008 | Chorismate synthase | 5.82/39,006.25 | See AroA: seventh (final) step | 179 | ||
| AroD | P05194 | 3-Dehydroquinate dehydratase | 5.19/27,466.65 | 5.31/27,332 (4.5-5.5) | See AroA: third step | 179, 287 | |
| AroF | P00888 | Phospho-2-dehydro-3-deoxyheptonate aldolase, Tyr sensitive | 5.42/38,803.96 | 5.38/37,715 (DIGE 4.5-6.5) | Stereospecific condensation of PEP and d-erythrose-4-phosphate, giving rise to 3-deoxy-d-arabino-heptulosonate-7-phosphate (DAHP); involved in first step of chorismate biosynthesis from d-erythrose 4-phosphate and PEP | 321 | |
| AroG | P0AB91 | Phospho-2-dehydro-3-deoxyheptonate aldolase, Phe sensitive | 6.14/38,009.52 | 6.12/39,462 | See AroF: first step | 179, 286 | |
| 6.13/38,005 | |||||||
| AroK | P0A6D7 | Shikimate kinase I | 5.26/19,406.85 | 5.30/17,959 | Involved in fifth step of chorismate biosynthesis from d-erythrose 4-phosphate and PEP; induced by high pH | 179, 274, 286, 287, 321 | |
| 5.30/19,078 (DIGE 4.5-6.5) | |||||||
| 5.18/21,250 (5-6) | |||||||
| ArtI | P30859 | Arginine-binding periplasmic protein 1 | 5.32/25,042.20 | 5.24/26,625 | Involved in the arginine periplasmic transport system (artPIQMJ gene products); increases after benzoic acid treatment | 179, 286, 287, 321 | |
| 5.27/24,964 (DIGE 4.5-6.5) | |||||||
| 5.07/31,478 (5-6) | |||||||
| ArtJ | P30860 | Arginine-binding periplasmic protein 2 | 5.97/24,908.11 | 5.94/25,009 (DIGE 4.5-6.5) | See ArtI; decreases after benzoic acid treatment | 321 | |
| 5.59/24,831 (DIGE 4.5-6.5) | |||||||
| ArtP | P0AAF6 | Arginine transport ATP-binding protein ArtP | 6.17/27,022.05 | See ArtI; probably responsible for energy coupling to the transport system | 179 | ||
| Asd | P0A9Q9 | Aspartate-semialdehyde dehydrogenase | 5.37/40,017.88 | 5.20/50,339 (5-6) | Involved in amino acid biosynthetic pathway: Lys, Met, and Thr | 179, 287 | |
| AsnA | P00963 | Aspartate-ammonia ligase | 5.45/36,650.55 | 5.45/37,876 (DIGE 4.5-6.5) | Involved in asparagine biosynthesis; decreases after benzoic acid treatment | 179, 321 | |
| 5.38/37,715 (DIGE 4.5-6.5) | |||||||
| AsnB | P22106 | Asparagine synthetase B (glutamine hydrolyzing) | 5.55/62,527.82 | Involved in asparagine biosynthesis | 179 | ||
| AsnS | P0A8M0 | Asparaginyl-tRNA synthetase | 5.17/52,439.25 | 5.64/92,858 | 228, 236, 287, 294, 321 | ||
| 4.69/50,800 (DIGE 4.5-6.5) | |||||||
| 5.01/38,992 (4.5-5.5) | |||||||
| AspA | P0AC38 | Aspartate ammonia-lyase | 5.19/52,356.13 | 179 | |||
| AspC | P00509 | Aspartate aminotransferase | 5.54/43,573.36 | 5.40/41,960 | 179, 228, 236, 287, 294 | ||
| 5.52/41,877 | |||||||
| 5.53/50,441 (5-6) | |||||||
| 5.56/49,421 (5-6) | |||||||
| AspS | P21889 | Aspartyl-tRNA synthetase | 5.47/65,913.45 | 5.42/61,614 | Changes very little at normal temp range (23-37°C) | 29, 109, 228, 294 | |
| AtoA | P76459 | Acetate CoA-transferase beta subunit | 5.65/22,959.65 | 5.41/22,852 | Involved in short-chain fatty acid metabolism; induced during phosphate limitation and at phosphonate growth | 293 | |
| AtpA | P0ABB0 | ATP synthase alpha chain | 5.80/55,222.08 | 5.98/43,053 | F-type ATPases consist of the two complex components CF (0), the membrane proton channel, and CF (1), the catalytic core; CF (1) has five subunits: alpha (3), beta (3), gamma (1); delta (1), and epsilon (1); CF (0) has three main subunits: a, b, and c; produces ATP from ADP in the presence of a proton gradient across the membrane; increases in the physiological short-term adaptation to glucose limitation | 179, 228, 286, 287, 294, 311, 321 | |
| 5.84/53,108 | |||||||
| 5.82/52,204 (DIGE 4.5-6.5) | |||||||
| 5.15/39,861 (DIGE 4.5-6.5) | |||||||
| 5.81/36,727 (5-6) | |||||||
| 6.11/57,637 (6-11) | |||||||
| 6.02/30,657 (6-11) | |||||||
| AtpC | P0A6E6 | ATP synthase epsilon chain | 5.46/14,937.07 | 5.48/14,811 | See AtpA | 179, 228, 287, 294 | |
| 5.34/10,379 (4.5-5.5) | |||||||
| 5.45/13,773 (5-6) | |||||||
| Theoreticalc | Experimentald | ||||||
| AtpD | P0ABB4 | ATP synthase beta chain | 4.90/50,194.23 | 4.90/47,721 | See AtpA; decreases during phosphate limitation | 179, 228, 287, 293, 294, 321 | |
| 4.96/48,103 (DIGE 4.5-6.5) | |||||||
| 4.99/48,103 (DIGE 4.5-6.5) | |||||||
| 4.95/43,643 (4-5) | |||||||
| 4.95/47,782 (4-5) | |||||||
| 4.55/43,745 (4-5) | |||||||
| 5.00/45,494 (4.5-5.5) | |||||||
| 4.96/42,894 (4.5-5.5) | |||||||
| AtpF | P0ABA0 | ATP synthase B chain | 5.99/17,263.96 | 5.53/19,289 (DIGE 4.5-6.5) | See AtpA; decreases after benzoic acid treatment | 179, 321 | |
| 5.53/18,722 (DIGE 4.5-6.5) | |||||||
| AtpG | P0ABA6 | ATP synthase gamma chain | 8.84/31,577.42 | See AtpA | 179 | ||
| AtpH | P0ABA4 | ATP synthase delta chain | 4.94/19,332.22 | See AtpA | 179 | ||
| Bcp | P0AE52 | Putative peroxiredoxin Bcp | 5.03/17,633.94 | 5.02/15,872 | 179, 228, 286, 294, 321 | ||
| 4.92/16,060 | |||||||
| 5.10/17,526 (DIGE 4.5-6.5) | |||||||
| Bfr | P0ABD3 | Bacterioferritin | 4.69/18,495.03 | 5.34/16,350 | May perform functions in iron detoxification and storage analogous to those of animal ferritins | 294 | |
| BglX | P33363 | Periplasmic beta-glucosidase | 5.77/81,406.5 | 179 | |||
| BioB | P12996 | Biotin synthase | 5.32/38,648.09 | Involved in final step of biotin biosynthesis: biotin from 6-carboxyhexanoyl-CoA | 179 | ||
| BioD | P13000 | Dethiobiotin synthetase | 5.56/24,008.4 | See BioB: third step | 179 | ||
| BtuB | Q93SE0 | Vitamin B12 transporter BtuB | 5.1/66,325.63 | Involved in the active translocation of vitamin B12 (cyanocobalamin) across the outer membrane to the periplasmic space; derives its energy for transport by interacting with the transperiplasmic membrane protein TonB | 179 | ||
| BtuE | P06610 | Vitamin B12 transport periplasmic protein BtuE | 4.81/20,469.56 | May be an auxiliary component of the transport system | 179 | ||
| CarA | P0A6F1 | Carbamoyl-phosphate synthase small chain | 5.91/41,431.01 | 5.91/44,088 | Involved in amino acid biosynthesis, l-arginine biosynthesis, and synthesis of carbamoyl phosphate from HCO3−; decreases during phosphate limitation early | 179, 228, 286, 293, 294, 321 | |
| 5.94/43,827 | |||||||
| 5.88/42,128 (DIGE 4.5-6.5) | |||||||
| 5.82/42,128 (DIGE 4.5-6.5) | |||||||
| 5.79/41,417 (DIGE 4.5-6.5) | |||||||
| CarB | P00968 | Carbamoyl-phosphate synthase large chain | 5.22/117,710.53 | 5.49/127,208 | See CarA; decreases significantly during phosphate limitation | 293 | |
| Cdd | P0ABF6 | Cytidine deaminase | 5.42/31,539.87 | 5.36/32,716 (DIGE 4.5-6.5) | Scavenges exogenous and endogenous cytidine and 2′-deoxycytidine for UMP synthesis; increases during high-cell-density cultivation and overexpression of recombinant protein in large scale | 97, 321, 325 | |
| CheA | P07363 | Chemotaxis protein | 4.78/71,382.39 | Involved in the transmission of sensory signals from the chemoreceptors to the flagellar motors; autophosphorylated; can transfer its phosphate group to either CheB or CheY; a histidine kinase | 179 | ||
| CheY | P0AE67 | Chemotaxis protein | 4.89/13,966.17 | 4.95/9,698 (4-5) | See CheA; the active CheY (phosphorylated or acetylated form) exhibits enhanced binding to a switch component, FliM, at the flagellar motor, which induces a change from counterclockwise to clockwise flagellar rotation. | 179, 287 | |
| CheZ | P0A9H9 | Chemotaxis protein | 4.44/23,976.03 | 4.51/28,668 | Involved in accelerating the dephosphorylation of phosphorylated CheY; involved in generating a regulating signal for bacterial flagellar rotation; increases at phosphonate growth | 228, 293, 294 | |
| CirA | P17315 | Colicin I receptor | 5.03/71,149.34 | 5.06/73,587 (DIGE 4.5-6.5) | Participates in iron transport; induced by iron and cAMP and increases after benzoic acid treatment | 89, 179, 321 | |
| ClpB | P63284 | Chaperone | 5.37/95,585.02 | 5.30/88,421 | Part of a stress-induced multichaperone system; involved in the recovery of the cell from heat-induced damage in cooperation with DnaK, DnaJ, and GrpE; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and after benzoic acid treatment; induced by heat shock, phosphate limitation, phosphonate growth, and other environmental stresses; controlled by RpoH | 71, 179, 228, 249, 287, 293, 294, 321 | |
| 5.38/73,900 | |||||||
| 5.33/88,745 (DIGE 4.5-6.5) | |||||||
| 5.44/34,853 (5-6) | |||||||
| ClpP | P0A6G7 | ATP-dependent Clp protease proteolytic subunit | 5.52/23,186.65 | 5.60/24,224 (5-6) | Cleaves peptides in various proteins in a process that requires ATP hydrolysis; has a chymotrypsin-like activity; plays a major role in the degradation of misfolded proteins; may play the role of a master protease which is attracted to different substrates by different specificity factors such as ClpA or ClpX; induced by heat shock; controlled by RpoH | 179, 249, 287 | |
| ClpS | P0A8Q6 | ATP-dependent Clp protease adaptor protein | 4.94/12,179.06 | 5.40/10,645 (4.5-5.5) | Involved in the modulation of the specificity of the ClpAP-mediated ATP-dependent protein degradation | 287 | |
| ClpX | P0A6H1 | ATP-dependent Clp protease ATP-binding subunit | 5.24/46,224.82 | ATP-dependent specificity component of the Clp protease; directs the protease to specific substrates; may bind to the lambda O substrate protein and present it to the ClpP protease in a form that can be recognized and readily hydrolyzed by ClpP; can perform chaperone functions in the absence of ClpP; induced by heat shock | 179 | ||
| Cmk | P0A6I0 | Cytidylate kinase | 5.56/24,746.34 | 179 | |||
| CoaBC | P0ABQ0 | CoA biosynthesis bifunctional protein | 7.04/43,306.95 | CoaBC catalyzes two steps in the biosynthesis of CoA; in the first step, cysteine is conjugated to 4′-phosphopantothenate to form 4-phosphopantothenoylcysteine; in the second, compound is decarboxylated to form 4′-phosphopantotheine | 179 | ||
| CoaE (YacE) | P0A6I9 | Dephospho-CoA kinase | 5.77/22,621.71 | Involved in CoA biosynthesis; catalyzes the phosphorylation of the 3′-hydroxyl group of dephospho-CoA to form CoA | 179 | ||
| CpdB | P08331 | 2′,3′-Cyclic-nucleotide 2′-phosphodiesterase | 5.38/68,914.86 | Catalyzes two consecutive reactions converting 2′,3′-cyclic nucleotide to 3′-nucleotide and then 3′-nucleotide to nucleic acid and phosphate | 179 | ||
| Crr | P69783 | Glucose-specific phosphotransferase enzyme IIA component | 4.73/18,119.88 | 4.68/18,985 | The phosphoenolpyruvate-dependent sugar phosphotransferase system (PTS), a major carbohydrate active-transport system, catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane; involved in glucose transport; plays an important role not only in the transcriptional control but also in the translational control of rpoS expression | 179, 228, 286, 287, 294 | |
| 4.57/20,069 | |||||||
| 4.95/14,761 (4-5) | |||||||
| 4.86/14,800 (4-5) | |||||||
| 4.95/13,865 (4-5) | |||||||
| 4.95/18,479 (4.5-5.5) | |||||||
| CspA | P0A9X9 | Cold shock protein | 5.57/7,272.09 | Induced by low temp; cold shock protein | 133, 163 | ||
| CspB | P36995 | Cold shock-like protein | 6.54/7,716.72 | Induced by low temp; cold shock protein | 163 | ||
| CspC | P0A9Y6 | Cold shock-like protein | 6.82/7,271.17 | 6.71/10,366 | Induced during a stationary phase and starvation | 54, 163, 228, 293, 294 | |
| CspD | P0A968 | Cold shock-like protein | 5.81/7,968.97 | Inhibits DNA replication at both initiation and elongation steps, most probably by binding to the opened, single-stranded regions at replication forks; plays a regulatory role in chromosomal replication in nutrient-depleted cells; induced by stationary phase and starvation | 163, 320 | ||
| CspE | P0A972 | Cold shock-like protein | 8.06/7,332.26 | 179 | |||
| CspG | P0A978 | Cold shock-like protein | 5.64/7,780.73 | Induced by low temp; cold shock protein | 207 | ||
| CysK | P0ABK5 | Cysteine synthase A | 5.83/34,358.46 | 5.81/36,027 | Involved in cysteine biosynthesis; repressed by sulfate or cysteine (protein induced by sulfate starvation); induced by high pH | 179, 228, 239, 274, 287, 294, 321 | |
| 5.83/34,342 (DIGE 4.5-6.5) | |||||||
| 4.95/24,032 (4-5) | |||||||
| 5.01/26,270 (4-5) | |||||||
| 4.95/25,586 (4-5) | |||||||
| 4.93/22,204 (4-5) | |||||||
| 5.06/29,410 (4.5-5.5) | |||||||
| 5.32/32,815 (4.5-5.5) | |||||||
| 5.04/28,256 (4.5-5.5) | |||||||
| 4.95/28,122 (4.5-5.5) | |||||||
| 5.78/42,978 (5-6) | |||||||
| 5.10/41,867 (5-6) | |||||||
| CysM | P16703 | Cysteine synthase B | 5.42/32,664.16 | 5.47/31,383 (4.5-5.5) | Like a CysK, catalyzes the same reaction of cysteine biosynthesis; can also use thiosulfate in place of sulfide to give cysteine thiosulfonate as a product | 287 | |
| CysP | P16700 | Thiosulfate-binding protein | 6.43/35,057.44 | 6.58/39,151 (6-11) | Part of the ABC transporter complex cysAWTP, involved in sulfate/thiosulfate import; specifically binds thiosulfate and is involved in its transmembrane transport | 287 | |
| CysQ | P22255 | CysQ protein | 5.59/27,175.86 | Could help control the pool of 3′-phosphoadenoside-5′-phosphosulfate or its use in sulfite synthesis | 179 | ||
| DacA | P0AEB2 | Penicillin-binding protein 5 | 6.68/41,337.2 | Removes C-terminal d-alanyl residues from sugar-peptide cell wall precursors | 179 | ||
| DacB | P24228 | Penicillin-binding protein 4 | 8.73/49,568.63 | Not involved in transpeptidation but in peptidoglycan synthesis | 179 | ||
| DadA | P0A6J5 | d-Amino acid dehydrogenase small subunit | 6.17/47,607.29 | Involved in oxidative deamination of d-amino acids such as alanine catabolism | 179 | ||
| DapA | P0A6L2 | Dihydrodipicolinate synthase | 5.98/31,269.97 | 6.10/32,361 | Involved in l-lysine biosynthesis via diaminopimelate pathway: tetrahydrodipicolinate from l-aspartate (third step); sensitive to lysine inhibition | 179, 228, 286, 294 | |
| 5.94/32,916 | |||||||
| DapB | P04036 | Dihydrodipicolinate reductase | 5.45/28,756.61 | 5.37/27,969 (DIGE 4.5-6.5) | See DapA: fourth step; its activity is repressed by lysine; it decreases after benzoic acid treatment | 287, 321 | |
| 5.11/37,922 (5-6) | |||||||
| 5.33/31,023 (5-6) | |||||||
| DapD | P0A9D8 | 2,3,4,5-Tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase | 5.56/29,892.10 | 5.51/31,680 | Involved in l-lysine biosynthesis via diaminopimelate pathway: dl-diaminopimelate from ll-diaminopimelate | 179, 228, 294, 321 | |
| 5.49/32,476 (DIGE 4.5-6.5) | |||||||
| DapF | P0A6K1 | Diaminopimelate epimerase | 5.86/30,208.56 | See DapD | 179 | ||
| DcrB | P37620 | Protein | 4.76/17,786.96 | 4.50/18,143 (DIGE 4.5-6.5) | Required for phage C1 adsorption; increases after benzoic acid treatment | 179, 321 | |
| DdlA | P0A6J8 | d-Alanine-d-alanine ligase A | 5.02/39,315.81 | Required for cell wall formation | 179 | ||
| DeaD | P0A9P6 | Cold shock DEAD-box protein A | 8.76/70,414.96 | 5.41/37,079 (DIGE 4.5-6.5) | Reassigned CsdA; plays a key role in optimal cell growth at low temp and required for normal cell division; exclusively localized in the ribosomal fraction and becomes a major ribosomal-associated protein in cells grown at low temp; decreases after benzoic acid treatment but induced by cold shock or acid condition | 132, 274, 321 | |
| DegP | P0C0V0 | Protease Do | 7.87/46,829.14 | 8.01/66,440 (6-11) | Serine protease; required at high temp and involved in the degradation of damaged proteins; induced by heat shock | 179, 287 | |
| DegQ | P39099 | Protease | 5.39/44,445.75 | 5.34/46,509 | Protease with a shared specificity with DegP; induced by high pH during anaerobic growth | 179, 286, 323 | |
| DeoB | P0A6K6 | Phosphopentomutase | 5.11/44,369.96 | Phosphotransfer between the C1 and C5 carbon atoms of pentose | 179 | ||
| DeoC | P0A6L0 | Deoxyribose-phosphate aldolase | 5.50/27,733.80 | 5.52/33,657 (5-6) | Involved in nucleotide and deoxyribonucleotide catabolism; increases during high-cell-density cultivation | 70, 287, 325 | |
| DeoD | P0ABP8 | Purine nucleoside phosphorylase | 5.42/25,818.72 | 5.40/25,098 (DIGE 4.5-6.5) | Cleaves guanosine or inosine to respective bases and sugar-1-phosphate molecules; decreases after benzoic acid treatment | 321 | |
| DhaK (YcgT) | P76015 | PTS-dependent dihydroxyacetone kinase, dihydroxyacetone-binding subunit | 4.93/39,494.75 | Involved in glycerol utilization; dihydroxyacetone-binding subunit of the dihydroxyacetone kinase, which is responsible for phosphorylating dihydroxyacetone; binds covalently dihydroxyacetone in hemiaminal linkage; acts also as a corepressor of DhaR by binding to its sensor domain in the absence of dihydroxyacetone; induced by high pH during anaerobic growth | 323 | ||
| DhaL (YcgS) | P76014 | PTS-dependent dihydroxyacetone kinase, ADP-binding subunit | 5.31/22,631.75 | Involved in glycerol utilization; ADP-binding subunit of the dihydroxyacetone kinase, which is responsible for phosphorylating dihydroxyacetone; DhaL-ADP receives a phosphoryl group from DhaM and transmits it to dihydroxyacetone; DhaL-ADP acts also as a coactivator by binding to the sensor domain of dhaR; DhaL-ATP is inactive; induced by high pH during anaerobic growth | 323 | ||
| DhaM (YcgC) | P37349 | PTS-dependent dihydroxyacetone kinase, phosphotransferase subunit | 4.61/51,579.81 | 4.69/50,800 (DIGE 4.5-6.5) | See DhaL; serves as the phosphoryl donor; induced by high pH during anaerobic growth | 179, 321, 323 | |
| DksA | P0ABS1 | DnaK suppressor protein | 5.06/17,527.75 | 4.90/18,723 | Dosage-dependent suppressor of a dnaK deletion mutation; suppressed not only the temp-sensitive growth but also the filamentous phenotype of the dnaK deletion strain, while the defect of lambda growth is not suppressed; induced by high pH | 179, 274, 286, 321 | |
| 5.01/17,853 | |||||||
| 5.08/19,656 (DIGE 4.5-6.5) | |||||||
| DnaB | P0ACB0 | Replicative DNA helicase | 4.95/52,390.08 | 4.94/49,355 | Participates in initiation and elongation during chromosome replication; exhibits DNA-dependent ATPase activity and contains distinct active sites for ATP binding, DNA binding, and interaction with DnaC protein, primase, and other prepriming proteins | 179, 228, 294 | |
| DnaJ | P08622 | Chaperone protein | 8.03/40,969.14 | Interacts with DnaK and GrpE to disassemble a protein complex at the origins of replication of phage lambda and several plasmids; participates actively in the response to hyperosmotic and heat shock by preventing the aggregation of stress-denatured proteins and by disaggregating proteins, also in an autonomous, DnaK-independent fashion; induced by heat shock under the control of the htpR regulatory protein | 179 | ||
| DnaK | P0A6Y8 | Chaperone protein | 4.83/68,983.76 | 4.81/69,647 | Involved in chromosomal DNA replication, possibly through an analogous interaction with the DnaA protein, and participates actively in the response to hyperosmotic shock; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and after benzoic acid treatment; induced by temp, ethanol, nalidixic acid, puromycin, and overexpression of bioproducts | 71, 97, 138, 179, 212, 228, 287, 294, 297, 321 | |
| 4.92/74,532 (DIGE 4.5-6.5) | |||||||
| 4.94/73,587 (DIGE 4.5-6.5) | |||||||
| 4.91/74,850 (DIGE 4.5-6.5) | |||||||
| 4.95/60,559 (4-5) | |||||||
| 4.98/67,563 (4-5) | |||||||
| 4.98/66,075 (4-5) | |||||||
| 4.96/61,267 (4-5) | |||||||
| 4.97/61,125 (4-5) | |||||||
| 4.95/60,700 (4-5) | |||||||
| 4.95/60,700 (4-5) | |||||||
| 4.48/59,582 (4-5) | |||||||
| 5.00/44,877 (4-5) | |||||||
| 4.93/52,435 (4-5) | |||||||
| 4.96/60,841 (4-5) | |||||||
| 4.96/60,234 (4.5-5.5) | |||||||
| 4.97/59,766 (4.5-5.5) | |||||||
| 4.95/53,032 (4.5-5.5) | |||||||
| DnaN | P0A988 | DNA polymerase III beta subunit | 5.25/40,586.60 | 4.97/41,400 | DNA polymerase III is a complex, multichain enzyme responsible for most of the replicative synthesis in bacteria; exhibits 3′-to-5′ exonuclease activity; required for initiation of replication once it is clamped onto DNA | 294 | |
| DnaQ | P03007 | DNA polymerase III epsilon subunit | 5.54/27,098.93 | 5.65/26,832 | See DnaN; contains the editing function and is a proofreading 3′-to-5′ exonuclease | 228, 294 | |
| DppA | P23847 | Periplasmic dipeptide transport protein | 5.75/57,407.06 | 5.47/52,481 | Dipeptide-binding protein of a transport system that can be subject to osmotic shock and is also required for peptide chemotaxis; increases in the physiological short-term adaptation to glucose limitation | 179, 286, 287, 311 | |
| 5.69/52,170 | |||||||
| 5.71/52,000 (5-6) | |||||||
| 5.71/50,118 (5-6) | |||||||
| 5.81/52,000 (5.5-6.7) | |||||||
| Dps | P0ABT2 | DNA protection during starvation protein | 5.72/18,564.11 | 5.53/18,722 (DIGE 4.5-6.5) | Unspecifically binds and protects DNA from oxidative damage mediated by hydrogen peroxide; induced by prolonged starvation, stationary phase, and acetic acid accumulation; controlled by ClpXP and ClpAP proteases, which affect log-phase stability and stationary-phase synthesis of Dps, respectively | 6, 99, 179, 276, 287, 308, 321 | |
| 5.78/17,897 (5-6) | |||||||
| 5.27/16,787 (5-6) | |||||||
| 5.21/16,164 (5-6) | |||||||
| 5.94/17,273 (5.5-6.7) | |||||||
| DsbA | P0AEG4 | Thiol:disulfide interchange protein | 5.42/21,132.07 | 5.34/22,426 | Transfers its disulfide bond to other proteins and is reduced in the process and then reoxidized by DsbB; induced by high pH during aerobic or anaerobic growth | 179, 228, 274, 294, 321, 323 | |
| 5.31/21,942 | |||||||
| 5.34/22,606 (DIGE 4.5-6.5) | |||||||
| DsbC | P0AEG6 | Thiol:disulfide interchange protein | 5.86/23,460 | See DsbA; required for disulfide bond formation in some periplasmic proteins | 179 | ||
| Dut | P06968 | Deoxyuridine 5′-triphosphate nucleotidohydrolase | 5.03/16,155.47 | 5.03/16,145 | Involved in de novo synthesis of thymidylate; produces dUMP, the immediate precursor of thymidine nucleotides, and decreases the intracellular concn of dUTP so that uracil cannot be incorporated into DNA | 179, 228, 294, 321 | |
| 5.10/17,526 (DIGE 4.5-6.5) | |||||||
| Eco | P23827 | Ecotin | 5.94/16,099.52 | General inhibitor of pancreatic serine proteases; inhibits chymotrypsin, trypsin, elastases, factor X, and kallikrein as well as a variety of other proteases | 179 | ||
| Eda | P0A955 | 2-Keto-4-hydroxyglutarate/2-keto-3-deoxy-6-phosphogluconate aldolase | 5.57/22,284.03 | 4.93/23,247 (DIGE 4.5-6.5) | Key enzyme in the Entner-Doudoroff pathway and participates in the regulation of the intracellular level of glyoxylate; induced at the stationary phase and in the presence of gluconate or hexuronic acids; increases after benzoic acid treatment, phosphate limitation, and phosphonate growth | 99, 179, 287, 293, 321 | |
| 5.43/27,937 (5-6) | |||||||
| Efp | P0A6N4 | Elongation factor P | 4.9/20,460.12 | Involved in peptide bond synthesis; stimulates efficient translation and peptide bond synthesis on native or reconstituted 70S ribosomes in vitro; probably functions acceptors for peptidyl transferase | 179 | ||
| ElbB (YhbL) | P0ABU5 | Enhancing lycopene biosynthesis protein 2 | 4.68/22,981.55 | 4.92/25,756 (4-5) | May be involved in the early steps of isoprenoid biosynthesis | 179, 287 | |
| EngD | P0ABU2 | GTP-dependent nucleic acid-binding protein | 4.87/39,536.13 | 5.03/42,217 (DIGE 4.5-6.5) | GTP-dependent nucleic acid-binding protein which may act as a translation factor | 287, 321 | |
| 5.02/42,547 (4.5-5.5) | |||||||
| Eno | P0A6P9 | Enolase | 5.32/45,523.75 | 5.34/46,509 | Involved in the pathway of glycolysis; also involved in the RNA degradosome, a multienzyme complex important in RNA processing and mRNA degradation; increases during anaerobic growth | 29, 179, 228, 271, 286, 287, 294, 321 | |
| 5.29/46,234 | |||||||
| 5.35/43,401 (DIGE 4.5-6.5) | |||||||
| 4.74/22,872 (4-5) | |||||||
| 5.46/45,494 (4.5-5.5) | |||||||
| 5.20/40,116 (4.5-5.5) | |||||||
| 5.16/31,510 (4.5-5.5) | |||||||
| 5.29/51,580 (5-6) | |||||||
| 5.27/51,301 (5-6) | |||||||
| 5.16/51,319 (5-6) | |||||||
| 5.00/50,698 (5-6) | |||||||
| 5.02/50,647 (5-6) | |||||||
| 5.26/50,000 (5-6) | |||||||
| 6.95/24,005 (6-11) | |||||||
| 6.72/25,314 (6-9) | |||||||
| EntA | P15047 | 2,3-Dihydro-2,3-dihydroxybenzoate (2,3-DHB) dehydrogenase | 4.97/26,249.66 | Involved in siderophore biosynthesis and enterobactin biosynthesis | 179 | ||
| EntB | P0ADI4 | Isochorismatase | 5.05/32,554.34 | 5.08/34,633 (DIGE 4.5-6.5) | Required for production of 2,3-DHB and serves as an aryl carrier protein; plays a role in enterobactin assembly: proteins EntB, EntD, EntE, and EntF form a multienzyme complex called enterobactin synthetase | 321 | |
| EvgA | P0ACZ4 | Positive transcription regulator | 6.83/22,690.2 | Member of the two-component regulatory system EvgS/EvgA; regulates the expression of emrKY operon and yfdX; also seems to control expression of at least one other multidrug efflux operon | 179 | ||
| FabB | P0A953 | 3-Oxoacyl-[acyl-carrier-protein] synthase I | 5.35/42,613.32 | 5.76/44,039 | Catalyzes the condensation reaction of fatty acid synthesis by the addition to an acyl acceptor of two carbons from malonyl-ACP | 294 | |
| FabD | P0AAI9 | Malonyl CoA-acyl carrier protein transacylase | 4.95/32,286.01 | 5.37/44,881 | Involved in fatty acid biosynthesis | 179, 228, 287, 294, 321 | |
| 4.95/33,409 | |||||||
| 5.03/33,449 (DIGE 4.5-6.5) | |||||||
| 5.03/32,221 (4.5-5.5) | |||||||
| FabF | P0AAI5 | 3-Oxoacyl-[acyl-carrier-protein] synthase II | 5.71/42,914.57 | See FabB; has a preference for short chain acid substrates and may function to supply the octanoic substrates for lipoic acid biosynthesis | 179 | ||
| FabG | P0AEK2 | 3-Oxoacyl-[acyl-carrier-protein] reductase | 6.76/25,560.29 | Involved in fatty acid biosynthesis pathway | 179 | ||
| FabH | P0A6R0 | 3-Oxoacyl-[acyl-carrier-protein] synthase III | 5.08/33,515.12 | See FabB; catalyzes the first condensation reaction, which initiates fatty acid synthesis and may therefore play a role in governing the total rate of fatty acid production; possesses both acetoacetyl-ACP synthase and acetyl transacylase activities | 179 | ||
| FabI | P0AEK4 | Enoyl-[acyl-carrier-protein] reductase (NADH) | 5.58/27,732.75 | 5.60/32,613 (DIGE 4.5-6.5) | Involved in second reduction step of fatty acid biosynthesis; decreases after benzoic acid treatment | 179, 287, 321 | |
| 5.40/32,959 (DIGE 4.5-6.5) | |||||||
| 5.37/38,366 (5-6) | |||||||
| 5.33/37,266 (5-6) | |||||||
| FabZ | P0A6Q6 | (3R)-hydroxymyristoyl-[acyl carrier protein] dehydratase | 6.84/17,032.95 | Involved in saturated fatty acid biosynthesis | 179 | ||
| FadA | P21151 | 3-Ketoacyl-CoA thiolase | 6.31/40,876.20 | Catalyzes the final step of fatty acid oxidation in which acetyl-CoA is released and the CoA ester of a fatty acid two carbons shorter is formed; involved in the aerobic and anaerobic degradation of long-chain fatty acids; induced in the presence of fatty acids and is under the control of the fadR repressor | 46 | ||
| FadB | P21177 | Fatty oxidation complex alpha subunit | 5.84/79,593.91 | Catalyzes the formation of a hydroxyacyl-CoA by addition of water on enoyl-CoA; also exhibits 3-hydroxyacyl-CoA epimerase and 3-hydroxyacyl-CoA dehydrogenase activities; involved in the aerobic and anaerobic degradation of long-chain fatty acids; induced in the presence of fatty acids and is under the control of the fadR repressor | 46 | ||
| FadL | P10384 | Long-chain fatty acid transport protein | 4.91/45,906.47 | Involved in translocation of long-chain fatty acids across the outer membrane; induced in the presence of long-chain fatty acids and under the control of the fadR repressor | 26 | ||
| FbaA | P0AB71 | Fructose-bisphosphate aldolase class II | 5.52/39,016.07 | 5.55/40,651 | Involved in the pathway of glycolysis; increases in poly(3-hydroxybutyrate)-accumulating cells | 99, 179, 286, 287, 321 | |
| 5.43/39,776 | |||||||
| 5.49/39,104 (DIGE 4.5-6.5) | |||||||
| 5.56/50,220 (5-6) | |||||||
| 5.56/49,421 (5-6) | |||||||
| Fbp | P0A993 | Fructose-1,6-bisphosphatase | 5.67/36,833.93 | 5.68/32,568 | Necessary for growth on substances such as glycerol, succinate, and acetate; inhibited by AMP, which affects the turnover of bound substrate and not the affinity for substrate | 179, 228, 294 | |
| FecA | P13036 | Iron (III) dicitrate transport protein | 5.36/81,707.21 | Outer membrane receptor protein in the Fe3+ dicitrate transport system; for induction, the TonB and the ExbB proteins have to be active | 179 | ||
| FepA | P05825 | Ferrienterobactin receptor | 5.23/79,771.07 | 5.24/79,447 (DIGE 4.5-6.5) | Involved in the initial step of iron uptake by binding ferrienterobactin, an iron chelatin siderophore that allows E. coli to extract iron from the environment; acts as a receptor for colicins B and D; increases after benzoic acid treatment | 321 | |
| FhuA | P06971 | Ferrichrome-iron receptor | 5.13/78,742.19 | Binds the ferrichrome-iron ligand; interacts with the TonB protein, which is responsible for energy coupling of the ferrichrome-promoted iron transport system; acts as a receptor for bacteriophage T5 as well as for T1, phi80, and colicin M; can also transport the antibiotic albomycin | 179 | ||
| Fis | P0A6R3 | DNA-binding protein | 9.34/11,239.93 | Activates rRNA transcription; plays a direct role in upstream activation of rRNA promoters; binds to a recombinational enhancer sequence that is required to stimulate Hin-mediated DNA inversion; prevents initiation of DNA replication from OriC | 179 | ||
| FklB | P0A9L3 | FKBP-type 22-kDa peptidyl-prolyl cis-trans isomerase | 4.85/22,085.00 | 4.84/24,436 (DIGE 4.5-6.5) | PPIases accelerate the folding of proteins; catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides; strongly inhibited by FK506 | 179, 321 | |
| FkpA | P45523 | FKBP-type peptidyl-prolyl cis-trans isomerase | 6.73/26,223.63 | 7.08/33,268 | See FklB | 179, 286 | |
| FldA | P61949 | Flavodoxin 1 | 4.21/19,605.75 | 4.65/21,200 | Low-potential electron donor to a no. of redox enzymes; induced at phosphonate growth | 293 | |
| FlgD | P75936 | Basal-body rod modification protein | 4.18/23,575.01 | Required for flagellar hook formation; may act as a scaffolding protein | 179 | ||
| FlgE | P75937 | Flagellar hook protein | 4.45/41,914 | 179 | |||
| FlgF | P75938 | Flagellar basal-body rod protein | 4.8/25,912.14 | The basal body constitutes a major portion of the flagellar organelle and consists of five rings (E, L, P, S, and M) mounted on a central rod; the rod consists of about 26 subunits of FlgG in the distal portion, and FlgB, FlgC, and FlgF are thought to build up the proximal portion of the rod with about 6 subunits each | 179 | ||
| FlgG | P0ABX5 | Flagellar basal-body rod protein | 4.68/27,743.89 | See FlgG | 179 | ||
| Theoreticalc | Experimentald | ||||||
| FlgL | P29744 | Flagellar hook-associated | 4.63/34,281.05 | 4.80/32,551 (4-5) | See FlgG. | 179, 287 | |
| protein 3 | 4.83/32,443 (4-5) | ||||||
| FliA | P0AEM6 | RNA polymerase sigma factor for flagellar operon | 5.2/27,521.11 | Sigma factors are initiation factors that promote the attachment of RNA polymerase to specific initiation sites and are then released; specific for class 3 flagellar operons | 179 | ||
| FliC | P04949 | Flagellin | 4.50/51,163.80 | 4.58/45,401 (4-5) | Polymerizes to form the filaments of bacterial flagella | 179, 287 | |
| 4.63/45,613 (4-5) | |||||||
| 4.55/45,507 (4-5) | |||||||
| 4.98/45,932 (4-5) | |||||||
| 4.61/45,507 (4-5) | |||||||
| 4.54/45,507 (4-5) | |||||||
| 5.00/44,877 (4-5) | |||||||
| FliD | P24216 | Flagellar hook-associated protein 2 | 4.82/48,325.26 | 4.96/42,894 (4.5-5.5) | Required for the morphogenesis and for the elongation of the flagellar filament by facilitating polymerization of the flagellin monomers at the tip of growing filament | 179, 287 | |
| FliG | P31067 | Flagellar motor switch protein | 4.69/36,775.98 | Three proteins (FliG, FliN, FliM) form a switch complex that is proposed to be located at the base of the basal body; interacts with the CheY and CheZ chemotaxis proteins, in addition to contacting components of the motor that determine the direction of flagellar rotation | 179 | ||
| FliH | P31068 | Flagellar assembly protein | 4.62/25,050.16 | Needed for flagellar regrowth and assembly | 179 | ||
| FliM | P06974 | Flagellar motor switch protein | 5.47/37,849.16 | See FliG | 179 | ||
| FliY | P39174 | Cystine-binding periplasmic protein | 5.29/26,068.48 | 5.01/26,213 | Part of a binding-protein-dependent transport system for cystine; repressed by sulfate or cysteine | 179, 286, 287, 321 | |
| 5.11/25,809 | |||||||
| 5.17/25,686 (DIGE 4.5-6.5) | |||||||
| 5.19/26,500 (4.5-5.5) | |||||||
| 5.23/28,594 (4.5-5.5) | |||||||
| Flu | P39180 | Antigen 43 | 5.37/101,278.83 | Controls colony form variation and autoaggregation; may function as an adhesin | 179 | ||
| Fmt | P23882 | Methionyl-tRNA formyltransferase | 5.56/34,037.27 | 5.49/35,885 | Modifies the free amino group of the aminoacyl moiety of methionyl-tRNA (fMet); the formyl group appears to play a dual role in the initiator identity of N-formylmethionyl-tRNA by promoting its recognition by IF2 and impairing its binding to EF-Tu-GTP | 179, 228, 236, 294 | |
| FolA | P0ABQ4 | Dihydrofolate reductase | 4.84/17,999.38 | 5.01/19,989 | Increases during phosphate limitation and phosphonate growth but decreases after benzoic acid treatment | 179, 228, 293, 294, 321 | |
| 5.02/21,096 (DIGE 4.5-6.5) | |||||||
| FrdA | P00363 | Fumarate reductase flavoprotein subunit | 5.87/65,840.41 | Two distinct membrane-bound FAD-containing enzymes are responsible for the catalysis of fumarate and succinate interconversion; the fumarate reductase is used in anaerobic growth, and the succinate dehydrogenase is used in aerobic growth | 179 | ||
| FrdB | P0AC47 | Fumarate reductase iron-sulfur protein | 6.09/26,991.87 | See FrdA | 179 | ||
| Frr | P0A805 | Ribosome recycling factor | 6.44/20,638.57 | 6.16/21,725 | Responsible for the release of ribosomes from mRNA at the termination of protein biosynthesis; may increase the efficiency of translation by recycling ribosomes from one round of translation to another | 179, 228, 294 | |
| FruB | P69811 | Multiphosphoryl transfer protein | 4.77/39,647.83 | 4.85/38,938 (DIGE 4.5-6.5) | The phosphoenolpyruvate-dependent sugar PTS, a major carbohydrate active-transport system, catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane; involved in fructose transport; induced by fructose; decreases after benzoic acid treatment | 179, 321 | |
| FtsA | P0ABH0 | Cell division protein | 5.84/45,329.97 | May be involved in anomalous filament growth; may be a component of the septum and interact with FtsZ; induced at the stationary phase | 103 | ||
| FtsZ | P0A9A6 | Cell division protein | 4.65/40,323.92 | 4.63/40,491 | Essential to the cell division process; seems to assemble into a dynamic ring on the inner surface of the cytoplasmic membrane at the place where division will occur, with the formation of the ring being the signal for septation to begin; induced at the stationary phase | 103, 179, 228, 287, 294 | |
| 4.83/39,220 (4-5) | |||||||
| 4.77/39,129 (4-5) | |||||||
| Fuck | P11553 | l-Fuculokinase | 5.38/53,235.87 | 4.73/55,145 | Involved in second step of fucose metabolism | 286 | |
| FucO | P0A9S1 | Lactaldehyde reductase | 5.1/40,644.56 | Involved in fourth step of fucose metabolism | 179 | ||
| FumB | P14407 | Fumarate hydratase class I, anaerobic | 5.88/60,105.32 | Functions in the generation of fumarate for use as an anaerobic electron acceptor | 179 | ||
| Fur | P0A9A9 | Ferric uptake regulation protein | 5.68/16,794.85 | 5.79/17,433 | Acts as a global negative controlling element, employing Fe2+ as a cofactor to bind the operator of the repressed genes; regulates the expression of several outer membrane proteins, including the iron transport operon; induced during phosphate limitation | 179, 228, 287, 293, 294 | |
| 5.78/17,897 (5-6) | |||||||
| 5.78/17,233 (5-6) | |||||||
| FusA | P0A6M8 | Elongation factor G | 5.24/77,450.11 | 5.29/85,046 (DIGE 4.5-6.5) | Promotes the GTP-dependent translocation of the nascent protein chain from the A site to the P site of the ribosome; decreases during phosphate limitation early | 179, 287, 293, 321 | |
| 5.20/84,325 (DIGE 4.5-6.5) | |||||||
| 5.51/46,525 (DIGE 4.5-6.5) | |||||||
| 5.20/38,691 (DIGE 4.5-6.5) | |||||||
| 5.78/32,101 (DIGE 4.5-6.5) | |||||||
| 5.21/75,514 (4.5-5.5) | |||||||
| 5.23/50,424 (5-6) | |||||||
| GadA | P69908 | Glutamate decarboxylase alpha | 5.22/52,685.16 | GadA converts glutamate to gamma-aminobutyrate, consuming one intracellular proton in the reaction; the Gad system helps to maintain a near-neutral intracellular pH when cells are exposed to extremely acidic conditions; the ability to survive transit through the acidic conditions of the stomach is essential for successful colonization of the mammalian host by commensal and pathogenic bacteria; induced by high pH during anaerobic growth | 27 | ||
| GalE | P09147 | UDP-glucose 4-epimerase | 5.89/37,265.11 | 5.91/35,787 (DIGE 4.5-6.5) | Involved in third step of galactose metabolism; increases after benzoic acid treatment | 179, 321 | |
| GalF | P0AAB6 | UTP-glucose-1-phosphate uridylyltransferase | 5.73/32,829.23 | Involved in lipopolysaccharide biosynthesis | 179 | ||
| GalM | P0A9C3 | Aldose-1-epimerase | 4.84/38,190.46 | 4.97/39,311 (4-5) | Converts alpha-aldose to the beta-anomer; active on d-glucose, l-arabinose, d-xylose, d-galactose, maltose, and lactose | 179, 287 | |
| 4.83/39,220 (4-5) | |||||||
| GalU | P0AEP3 | UTP-glucose-1-phosphate uridylyltransferase | 5.11/32,811.07 | May play a role in stationary-phase survival | 179 | ||
| GapA | P0A9B2 | Glyceraldehyde 3-phosphate dehydrogenase A | 6.58/35,401.30 | 6.93/23,085 | Involved in the pathway of glycolysis; induced by high pH during anaerobic growth | 179, 228, 286, 287, 294, 321, 323 | |
| 6.28/36,748 | |||||||
| 6.58/36,386 | |||||||
| 5.93/22,606 (DIGE 4.5-6.5) | |||||||
| 5.94/36,106 (DIGE 4.5-6.5) | |||||||
| 5.32/9,085 (4.5-5.5) | |||||||
| 5.03/10,612 (4.5-5.5) | |||||||
| 5.74/40,430 (5-6) | |||||||
| 6.11/34,907 (6-11) | |||||||
| GatY | P37192 | Tagatose-1,6-bisphosphate aldolase | 5.87/30,811.93 | Catalyzes the reversible condensation of dihydroxyacetone phosphate with glyceraldehyde 3-phosphate to produce tagatose-1,6-bisphosphate; induced by low pH during aerobic or anaerobic growth; increases in the physiological short-term adaptation to glucose limitation | 27, 311, 323 | ||
| Gcd | P15877 | Quinoprotein glucose dehydrogenase | 5.4/86,747.35 | Probably involved in energy conservation rather than in sugar metabolism | 179 | ||
