Abstract
Transient influx of Ca2+ constitutes an early element of signaling cascades triggering pathogen defense responses in plant cells. Treatment with the Phytophthora sojae–derived oligopeptide elicitor, Pep-13, of parsley cells stably expressing apoaequorin revealed a rapid increase in cytoplasmic free calcium ([Ca2+]cyt), which peaked at ∼1 μM and subsequently declined to sustained values of 300 nM. Activation of this biphasic [Ca2+]cyt signature was achieved by elicitor concentrations sufficient to stimulate Ca2+ influx across the plasma membrane, oxidative burst, and phytoalexin production. Sustained concentrations of [Ca2+]cyt but not the rapidly induced [Ca2+]cyt transient peak are required for activation of defense-associated responses. Modulation by pharmacological effectors of Ca2+ influx across the plasma membrane or of Ca2+ release from internal stores suggests that the elicitor-induced sustained increase of [Ca2+]cyt predominantly results from the influx of extracellular Ca2+. Identical structural features of Pep-13 were found to be essential for receptor binding, increases in [Ca2+]cyt, and activation of defense-associated responses. Thus, a receptor-mediated increase in [Ca2+]cyt is causally involved in signaling the activation of pathogen defense in parsley.
INTRODUCTION
Cytoplasmic free Ca2+ ([Ca2+]cyt) serves as a second messenger in plant processes as diverse as root nodule formation, phytochrome phototransduction, stomatal closure, geotropism, circadian rhythm, pollen tube growth, and stress adaptation (Rudd and Franklin-Tong, 1999). Stimulus-specific and spatially and temporally defined Ca2+ signatures of characteristic magnitude, frequency, and duration are assumed to maintain signal specificity of transduction cascades (Thuleau et al., 1998; Trewavas, 1999). Subsequently, the binding of [Ca2+]cyt to calmodulin, Ca2+-dependent protein kinases, Ca2+-dependent protein phosphatases, Ca2+-gated ion channels, or Ca2+-activated phospholipases facilitates downstream signal transduction directed toward activation of a signal-specific cellular response (Blumwald et al., 1998).
Expression of the Aequorea aequorea apoaequorin gene in the cytoplasm of plant cells provides a means for accurate, noninvasive quantification of changes in [Ca2+]cyt (Knight et al., 1991). When reconstituted with coelenterazine, holoaequorin acts as a bioluminescent indicator of [Ca2+]cyt. Since the pioneering work of Knight et al. (1991), aequorin technology has been widely applied in plants to report changes in [Ca2+]cyt in response to abiotic stimuli, such as touch, wind, cold, heat, and drought (Knight et al., 1991, 1992, 1996, 1997; Haley et al., 1995; Gong et al., 1998; Plieth et al., 1999); blue light (Lewis et al., 1997); circadian rhythm (Johnson et al., 1995); ozone (Clayton et al., 1999); anoxia (Sedbrook et al., 1996); oxidative stress (Price et al., 1994); and hypoosmotic shock (Chandra and Low, 1997; Takahashi et al., 1997; Cessna et al., 1998).
Numerous recent studies have provided evidence that Ca2+ plays a pivotal role in activating the plant's surveillance system against attempted microbial invasion (Yang et al., 1997; Scheel, 1998). Activation of plant defense is believed to be receptor mediated through recognition of pathogen-derived elicitors (Yang et al., 1997; Scheel, 1998; Nürnberger, 1999). In contrast to elicitors from phytopathogenic bacteria, elicitors of fungal or oomycete origin appear to be recognized by high-affinity receptors residing in the plasma membrane of plant cells. Although several such plasma membrane binding sites have been characterized kinetically and structurally, our knowledge of the molecular mode of fungal pathogen perception in plants remains fragmentary: only one elicitor receptor has been isolated thus far, a soybean 70-kD plasma membrane protein that binds Phytophthora sojae–derived β-glucans (Umemoto et al., 1997).
Receptor–ligand interaction initiates an intracellular signal transduction cascade that mediates activation of the defense against the pathogen. Cellular components shown to be modulated by elicitor treatment include GTP binding proteins (Bischoff et al., 1999); plasma membrane ion channels (Thuleau et al., 1998); reactive oxygen intermediates (Lamb and Dixon, 1997); nitric oxide (Delledonne et al., 1998); lipid-derived metabolites (Chandra et al., 1997); and alterations in the phosphorylation status of various proteins catalyzed by serine/threonine protein kinases, protein phosphatases, and post-translationally activated mitogen-activated protein kinase (MAP kinase) cascades (Sopory and Munshi, 1998). Remarkably, many of these elements thought to be implicated in elicitor signaling have been identified in various plants, suggesting evolutionary conservation of signaling modules in plant pathogen defense (Yang et al., 1997; Scheel, 1998).
Plasma membrane ion channels are rapidly activated by pathogen infection or elicitor treatment of plant cells. In particular, extracellular alkalinization, Ca2+ influx, and effluxes of K+ and Cl− lead to depolarization of the plasma membrane (Scheel, 1998). Extracellular Ca2+ appears to be crucial to induction of plant defense against pathogens (Yang et al., 1997; Scheel, 1998). Elicitor-responsive Ca2+-permeable ion channels residing in the plasma membrane of plant cells may mediate elicitor-induced Ca2+ influx and subsequently lead to increased amounts of [Ca2+]cyt (Gelli et al., 1997; Zimmermann et al., 1997).
Rapidly induced transient increase of [Ca2+]cyt was previously monitored in apoaequorin-transformed tobacco cells that had been treated with crude elicitors derived from yeast or Gliocladium deliquescens (Knight et al., 1991). In contrast, harpin from Erwinia amylovora, which induces an oxidative burst, defense-related gene expression, and hypersensitive cell death in tobacco, did not affect [Ca2+]cyt in this plant (Chandra et al., 1997). Only recently were defined fungal chitin fragments or oomycete-derived glucan fragments shown to increase [Ca2+]cyt in soybean cells (Mithöfer et al., 1999). Crucial questions, however, as to whether changes in [Ca2+]cyt are receptor mediated and thus involved in signal activation of defense responses to pathogens remain to be resolved.
The nonhost resistance response of parsley (Petroselinum crispum) leaves to infection with zoospores of the phytopathogenic oomycete P. sojae was found to be closely mimicked in parsley cell cultures treated with cell wall–derived elicitors (Hahlbrock et al., 1995). An oligopeptide fragment (Pep-13) of a 42-kD P. sojae cell wall glycoprotein stimulates transcriptional activation of defense-related genes and phytoalexin production (Nürnberger et al., 1994). The binding of Pep-13 to a 100-kD parsley plasma membrane receptor (Nürnberger et al., 1995; Nennstiel et al., 1998) rapidly stimulates Ca