| GcvT | P27248 | Aminomethyltransferase; glycine cleavage system T protein | 5.36/40,015.52 | The glycine cleavage system catalyzes the degradation of glycine | 179 | ||
| GdhA | P00370 | NADP-specific glutamate dehydrogenase | 5.98/48,581.37 | 5.96/45,327 | Decreases during phosphate limitation; may be directly degraded by ClpAP or ClpXP, respectively, or be modulated by a protease-dependent mechanism | 179, 228, 293, 294, 308, 321 | |
| 5.81/46,385 (DIGE 4.5-6.5) | |||||||
| 5.94/44,524 (DIGE 4.5-6.5) | |||||||
| GldA | P0A9S5 | Glycerol dehydrogenase | 4.81/38,712.2 | Induced by hydroxyacetone and stationary-phase growth conditions | 179 | ||
| Glf | P37747 | UDP-galactopyranose mutase | 6.62/42,965.88 | Involved in the conversion of UDP-galactose proton symporter (GalP) into UDP-GalF through a 2-keto intermediate for lipopolysaccharide biosynthesis | 179 | ||
| GlgS | P26649 | Glycogen synthesis protein | 5.38/7,891.88 | Involved in glycogen synthesis; induced strongly at the early stationary phase and also less osmotic induction; regulated by σS and cAMP-CRP | 103, 105 | ||
| GlmM | P31120 | Phosphoglucosamine mutase | 5.71/47,412.38 | Catalyzes the conversion of glucosamine-6-phosphate to glucosamine-1-phosphate | 179 | ||
| GlmU | P0ACC7 | Bifunctional protein | 6.09/49,190.08 | Responsible for the acetylation of glucosamine-1-phosphate (Glc-N-1-P) to give N-acetylglucosamine-1-phosphate (GlcNAc-1-P) and the synthesis of UDP-GlcNAc | 179 | ||
| GlnA | P0A9C5 | Glutamine synthetase | 5.26/51,772.57 | 5.25/53,849 | Controlled by adenylation under conditions of abundant glutamine; decreases during phosphate limitation and after benzoic acid treatment; regulated positively by Lrp; may be directly degraded by ClpAP or ClpXP, respectively, or be modulated by a protease-dependent mechanism | 61, 179, 228, 286, 287, 293, 294, 308, 321 | |
| 5.18/53,743 | |||||||
| 5.39/36,675 | |||||||
| 5.30/53,162 (DIGE 4.5-6.5) | |||||||
| 5.00/26,795 (4-5) | |||||||
| GlnB | P0A9Z1 | Nitrogen regulatory protein P-II 1 | 5.17/12,425.45 | P-II indirectly controls the transcription of the glutamine synthetase gene (glnA); prevents nitrogen regulatory (NR)-II-catalyzed conversion of NR-I to NR-I-phosphate, the transcriptional activator of glnA; when P-II is uridylylated to P-II-UMP, these events are reversed; when the ratio of Gln to 2-ketoglutarate decreases, P-II is uridylylated to P-II-UMP, which causes the deadenylylation of glutamine synthetase by GlnE, thus activating the enzyme. | 179 | ||
| GlnH | P0AEQ3 | Glutamine-binding periplasmic protein | 6.87/24,963.41 | 7.04/25,213 | Involved in a glutamine-transport system GlnHPQ; induced by lack of glutamine | 48, 179, 286, 287 | |
| 6.93/24,504 | |||||||
| 7.32/30,106 (6-11) | |||||||
| 7.13/29,388 (6-11) | |||||||
| GlnK | P0AC55 | Nitrogen regulatory protein P-II 2 | 5.84/12,259.22 | 5.78/12,732 (5-6) | See GlnB | 179, 287 | |
| 5.94/11,914 (5.5-6.7) | |||||||
| GlnQ | P10346 | Glutamine transport ATP-binding protein | 6.08/26,730.99 | Part of the binding-protein-dependent transport system for glutamine; probably responsible for energy coupling to the transport system; induced by lack of glutamine | 179 | ||
| GlnS | P00962 | Glutaminyl-tRNA synthetase | 5.89/63,346.70 | 5.88/63,346 | Changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate | 29, 109, 179, 228, 230, 294 | |
| 5.91/74,850 (DIGE 4.5-6.5) | |||||||
| GlpB | P13033 | Anaerobic glycerol-3-phosphate dehydrogenase subunit B | 5.75/45,357.24 | Conversion of glycerol-3-phosphate to dihydroxyacetone; uses fumarate or nitrate as the electron acceptor | 179 | ||
| GlpC | P0A996 | Anaerobic glycerol-3-phosphate dehydrogenase subunit C | 8.78/44,108.04 | Electron transfer protein; may also function as the membrane anchor for the GlpAB dimer | 179 | ||
| GlpD | P13035 | Aerobic glycerol-3-phosphate dehydrogenase | 6.96/56,750.54 | 5.38/49,649 | Converts glycerol-3-phosphate to dihydroxyacetone by using molecular oxygen or nitrate as the electron acceptor | 179, 228, 294 | |
| GlpK | P0A6F3 | Glycerol kinase | 5.36/56,099.57 | 5.30/50,642 | Key enzyme in the regulation of glycerol uptake and metabolism; the activity of this regulatory enzyme is affected by several metabolites; the inhibition by fructose 1,6-biphosphate causes alterations in the quaternary structure of the enzyme; induced by l-alpha-glycerol-3-phosphate; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol | 29, 71, 179, 228, 296 | |
| GlpQ | P09394 | Glycerophosphoryl diester phosphodiesterase | 5.22/38,200 | Hydrolyzes deacylated phospholipids to glycerol-3-phosphate and the corresponding alcohols | 179 | ||
| GlpR | P0ACL0 | Glycerol-3-phosphate regulon repressor | 5.82/28,047.84 | 5.82/28,724 | Repressor of the glycerol-3-phosphate regulon | 228, 294 | |
| GltA | P0ABH7 | Citrate synthase | 6.21/48,014.99 | 6.85/45,571 | Involved in tricarboxylic acid cycle and is allosterically inhibited by NADH | 179, 294 | |
| GltD | P09832 | Glutamate synthase (NADPH) small chain | 5.54/51,884.08 | 5.47/52,481 | Involved in nitrogen metabolism and glutamate biosynthesis; has low activity in the presence of exogenous leucine; regulated positively by Lrp | 61, 212, 228, 286, 287, 294 | |
| 5.40/52,585 | |||||||
| 5.48/53,050 (5-6) | |||||||
| GltI | P37902 | Glutamate/aspartate periplasmic binding protein | 7.82/31,229.49 | 5.67/15,568 (F) | Part of the binding-protein-dependent transport system for glutamate and aspartate | 179, 228, 287 | |
| 7.87/36,857 (6-11) | |||||||
| GltX | P04805 | Glutamyl-tRNA synthetase | 5.59/53,815.73 | 179 | |||
| GlyA | P0A825 | Serine hydroxymethyltransferase | 6.03/45,316.59 | 6.04/45,960 | Interconversion of serine and glycine and a key enzyme in the biosynthesis of purines, lipids, hormones, and other components; decreases during overexpression of recombinant protein in large scale | 97, 179, 228, 286, 294, 321 | |
| 5.94/46,142 | |||||||
| 6.02/45,274 (DIGE 4.5-6.5) | |||||||
| 6.00/45,000 (DIGE 4.5-6.5) | |||||||
| 6.00/43,217 (DIGE 4.5-6.5) | |||||||
| GlyS | P00961 | Glycyl-tRNA synthetase beta chain | 5.29/76,681.76 | 179 | |||
| GmhA | P63224 | Phosphoheptose isomerase | 5.97/20,814.75 | Catalyzes the isomerization of sedoheptulose 7-phosphate in d-glycero-d-manno-heptose 7-phosphate | 179 | ||
| Gnd | P37754 | 6-Phosphogluconate dehydrogenase, decarboxylating | 5.13/51,625.39 | 5.33/43,690 | Involved in the hexose monophosphate shunt; increases during phosphate limitation | 179, 293 | |
| GntR | P0ACP5 | HTH-type transcriptional regulator | 6.41/36,422.02 | Negative regulator for the gluconate utilization system GNT-I, the gntUKR operon | 179 | ||
| GntY (YhgI) | P63020 | Protein GntY | 4.52/20,997.70 | 4.72/19,718 (4-5) | Could be involved in gluconate metabolism | 179, 287 | |
| 4.96/19,460 (4-5) | |||||||
| Gor | P06715 | Glutathione reductase | 5.64/48,772.51 | 5.65/48,676 | Maintains high levels of reduced glutathione in the cytosol | 29, 179, 228, 294 | |
| Gph | P32662 | Phosphoglycolate phosphatase | 4.58/27,389.17 | 4.52/30,891 (DIGE 4.5-6.5) | Specifically catalyzes the dephosphorylation of 2-phosphoglycolate; involved in the dissimilation of the intracellular 2-phosphoglycolate formed during the DNA repair of 3′ phosphoglycolate ends, a major class of DNA lesions induced by oxidative stress; constitutively expressed | 179, 321 | |
| GpmA | P62707 | 2,3-Bisphosphoglycerate-dependent phosphoglycerate mutase | 5.86/28,425.21 | 5.86/28,425 | Catalyzes the interconversion of 2-phosphoglycerate and 3-phosphoglycerate; strongly inhibited by vanadate and regulated by the Fur protein | 179, 228, 287, 294, 321 | |
| 5.86/27,000 (DIGE 4.5-6.5) | |||||||
| 5.82/32,032 (5-6) | |||||||
| GpmI | P37689 | 2,3-Bisphosphoglycerate-independent phosphoglycerate mutase | 5.14/56,193.89 | 5.15/52,681 (DIGE 4.5-6.5) | See GpmA; insensitive to vanadate | 287, 321 | |
| 5.11/52,363 (DIGE 4.5-6.5) | |||||||
| 5.15/52,348 (4.5-5.5) | |||||||
| Gpt | P0A9M5 | Xanthine-guanine phosphoribosyltransferase | 5.52/16,970.59 | 5.44/13,981 (4.5-5.5) | Involved in purine salvage pathway; acts on guanine, on xanthine, and to a lesser extent on hypoxanthine | 287 | |
| 5.21/16,164 (5-6) | |||||||
| GrcA (YfiD) | P68066 | Autonomous glycyl radical cofactor | 5.09/14,284.19 | 5.02/15,065 | Acts as a radical domain for damaged PFL and possibly other radical proteins; induced by low pH; a strong candidate for response to internal acidification | 27, 179, 274, 286, 287 | |
| 5.00/12,390 | |||||||
| 5.13/11,675 | |||||||
| 5.01/11,079 (4-5) | |||||||
| 4.96/10,626 (4-5) | |||||||
| 5.05/9,834 (4.5-5.5) | |||||||
| 5.21/9,497 (4.5-5.5) | |||||||
| 5.09/11,523 (4.5-5.5) | |||||||
| 5.23/9,710 (4.5-5.5) | |||||||
| GreA | P0A6W5 | Transcription elongation factor | 4.71/17,640.96 | 4.68/15,984 | Necessary for efficient RNA polymerase transcription elongation past template-encoded arresting sites; cleavage of the nascent transcript by cleavage factors such as GreA or GreB allows the resumption of elongation from the new 3′ terminus | 179, 286, 287 | |
| 4.95/13,865 (4-5) | |||||||
| GroL | P0A6F5 | 60-kDa chaperonin | 4.85/57,197.66 | 4.85/56,695 | Prevents misfolding and promotes the refolding and proper assembly of unfolded polypeptides generated under stress conditions; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and overexpression of bioproducts; induced by temp, ethanol, nalidixic acid, and puromycin | 71, 97, 138, 179, 228, 287, 294, 297, 321 | |
| 4.93/61,278 (DIGE 4.5-6.5) | |||||||
| 4.98/60,758 (DIGE 4.5-6.5) | |||||||
| 4.96/59,987 (DIGE 4.5-6.5) | |||||||
| 4.99/56,877 (4-5) | |||||||
| 4.95/55,185 (4-5) | |||||||
| 4.97/61,125 (4-5) | |||||||
| 4.93/55,185 (4-5) | |||||||
| 4.95/55,185 (4-5) | |||||||
| 4.84/52,802 (4-5) | |||||||
| 4.96/54,146 (4.5-5.5) | |||||||
| 4.99/55,284 (4.5-5.5) | |||||||
| GroS | P0A6F9 | 10-kDa chaperonin | 5.15/10,386.95 | 5.15/15,657 | Binds to GroL in the presence of Mg-ATP and suppresses the ATPase activity of the latter; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and is induced by temp, ethanol, nalidixic acid, puromycin, and phosphate limitation | 71, 179, 228, 287, 293, 294, 297, 321 | |
| 5.15/10,500 (DIGE 4.5-6.5) | |||||||
| 5.22/12,998 (4.5-5.5) | |||||||
| GrpE | P09372 | Protein | 4.68/21,797.83 | 4.68/25,542 | Participates actively in the response to hyperosmotic and heat shock by preventing the aggregation of stress-denatured proteins in association with DnaK; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol; induced by temp, ethanol, nalidixic acid, CdCl2, and puromycin | 71, 179, 228, 294, 297 | |
| GshB | P04425 | Glutathione synthetase | 5.11/35,560.9 | Inhibited by 7,8-dihydrofolate, methotrexate, and trimethoprim | 179 | ||
| Gst | P0A9D2 | Glutathione S-transferase | 5.85/22,868.37 | 5.76/27,614 (5-6) | Conjugates reduced glutathione to a large no. of exogenous and endogenous hydrophobic electrophiles | 287 | |
| GuaB | P0ADG7 | Inosine-5′-monophosphate dehydrogenase | 6.02/52,022.45 | 6.01/55,036 | Involved in first step of GMP biosynthesis from IMP; induced by low pH during anaerobic growth | 179, 228, 286, 287, 294, 321, 323 | |
| 5.76/56,695 | |||||||
| 5.99/55,298 (DIGE 4.5-6.5) | |||||||
| 5.49/38,144 (5-6) | |||||||
| GuaC | P60560 | GMP reductase | 6.1/37,383.67 | Catalyzes the irreversible NADPH-dependent deamination of GMP to IMP; functions in the conversion of nucleobase, nucleoside, and nucleotide derivatives of G-to-A nucleotides and in maintaining the intracellular balance of A and G nucleotides | 179 | ||
| GyrA | P0AES4 | DNA gyrase subunit A | 5.09/96,963.51 | 5.28/100,213 | DNA gyrase negatively supercoils closed circular double-stranded DNA in an ATP-dependent manner and also catalyzes the interconversion of other topological isomers of double-stranded DNA rings, including catenanes and knotted rings | 228, 294 | |
| GyrB | P0AES6 | DNA gyrase subunit B | 5.72/89,818.72 | See GyrA | 179 | ||
| HchA | P31658 | Chaperone protein | 5.63/31,059.27 | 5.44/33,098 (DIGE 4.5-6.5) | Uses temp-induced exposure of structured hydrophobic domains to capture and stabilize early unfolding protein intermediates under severe thermal stress; rapidly releases them once stress has abated and is induced by heat shock | 321 | |
| HdeA | P0AES9 | Protein | 4.68/9,740.91 | 4.56/10,724 | Induced by low pH during anaerobic growth and at the stationary phase | 103, 179, 228, 287, 294, 323 | |
| 4.84/9,424 (4-5) | |||||||
| HdeB | P0AET2 | Protein | 4.94/9,065.24 | 4.85/11,285 | Periplasmic protein that may be involved in acid resistance; enhanced at the stationary phase and in the acnB mutant but repressed by H-NS | 103, 179, 228, 278, 287, 294 | |
| 5.00/9,588 (4.5-5.5) | |||||||
| HdhA | P0AET8 | 7-Alpha-hydroxysteroid dehydrogenase | 5.22/26,778.59 | 5.17/25,083 | 7-Alpha-dehydroxylation of cholic acid, yielding deoxycholic acid and lithocholic acid, respectively; highest affinity with taurochenodeoxycholic acid | 179, 286 | |
| HemB | P0ACB2 | Delta-aminolevulinic acid dehydratase | 5.25/35,493.60 | 5.20/38,691 (DIGE 4.5-6.5) | Involved in porphyrin biosynthesis | 321 | |
| HemC | P06983 | Porphobilinogen deaminase | 5.31/33,851.83 | Tetrapolymerization of the monopyrrole porphobilinogen into the hydroxymethylbilane preuroporphyrinogen in several discrete steps | 179 | ||
| HemE | P29680 | Uroporphyrinogen decarboxylase | 5.88/39,248.12 | Involved in porphyrin biosynthesis | 179 | ||
| HemX | P09127 | Putative uroporphyrin-III C-methyltransferase | 4.68/42,963.02 | Involved in siroheme and cobalamin biosynthesis | 179 | ||
| HflB | P0AAI3 | Cell division protein FtsH | 5.91/70,708.09 | Seems to act as an ATP-dependent zinc metallopeptidase; involved in the degradation of σ32 | 179 | ||
| HflC | P0ABC3 | Protein | 6.3/37,649.88 | HflC and HflK govern the stability of phage lambda cII protein and have been proposed to encode or regulate a cII-specific protease | 179 | ||
| Hfq | P0A6X3 | Protein | 7.20/11,035.19 | RNA-binding protein that stimulates the elongation of poly(A) tails and targets several mRNAs for degradation, possibly by increasing polyadenylation or by interfering with ribosome binding. More than 30 proteins are altered in the hfq null mutant; some of these proteins are σS-dependent genes | 204 | ||
| HisA | P10371 | 1-(5-Phosphoribosyl)-5- | 4.94/26,032.65 | 4.99/24,831 (DIGE 4.5-6.5) | Involved in fourth step of l- | 179, 321 | |
| [(5-phosphoribosylamino) | 5.46/23,581 (DIGE 4.5-6.5) | histidine from 5-phospho-alpha- | |||||
| methylideneamino] | d-ribose 1-diphosphate | ||||||
| imidazole-4-carboxamide | |||||||
| isomerase | |||||||
| HisB | P06987 | Histidine biosynthesis bifunctional protein | 5.76/40,277.96 | 5.43/35,112 | See HisA: steps 6 and 8 | 286 | |
| HisC | P06986 | Histidinol-phosphate aminotransferase | 5.01/39,360.14 | See HisA: step 7; induced by high pH during anaerobic growth | 323 | ||
| HisD | P06988 | Histidinol dehydrogenase | 5.06/45,979.15 | 5.07/48,837 (DIGE 4.5-6.5) | See HisA: final step; catalyzes the sequential NAD-dependent oxidations of l-histidinol to l-histidinaldehyde and then to l-histidine; induced by high pH during anaerobic growth | 179, 321, 323 | |
| HisF | P60664 | Imidazole glycerol phosphate synthase subunit | 5.03/28,454.45 | See HisA: step 5 | 179 | ||
| HisG | P60757 | ATP phosphoribosyltransferase | 5.47/33,366.72 | 5.40/32,959 (DIGE 4.5-6.5) | Has a crucial role in the pathway because the rate of histidine biosynthesis seems to be controlled primarily by regulation of hisG enzymatic activity; feedback inhibited by histidine or inhibited by AMP and N′-5′-phosphoribosyl-ATP | 179, 321 | |
| HisH | P60595 | Imidazole glycerol phosphate synthase subunit | 5.33/21,652.86 | 5.24/27,057 (5-6) | See HisA: step 1 | 287 | |
| HisI | P06989 | Histidine biosynthesis bifunctional protein HisIE | 5.24/22,755.82 | See HisA: steps 2 and 3 | 179 | ||
| HisJ | P0AEU0 | Histidine-binding periplasmic protein | 5.17/26,232.61 | 5.05/28,668 | Component of the high-affinity histidine permease, a binding-protein-dependent transport system (HisJ, HisQ, HisM, and HisP) | 179, 286, 287, 321 | |
| 5.28/27,969 (DIGE 4.5-6.5) | |||||||
| 5.14/27,701 (DIGE 4.5-6.5) | |||||||
| 4.99/10,461 (4-5) | |||||||
| 5.14/28,391 (4.5-5.5) | |||||||
| HisS | P60906 | Histidyl-tRNA synthetase | 5.65/46,898.25 | 5.59/50,642 | 29, 179, 228, 294 | ||
| HldE | P76658 | Bifunctional protein | 5.29/51,050.62 | Catalyzes the phosphorylation of d-glycero-d-manno-heptose 7-phosphate at the C-1 position to form d,d-heptose-1,7-bisphosphate; also catalyzes the ADP transfer to d-glycero-d-manno-heptose 1-phosphate, yielding ADP-d,d-heptose | 179 | ||
| Hmp | P24232 | Flavohemoprotein | 5.48/43,867.66 | Involved in NO detoxification in an aerobic process, termed nitric oxide dioxygenase reaction, that utilizes O2 and NAD(P)H to convert NO to nitrate, which protects the bacterium from various noxious nitrogen compounds; induced by nitric oxide NO (under aerobic conditions), nitrite, nitrate (under anaerobic conditions), nitroso compounds, and paraquat; induced by low pH during anaerobic growth | 323 | ||
| Hns | P0ACF8 | DNA-binding protein H-NS | 5.44/15,408.44 | 5.45/15,612 | Has possible histone-like function and may be a global transcriptional regulator through its ability to bind to curved DNA sequences, which are found in regions upstream of a certain subset of promoters; subject to transcriptional autorepression by binding preferentially to the upstream region of its own gene recognizing two segments of DNA on both sides of a bend centered around position −150; the hns mutant exhibit derepression not only of csgBA, hdeAB, mcc, and osmY but also of the expression of at least 22 σS-controlled genes, including σS itself; increases during phosphate limitation and phosphonate growth | 19, 179, 228, 286, 293, 294, 315 | |
| 5.00/12,390 | |||||||
| 5.38/15,657 | |||||||
| 5.02/9,935 | |||||||
| Hpt | P0A9M2 | Hypoxanthine phosphoribosyltransferase | 5.09/20,115.24 | 5.38/12,316 (4.5-5.5) | Involved in purine salvage pathway and acts exclusively on hypoxanthine | 179, 287 | |
| HscB | P0A6L9 | Cochaperone protein | 5.05/20,137.71 | Involved in the maturation of iron-sulfur cluster-containing proteins; seems to help targeting proteins to be folded toward HscA | 179 | ||
| HslO | P0A6Y5 | 33-kDa chaperonin | 4.35/32,534.48 | Redox regulated molecular chaperone; protects both thermally unfolding and oxidatively damaged proteins from irreversible aggregation; plays an important role in the bacterial defense system toward oxidative stress; induced by heat shock | 179 | ||
| HslU | P0A6H5 | ATP-dependent hsl protease ATP-binding subunit | 5.24/49,593.80 | 5.30/48,579 | Chaperone subunit of a proteasome-like degradation complex; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol | 71, 179, 228, 294 | |
| HslV | P0A7B8 | ATP-dependent protease | 5.95/18,961.66 | 5.93/22,161 | Protease subunit of a proteasome-like degradation complex; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol | 71, 228, 294 | |
| HtpG | P0A6Z3 | Chaperone protein | 5.09/71,422.53 | 5.06/65,639 | Molecular chaperone; has ATPase activity; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol but decreases during phosphate limitation late | 71, 179, 228, 293, 294 | |
| HybA | P0AAJ8 | Hydrogenase-2 operon protein | 6.38/33,263.79 | 6.38/33,263.79 | Participates in the periplasmic electron-transferring activity of hydrogenase 2 during its catalytic turnover | 179 | |
| HypB | P0AAN3 | Hydrogenase isoenzymes nickel incorporation protein | 5.42/31,565.04 | 6.32/32,224 | Required for the maturation of the three [NiFe] hydrogenases; exhibits a low intrinsic GTPase activity; affects some aspect of the processing of hydrogenases 1 and 2, perhaps nickel incorporation into the apoenzymes, since hypB gene lesions can be complemented by high nickel ion concn in the medium | 286 | |
| HypD | P24192 | Hydrogenase isoenzymes formation protein | 5.80/41,363.38 | 5.81/41,711 | Required for the formation of all three hydrogenase isoenzymes | 228, 294 | |
| IbpA | P0C054 | Small heat shock protein | 5.57/15,773.71 | 5.35/15,600 | Associates with aggregated proteins, together with IbpB, to stabilize and protect them from irreversible denaturation and extensive proteolysis during heat shock and oxidative stress; aggregated proteins bound to the IbpAB complex are more efficiently refolded and reactivated by the ATP-dependent chaperone systems ClpB and DnaK/DnaJ/GrpE; its activity is ATP independent; IbpAB increase in overexpression of heterologous proteins and biopolymers | 4, 97, 98, 228, 287, 296 | |
| 5.68/15,600 | |||||||
| 5.63/15,600 | |||||||
| 5.63/15,384 (5-6) | |||||||
| 5.63/14,237 (5.5-6.7) | |||||||
| IbpB | P0C058 | Small heat shock protein | 5.19/16,093.20 | 5.02/15,600 | See IbpA; decreases during phosphate limitation early | 4, 97, 98, 295 | |
| 5.20/15,600 | |||||||
| Icd | P08200 | Isocitrate dehydrogenase (NADP) | 5.15/45,756.71 | 5.06/46,200 | Inhibition of this enzyme by phosphorylation regulates the branch point between the Krebs cycle and the glyoxylate bypass, which is an alternate route that accumulates carbon for biosynthesis when acetate is the sole carbon source for growth; increases during high-cell-density cultivation but decreases during phosphate limitation | 179, 228, 236, 287, 293, 294, 321, 325 | |
| 5.15/46,200 | |||||||
| 5.02/46,100 | |||||||
| 5.11/44,335 (DIGE 4.5-6.5) | |||||||
| 5.15/44,053 (DIGE 4.5-6.5) | |||||||
| 4.70/22,351 (4-5) | |||||||
| 5.15/43,951 (4.5-5.5) | |||||||
| 5.00/74,518 (5-6) | |||||||
| IdhA | P52643 | d-Lactate dehydrogenase | 5.29/36,534.79 | 5.59/34,100 | Fermentative lactate dehydrogenase; increases during phosphate limitation and at low pH under anaerobic conditions | 128, 293 | |
| IlvA | P04968 | Threonine dehydratase biosynthetic | 5.57/56,195.25 | 6.16/54,999 | Catalyzes the formation of alpha-ketobutyrate from threonine in isoleucine biosynthesis; isoleucine allosterically inhibits whereas valine allosterically activates this enzyme; increases during phosphate limitation and phosphonate growth | 293 | |
| IlvB | P08142 | Acetolactate synthase isozyme I large subunit | 5.30/60,440.57 | 5.22/56,152 (4.5-5.5) | Involved in valine and isoleucine biosynthesis: step 1 | 287 | |
| IlvC | P05793 | Ketol-acid reductoisomerase | 5.20/53,937.83 | 5.26/52,066 | See IlvB: step 2; induced in the presence of acetohydroxybutyrate and acetolactate; decreases after benzoic acid treatment | 286, 321 | |
| 5.22/52,046 (DIGE 4.5-6.5) | |||||||
| 5.29/51,264 (DIGE 4.5-6.5) | |||||||
| IlvD | P05791 | Dihydroxy-acid dehydratase | 5.59/65,400.37 | 5.54/62,065 (DIGE 4.5-6.5) | See IlvB: step 3; increases after benzoic acid treatment | 321 | |
| IlvE | P0AB80 | Branched-chain amino acid aminotransferase | 5.54/33,962.46 | 5.43/35,112 | See IlvB: step 4 (final); acts on leucine, isoleucine, and valine; decreases during phosphate limitation | 228, 236, 293, 294 | |
| IlvH | P00894 | Acetolactate synthase isozyme III small subunit | 8.01/17,976.76 | 8.11/15,587 (6-11) | See IlvB: step 1; sensitive to valine inhibition | 287 | |
| Imp | P31554 | Organic solvent tolerance protein | 4.85/87,068.95 | 4.91/88,368 (DIGE 4.5-6.5) | Determines N-hexane tolerance; involved in outer membrane permeability; essential for envelope biogenesis; could be part of a targeting/usher system for outer membrane components | 179, 321 | |
| IscA (YfhF) | P0AAC8 | Iron-binding protein | 4.75/11,556.05 | Could transfer iron-sulfur clusters to apo-ferredoxin; multiple cycles of [2Fe2S] cluster formation and transfer are observed; acts catalytically; recruits intracellular free iron so as to provide iron for the assembly of transient iron-sulfur cluster in IscU in the presence of IscS, l-cysteine, and the thioredoxin reductase system TrxA/TrxB | 179 | ||
| IscU | Involved in Fe-S biosynthesis | 4.82/13,848.59 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16130454) | 179 | |||
| IspA | P22939 | Geranyltranstransferase | 5.27/32,159.63 | 5.37/26,625 | 179, 286 | ||
| IspG | P62620 | 4-Hydroxy-3-methylbut-2-en-1-yl diphosphate synthase | 5.87/40,683.61 | Converts 2C-methyl-d-erythritol 2,4-cyclodiphosphate into 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate | 179 | ||
| IspH | P62623 | 4-Hydroxy-3-methylbut-2-enyl diphosphate reductase | 5.2/34,774.56 | Converts 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into isopentenyl diphosphate and dimethylallyl diphosphate; also involved in penicillin tolerance, control of the stringent response, and isoprenoid biosynthesis | 179 | ||
| Ivy | P0AD59 | Inhibitor of vertebrate lysozyme | 5.51/14,102.90 | 5.46/15,065 | Strong inhibitor of lysozyme C | 228, 287 | |
| 5.41/14,304 (5-6) | |||||||
| 5.33/12,548 (5-6) | |||||||
| KatE | P21179 | Catalase HPII | 5.54/84,162.61 | 5.47/86,507 (DIGE 4.5-6.5) | Decomposes hydrogen peroxide into water and oxygen; serves to protect cells from the toxic effects of hydrogen peroxide; induced by entry into stationary phase and after benzoic acid treatment | 103, 321 | |
| KatG | P13029 | Peroxidase/catalase HPI | 5.14/80,023.82 | 5.18/77,774 (DIGE 4.5-6.5) | Bifunctional, exhibiting both catalase and broad-spectrum peroxidase activities; induced by hydrogen peroxide and by entry into stationary phase but decreases after benzoic acid treatment | 103, 287, 321 | |
| 5.16/74,539 (4.5-5.5) | |||||||
| Kba | P0AB74 | Tagatose-1,6-bisphosphate aldolase AgaY | 5.37/31,293.68 | 5.98/31,100 | Catalyzes the reversible condensation of dihydroxyacetone phosphate with glyceraldehyde 3-phosphate to produce tagatose-1,6-bisphosphate; repressed in the presence of glucose | 99 | |
| Kbl | P0AB77 | 2-Amino-3-ketobutyrate CoA ligase | 5.64/43,117.04 | -/42,200 | Negatively regulated by Lrp | 61 | |
| KdgK | P37647 | 2-Dehydro-3-deoxygluconokinase | 4.92/33,962.36 | 179 | |||
| KdsA | P0A715 | 2-Dehydro-3-deoxyphosphooctonate aldolase | 6.32/30,832.69 | 6.10/33,261 (6-11) | Synthesis of 2-keto-3-deoxyoctulosonic acid 8-phosphate, which is required for lipid A maturation and cellular growth | 179, 287 | |
| KdsB | P04951 | 3-Deoxy-manno-octulosonate cytidylyltransferase | 5.15/27,483.25 | 5.06/33,695 | Activates 2-keto-3-deoxyoctulosonic acid for incorporation into bacterial lipopolysaccharide in gram-negative bacteria | 228, 294 | |
| LamB | P02943 | Maltoporin | 4.72/47,385.03 | Involved in the transport of maltose and maltodextrins; indispensable for translocation of dextrins containing more than three glucosyl moieties; acts as a receptor for several bacteriophages including lambda; induced by maltose | 179 | ||
| LdhA | P52643 | d-Lactate dehydrogenase | 5.29/36,534.79 | 5.59/34,100 | Fermentative lactate dehydrogenase; increases during phosphate limitation and at low pH under anaerobic conditions | 128, 293 | |
| LeuA | P09151 | 2-Isopropylmalate synthase | 5.47/57,166.71 | Catalyzes the condensation of the acetyl group of acetyl-CoA with 3-methyl-2-oxobutanoate (2-oxoisovalerate) to form 3-carboxy-3-hydroxy-4-methylpentanoate (2-isopropylmalate); may be directly degraded by ClpAP and ClpXP, respectively, or be modulated by a protease-dependent mechanism | 308 | ||
| LeuC | P0A6A6 | 3-Isopropylmalate dehydratase large subunit | 5.90/49,750.64 | 5.95/51,656 | Catalyzes the isomerization between 2-isopropylmalate and 3-isopropylmalate via the formation of 2-isopropylmaleate | 179, 286, 321 | |
| 5.77/51,451 | |||||||
| 5.42/44,439 | |||||||
| 5.93/50,340 (DIGE 4.5-6.5) | |||||||
| LeuD | P30126 | 3-Isopropylmalate dehydratase small subunit | 5.16/22,356.29 | 5.20/23,000 (DIGE 4.5-6.5) | See LeuC; increases after benzoic acid treatment | 179, 321 | |
| LeuO | P10151 | Probable HTH-type transcriptional regulator | 5.80/35,694.73 | 5.77/34,515 | Increases late during phosphate limitation | 293 | |
| LeuS | P07813 | Leucyl-tRNA synthetase | 5.16/97,233.76 | 5.11/98,831 | Changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate | 29, 109, 228, 230, 294 | |
| LexA | P0A7C2 | LexA repressor | 6.23/22,357.72 | Represses a no. of genes involved in the response to DNA damage (SOS response), including RecA and LexA; in the presence of single-stranded DNA, RecA interacts with LexA, causing an autocatalytic cleavage which disrupts the DNA-binding part of LexA, leading to derepression of the SOS regulon and eventually DNA repair | 179 | ||
| LigA | P15042 | DNA ligase | 5.39/73,606.07 | Catalyzes the formation of phosphodiester linkages between 5′-phosphoryl and 3′-hydroxyl groups in double-stranded DNA using NAD as a coenzyme and as the energy source for the reaction; essential for DNA replication and repair of damaged DNA | 179 | ||
| LivJ | P0AD96 | Leu/Ile/Val-binding protein | 5.28/36,772.53 | 5.28/41,960 | Component of the leucine, isoleucine, valine (threonine) transport system, which is one of the two periplasmic binding-protein-dependent transport systems of the high-affinity transport of the branched-chain amino acids; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and in the physiological short-term adaptation to glucose limitation; decreases after benzoic acid treatment and phosphate limitation late and in the presence of leucine | 29, 61, 71, 179, 228, 258, 293, 294, 311, 321 | |
| 5.18/39,439 (DIGE 4.5-6.5) | |||||||
| 5.30/38,773 (DIGE 4.5-6.5) | |||||||
| 5.31/40,279 (4.5-5.5) | |||||||
| 5.21/16,964 (4.5-5.5) | |||||||
| 5.09/50,544 (5-6) | |||||||
| 5.23/50,424 (5-6) | |||||||
| 5.67/30,753 (5-6) | |||||||
| 5.33/23,392 (5-6) | |||||||
| 5.73/30,106 (5.5-6.7) | |||||||
| LivK | P04816 | Leucine-specific binding protein | 5.07/36,982.71 | 5.00/41,464 | Component of the leucine-specific transport system, which is one of the two periplasmic binding-protein-dependent transport systems of the high-affinity transport of the branched-chain amino acids; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol; decreases after benzoic acid treatment | 71, 179, 212, 228, 258, 287, 294, 321 | |
| 5.09/39,439 (DIGE 4.5-6.5) | |||||||
| 5.08/39,792 (4.5-5.5) | |||||||
| 5.56/28,995 (4.5-5.5) | |||||||
| 5.25/59,333 (5-6) | |||||||
| LldD | P33232 | l-Lactate dehydrogenase | 6.33/42,728.19 | Induced by l-lactate aerobically | 179 | ||
| LolA | P61316 | Outer-membrane lipoprotein carrier protein | 5.55/20,322.37 | 5.52/21,066 (5-6) | Participates in the translocation of lipoproteins from the inner membrane to the outer membrane | 179, 287 | |
| LolB | P61320 | Outer-membrane lipoprotein | 8.58/21,234.84 | Plays a critical role in the incorporation of lipoproteins in the outer membrane after they are released by the LolA protein; essential for E. coli viability | 179 | ||
| Lon | P0A9M0 | ATP-dependent protease La | 6.01/87,438.12 | —/94,000 | Degrades short-lived regulatory and abnormal proteins in the presence of ATP; induced by stress conditions such as high temperatures, nalidixic acid, and accumulation of abnormal proteins; positively regulated by htpR | 237, 297 | |
| LpdA | P0A9P0 | Dihydrolipoyl dehydrogenase | 5.79/50,557.30 | 5.81/53,637 | Component of the glycine cleavage system as well as of the alpha-ketoacid dehydrogenase complexes; increases during aerobic growth and the low temp at 10°C; induced by low pH during anaerobic growth | 133, 179, 228, 270, 294, 321, 323 | |
| 5.82/50,800 | |||||||
| 5.90/28,059 (DIGE 4.5-6.5) | |||||||
| Lpp | P69776 | Major outer membrane lipoprotein | 8.12/6,385.04 | Interacts with the peptidoglycan both covalently and noncovalently; contributes to the maintenance of the structural and functional integrity of the cell envelope | 179 | ||
| LuxS (YgaG) | P45578 | S-Ribosylhomocysteine lyase | 5.18/19,285.00 | 5.20/18,715 (4.5-5.5) | Catalyzes the transformation of S-ribosylhomocysteine to homocysteine and 4,5-dihydroxy-2,3-pentadione; involved in the synthesis of autoinducer 2, which is secreted by bacteria and is used to communicate both the cell density and the metabolic potential of the environment; changes in cell density in a process called quorum sensing; induced by low pH | 179, 274, 287 | |
| 5.07/18,656 (4.5-5.5) | |||||||
| LysA | P00861 | Diaminopimelate decarboxylase | 5.63/46,177.26 | 5.76/44,970 | Involved in lysine biosynthesis | 228, 294 | |
| LysS | P0A8N3 | Lysyl-tRNA synthetase | 5.11/57,472.23 | 5.14/58,637 | Changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate | 29, 109, 228, 230, 294 | |
| LysU | P0A8N5 | Lysyl-tRNA synthetase, heat inducible | 5.10/57,695.38 | —/60,500 | Synthesizes a no. of adenyl dinucleotides, which have been proposed to act as modulators of the heat shock response and stress response; induced by temp and ethanol; negatively regulated by Lrp but positively regulated by htpR | 61, 297 | |
| Maa | P77791 | Maltose O-acetyltransferase | 6.19/19,964.80 | 6.04/22,694 | Acetylates maltose and other sugars | 286 | |
| MacA | P75830 | Macrolide-specific efflux protein | 5.49/37,032.35 | Efflux transporter for macrolide antibiotics | 179 | ||
| MalE | P0AEX9 | Maltose-binding periplasmic protein | 5.22/40,707.32 | 5.08/41,137 | Involved in the high-affinity maltose membrane transport system malEFGK and is the initial receptor for the active transport of chemotaxis toward maltooligosaccharides; induced by high pH during aerobic or anaerobic growth; increases in the physiological short-term adaptation to glucose limitation but reduces at the acnA or/and acnB mutants | 27, 179, 274, 278, 286, 287, 311, 321, 323 | |
| 5.20/38,691 (DIGE 4.5-6.5) | |||||||
| 5.23/38,521 (4.5-5.5) | |||||||
| 5.21/39,072 (4.5-5.5) | |||||||
| 5.09/50,544 (5-6) | |||||||
| 5.00/50,085 (5-6) | |||||||
| MalK | P68187 | Maltose/maltodextrin import ATP-binding protein | 6.23/40,990.45 | Part of the ABC transporter complex MalEFGK, involved in maltose/maltodextrin import; responsible for energy coupling to the transport system | 179 | ||
| ManA | P00946 | Mannose-6-phosphate isomerase | 5.29/42,849.95 | 5.16/50,629 (5-6) | Involved in the conversion of glucose to GDP-l-fucose, which can be converted to l-fucose, a capsular polysaccharide | 287 | |
| ManX | P69797 | PTS system mannose-specific IIAB component | 5.74/34,916.36 | 5.17/26,187 (4.5-5.5) | The phosphoenolpyruvate-dependent sugar PTS, a major carbohydrate active-transport system, catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane; involved in mannose transport; induced by low pH | 27, 179, 287 | |
| Map | P0AE18 | Methionine aminopeptidase | 5.64/29,330.80 | 5.66/33,838 | Removes the amino-terminal methionine from nascent proteins | 179, 228, 287, 294 | |
| 5.71/38,478 (5-6) | |||||||
| 5.81/37,872 (5.5-6.7) | |||||||
| Mdh | P61889 | Malate dehydrogenase | 5.61/32,337.30 | 5.55/35,532 | Catalyzes the reversible oxidation of malate to oxaloacetate | 179, 228, 286, 287, 294, 321 | |
| 5.62/33,338 | |||||||
| 5.43/35,112 | |||||||
| 5.56/35,156 (DIGE 4.5-6.5) | |||||||
| 5.57/40,903 (5-6) | |||||||
| 5.49/38,144 (5-6) | |||||||
| 5.25/37,049 (5-6) | |||||||
| 6.86/29,566 (6-11) | |||||||
| MdoG | P33136 | Glucans biosynthesis protein G | 6.26/55,365.38 | 8.04/24,966 (6-11) | Involved in the biosynthesis of osmoregulated periplasmic glucans | 179, 287 | |
| 6.28/87,397 (6-9) | |||||||
| MenB | P0ABU0 | Naphthoate synthase | 5.99/31,633.08 | Converts O-succinylbenzoyl-CoA to 1,4-dihydroxy-2-naphthoic acid; involved in menaquinone biosynthesis | 179 | ||
| MetE | P25665 | 5-Methyltetrahydropteroyl triglutamate-homocysteine methyltransferase | 5.61/84,542.35 | Catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine, resulting in methionine formation; increases during growth in the absence of methionine | 294 | ||
| MetG | P00959 | Methionyl-tRNA synthetase | 5.56/76,123.54 | 5.56/76,123 | Required not only for elongation of protein synthesis but also for the initiation of all mRNA translation through initiator tRNA (fMet) aminoacylation; decreases during phosphate limitation | 179, 228, 236, 293, 294 | |
| MetH | P13009 | Methionine synthase | 4.97/135,865.85 | 4.96/134,153 | Involved in terminal step in the de novo biosynthesis of methionine; decreases during phosphate limitation | 228, 293, 294 | |
| MetK | P0A817 | S-Adenosylmethionine synthetase | 5.10/41,820.43 | 5.03/44,970 | Catalyzes the formation of S-adenosylmethionine from methionine and ATP; may be directly degraded by ClpAP and ClpXP, respectively, or be modulated by a protease-dependent mechanism | 29, 179, 228, 287, 294, 308 | |
| 5.13/43,069 (4.5-5.5) | |||||||
| 5.00/30,257 (4.5-5.5) | |||||||
| MetQ | P28635 | d-Methionine-binding lipoprotein | 4.93/27,236.94 | 4.90/27,000 (DIGE 4.5-6.5) | Component of a d-methionine permease, a binding-protein-dependent, ATP-driven transport system; decreases after benzoic acid treatment | 287, 321 | |
| 4.95/11,887 (4-5) | |||||||
| 4.95/11,491 (4-5) | |||||||
| MglB | P0AEE5 | d-Galactose-binding periplasmic protein | 5.25/33,367.70 | 5.16/31,345 | Involved in the active transport of galactose and glucose; plays a role in the chemotaxis towards the two sugars by interacting with the Trg chemoreceptor; induced by high pH during anaerobic growth; increases in the physiological short-term adaptation to glucose limitation | 179, 228, 287, 294, 311, 323 | |
| 5.19/31,192 (4.5-5.5) | |||||||
| MgsA | P0A731 | Methylglyoxal synthase | 6.12/16,918.58 | 179 | |||
| MinD | P0AEZ3 | Septum site-determining protein | 5.25/29,482.84 | 5.28/29,709 (4.5-5.5) | Cell division inhibitors MinC and MinD act in concert to form an inhibitor capable of blocking formation of the polar Z ring septa; rapidly oscillates between the poles of the cell to destabilize ftsZ filaments that have formed before they mature into polar Z rings | 179, 287 | |
| MinE | P0A734 | Cell division topological specificity factor | 5.15/10,234.91 | Prevents the cell division inhibition by proteins MinC and MinD at internal division sites while permitting inhibition at polar sites; ensures cell division at the proper site by restricting the formation of a division septum at the midpoint of the long axis of the cell | 179 | ||
| MipA | P0A908 | MltA-interacting protein | 5.03/25,670.3 | May serve as a scaffold protein required for the formation of a complex with MrcB/PonB and MltA; this complex could play a role in enlargement and septation of the murein sacculus | 179 | ||
| MoaB | P0AEZ9 | Molybdenum cofactor biosynthesis protein B | 5.73/18,533.90 | 5.74/18,915 (5-6) | Involved in the biosynthesis of molybdopterin precursor Z from guanosine; induced by anaerobiosis but repressed by the molybdenum cofactor | 179, 287 | |
| 5.87/18,128 (5.5-6.7) | |||||||
| ModA | P37329 | Molybdate-binding periplasmic protein | 6.38/24,918.32 | 6.70/29,299 (6-11) | Involved in the transport of molybdenum into the cell; binds molybdate with high specificity and affinity | 179, 287 | |
| MoeB | P12282 | Molybdopterin biosynthesis protein | 4.91/26,718.83 | Involved in the biosynthesis of a demolybdo cofactor (molybdopterin) necessary for molybdoenzymes; plays a role in the activation of the small subunit of the molybdopterin-converting factor (MoaD) | 179 | ||
| Mog | P28694 | Molybdopterin biosynthesis protein | 4.97/21,222.35 | 4.84/9,749 (4-5) | Involved in the biosynthesis of a demolybdo cofactor (molybdopterin) necessary for molybdoenzymes | 287 | |
| MotB | P0AF06 | Chemotaxis protein | 9/34,186.08 | Required for the rotation of the flagellar motor; may be a linker that fastens the torque-generating machinery to the cell wall | 179 | ||
| MppA | P77348 | Periplasmic murein peptide-binding protein | 8.32/57,618.48 | 8.55/73,914 (6-11) | Essential for the uptake of the murein peptide l-alanyl-gamma-d-glutamyl-meso-diaminopimelate and also transports some alpha-linked peptides such as Pro-Phe-Lys with low affinity; affected by the oligopeptide permease system | 179, 287 | |
| MrcA | P02918 | Penicillin-binding protein 1A | 6.15/93,636.17 | Cell wall formation; synthesis of cross-linked peptidoglycan from the lipid intermediates; has a penicillin-insensitive transglycosylase N-terminal domain (formation of linear glycan strands) and a penicillin-sensitive transpeptidase C-terminal domain (cross-linking of the peptide subunits) | 179 | ||
| MreB | P0A9X4 | Rod shape-determining protein | 5.19/36,952.4 | Involved in formation of the rod shape of the cell; may act as a negative regulator of FtsI | 179 | ||
| Mrp | P0AF08 | Protein | 5.85/39,938.08 | 179 | |||
| MsbA | P60752 | Lipid A export ATP-binding/permease protein | 8.62/64,460.71 | Involved in lipid A export and possibly also in glycerophospholipid export and biogenesis of the outer membrane | 179 | ||
| MscS (YggB) | P0C0S1 | Small-conductance mechanosensitive channel | 7.9/30,896.02 | Participates in the regulation of osmotic pressure changes within the cell, opening in response to stretch forces in the membrane lipid bilayer, without the need for other proteins; forms an ion channel of 1.0-nanosiemen conductance with a slight preference for anions; sensitive to voltage: as the membrane is depolarized, less tension is required to open the channel and vice versa; characterized by short bursts of activity that last for a few seconds | 179 | ||
| MsrA | P0A744 | Peptide methionine sulfoxide reductase | 4.99/23,183.84 | Could have an important function as a repair enzyme for proteins that have been inactivated by oxidation; catalyzes the reversible oxidation-reduction of methionine sulfoxide in proteins to methionine | 179 | ||
| MtlA | P00550 | PTS system mannitol-specific EIICBA component | 6.05/67,972.26 | The phosphoenolpyruvate-dependent sugar PTS, a major carbohydrate active-transport system, catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane; involved in mannitol transport | 179 | ||
| MtnN | P0AF12 | Methylthioadenosine/S-adenosylhomocysteine nucleosidase | 5.09/24,353.97 | 5.06/25,943 | Responsible for cleavage of the glycosidic bond in both 5′-methylthioadenosine and S-adenosylhomocysteine | 179, 228, 294 | |
| MurA | P0A749 | UDP-N-acetylglucosamine-1-carboxyvinyltransferase | 5.81/44,817.65 | Cell wall formation; adds enolpyruvyl to UDP-N-acetylglucosamine; target for the antibiotic phosphomycin | 179 | ||
| MurE | P22188 | UDP-N-acetylmuramoylalanyl-d-glutamate-2,6-diaminopimelate ligase | 5.43/53,212.40 | 5.44/54,493 | Involved in peptidoglycan biosynthesis forming cell wall | 228, 294 | |
| MurG | P17443 | UDP-N-acetylglucosamine-N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase | 9.74/37,683.53 | Cell wall formation; catalyzes the transfer of a GlcNAc subunit on undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide (lipid intermediate I) to form undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide) GlcNAc (lipid intermediate II) | 179 | ||
| MutS | P23909 | DNA mismatch repair protein | 5.39/95,246.90 | 5.42/100,213 | Involved in the repair of mismatches in DNA; possibly carries out the mismatch recognition step | 228, 294 | |
| NadA | P11458 | Quinolinate synthetase A | 5.19/38,240.8 | Involved in NAD biosynthesis: step 2; catalyzes the condensation of iminoaspartate with dihydroxyacetone phosphate to form quinolinate | 179 | ||
| NadC | P30011 | Nicotinate-nucleotide pyrophosphorylase (carboxylating) | 5.07/32,630.87 | See NadA: step 3 (final) | 179 | ||
| NadE | P18843 | NH3-dependent NAD+ synthetase | 5.41/30,636.83 | 5.43/45,820 (5-6) | Catalyzes a key step in NAD biosynthesis, transforming deamido-NAD into NAD by a two-step reaction | 179, 287 | |
| 5.34/42,978 (5-6) | |||||||
| NagE | P09323 | PTS system N-acetylglucosamine-specific EIICBA component | 5.78/68,346.89 | The phosphoenolpyruvate-dependent sugar PTS, a major carbohydrate active-transport system, catalyzes the phosphorylation of incoming sugar substrates concomitant with their translocation across the cell membrane; involved in N-acetylglucosamine transport | 179 | ||
| NagZ | P75949 | Beta-hexosaminidase | 5.86/37,594.73 | Involved in cell wall synthesis | 179 | ||
| NarH | P11349 | Respiratory nitrate reductase 1 beta chain | 6.36/58,066.41 | The nitrate reductase enzyme complex allows E. coli to use nitrate as an electron acceptor during anaerobic growth; the beta chain is an electron transfer unit containing four cysteine clusters involved in the formation of iron-sulfur centers; electrons are transferred from the gamma chain to the molybdenum cofactor of the alpha subunit; induced by nitrate | 179 | ||
| NarW | P19317 | Respiratory nitrate reductase 2 delta chain | 4.45/26,160.59 | Required for the assembly of the nitrate reductase-cytochrome b-nitrate reductase complex to be fully active in the membrane | 179 | ||
| Ndk | P0A763 | Nucleoside diphosphate kinase | 5.55/15,332.25 | 5.59/15,260 | Plays major role in the synthesis of nucleoside triphosphates other than ATP; the ATP gamma phosphate is transferred to the NDP beta phosphate via a ping-pong mechanism by use of a phosphorylated active-site intermediate | 179, 228, 287, 294 | |
| 5.19/14,514 (5-6) | |||||||
| 5.53/14,556 (5-6) | |||||||
| NemA | P77258 | N-Ethylmaleimide reductase | 5.80/39,516.41 | 5.78/39,021 (DIGE 4.5-6.5) | 179, 321 | ||
| NfnB | P38489 | Oxygen-insensitive NAD(P)H nitroreductase | 5.80/23,905.19 | 5.83/24,964 (DIGE 4.5-6.5) | Involved in reduction of a variety of nitroaromatic compounds using NADH (and to a lesser extent NADPH) as a source of reducing equivalents: two electrons are transferred; capable of reducing nitrofurazone, quinones, and the antitumor agent CB1954 [5-(aziridin-1-yl)-2,4-dinitrobenzamide] | 179, 287, 321 | |
| 5.09/14,115 (4.5-5.5) | |||||||
| 5.55/25,091 (4.5-5.5) | |||||||
| 5.47/32,502 (5-6) | |||||||
| 5.32/28,018 (5-6) | |||||||
| Nfo | P0A6C1 | Endonuclease IV | 5.43/31,479.53 | Plays a role in DNA repair; induced by agents which generate superoxide radical anions such as menadione; controlled by soxR | 88 | ||
| NikA | P33590 | Nickel-binding periplasmic protein | 5.51/56,302.05 | Involved in a nickel transport system, probably represents the nickel binder; induced by low pH during anaerobic growth | 323 | ||
| NlpA | P04846 | Lipoprotein 28 | 5.29/27,069.69 | 5.15/17,637 (DIGE 4.5-6.5) | 179, 321 | ||
| NlpB | P0A903 | Lipoprotein 34 | 4.96/34,371.39 | 4.88/35,000 (DIGE 4.5-6.5) | 179, 321 | ||
| NlpD | P0ADA3 | Lipoprotein | 9.26/37,534.85 | 8.84/38,798 (6-11) | May be involved in stationary-phase survival | 287 | |
| NuoB | P0AFC7 | NADH-quinone oxidoreductase chain B | 5.59/25,055.82 | 5.46/25,413 (DIGE 4.5-6.5) | NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain; the immediate electron acceptor for the enzyme in this species is believed to be ubiquinone; couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane) and thus conserves the redox energy in a proton gradient | 179, 287, 321 | |
| 5.42/30,843 (5-6) | |||||||
| NuoE | P0AFD1 | NADH-quinone oxidoreductase chain E | 5.4/18,590.05 | See NuoB | 179 | ||
| NuoG | P33602 | NADH-quinone oxidoreductase chain G | 5.85/100,168.01 | See NuoB | 179 | ||
| NusA | P0AFF6 | Transcription elongation protein | 4.53/54,870.92 | 4.55/61,127 | Participates in both the termination and antitermination of transcription; binds directly to the core enzyme of the DNA-dependent RNA polymerase and also interacts with lambda N protein, RNA, Rho factor, and perhaps NusB; increases during the low temp at 10°C | 133, 179, 212, 228, 294 | |
| NusB | P0A780 | N utilization substance protein B | 6.6/15,689.06 | One of the proteins essential for the formation of the RNA polymerase antitermination complex in the presence of lambda phage N protein; involved in the transcription termination process at certain sites during normal bacterial growth | 179 | ||
| NusG | P0AFG0 | Transcription antitermination protein | 6.33/20,400.32 | 5.93/22,161 | Influences transcription termination and antitermination; acts as a component of the transcription complex and interacts with the termination factor Rho and RNA polymerase | 179, 286, 287 | |
| 5.44/14,684 (5-6) | |||||||
| 6.40/21,860 (6-11) | |||||||
| OmpA | P0A910 | Outer membrane protein A | 5.60/35,172.28 | 5.55/34,419 | Required for the action of colicins K and L and for the stabilization of mating aggregates in conjugation; serves as a receptor for a no. of T-even like phages and also acts as a porin with low permeability that allows slow penetration of small solutes; induced by low pH during aerobic or anaerobic growth but decreases by phosphate limitation and SOS induction | 75, 179, 228, 274, 287, 293, 294, 321, 323 | |
| 5.36/32,707 | |||||||
| 5.34/28,591 | |||||||
| 5.50/33,875 (DIGE 4.5-6.5) | |||||||
| 5.38/33,875 (DIGE 4.5-6.5) | |||||||
| 5.23/14,346 (5-6) | |||||||
| 5.25/13,108 (5-6) | |||||||
| OmpC | P06996 | Outer membrane protein C | 4.48/38,307.50 | —/36,000 | Forms passive diffusion pores which allow small-molecular-wt hydrophilic materials across the outer membrane; negatively regulated by Lrp | 61 | |
| OmpF | P02931 | Outer membrane protein F | 4.64/37,084.49 | 4.61/36,170 | Forms passive diffusion pores which allow low-mol wt hydrophilic materials across the outer membrane; receptor for the bacteriophage T2; positively regulated by Lrp; decreases during phosphate limitation and after benzoic acid treatment and treatment with superoxide-generating agents such as menadione | 61, 88, 179, 228, 293, 294, 321 | |
| 4.56/37,316 (DIGE 4.5-6.5) | |||||||
| 4.64/37,000 (DIGE 4.5-6.5) | |||||||
| 4.71/37,000 (DIGE 4.5-6.5) | |||||||
| OmpR | P0AA16 | Transcriptional regulatory protein | 6.04/27,353.62 | The N terminus of this protein is required for the transcriptional expression of both major outer membrane protein genes ompF and ompC; its carboxyl-terminal moiety mediates the multimerization of the ompR protein | 179 | ||
| OmpT | P09169 | Protease VII | 5.38/33,477.72 | 5.36/37,000 (DIGE 4.5-6.5) | Protease that can cleave T7 RNA polymerase, ferric enterobactin receptor protein (FEP), antimicrobial peptide protamine, and other proteins | 179, 321 | |
| OmpX | P0A917 | Outer membrane protein X | 5.30/16,382.89 | 5.25/16,126 (DIGE 4.5-6.5) | Induced by low or high pH | 179, 274, 321 | |
| 5.15/16,000 (DIGE 4.5-6.5) | |||||||
| OppA | P23843 | Periplasmic oligopeptide-binding protein | 5.85/58,359.84 | 5.93/56,137 | Component of the oligopeptide permease and binding-protein-dependent transport system; binds peptides up to 5 amino acids long with high affinity; increases by high pH during anaerobic growth and after benzoic acid treatment | 179, 228, 286, 287, 294, 321, 323 | |
| 5.70/57,487 | |||||||
| 5.64/56,808 | |||||||
| 5.44/54,493 | |||||||
| 5.93/55,635 (DIGE 4.5-6.5) | |||||||
| 5.82/55,131 (DIGE 4.5-6.5) | |||||||
| 5.71/52,681 (DIGE 4.5-6.5) | |||||||
| 5.13/50,973 (5-6) | |||||||
| 5.47/50,921 (5-6) | |||||||
| 5.63/50,921 (5-6) | |||||||
| 5.65/50,915 (5.5-6.7) | |||||||
| OppD | P76027 | Oligopeptide transport ATP-binding protein | 5.78/37,188.43 | Part of the binding-protein-dependent transport system for oligopeptides; probably responsible for energy coupling to the transport system | 179 | ||
| OsmB | P0ADA7 | Osmotically inducible lipoprotein B | 9.31/4,580.12 | Provides resistance to osmotic stress; may be important for stationary-phase survival; induced by elevated osmotic pressure in the growth medium | 103, 137 | ||
| OsmC | P0C0L2 | Peroxiredoxin | 5.57/14,956.95 | Preferentially metabolizes organic hydroperoxides over inorganic hydrogen peroxide; induced by elevated osmotic pressure and during the decelerating phase, before entry into stationary phase; regulated by a complex network of several global regulators, including at least σS, Lrp, and H-NS | 30, 82, 103 | ||
| OsmE | P0ADB1 | Osmotically inducible lipoprotein E | 6.9/10,061.26 | Induced by elevated osmotic pressure | 179 | ||
| OsmY | P0AFH8 | Osmotically inducible protein Y | 5.42/18,161.15 | Osmotically and stationary phase inducible and controlled by several global regulators, including σS, cAMP-CRP, IHF, and Lrp | 103, 156, 179 | ||
| OtsA | P31677 | α,α-Trehalose-phosphate synthase (UDP-forming) | 6.37/53,479.99 | Induced by growth in glucose-mineral medium of elevated osmotic strength; controlled by σS | 79, 103 | ||
| OtsB | P31678 | Trehalose-phosphatase | 5.38/29,175.28 | 5.32/29,821 (DIGE 4.5-6.5) | Induced by growth in glucose-mineral medium of elevated osmotic strength; controlled by σS | 79, 103, 321 | |
| Pal | P0A912 | Peptidoglycan-associated lipoprotein | 5.59/16,616.32 | May play a role in bacterial envelope integrity; strongly associated with the peptidoglycan | 179 | ||
| PanB | P31057 | 3-Methyl-2-oxobutanoate hydroxymethyltransferase | 5.15/28,237.44 | Involved in pantothenate biosynthesis: first step | 179 | ||
| PanC | P31663 | Pantoate-beta-alanine ligase | 5.92/31,597.67 | 5.63/32,442 (DIGE 4.5-6.5) | See PanB: final step. | 179, 287, 321 | |
| 5.25/35,056 (5-6) | |||||||
| PckA | P22259 | Phosphoenolpyruvate carboxykinase (ATP) | 5.46/59,643.48 | Rate-limiting gluconeogenic enzyme | 179 | ||
| PdhR | P0ACL9 | Pyruvate dehydrogenase complex repressor | 6.04/29,425.47 | Transcriptional repressor for the pyruvate dehydrogenase complex genes aceEF and lpd | 179 | ||
| PdxB | P05459 | Erythronate-4-phosphate dehydrogenase | 6.23/41,367.65 | Involved in de novo synthesis of pyridoxine (vitamin B6) and pyridoxal phosphate; induced as growth rate increases | 179 | ||
| PdxK | P40191 | Pyridoxine kinase | 5.14/30,847.4 | Phosphorylates B6 vitamers; functions in a salvage pathway; uses pyridoxal, pyridoxine, and pyridoxamine as substrates | 179 | ||
| PdxY | P77150 | Pyridoxamine kinase | 6.04/31,322.2 | See PdxK; uses pyridoxamine but has negligible activity toward pyridoxal and pyridoxine as substrates | 179 | ||
| PepB | P37095 | Peptidase B | 5.6/46,180.17 | Probably plays an important role in intracellular peptide degradation | 179 | ||
| PepD | P15288 | Aminoacyl-histidine dipeptidase | 5.20/52,784.22 | 5.22/52,046 (DIGE 4.5-6.5) | Has specificity for the unusual dipeptide beta-alanyl-l-histidine | 179, 321 | |
| PepQ | P21165 | Xaa-Pro dipeptidase | 5.60/50,176.17 | 5.62/50,340 (DIGE 4.5-6.5) | Hydrolyzes Xaa-Pro dipeptides and also acts on aminoacyl-hydroxyproline analogs; increases after benzoic acid treatment | 179, 321 | |
| PfkA | P0A796 | 6-Phosphofructokinase isozyme I | 5.47/34,842.04 | 5.45/38,156 | Involved in key control step of glycolysis; subject to allosteric activation by ADP and other diphosphonucleosides and inhibition by phosphoenolpyruvate | 179, 212, 228, 286, 287, 294, 321 | |
| 5.43/36,348 (DIGE 4.5-6.5) | |||||||
| 5.43/45,820 (5-6) | |||||||
| PfkB | P06999 | 6-Phosphofructokinase isozyme 2 | 5.25/32,455.99 | 5.30/38,005 | See PfkA; sensitive to inhibition by fructose 1,6-diphosphate and increases during phosphate limitation and phosphonate growth | 179, 212, 228, 293, 294 | |
| PflA | P0A9N7 | Pyruvate formate-lyase 1 activating enzyme | 6/28,073.1 | Activates pyruvate formate-lyase 1 under anaerobic conditions by generation of an organic free radical, using S-adenosylmethionine and reduced flavodoxin as cosubstrates to produce 5′-deoxy-adenosine | 179 | ||
| PflB | P09373 | Formate acetyltransferase 1 | 5.69/85,226.01 | 5.62/85,417 | Induced by pfl-activating enzyme under anaerobic conditions by generation of an organic free radical; decreases significantly during phosphate limitation | 179, 212, 228, 293 | |
| 5.61/82,279 | |||||||
| Pgk | P0A799 | Phosphoglycerate kinase | 5.08/40,987.03 | 5.07/41,960 | Involved in the pathway of glycolysis | 179, 228, 286, 287, 294, 321 | |
| 5.02/41,794 | |||||||
| 5.00/41,464 | |||||||
| 5.30/52,681 (DIGE 4.5-6.5) | |||||||
| 5.14/39,792 (4.5-5.5) | |||||||
| 5.15/40,443 (4.5-5.5) | |||||||
| PgmA | P62707 | 2,3-Bisphosphoglycerate-dependent phosphoglycerate mutase | 5.43/58,360.95 | Catalyzes the interconversion of 2-phosphoglycerate and 3-phosphoglycerate; regulated by the Fur protein | 179 | ||
| PheS | P08312 | Phenylalanyl-tRNA synthetase alpha chain | 5.79/36,831.81 | 5.85/38,689 | Changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate | 29, 109, 179, 228, 230, 287, 294 | |
| 5.81/45,820 (5-6) | |||||||
| 5.74/40,430 (5-6) | |||||||
| 5.88/40,109 (5.5-6.7) | |||||||
| PheT | P07395 | Phenylalanyl-tRNA synthetase beta chain | 5.17/87,378.11 | 5.14/94,205 | 29, 228, 294 | ||
| PhnA | P0AFJ1 | Protein | 4.97/12,345.05 | 179 | |||
| PhoA | P00634 | Alkaline phosphatase | 5.54/47,199.79 | 5.54/47,200 | Significantly induced during phosphate limitation and phosphonate growth | 293, 325 | |
| PhoE | P02932 | Outer membrane pore protein E | 4.80/36,835.42 | 4.71/35,743 | Uptakes inorganic phosphate, phosphorylated compounds, and some other negatively charged solutes; induced during phosphate limitation and phosphonate growth | 228, 293, 294 | |
| PhoP | P23836 | Transcriptional regulatory protein | 5.1/25,535.22 | Member of the two-component regulatory system PhoQ/PhoP, involved in the regulation of acid phosphatase | 179 | ||
| PmbA | P0AFK0 | Protein | 5.4/48,369.63 | May facilitate the secretion of the peptide antibiotic microcin B17 (MccB17) by completing its maturation; suppresses the inhibitory activity of the carbon storage regulator (CsrA) | 179 | ||
| Pnp | P05055 | Polyribonucleotide nucleotidyltransferase | 5.11/77,100.97 | 5.10/83,954 | Involved in the RNA degradosome, a multienzyme complex important in RNA processing and mRNA degradation; induced by low temp at 10°C but decreases after benzoic acid treatment | 133, 179, 212, 228, 287, 294, 321 | |
| 5.16/80,468 (DIGE 4.5-6.5) | |||||||
| 5.15/78,105 (DIGE 4.5-6.5) | |||||||
| 5.13/73,960 (4.5-5.5) | |||||||
| PntB | P0AB67 | NAD(P) transhydrogenase subunit beta | 5.72/48,723.03 | The transhydrogenation between NADH and NADP is coupled to respiration and ATP hydrolysis and functions as a proton pump across the membrane. | 179 | ||
| PolA | P00582 | DNA polymerase I | 5.40/103,118.12 | 5.37/108,419 | In addition to polymerase activity, this protein exhibits 3′-to-5′ and 5′-to-3′ exonuclease activity; it can utilize nicked circular duplex DNA as a template and can unwind the parental DNA strand from its template | 228, 294 | |
| PotA | P69874 | Spermidine/putrescine import ATP-binding protein | 5.19/43,028.21 | Part of the ABC transporter complex PotABCD, involved in spermidine/putrescine import; responsible for energy coupling to the transport system | 179 | ||
| PotD | P0AFK9 | Spermidine/putrescine-binding periplasmic protein (SPBP) | 4.86/36,493.22 | 4.77/35,814 | Required for the activity of the bacterial periplasmic transport system of putrescine and spermidine | 179, 228, 287, 294, 321 | |
| 4.88/35,000 (DIGE 4.5-6.5) | |||||||
| 4.95/35,000 (4-5) | |||||||
| 4.76/34,885 (4-5) | |||||||
| 4.97/35,017 (4.5-5.5) | |||||||
| PotF | P31133 | Putrescine-binding periplasmic protein | 5.53/38,254.52 | 5.48/36,267 (DIGE 4.5-6.5) | Required for the activity of the bacterial periplasmic transport system of putrescine; polyamine-binding protein | 179, 321 | |
| PoxB | P07003 | Pyruvate dehydrogenase | 5.86/62,011.38 | 5.86/56,583 | Dependent on a functional rpoS (katF) gene which encodes a sigma factor required to transcribe a no. of stationary-phase-induced genes and reaches a maximum at early stationary phase; decreases under anaerobiosis | 41, 103, 228, 294 | |
| Ppa | P0A7A9 | Inorganic pyrophosphatase | 5.03/19,572.36 | 5.01/21,554 | Induced by low pH during anaerobic growth | 179, 286, 287, 321, 323 | |
| 5.00/22,748 (DIGE 4.5-6.5) | |||||||
| 5.06/21,705 (4.5-5.5) | |||||||
| Ppc | P00864 | Phosphoenolpyruvate carboxylase | 5.52/99,062.61 | Forms oxaloacetate, a four-carbon dicarboxylic acid source for the tricarboxylic acid cycle through the carboxylation of PEP | 179 | ||
| PpiA | P0AFL3 | Peptidyl-prolyl cis-trans isomerase A | 8.17/18,077.44 | 8.52/17,483 (6-11) | PPIases accelerate the folding of proteins. Catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and inhibited by cyclosporin A | 287 | |
| PpiB | P23869 | Peptidyl-prolyl cis-trans isomerase B | 5.51/18,153.47 | 5.51/17,747 | See PpiA | 179, 286, 287 | |
| 5.51/19,192 (5-6) | |||||||
| PpiD | P77241 | Peptidyl-prolyl cis-trans isomerase D | 4.94/68,149.86 | See PpiA; it seems to be involved in the folding of outer membrane proteins. Its preference at the P1 position of the peptide substrate is Glu > Leu > Ala > His > Val > Phe > Ile > Gly > Lys > Thr; induced by heat shock | 179 | ||
| PpsA | P23538 | Phosphoenolpyruvate synthase | 4.93/87,303.88 | 4.94/86,407 | Involved in essential step in gluconeogenesis when pyruvate and lactate are used as a carbon source | 212, 228, 294 | |
| PqqL | P31828 | Probable zinc protease | 5.95/104,656.47 | 179 | |||
| Prc | P23865 | Tail-specific protease | 6.04/74,323.25 | Involved in the cleavage of a C-terminal peptide of 11 residues from the precursor form of penicillin-binding protein 3; may be involved in protection of the bacterium from thermal and osmotic stresses | 179 | ||
| PrfB | P07012 | Peptide chain release factor 2 (RF-2) | 4.64/41,250.73 | Directs the termination of translation in response to the peptide chain termination codons UGA and UAA | 179 | ||
| PrfC | P0A7I4 | Peptide chain RF-3 | 5.65/59,442.89 | Increases the formation of ribosomal termination complexes and stimulates activities of RF-1 and RF-2; binds guanine nucleotides and has strong preference for UGA stop codons; may interact directly with the ribosome; the stimulation of RF-1 and RF-2 is significantly reduced by GTP and GDP but not by GMP | 179 | ||
| ProA | P07004 | Gamma-glutamyl phosphate reductase | 5.42/44,630.05 | 5.39/51,249 (5-6) | Catalyzes the NADPH dependent reduction of l-gamma-glutamyl 5-phosphate into l-glutamate 5-semialdehyde and phosphate in proline biosynthesis; spontaneously undergoes cyclization to form 1-pyrroline-5-carboxylate; | 179, 287 | |
| ProC | P0A9L8 | Pyrroline-5-carboxylate reductase | 5.64/28,144.88 | Involved in proline biosynthesis | 179 | ||
| ProS | P16659 | Prolyl-tRNA synthetase | 5.12/63,692.60 | 5.06/55,572 (4.5-5.5) | 179, 287 | ||
| ProX | P0AFM2 | Glycine betaine-binding | 5.65/33,726.86 | 5.65/33,203 (DIGE 4.5-6.5) | Member of a multicomponent | 103, 179, 191, 321, | |
| periplasmic protein | 5.44/33,098 (DIGE 4.5-6.5) | binding-protein-dependent | 323 | ||||
| transport system (the ProU | |||||||
| transporter) which serves as | |||||||
| the glycine betaine/l-proline | |||||||
| transporter; induced by high | |||||||
| pH during anaerobic growth, | |||||||
| osmotic stress, and the | |||||||
| stationary phase | |||||||
| Prs | P0A717 | Ribose-phosphate pyrophosphokinase | 5.23/34,087.08 | Utilized by both the de novo and the salvage pathways by which endogenously formed or exogenously added pyrimidine, purine, or pyridine bases are converted to the corresponding ribonucleoside monophosphates | 179 | ||
| Psd | P0A8K1 | Phosphatidylserine decarboxylase proenzyme | 5.51/35,934.42 | 179 | |||
| PstS | P0AG82 | Phosphate-binding periplasmic protein | 6.92/34,421.76 | 6.85/45,258 (6-11) | Required for binding-protein-mediated phosphate transport; induced by phosphate deprivation; subject to positive control by PhoB and to negative control by PhoR | 38, 179, 287 | |
| Pta | P0A9M8 | Phosphate acetyltransferase | 5.28/77,040.90 | 5.29/85,417 | Involved in conversion of acetate to acetyl-CoA; induced by high pH but decreases during phosphate limitation | 179, 228, 274, 293, 294 | |
| PtrA | P05458 | Protease III | 5.66/105,113.29 | Endopeptidase that degrades small peptides of less than 7 kDa, such as glucagon and insulin | 179 | ||
| PtsH | P0AA04 | Phosphocarrier protein HPr | 5.65/9,119.37 | 5.65/10,366 | Component of the phosphoenolpyruvate-dependent sugar PTS, a major carbohydrate active-transport system. The phosphoryl group from PEP is transferred to the phosphoryl carrier protein HPr by enzyme I (PtsI); phospho-HPr then transfers it to the permease (enzymes II/III); HPr is common to all PTS; regulated by the cAMP-CRP complex and also by growth on glucose; induced by low pH and phosphonate growth | 27, 228, 293, 294 | |
| PtsI | P08839 | Phosphoenolpyruvate-protein phosphotransferase | 4.78/63,561.84 | 4.78/59,810 | See PtsH; decreases after benzoic acid treatment but increases at phosphonate growth | 56, 179, 228, 293, 294, 321 | |
| 4.91/63,671 (DIGE 4.5-6.5) | |||||||
| PurA | P0A7D4 | Adenylosuccinate synthetase | 5.32/47,213.76 | 5.30/44,524 (DIGE 4.5-6.5) | Plays an important role in de novo pathway of purine nucleotide biosynthesis: AMP biosynthesis; increases after benzoic acid treatment | 179, 321 | |
| 5.30/43,494 (DIGE 4.5-6.5) | |||||||
| PurB | P0AB89 | Adenylosuccinate lyase | 5.68/51,542.81 | Involved in IMP biosynthesis: 5-amino-1-(5-phospho-d-ribosyl)imidazole-4-carboxamide from N(2)-formyl-N(1)-(5-phospho-dribosyl)glycinamide (step 5) | 179 | ||
| PurC | P0A7D7 | Phosphoribosylaminoimidazole-succinocarboxamide synthase | 5.07/26,995.00 | 5.09/26,148 (DIGE 4.5-6.5) | See PurB: step 4; increases after benzoic acid treatment | 179, 321 | |
| PurD | P15640 | Phosphoribosylamine-glycine ligase | 4.96/45,940.36 | 5.06/47,667 (DIGE 4.5-6.5) | See PurB: step 2; increases after benzoic acid treatment | 179, 321 | |
| PurE | P0AG18 | Phosphoribosylaminoimidazole carboxylase catalytic subunit | 6.03/17,649.13 | 6.05/18,899 (DIGE 4.5-6.5) | See PurB: step 3 | 179, 321 | |
| PurH | P15639 | Bifunctional purine biosynthesis protein | 5.53/57,329.21 | 5.50/57,000 (DIGE 4.5-6.5) | See PurB: final step; increases after benzoic acid treatment | 179, 321 | |
| PurK | P09029 | Phosphoribosylaminoimidazole carboxylase ATPase subunit | 5.60/39,461.12 | 5.75/28,100 (5-6) | See PurB: step 3; possesses an ATPase activity that is dependent on the presence of AIR (aminoimidazole ribonucleotide); the association of PurK and PurE produces an enzyme complex capable of converting AIR to CAIR efficiently under physiological condition | 287 | |
| PurM | P08178 | Phosphoribosylformylglycinamidine cyclo-ligase | 4.83/36,722.85 | See PurB: step 2 | 179 | ||
| PurR | P0ACP7 | HTH-type transcriptional repressor | 6.3/38,043.62 | Repressor that binds to the purF operator and coregulates other genes for de novo purine nucleotide synthesis; involved in regulation of purB, purC, purEK, purHD, purL, purMN, and guaBA expression; binds hypoxanthine and guanine as inducers | 179 | ||
| PutA | P09546 | Bifunctional protein | 5.69/143,815.16 | Oxidizes proline to glutamate for use as a carbon and nitrogen source and also functions as a transcriptional repressor of the put operon; induced by proline | 179 | ||
| PykF | P0AD61 | Pyruvate kinase I | 5.77/50,729.42 | 5.65/56,143 (DIGE 4.5-6.5) | Involved in final step of glycolysis | 179, 321 | |
| PyrB | P0A786 | Aspartate carbamoyltransferase catalytic chain | 6.13/34,296.17 | 5.70/93,664 | Involved in UMP biosynthesis: UMP from HCO3− (step 2) | 179, 212, 228, 294 | |
| 6.13/35,321 | |||||||
| PyrC | P05020 | Dihydroorotase | 5.77/38,696.19 | See PyrB: step 3 | 179 | ||
| PyrD | P0A7E1 | Dihydroorotate dehydrogenase | 7.66/36,774.52 | 7.28/47,786 (6-11) | See PyrB: step 4 | 179, 287 | |
| PyrF | P08244 | Orotidine 5′-phosphate decarboxylase | 5.81/26,350.24 | 5.76/56,695 | See PyrB: step 6 (final) | 179, 228, 294, 321 | |
| 5.83/24,964 (DIGE 4.5-6.5) | |||||||
| PyrG | P0A7E5 | CTP synthase | 5.63/60,242.96 | 5.61/58,475 (DIGE 4.5-6.5) | Catalyzes the ATP-dependent amination of UTP to CTP with either l-glutamine or ammonia as the source of nitrogen; allosterically activated by GTP when glutamine is the substrate and also activated by magnesium but inhibited by CTP and by divalent metal ions such as copper and zinc | 179, 321 | |
| PyrH | P0A7E9 | Uridylate kinase | 7.07/25,839.07 | Catalyzes the phosphorylation of UMP to UDP, with ATP as the preferred donor | 179 | ||
| PyrI | P0A7F3 | Aspartate carbamoyltransferase regulatory chain | 6.84/16,989.44 | 6.85/17,023 | Involved in allosteric regulation of aspartate carbamoyltransferase | 179, 212, 228, 287, 294 | |
| 7.01/17,642 (6-11) | |||||||
| RbfA | P0A7G2 | Ribosome-binding factor A | 5.96/15,023.29 | 5.79/17,433 | Essential for efficient processing of 16S rRNA; has affinity for free ribosomal 30S subunits but not for 70S ribosomes; cold shock protein essential for E. coli cells to adapt to low temp | 286, 319 | |
| 6.00/15,635 | |||||||
| RbsB | P02925 | d-Ribose-binding periplasmic protein | 5.99/28,474.47 | 6.85/30,900 | Involved in the high-affinity d-ribose membrane transport system and also serves as the primary chemoreceptor for chemotaxis; repressed in the presence of glucose but induced in the physiological short-term adaptation to glucose limitation | 99, 179, 311, 321 | |
| 5.92/29,066 (DIGE 4.5-6.5) | |||||||
| RbsK | P0A9J6 | Ribokinase | 4.99/32,290.52 | Involved in ribose metabolism | 179 | ||
| RecA | P0A7G6 | Protein RecA | 5.09/37,842.18 | 5.08/41,137 | Catalyzes the hydrolysis of ATP in the presence of single-stranded DNA, the ATP-dependent uptake of single-stranded DNA by duplex DNA, and the ATP-dependent hybridization of homologous single-stranded DNAs; interacts with LexA, causing its activation and leading to its autocatalytic cleavage; induced by low temp at 10°C as well as by cadmium chloride, hydrogen peroxide, and ACDQ | 133, 179, 228, 294, 297 | |
| RelB | P0C079 | Antitoxin | 4.81/9,071.48 | 4.81/9,100 | Counteracts the effect of relE by means of direct protein-protein interaction, enabling the reversion of translation inhibition; acts as an autorepressor of relBE transcription; increased transcription rate of relBE and activation of relE is consistent with a lower level of relB in starved cells due to degradation of relB by protease lon; induced by amino acid starvation | 99 | |
| RfaD | P67910 | ADP-l-glycero-d-manno-heptose-6-epimerase | 4.80/34,893.17 | 4.85/36,820 | Catalyzes the interconversion between ADP-d-glycero-beta-d-manno-heptose and ADP-l-glycero-beta-d-manno-heptose via an epimerization at carbon 6 of the heptose; completely inhibited by ADP and ADP-glucose and partially inhibited by ATP and NADH; induced by heat shock | 179, 228, 287, 294, 321 | |
| 4.94/36,106 (DIGE 4.5-6.5) | |||||||
| 4.98/35,245 (4-5) | |||||||
| RfbB | P37759 | dTDP-glucose 4,6-dehydratase | 5.47/40,558.33 | Involved in carbohydrate biosynthesis and dTDP-l-rhamnose biosynthesis | 179 | ||
| RfbC | P37745 | dTDP-4-dehydrorhamnose 3,5-epimerase | 5.48/21,270.11 | See RfbB | 179 | ||
| Rho | P0AG30 | Transcription termination factor | 6.75/47,004.21 | Facilitates transcription termination by a mechanism that involves rho binding to the nascent RNA, activation of rho's RNA-dependent ATPase activity, and release of the mRNA from the DNA template | 179 | ||
| RibB | P0A7J0 | 3,4-Dihydroxy-2-butanone 4-phosphate synthase | 4.90/23,353.47 | Involved in riboflavin biosynthesis; repressed by heat shock but induced by low pH | 274 | ||
| RibE | P0AFU8 | Riboflavin synthase alpha chain | 5.64/23,444.90 | 5.67/26,821 (5-6) | Involved in final steps of riboflavin synthesis; riboflavin synthase is a bifunctional enzyme complex catalyzing the formation of riboflavin from 5-amino-6-(1′-d)-ribityl-amino-2,4(1H,3H)-pyrimidinedione and l-3,4-dihydrohy-2-butanone-4-phosphate via 6,7-dimethyl-8-lumazine; the alpha subunit catalyzes the dismutation of 6,7-dimethyl-8-lumazine to riboflavin and 5-amino-6-(1′-D)-ribityl-amino-2,4(1H,3H)-pyrimidinedione | 179, 287 | |
| 5.73/25,731 (5.5-6.7) | |||||||
| RibH | P61714 | 6,7-Dimethyl-8-ribityllumazine synthase | 5.15/16,156.51 | 5.15/16,000 (DIGE 4.5-6.5) | See RibE; decreases after benzoic acid treatment | 179, 287, 321 | |
| 5.19/12,875 (4.5-5.5) | |||||||
| RimL | P13857 | Ribosomal protein-serine acetyltransferase | 5.86/20,680.55 | 5.42/40,651 | Acetylates the N-terminal serine of ribosomal protein L7/L12; increases during phosphate limitation and phosphonate growth | 228, 293, 294 | |
| RimM | P0A7X6 | 16S rRNA-processing protein | 4.61/20,605.43 | 4.68/24,436 (DIGE 4.5-6.5) | Essential for efficient processing of 16S rRNA; probably part of the 30S subunit prior to or during the final step in the processing of 16S free 30S ribosomal subunits; could be some accessory protein needed for efficient assembly of the 30S subunit; needed in a step prior to RbfA during the maturation of 16S rRNA; has affinity for free ribosomal 30S subunits but not for 70S ribosomes; decreases after benzoic acid treatment | 321 | |
| RlpA | P10100 | Rare lipoprotein A | 5.09/35,712.71 | 179 | |||
| RmlA1 | P37744 | Glucose-1-phosphate thymidylyltransferase 1 | 5.39/32,693.56 | 5.41/32,544 (DIGE 4.5-6.5) | Catalyzes the formation of dTDP-glucose from dTTP and glucose-1-phosphate as well as its pyrophosphorolysis; decreases after benzoic acid treatment | 321 | |
| Rnc | P0A7Y0 | RNase III | 6.4/25,550.04 | Digests double-stranded RNA; involved in the processing of rRNA precursors and of some mRNAs | 179 | ||
| Rne | P21513 | RNase E | 5.48/118,196.73 | Matures 5S rRNA from its precursors from all the rRNA genes; cleaves RNA I, a molecule that controls the replication of colE1 plasmid DNA; the major endoribonuclease participating in mRNA turnover in E. coli | 179 | ||
| RnfG | P77285 | Electron transport complex protein | 6.59/21,911.91 | May be part of a membrane complex involved in electron transport | 179 | ||
| Rnt | P30014 | RNase T | 5.19/23,522.7 | Responsible for the end turnover of tRNA: specifically removes the terminal AMP residue from uncharged tRNA (tRNA-C-C-A); involved in tRNA biosynthesis, especially in strains lacking other exoribonucleases | 179 | ||
| Rpe | P0AG07 | Ribulose-phosphate 3-epimerase | 5.13/24,554.25 | 179 | |||
| RpiA | P0A7Z0 | Ribose-5-phosphate isomerase A | 5.20/22,860.40 | 5.06/25,943 | Involved in nonoxidative branch of the pentose phosphate pathway; base-induced protein | 179, 274, 286 | |
| RplA | P0A7L0 | 50S ribosomal protein L1 | 9.64/24,598.44 | 8.24/25,577 (6-11) | One of the primary rRNA-binding proteins; binds very close to the 3′ end of the 23S rRNA and forms part of the L1 stalk; translational repressor protein; controls the translation of the L11 operon by binding to its mRNA | 179, 287 | |
| RplI | P0A7R1 | 50S ribosomal protein L9 | 6.17/15,769.06 | 6.20/19,831 | See RplA; increases at phosphonate growth | 29, 179, 228, 286, 287, 293, 294 | |
| 6.17/15,769 | |||||||
| 6.71/10,366 | |||||||
| 5.10/8,830 (4.5-5.5) | |||||||
| 6.36/15,124 (6-11) | |||||||
| RplJ | P0A7J3 | 50S ribosomal protein L10 | 9.04/17,580.4 | 9.04/17,580.4 | Translational repressor protein; controls the translation of the rplJL-rpoBC operon by binding to its mRNA | 179 | |
| RplL | P0A7K2 | 50S ribosomal protein L7/L12 (L8) | 4.60/12,164.00 | 4.87/9,800 (4-5) | Seems to be the binding site for several of the factors involved in protein synthesis and appears to be essential for accurate translation; decreases during phosphate limitation | 179, 287, 293 | |
| 4.71/9,724 (4-5) | |||||||
| 4.74/9,800 (4-5) | |||||||
| RplO | P02413 | 50S ribosomal protein L15 | 11.18/14,980.42 | Binds the 5S rRNA; required for the late stages of subunit assembly | 179 | ||
| RplU | P0AG48 | 50S ribosomal protein L21 | 9.85/11,564.35 | 6.71/10,366 | Binds to 23S rRNA in the presence of protein L20 | 29, 228, 294 | |
| RplY | P68919 | 50S ribosomal protein L25 | 9.60/10,693.44 | 10.61/11,560 (6-11) | One of the proteins that binds to the 5S RNA in the ribosome, where it forms part of the central protuberance; binds to the 5S rRNA independently of L5 and L18; not required for binding of the 5S rRNA/L5/L18 subcomplex to 23S rRNA | 287 | |
| RpmE2 | P0A7N1 | 50S ribosomal protein L31 type B | 9.30/9,920.20 | 8.30/9,673 (6-11) | May be induced under zinc-limiting conditions; may be repressed in that case by the zinc uptake regulation protein Zur | 287 | |
| RpoA | P0A7Z4 | DNA-directed RNA polymerase alpha chain | 4.98/36,511.72 | 5.03/39,691 (DIGE 4.5-6.5) | DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates. Plays an important role in subunit assembly, since its dimerization is the first step in the sequential assembly of subunits to form the holoenzyme; decreases during phosphate limitation | 179, 287, 293, 321 | |
| 5.07/39,523 (DIGE 4.5-6.5) | |||||||
| 5.03/39,792 (4.5-5.5) | |||||||
| 5.06/39,631 (4.5-5.5) | |||||||
| RpoB | P0A8V2 | DNA-directed RNA polymerase beta chain | 5.15/150,632.35 | 5.12/151,314 | See RpoA; decreases during phosphate limitation | 29, 179, 228, 293, 294 | |
| RpoE | P0AGB6 | RNA polymerase sigma-E factor | 5.38/21,695.74 | Involved in heat shock and oxidative stress response | 179 | ||
| RpoS | P13445 | RNA polymerase sigma factor | 4.89/37,971.86 | Induced during transition into stationary phase and in response to a variety of other stress conditions; H-NS and HF-I reduce the expression of sigma S itself by a mechanism that acts at the posttranscriptional level; at present, more than 70 genes or operons are known to be under sigma S control | 19, 204 | ||
| RpoZ | P0A800 | DNA-directed RNA polymerase omega chain | 4.87/10,236.57 | Promotes RNA polymerase assembly | 179 | ||
| RpsA | P0AG67 | 30S ribosomal protein S1 | 4.89/61,158.07 | 4.87/67,214 | Binds mRNA, thus facilitating recognition of the initiation point; needed to translate mRNA with a short Shine-Dalgarno purine-rich sequence; changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate; reduces in the acnA or/and acnB mutants | 29, 109, 179, 228, 230, 278, 287, 294, 321 | |
| 4.97/68,742 (DIGE 4.5-6.5) | |||||||
| 4.99/64,780 (4-5) | |||||||
| 4.99/64,444 (4.5-5.5) | |||||||
| 4.99/42,461 (4.5-5.5) | |||||||
| RpsB | P0A7V0 | 30S ribosomal protein S2 | 6.69/26,612.45 | 4.83/19,048 (DIGE 4.5-6.5) | RpsB is part of the 30S ribosomal subunit. Some nascent polypeptide chains are able to cross-link to this protein in situ | 179, 321 | |
| RpsF | P02358 | 30S ribosomal protein S6 | 4.93/15,703.50 | 5.31/15,844 | Binds together with S18 to 16S rRNA; increases with increasing growth rate but decreases during phosphate limitation | 29, 179, 228, 230, 286, 293, 294, 321 | |
| 5.15/15,862 | |||||||
| 5.26/15,844 | |||||||
| 5.30/16,076 (DIGE 4.5-6.5) | |||||||
| 5.20/16,000 (DIGE 4.5-6.5) | |||||||
| RseB | P0AFX9 | Sigma-E factor regulatory protein | 7.71/33,293.82 | Seems to modulate the activity of RpoE (σE) | 179 | ||
| RsuA | P0AA43 | Ribosomal small-subunit | 5.75/25,865.30 | 5.78/32,101 (DIGE 4.5-6.5) | Responsible for synthesis of | 179, 321 | |
| pseudouridine synthase A | 5.63/32,442 (DIGE 4.5-6.5) | pseudouridine from uracil-516 | |||||
| in 16S rRNA | |||||||
| RtcB | P46850 | Protein | 5.92/45,221.72 | 5.65/34,051 (5-6) | 287 | ||
| 5.68/33,161 (5.5-6.7) | |||||||
| Sbp | P0AG78 | Sulfate-binding protein | 6.38/34,666.89 | 6.49/49,549 (6-11) | Specifically binds sulfate and is involved in its transmembrane transport; repressed by sulfate or cysteine | 287 | |
| SdaB | P30744 | l-Serine dehydratase 2 | 5.51/48,752.92 | Deaminates threonine, particularly when it is present in high concn; transcribed in rich medium, particularly in the absence of glucose and under the control of catabolite activator protein | 179 | ||
| SdhA | P0AC41 | Succinate dehydrogenase flavoprotein subunit | 5.85/64,421.84 | 5.82/64,101 | SdhA is involved in tricarboxylic acid cycle; two distinct, membrane-bound, FAD-containing enzymes are responsible for the catalysis of fumarate and succinate interconversion; the fumarate reductase is used in anaerobic growth, and the succinate dehydrogenase is used in aerobic growth | 179, 228, 286, 294 | |
| 5.74/63,723 | |||||||
| 5.88/63,346 | |||||||
| SdhB | P07014 | Succinate dehydrogenase iron-sulfur protein | 6.31/26,769.86 | See SdhA | 179 | ||
| SelA | P0A821 | l-Seryl-tRNA (Sec) selenium transferase | 6.05/50,607.19 | Converts seryl-tRNA to selenocysteinyl-tRNA during selenoprotein biosynthesis | 179 | ||
| SelD | P16456 | Selenide, water dikinase | 5.3/36,687.26 | Synthesizes selenophosphate from selenide and ATP | 179 | ||
| SerC | P23721 | Phosphoserine aminotransferase | 5.37/39,652.12 | 5.34/40,251 | Required both in major phosphorylated pathway of serine biosynthesis and in the biosynthesis of pyridoxine | 179, 286, 287, 321 | |
| 5.34/38,691 (DIGE 4.5-6.5) | |||||||
| 5.47/39,231 (4.5-5.5) | |||||||
| 5.18/32,682 (4.5-5.5) | |||||||
| 5.02/50,647 (5-6) | |||||||
| 5.26/50,000 (5-6) | |||||||
| SerS | P0A8L1 | Seryl-tRNA synthetase | 5.34/48,414.02 | 5.34/51,045 | 29, 179, 228, 294 | ||
| SgaH | P39304 | Probable hexulose-6-phosphate synthase | 5.02/23,578.08 | 5.14/19,597 | Condensation of d-ribulose 5-phosphate with formaldehyde to form d-arabino-6-hexulose 3-phosphate; probably part of a sugar metabolic pathway along with SgaU and SgaE | 286 | |
| Slp | P37194 | Outer membrane protein | 6.32/19,087.57 | May help to stabilize the outer membrane during carbon starvation and stationary phase; induced upon starvation and slowed growth | 179 | ||
| Slt | P0AGC3 | Soluble lytic murein transglycosylase | 8.48/70,468.66 | Murein-degrading enzyme; catalyzes the cleavage of the glycosidic bonds between N-acetylmuramic acid and N-acetylglucosamine residues in peptidoglycan; may play a role in recycling of muropeptides during cell elongation and/or cell division | 179 | ||
| SlyB | P0A905 | Outer membrane lipoprotein | 8.12/13,818.36 | 179 | |||
| SlyD | P0A9K9 | FKBP-type peptidyl-prolyl cis-trans isomerase | 4.86/20,852.83 | PPIases accelerate the folding of proteins; the activity of SlyD is considerably smaller than the one found in other PPIases with the same substrate; the substrate specificity carried out with suc-Ala-Xaa-Pro-Phe-4NA, where Xaa is the amino acid tested, was found to be Phe > Ala > Leu | 179 | ||
| SodA | P00448 | Superoxide dismutase (Mn) | 6.44/22,965.91 | 6.44/22,920 | Destroys radicals which are normally produced within the cells and which are toxic to biological systems; increases during aerobic growth; enhanced by acnB and acnAB mutations and exposure to methyl viologen or menadione; controlled positively by soxR | 29, 88, 179, 228, 270, 278, 294 | |
| SodB | P0AGD3 | Superoxide dismutase (Fe) | 5.58/21,134.59 | 5.53/22,117 | See SodA; induced by low pH | 29, 179, 274, 286, 287 | |
| 5.49/25,158 (5-6) | |||||||
| 5.45/21,312 (5-6) | |||||||
| SodC | P0AGD1 | Superoxide dismutase (Cu-Zn) | 5.58/15,738.58 | See SodA | 179 | ||
| SohB | P0AG14 | Possible protease | 9.24/39,366.44 | Multicopy suppressor of the htrA (degP) null phenotype; possibly a protease; not essential for bacterial viability | 179 | ||
| SpeB | P60651 | Agmatinase | 5.14/33,557.04 | 5.14/32,339 (DIGE 4.5-6.5) | Catalyzes the formation of putrescine from agmatine; the AUH activity is antagonistically regulated by cyclic AMP-CRP and agmatine; decreases after benzoic acid treatment | 321 | |
| SpeE | P09158 | Spermidine synthase | 5.33/32,190.20 | 5.30/32,000 (DIGE 4.5-6.5) | Regulated mainly by the availability of decarboxylated S-adenosylmethionine; decreases after benzoic acid treatment | 179, 321 | |
| SppA | P08395 | Protease IV | 5.72/67,219.34 | Digests the cleaved signal peptides; necessary to maintain proper secretion of mature proteins across the membrane | 179 | ||
| Ssb | P0AGE0 | Single-strand binding protein | 5.45/18,843.80 | 5.41/23,874 (5-6) | Essential for replication of the chromosomes and its single-stranded DNA phages; involved in DNA recombination and repair; induced by high pH; decreases during phosphate limitation | 274, 287, 293 | |
| 5.46/19,137 (5-6) | |||||||
| SseA | P31142 | 3-Mercaptopyruvate sulfurtransferase | 4.56/30,680.65 | Transfers a sulfur ion to cyanide or to other thiol compounds; has weak rhodanese activity (130-fold lower); participation in detoxification of cyanide perhaps small; may be involved in the enhancement of serine sensitivity | 179 | ||
| SspA | P0ACA3 | Stringent starvation protein A | 5.22/24,173.72 | 5.24/26,625 | Forms an equimolar complex with the RNA polymerase holoenzyme (RNAP) but not with the core enzyme; synthesized predominantly when cells are exposed to amino acid starvation, at which time it accounts for over 50% of the total protein synthesized | 179, 228, 294 | |
| SspB | P0AFZ3 | Stringent starvation protein B | 4.38/18,262.42 | 5.01/20,245 (4-5) | Seems to act in concert with SspA in the regulation of several proteins during exponential- and stationary-phase growth; the exact function of SspB is not yet known, but it is induced by amino acid starvation. | 179, 287 | |
| SucA | P0AFG3 | 2-Oxoglutarate dehydrogenase E1 component | 6.04/105,061.72 | 6.01/103,036 | The 2-oxoglutarate dehydrogenase complex catalyzes the overall conversion of 2-oxoglutarate to succinyl-CoA and CO2; it contains multiple copies of three enzymatic components: 2-oxoglutarate dehydrogenase (E1), dihydrolipoamide succinyltransferase (E2), and lipoamide dehydrogenase (E3); increases during aerobic growth | 228, 270, 294 | |
| SucB | P0AFG6 | Dihydrolipoyllysine residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex | 5.58/43,880.20 | 5.40/52,585 | See SucA; increases during aerobic growth and low pH but decreases during phosphate limitation early and phosphonate growth | 179, 228, 270, 274, 293, 294 | |
| SucC | P0A836 | Succinyl-CoA synthetase beta chain | 5.37/41,392.65 | 5.30/42,377 | Exhibits two interesting properties: “substrate synergism,” in which the enzyme is most active for the catalysis of its partial reactions only when all the substrate-binding sites are occupied, and “catalytic cooperativity” between alternating active sites in the tetramer, whereby the interaction of substrates (particularly ATP) at one site is needed to promote catalysis at the other; induced by low pH; decreases during phosphate limitation | 29, 228, 274, 293, 294 | |
| SucD | P0AGE9 | Succinyl-CoA synthetase alpha chain | 6.31/29,646.28 | 6.16/31,613 | See SucC | 29, 179, 228, 287, 294 | |
| 5.73/29,525 (5-6) | |||||||
| 5.86/28,860 (5.5-6.7) | |||||||
| SufC | P77499 | Probable ATP-dependent transporter | 4.84/27,582.37 | 179 | |||
| SufI | P26648 | Protein | 5.49/49,128.91 | Involved in cell division; suppresses an ftsI mutation | 179 | ||
| SuhB | P0ADG4 | Inositol-1-monophosphatase | 6.45/29,172.13 | 5.71/35,882 (5-6) | 179, 287 | ||
| 5.82/35,119 (5.5-6.7) | |||||||
| SurA | P0ABZ6 | Chaperone | 6.12/45,078.01 | 5.83/45,411 (DIGE 4.5-6.5) | Assists in the folding of extracytoplasmic proteins; essential for the survival of E. coli in stationary phase | 179, 321 | |
| TalA | P0A867 | Transaldolase A | 5.89/35,658.8 | Important for the balance of metabolites in the pentose-phosphate pathway | 179 | ||
| TalB | P0A870 | Transaldolase B | 5.11/35,088.05 | 5.01/35,814 | See TalA | 179, 228, 287, 294, 321 | |
| 5.10/35,000 (DIGE 4.5-6.5) | |||||||
| 5.08/34,734 (4.5-5.5) | |||||||
| Tar | P07017 | Methyl-accepting chemotaxis protein II | 5.39/59,943.7 | Receptor for the attractant l-aspartate and related amino and dicarboxylic acids; mediates taxis to the attractant maltose via an interaction with the periplasmic maltose-binding protein | 179 | ||
| TatA | P69428 | Sec-independent protein translocase protein | 5.73/9,663.98 | Required for correct localization of precursor proteins bearing signal peptides with the twin arginine-conserved motif S/T-R-R-X-F-L-K; this sec-independent pathway is termed TAT for twin arginine translocation system; mainly transports proteins with bound cofactors that require folding prior to export | 179 | ||
| TatB | P69425 | Sec-independent protein translocase protein | 5.13/18,420.87 | See TatA | 179 | ||
| Tdh | P07913 | l-Threonine 3-dehydrogenase | 5.94/37,239.04 | —/38,800 | Involved in threonine catabolism and activated by manganese or cobalt ions | 61 | |
| TehB | P25397 | Tellurite resistance protein | 6.84/22,530.73 | Responsible for potassium tellurite resistance when present in high copy no., probably by increasing the reduction rate of tellurite to metallic tellurium within the bacterium | 179 | ||
| TesA | P0ADA1 | Acyl-CoA thioesterase I | 5.91/20,470.33 | 5.97/19,936 (DIGE 4.5-6.5) | Hydrolyzes only long-chain acyl thioesters (C12-C18); has specificity similar to that of chymotrypsin | 321 | |
| ThiD | P76422 | Phosphomethylpyrimidine kinase | 5.73/28,633.61 | Involved in thiamine pyrophosphate biosynthesis; catalyzes the phosphorylation of 4-amino-5-hydroxymethyl pyrimidine monophosphate to HMP-pyrophosphate (HMP-PP) | 179 | ||
| ThiE | P30137 | Thiamine-phosphate pyrophosphorylase | 5.54/23,015.28 | 5.51/24,964 (DIGE 4.5-6.5) | Involved in thiamine pyrophosphate biosynthesis; condenses 4-methyl-5-(beta-hydroxyethyl)-thiazole monophosphate and HMP-PP to form thiamine monophosphate (TMP) | 179, 321 | |
| ThiG | P30139 | Thiazole biosynthesis protein | 5.36/26,896.1 | Involved in thiamine biosynthesis; required for the synthesis of the thiazole moiety of thiamine | 179 | ||
| ThiJ | Q46948 | Protein | 5.24/20,777.09 | 179 | |||
| ThrA | P00561 | Bifunctional aspartokinase/homoserine dehydrogenase | 5.47/89,120.24 | Involved in amino acid biosynthesis: Lys, Met, or Thr | 179 | ||
| ThrB | P00547 | Homoserine kinase | 5.45/33,623.65 | 5.38/32,000 (DIGE 4.5-6.5) | Involved in threonine biosynthesis from aspirate: step 4; decreases after benzoic acid treatment | 179, 287, 321 | |
| 5.33/37,266 (5-6) | |||||||
| ThrC | P00934 | Threonine synthase | 5.24/47,113.84 | 5.30/48,579 | See ThrB: step 5 (final) | 179, 286, 321 | |
| 5.27/46,667 (DIGE 4.5-6.5) | |||||||
| ThyA | P0A884 | Thymidylate synthase | 5.62/30,479.69 | 6.00/28,285 | Provides the sole de novo source of dTMP for DNA biosynthesis; binds to its mRNA, thus repressing its own translation | 294 | |
| Tig | P0A850 | Trigger factor | 4.83/48,192.67 | 4.83/51,045 | Involved in protein export and acts as a chaperone by maintaining the newly synthesized protein in an open conformation; increases by high pH during anaerobic growth and overproduction of recombinant protein but decreases during phosphate limitation | 138, 179, 228, 287, 293, 294, 321, 323 | |
| 4.94/52,681 (DIGE 4.5-6.5) | |||||||
| 4.96/49,707 (4-5) | |||||||
| 4.58/49,476 (4-5) | |||||||
| 4.97/47,893 (4-5) | |||||||
| 4.87/45,401 (4-5) | |||||||
| 4.93/48,928 (4.5-5.5) | |||||||
| 4.97/48,171 (4.5-5.5) | |||||||
| 4.95/47,057 (4.5-5.5) | |||||||
| TkrA | P37666 | 2-Ketogluconate reductase | 5.5/35,395.5 | Catalyzes the NADPH-dependent reductions of 2,5-diketo-d-gluconate to 5-keto-d-gluconate, 2-keto-d-gluconate to d-gluconate, and 2-keto-l-gulonate to l-idonate | 179 | ||
| TldD | P0AGG8 | Protein | 4.93/51,364.09 | Suppresses the inhibitory activity of the carbon storage regulator csrA | 179 | ||
| TnaA | P0A853 | Tryptophanase | 5.88/52,773.46 | 5.81/48,395 (DIGE 4.5-6.5) | Involved in tryptophan catabolism; increases by high pH during aerobic or anaerobic growth but decreases after benzoic acid treatment | 27, 179, 274, 321, 323 | |
| TolB | P0A855 | Protein | 6.14/43,601.64 | 5.98/43,053 | Involved in the TonB-independent uptake of group A colicins (colicins A, E1, E2, E3, and K); necessary for the colicins to reach their respective targets after initial binding to the bacteria | 179, 228, 286, 287, 294 | |
| 5.91/44,088 | |||||||
| 7.05/35,331 (6-11) | |||||||
| TolC | P02930 | Outer membrane protein | 5.23/51,468.93 | 5.16/50,036 (DIGE 4.5-6.5) | Required for proper expression of outer membrane protein genes such as ompF and nmpC and those encoding protein 2, hemolysin, colicin V, and colicin E1; induced by low pH during anaerobic growth | 179, 321, 323 | |
| TpiA | P0A858 | Triosephosphate isomerase | 5.64/26,971.81 | 5.57/26,972 | Plays an important role in several metabolic pathways; decreases after benzoic acid treatment | 179, 228, 287, 294, 321 | |
| 5.51/25,962 (DIGE 4.5-6.5) | |||||||
| 5.01/11,079 (4-5) | |||||||
| Tpx | P0A862 | Thiol peroxidase | 4.75/17,704.12 | 4.90/18,723 | Has antioxidant activity; could remove peroxides or H2O2 within the catalase- and peroxidase-deficient periplasmic space; induced by low or high pH; decreases after benzoic acid treatment | 179, 228, 274, 287, 321 | |
| 4.99/19,656 (DIGE 4.5-6.5) | |||||||
| 5.02/15,046 (4-5) | |||||||
| 5.00/17,510 (4.5-5.5) | |||||||
| TreA | P13482 | Periplasmic trehalase | 5.36/60,463.80 | Provides the cells with the ability to utilize trehalose at high osmolarity by splitting it into glucose molecules that can subsequently be taken up by the phosphotransferase-mediated uptake system; induced by growth at high osmolarity or by entry into stationary phase; regulated by cAMP-CRP | 103, 244 | ||
| TrmA | P23003 | tRNA (uracil-5-)-methyltransferase | 5.71/41,966.95 | Catalyzes the formation of 5-methyl-uridine at position 54 (M-5-U54) in all tRNA; induced during growth rate-dependent regulation of transcription | 179 | ||
| TrpA | P0A877 | Tryptophan synthase alpha chain | 5.31/28,724.16 | 5.30/28,724 | Responsible for the aldol cleavage of indoleglycerol phosphate to indole and glyceraldehyde 3-phosphate in tryptophan biosynthetic pathway | 29, 179, 228, 287, 294 | |
| 5.38/28,867 (4.5-5.5) | |||||||
| 5.36/27,856 (4.5-5.5) | |||||||
| 5.18/32,691 (5-6) | |||||||
| TrpB | P0A879 | Tryptophan synthase beta chain | 5.71/42,851.82 | 5.75/41,241 (DIGE 4.5-6.5) | Responsible for the synthesis of l-tryptophan from indole and l-serine in tryptophan biosynthetic pathway | 179, 287, 321 | |
| 5.03/33,559 (5-6) | |||||||
| TrpD | P00904 | Anthranilate synthase component II | 6.05/56,738.75 | 6.08/55,915 | Involved in tryptophan biosynthesis | 179, 228, 294 | |
| TrpS | P00954 | Tryptophanyl-tRNA synthetase | 6.27/37,437.82 | 179 | |||
| TrxA | P0AA25 | Thioredoxin 1 | 4.67/11,675.43 | 4.67/11,576 | Participates in various redox reactions through the reversible oxidation of its active center dithiol to a disulfide and catalyzes dithiol-disulfide exchange reactions | 29, 179, 228, 287, 294 | |
| 4.80/9,648 (4-5) | |||||||
| 4.96/9,467 (4.5-5.5) | |||||||
| TrxB | P0A9P4 | Thioredoxin reductase | 5.30/34,491.84 | 5.30/34,419 | May be directly degraded by ClpXP or modulated by a protease-dependent mechanism; increases in the acnA and/or acnB mutants but decreases late during phosphate limitation | 29, 179, 228, 255, 278, 293, 294, 308 | |
| TrxC | P0AGG4 | Thioredoxin 2 | 5/15,554.77 | Efficient electron donor for the essential enzyme ribonucleotide reductase | 179 | ||
| Tsf | P0A6P1 | EF-Ts | 5.22/30,291.79 | 5.15/33,623 | Associates with the EF-Tu-GDP complex and induces the exchange of GDP to GTP; changes very little throughout the normal temp (23-37°C) and increases with increasing growth rate, after benzoic acid treatment, and at low pH during anaerobic growth; decreases during phosphate limitation | 29, 109, 179, 228, 230, 286, 287, 293, 294, 321, 323 | |
| 5.06/33,695 | |||||||
| 5.14/34,596 (DIGE 4.5-6.5) | |||||||
| 5.20/34,415 (DIGE 4.5-6.5) | |||||||
| 5.08/34,633 (DIGE 4.5-6.5) | |||||||
| 5.22/32,484 (4.5-5.5) | |||||||
| Tsr | P02942 | Methyl-accepting chemotaxis protein I | 4.88/59,442.99 | Receptor for the attractant l-serine and related amino acids; responsible for chemotaxis away from a wide range of repellents, including leucine, indole, and weak acids | 179 | ||
| Tsx | P0A927 | Nucleoside-specific channel-forming protein | 4.87/31,413.41 | Constitutes the receptor for colicin K and phage T6 and functions as substrate-specific channel for nucleosides and deoxynucleosides | 179 | ||
| TufA | P0A6N1 | EF-Tu | 5.30/43,182.39 | 5.32/44,615 | Promotes the GTP-dependent binding of aminoacyl-tRNA to the A site of ribosomes during protein biosynthesis; may play an important regulatory role in cell growth and in the bacterial response to nutrient deprivation; changes very little throughout the normal temp (23-37°C) and increases in level with increasing growth rate and after benzoic acid treatment | 29, 109, 179, 228, 230, 287, 294, 321 | |
| 5.38/41,860 | |||||||
| 5.07/32,442 (DIGE 4.5-6.5) | |||||||
| 5.69/25,098 (DIGE 4.5-6.5) | |||||||
| 5.40/42,307 (DIGE 4.5-6.5) | |||||||
| 5.58/45,310 (4.5-5.5) | |||||||
| 5.14/30,073 (4.5-5.5) | |||||||
| 5.39/51,249 (5-6) | |||||||
| 5.20/51,059 (5-6) | |||||||
| 5.32/50,990 (5-6) | |||||||
| 5.35/50,921 (5-6) | |||||||
| 5.34/50,034 (5-6) | |||||||
| 5.31/48,144 (5-6) | |||||||
| 5.33/47,448 (5-6) | |||||||
| TyrB | P04693 | Aromatic amino acid aminotransferase | 5.32/43,537.81 | Involved in amino acid biosynthesis of Phe, Tyr, Asp, and Leu | 179 | ||
| TyrR | P07604 | Transcriptional regulatory protein | 5.54/57,656.14 | Involved in transcriptional regulation of aromatic amino acid biosynthesis and transport; modulates the expression of at least eight unlinked operons; in most cases, causes negative regulation but has positive effects on the TyrP gene at high phenylalanine concentrations | 179 | ||
| TyrS | P0AGJ9 | Tyrosyl-tRNA synthetase | 5.59/47,395.78 | 5.59/44,527 | Decreases late during phosphate limitation | 228, 236, 293, 294 | |
| UbiE | P0A887 | Ubiquinone/ | 7.77/28,073.21 | Involved in menaquinone and ubiquinone | 179 | ||
| menaquinone | biosynthesis | ||||||
| biosynthesis | |||||||
| methyltransferase | |||||||
| UcpA | P37440 | Oxidoreductase | 5.13/27,849.97 | 179 | |||
| Udk | P0A8F4 | Uridine kinase | 6.39/24,353.11 | Involved in pyrimidine salvage pathway | 179 | ||
| Udp | P12758 | Uridine phosphorylase | 5.81/27,027.89 | 5.86/27,960 | Udp catalyzes the reversible phosphorylytic cleavage of uridine and deoxyuridine to uracil and ribose- or deoxyribose-1-phosphate; the produced molecules are then utilized as carbon and energy sources or in the rescue of pyrimidine bases for nucleotide synthesis; increases during high-cell-density cultivation | 179, 228, 287, 294, 325 | |
| 5.66/31,754 (5-6) | |||||||
| 5.71/30,842 (5.5-6.7) | |||||||
| UgpB | P0AG80 | Glycerol-3-phosphate-binding periplasmic protein | 5.98/46,123.95 | 5.98/46,123.95 | SN-Glycerol-3-phosphate and glycerophosphoryl diester-binding protein interacts with the binding-protein-dependent transport system UgpACE; increases in the physiological short-term adaptation to glucose limitation | 311 | |
| Upp | P0A8F0 | Uracil phosphoribosyltransferase | 5.32/22,533.26 | 5.29/23,815 | Involved in pyrimidine salvage pathway; induced by pyrimidine starvation | 179, 228, 294 | |
| Usg | P08390 | USG-1 protein | 4.38/36,364.13 | 179 | |||
| UshA | P07024 | Protein | 5.4/58,208.8 | Degradation of external UDP-glucose to UMP and glucose-1-phosphate, which can then be used by the cell | 179 | ||
| UspA | P0AED0 | Universal stress protein A | 5.12/15,935.18 | 5.14/15,108 | Required for resistance to DNA-damaging agents; induced during growth inhibition caused by the exhaustion of any of a variety of nutrients (carbon, nitrogen, phosphate, sulfate, required amino acid) or by the presence of a variety of toxic agents; positively regulated by guanosine 3′,5′-bisphosphate (ppGpp) and by a recA/ftsK-dependent regulatory pathway but negatively regulated by FadR; also regulated by CspC and CspE; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol and phosphonate growth and at high pH during anaerobic growth | 71, 179, 228, 287, 293, 294, 323 | |
| 5.20/11,633 (4.5-5.5) | |||||||
| UspE | P0AAC0 | Universal stress protein E | 5.16/35,575.69 | 5.30/34,419 | See UspA | 286 | |
| UspF | P37903 | Universal stress protein F | 5.60/16,016.53 | 7.40/30,751 | 228 | ||
| UspG | P39177 | Universal stress protein G | 6.03/15,935.18 | 6.08/15,524 | Interacts with GroEL; induced by starvation and heat shock and in response to some toxic agents | 228, 287 | |
| 6.25/10,623 (6-11) | |||||||
| WrbA | P0A8G6 | Flavoprotein | 5.60/20,714.36 | 5.48/24,005 (DIGE 4.5-6.5) | Seems to enhance the formation and/or stability of noncovalent complexes between the Trp repressor protein and operator-bearing DNA; alone does not interact with the operator-bearing DNA; major target species probably the TrpR/TrpO complex; may function as an accessory element in blocking TrpR-specific transcriptional processes that might be physiologically disadvantageous in the stationary phase of the bacterial life cycle; induced by entry into stationary phase | 103, 179, 287, 321 | |
| 5.51/28,018 (5-6) | |||||||
| WzzB | P76372 | Chain length determinant protein | 5.43/36,454.74 | Confers a modal distribution of chain length on the O-antigen component of lipopolysaccharide; gives rise to a reduced no. of short-chain molecules and increases in numbers of longer molecules | 179 | ||
| WzzE | P0AG00 | Lipopolysaccharide biosynthesis protein | 6.25/39,620.29 | 179 | |||
| XthA | P09030 | Exodeoxyribonuclease III | 5.80/30,969.17 | 5.82/32,430 | Major apurinic-apyrimidinic endonuclease of E. coli; removes the damaged DNA at cytosines and guanines by cleaving on the 3′ side of the AP site by a beta-elimination reaction; induced during the stationary phase | 103, 179, 212, 228, 287, 294 | |
| 5.80/37,049 (5-6) | |||||||
| YadG | P36879 | Hypothetical ABC transporter ATP-binding protein | 8.44/34,647.07 | 179 | |||
| YadK | P37016 | Protein | 5.87/21,112.01 | 5.55/28,425 | 228, 294 | ||
| YadR | P0ACC3 | Hypothetical protein | 4.11/12,100.48 | 179 | |||
| YaeC | Q9F577 | YaeC protein | 9.75/22,948.86 | 179 | |||
| YaeH | P62768 | Hypothetical UPF0325 protein | 6.61/15,096.17 | 179 | |||
| YaeT | P0A940 | Outer membrane protein assembly factor | 4.87/88,426.12 | 5.33/47,448 (5-6) | Involved in the assembly of outer membrane proteins; does not play a direct role in the export of outer membrane lipids | 179, 287 | |
| YahK | P75691 | Zinc-type alcohol dehydrogenase-like protein | 5.80/37,978.38 | 5.82/42,128 (DIGE 4.5-6.5) | 179, 321 | ||
| YajD | P0AAQ2 | Hypothetical protein | 6.14/13,363.99 | 179 | |||
| YajG | P0ADA5 | Hypothetical lipoprotein | 6.79/19,028.51 | 179 | |||
| YajO | P77735 | Hypothetical oxidoreductase | 5.19/36,420.17 | 179 | |||
| YajQ | P0A8E7 | UPF0234 protein | 5.99/19,046.68 | 179 | |||
| YbbL | P77279 | Hypothetical ABC transporter ATP-binding protein | 5.27/25,382.01 | 179 | |||
| YbbN | P77395 | Protein | 4.50/31,791.06 | 4.45/34,596 (DIGE 4.5-6.5) | 179, 321 | ||
| YbdQ | Conserved hypothetical protein, adenine nucleotide-binding domain | 6.03/15,935.18 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16128590) | 179 | |||
| YbeZ | P0A9K3 | PhoH-like protein | 6.24/40,654.52 | 179 | |||
| YbfF | P75736 | Esterase | 5.86/28,437.26 | Displays esterase activity toward palmitoyl-CoA and malonyl-CoA | 179 | ||
| YbgF | P45955 | Hypothetical protein | 7.98/25,448.12 | 179 | |||
| YbgI | P0AFP6 | Hypothetical UPF0135 protein | 5.07/26,892.48 | 179 | |||
| YbhC | P46130 | Acyl-CoA thioester hydrolase | 5.49/43,916.62 | Catalyzes the hydrolysis of the thioester bond in palmitoyl-CoA | 179 | ||
| YbiL | P75780 | Probable TonB-dependent receptor | 5.43/78,341.86 | Probable receptor, TonB dependent, that participates in iron transport | 179 | ||
| YbiS | P0AAX8 | Protein YbiS | 5.6/30,863.3 | 179 | |||
| YbjP | P75818 | Putative lipoprotein | 5.48/16,984.75 | 179 | |||
| YcbL | P75849 | Hypothetical protein | 4.95/23,784.05 | 179 | |||
| YcbY | P75864 | Hypothetical UPF0020/UPF0064 protein | 8.96/78,854.1 | 179 | |||
| YccU | P75874 | Protein | 6.72/14,701.12 | 179 | |||
| YcdO | P0AB24 | Protein | 4.98/41,137.63 | Induced by low pH | 274 | ||
| YceB | P0AB26 | Putative lipoprotein | 5.76/18,653.43 | 5.63/15,568 | 286 | ||
| YceD | P0AB28 | Hypothetical protein | 4.45/19,314.91 | 179 | |||
| YceI | P0A8X2 | Protein | 5.20/18,699.78 | 5.17/21,096 (DIGE 4.5-6.5) | Induced by high pH | 274, 287, 321 | |
| 5.20/19,751 (4.5-5.5) | |||||||
| YcfH | P0AFQ7 | Putative DNase | 5.19/29,808.78 | 179 | |||
| YcfP | P0A8E1 | Hypothetical UPF0227 protein | 6.13/21,226.18 | 179 | |||
| YcgK | P76002 | Protein | 9.01/12,518.82 | 9.31/13,566 (6-11) | 287 | ||
| 9.14/12,279 (6-11) | |||||||
| YchF | Putative GTP-binding protein | 4.87/39,667.32 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16129166) | 179 | |||
| YciD (OmpW) | P0A915 | Outer membrane protein W | 6.03/22,927.83 | Acts as a receptor for colicin S4 | 179 | ||
| YciF | P21362 | Protein | 5.47/18,597.16 | 179 | |||
| YciI | P0AB55 | Protein | 5.19/10,602.04 | 5.29/9,467 (4.5-5.5) | 179, 287 | ||
| YciO | P0AFR4 | Protein | 5.97/23,211.76 | 179 | |||
| YciT | P76034 | Putative HTH-type transcriptional regulator | 5.99/27,602.61 | 179 | |||
| YdaA | Q6SJ49 | Protein | 11.79/6,574.73 | 179 | |||
| YdbC | P25906 | Putative oxidoreductase | 5.32/30,705.98 | 5.32/29,821 (DIGE 4.5-6.5) | 179, 321 | ||
| YdbH | P52645 | Hypothetical protein | 5.67/96,834.76 | 179 | |||
| YdcG | Putative glycoprotein | 5.89/62,757.95 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16129383) | 179 | |||
| YdcS | P76108 | Putative ABC transporter periplasmic binding protein | 6.27/39,954.37 | Probably part of the binding-protein-dependent transport system YdcSTUV; increases in the physiological short-term adaptation to glucose limitation | 311 | ||
| YdeN | P77318 | Putative sulfatase | 5.38/59,928.8 | 179 | |||
| YdgA | P77804 | Protein | 5.07/54,689 | 179 | |||
| YdgH | P76177 | Protein | 9.1/31,910.83 | 179 | |||
| YdhD | P0AC69 | Probable monothiol glutaredoxin | 4.75/12,878.76 | 4.96/9,928 (4.5-5.5) | 179, 287 | ||
| YdhQ | P77552 | Hypothetical protein | 4.42/42,876.01 | 179 | |||
| YdhR | P0ACX3 | Protein | 5.09/9,153.36 | 179 | |||
| YdiA | P0A8A4 | Hypothetical UPF0085 protein | 5.99/31,210.87 | 179 | |||
| YdiJ | P77748 | Hypothetical protein | 6.68/113,248.01 | 179 | |||
| YdiY | P76206 | Hypothetical protein | 4.87/25,248.29 | Induced by low pH | 274 | ||
| YdjA | P0ACY1 | Protein | 6.31/20,059.01 | 179 | |||
| YeaD | P39173 | UPF0010 protein | 5.89/32,666.09 | 179 | |||
| YeaZ | P76256 | Hypothetical M22 peptidase homolog | 5.02/25,180.81 | 179 | |||
| YebC | P0A8A0 | UPF0082 protein | 4.71/26,422.56 | 179 | |||
| YebL | Putative adhesin | 5.8/35,885.06 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16129810) | 179 | |||
| YebR | P76270 | UPF0067 protein | 4.68/20,277.21 | 4.88/20,670 (DIGE 4.5-6.5) | Decreases after benzoic acid treatment | 179, 321 | |
| YebT | P76272 | Hypothetical protein | 5.77/94,970.49 | 179 | |||
| YecM | P52007 | Protein | 5.33/21,205.01 | 179 | |||
| YecO | P76290 | Protein | 5.36/27,776.64 | 5.80/31,295 (5-6) | 179, 287 | ||
| YedD | P31063 | Hypothetical lipoprotein | 4.62/13,518.34 | 179 | |||
| YedO | Putative 1-aminocyclopropane-1-carboxylate deaminase | 5.24/38,705.44 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16129866) | 179 | |||
| YedU | Hypothetical protein b1967 | 5.63/31,190.46 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16129913) | 179 | |||
| YehZ | P33362 | Hypothetical protein | 5.56/30,204.26 | 179 | |||
| YeiP | P0A6N8 | Elongation factor P-like protein | 4.92/21,532.63 | 179 | |||
| YfbB | P37355 | Acyl-CoA thioester hydrolase | 5.97/27,682.36 | Catalyzes the hydrolysis of the thioester bond in palmitoyl-CoA | 179 | ||
| YfbU | P0A8W8 | UPF0304 protein | 6.07/19,536.2 | 179 | |||
| YfcB | P39199 | Hypothetical adenine-specific methylase | 4.62/35,001.73 | 179 | |||
| YfcD | P65556 | Putative Nudix hydrolase | 4.7/20,375.86 | 179 | |||
| YfcE | P67095 | Phosphodiesterase | 5.63/20,122.08 | Shows phosphodiesterase activity, hydrolyzing phosphodiester bonds in the artificial chromogenic substrates bis-p-nitrophenyl phosphate (bis-pNPP), and, less efficiently, dTMP p-nitrophenyl ester (pNP-TMP) and p-nitrophenylphosphorylcholine (pNPPC) | 179 | ||
| YfeX | P76536 | Hypothetical protein | 5.34/33,052.26 | 179 | |||
| YfgC | P66948 | Hypothetical protein | 6.23/51,021.37 | 179 | |||
| YfgL | P77774 | Lipoprotein | 4.72/41,887.21 | May play a role in a homeostatic control mechanism that coordinates the overall outer membrane assembly process | 179 | ||
| YfgM | P76576 | Hypothetical UPF0070 protein | 5.07/22,176.06 | 179 | |||
| YfhQ | P0AE01 | Hypothetical tRNA/rRNA methyltransferase | 5.69/27,047.94 | 179 | |||
| YfiA | P0AD49 | Protein | 6.19/12,653.39 | 6.16/15,238 | 228, 294 | ||
| YfiO | P0AC02 | Hypothetical UPF0169 lipoprotein | 5.48/25,789.87 | 179 | |||
| YfjA | Q9JMR3 | YfjA protein | 6.83/9,376.64 | 179 | |||
| YgaU | P0ADE6 | Protein | 5.72/15,931.92 | 5.70/16,000 (DIGE 4.5-6.5) | Increases after benzoic acid treatment | 179, 321 | |
| YgfZ | P0ADE8 | Protein | 5.18/35,962.93 | 6.11/34,907 (6-11) | 179, 287 | ||
| YggN | P0ADS9 | Hypothetical protein | 8.97/26,429.21 | 179 | |||
| YggS | P67080 | Hypothetical UPF0001 protein | 6.09/25,787.44 | 179 | |||
| YggX | P0A8P3 | Probable Fe2+-trafficking protein | 5.91/10,821.34 | 5.46/12,295 (5-6) | Could be a mediator in iron transactions between iron acquisition and iron-requiring processes, such as synthesis and/or repair of Fe-S clusters in biosynthetic enzymes; necessary to maintain high levels of aconitase under oxidative stress; may have functions related to oxidative stress; increases significantly in the acnB mutant | 278, 287 | |
| YgiN | P0ADU2 | Protein | 5.79/11,532.39 | 5.57/9,851 | 286, 287 | ||
| 5.77/11,202 (5-6) | |||||||
| 5.93/10,432 (5.5-6.7) | |||||||
| YgiW | P0ADU5 | Protein | 4.73/11,976.13 | 179 | |||
| YgjD | Putative O-sialoglycoprotein endopeptidase | 5.92/36,008.4 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16130960) | 179 | |||
| YhbG | P0A9V1 | Probable ABC transporter ATP-binding protein | 5.64/26,669.46 | 179 | |||
| YhbN | P0ADV1 | Protein | 7/17,295.43 | 179 | |||
| YhbS | P63417 | Hypothetical acetyltransferase | 4.57/18,533.79 | 179 | |||
| YhdE | P25536 | Maf-like protein | 5.55/21,515.44 | 179 | |||
| YhdH | P26646 | Protein | 5.63/34,723.76 | 5.58/33,803 (DIGE 4.5-6.5) | 179, 287, 321 | ||
| 5.60/40,548 (5-6) | |||||||
| YhgB | Putative ATP-binding component of a transport system | 5.64/26,800.65 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131091) | 179 | |||
| YhhF | P0ADX9 | Putative methylase | 5.96/21,677.60 | 6.04/22,694 | 228, 294 | ||
| YhiU | Multidrug resistance protein (lipoprotein) | 5.73/41,190.57 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131385) | 179 | |||
| YhjG | P37645 | Hypothetical protein | 8.98/75,130.33 | 179 | |||
| YiaF | P0ADK0 | Hypothetical protein | 9.35/30,158.6 | 179 | |||
| YibO | Phosphoglycerate mutase III, cofactor independent | 5.14/56,193.89 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131483) | 179 | |||
| YidA | P0A8Y5 | Phosphatase | 5.11/29,721.14 | Catalyzes the dephosphorylation of the artificial chromogenic substrate p-nitrophenyl phosphate (pNPP) and of the natural substrates erythrose 4-phosphate and mannose 1-phosphate | 179 | ||
| YieL | P31471 | Protein | 5.23/63,739.36 | 5.78/39,021 (DIGE 4.5-6.5) | Decreases after benzoic acid treatment | 321 | |
| YifE | P0ADN2 | Protein | 6.13/13,002.38 | 179 | |||
| YifL | P0ADN6 | Hypothetical lipoprotein | 6.19/5,098.63 | 179 | |||
| YigL | P27848 | Hypothetical protein | 5.23/29,707.73 | 179 | |||
| YihK | Putative GTP-binding factor | 5.1/65,446.4 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131711) | 179 | |||
| YiiU | P0AF36 | Hypothetical protein | 4.69/9,634.81 | 179 | |||
| YjcF | P32704 | Hypothetical protein | 6.7/49,378.33 | 179 | |||
| YjdC | P0ACU7 | Putative HTH-type transcriptional regulator | 4.95/21,931.08 | 5.00/20,924 (4-5) | 321 | ||
| YjeR | ORF product, hypothetical protein | 5/23,463.6 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131987) | 179 | |||
| YjfH | Gm2251 methyltransferase of 23S rRNA | 6.17/26,556.63 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16132002) | 179 | |||
| YjgF | P0AF93 | UPF0076 protein | 5.36/13,480.40 | 5.29/13,038 | Induced by high pH during anaerobic growth | 179, 228, 294, 323 | |
| YkfE | Inhibitor of vertebrate C-type lysozyme, periplasmic | 6.27/16,872.29 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16128206) | 179 | |||
| YkgG | P77433 | Hypothetical protein | 4.96/25,212.71 | 179 | |||
| YliB | P75797 | Putative binding protein | 7.14/54,291.58 | 7.32/73,914 (6-11) | Probably part of the binding-protein-dependent transport system YliABCD | 179, 287 | |
| YncE | P76116 | Hypothetical protein | 8.8/35,317.06 | 179 | |||
| YneI | P76149 | Aldehyde-dehydrogenase-like protein | 5.44/49,717.86 | 5.38/52,838 (5-6) | 287 | ||
| YnfB | P76170 | Hypothetical protein | 8.07/9,977.98 | 179 | |||
| YniC | P77247 | Protein | 5.75/44,086.36 | 4.84/24,436 (DIGE 4.5-6.5) | Catalyzes the dephosphorylation of the artificial chromogenic substrate pNPP and of the natural substrates 2-deoxyglucose 6-phosphate and mannose 6-phosphate | 321 | |
| YnjE | P78067 | Putative thiosulfate sulfurtransferase | 5.97/45,848.67 | 179 | |||
| YodA | P76344 | Metal-binding protein | 5.66/22,341.86 | 5.51/24,964 (DIGE 4.5-6.5) | May be involved in stress response; induced by cadmium but decreases after benzoic acid treatment | 321 | |
| 5.37/24,787 (DIGE 4.5-6.5) | |||||||
| YqgE | P0A8W5 | UPF0301 protein | 5.34/20,685.91 | 179 | |||
| YqhD | Q46856 | Hypothetical oxidoreductase | 5.72/42,097.02 | 179 | |||
| YqhE | ORF product, hypothetical protein | 6.31/26,951.84 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16130910) | 179 | |||
| YqiC | Q46868 | Hypothetical protein | 6.62/13,762.78 | 179 | |||
| YqiK | P77306 | Inner membrane protein | 5.07/60,699.66 | 179 | |||
| YqjD | P64581 | Hypothetical protein | 9.05/11,051.49 | 179 | |||
| YraM | P45464 | Hypothetical protein | 5.26/72,825.16 | 179 | |||
| YraP | P64596 | Hypothetical protein | 8.9/17,792.13 | 179 | |||
| YraR | P45469 | Hypothetical protein | 8.66/23,197.88 | 179 | |||
| YrbC | P0ADV7 | Protein | 9.12/21,732.74 | 10.28/27,749 (6-11) | 179, 287 | ||
| YrbF | P63386 | Hypothetical ABC transporter ATP-binding protein | 6.16/29,096.81 | 179 | |||
| YrdA | P0A9W9 | Protein | 5.26/20,245.02 | 179 | |||
| YrdC | P45748 | Protein | 4.94/20,767.74 | Binds preferentially to double-stranded RNA | 179 | ||
| YrfE | Conserved protein, MutT-like | 4.85/21,153.17 | Not found in SWISS-PROT/TrEMBL database but available in NCBI database (GI: 16131274) | 179 | |||
| YtfJ | P39187 | Protein | 6.35/18,247.7 | 179 | |||
| YtfQ | P39325 | ABC transporter periplasmic binding protein | 5.77/32,125.88 | 179 | |||
| ZnuA | P39172 | High-affinity zinc uptake system protein | 5.44/31,138.26 | 4.81/40,171 | Decreases after benzoic acid treatment | 286, 287, 321 | |
| 5.41/32,846 | |||||||
| 5.39/31,951 | |||||||
| 5.47/32,751 (DIGE 4.5-6.5) | |||||||
| 5.49/32,476 (DIGE 4.5-6.5) | |||||||
| 5.58/34,244 (4.5-5.5) | |||||||
| 5.51/37,702 (5-6) | |||||||
| Zwf | P0AC53 | Glucose-6-phosphate 1-dehydrogenase | 5.56/55,704.44 | 5.61/51,553 | Involved in first step of pentose phosphate pathway; increases following exposure to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol by superoxide-generating agents such as menadione; controlled by soxR | 71, 88, 228, 294 | |
The table presents a nonredundant list of 715 proteins found in SWISS-2DPAGE (data set I) and other data sources from references (data set II). Entries found in both data sources (234 proteins) are shown in bold. The proteins are all arranged in alphabetical order, and conditions for their expression and induction are described in more detail in the references provided, allowing us to predict proteins induced under different genotypic and/or environmental conditions.
Protein names, accession numbers, and descriptions are from ExPASy Proteomics Server (http://kr.expasy.org/). The search is performed on the current UniProt Knowledgebase release (SWISS-PROT [release 48.8 of 10 January 2006] and TrEMBL [release 31.8 of 10 January 2006]). ORF, open reading.
Theoretical pI/MW ratios were calculated using the Compute pI/Mw tool (http://www.kr.expasy.org/tools/pi_tool.html).
Experimental pI/MW ratios were derived from SWISS-2DPAGE (http://www.kr.expasy.org/cgi-bin/map1) or the references. The narrow pH ranges of IPG strips used are indicated in parentheses.
Protein function and expression characteristics are derived from ExPASy Proteomics Server or the indicated references. The changes in expression levels of proteins under different genotypic and/or environmental conditions are described in more detail mainly as increases (or inductions) and/or as decreases. PFL, pyruvate-formate-lyase.
As another interesting strategy for enhancing the separation capacity of 2-D gels, researchers have employed sample prefractionation methods, such as sequential extractions with increasingly stronger solubilization solutions, subcellular fractionation, selective removal of the most abundant protein components, preparative isoelectric focusing (IEF) separations, and chromatographic fractionation of sample mixtures. This strategy offers the benefits of high protein-loading capability along with the ability to discriminate two or more proteins migrating together. For example, since membrane proteins have proven difficult to solubilize with common solubilization agents such as urea, thiourea, 3-[(3-cholamidopropryl)dimethylammonio]-1-propanesulfonic acid (CHAPS), and dithiothreitol, Molloy et al. (201) introduced a new isolation method of sequential extractions with increasing concentrations of sodium carbonate in analyzing E. coli outer membrane proteins. This led to the successful identification of 21 out of 26 of the predicted integral outer membrane proteins. Similarly, Lai et al. (153) identified more than 200 E. coli membrane proteins by use of the method described by Molloy et al. (201), after modifying it to minimize nonmembrane protein contamination. The largest database of E. coli membrane proteins constructed to date is that reported by Fountoulakis and Gasser (68), who identified 394 different gene products using a method identical to that described by Molloy et al. (201). Notably, these studies demonstrate that membrane proteins, which are commonly absent from 2-D gel maps, are amenable to 2-DE separation using specific techniques.
As an alternative method, high-resolution preparative IEF separation can be combined with the use of narrow-pH-range IPG strips. Several preparative electrophoresis devices, such as Rotofor (Bio-Rad, Hercules, CA), IsoPrime (Amersham Biosciences, Uppsala, Sweden), and the ZOOM IEF fractionator (Invitrogen, Carlsbad, CA), have been developed for increasing the number of proteins separated and detecting less abundant proteins (334). For example, Herbert and Righetti (108) used a multicompartment electrolyzer (MCE) to prefractionate E. coli prior to 2-DE analysis and observed many more spots than with the standard maps available in databases such as SWISS-2DPAGE. This device appears simple, but it still contains large sample chambers (∼100 ml), which are not compatible with samples available in small quantities. Zuo and Speicher (333) prefractionated E. coli using a ZOOM IEF fractionator and found that this initial step greatly enhanced the loading ability, resolution, and detection sensitivity of their 2-D gels. This method greatly conserves proteome samples compared with direct analyses of unfractionated samples on a series of narrow-pH-range 2-D gels. Most interestingly, MicroSol IEF prefractionation is compatible with most downstream proteome-profiling methods, including 1-DE, narrow-pH-range 2-DE, 2-D difference gel electrophoresis (2-D DIGE), and liquid chromatography (LC)-MS/MS methods.
Sample fractionation by chromatography can generate hundreds of fractions for individual 2-DE analysis, allowing enrichment of low-abundance proteins. This results in better qualitative and quantitative analysis of 2-D gels. The combination of LC, 2-DE, and MS/MS has expanded the upper limits of protein visibility typically obtainable by gel-based approaches, but this method has higher costs in terms of price, labor, and time.
Recently, some researchers have focused on subcellular proteomics (or organelle proteomics), which is proteome analysis of the macromolecular architecture of a cell, e.g., subcellular compartments, organelles, macromolecular structures, and multiprotein complexes. This technique has the added benefits of reducing sample complexity, identifying additional unique proteins, localizing newly discovered proteins to specific organelles, and, in some cases, allowing functional validation (121, 281). In terms of the E. coli proteome, subcellular proteomics based on 2-DE can be used to assign various proteins to the cytosol, periplasm, inner membrane, or outer membrane by biochemical fractionation; this method was used to assemble the largest proteome database to date, as shown in Table 2 (179). Analysis of 2,160 spots revealed 575 unique ORF entries, including 151 hypothetical ORF entries, 76 proteins of completely unknown functions, and 222 proteins currently not assigned in the SWISS-PROT database. Of the 575 different entries identified, 241 (42%) were found to exist in more than 1 form, at an average of 7.5 forms per entry. These findings indicate that proteomics involving sample fractionation and 2-DE can be a valuable research technique. However, we have to choose carefully an appropriate fractionation method that prevents substantial and variable protein cross-contamination among the multiple fractions, as this severely complicates the quantitative comparison of protein profiles. A more important factor for quantitative proteome analysis is the need to control separation quality and reproducibility.
The development of improved methodologies for the detection of protein spots has formed the basis for a number of remarkable advances in 2-DE research. A number of general protein detection methods have been developed using organic dyes, silver staining, radiolabeling, reverse staining, fluorescent staining, and chemiluminescent staining. Typically, the majority of researchers have used Coomassie brilliant blue and silver staining for protein detection, but these stains have low sensitivity and narrow linearity, respectively. In case of a radiolabeling method, which is the most sensitive detection method, the potential hazards of working with radioactive material, the limited shelf life, the costs of disposal, and problems with handling mixed waste have decreased its popularity.
Fluorescent dyes provide great sensitivity and broad, linear, dynamic responses compared to their colorimetric counterparts and are compatible with modern downstream protein identification and characterization procedures, such as MS. In comparison to their colorimetric counterparts, fluorophores are easy to handle, have long shelf lives, and have minimal disposal issues. Thus, fluorescence-based protein detection has become a more common practice in recent years. For example, 2-D DIGE was first introduced by Ünlü et al. (289) in 1997 and has been further developed by GE Healthcare (Chalfont St. Giles, Bucks, United Kingdom; formerly Amersham Biosciences, Uppsala, Sweden). The basis of the technique is the use of two or three mass- and charge-matched N-hydroxy succinimidyl ester derivatives of the fluorescent cyanine dyes Cy2, Cy3, and Cy5, which possess distinct excitation and emission spectra. Each labeled sample is then mixed and run simultaneously on a single 2-D gel. However, it should be noted that the use of amino group labels will favor detection of basic proteins over acidic proteins. This technology allows two or three samples to be coseparated under identical electrophoretic conditions, reducing the number of gels required while allowing more-accurate comparative proteome profiling (100). In a case study on the E. coli proteome after benzoic acid treatment (321), 2-D DIGE was shown to produce quantitative results more accurate than those produced with conventional 2-DE. As shown in Table 2 (DIGE pH range, 4.5 to 6.5), a total of 179 differentially expressed E. coli protein spots could be identified by use of matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) and quadrupole-time of flight MS, indicating that this technique not only avoids the complications of gel-to-gel variation but also enables a more accurate and rapid analysis of differences and reduces the number of gels that need to be run. Furthermore, since the gels can be directly scanned and imaged after electrophoresis, this process reduces artifactual features, and the image has a wider dynamic range and more sensitivity than other detection methods.
Recently, researchers have sought to develop detection methods suitable for revealing posttranslational protein modifications, such as glycosylation, phosphorylation, proteolytic modification, S nitrosylation, arginine methylation, and ADP ribosylation (229). For example, the multiplexed proteomics platform allows different samples to be run on separate 2-D gels that are individually stained, thus allowing parallel determination of protein expression levels and certain functional attributes, such as levels of glycosylation or drug-binding and-metabolizing capabilities. These multiplexing techniques have facilitated the use of 2-DE to examine fundamental proteome-wide changes in protein expression and posttranslational modifications in the past few years.
Together, the gel-based methods form the core of proteomic technology and the source of most of the published work on the E. coli proteome, despite their technical shortcomings. To date, 715 E. coli proteins (336 proteins available in the current E. coli SWISS-2DPAGE database plus an additional 379 nonredundant proteins reported in the literature) have been identified on 2-D gels (Fig. 3 and Table 2), with the number of identified proteins continuously increasing. However, it is important to note that an organism will not synthesize all the proteins under a given condition; for example, alkaline phosphatase (PhoA) is not synthesized by E. coli grown in normal growth medium but is significantly induced under a phosphate-limited condition (Table 2). While a great deal of progress in elucidating the E. coli proteome has been made, it is still extremely difficult (if not impossible) to examine the whole proteome of an organism under a given condition. More importantly, 2-DE will likely remain a key technology for the detection of protein variants that undergo proteolytic processing and posttranslational modifications such as phosphorylation or glycosylation. More protein spots will be identified as advanced MS technologies such as MALDI-TOF-MS, electrospray ionization (ESI)-MS, and MS/MS are paired with functional genomic studies based on the complete genome sequence. Thus, the gel-based techniques are, and will likely remain, highly useful tools for assessing differential protein expression.
FIG. 3.
Distribution of E. coli proteins identified by gel-based and non-gel-based approaches. These figures plot the theoretical pI versus the theoretical MW (Mw) of the open reading frame products in E. coli. Shown are images of E. coli proteins identified by gel-based approaches (a) and non-gel-based approaches (b) and the virtual 2-D image of 4,237 E. coli K-12 ORF entries predicted by a predictive proteomic tool (c). Each crossbar represents a protein spot. The numbers of proteins found by gel-based and/or non-gel-based approaches and by predictive proteomic tools are compared in panel d. The total number of E. coli proteins nonredundantly identified by experiments is 1,627 (∼38% of 4,237 ORF entries). For alkaline proteins (pI, >8.0), only 253 proteins (∼19%) out of 1,356 ORF entries were identified so far. For the names and the exact locations of all these protein spots, see Fig. S1 in the supplemental material. The theoretical pI/MW ratios were calculated using the Compute pI/Mw tool (http://www.kr.expasy.org/tools/pi_tool.html).
Non-Gel-Based Approaches
MS has been used for identifying proteins resolved by 2-DE and other methods and also for direct analysis of complex protein mixtures. MS has essentially replaced the classical technique of Edman degradation, even in traditional protein chemistry (1, 111), because it is much more sensitive, can deal with protein mixtures, and offers much higher throughput. The use of MS techniques to identify proteins in complex samples depends on the existence of large protein sequence databases generally derived from DNA-sequencing efforts. There are two main approaches for mass spectrometric protein identification. First, the peptide mass fingerprinting method, initially suggested by Henzel and coworkers (107), involves measurement of the mass spectrum of an eluted peptide mixture, which is then compared with theoretically derived peptide mass databases generated by applying specific enzymatic cleavage rules to predicted and known protein sequences. Typically, protein mixtures are first separated by use of 2-DE, and protein spots are subsequently excised from the gel (251). The proteins contained in the gel pieces are digested using a sequence-specific protease, such as trypsin, and then the resulting peptides are analyzed by MS. When MALDI is used, the samples of interest are solidified within an acidified matrix, which absorbs energy in a specific UV range and dissipates the energy thermally. This rapidly transferred energy generates a vaporized plume of matrix and thereby simultaneously ejects the analytes into the gas phase, where they acquire charge. A strong electrical field between the MALDI plate and the entrance of the MS tube forces the charged analytes to rapidly reach the entrance at different speeds based on their mass-to-charge (m/z) ratios. Because trypsin cleaves the protein backbone at the arginine and lysine residues, the masses of tryptic peptides can be predicted theoretically from protein sequence databases. These predicted peptide masses are compared with those obtained experimentally by MALDI analysis. The protein can be identified correctly if there are sufficient peptide matches with a protein in the databases, resulting in a high score. A high degree of mass accuracy is critical for the unambiguous identification and elimination of the false positives. This technique allows rapid identification of proteins when a fully decoded genome is available. A disadvantage of this approach is that it does not directly provide a sequence-based identification, which results in clustering of proteins with similar masses and necessitates additional effort for the identification.
To solve this problem, a sequence-based approach has been applied to protein identification. In this method, there are two major mass spectrometric strategies that use ESI. The unique feature of ESI is that at atmospheric pressure it allows the rapid transfer of analytes from the liquid phase to the gas phase. The spray device creates droplets, which once in the MS go through a repetitive process of solvent evaporation until the solvent disappears and charged analytes are left in the gas phase. In one strategy, the unseparated mixture of peptides is applied to a low-flow nanoelectrospray device. The peptide mixture is electrosprayed from a very fine needle into the mass spectrometer. Individual peptides from the mixture are isolated in the first step and fragmented during the second step to sequence the peptides (hence MS/MS). Peptide fragments obtained by this method are derived from the N or C terminus of the protein and are designated “b” and “y” ions, respectively (322). The other strategy uses liquid chromatography for initial separation of peptides followed by sequencing as they elute into the electrospray ion source. This method can also be used without gel electrophoresis; in this case, a mixture of proteins is digested in solution and the scrambled sets of peptides are sequenced, ideally resulting in the mixture. A great deal of data can be obtained from a single run done in an automated fashion. The fragmentation data can be used to find matches in various protein and nucleotide sequence databases, including the expressed sequence tag and raw genomic sequence databases.
The most significant breakthrough in non-gel-based approaches was the development of methods involving the combination of n-dimensional prefractionation methods (1-D or 2-D LC) with MS, as shown in Table 1. In these methods, chromatographic separations by affinity, covalent chromatography, strong anion/cation exchange, size exclusion, or the use of packed reactive dye compound or reverse-phase columns are used to reduce the complexity of digested protein mixtures, and this is followed by an MS technique such as MALDI-TOF-MS, ESI-MS, or MS/MS for high-throughput identification of the fractionated peptides. Gevaert et al. (78) identified 800 E. coli proteins from sorted methionine-containing peptides by use of a combination of technologies consisting of combined fractional diagonal chromatography (COFRADIC), LC-MS/MS, and MALDI-TOF-MS (78). More than 1,100 E. coli proteins (a quarter of those encoded in the E. coli genome) were identified by high-performance liquid chromatography (HPLC)-MS/MS analysis (49). Perhaps the most popular of these techniques to date is multidimensional protein identification technology, often referred to as MudPIT (193). In this method, mixtures of trypsin-digested peptides are loaded onto a biphasic microcapillary column containing a strong-cation-exchange resin upstream of a reverse-phase resin directly coupled to an MS/MS. Peptides are displaced from the strong-cation-exchange resin using a salt step gradient and subsequently bind to the reverse-phase resin. Elution from the reverse-phase resin is accomplished using an acetonitrile gradient, and the peptides are analyzed online by MS/MS. Repeated rounds of step and gradient elutions can result in analysis and identification of a large number of peptides in a single run. Vollmer et al. (301) used this approach for the analysis of E. coli cellular extracts originating from lactose- and glucose-grown cultures, which resulted in the identification of 305 and 450 proteins, respectively, from a single experiment within the 95% confidence level. Results with these approaches can be achieved rapidly with small amounts of cell extract, and the software can quickly and accurately analyze the mass and/or sequence data. However, because of the complexity of any given proteome and the separation limits of 1-D or 2-D LC, it is still required to reduce the complexity prior to protein separation and characterization.
An advanced instrument that combines the benefits of high mass accuracy and highly sensitive detection is the Fourier transform ion cyclotron resonance (FTICR) mass spectrometer. FTICR-MS has recently been applied to identify low-abundance compounds or proteins in complex mixtures and to resolve species of closely related m/z ratios (261). Coupled with HPLC and ESI, FTICR-MS is able to characterize single compounds (up to 500 Da) from large combinatorial chemistry libraries and to accurately detect the masses of peptides in a complex protein sample in a high-throughput mode. Jensen and colleagues identified more than 1,000 E. coli proteins using capillary IEF (CIEF) combined with FTICR-MS (126, 127).
Another strategy for monitoring differential protein expression and identifying low-abundance proteins was introduced by Weinberger et al. (309). In this approach, proteins of E. coli lysates were digested, and the resultant peptides were selectively extracted by covalent attachment of methionine residues with bromoacetyl-reactive groups tethered to the surface of glass beads packed in small reaction vessels. The recovered methionine-containing peptides were profiled using the surface-enhanced laser desorption ionization retentate chromatography-MS method. The parent proteins of the selected peptides were then identified using ProteinChip MS/MS (Ciphergen Biosystems, Inc.). Of 34 proteins identified by this method (309), at least 5 (BglX, ParD, YeaM, YfiO, and YhgF; 12% of the total) were low-abundance proteins, demonstrating that this method is capable of visualizing proteins having low expression levels. However, this method does not seem to be suitable for detecting proteins with posttranslational modifications, such as proteolytic truncation, glycosylation, and phosphorylation.
In non-gel-based approaches, it should be noted that the quantities of extracted peptides described above may not truly represent nascent protein abundance, as it is possible that the peptide extraction and liberation steps could be biased by peptide properties such as hydrophobicity. For quantitative comparison, two samples may be labeled with stable isotopes prior to sample separation, either by metabolic incorporation or through chemical derivatization. In this way, proteins derived from the different samples (e.g., normal versus abnormal or untreated versus treated samples) can be directly separated, identified, and quantified using n-D LC-MS/MS (36, 303, 305). A recently developed, attractive method for quantitative comparison of two proteomes is the isotope-coded affinity tag (ICAT) method (331). The ICAT reagent has a protein-reactive group, a biotin tag, and an ethylene glycol linker connecting the two functional groups, which can be synthesized with hydrogen (light ICAT) or deuterium (heavy ICAT). For comparison, one sample is reacted with the light reagent and the second sample is reacted with the heavy reagent under identical labeling conditions. After trypsin digestion, the extremely complex tryptic peptide mixture is simplified by affinity purification of the cysteine-containing derivatized peptides on an avidin affinity resin. The eluted peptides are then analyzed using LC-MS/MS for simpler samples or LC/LC-MS/MS for more-complex samples. The ratios of MS signals from the light and heavy ICAT-labeled forms of the same peptide are compared to determine the relative abundances of the parent protein in the respective samples, and MS/MS is used to identify the proteins. A typical ICAT-MS experiment was used to measure proteome changes in E. coli cells treated with triclosan, an inhibitor of fatty acid biosynthesis (202). The technique provided good quantitative reproducibility and on average identified more than 450 unique proteins per experiment. Furthermore, ICAT-MS identified a number of E. coli proteins that had not previously been identified on 2-DE gels. However, the method was limited in that it was strongly biased to detect acidic proteins (pI, <7), underrepresented small proteins (MW, >10), and failed to detect hydrophobic proteins. Another weakness of the current ICAT method is that it requires the proteins to contain cysteine residues flanked by appropriately spaced protease cleavage sites (102). This problem was highlighted in the study of a multisubunit membrane protein, E. coli FoF1 ATP synthase (20), in which none of the membrane-embedded proteins in the Fo complex could be visualized by ICAT. In the E. coli genome, about 10 to 15% of the proteins do not contain cysteine residues, obviating the use of a cysteine-specific technology as a total-protein indicator. This cysteine-labeling problem could be overcome by devising ICAT reagents that react with other amino acid residues. Chakraborty and Regnier (36) introduced a new isotope-labeling method as a global internal standard technology for identifying and quantifying protein changes during overexpression of β-galactosidase in E. coli. They used N-acetoxysuccinimide and N-acetoxy-[2H3]succinimide to differentially derivatize primary amino groups in peptides extracted and tryptic digested from cultures treated with 0.5 nM or 2 mM isopropyl-β-d-thiogalactopyranoside. However, these authors tested the efficacy of their strategy only with β-galactosidase; this work has not yet been extended to a large-scale proteomic analysis. In another use of the isotopic labeling method, Veenstra et al. (300) identified intact proteins from genomic databases with a combination of accurate molecular mass measurements and partial amino acid content analysis. Proteins extracted from E. coli cells grown in natural-isotopic-abundance minimal medium or minimal medium containing isotopically labeled leucine (Leu-D10) were mixed and analyzed by CIEF coupled with FTICR. The difference in the molecular masses between proteins labeled with the natural isotope or Leu-D10 was used to determine the number of Leu residues present in each protein. Information on the molecular mass and the number of Leu residues present could be used to unambiguously identify intact proteins (e.g., CspE, Mdh, and YggX).
Recently, a multiplexed protein quantitation strategy that provides relative and absolute measurements of proteins in complex mixtures was developed by Ross et al. (253). The multiplex strategy simultaneously determines the relative levels of proteins at multiple states (e.g., several experimental controls or time-course studies) for up to four samples in parallel. A multiplexed set of isobaric reagents that yield amine-derivatized peptides (iTRAQ reagents; Applied Biosystems, CA) was used for labeling at the N termini and lysine side chains of peptides in a digest mixture. The derivatized peptides are indistinguishable in MS but exhibit intense low-mass MS/MS signature ions that support quantitation. Absolute quantitation of targeted proteins can also be achieved using synthetic peptides tagged with one of the members of the multiplex reagent set. Aggarwal et al. (2) used this approach to study rhsA expression in E. coli. They were able to quantify 780 proteins, including several low-abundance proteins, such as transcription factors (DnaB and DnaG).
In addition to identifying proteins, characterizing interactions among proteins is important to understand dynamic biological processes in response to changes in cellular environment, since proteins often function as components of multisubunit complexes. Indeed, protein interactions are observed in nearly all cellular processes, and protein complexes are so ubiquitous that the biological function of an unknown protein can often be predicted from the functions of the proteins with which it is associated. Classically, ligand-binding methods, such as radioreceptor assays, were standard methods of determining protein interactions. Additionally, coimmunoprecipitation studies are commonly used to assess protein-protein interactions. High-throughput analysis of protein-protein interactions is now possible by pulldown assay coupled with MS; this method serves as an important alternative to the yeast two-hybrid system. In pulldown assays, a target is expressed in a cell (in vitro) or added to a cell lysate (in vitro), usually fused with a tag, such as glutathione S-transferase (269), polyhistidine (43, 180), or a tandem affinity purification (TAP) tag (34, 93) or its various relatives, including the sequential peptide affinity tags (34, 327) and split tag (92). The glutathione S-transferase or polyhistidine tag is immunoprecipitated, and associating proteins are then identified by immunological methods, sequencing, or MS. The TAP method was first used to purify complexes containing the acyl carrier protein (ACP) from E. coli. Besides the identification of several known partners of ACP, three proteins, including SpoT, IscS, and MukB, were found to interact with ACP. This method has recently been used to its full potential to build the interaction network of E. coli (34). The TAP procedure for isolating protein complexes makes use of site-specific recombination to introduce a dual tagging cassette into chromosomal loci. E. coli does not readily recombine exogenous linear DNA fragments into its chromosome, but the expression of the lambda general recombination system (λ-Red) markedly enhances integration. This system consists of a DNA cassette bearing a selectable marker and either the TAP or sequential peptide affinity tag into the C termini of ORF products in E. coli. A total of 857 proteins, including 198 proteins that are most highly conserved and soluble nonribosomal ones essential in at least one bacterial species, were tagged successfully. Also, 648 proteins could be purified to homogeneity, and their interacting protein partners were identified by using MS and MS/MS. This network includes many new interactions as well as interactions predicted based solely on genomic inference or limited phenotypic data. However, it is important to verify various interactions observed this way, as there may be false positives.
Taken together, many of the proteins in the E. coli proteome have been identified by using more than one method, whereas others have been uniquely identified by one particular method, indicating that these techniques are complementary to each other. More than 1,486 E. coli proteins from the two major databases (49, 78) were identified using non-gel-based approaches (Fig. 3). A total of 1,627 proteins, which correspond to more than one-third of the E. coli proteins (e.g., the ∼4,237 proteins of E. coli K-12 from the NCBI database), were identified by gel-based and non-gel-based approaches. Among them, 574 proteins were identified in common by gel-based and non-gel-based approaches. The non-gel-based approaches showed clear superiority over 2-DE methods in monitoring alkaline proteins (pI, >8.0) but still need technical improvement.
Non-gel-based analyses can be done for the samples with or without tags, which cause different problems. The former condition results in poor recovery of peptides and proteins by specific amino acid residue labeling, while the latter causes higher complexity and inaccurate quantitation. These problems can lead to the identification of proteins with low confidence (or false positives). Thus, it would be helpful to develop a multiple labeling system for a given sample, which would allow MS analyses of each tag to eliminate false positives and increase confidence. The development of search algorithms and databases with high accuracy is of continued interest and importance. They have been continuously developed and updated, as shown in Table 3. The proper assignment of the MS/MS data to the sequences in the databases would enhance the quality and quantity of data collected by non-gel-based approaches.
TABLE 3.
Useful databases for proteomic and related studies
| Procedure | Database | Description | Website | Reference or source |
|---|---|---|---|---|
| 2-D PAGEa | GELBANK | Database of virtual 2-D gel protein maps and exptl 2-D gel images gallery | http://gelbank.anl.gov | 15 |
| JVirGel | Virtual 2-D gel protein maps | http://www.jvirgel.de/ | 112 | |
| SWISS-2DPAGE | Database containing ∼1,265 entries in 36 reference maps | http://www.expasy.org/ch2d/ | 7 | |
| Protein sequencing | Integr8 | Browser for information relating to completed genomes and proteomes, based on data contained in Genome Reviews and the UniProt proteome sets | http://www.ebi.ac.uk/integr8 | 144 |
| NCBI | Integrated database of gene and protein sequence information, the scientific literature (MEDLINE), molecular structures, and a large number of related resources, including Basic Local Alignment Search Tool (BLAST), protein-protein BLAST (blastp), search results from the conserved domain database (rpsblast), or protein homology by domain architecture (cdart) | http://www.ncbi.nlm.nih.gov | Web page | |
| SWISS-PROT | Curated protein sequence database with a high level of annotation | http://www.expasy.org/sprot | Web page | |
| UniProt | Central database of protein sequence and function created by joining the information contained in UniProtKB/SWISS-PROT, UniProtKB/TrEMBL, and PIR databases | http://www.pir.uniprot.org/ | 18 | |
| Topology prediction | ConPred II | Consensus prediction method for obtaining transmembrane topology models with high reliability | http://bioinfo.si.hirosaki-u.ac.jp/∼ConPred2/ | 8 |
| DAS | Simple method for predicting transmembrane segments in integral membrane proteins based on low-stringency dot plots of the query sequence | http://www.sbc.su.se/%7Emiklos/DAS/ | 52 | |
| HMMTOP | Prediction of both the localization of helical transmembrane segments and the topology of transmembrane proteins | http://www.enzim.hu/hmmtop/ | 288 | |
| MEMSAT2 | Method for the prediction of the secondary structure and topology of integral membrane proteins based on the recognition of topological models | http://bioinf.cs.ucl.ac.uk/psipred/ | 129 | |
| SOSUI | Discrimination of membrane proteins and soluble ones together with the prediction of transmembrane helices | http://sosui.proteome.bio.tuat.ac.jp/%7Esosui/proteome/sosuiframe0E.html | 113 | |
| THUMBUP | Topology prediction of transmembrane helical proteins with mean burial propensity and a hidden Markov model-based method | http://phyyz4.med.buffalo.edu/service.htm | 330 | |
| TMBETA-NET | Discrimination of outer membrane proteins and prediction their membrane spanning β-strand segments | http://psfs.cbrc.jp/tmbeta-net/ | 90 | |
| TMHMM | Prediction of transmembrane regions in proteins based on a global approach implemented by circular hidden Markov models | http://www.cbs.dtu.dk/services/TMHMM/ | 199 | |
| TMPDB | Database of experimentally characterized transmembrane topologies | http://bioinfo.si.hirosaki-u.ac.jp/∼TMPDB/ | 124 | |
| TMpred | Prediction of membrane-spanning regions and their orientations | http://www.ch.embnet.org/software/TMPRED_form.html | 117 | |
| TopPred II | Topology prediction of membrane proteins | http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html | 47 | |
| Prediction of subcellular localization | Cello | Use of the Support Vector Machine trained by multiple feature vectors based on n-peptide compositions | http://cello.life.nctu.edu.tw/ | 326 |
| NNPSL | Use of neural networks trained to predict the subcellular location of proteins in prokaryotic or eukaryotic cells from their amino acid composition | http://predict.sanger.ac.uk/nnpsl | 243 | |
| Proteome Analyst | Use of database text annotations from homologs and machine learning to substantially improve the prediction of subcellular location | http://www.cs.ualberta.ca/∼bioinfo/PA/Sub/ | 181 | |
| PSORT-B | Probabilistic method integrated the analyses of a given protein sequence for amino acid composition, similarity to proteins of known localization, and presence of a signal peptide, transmembrane α-helices, and motifs corresponding to specific localizations | http://www.psort.org/psortb/ | 74 | |
| SignalP | Use of neural networks trained on separate sets of prokaryotic and eukaryotic sequences to predict presence and locations of signal peptide cleavage sites in proteins of different organisms | http://www.cbs.dtu.dk/services/SignalP/ | 216 | |
| SubLoc | Use of Support Vector Machine to predict the subcellular localizations of proteins from their amino acid compositions | http://www.bioinfo.tsinghua.edu.cn/SubLoc/ | 120 | |
| Protein function and interaction | BIND | Expanding database storing full descriptions of molecular interactions, complexes, and pathways | http://www.blueprint.org/ | 16 |
| BLOCKS | Tool for the detection and analysis of protein homology based on alignment blocks representing conserved regions of proteins | http://blocks.fhcrc.org/ | 106 | |
| CluSTr | Clustering of all proteins in TrEMBL and SWISS-PROT based on pairwise similarity | http://www.ebi.ac.uk/clustr/ | 150 | |
| COGS | Clustering of similar proteins in at least three species collected from available genomic sequences | http://www.ncbi.nlm.nih.gov/COG | 280 | |
| CONSURF | Mapping of functional regions on surfaces of proteins using conserved amino acid patterns | http://consurf.tau.ac.il/ | 9 | |
| DiffTool | Clustering of proteins based in similarity | http://bioweb.pasteur.fr/seqanal/difftool/ | 44 | |
| DIP | Documents experimentally determined protein-protein interactions | http://dip.doe-mbi.ucla.edu | 318 | |
| eMOTIF | Database of highly specific and sensitive protein sequence motifs representing conserved biochemical properties and biological functions based on the BLOCKS database and the PRINTS database | http://motif.stanford.edu/emotif/ | 214 | |
| InterPro | Integrated documentation resource for protein families, domains, and functional sites from PROSITE, PRINTS, Pfam, ProDom, SMART, TIGRFAMs, PDB, SUPERFAMILY, and PIR superfamily | http://www.ebi.ac.uk/interpro | 206 | |
| Procedure | Database | Description | Website | Reference or source |
| Pfam | Profiles derived from alignment of protein families, each one composed of similar sequences and analyzed by hidden Markov models | http://www.sanger.ac.uk/Pfam | 273 | |
| PIR | Family and superfamily classification based on sequence alignment | http://www-nbrf.georgetown.edu/ | 76 | |
| PRINTS | Protein fingerprints or sets of unweighted sequence motifs from aligned sequence families | http://www.bioinf.man.ac.uk/dbbrowser/PRINTS/ | 14 | |
| iproClass | Database organized by Prosite patterns and PIR superfamily; neural network system for protein classification into superfamily | http://pir.georgetown.edu/pirwww/dbinfo/iproclass.shtml | 317 | |
| PRODOM | Groups of sequence segments or domains from similar sequences found in SWISS-PROT database by BLASTP algorithm | http://protein.toulouse.inra.fr/prodom.html | 50 | |
| PROSITE | Groups of proteins of similar biochemical function on basis of amino acid patterns | http://www.expasy.ch/prosite | 17 | |
| ProtoMap | Classification of SWISS-PROT and TrEMBL proteins into clusters | http://protomap.cornell.edu | 324 | |
| ProtoNet | Automatic hierarchical clustering of SWISS-PROT proteins | http://www.protonet.cs.huji.ac.il/ | 259 | |
| SMART | Database of signaling domain sequences with accurate alignments | http://smart.embl-heidelberg.de | 264 | |
| STRING | A tool to retrieve and display the genes; a query gene repeatedly occurs within clusters on the genome for predicting functional associations between proteins | http://www.bork.embl-heidelberg.de/STRING | 272 | |
| SYSTERS | Classification of all sequences in the SWISS-PROT and PIR databases into clusters based on sequence similarity | http://systers.molgen.mpg.de/ | 148 | |
| Protein structure determination | 3D-Ali | Aligned protein structures and related sequences using only secondary structures assigned with the known three-dimensional architectures | http://139.91.72.10/def2/def2.html | 227 |
| 3DCA | Cluster analysis integrating structural and sequence information to obtain predictions about functionally relevant clusters of residues | http://www.rlandgraf.med.ucla.edu/3DCA.html | Website | |
| 3D-PSSM | Structural alignments of homologous proteins of similar three-dimensional structure in the SCOP (structural classification of proteins) database | http://www.sbg.bio.ic.ac.uk/3dpssm | 143 | |
| HOMSTRAD | Structure-based alignments organized at the level of homologous families | http://www-cryst.bioc.cam.ac.uk/homstrad/ | 197 | |
| HSSP | Homology-derived secondary structure of proteins (HSSP) by aligning to each protein of known structure | http://swift.embl-heidelberg.de/hssp/ | 257 | |
| LPFC | Structural alignments of protein families and computed avg core structures for each family | http://smi-web.stanford.edu/projects/helix/LPFC/ | 262 | |
| Protein identification from MS and MS/MS data | BioWorks | Redesigned and enhanced software based on SEQUEST | http://www.thermo.com/com/cda/product/detail/1,1055,16483,00.html | Website |
| Mascot | Software of probability-based protein identification by searching sequence databases using MS/MS data | http://www.matrixscience.com/ | 232 | |
| MS-Fit/MS-Tag/MS-Seq | Software with peptide-mass fingerprinting data from MS, fragment-ion tag data, or sequence tag data from MS/MS data | http://prospector.ucsf.edu/ | Website | |
| PepFrag | Software for searching protein and DNA sequences | http://prowl.rockefeller.edu/prowl/pepfragch.html | 63 | |
| PeptideSearch | Search engine from MS and MS/MS data | http://www.narrador.embl-heidelberg.de/GroupPages/PageLink/peptidesearchpage.html | 80 | |
| ProbID | Software tool designed to identify peptides from MS/MS data on the basis of a sequence database. Score function based on a probabilistic model and the Bayesian method | http://projects.systemsbiology.net/probid/ | 329 | |
| SEQUEST | Software that automatically identifies proteins by comparing exptl MS/MS data with standard protein and DNA databases | http://www.thermo.com/or http://fields.scripps.edu/sequest/ | 60 | |
| Spectrum Mill | Software introduced the intelligent extraction and processing of data before search begins | http://www.chem.agilent.com/scripts/pds.asp?Lpage=7771 | Website | |
| X!Tandem | Software that can match MS/MS spectra with peptide sequences in a process that has come to be known as protein identification | http://www.thegpm.org/TANDEM/ | Website |
PAGE, polyacrylamide gel electrophoresis.
Predictive Proteomics
Although the complete proteome of an organism cannot be obtained by gel-based or non-gel-based approaches, it can be predicted from the complete genome sequence. The predictive proteome of E. coli MG1655 was examined in this manner and was found to consist of 4,288 ORF products (28). The predictive proteome can be displayed like a 2-D gel, as shown in Fig. 3c, represented by the predicted isoelectric point (pI) versus the predicted molecular masses of the putative ORF products by use of the Compute pI/Mw tool in ExPASy (http://www.expasy.org/tools/pi_tool.html) (295) or virtual 2-D databases (Table 3). Predictive data readily can be compared with the experimental data from actual proteome analyses. For example, alkaline proteins (pI, >8.0), which include 253 proteins (∼19%) out of 1,356 ORF entries identified, are currently underestimated. Recently, a more realistic virtual 2-D gel was created based on the relationship between expression-level-dependent features in codon usage and protein abundance (194). Compared with results from a real 2-D gel experiment conducted with a protein extract from exponentially growing E. coli cells, many abundant proteins identified in the real gel corresponded to abundant proteins in the virtual 2-D gel. This computational approach can help researchers to determine the appropriate 2-D gel composition for optimal separation of proteins. Thus, predictive proteomics can be used to extract valuable information on the function, topology, localization, and structure of E. coli proteins. In recent years, many bioinformatics researchers have created and developed computer-based tools and databases, as shown in Table 3. For example, protein topology prediction methods allow identification of possible membrane-bound proteins, allowing researchers to predict protein location and sometimes even function and structure. Several programs for predicting transmembrane segments exist, with prediction accuracies reportedly as high as 80% (124, 199, 330). Predicting the subcellular localization of proteins by computational method has been attracting much research interest during recent years. Computational methods for predicting protein subcellular localization can generally be divided into the following four categories based on the prediction method (58): (i) by the overall amino acid composition, (ii) by known targeting sequences, (iii) by sequence homology and/or motifs, and (iv) by a hybrid method which combines the above three elements. Several tools listed in Table 3 allow researchers to readily identify protein localizations and functions and to estimate the efficiencies of different methods, such as subcellular fractionation.
Recently, a neural network-based method was used to predict the bonding state of cysteines from the protein sequence (187), allowing researchers to predict the entire content of disulfide-rich proteins in a proteome (the so-called disulfide proteome). The formation of disulfide bonds between the paired cysteine residues is a key step in the folding process of many proteins. This method predicted the percentage of proteins with disulfide bonds (6% of 4,173 proteins) in E. coli K-12 with 86% accuracy. The percentage of proteins with disulfide bonds is higher in the extracytoplasmic compartment (18% of 405 proteins) than in the cytoplasmic space (5% of 2,796 proteins), confirming that the extracytoplasmic proteins are more likely to form disulfide bridges due to a more oxidizing environment.
In addition, predictive proteomics can identify a significant number of previously unknown candidate proteins within an organism or might reveal interesting characteristics of the organism. For example, the histograms of pI values computationally estimated for all predicted ORF products encoded by the fully sequenced genomes revealed bimodality in bacterial and archaeal genomes and trimodality in eukaryotic genomes (265). The nuclear proteins have a broader distribution that accounts for the third mode observed in eukaryotes. This distribution suggests that whole-proteome pI values correlate with subcellular localization of proteins. However, even with all the benefits of computational approaches, the probable functional relations obtained in silico must still be confirmed at least in vitro and ideally in vivo.
CURRENT STATUS OF THE E. COLI PROTEOME
The recent studies on the E. coli proteome can be classified into two main topics: proteomics for biology and proteomics for biotechnology. An enormous number of E. coli proteome studies have focused on improving our biological knowledge regarding proteins and finding members of regulons and/or stimulons under particular conditions (290, 292); these studies are referred to as “proteomics for biology.” Other groups have studied the E. coli proteome under various genetic and/or environmental perturbations in an effort to develop strategies for improving cellular properties and enhancing the production of bioproducts based on comparative proteome profiles (95); these studies are referred to as “proteomics for biotechnology.”
Proteomics for Biology
Proteomics has changed the way in which cellular physiology is studied. Previously, one or more proteins were chosen as models for understanding local physiological phenomena. These days, proteomic studies allow researchers to identify large members of stimulons or regulons and to obtain information that indicates which specific proteins should be studied further. When subjected to environmental perturbations, E. coli cells undergo fundamental changes in cellular physiology and/or morphology, as reflected and directed by changes in the global gene and protein expression patterns. Up- and downregulation of specific protein sets is seen in response to a number of chemical and physical stresses, such as heat, oxidative agents, and hyperosmotic shock; these responses are thought to act as protective mechanisms leading to elimination of the stress agent and/or repair of cellular damage. Thus, the cellular responses, as reflected by the proteome, can differ widely according to the stresses imposed. Comparative proteome profiling under various genotypic and environmental conditions can reveal new regulatory circuits and the relative abundances of protein sets at the system-wide level.
In one of the first studies using proteomics, comparison of 2-D gels allowed identification of a large group of E. coli heat shock proteins (166, 208, 210, 296). In the following years, many E. coli proteomic studies revealed changes in proteome profiles in response to various stresses, such as changes in pH (24, 27, 274, 323), cell density (70, 325), and temperature (109, 291); organic solvents (321); nutrient starvation (293, 311); and anaerobic conditions (271) (Table 2). These studies resulted in the identification of various E. coli stress-induced stimulons (Fig. 4). The applied stresses were found to affect the observable proteome size by anywhere from a few proteins to nearly half of the proteins in the cell. Some of the altered proteins appear to be general stress-induced proteins, while others appear specific to particular environmental stimuli. More importantly, these studies also showed that the responses of an organism to an environmental stimulus are not simply the sum of independent responses of individual genes but rather seem to be a coordinated series of linked events leading to cross-adaptation among the stress responses.
FIG. 4.
The cascade-like regulation observed with various stimulons and/or regulons in a complex regulatory network. The circles indicate regulons, while the rectangles indicate stimulons. Stimulons in which proteins are induced by stimuli such as stationary phase, temperature shock, pH variation, oxidative stress, and starvation are shown in the respectively labeled panels. Regulons shown in large circles are accompanied by small circles which represent major regulators for the corresponding stimuli. One signal activates or represses many regulators, as shown in small circles, to control the transcription and translation of various genes, leading to complex interactions in the cell. For example, E. coli cells enter the stationary phase in response to complex stresses such as cell growth, increased cell density, the presence of byproducts or toxic substances, and inappropriate conditions (restriction of oxygen, low/high temperature and pH, and limitation of nutrients). This complex response is mediated by a variety of specific regulators in addition to the master regulator, RpoS, which is controlled by itself or by other proteins (see the text for a detailed explanation). Abbreviations: HNS, nucleoid-associated protein; IHF, integration host factor; Lrp, leucine-responsive protein; Fis, factor for inversion stimulation.
The complex and physiologically far-reaching responses of E. coli are often under the control of master regulators located at the interface between upstream signal processing and downstream regulatory mechanisms. These master regulators, which serve as the decisive information-processing units, connect complex signaling networks with the downstream regulatory cascades or networks that ultimately control expression of the response-associated genes. A regulon is a set of proteins whose synthesis is regulated by the same regulatory proteins, while a stimulon is a set of proteins whose amount or synthesis rate changes in response to a certain stimulus. At the molecular level, each stimulon may be composed of more than one regulon, each controlled by a different molecular factor. The dissection of stimulons into regulons is based on comparison of the induction patterns in wild-type cells versus those of strains having mutations in known regulatory elements. In this way, regulators such as the RNA polymerase sigma factor RpoS (192), the histone-like protein H-NS (19, 118, 158), and the leucine-responsive regulatory protein Lrp (61) have been studied based on the comparative analysis of wild-type and mutant or double mutant strains.
The highly complex and nonlinear behaviors of these networks have complicated their studies, but proteomic analysis of cells stimulated by one or more signals (stimulons) or cells lacking one or more global regulators (regulons) has provided new insight into the stimulus-response processes in E. coli. Proteomics has allowed researchers to isolate new stress-associated and stress-specific protein markers, identify all proteins controlled by a certain regulatory protein, and understand the integrated cellular metabolic and regulatory networks. Here, several main cellular responses of E. coli under different conditions are described in more detail based on proteome analyses. Moreover, a main regulator and/or its coordinated regulators in response to each stress are discussed.
Stationary-phase response.
At the onset of the stationary phase, E. coli cells undergo a global modification of their protein expression pattern, leading to the acquisition of resistance to complex stresses such as increased cell density, the presence of toxic byproducts, and nutrient limitation. Overall, these properties result in better cell survival under adverse conditions. One top-level master regulator of this genetic program is an RNA polymerase sigma factor called RpoS (σS, σ38, or KatF), which is encoded by the rpoS gene (104, 155). This σS has been reported to control an E. coli regulon comprised of 70 or more genes expressed in response to starvation or during the transition to stationary phase (104). These genes were identified by transcriptional analysis of specific genes as well as by proteomic approaches (Fig. 4).
In terms of upstream signal processing, the σS regulon can be divided into subfamilies of genes regulated by specific stresses and/or additional global regulatory proteins. As shown in Fig. 4, many of these subsets of σS-dependent genes or proteins may also be induced by stresses such as anaerobiosis (12), oxidative stress (6), and osmotic stress (105). Additionally, these genes are regulated by transcription factors specific for certain stress responses (e.g., OxyR, which is involved in the oxidative stress response) or more-global regulators, such as H-NS, IHF, cyclic AMP (cAMP)-cAMP receptor protein (CRP), and Lrp (104). These regulators can individually or coordinately affect many σS-dependent stationary-phase-responsive proteins. For instance, comparative proteome analysis of an H-NS deletion mutant and the wild-type strain revealed that some of the σS-dependent proteins or genes, including rpoS itself, were controlled by the H-NS regulon (19).
In terms of downstream regulatory mechanisms, the starvation-induced DNA protection protein Dps, which is one of the σS-dependent stationary-phase-responsive proteins, was also found to affect the expression of other proteins in E. coli (5). Dps was rapidly degraded during exponential growth by the protease ClpXP (which is regulated by σ32 or RpoH) but was stabilized under conditions of carbon starvation or oxidative stress. This, along with increased Dps synthesis, results in the high-level accumulation of Dps during the stationary phase (276), showing that Dps levels are specifically controlled under certain stress conditions. In addition, studies have shown that σS itself is also controlled by the subregulatory protease ClpXP and the recognition factor RssB (332). Collectively, these findings indicate that σS is controlled by a complex signal transduction network with redundancy, additivity, and internal feedback regulatory loops, resulting in its sophisticated regulations.
Temperature response.
Protein expression in E. coli can be altered significantly when cells are grown at temperatures outside the normal range. This response plays a critical role in protecting the cells from temperature stress, producing tolerance, or repairing cellular systems. The E. coli cellular response to high temperature includes the synthesis of a set of highly conserved proteins known as the heat shock proteins (249). Similarly, a separate, nonoverlapping group of proteins known as cold shock proteins are produced during the period of growth cessation following a shift from 37°C to 10°C (283).
Many of the heat shock proteins are molecular chaperones that function to bind newly synthesized partially folded or unfolded proteins and promote their folding and refolding by limiting the nonproductive interactions that lead to aggregation and misfolding. Some of the other heat shock proteins are proteases that function to degrade misfolded or abnormal proteins (209). These proteins were first recognized as being highly abundant when examined with 2-D gels in 1978 (166). Later, Neidhardt and colleagues (208, 210, 211, 296) used 2-D gels to monitor the synthesis rates of individual proteins before and after heat shock and identified a number of proteins whose synthesis rates were dramatically increased following the temperature increase. Initially, these proteins were named by their positions on 2-D gels. Later, many of the spots were identified as known gene products, including DnaK (77), GroEL (211), GroES (284), GrpE (335), La (lon) protease (237), and LysU (168). For E. coli, at least 34 heat shock proteins have been identified to date by use of a combination of genomics, transcriptional analysis of specific genes, and proteomics (Fig. 4). The characterized proteins include the main cellular chaperones, DnaK and GroELS; the ATP-dependent proteases ClpP, DegP, FtsH (FhlB), HslVU, and La; and other proteins involved in protein folding, refolding, quality control, and degradation (249). Other important heat shock proteins include HTS (homoserine transsuccinylase), which is a key enzyme in methionine biosynthesis (23); protein pairs involved in protein isomerization, such as HtrM (240) and PpiD (55); and the vegetative sigma factor σ70 (33, 91, 282).
The synthesis of the major heat shock protein regulon is controlled by the alternative sigma factor σ32 (encoded by the rpoH gene), which guides RNA polymerase to the heat shock promoters (91). In addition, E. coli contains a second heat-responsive regulon, which is controlled by an alternative sigma factor, σE (encoded by the rpoE gene) (91). It is thus possible that the heat-mediated induction of some genes may occur via other mechanisms and regulators that remain to be elucidated. For example, members of the phage shock protein (Psp) family, which are induced in response to filamentous phage infection as well as in response to heat, ethanol, and osmotic shock, do not require the action of σ32 (32). Furthermore, a large set of heat shock proteins was found to be induced by other stimuli, such as exposure to denaturing conditions (i.e., the presence of alcohols or of heavy metals) (91). The proteins induced under various stress conditions can overlap with one another to degrees ranging from complete overlap to no overlap at all. For example, in E. coli, the heat shock and ethanol stimulons overlap, while the heat shock and cold shock responses have no shared proteins (133).
Additional information on the heat shock response has been obtained by examination of subproteomes. For example, proteins damaged or unfolded by elevated temperatures during heat shock tend to aggregate (198); thus, a proteomic study of the aggregates can be used to define the thermally unstable proteins. This study is also important for the elucidation of cellular protein quality control mechanisms, because damaged proteins can be refolded with the aid of chaperones or can be degraded by proteases (84). An example of one such study is the investigation of E. coli aggregates at various temperatures, which contained 350 to 400 protein species that were all classifiable as substrates of the ClpB and DnaK chaperones (285). Another proteomic study on the DnaKJ- and ribosome-associated trigger factor mutant strains revealed approximately 340 spots of aggregated proteins in two mutant strains. All major aggregated proteins were shared between the two mutants, indicating that they cooperatively assist the folding of newly synthesized proteins in E. coli (57). A similar study indicated that the major cytosolic energy-dependent proteases are involved in preventing aggregation, because protein aggregates formed in their absence showed increased concentrations and contained more protein species (250). These studies suggest that it may be possible to use proteomic analysis of aggregates to identify specific substrates for the various chaperones and proteases.
One major advantage of using 2-D gels to analyze the heat shock proteome is the ability to discriminate posttranslationally modified proteins from their nonmodified forms. The major chaperones, DnaK and GroEL, appear as multiple spots on a narrow range of 2-D gels (pH 4.5 to 5.5), indicating that they may be present in several forms within the E. coli cell (Table 2). In addition, MALDI-TOF peptide mass fingerprinting allowed identification of E. coli ribosomal proteins with posttranslational modifications such as acetylation, methylation, β-methylthiolation, multiple methylations, and amino acid cleavage (11). These findings provide important insights into the regulatory mechanisms of heat shock response by protein modification.
Proteome analysis of E. coli cells adapting to low temperatures has also been carried out. In E. coli, a downshift in temperature causes transient inhibition of most protein synthesis, resulting in a growth lag called the acclimation phase. During the acclimation phase, a group of cold shock proteins is dramatically induced (Fig. 4), while the heat shock proteins remain repressed (133). A single regulatory factor for the cold shock response has not been identified, but some cold-induced proteins are essential for the cells to resume growth at low temperature and have been shown to function in various cellular processes. CspA, CspB, and CspG, which belong to a family of structurally related cold shock proteins, show the highest induction in response to cold shock (207). CspA has long been suspected to play a regulatory role in this response by destabilizing mRNA secondary structures, allowing more-efficient translation at low temperatures (81). The CspA mRNA appears to become more stable during a shift to temperatures below 30°C. In addition members of to the Csp family, 12 out of 16 cold shock proteins in E. coli have been further identified by 2-DE (283). As shown in Fig. 4, these include the following proteins: GyrA, the a-subunit of the topoisomerase DNA gyrase (132, 134); H-NS, a nucleoid-associated DNA-binding protein required for optimal growth at low temperature (157); Hsc66, a homolog of Hsp70 (165); NusA, which is involved in both termination and antitermination of transcription (132); PNP, which is an exoribonuclease (132); and RecA, which is involved in recombination and induction of the cold shock response (283). In addition, three cold shock proteins have been shown to be ribosome associated: initiation factor 2 (IF2); CsdA, which is an RNA-unwinding protein (132); and ribosome-binding factor A (RbfA) (130, 132).
The latter group is interesting in that cells experiencing the transition to a low temperature showed accumulation of 70S monosomes, decreased the number of polysomes, and stabilized RNA and DNA secondary structures (283). This transition decreases efficient mRNA translation and leads to a transient reduction in cell growth rate (131). Both CsdA and RbfA are required for optimal growth at low temperatures, indicating that these two newly produced ribosome-associated proteins, along with enhanced synthesis of IF2, are required for efficient ribosomal function at low temperatures. Comparative 2-DE proteomic analyses of E. coli cultures treated with various antibiotics or with temperature shock (291) showed that the heat and cold shock responses could be mimicked by different sets of ribosome-targeting antibiotics. For example, streptomycin, puromycin, and kanamycin induced protein expression patterns that resemble the heat shock and stringent responses, while tetracycline, chloramphenicol, erythromycin, fusidic acid, and spiramycin invoked the cold shock and relaxed ribosomal responses (291). Based on these findings, researchers have suggested that translational blocks induce heat shock-like or cold shock-like responses, indicating that the state of the ribosome or a ribosomal product may signal these responses.
A recent study on the E. coli cells experiencing a temperature shift from 37°C to 4°C using improved 2-DE methods showed that 69 proteins were overexpressed and that the total number of proteins decreased by 40% (233). Further 2-DE studies on cold shock responses were carried out under several different conditions, including suspended cells at the exponential phase, suspended cells at the stationary phase, and cells immobilized on 2% (wt/vol) agar. Comparative analysis of the proteomes obtained with and without cold shock allowed identification of 203 protein spots showing expression changes during the temperature downshift (between 10 °C and 4°C or when 4°C was reached) compared with synthesis at 37°C using a principal component analysis (234). In suspended E. coli cells, the synthesis levels of 91 proteins (71 newly synthesized proteins and 26 induced proteins) were altered at the exponential phase after cold shock, and the synthesis levels of 59 proteins (34 newly synthesized proteins and 25 induced proteins) were changed at the stationary phase after cold shock. In immobilized E. coli cells, the synthesis of 53 proteins (33 newly synthesized proteins and 20 induced proteins) was induced by cold shock. These results suggest that the number of cold shock-induced proteins was originally underestimated and that further proteomics work will likely uncover a large cohort of cold shock response proteins.
pH response.
Since E. coli requires homeostasis of internal pH in the range from 7.4 to 7.9, the pH response is important for cellular growth and survival under conditions of fluctuating pH levels. In addition, pH plays an important role in pathogenic bacteria. Pathogenic strains of E. coli, Salmonella enterica serovar Typhimurium, Shigella flexneri, and Vibrio cholerae often encounter extreme pH conditions both within and outside human hosts. During pathogenesis, cells are exposed to low pH in the stomach or within the phagosomes and phagolysosomes of intestinal epithelial cells or macrophages. Consequently, low pH induces virulence factors that contribute to pathogenesis, such as the virulence regulator ToxR in V. cholerae (268). Perturbations in pH can exert many significant effects on cell growth and induce different classes of proteins, such as stress proteins, redox modulators, and envelope proteins (223). The major pH-responsive proteins that have been identified by genomics, proteomics, and other technologies (268) are shown in Fig. 4. Among them, the acid stress chaperones HdeA and HdeB enhance survival under extremely acidic conditions (10, 72), while the membrane-bound Na+/H+ antiporter NhaA protects cells from excess Na+ under high-external-pH conditions (141). Proteome analyses have allowed identification of a number of other pH-responsive proteins in E. coli. Initially, 2-D gels were used to elucidate individually the cellular protein responses to changes in pH (294), to aerobiosis, and to anaerobiosis (270, 271). The pH-dependent response is often coinduced by other environmental factors, such as growth phase, anaerobiosis, and various metabolites. Thus, most of the proteomic studies on pH responses have been performed under specific aerobic and/or anaerobic conditions, allowing identification of new classes of acid- and base-dependent regulators and dissection of the relationship between pH and oxygen levels. For example, Blankenhorn et al. (27) identified a total of nine pH-responsive proteins from 18 spots during aerobic or anaerobic growth: five acid-induced proteins (GatY, ManX, PtsH, YfiD, and the aerobic acid-induced protein AhpC); three base-induced proteins (MalE, TnaA, and the anaerobic base-induced protein GadA); and one aerobic acid- or base-induced protein (AceA). Stancik et al. (274) identified a total of 22 pH-dependent proteins from 44 spots during aerobic or anaerobic growth: 9 acid-induced proteins (DeaD, LuxS, RibB, SodB, SucB, SucC, YcdO, YdiY, and YfiD); 11 base-induced proteins (AroK, CysK, DksA, DsbA, MalE, OmpA, Pta, RpiA, Ssb, TnaA, and YceI); and 2 acid- or base-induced proteins (OmpX and Tpx). Furthermore, Yohannes et al. (323) identified a total of 28 pH-dependent proteins from 32 spots in anaerobic cultures: 11 acid-induced proteins (AckA, GatY, GuaB, HdeA, Hmp, Lpd, NikA, OmpA, Ppa, TolC, and Tsf) and 17 base-induced proteins (AccB, DegQ, DhaK [YcgT], DhaL [YcgS], DhaM [YcgC], DsbA, GapA, HisC, HisD, MalB, MglB, OppA, ProX, Tig, TnaA, UspA, and YjgF). These findings indicate that low pH accelerates acid consumption and proton export while coinducing the oxidative stress and heat shock regulons. In contrast, high pH accelerates proton import while repressing the energy-expensive flagellar and chemotaxis regulons. Furthermore, pH differentially regulates a large number of periplasmic and envelope proteins as well as enzymes involved in several pH-dependent amino acid and carbohydrate catabolism pathways. High pH was shown to favor the catabolic pathways that generate NH3 and fermentation acids (AstD, CysK, DhaKLM, GabT, GadAB, GapA, SdaA, and TnaA), whereas low pH favored pathways that generate CO2 and amines (Adi, CadA, GadAB, and SpeF). Among these, Adi, AstD, CadA, CysK, GabT, SpeF, and TnaA were also significantly induced by anaerobiosis. Recently, researchers have used enrichment by column chromatography on reactive dye columns for the proteomic investigation of low-abundance acid-responsive proteins in E. coli grown at either pH 7.0 or pH 5.8 (24). This work allowed identification of new pH-responsive proteins: six acid-induced proteins (EF-Ts, GdhA, PanC, ProC, TkrA, and YodA) and five acid-repressed proteins (AroG, EF-Tu, FabI, GlyA, and PurA).
During the growth of E. coli, the external pH can be substantially changed by fermentative generation of acids or through aerobic consumption of acids. External acids which show an amplified uptake in response to increased pH gradients, such as acetic and formic acids, have been shown to induce heat shock proteins, oxidative stress proteins, and the RpoS regulon (146, 256). Several studies using proteomic approaches have revealed that benzoate induced heat shock and universal stress proteins (154), while propionate induced pH-responsive proteins such as AhpC, GatY, ManX, and YfiD (27). Treatment of E. coli cells with acetic acid increased the expression levels of 37 proteins, including periplasmic transporters for amino acids and peptides (ArtI, FliY, OppA, and ProX), metabolic enzymes (GatY and YfiD), the RpoS growth phase regulon, and the autoinducer synthesis protein LuxS (146). In contrast, acetic acid repressed 17 proteins, including phosphotransferase (Pta) (146). Similarly, an ackA-pta deletion, which abrogated the interconversion between acetate and acetyl-coenzyme A (CoA), led to elevated basal levels of 16 of the acetate-inducible proteins, including the RpoS regulon. Consistent with RpoS activation, the ackA-pta strain also showed constitutive extreme-acid resistance (146). On the other hand, treatment of E. coli cells with formic acid repressed 10 of the acetate-inducible proteins, including the RpoS regulon (146). Acetic and formic acids appear to exert opposite effects on proteins such as arginine-binding periplasmic protein 1 (ArtI), DNA-protecting protein during starvation (Dps), cysteine-binding periplasmic protein (FliY), tagatose-bisphosphate aldolase (GatY), extreme-acid periplasmic chaperone (HdeA and HdeB), hyperosmotically inducible protein Y (OsmY), and 6-phosphofructokinase isozyme 2 (PfkB). Membrane-permeable acids also induce the Mar multiple drug resistance regulon, which is coregulated by the SoxRS superoxide stress system (252). Several genetic systems are coregulated by pH and growth phase; for example, the RpoS growth phase sigma factor regulates several components of resistance to both acids and bases. Thus, the effects of pH on global cellular regulation are complex because they overlap with other environmental factors such as oxygenation, growth phase, and various metabolites. Current proteomic studies continue in an effort to dissect the relationships among the effects of pH, oxygen level, and osmolarity from combinatorial stimuli.
Oxidative stress response.
Reactive oxygen species (ROS) are produced as an inescapable consequence of aerobic life and are maintained at low, tolerable levels within cells by the actions of specific enzymes, such as superoxide dismutase (SodA). The expression levels of these defense enzymes are modulated in response to the environmental oxidative threat. However, this basic protection is not sufficient to protect cells against sudden large increases in ROS, which can act negatively on important cellular materials, including lipids, proteins, certain enzyme prosthetic groups, and DNA (183). To cope with oxidative stress, E. coli cells trigger rapid global responses designed to eliminate ROS, repair oxidative damage, bypass damaged functions, and induce adapted metabolism, thus allowing the cells to persist under high-ROS conditions. In E. coli, the SoxRS and OxyRS regulatory systems are known to control many of the oxidative stress-responsive proteins.
The soxRS regulon is induced in a two-stage process. Upon activation, SoxR induces expression of the soxS gene in response to superoxide-generating agents, and then SoxS activates transcription of genes within the regulon. About 40 E. coli proteins are induced, including the following: superoxide dismutase (SodA), which might associate with DNA to provide special protection from superoxide damage (275); endonuclease IV (Nfo), which is involved in DNA repair (40, 169); glucose-6-phosphate dehydrogenase (Zwf), whose increase is expected to elevate the pool of NADPH (254); fumarase (FumC) (174); aconitase (AcnA) (73); and NADPH ferredoxin:oxidoreductase (Fpr), which may serve to maintain FeS groups in the reduced form (173). The E. coli acn mutants were shown to be hypersensitive to the redox stress reagents H2O2 and methyl viologen (278). Physiological and enzymological studies have shown that AcnB is a major citric acid cycle enzyme synthesized during the exponential phase, whereas AcnA is a more stable stationary-phase enzyme, which is also specifically induced by iron and oxidative stress (53). Proteomic analyses have further revealed that the level of SodA is enhanced in acnB and acnAB mutants and by exposure to methyl viologen (278). The amounts of other proteins, including thioredoxin reductase, 2-oxoglutarate dehydrogenase, succinyl-CoA synthetase, and chaperones, were also affected in the acn mutants. These studies demonstrated that AcnA enhances the stability of the sodA transcript, whereas AcnB lowers its stability. Thus, aconitases serve as a protective buffer against the basal level of oxidative stress that accompanies aerobic growth by acting as a sink for ROS and modulating translation of the sodA transcript.
Similarly, the OxyRS regulon is also induced in a two-stage process. Exposure of cells to H2O2 in a range from 5 to 200 μM activates OxyR and enhances the synthesis of ∼40 proteins (183), including HPI catalase (KatG) (279), an NADPH-dependent alkyl hydroperoxide reductase (AhpCF) (279), glutathione reductase (GorA) (183), and Dps, which nonspecifically binds DNA to protect cells from H2O2 toxicity (6). In an oxyR-deleted mutant strain, 20 to 30 enzymes were found to remain H2O2 inducible (86). Some of these enzymes are also elevated during other stress responses, including exposure to redox cycling agents and heat shock.
These enzyme responses to oxidative stress are underpinned by metabolites or proteins such as NADPH, NADH, thioredoxin, and glutathione, which remove harmful oxygen species by stoichiometric reactions. In particular, thioredoxin, a ubiquitous and evolutionarily conserved protein, modulates the structure and activity of proteins involved in a spectrum of processes, such as gene expression and the oxidative stress response (183). A comprehensive analysis of the thioredoxin-linked E. coli proteome was performed by using tandem affinity purification and nanospray microcapillary MS/MS (151). A total of 80 thioredoxin-associated proteins were identified, and their various functions suggest that thioredoxin is involved in at least 26 distinct cellular processes, including transcription regulation, cell division, energy transduction, and several biosynthetic pathways. These thioredoxin-associated proteins either participate directly (AhpC, KacG, and SodA) or have key regulatory functions (AcnB and Fur) in cellular detoxification. Transcription factors including NusG, OmpR, and RcsB, which are generally not considered to be under redox control, were also associated with thioredoxin, providing compelling evidence for an extensively coupled network of redox regulation of E. coli.
E. coli cells treated with nontoxic levels of the superoxide-generating redox cycling agents menadione and paraquat showed dramatic changes in protein composition as monitored by 2-D gel analysis (87). The distribution of proteins synthesized after treatment with these agents overlapped significantly with that seen after H2O2 treatment. In addition, the redox cycling agents elicited the synthesis of at least 33 other proteins that were not H2O2 responsive. These include three heat shock proteins (C41.7, C62.5, and GroES), the Mn-containing superoxide dismutase (SodA), the DNA repair protein endonuclease IV (Nfo), and glucose-6-phosphate dehydrogenase (Zwf). At least some of these redox inducible proteins appear to be part of a specific response to intracellular superoxide, indicating that E. coli cells are equipped with a network of inducible responses against oxidative damage which are controlled via multiple regulatory pathways.
Starvation response.
The complex response of E. coli to nutrient starvation includes the sequential synthesis of starvation-inducible proteins. Although starvation for different individual nutrients generally provokes unique and individual patterns of protein expression, there are some overlaps among the starvation stimulons. Proteome analyses revealed that the subset of proteins involved in protein synthesis in E. coli was greatly increased during growth inhibition caused by depletion of various nutrients, such as carbon, nitrogen, phosphate, sulfate, and amino acids. For example, SspA expression increased with decreasing growth rate and was induced by glucose, nitrogen, phosphate, or amino acid starvation. Furthermore, the proteome profiles during the exponential growth phase showed that the expression levels of at least 11 proteins were altered in sspA mutant strains (314). These findings indicate that SspA acts as a transcription factor and is essential for starvation stress-induced tolerance (e.g., stationary phase) in E. coli.
At the onset of glucose starvation, cyclic AMP and its receptor protein (cAMP-CRP) were found to play important roles in the expression of a number of genes. An early 2-DE study identified five glucose-responsive outer membrane proteins (four upregulated and one downregulated) (186). A comparison with membrane proteins from mutant strains revealed that two of the upregulated proteins were the receptors for λ and T6, and coelectrophoresis of the outer membrane fraction identified the downregulated protein as OmpA. The glucose starvation stimulon was further examined using 2-DE followed by comparison to the E. coli gene-protein database (218). Members of this stimulon were found to include enzymes of the Embden-Meyerhof-Parnas pathway, phosphotransacetylase (Pta) and acetate kinase (AckA) in the acetic acid pathway, and formate transacetylase. Trichloroacetic acid cycle enzymes were repressed, whereas enzymes involved in acetate and formate production and the Embden-Meyerhof-Parnas pathway were induced. These modulations suggest that a glucose-starved cell increases the relative flow of carbon through the Pta-AckA pathway. Indeed, pta and pta-ackA mutants were found to be impaired in their abilities to survive glucose starvation, indicating that the capacity to synthesize acetyl phosphate, an intermediate of this pathway, is indispensable for glucose-starved cells. The pta mutant failed to induce several proteins of the glucose starvation stimulon. More recently, proteome studies revealed that glucose limitation upregulates the levels of proteins such as AceA, AldA, ArgT, AtpA, DppA, GatY, LivJ, MalE, MglB, RbsB, UgpB, and YdcS (311). Of these, ArgT, DppA, LivJ, MalE, MglB, RbsB, UgpB, and YdcS are periplasmic binding proteins of the ABC transporters, suggesting that in addition to the central metabolism proteins, periplasmic binding proteins are involved in the carbohydrate and amino acid uptakes that are important during glucose limitation.
Inorganic phosphate is the preferred source of phosphorus for E. coli. Phosphonates are commonly found in nature and can serve as an alternate phosphorus source when inorganic phosphate is depleted, but this causes a significant decrease in growth rate. Researchers have used 2-DE to examine the effects of inorganic phosphate limitation and the use of phosphonates as the sole phosphorus source (293). Depletion of inorganic phosphate was shown to induce the expression of 208 proteins and reduce the levels of 205 proteins, whereas growth on phosphonate induced 227 proteins and reduced the levels of 30. Comparison of these stimulons revealed that 118 of the induced proteins and 19 of the proteins with reduced levels were shared, suggesting that these may be involved in the adaptive response to phosphate limitation. The large number of downregulated proteins (205 proteins) involved in inorganic phosphate starvation compared with the number involved in growth on phosphonate indicates that the starvation response is more strongly characterized by repression.
In E. coli, sulfur limitation leads to derepression of the cysteine regulon (cysB, cysE, cysDNC, cysJIH, cysK cysM, cysPTWA, and sbp) and subsequent upregulation of cysteine biosynthesis (149). Maximal expression of the cys genes is seen during growth in limiting sulfur sources such as glutathione or l-djenkolic acid. On the other hand, growth in sulfate, sulfite, or thiosulfate leads to partial repression of these genes, and growth with sulfide, l-cysteine, and l-cystine leads to full repression (149). Comparative proteomic analyses have revealed that several proteins are induced in E. coli grown in media offering compounds other than sulfate or cysteine as the sole sulfur source (239, 299). Wild-type E. coli cells showed upregulation of sulfate starvation-induced proteins, such as CysK, Sbp, Ssi4, Ssi6, TauA, and TauD, during growth with lanthionine or glutathione as the sulfur source (Fig. 4). These sulfate-starvation-induced proteins were significantly reduced or wholly absent in cbl mutants (299), indicating that the cbl gene product, a transcription factor governing the genes required for sulfonate-sulfur utilization, is required for the synthesis of sulfate-regulated proteins. Interestingly, although the cbl mutant grew on sulfate, it lacked production of CysK and Sbp, which are involved in the sulfate assimilation pathway. The ability of the cbl mutant to assimilate sulfate may be explained by the fact that E. coli contains CysM and CysP, which act as functional backups for CysK and Sbp, respectively (149). Additional sulfate starvation-induced proteins include the products of the tauABCD genes, which are required for utilization of taurine as the sulfur source for growth (298). These findings indicate that most of the genes involved are coordinately regulated as the cysteine regulon, and high-level expression of these genes requires sulfur limitation and transcriptional regulator(s) CysB and/or Cbl.
The leucine-responsive regulatory protein (Lrp) has been shown to both positively and negatively regulate transcription of a number of genes in response to exogenous leucine (215); Lrp action is significantly activated by the absence of l-leucine in the growth medium, whereas it is repressed in the presence of l-leucine. On the other hand, exogenous leucine has little or no effect on the expression of some other Lrp-responsive proteins, such as glutamine synthetase (GlnA) and glutamate synthase (GltD) (61, 215). The total number of genes in the l-leucine/Lrp regulon was estimated to be between 35 and 75. The lower estimate comes from a comparison of 2-D gels from extracts of wild-type and lrp mutant strains grown with and without leucine. Some 30 proteins were clearly affected up or down by the absence of Lrp (61, 66, 172). The higher estimate is from a study of random λ placMu insertions in the E. coli genome with subsequent screening for Lrp-responsive proteins affected by l-leucine (171). Among the well-known proteins that are regulated by Lrp (Fig. 4) are upregulated proteins including DaaABCDE, FanABC, FimB, GcvTHP, GltBDF, IlvIH, LacZYA, LeuABCD, MalEFG, MalK-LamB-MalM, MalT, OmpF, PapBA, PapI, PntAB, SdaC, SerA, and SfaA and downregulated proteins including Fae, GlyA, Kbl-Tdh, LivJ, LivKHMG, Lrp, LysU, OmpC, OppABCDF, OsmY, and SdaA. These findings collectively indicate that the expression of many genes required for the transport and catabolism of amino acids and peptides is negatively regulated by Lrp, while the expression of genes required for amino acid biosynthesis and ammonia assimilation in a nitrogen-poor environment is positively regulated by Lrp (215).
Finally, the stringent response is a general starvation response mediated by guanosine 3′,5′-bispyrophosphate (ppGpp). RelA and SpoT strictly regulate the levels of ppGpp during growth-favorable conditions (263), while starvation increases the levels of ppGpp, leading to an abrupt decrease in rRNA and tRNA transcription and blockade of purine biosynthesis. Early studies showed that starvation and subsequent increases of ppGpp decreased the fidelity of protein translation (221). Later mutant studies suggested that the stringent response reduces the concentration of mistranslated proteins, which is critical for survival (185, 218). High ppGpp levels also increase the stationary-phase regulator RpoS (σS), accelerate protein degradation, and impair initiation of DNA replication (104). In contrast, depletion of ppGpp induces the so-called “relaxed response,” where transcription and translation factor synthesis remains high despite a growth lag. RpoS is involved in the signaling of many cell responses, including starvation, multiple stress responses, and inhibition of glycogen and trehalose synthesis (155, 192, 205). Induction of the stationary phase in response to starvation is also dependent on the ClpAP protease, which plays a key role in the degradation of growth phase proteins (308).
Other environmental responses.
Cadmium is used in a variety of industrial applications and is a potential source of environmental contamination. Cadmium is readily taken up by bacterial cells, presumably by the Mn2+ uptake system, and can seriously damage the cell via its activity as a potent oxidative agent (297) and inhibitor of DNA replication (196, 219). At low cadmium concentrations, cells are able to adapt and resume growth after a period of stasis. This period appears to involve the repair of cadmium-mediated cellular damage and adjustment of the cell physiology to limit the distribution of the toxic ion in the cell (196). During cadmium-induced growth arrest, E. coli cells increase the synthesis of the cadmium-induced proteins (CDPs), which form the cadmium stress stimulon. Most of the CDPs are of unknown function, and only limited information as to the identities of the specific sensors or signals responsible for triggering the synthesis of these proteins is available (297). Some CDPs are members of well-characterized stress regulons (297). Only a limited number of proteins in these regulons are induced during cadmium exposure, and the synthesis of these CDPs constitutes a minor fraction of the overall cellular response (297). The CDPs identified by 2-DE include Adk, ArgI, ClpB, DnaK, H-NS, HtpG, MaoA, MetK, RecA, Tig, TyrA, UspA, W-protein, XthA, the cold shock protein G041.2, and five unknown proteins (64). Some CDPs were found to be induced by the heat shock, oxidation stress, SOS, and stringent response regulons, while others appeared to be general stress-inducible proteins (e.g., H-NS, UspA). The synthesis rates of most of the immediate responders to cadmium exposure decreased when cell growth resumed, but seven CDPs, including ArgI, TyrA, and XthA, were found to maintain a high production rate during growth in the presence of cadmium (64). This type of E. coli response to cadmium may be employed to monitor cadmium contamination in the environment.
The effects of low concentrations of monochlorophenol, pentachlorophenol, and cadmium chloride as industrial pollutants on total cellular proteins in E. coli have been studied using 2-DE (62). Induction of previously identified stress-responsive proteins was noted, as were transient decreases in the synthesis rates of several other proteins, including OmpF and aspartate transcarbamoylase (ATCase). Their transient repression appears to be an overall response to stress elicited by different pollutants and may prove useful as a general and sensitive early warning system for pollutant stress.
Proteomics for Biotechnology
Researchers have found engineered E. coli to be of enormous value for both scientific and practical applications. To enhance the production of bioproducts and improve the performance of E. coli strains in various biotechnological processes, native or foreign genes have been amplified or deleted through recombinant DNA technology. These efforts initially involved trial-and-error approaches, in which various genetic modifications are repeatedly tried until a desired objective is achieved. However, since bioproducts are formed by coordinated enzyme functions acting through the metabolic pathways, it is essential to understand the metabolism and regulation that occur during cell growth and product formation. Recently, these investigations have been streamlined with the use of new high-throughput analytical, molecular biological, and mathematical tools, all of which have been combined to facilitate the development of “custom-made” production systems in E. coli. In this important context, proteome analysis enables measurement of whole-protein (enzyme) expression levels, facilitating the construction of metabolic pathways that researchers can use to elucidate which molecules supply the energy and building blocks or precursors (e.g., amino acids and other metabolic intermediates) necessary for cell function and product formation.
As described by VanBogelen et al. (292), several E. coli proteomic signatures can be used to monitor cellular states. First, the L7 (modified form)-to-L12 (unmodified form) ratio of ribosomal protein RplL, which is highly correlated with the growth rate, can serve as specific biospectrophotometry marker for monitoring cell growth. Second, some heat and cold shock proteins, which are increased at the temperature extremes, can be used as cellular thermometers. Third, the RecA protein can be used as an initial indicator of loss of chromosome function. Furthermore, conditional promoters activated by environmental changes such as stationary phase, pH, temperature, and nutrient limitation may be used for efficient production of heterologous proteins in bacteria and also for developing strains for bioremediation purposes (190). Indeed, the heat-inducible and inorganic phosphorus-responsive promoters have been widely used in numerous laboratories (3, 37, 38, 115, 139). In addition, the genes encoding proteins that confer tolerances to acid, heat, and toxic substances have been successfully used for the improvement of cellular properties and enhanced degradation of toxic chemicals. Survival under extremely acidic conditions may be associated with the viability of pathogenic bacteria in the stomach (195), so an improved understanding of pH sensors in virulence may lead to the development of therapeutic strategies targeting these functions.
Proteomic analysis has been used to directly monitor cellular changes occurring during the production of heterologous proteins in E. coli and develop efficient strains for the enhanced production of bioproducts (3, 37, 38, 97, 98, 114, 115, 116, 135, 138, 160, 162, 235, 306) and biodegradable polymers (99, 139). Furthermore, many of these proteomic studies have been performed in large-scale processes employing E. coli and recombinant E. coli for industrial applications (3, 37, 38, 97, 114, 115, 116, 135, 138, 139, 145, 241, 245, 325). In addition, proteomic studies for analyzing the composition of inclusion bodies (IBs) (98, 101, 135, 138, 246, 247) have been carried out in order to improve the quality (or uniformity) of the desired product and the downstream process of recombinant proteins such as protein purification and refolding. Unfortunately, most of the results from proteome analysis cannot be clearly compared, since they differ in terms of growth conditions, strains and genotypes, target products, sampling times, and bioprocesses.
Among these studies, those that led to the enhanced production of recombinant proteins, including IBs and secretory proteins, and improved industrial processes are described below. Jordan and Harcum (135) analyzed the proteome profiles of soluble and insoluble IB fractions to detect and characterize proteases upregulated during the production of Axokine in recombinant E. coli cells. Exposure to EDTA reduced protease activity, indicating that Axokine degradation was likely mediated by metalloproteases. In addition, two small heat shock proteins (sHsps), IbpA and IbpB, were first identified by the conventional biochemical technique as the major proteins associated with the IBs of recombinant proteins produced in E. coli (4). Furthermore, IbpA and IbpB were recently first demonstrated to facilitate the production of recombinant proteins in E. coli and play important roles in protecting recombinant proteins from degradation by cytoplasmic proteases (98). Amplification of the ibpA and/or ibpB genes enhanced production of recombinant proteins as IBs, whereas ibpAB gene knockout enhanced the secretory production of recombinant proteins as soluble forms (98). More recently, LeThanh et al. (167) reported results similar to those of Han et al. (98), i.e., that α-glucosidase production was enhanced at elevated IbpA and IbpB levels and reduced in an ibpAB-negative mutant strain in a temperature-dependent manner. Also, it was revealed that IbpA and IbpB prevent IBs of α-glucosidase from degradation in a temperature-dependent manner. These findings suggest that manipulation of ibpAB gene expression may prove to be a valuable new technique for fine-tuning the production of recombinant proteins in E. coli. In addition, these results demonstrate the effectiveness of employing proteome profiling in the development of production strains suitable for industrial applications.
The use of sHsps has recently been extended for significantly enhancing the performance of 2-DE (96). Proteolytic degradation is one of the critical problems in 2-DE. Loss of protein spots in 2-D gels due to residual protease activity is commonly observed when using immobilized pH gradient gels for isoelectric focusing. Three sHsps, IbpA and IbpB from E. coli and Hsp26 from Saccharomyces cerevisiae, were found to be able to protect proteins in vitro from proteolytic degradation. The addition of sHsps during 2-DE of human serum or whole-cell extracts of bacteria (E. coli, Mannheimia succinciproducens), plant Arabidopsis thaliana, and human kidney cells allowed detection of up to 50% more protein spots than were obtainable with currently available protease inhibitors. This may change the way proteome profiling is carried out by generally enabling the detection of many protein spots that could not be seen previously.
Recently, the physiological changes of recombinant E. coli during secretory production of a recombinant humanized antibody fragment were monitored by 2-DE (3). Twenty-five protein spots were differentially expressed in the control and production fermentations at 72 h, while 19 other protein spots were present only in the control or production fermentation at this time. The synthesis of the stress protein phage shock protein A (PspA) was strongly correlated with the synthesis of a recombinant product. Coexpression of the pspA gene with a recombinant antibody fragment in E. coli significantly improved the yield of the secreted biopharmaceutical (3). In another study, a combined analysis of proteome, transcriptome, and mathematical models was used to engineer an E. coli strain (162). This E. coli mutant strain, obtained by random mutagenesis and secreting fourfold more active alpha-hemolysin (HlyA) than its parent strain, was characterized using both high-density microarrays for mRNA profiling and a proteomic strategy for protein expression. The relative mRNA and protein expression levels of tRNA synthetases, including AsnS, AspS, LysS, PheT, and TrpS, were lower in the mutant than in the parent. This combined examination of the mRNA and protein expression profiles showed that downregulation of the tRNA synthetases in the mutant lowered the general translation rate and, more specifically, lowered the rate of HlyA synthesis. Better secretion of alpha-hemolysin at a low synthesis rate is attributable to a balance between translation and secretion. The use of rare codons in the hlyA gene has been shown to reduce its rate of translation, because the number of available aminoacyl tRNAs is limited. A variant of the hlyA gene involving the alteration of five bases but encoding the same amino acid sequence was designed using a mathematical model of prokaryotic translation. In this way, the rate of translation could be artificially slowed down, leading to further improved secretory production of alpha-hemolysin.
An important factor to be considered in the production of recombinant proteins is the direct and indirect influences of the metabolic pathways that supply the energy and precursors required for the synthesis of proteins. Global proteome profiling of recombinant E. coli during the overproduction of human leptin was used to identify a target gene, leading to successful metabolic engineering for increased productivity of leptin and other serine-rich proteins by coexpression of the cysK gene (97). Thus, proteomic analysis can be used to examine changes in protein (enzyme) expression levels or to identify rate-controlling steps in metabolic pathways and develop a systematic strategy for optimizing the relevant metabolic pathways (95). It should be noted that the amount of protein is not always proportional to the protein activity, which in turn does not necessarily correlate with the corresponding metabolic reaction rate. However, it has been reported that protein abundance data obtained from proteome profiles appear to correlate to some extent with the enzyme activities in E. coli, with a few exceptions (231). Thus, it appears as though proteomics can be effectively used to identify candidates for successful metabolic engineering for improved bioproduct yield.
Proteomic analysis can also be used to detect the presence or absence of host proteins in the recombinant protein products. A proteomic study of recombinant E. coli cells expressing different biopharmaceutical proteins showed that the host cell protein profiles were highly similar (85 to 90%) at the end of their production runs, indicating that the multiproduct host cell immunoassay is a feasible method for the detection of host cell protein contaminants during the downstream processing of recombinant protein products (37, 38). These findings and other reports continue to emphasize the fact that E. coli proteomics is likely to become increasingly important not only in the biological research fields but also in various biotechnological applications.
CONCLUSIONS AND FUTURE PROSPECTS
A major goal of proteomics is the complete description of the entire protein spectrum underlying cell physiology. A large number of small-scale and more-recent large-scale experiments have contributed to expanding our understanding of the nature of whole-protein networks, though there are still some limitations regarding the use of proteomic methods. Many initial proteome studies were applied to E. coli, yielding a collection of extremely well characterized proteome databases including 1,627 proteins identified using gel-based or non-gel-based approaches. Extensive gel-based studies have given researchers a solid understanding of the global protein network and well-established 2-D gel databanks. Recently, many non-gel-based approaches have been validated with E. coli strains, leading to the identification of additional proteomic components. As these two approaches are complementary, they will likely contribute to identifying more proteins in the future. A well-defined E. coli proteome will have direct applications in biochemical, biological, and biotechnological research fields in the following ways. (i) The E. coli proteome underpins our understanding not only of the prokaryotic regulatory network but also of complex eukaryotic regulatory networks including stimulon, regulon, and cascade-like networks. (ii) The E. coli proteome can provide invaluable information for designing metabolic engineering strategies to enhance production of various bioproducts, including recombinant proteins, biopolymers, and metabolites. (iii) The E. coli proteome can be used as a model system to help accelerate the development of advanced high-resolution, high-throughput, and high-sensitivity proteomic technologies.
As we peek at the future, we see that proteomic studies will likely evolve in a number of ways. First, proteomic studies can be expected to transition toward a miniaturized platform, allowing scale-down analysis. In the case of microorganisms such as E. coli, single-cell proteome analysis (rather than that of a population of cells) may be realized. Towards these goals, new analytical protocols capable of processing nanoliter to picoliter volumes and femtomole to attomole quantities of proteins or peptides are being developed (170, 260). Advances in microfluidics and processes for handling minute sample volumes without adsorptive losses and with improved reaction kinetics should make it possible to carry out proteome analysis on a microchip (175). Second, proteomic analysis will become more automated. So far, the 2-DE technologies have proven difficult to automate due to several issues, including sample contamination and degradation, loss of proteins, and generally poor-quality data. However, progress has been made in recent years, including the development of programmable IEF units for automated overnight IPG strip rehydration and focusing and even partially or fully automated 2-DE units. Even greater progress is being made in the post-gel-handling steps, including the use of robots for spot excision, in-gel trypsin digestion, postdigestion cleanup and concentration, sample mounting onto MALDI-MS targets, and sample injection for LC-MS analysis. Non-gel-based methods can be more easily automated using nanoscale-compatible autosamplers, sophisticated HPLC pumping systems, and automated switching valves for multidimensional separations. As automation methods become more robust, they are expected to enhance the throughput and reproducibility, particularly among different laboratories. Third, instruments of higher sensitivity and accuracy for the detection of proteins will be continuously developed. For example, the tremendous volumes of data generated from traditional mass spectrometers include large numbers of false positives and/or true negatives (at least 20%, depending on the mass spectrometer). Recently developed or in-production instruments are expected to improve the future accuracy of MS data. Finally, more-solid bioinformatic tools will be developed for the analysis of large data sets generated by proteomics. High-quality software is required for the accurate detection, quantification, and identification of protein spots. In the future, the software will likely be equipped with algorithms for heuristic clustering and neural network analysis, which are currently used in other disciplines to analyze large data sets. These improved data analysis techniques can be expected to yield more-accurate mass measurements, unambiguous protein identification, and discrimination between artifactual modifications and true posttranslational modifications.
Many cutting-edge biological and biotechnological studies are currently driven by the high-throughput acquisition and examination of proteomic data supported by systematic biological and bioinformatic analyses (164). E. coli has been and will continue to be a model organism for these global-scale studies, which are aimed toward understanding the cell and organism as a whole. Considering that proteins mediate most cellular activities, proteomics will play a central role in achieving this ambitious goal. During this exciting expansion of data and understanding in the coming years, the E. coli proteome will continue to stand strong as a standard platform and the gold standard of model organisms.
Supplementary Material
Acknowledgments
This work was supported by a Korean Systems Biology Research Grant (M10309020000-03B5002-00000) of the Ministry of Science and Technology. Further support by the LG Chem Chair Professorship, the IBM SUR program, Microsoft, KOSEF through the Center for Ultramicrochemical Process Systems, and the Brain Korea 21 project is appreciated.
Footnotes
Supplemental material for this article may be found at http://mmbr.asm.org/.
REFERENCES
- 1.Aebersold, R. H., J. Leavitt, R. A. Saavedra, L. E. Hood, and S. B. Kent. 1987. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc. Natl. Acad. Sci. USA 84:6970-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal, K., L. H. Choe, and K. H. Lee. 2005. Quantitative analysis of protein expression using amine-specific isobaric tags in Escherichia coli cells expressing rhsA elements. Proteomics 5:2297-2308. [DOI] [PubMed] [Google Scholar]
- 3.Aldor, I. S., D. C. Krawitz, W. Forrest, C. Chen, J. C. Nishihara, J. C. Joly, and K. M. Champion. 2005. Proteomic profiling of recombinant Escherichia coli in high-cell-density fermentations for improved production of an antibody fragment biopharmaceutical. Appl. Environ. Microbiol. 71:1717-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen, S. P., J. O. Polazzi, J. K. Gierse, and A. M. Easton. 1992. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J. Bacteriol. 174:6938-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almirón, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 6.Altuvia, S., M. Almiron, G. Huisman, R. Kolter, and G. Storz. 1994. The dps promoter is activated by OxyR during growth and by IHF and σS in stationary phase. Mol. Microbiol. 13:265-272. [DOI] [PubMed] [Google Scholar]
- 7.Appel, R. D., J. C. Sanchez, A. Bairoch, O. Golaz, F. Ravier, C. Pasquali, G. J. Hughes, and D. F. Hochstrasser. 1996. The SWISS-2DPAGE database of two-dimensional polyacrylamide gel electrophoresis, its status in 1995. Nucleic Acids Res. 24:180-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai, M., H. Mitsuke, M. Ikeda, J. X. Xia, T. Kikuchi, M. Satake, and T. Shimizu. 2004. ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. 32:W390-W393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armon, A., D. Graur, and N. Ben-Tal. 2001. ConSurf: an algorithmic tool for the identification of functional regions in proteins by surface mapping of phylogenetic information. J. Mol. Biol. 307:447-463. [DOI] [PubMed] [Google Scholar]
- 10.Arnold, C. N., J. McElhanon, A. Lee, R. Leonhart, and D. A. Siegele. 2001. Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J. Bacteriol. 183:2178-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold, R. J., and J. P. Reilly. 2002. Analysis of methylation and acetylation in E. coli ribosomal proteins. Methods Mol. Biol. 194:205-210. [DOI] [PubMed] [Google Scholar]
- 12.Atlung, T., and L. Brøndsted. 1994. Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. J. Bacteriol. 176:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atlung, T., K. Knudsen, L. Heerfordt, and L. Brøndsted. 1997. Effects of σS and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J. Bacteriol. 179:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attwood, T. K., M. E. Beck, A. J. Bleasby, K. Degtyarenko, A. D. Michie, and D. J. Parry-Smith. 1997. Novel developments with the PRINTS protein fingerprint database. Nucleic Acids Res. 25:212-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babnigg, G., and C. S. Giometti. 2004. GELBANK: a database of annotated two-dimensional gel electrophoresis patterns of biological systems with completed genomes. Nucleic Acids Res. 32:D582-D585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bader, G. D., I. Donaldson, C. Wolting, B. F. Ouellette, T. Pawson, and C. W. Hogue. 2001. BIND-The Biomolecular Interaction Network Database. Nucleic Acids Res. 29:242-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bairoch, A. 1991. PROSITE: a dictionary of sites and patterns in proteins. Nucleic Acids Res. 19(Suppl.):2241-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bairoch, A., R. Apweiler, C. H. Wu, W. C. Barker, B. Boeckmann, S. Ferro, E. Gasteiger, H. Huang, R. Lopez, M. Magrane, M. J. Martin, D. A. Natale, C. O'Donovan, N. Redaschi, and L. S. Yeh. 2005. The Universal Protein Resource (UniProt). Nucleic Acids Res. 33:D154-D159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barth, M., C. Marschall, A. Muffler, D. Fischer, and R. Hengge-Aronis. 1995. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of σS and many σS-dependent genes in Escherichia coli. J. Bacteriol. 177:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berggren, K. N., E. Chernokalskaya, M. F. Lopez, J. M. Beechem, and W. F. Patton. 2001. Comparison of three different fluorescent visualization strategies for detecting Escherichia coli ATP synthase subunits after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteomics 1:54-65. [DOI] [PubMed] [Google Scholar]
- 21.Bertone, P., and M. Snyder. 2005. Advances in functional protein microarray technology. FEBS J. 272:5400-5411. [DOI] [PubMed] [Google Scholar]
- 22.Bienvenut, W. V., J. C. Sanchez, A. Karmime, V. Rouge, K. Rose, P. A. Binz, and D. F. Hochstrasser. 1999. Toward a clinical molecular scanner for proteome research: parallel protein chemical processing before and during western blot. Anal. Chem. 71:4800-4807. [DOI] [PubMed] [Google Scholar]
- 23.Biran, D., N. Brot, H. Weissbach, and E. Z. Ron. 1995. Heat shock-dependent transcriptional activation of the metA gene of Escherichia coli. J. Bacteriol. 177:1374-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birch, R. M., C. O'Byrne, I. R. Booth, and P. Cash. 2003. Enrichment of Escherichia coli proteins by column chromatography on reactive dye columns. Proteomics 3:764-776. [DOI] [PubMed] [Google Scholar]
- 25.Bjellqvist, B., K. Ek, P. G. Righetti, E. Gianazza, A. Görg, R. Westermeier, and W. Postel. 1982. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J. Biochem. Biophys. Methods 6:317-339. [DOI] [PubMed] [Google Scholar]
- 26.Black, P. N. 1991. Primary sequence of the Escherichia coli fadL gene encoding an outer membrane protein required for long-chain fatty acid transport. J. Bacteriol. 173:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blankenhorn, D., J. Phillips, and J. L. Slonczewski. 1999. Acid- and base-induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. J. Bacteriol. 181:2209-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 29.Bloch, P. L., T. A. Phillips, and F. C. Neidhardt. 1980. Protein identifications of O'Farrell two-dimensional gels: locations of 81 Escherichia coli proteins. J. Bacteriol. 141:1409-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouvier, J., S. Gordia, G. Kampmann, R. Lange, R. Hengge-Aronis, and C. Gutierrez. 1998. Interplay between global regulators of Escherichia coli: effect of RpoS, Lrp and H-NS on transcription of the gene osmC. Mol. Microbiol. 28:971-980. [DOI] [PubMed] [Google Scholar]
- 31.Bradbury, A., and A. Cattaneo. 1995. The use of phage display in neurobiology. Trends Neurosci. 18:243-249. [PubMed] [Google Scholar]
- 32.Brissette, J. L., M. Russel, L. Weiner, and P. Model. 1990. Phage shock protein, a stress protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:862-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burton, Z., R. R. Burgess, J. Lin, D. Moore, S. Holder, and C. A. Gross. 1981. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E. coli K12. Nucleic Acids Res. 9:2889-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butland, G., J. M. Peregrin-Alvarez, J. Li, W. Yang, X. Yang, V. Canadien, A. Starostine, D. Richards, B. Beattie, N. Krogan, M. Davey, J. Parkinson, J. Greenblatt, and A. Emili. 2005. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433:531-537. [DOI] [PubMed] [Google Scholar]
- 35.Butt, A., M. D. Davison, G. J. Smith, J. A. Young, S. J. Gaskell, S. G. Oliver, and R. J. Beynon. 2001. Chromatographic separations as a prelude to two-dimensional electrophoresis in proteomics analysis. Proteomics 1:42-53. [DOI] [PubMed] [Google Scholar]
- 36.Chakraborty, A., and F. E. Regnier. 2002. Global internal standard technology for comparative proteomics. J. Chromatogr. A 949:173-184. [DOI] [PubMed] [Google Scholar]
- 37.Champion, K. M., J. C. Nishihara, I. S. Aldor, G. T. Moreno, D. Andersen, K. L. Stults, and M. Vanderlaan. 2003. Comparison of the Escherichia coli proteomes for recombinant human growth hormone producing and nonproducing fermentations. Proteomics 3:1365-1373. [DOI] [PubMed] [Google Scholar]
- 38.Champion, K. M., J. C. Nishihara, J. C. Joly, and D. Arnott. 2001. Similarity of the Escherichia coli proteome upon completion of different biopharmaceutical fermentation processes. Proteomics 1:1133-1148. [DOI] [PubMed] [Google Scholar]
- 39.Champion, M. M., C. S. Campbell, D. A. Siegele, D. H. Russell, and J. C. Hu. 2003. Proteome analysis of Escherichia coli K-12 by two-dimensional native-state chromatography and MALDI-MS. Mol. Microbiol. 47:383-396. [DOI] [PubMed] [Google Scholar]
- 40.Chan, E., and B. Weiss. 1987. Endonuclease IV of Escherichia coli is induced by paraquat. Proc. Natl. Acad. Sci. USA 84:3189-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang, Y. Y., A. Y. Wang, and J. E. Cronan, Jr. 1994. Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS (katF) gene. Mol. Microbiol. 11:1019-1028. [DOI] [PubMed] [Google Scholar]
- 42.Chapal, N., M. Laplanche, G. Ribes, B. Pau, J. Garin, and P. Petit. 2002. Pharmaco-proteomic analysis: application of proteomic analysis to the discovery and development of new drugs. J. Soc. Biol. 196:317-322. (In French with English summary.) [PubMed] [Google Scholar]
- 43.Chen, B. P., and T. Hai. 1994. Expression vectors for affinity purification and radiolabeling of proteins using Escherichia coli as host. Gene 139:73-75. [DOI] [PubMed] [Google Scholar]
- 44.Chetouani, F., P. Glaser, and F. Kunst. 2002. DiffTool: building, visualizing and querying protein clusters. Bioinformatics 18:1143-1144. [DOI] [PubMed] [Google Scholar]
- 45.Choe, L. H., and K. H. Lee. 2000. A comparison of three commercially available isoelectric focusing units for proteome analysis: the multiphor, the IPGphor and the protean IEF cell. Electrophoresis 21:993-1000. [DOI] [PubMed] [Google Scholar]
- 46.Clark, D. P., and J. E. Cronan, Jr. 1996. Two-carbon compounds and fatty acids as carbon sources, p. 343-357. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 47.Claros, M. G., and G. von Heijne. 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10:685-686. [DOI] [PubMed] [Google Scholar]
- 48.Copeland, B. R., R. J. Richter, and C. E. Furlong. 1982. Renaturation and identification of periplasmic proteins in two-dimensional gels of Escherichia coli. J. Biol. Chem. 257:15065-15071. [PubMed] [Google Scholar]
- 49.Corbin, R. W., O. Paliy, F. Yang, J. Shabanowitz, M. Platt, C. E. Lyons, Jr., K. Root, J. McAuliffe, M. I. Jordan, S. Kustu, E. Soupene, and D. F. Hunt. 2003. Toward a protein profile of Escherichia coli: comparison to its transcription profile. Proc. Natl. Acad. Sci. USA 100:9232-9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corpet, F., J. Gouzy, and D. Kahn. 1998. The ProDom database of protein domain families. Nucleic Acids Res. 26:323-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costanzo, M. C., M. E. Crawford, J. E. Hirschman, J. E. Kranz, P. Olsen, L. S. Robertson, M. S. Skrzypek, B. R. Braun, K. L. Hopkins, P. Kondu, C. Lengieza, J. E. Lew-Smith, M. Tillberg, and J. I. Garrels. 2001. YPD, PombePD and WormPD: model organism volumes of the BioKnowledge library, an integrated resource for protein information. Nucleic Acids Res. 29:75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham, L., M. J. Gruer, and J. R. Guest. 1997. Transcriptional regulation of the aconitase genes (acnA and acnB) of Escherichia coli. Microbiology 143:3795-3805. [DOI] [PubMed] [Google Scholar]
- 54.Dai, Y., L. Li, D. C. Roser, and S. R. Long. 1999. Detection and identification of low-mass peptides and proteins from solvent suspensions of Escherichia coli by high performance liquid chromatography fractionation and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 13:73-78. [DOI] [PubMed] [Google Scholar]
- 55.Dartigalongue, C., and S. Raina. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Reuse, H., and A. Danchin. 1988. The ptsH, ptsI, and crr genes of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: a complex operon with several modes of transcription. J. Bacteriol. 170:3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deuerling, E., H. Patzelt, S. Vorderwülbecke, T. Rauch, G. Kramer, E. Schaffitzel, A. Mogk, A. Schulze-Specking, H. Langen, and B. Bukau. 2003. Trigger factor and DnaK possess overlapping substrate pools and binding specificities. Mol. Microbiol. 47:1317-1328. [DOI] [PubMed] [Google Scholar]
- 58.Dönnes, P., and A. Höglund. 2004. Predicting protein subcellular localization: past, present, and future. Genomics Proteomics Bioinformatics 2:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunlop, K. Y., and L. Li. 2001. Automated mass analysis of low-molecular-mass bacterial proteome by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 925:123-132. [DOI] [PubMed] [Google Scholar]
- 60.Eng, J. K., A. L. McCormack, and J. R. Yates III. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 61.Ernsting, B. R., M. R. Atkinson, A. J. Ninfa, and R. G. Matthews. 1992. Characterization of the regulon controlled by the leucine-responsive regulatory protein in Escherichia coli. J. Bacteriol. 174:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faber, F., T. Egli, and W. Harder. 1993. Transient repression of the synthesis of OmpF and aspartate transcarbamoylase in Escherichia coli K12 as a response to pollutant stress. FEMS Microbiol. Lett. 111:189-195. [DOI] [PubMed] [Google Scholar]
- 63.Fenyo, D., J. Qin, and B. T. Chait. 1998. Protein identification using mass spectrometric information. Electrophoresis 19:998-1005. [DOI] [PubMed] [Google Scholar]
- 64.Ferianc, P., A. Farewell, and T. Nyström. 1998. The cadmium-stress stimulon of Escherichia coli K-12. Microbiology 144:1045-1050. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez, L. A. 2004. Prokaryotic expression of antibodies and affibodies. Curr. Opin. Biotechnol. 15:364-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrario, M., B. R. Ernsting, D. W. Borst, D. E. Wiese II, R. M. Blumenthal, and R. G. Matthews. 1995. The leucine-responsive regulatory protein of Escherichia coli negatively regulates transcription of ompC and micF and positively regulates translation of ompF. J. Bacteriol. 177:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 68.Fountoulakis, M., and R. Gasser. 2003. Proteomic analysis of the cell envelope fraction of Escherichia coli. Amino Acids 24:19-41. [DOI] [PubMed] [Google Scholar]
- 69.Fountoulakis, M., M. F. Takács, P. Berndt, H. Langen, and B. Takács. 1999. Enrichment of low abundance proteins of Escherichia coli by hydroxyapatite chromatography. Electrophoresis 20:2181-2195. [DOI] [PubMed] [Google Scholar]
- 70.Franzén, B., S. Becker, R. Mikkola, K. Tidblad, A. Tjernberg, and S. Birnbaum. 1999. Characterization of periplasmic Escherichia coli protein expression at high cell densities. Electrophoresis 20:790-797. [DOI] [PubMed] [Google Scholar]
- 71.Gage, D. J., and F. C. Neidhardt. 1993. Adaptation of Escherichia coli to the uncoupler of oxidative phosphorylation 2,4-dinitrophenol. J. Bacteriol. 175:7105-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gajiwala, K. S., and S. K. Burley. 2000. HDEA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. J. Mol. Biol. 295:605-612. [DOI] [PubMed] [Google Scholar]
- 73.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 266:19328-19333. [PubMed] [Google Scholar]
- 74.Gardy, J. L., C. Spencer, K. Wang, M. Ester, G. E. Tusnady, I. Simon, S. Hua, K. deFays, C. Lambert, K. Nakai, and F. S. Brinkman. 2003. PSORT-B: improving protein subcellular localization prediction for Gram-negative bacteria. Nucleic Acids Res. 31:3613-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garvey, N., A. C. St. John, and E. M. Witkin. 1985. Evidence for RecA protein association with the cell membrane and for changes in the levels of major outer membrane proteins in SOS-induced Escherichia coli cells. J. Bacteriol. 163:870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.George, D. G., R. J. Dodson, J. S. Garavelli, D. H. Haft, L. T. Hunt, C. R. Marzec, B. C. Orcutt, K. E. Sidman, G. Y. Srinivasarao, L. S. Yeh, L. M. Arminski, R. S. Ledley, A. Tsugita, and W. C. Barker. 1997. The Protein Information Resource (PIR) and the PIR-International Protein Sequence Database. Nucleic Acids Res. 25:24-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georgopoulos, C., K. Tilly, D. Drahos, and R. Hendrix. 1982. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J. Bacteriol. 149:1175-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gevaert, K., J. Van Damme, M. Goethals, G. R. Thomas, B. Hoorelbeke, H. Demol, L. Martens, M. Puype, A. Staes, and J. Vandekerckhove. 2002. Chromatographic isolation of methionine-containing peptides for gel-free proteome analysis: identification of more than 800 Escherichia coli proteins. Mol. Cell. Proteomics 1:896-903. [DOI] [PubMed] [Google Scholar]
- 79.Giaever, H. M., O. B. Styrvold, I. Kaasen, and A. R. Strøm. 1988. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J. Bacteriol. 170:2841-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ginter, J. M., F. Zhou, and M. V. Johnston. 2004. Generating protein sequence tags by combining cone and conventional collision induced dissociation in a quadrupole time-of-flight mass spectrometer. J. Am. Soc. Mass Spectrom. 15:1478-1486. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein, J., N. S. Pollitt, and M. Inouye. 1990. Major cold shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 87:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gordia, S., and C. Gutierrez. 1996. Growth-phase-dependent expression of the osmotically inducible gene osmC of Escherichia coli K-12. Mol. Microbiol. 19:729-736. [DOI] [PubMed] [Google Scholar]
- 83.Görg, A., C. Obermaier, G. Boguth, A. Harder, B. Scheibe, R. Wildgruber, and W. Weiss. 2000. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis 21:1037-1053. [DOI] [PubMed] [Google Scholar]
- 84.Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 30:465-506. [DOI] [PubMed] [Google Scholar]
- 85.Gottschlich, N., C. T. Culbertson, T. E. McKnight, S. C. Jacobson, and J. M. Ramsey. 2000. Integrated microchip-device for the digestion, separation and postcolumn labeling of proteins and peptides. J. Chromatogr. B 745:243-249. [DOI] [PubMed] [Google Scholar]
- 86.Greenberg, J. T., and B. Demple. 1988. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 7:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Greenberg, J. T., and B. Demple. 1989. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J. Bacteriol. 171:3933-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greenberg, J. T., P. Monach, J. H. Chou, P. D. Josephy, and B. Demple. 1990. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Griggs, D. W., K. Kafka, C. D. Nau, and J. Konisky. 1990. Activation of expression of the Escherichia coli cir gene by an iron-independent regulatory mechanism involving cyclic AMP-cyclic AMP receptor protein complex. J. Bacteriol. 172:3529-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gromiha, M. M., S. Ahmad, and M. Suwa. 2005. TMBETA-NET: discrimination and prediction of membrane spanning beta-strands in outer membrane proteins. Nucleic Acids Res. 33:W164-W167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 92.Gully, D., and E. Bouveret. 2005. A protein network for phospholipid synthesis uncovered by a variant of the tandem affinity purification method in Escherichia coli. Proteomics 6:282-293. [DOI] [PubMed] [Google Scholar]
- 93.Gully, D., D. Moinier, L. Loiseau, and E. Bouveret. 2003. New partners of acyl carrier protein detected in Escherichia coli by tandem affinity purification. FEBS Lett. 548:90-96. [DOI] [PubMed] [Google Scholar]
- 94.Gygi, S. P., B. Rist, S. A. Gerber, F. Turecek, M. H. Gelb, and R. Aebersold. 1999. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat. Biotechnol. 17:994-999. [DOI] [PubMed] [Google Scholar]
- 95.Han, M.-J., and S. Y. Lee. 2003. Proteome profiling and its use in metabolic and cellular engineering. Proteomics 3:2317-2324. [DOI] [PubMed] [Google Scholar]
- 96.Han, M.-J., J. W. Lee, and S. Y. Lee. 2005. Enhanced proteome profiling by inhibiting proteolysis with small heat shock proteins. J. Proteome Res. 4:2429-2434. [DOI] [PubMed] [Google Scholar]
- 97.Han, M.-J., K. J. Jeong, J.-S. Yoo, and S. Y. Lee. 2003. Engineering Escherichia coli for increased productivity of serine-rich proteins based on proteome profiling. Appl. Environ. Microbiol. 69:5772-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han, M.-J., S. J. Park, T. J. Park, and S. Y. Lee. 2004. Roles and applications of small heat shock proteins in the production of recombinant proteins in Escherichia coli. Biotechnol. Bioeng. 88:426-436. [DOI] [PubMed] [Google Scholar]
- 99.Han, M.-J., S. S. Yoon, and S. Y. Lee. 2001. Proteome analysis of metabolically engineered Escherichia coli producing poly(3-hydroxybutyrate). J. Bacteriol. 183:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hanlon, W. A., and P. R. Griffin. 2004. Protein profiling using two-dimensional gel electrophoresis with multiple fluorescent tags, p. 75-91. In D. W. Speicher (ed.), Proteome analysis: interpreting the genome. Elsevier Science B.V., Amsterdam, The Netherlands.
- 101.Hart, R. A., U. Rinas, and J. E. Bailey. 1990. Protein composition of Vitreoscilla hemoglobin inclusion bodies produced in Escherichia coli. J. Biol. Chem. 265:12728-12733. [PubMed] [Google Scholar]
- 102.Haynes, P. A., and J. R. Yates III. 2000. Proteome profiling—pitfalls and progress. Yeast 17:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hengge-Aronis, R. 1996. Regulation of gene expression during entry into stationary phase, p. 1497-1512. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 104.Hengge-Aronis, R. 2002. Signal transduction and regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hengge-Aronis, R., R. Lange, N. Henneberg, and D. Fischer. 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Henikoff, S., S. Pietrokovski, and J. G. Henikoff. 1998. Superior performance in protein homology detection with the Blocks Database servers. Nucleic Acids Res. 26:309-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henzel, W. J., T. M. Billeci, J. T. Stults, S. C. Wong, C. Grimley, and C. Watanabe. 1993. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc. Natl. Acad. Sci. USA 90:5011-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Herbert, B., and P. G. Righetti. 2000. A turning point in proteome analysis: sample prefractionation via multicompartment electrolyzers with isoelectric membranes. Electrophoresis 21:3639-3648. [DOI] [PubMed] [Google Scholar]
- 109.Herendeen, S. L., R. A. VanBogelen, and F. C. Neidhardt. 1979. Levels of major proteins of Escherichia coli during growth at different temperatures. J. Bacteriol. 139:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herr, A. E., J. I. Molho, K. A. Drouvalakis, J. C. Mikkelsen, P. J. Utz, J. G. Santiago, and T. W. Kenny. 2003. On-chip coupling of isoelectric focusing and free solution electrophoresis for multidimensional separations. Anal. Chem. 75:1180-1187. [DOI] [PubMed] [Google Scholar]
- 111.Hewick, R. M., M. W. Hunkapiller, L. E. Hood, and W. J. Dreyer. 1981. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 256:7990-7997. [PubMed] [Google Scholar]
- 112.Hiller, K., M. Schobert, C. Hundertmark, D. Jahn, and R. Munch. 2003. JVirGel: calculation of virtual two-dimensional protein gels. Nucleic Acids Res. 31:3862-3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hirokawa, T., S. Boon-Chieng, and S. Mitaku. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14:378-379. [DOI] [PubMed] [Google Scholar]
- 114.Hoffmann, F., and U. Rinas. 2001. On-line estimation of the metabolic burden resulting from synthesis of plasmid-encoded and heat-shock proteins by monitoring respiratory energy generation. Biotechnol. Bioeng. 76:333-340. [DOI] [PubMed] [Google Scholar]
- 115.Hoffmann, F., and U. Rinas. 2000. Kinetics of heat-shock response and inclusion body formation during temperature-induced production of basic fibroblast growth factor in high-cell-density cultures of recombinant Escherichia coli. Biotechnol. Prog. 16:1000-1007. [DOI] [PubMed] [Google Scholar]
- 116.Hoffmann, F., J. Weber, and U. Rinas. 2002. Metabolic adaptation of Escherichia coli during temperature induced recombinant protein synthesis: 1. Readjustment of metabolic enzyme synthesis. Biotechnol. Bioeng. 80:313-319. [DOI] [PubMed] [Google Scholar]
- 117.Hofmann, K., and W. Stoffel. 1993. TMbase—a database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 118.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 119.Hu, J. C., M. G. Kornacker, and A. Hochschild. 2000. Escherichia coli one- and two-hybrid systems for the analysis and identification of protein-protein interactions. Methods 20:80-94. [DOI] [PubMed] [Google Scholar]
- 120.Hua, S., and Z. Sun. 2001. Support vector machine approach for protein subcellular localization prediction. Bioinformatics 17:721-728. [DOI] [PubMed] [Google Scholar]
- 121.Huber, L. A., K. Pfaller, and I. Vietor. 2003. Organelle proteomics: implications for subcellular fractionation in proteomics. Circ. Res. 92:962-968. [DOI] [PubMed] [Google Scholar]
- 122.Human Proteome Organization. 2002. Abstr. 1st World Congress, Versailles, France. Mol. Cell. Proteomics 1:651-752. [PubMed] [Google Scholar]
- 123.Hutchens, T. W., and T. T. Yip. 1993. New desorption strategies for the mass spectrometric analysis of macromolecules. Rapid Commun. Mass. Spectrom. 7:576-580. [Google Scholar]
- 124.Ikeda, M., M. Arai, T. Okuno, and T. Shimizu. 2003. TMPDB: a database of experimentally-characterized transmembrane topologies. Nucleic Acids Res. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iuchi, S., and E. C. Lin. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. USA 85:1888-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jensen, P. K., L. Paša-Tolić, G. A. Anderson, J. A. Horner, M. S. Lipton, J. E. Bruce, and R. D. Smith. 1999. Probing proteomes using capillary isoelectric focusing-electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 71:2076-2084. [DOI] [PubMed] [Google Scholar]
- 127.Jensen, P. K., L. Paša-Tolić, K. K. Peden, S. Martinović, M. S. Lipton, G. A. Anderson, N. Tolic, K. K. Wong, and R. D. Smith. 2000. Mass spectrometric detection for capillary isoelectric focusing separations of complex protein mixtures. Electrophoresis 21:1372-1380. [DOI] [PubMed] [Google Scholar]
- 128.Jiang, G. R., S. Nikolova, and D. P. Clark. 2001. Regulation of the ldhA gene, encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 147:2437-2446. [DOI] [PubMed] [Google Scholar]
- 129.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1994. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 33:3038-3049. [DOI] [PubMed] [Google Scholar]
- 130.Jones, P. G., and M. Inouye. 1996. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol. Microbiol. 21:1207-1218. [DOI] [PubMed] [Google Scholar]
- 131.Jones, P. G., M. Cashel, G. Glaser, and F. C. Neidhardt. 1992. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J. Bacteriol. 174:3903-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones, P. G., M. Mitta, Y. Kim, W. Jiang, and M. Inouye. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones, P. G., R. A. VanBogelen, and F. C. Neidhardt. 1987. Induction of proteins in response to low temperature in Escherichia coli. J. Bacteriol. 169:2092-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jones, P. G., R. Krah, S. R. Tafuri, and A. P. Wolffe. 1992. DNA gyrase, CS7.4, and the cold shock response in Escherichia coli. J. Bacteriol. 174:5798-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jordan, G. L., and S. W. Harcum. 2002. Characterization of up-regulated proteases in an industrial recombinant Escherichia coli fermentation. J. Ind. Microbiol. Biotechnol. 28:74-80. [DOI] [PubMed] [Google Scholar]
- 136.Jung, E., M. Heller, J. C. Sanchez, and D. F. Hochstrasser. 2000. Proteomics meets cell biology: the establishment of subcellular proteomes. Electrophoresis 21:3369-3377. [DOI] [PubMed] [Google Scholar]
- 137.Jung, J. U., C. Gutierrez, and M. R. Villarejo. 1989. Sequence of an osmotically inducible lipoprotein gene. J. Bacteriol. 171:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jürgen, B., H. Y. Lin, S. Riemschneider, C. Scharf, P. Neubauer, R. Schmid, M. Hecker, and T. Schweder. 2000. Monitoring of genes that respond to overproduction of an insoluble recombinant protein in Escherichia coli glucose-limited fed-batch fermentations. Biotechnol. Bioeng. 70:217-224. [DOI] [PubMed] [Google Scholar]
- 139.Kabir, M., and K. Shimizu. 2001. Proteome analysis of a temperature-inducible recombinant Escherichia coli for poly-β-hydroxybutyrate production. J. Biosci. Bioeng. 92:277-284. [DOI] [PubMed] [Google Scholar]
- 140.Kaji, H., H. Saito, Y. Yamauchi, T. Shinkawa, M. Taoka, J. Hirabayashi, K. Kasai, N. Takahashi, and T. Isobe. 2003. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat. Biotechnol. 21:667-672. [DOI] [PubMed] [Google Scholar]
- 141.Karpel, R., T. Alon, G. Glaser, S. Schuldiner, and E. Padan. 1991. Expression of a sodium proton antiporter (NhaA) in Escherichia coli is induced by Na+ and Li+ ions. J. Biol. Chem. 266:21753-21759. [PubMed] [Google Scholar]
- 142.Keller, B. O., Z. Wang, and L. Li. 2002. Low-mass proteome analysis based on liquid chromatography fractionation, nanoliter protein concentration/digestion, and microspot matrix-assisted laser desorption ionization mass spectrometry. J. Chromatogr. B 782:317-329. [DOI] [PubMed] [Google Scholar]
- 143.Kelley, L. A., R. M. MacCallum, and M. J. Sternberg. 2000. Enhanced genome annotation using structural profiles in the program 3D-PSSM. J. Mol. Biol. 299:499-520. [DOI] [PubMed] [Google Scholar]
- 144.Kersey, P., L. Bower, L. Morris, A. Horne, R. Petryszak, C. Kanz, A. Kanapin, U. Das, K. Michoud, I. Phan, A. Gattiker, T. Kulikova, N. Faruque, K. Duggan, P. Mclaren, B. Reimholz, L. Duret, S. Penel, I. Reuter, and R. Apweiler. 2005. Integr8 and Genome Reviews: integrated views of complete genomes and proteomes. Nucleic Acids Res. 33:D297-D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim, Y. H., K. Y. Han, K. Lee, and J. Lee. 2005. Proteome response of Escherichia coli fed-batch culture to temperature downshift. Appl. Microbiol. Biotechnol. 68:786-793. [DOI] [PubMed] [Google Scholar]
- 146.Kirkpatrick, C., L. M. Maurer, N. E. Oyelakin, Y. N. Yoncheva, R. Maurer, and J. L. Slonczewski. 2001. Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J. Bacteriol. 183:6466-6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Klose, J. 1975. Protein mapping by combined isoelectric focusing and electrophoresis of mouse tissues. A novel approach to testing for induced point mutations in mammals. Humangenetik 26:231-243. [DOI] [PubMed] [Google Scholar]
- 148.Krause, A., J. Stoye, and M. Vingron. 2000. The SYSTERS protein sequence cluster set. Nucleic Acids Res. 28:270-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kredich, N. M. 1996. Biosynthesis of cysteine, p. 514-527. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 150.Kriventseva, E. V., W. Fleischmann, E. M. Zdobnov, and R. Apweiler. 2001. CluSTr: a database of clusters of SWISS-PROT+TrEMBL proteins. Nucleic Acids Res. 29:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar, J. K., S. Tabor, and C. C. Richardson. 2004. Proteomic analysis of thioredoxin-targeted proteins in Escherichia coli. Proc. Natl. Acad. Sci. USA 101:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 153.Lai, E. M., U. Nair, N. D. Phadke, and J. R. Maddock. 2004. Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol. Microbiol. 52:1029-1044. [DOI] [PubMed] [Google Scholar]
- 154.Lambert, L. A., K. Abshire, D. Blankenhorn, and J. L. Slonczewski. 1997. Proteins induced in Escherichia coli by benzoic acid. J. Bacteriol. 179:7595-7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 156.Lange, R., M. Barth, and R. Hengge-Aronis. 1993. Complex transcriptional control of the σS-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for Lrp, cyclic AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J. Bacteriol. 175:7910-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.La Teana, A., A. Brandi, M. Falconi, R. Spurio, C. L. Pon, and C. O. Gualerzi. 1991. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc. Natl. Acad. Sci. USA 88:10907-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Laurent-Winter, C., S. Ngo, A. Danchin, and P. Bertin. 1997. Role of Escherichia coli histone-like nucleoid-structuring protein in bacterial metabolism and stress response-identification of targets by two-dimensional electrophoresis. Eur. J. Biochem. 244:767-773. [DOI] [PubMed] [Google Scholar]
- 159.le Coutre, J., J. P. Whitelegge, A. Gross, E. Turk, E. M. Wright, H. R. Kaback, and K. F. Faull. 2000. Proteomics on full-length membrane proteins using mass spectrometry. Biochemistry 39:4237-4242. [DOI] [PubMed] [Google Scholar]
- 160.Lee, J. H., D. E. Lee, B. U. Lee, and H. S. Kim. 2003. Global analyses of transcriptomes and proteomes of a parent strain and an l-threonine-overproducing mutant strain. J. Bacteriol. 185:5442-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee, P. S., and K. H. Lee. 2003. Escherichia coli—a model system that benefits from and contributes to the evolution of proteomics. Biotechnol. Bioeng. 84:801-814. [DOI] [PubMed] [Google Scholar]
- 162.Lee, P. S., and K. H. Lee. 2005. Engineering HlyA hypersecretion in Escherichia coli based on proteomic and microarray analyses. Biotechnol. Bioeng. 89:195-205. [DOI] [PubMed] [Google Scholar]
- 163.Lee, S. J., A. Xie, W. Jiang, J. P. Etchegaray, P. G. Jones, and M. Inouye. 1994. Family of the major cold-shock protein, CspA (CS7.4), of Escherichia coli, whose members show a high sequence similarity with the eukaryotic Y-box binding proteins. Mol. Microbiol. 11:833-839. [DOI] [PubMed] [Google Scholar]
- 164.Lee, S. Y., D.-Y. Lee, and T. Y. Kim. 2005. Systems biotechnology for strain improvement. Trends Biotechnol. 23:349-358. [DOI] [PubMed] [Google Scholar]
- 165.Lelivelt, M. J., and T. H. Kawula. 1995. Hsc66, an Hsp70 homolog in Escherichia coli, is induced by cold shock but not by heat shock. J. Bacteriol. 177:4900-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lemaux, P. G., S. L. Herendeen, P. L. Bloch, and F. C. Neidhardt. 1978. Transient rates of synthesis of individual polypeptides in E. coli following temperature shifts. Cell 13:427-434. [DOI] [PubMed] [Google Scholar]
- 167.LeThanh, H., P. Neubauer, and F. Hoffmann. 2005. The small heat-shock proteins IbpA and IbpB reduce the stress load of recombinant Escherichia coli and delay degradation of inclusion bodies. Microb. Cell Fact. 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Leveque, F., P. Plateau, P. Dessen, and S. Blanquet. 1990. Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 18:305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Levin, J., A. Johnson, and B. Demple. 1988. Homogenous Escherichia coli endonuclease IV: characterization of an enzyme that recognizes free-radical-induced damage in DNA. J. Biol. Chem. 263:8066-8071. [PubMed] [Google Scholar]
- 170.Li, J., T. LeRiche, T. L. Tremblay, C. Wang, E. Bonneil, D. J. Harrison, and P. Thibault. 2002. Application of microfluidic devices to proteomics research: identification of trace-level protein digests and affinity capture of target peptides. Mol. Cell. Proteomics 1:157-168. [DOI] [PubMed] [Google Scholar]
- 171.Lin, R., R. D'Ari, and E. B. Newman. 1992. λ placMu insertions in genes of the leucine regulon: extension of the regulon to genes not regulated by leucine. J. Bacteriol. 174:1948-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lin, R., B. Ernsting, I. N. Hirshfield, R. G. Matthews, F. C. Neidhardt, R. L. Clark, and E. B. Newman. 1992. The lrp gene product regulates expression of lysU in Escherichia coli K-12. J. Bacteriol. 174:2779-2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liochev, S. I., A. Hausladen, F. B. Beyer, and I. Fridovich. 1994. NADPH:ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc. Natl. Acad. Sci. USA 91:1328-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liochev, S. I., and I. Fridovich. 1992. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc. Natl. Acad. Sci. USA 89:5892-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lion, N., T. C. Rohner, L. Dayon, I. L. Arnaud, E. Damoc, N. Youhnovski, Z. Y. Wu, C. Roussel, J. Josserand, H. Jensen, J. S. Rossier, M. Przybylski, and H. H. Girault. 2003. Microfluidic systems in proteomics. Electrophoresis 24:3533-3562. [DOI] [PubMed] [Google Scholar]
- 176.Lockhart, D. J., and E. A. Winzeler. 2000. Genomics, gene expression and DNA arrays. Nature 405:827-836. [DOI] [PubMed] [Google Scholar]
- 177.Loo, J. A., C. G. Edmonds, and R. D. Smith. 1990. Primary sequence information from intact proteins by electrospray ionization tandem mass spectrometry. Science 248:201-204. [DOI] [PubMed] [Google Scholar]
- 178.Loo, R. R., J. D. Cavalcoli, R. A. VanBogelen, C. Mitchell, J. A. Loo, B. Moldover, and P. C. Andrews. 2001. Virtual 2-D gel electrophoresis: visualization and analysis of the E. coli proteome by mass spectrometry. Anal. Chem. 73:4063-4070. [DOI] [PubMed] [Google Scholar]
- 179.Lopez-Campistrous, A., P. Semchuk, L. Burke, T. Palmer-Stone, S. J. Brokx, G. Broderick, D. Bottorff, S. Bolch, J. H. Weiner, and M. J. Ellison. 2005. Localization, annotation and comparison of the Escherichia coli K-12 proteome under two states of growth. Mol. Cell. Proteomics 4:1205-1209. [DOI] [PubMed] [Google Scholar]
- 180.Lu, T., M. Van Dyke, and M. Sawadogo. 1993. Protein-protein interaction studies using immobilized oligohistidine fusion proteins. Anal. Biochem. 213:318-322. [DOI] [PubMed] [Google Scholar]
- 181.Lu, Z., D. Szafron, R. Greiner, P. Lu, D. S. Wishart, B. Poulin, J. Anvik, C. Macdonell, and R. Eisner. 2004. Predicting subcellular localization of proteins using machine-learned classifiers. Bioinformatics 20:547-556. [DOI] [PubMed] [Google Scholar]
- 182.Lueking, A., M. Horn, H. Eickhoff, K. Bussow, H. Lehrach, and G. Walter. 1999. Protein microarrays for gene expression and antibody screening. Anal. Biochem. 270:103-111. [DOI] [PubMed] [Google Scholar]
- 183.Lynch, A. S., and E. C. C. Lin. 1996. Responses to molecular oxygen, p. 1526-1538. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 184.MacNair, J. E., G. J. Opiteck, J. W. Jorgenson, and M. A. Moseley III. 1997. Rapid separation and characterization of protein and peptide mixtures using 1.5 microns diameter non-porous silica in packed capillary liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 11:1279-1285. [DOI] [PubMed] [Google Scholar]
- 185.Magnusson, L. U., A. Farewell, and T. Nyström. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 13:236-242. [DOI] [PubMed] [Google Scholar]
- 186.Mallick, U., and P. Herrlich. 1979. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc. Natl. Acad. Sci. USA 76:5520-5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martelli, P. L., P. Fariselli, and R. Casadio. 2004. Prediction of disulfide-bonded cysteines in proteomes with a hidden neural network. Proteomics 4:1665-1671. [DOI] [PubMed] [Google Scholar]
- 188.Martinović, S., L. Paša-Tolić, and R. D. Smith. 2004. Capillary isoelectric focusing-mass spectrometry of proteins and protein complexes. Methods Mol. Biol. 276:291-304. [DOI] [PubMed] [Google Scholar]
- 189.Martinović, S., T. D. Veenstra, G. A. Anderson, L. Paša-Tolić, and R. D. Smith. 2002. Selective incorporation of isotopically labeled amino acids for identification of intact proteins on a proteome-wide level. J. Mass Spectrom. 37:99-107. [DOI] [PubMed] [Google Scholar]
- 190.Matin, A. 1994. Starvation promoters of Escherichia coli. Their function, regulation, and use in bioprocessing and bioremediation. Ann. N. Y. Acad. Sci. 721:277-291. [DOI] [PubMed] [Google Scholar]
- 191.May, G., E. Faatz, M. Villarejo, and E. Bremer. 1986. Binding protein dependent transport of glycine betaine and its osmotic regulation in Escherichia coli K12. Mol. Gen. Genet. 205:225-233. [DOI] [PubMed] [Google Scholar]
- 192.McCann, M. P., J. P. Kidwell, and A. Matin. 1991. The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J. Bacteriol. 173:4188-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McDonald, W. H., and J. R. Yates. 2002. Shotgun proteomics and biomarker discovery. Dis. Markers 18:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McHardy, A. C., A. Puhler, J. Kalinowski, and F. Meyer. 2004. Comparing expression level-dependent features in codon usage with protein abundance: an analysis of “predictive proteomics.” Proteomics 4:46-58. [DOI] [PubMed] [Google Scholar]
- 195.Miller, J. F., J. J. Mekalanos, and S. Falkow. 1989. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243:916-922. [DOI] [PubMed] [Google Scholar]
- 196.Mitra, R. S., R. H. Gray, B. Chin, and I. A. Bernstein. 1975. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J. Bacteriol. 121:1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mizuguchi, K., C. M. Deane, T. L. Blundell, and J. P. Overington. 1998. HOMSTRAD: a database of protein structure alignments for homologous families. Protein Sci. 7:2469-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mogk, A., T. Tomoyasu, P. Goloubinoff, S. Rüdiger, D. Röder, H. Langen, and B. Bukau. 1999. Identification of thermolabile Escherichia coli proteins: prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 18:6934-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Möller, S., M. D. Croning, and R. Apweiler. 2001. Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646-653. [DOI] [PubMed] [Google Scholar]
- 200.Molloy, M. P., B. R. Herbert, K. L. Williams, and A. A. Gooley. 1999. Extraction of Escherichia coli proteins with organic solvents prior to two-dimensional electrophoresis. Electrophoresis 20:701-704. [DOI] [PubMed] [Google Scholar]
- 201.Molloy, M. P., B. R. Herbert, M. B. Slade, T. Rabilloud, A. S. Nouwens, K. L. Williams, and A. A. Gooley. 2000. Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 267:2871-2881. [DOI] [PubMed] [Google Scholar]
- 202.Molloy, M. P., S. Donohoe, E. E. Brzezinski, G. W. Kilby, T. I. Stevenson, J. D. Baker, D. R. Goodlett, and D. A. Gage. 2005. Large-scale evaluation of quantitative reproducibility and proteome coverage using acid cleavable isotope coded affinity tag mass spectrometry for proteomic profiling. Proteomics 5:1204-1208. [DOI] [PubMed] [Google Scholar]
- 203.Moreau, P. L., F. Gerard, N. W. Lutz, and P. Cozzone. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol. Microbiol. 39:1048-1060. [DOI] [PubMed] [Google Scholar]
- 204.Muffler, A., D. D. Traulsen, D. Fischer, R. Lange, and R. Hengge-Aronis. 1997. The RNA-binding protein HF-I plays a global regulatory role which is largely, but not exclusively, due to its role in expression of the σS subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 179:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Muffler, A., M. Barth, C. Marschall, and R. Hengge-Aronis. 1997. Heat shock regulation of σS turnover: a role for DnaK and relationship between stress responses mediated by σS and σ32 in Escherichia coli. J. Bacteriol. 179:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mulder, N. J., R. Apweiler, T. K. Attwood, A. Bairoch, A. Bateman, D. Binns, P. Bradley, P. Bork, P. Bucher, L. Cerutti, R. Copley, E. Courcelle, U. Das, R. Durbin, W. Fleischmann, J. Gough, D. Haft, N. Harte, N. Hulo, D. Kahn, A. Kanapin, M. Krestyaninova, D. Lonsdale, R. Lopez, I. Letunic, M. Madera, J. Maslen, J. McDowall, A. Mitchell, A. N. Nikolskaya, S. Orchard, M. Pagni, C. P. Ponting, E. Quevillon, J. Selengut, C. J. Sigrist, V. Silventoinen, D. J. Studholme, R. Vaughan, and C. H. Wu. 2005. InterPro, progress and status in 2005. Nucleic Acids Res. 33:D201-D205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nakashima, K., K. Kanamaru, T. Mizuno, and K. Horikoshi. 1996. A novel member of the cspA family of genes that is induced by cold shock in Escherichia coli. J. Bacteriol. 178:2994-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neidhardt, F. C., and R. A. VanBogelen. 1981. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem. Biophys. Res. Commun. 100:894-900. [DOI] [PubMed] [Google Scholar]
- 209.Neidhardt, F. C., and R. A. VanBogelen. 2000. Proteomic analysis of bacterial stress responses, p. 445-452. In G. Storz and H. Hennecke (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 210.Neidhardt, F. C., R. A. VanBogelen, and E. T. Lau. 1983. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J. Bacteriol. 153:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Neidhardt, F. C., T. A. Phillips, R. A. VanBogelen, M. W. Smith, Y. Georgalis, and A. R. Subramanian. 1981. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J. Bacteriol. 145:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Neidhardt, F. C., V. Vaughn, T. A. Phillips, and P. L. Bloch. 1983. Gene-protein index of Escherichia coli K-12. Microbiol. Rev. 47:231-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nelson, R. W., J. R. Krone, and O. Jansson. 1997. Surface plasmon resonance biomolecular interaction analysis mass spectrometry. 1. Chip-based analysis. Anal. Chem. 69:4363-4368. [DOI] [PubMed] [Google Scholar]
- 214.Nevill-Manning, C. G., T. D. Wu, and D. L. Brutlag. 1998. Highly specific protein sequence motifs for genome analysis. Proc. Natl. Acad. Sci. USA 95:5865-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Newman, E. B., R. T. Lin, and R. D'Ari. 1996. The leucine/Lrp regulon, p. 1513-1525. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 216.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 217.Nielsen, J., and L. Olsson. 2002. An expanded role for microbial physiology in metabolic engineering and functional genomics: moving towards systems biology. FEMS Yeast Res. 2:175-181. [DOI] [PubMed] [Google Scholar]
- 218.Nyström, T. 1994. Role of guanosine tetraphosphate in gene expression and the survival of glucose or seryl-transfer-RNA starved cells of Escherichia coli K-12. Mol. Gen. Genet. 245:355-362. [DOI] [PubMed] [Google Scholar]
- 219.Nyström, T., and S. Kjelleberg. 1987. The effect of cadmium on starved heterotrophic bacteria isolated from marine waters. FEMS Microbiol. Ecol. 45:143-153. [Google Scholar]
- 220.O'Farrell, P. H. 1975. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 250:4007-4021. [PMC free article] [PubMed] [Google Scholar]
- 221.O'Farrell, P. H. 1978. The suppression of defective translation by ppGpp and its role in the stringent response. Cell 14:545-557. [DOI] [PubMed] [Google Scholar]
- 222.O'Farrell, P. Z., H. M. Goodman, and P. H. O'Farrell. 1977. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell 12:1133-1141. [DOI] [PubMed] [Google Scholar]
- 223.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 224.Opiteck, G. J., K. C. Lewis, J. W. Jorgenson, and R. J. Anderegg. 1997. Comprehensive on-line LC/LC/MS of proteins. Anal. Chem. 69:1518-1524. [DOI] [PubMed] [Google Scholar]
- 225.Opiteck, G. J., S. M. Ramirez, J. W. Jorgenson, and M. A. Moseley III. 1998. Comprehensive two-dimensional high-performance liquid chromatography for the isolation of overexpressed proteins and proteome mapping. Anal. Biochem. 258:349-361. [DOI] [PubMed] [Google Scholar]
- 226.Pandey, A., and M. Mann. 2000. Proteomics to study genes and genomes. Nature 405:837-846. [DOI] [PubMed] [Google Scholar]
- 227.Pascarella, S., and P. Argos. 1992. A data bank merging related protein structures and sequences. Protein Eng. 5:121-137. [DOI] [PubMed] [Google Scholar]
- 228.Pasquali, C., S. Frutiger, M. R. Wilkins, G. J. Hughes, R. D. Appel, A. Bairoch, D. Schaller, J. C. Sanchez, and D. F. Hochstrasser. 1996. Two-dimensional gel electrophoresis of Escherichia coli homogenates: the Escherichia coli SWISS-2DPAGE database. Electrophoresis 17:547-555. [DOI] [PubMed] [Google Scholar]
- 229.Patton, W. F. 2002. Detection technologies in proteome analysis. J. Chromatogr. B 771:3-31. [DOI] [PubMed] [Google Scholar]
- 230.Pedersen, S., P. L. Bloch, S. Reeh, and F. C. Neidhardt. 1978. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell 14:179-190. [DOI] [PubMed] [Google Scholar]
- 231.Peng, L., and K. Shimizu. 2003. Global metabolic regulation analysis for Escherichia coli K12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl. Microbiol. Biotechnol. 61:163-178. [DOI] [PubMed] [Google Scholar]
- 232.Perkins, D. N., D. J. Pappin, D. M. Creasy, and J. S. Cottrell. 1999. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 20:3551-3567. [DOI] [PubMed] [Google Scholar]
- 233.Perrot, F., M. Hébraud, G. A. Junter, and T. Jouenne. 2000. Protein synthesis in Escherichia coli at 4°C. Electrophoresis 21:1625-1629. [DOI] [PubMed] [Google Scholar]
- 234.Perrot, F., M. Hébraud, R. Charlionet, G. A. Junter, and T. Jouenne. 2001. Cell immobilization induces changes in the protein response of Escherichia coli K-12 to a cold shock. Electrophoresis 22:2110-2119. [DOI] [PubMed] [Google Scholar]
- 235.Pferdeort, V. A., T. K. Wood, and K. F. Reardon. 2003. Proteomic changes in Escherichia coli TG1 after metabolic engineering for enhanced trichloroethene biodegradation. Proteomics 3:1066-1069. [DOI] [PubMed] [Google Scholar]
- 236.Phillips, T. A., P. L. Bloch, and F. C. Neidhardt. 1980. Protein identifications on O'Farrell two-dimensional gels: locations of 55 additional Escherichia coli proteins. J. Bacteriol. 144:1024-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Phillips, T. A., R. A. VanBogelen, and F. C. Neidhardt. 1984. lon gene product of Escherichia coli is a heat-shock protein. J. Bacteriol. 159:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pinto, D. M., Y. Ning, and D. Figeys. 2000. An enhanced microfluidic chip coupled to an electrospray Qstar mass spectrometer for protein identification. Electrophoresis 21:181-190. [DOI] [PubMed] [Google Scholar]
- 239.Quadroni, M., W. Staudenmann, M. Kertesz, and P. James. 1996. Analysis of global responses by protein and peptide fingerprinting of proteins isolated by two-dimensional gel electrophoresis. Application to the sulfate-starvation response of Escherichia coli. Eur. J. Biochem. 239:773-781. [DOI] [PubMed] [Google Scholar]
- 240.Raina, S., and C. Georgopoulos. 1991. The htrM gene, whose product is essential for Escherichia coli viability only at elevated temperatures, is identical to the rfaD gene. Nucleic Acids Res. 19:3811-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Raman, B., M. P. Nandakumar, V. Muthuvijayan, and M. R. Marten. 2005. Proteome analysis to assess physiological changes in Escherichia coli grown under glucose-limited fed-batch conditions. Biotechnol. Bioeng. 92:384-392. [DOI] [PubMed] [Google Scholar]
- 242.Ramsey, J. D., S. C. Jacobson, C. T. Culbertson, and J. M. Ramsey. 2003. High-efficiency, two-dimensional separations of protein digests on microfluidic devices. Anal. Chem. 75:3758-3764. [DOI] [PubMed] [Google Scholar]
- 243.Reinhardt, A., and T. Hubbard. 1998. Using neural networks for prediction of the subcellular location of proteins. Nucleic Acids Res. 26:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Repoila, F., and C. Gutierrez. 1991. Osmotic induction of the periplasmic trehalase in Escherichia coli K12: characterization of the treA gene promoter. Mol. Microbiol. 5:747-755. [DOI] [PubMed] [Google Scholar]
- 245.Rinas, U., and F. Hoffmann. 2004. Selective leakage of host cell proteins during high-cell density cultivation of recombinant and non-recombinant Escherichia coli. Biotechnol. Prog. 20:679-687. [DOI] [PubMed] [Google Scholar]
- 246.Rinas, U., and J. E. Bailey. 1992. Protein compositional analysis of inclusion bodies produced in recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 37:609-614. [DOI] [PubMed] [Google Scholar]
- 247.Rinas, U., T. C. Boone, and J. E. Bailey. 1993. Characterization of inclusion bodies in Escherichia coli producing high levels of recombinant porcine somatotropin. J. Biotechnol. 28:313-320. [DOI] [PubMed] [Google Scholar]
- 248.Ros, A., M. Faupel, H. Mees, J. Oostrum, R. Ferrigno, F. Reymond, P. Michel, J. S. Rossier, and H. H. Girault. 2002. Protein purification by off-gel electrophoresis. Proteomics 2:151-156. [DOI] [PubMed] [Google Scholar]
- 249.Rosen, R., and E. Z. Ron. 2002. Proteome analysis in the study of the bacterial heat-shock response. Mass Spectrom. Rev. 21:244-265. [DOI] [PubMed] [Google Scholar]
- 250.Rosen, R., D. Biran, E. Gur, D. Becher, M. Hecker, and E. Z. Ron. 2002. Protein aggregation in Escherichia coli: role of proteases. FEMS Microbiol. Lett. 207:9-12. [DOI] [PubMed] [Google Scholar]
- 251.Rosenfeld, J., J. Capdevielle, J. C. Guillemot, and P. Ferrara. 1992. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203:173-179. [DOI] [PubMed] [Google Scholar]
- 252.Rosner, J. L., and J. L. Slonczewski. 1994. Dual regulation of inaA by the multiple antibiotic resistance (mar) and superoxide (soxRS) stress response systems of Escherichia coli. J. Bacteriol. 176:6262-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ross, P. L., Y. N. Huang, J. N. Marchese, B. Williamson, K. Parker, S. Hattan, N. Khainovski, S. Pillai, S. Dey, S. Daniels, S. Purkayastha, P. Juhasz, S. M. Martin, Bartlet-Jones, F. He, A. Jacobson, and D. J. Pappin. 2004. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 3:1154-1169. [DOI] [PubMed] [Google Scholar]
- 254.Rowley, D. L., and R. E. Wolf. 1991. Molecular characterization of the Escherichia coli K-12 zwf gene encoding glucose 6-phosphate dehydrogenase. J. Bacteriol. 173:968-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Russel, M., and P. Model. 1988. Sequence of thioredoxin reductase from Escherichia coli. Relationship to other flavoprotein disulfide oxidoreductases. J. Biol. Chem. 263:9015-9019. [PubMed] [Google Scholar]
- 256.Russell, J. B., and F. Diez-Gonzalez. 1998. The effects of fermentation acids on bacterial growth. Adv. Microb. Physiol. 39:205-234. [DOI] [PubMed] [Google Scholar]
- 257.Sander, C., and R. Schneider. 1991. Database of homology-derived protein structures and the structural meaning of sequence alignment. Proteins 9:56-68. [DOI] [PubMed] [Google Scholar]
- 258.Sankar, P., M. E. Hutton, R. A. VanBogelen, R. L. Clark, and F. C. Neidhardt. 1993. Expression analysis of cloned chromosomal segments of Escherichia coli. J. Bacteriol. 175:5145-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sasson, O., A. Vaaknin, H. Fleischer, E. Portugaly, Y. Bilu, N. Linial, and M. Linial. 2003. ProtoNet: hierarchical classification of the protein space. Nucleic Acids Res. 31:348-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schasfoort, R. B. 2004. Proteomics-on-a-chip: the challenge to couple lab-on-a-chip unit operations. Expert Rev. Proteomics 1:123-132. [DOI] [PubMed] [Google Scholar]
- 261.Schmid, D. G., P. Grosche, H. Bandel, and G. Jung. 2001 2000. FTICR-mass spectrometry for high-resolution analysis in combinatorial chemistry. Biotechnol. Bioeng. 71:149-161. [PubMed] [Google Scholar]
- 262.Schmidt, R., M. Gerstein, and R. B. Altman. 1997. LPFC: an Internet library of protein family core structures. Protein Sci. 6:246-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Schreiber, G., S. Metzger, E. Aizenman, S. Roza, M. Cashel, and G. Glaser. 1991. Overexpression of the relA gene in Escherichia coli. J. Biol. Chem. 266:3760-3767. [PubMed] [Google Scholar]
- 264.Schultz, J., F. Milpetz, P. Bork, and C. P. Ponting. 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95:5857-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Schwartz, R., C. S. Ting, and J. King. 2001. Whole proteome pI values correlate with subcellular localizations of proteins for organisms within the three domains of life. Genome Res. 11:703-709. [DOI] [PubMed] [Google Scholar]
- 266.Seaver, L. C., and J. A. Imlay. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Slentz, B. E., N. A. Penner, and F. E. Regnier. 2003. Protein proteolysis and the multi-dimensional electrochromatographic separation of histidine-containing peptide fragments on a chip. J. Chromatogr. A 984:97-107. [DOI] [PubMed] [Google Scholar]
- 268.Slonczewski, J. L., and J. W. Foster. 1996. pH-regulated genes and survival at extreme pH, p. 1539-1552. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 269.Smirnova, E., D. L. Shurland, and A. M. van der Bliek. 2001. Mapping dynamin interdomain interactions with yeast two-hybrid and glutathione S-transferase pulldown experiments. Methods Enzymol. 329:468-477. [DOI] [PubMed] [Google Scholar]
- 270.Smith, M. W., and F. C. Neidhardt. 1983. Proteins induced by aerobiosis in Escherichia coli. J. Bacteriol. 154:344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Smith, M. W., and F. C. Neidhardt. 1983. Proteins induced by anaerobiosis in Escherichia coli. J. Bacteriol. 154:336-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Snel, B., G. Lehmann, P. Bork, and M. A. Huynen. 2000. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28:3442-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sonnhammer, E. L., S. R. Eddy, E. Birney, A. Bateman, and R. Durbin. 1998. Pfam: multiple sequence alignments and HMM-profiles of protein domains. Nucleic Acids Res. 26:320-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stancik, L. M., D. M. Stancik, B. Schmidt, D. M. Barnhart, Y. N. Yoncheva, and J. L. Slonczewski. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Steinman, H. M., L. Weinstein, and M. Brenowitz. 1994. The manganese superoxide dismutase of Escherichia coli K-12 associates with DNA. J. Biol. Chem. 269:28629-28634. [PubMed] [Google Scholar]
- 276.Stephani, K., D. Weichart, and R. Hengge. 2003. Dynamic control of Dps protein levels by ClpXP and ClpAP proteases in Escherichia coli. Mol. Microbiol. 49:1605-1614. [DOI] [PubMed] [Google Scholar]
- 277.Stern, D. F. 2001. Phosphoproteomics. Exp. Mol. Pathol. 70:327-331. [DOI] [PubMed] [Google Scholar]
- 278.Tang, Y., M. A. Quail, P. J. Artymiuk, J. R. Guest, and J. Green. 2002. Escherichia coli aconitases and oxidative stress: post-transcriptional regulation of sodA expression. Microbiology 148:1027-1037. [DOI] [PubMed] [Google Scholar]
- 279.Tartaglia, L. A., G. Storz, and B. N. Ames. 1989. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J. Mol. Biol. 210:709-719. [DOI] [PubMed] [Google Scholar]
- 280.Tatusov, R. L., E. V. Koonin, and D. J. Lipman. 1997. A genomic perspective on protein families. Science 278:631-637. [DOI] [PubMed] [Google Scholar]
- 281.Taylor, S. W., E. Fahy, and S. S. Ghosh. 2003. Global organellar proteomics. Trends Biotechnol. 21:82-88. [DOI] [PubMed] [Google Scholar]
- 282.Taylor, W. E., D. B. Straus, A. D. Grossman, Z. F. Burton, C. A. Gross, and R. R. Burgess. 1984. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell 38:371-381. [DOI] [PubMed] [Google Scholar]
- 283.Thieringer, H. A., P. G. Jones, and M. Inouye. 1998. Cold shock and adaptation. Bioessays 20:49-57. [DOI] [PubMed] [Google Scholar]
- 284.Tilly, K., R. A. VanBogelen, C. Georgopoulos, and F. C. Neidhardt. 1983. Identification of the heat-inducible protein C15.4 as the groES gene product in Escherichia coli. J. Bacteriol. 154:1505-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Tomoyasu, T., A. Mogk, H. Langen, P. Goloubinoff, and B. Bukau. 2001. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 40:397-413. [DOI] [PubMed] [Google Scholar]
- 286.Tonella, L., B. J. Walsh, J. C. Sanchez, K. Ou, M. R. Wilkins, M. Tyler, S. Frutiger, A. A. Gooley, I. Pescaru, R. D. Appel, J. X. Yan, A. Bairoch, C. Hoogland, F. S. Morch, G. J. Hughes, K. L. Williams, and D. F. Hochstrasser. 1998. '98 Escherichia coli SWISS-2DPAGE database update. Electrophoresis 19:1960-1971. [DOI] [PubMed] [Google Scholar]
- 287.Tonella, L., C. Hoogland, P. A. Binz, R. D. Appel, D. F. Hochstrasser, and J. C. Sanchez. 2001. New perspectives in the Escherichia coli proteome investigation. Proteomics 1:409-423. [DOI] [PubMed] [Google Scholar]
- 288.Tusnady, G. E., and I. Simon. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849-850. [DOI] [PubMed] [Google Scholar]
- 289.Ünlü, M., M. E. Morgan, and J. S. Minden. 1997. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18:2071-2077. [DOI] [PubMed] [Google Scholar]
- 290.VanBogelen, R. A. 2003. Probing the molecular physiology of the microbial organism, Escherichia coli using proteomics. Adv. Biochem. Eng. Biotechnol. 83:27-55. [PubMed] [Google Scholar]
- 291.VanBogelen, R. A., and F. C. Neidhardt. 1990. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:5589-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.VanBogelen, R. A., E. E. Schiller, J. D. Thomas, and F. C. Neidhardt. 1999. Diagnosis of cellular states of microbial organisms using proteomics. Electrophoresis 20:2149-2159. [DOI] [PubMed] [Google Scholar]
- 293.VanBogelen, R. A., E. R. Olson, B. L. Wanner, and F. C. Neidhardt. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J. Bacteriol. 178:4344-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Vanbogelen, R. A., K. Z. Abshire, A. Pertsemlidis, R. L. Clark, and F. C. Neidhardt. 1996. Gene-protein database of Escherichia coli K-12, p. 2067-2117. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 295.VanBogelen, R. A., K. Z. Abshire, B. Moldover, E. R. Olson, and F. C. Neidhardt. 1997. Escherichia coli proteome analysis using the gene-protein database. Electrophoresis 18:1243-1251. [DOI] [PubMed] [Google Scholar]
- 296.VanBogelen, R. A., M. A. Acton, and F. C. Neidhardt. 1987. Induction of the heat-shock regulon does not produce thermotolerance in Escherichia coli. Genes Dev. 1:525-531. [DOI] [PubMed] [Google Scholar]
- 297.VanBogelen, R. A., P. M. Kelley, and F. C. Neidhardt. 1987. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J. Bacteriol. 169:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.van der Ploeg, J. R., M. A. Weiss, E. Saller, H. Nashimoto, N. Saito, M. A. Kertesz, and T. Leisinger. 1996. Identification of sulfate starvation-regulated genes in Escherichia coli: a gene cluster involved in the utilization of taurine as a sulfur source. J. Bacteriol. 178:5438-5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.van der Ploeg, J. R., R. Iwanicka-Nowicka, M. A. Kertesz, T. Leisinger, and M. M. Hryniewicz. 1997. Involvement of CysB and Cbl regulatory proteins in expression of the tauABCD operon and other sulfate starvation-inducible genes in Escherichia coli. J. Bacteriol. 179:7671-7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Veenstra, T. D., S. Martinović, G. A. Anderson, L. Paša-Tolić, and R. D. Smith. 2000. Proteome analysis using selective incorporation of isotopically labeled amino acids. J. Am. Soc. Mass Spectrom. 11:78-82. [DOI] [PubMed] [Google Scholar]
- 301.Vollmer, M., E. Nägele, and P. Hörth. 2003. Differential proteome analysis: two-dimensional nano-LC/MS of E. coli proteome grown on different carbon sources. J. Biomol. Tech. 14:128-135. [PMC free article] [PubMed] [Google Scholar]
- 302.Vollmer, M., P. Hörth, and E. Nägele. 2004. Optimization of two-dimensional off-line LC/MS separations to improve resolution of complex proteomic samples. Anal. Chem. 76:5180-5185. [DOI] [PubMed] [Google Scholar]
- 303.Wang, S., and F. E. Regnier. 2001. Proteomics based on selecting and quantifying cysteine containing peptides by covalent chromatography. J. Chromatogr. A 924:345-357. [DOI] [PubMed] [Google Scholar]
- 304.Wang, S., and F. E. Regnier. 2001. Proteolysis of whole cell extracts with immobilized enzyme columns as part of multidimensional chromatography. J. Chromatogr. A 913:429-436. [DOI] [PubMed] [Google Scholar]
- 305.Wang, S., X. Zhang, and F. E. Regnier. 2002. Quantitative proteomics strategy involving the selection of peptides containing both cysteine and histidine from tryptic digests of cell lysates. J. Chromatogr. A 949:153-162. [DOI] [PubMed] [Google Scholar]
- 306.Wang, Y., S. L. Wu, W. S. Hancock, R. Trala, M. Kessler, A. H. Taylor, P. S. Patel, and J. C. Aon. 2005. Proteomic profiling of Escherichia coli proteins under high cell density fed-batch cultivation with overexpression of phosphogluconolactonase. Biotechnol. Prog. 21:1401-1411. [DOI] [PubMed] [Google Scholar]
- 307.Washburn, M. P., D. Wolters, and J. R. Yates III. 2001. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19:242-247. [DOI] [PubMed] [Google Scholar]
- 308.Weichart, D., N. Querfurth, M. Dreger, and R. Hengge-Aronis. 2003. Global role for ClpP-containing proteases in stationary-phase adaptation of Escherichia coli. J. Bacteriol. 185:115-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Weinberger, S. R., R. I. Viner, and P. Ho. 2002. Tagless extraction-retentate chromatography: a new global protein digestion strategy for monitoring differential protein expression. Electrophoresis 23:3182-3192. [DOI] [PubMed] [Google Scholar]
- 310.Whitelegge, J. P., J. le Coutre, J. C. Lee, C. K. Engel, G. G. Prive, K. F. Faull, and H. R. Kaback. 1999. Toward the bilayer proteome, electrospray ionization-mass spectrometry of large, intact transmembrane proteins. Proc. Natl. Acad. Sci. USA 96:10695-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wick, L. M., M. Quadroni, and T. Egli. 2001. Short- and long-term changes in proteome composition and kinetic properties in a culture of Escherichia coli during transition from glucose-excess to glucose-limited growth conditions in continuous culture and vice versa. Environ. Microbiol. 3:588-599. [DOI] [PubMed] [Google Scholar]
- 312.Wilkins, M. R., E. Gasteiger, L. Tonella, K. Ou, M. Tyler, J. C. Sanchez, A. A. Gooley, B. J. Walsh, A. Bairoch, R. D. Appel, K. L. Williams, and D. F. Hochstrasser. 1998. Protein identification with N and C-terminal sequence tags in proteome projects. J. Mol. Biol. 278:599-608. [DOI] [PubMed] [Google Scholar]
- 313.Wilkins, M. R., J. C. Sanchez, A. A. Gooley, R. D. Appel, I. Humphery-Smith, D. F. Hochstrasser, and K. L. Williams. 1996. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 13:19-50. [DOI] [PubMed] [Google Scholar]
- 314.Williams, M. D., T. X. Ouyang, and M. C. Flickinger. 1994. Starvation-induced expression of SspA and SspB: the effects of a null mutation in sspA on Escherichia coli protein synthesis and survival during growth and prolonged starvation. Mol. Microbiol. 11:1029-1043. [DOI] [PubMed] [Google Scholar]
- 315.Williams, R. M., and S. Rimsky. 1997. Molecular aspects of the E. coli nucleoid protein, H-NS: a central controller of gene regulatory networks. FEMS Microbiol. Lett. 156:175-185. [DOI] [PubMed] [Google Scholar]
- 316.Witzmann, F. A., and J. Li. 2002. Cutting-edge technology. II. Proteomics: core technologies and applications in physiology. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G735-G741. [DOI] [PubMed] [Google Scholar]
- 317.Wu, C. H., H. Huang, A. Nikolshaya, Z. Hu, and W. C. Barker 2004. The iProClass integrated database for protein functional analysis. J. Comput. Biol. Chem. 28:87-96. [DOI] [PubMed] [Google Scholar]
- 318.Xenarios, I., D. W. Rice, L. Salwinski, M. K. Baron, E. M. Marcotte, and D. Eisenberg. 2000. DIP: the database of interacting proteins. Nucleic Acids Res. 28:289-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Xia, B., H. Ke, U. Shinde, and M. Inouye. 2003. The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J. Mol. Biol. 332:575-584. [DOI] [PubMed] [Google Scholar]
- 320.Yamanaka, K., W. Zheng, E. Crooke, Y. H. Wang, and M. Inouye. 2001. CspD, a novel DNA replication inhibitor induced during the stationary phase in Escherichia coli. Mol. Microbiol. 39:1572-1584. [DOI] [PubMed] [Google Scholar]
- 321.Yan, J. X., A. T. Devenish, R. Wait, T. Stone, S. Lewis, and S. Fowler. 2002. Fluorescence two-dimensional difference gel electrophoresis and mass spectrometry based proteomic analysis of Escherichia coli. Proteomics 2:1682-1698. [DOI] [PubMed] [Google Scholar]
- 322.Yates, J. R. 2000. Mass spectrometry. From genomics to proteomics. Trends Genet. 16:5-8. [DOI] [PubMed] [Google Scholar]
- 323.Yohannes, E., D. M. Barnhart, and J. L. Slonczewski. 2004. pH-dependent catabolic protein expression during anaerobic growth of Escherichia coli K-12. J. Bacteriol. 186:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yona, G., N. Linial, and M. Linial. 1999. ProtoMap: automatic classification of protein sequences, a hierarchy of protein families, and local maps of the protein space. Proteins 37:360-378. [PubMed] [Google Scholar]
- 325.Yoon, S. H., M.-J. Han, S. Y. Lee, K. J. Jeong, and J.-S. Yoo. 2003. Combined transcriptome and proteome analysis of Escherichia coli during high cell density culture. Biotechnol. Bioeng. 81:753-767. [DOI] [PubMed] [Google Scholar]
- 326.Yu, C. S., C. J. Lin, and J. K. Hwang. 2004. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 13:1402-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Zeghouf, M., J. Li, G. Butland, A. Borkowska, V. Canadien, D. Richards, B. Beattie, A. Emili, and J. F. Greenblatt. 2004. Sequential peptide affinity (SPA) system for the identification of mammalian and bacterial protein complexes. J. Proteome Res. 3:463-468. [DOI] [PubMed] [Google Scholar]
- 328.Zhang, N., A. Doucette, and L. Li. 2001. Two-layer sample preparation method for MALDI mass spectrometric analysis of protein and peptide samples containing sodium dodecyl sulfate. Anal. Chem. 73:2968-2975. [DOI] [PubMed] [Google Scholar]
- 329.Zhang, N., R. Aebersold, and B. Schwikowski. 2002. ProbID: a probabilistic algorithm to identify peptides through sequence database searching using tandem mass spectral data. Proteomics 2:1406-1412. [DOI] [PubMed] [Google Scholar]
- 330.Zhou, H., and Y. Zhou. 2003. Predicting the topology of transmembrane helical proteins using mean burial propensity and a hidden-Markov-model-based method. Protein Sci. 12:1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhou, H., J. A. Ranish, J. D. Watts, and R. Aebersold. 2002. Quantitative proteome analysis by solid-phase isotope tagging and mass spectrometry. Nat. Biotechnol. 20:512-515. [DOI] [PubMed] [Google Scholar]
- 332.Zhou, Y., S. Gottesman, J. R. Hoskins, M. R. Maurizi, and S. Wickner. 2001. The RssB response regulator directly targets σS for degradation by ClpXP. Genes Dev. 15:627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zuo, X., and D. W. Speicher. 2000. A method for global analysis of complex proteomes using sample prefractionation by solution isoelectrofocusing prior to two-dimensional electrophoresis. Anal. Biochem. 284:266-278. [DOI] [PubMed] [Google Scholar]
- 334.Zuo, X., K. Lee, and D. W. Speicher. 2004. Electrophoretic prefractionation for comprehensive analysis of proteomes, p. 93-118. In D. W. Speicher (ed.), Proteome analysis: interpreting the genome. Elsevier Science B.V., Amsterdam, The Netherlands.
- 335.Zylicz, M., D. Ang, and C. Georgopoulos. 1987. The grpE protein of Escherichia coli. Purification and properties. J. Biol. Chem. 262:17437-17442. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.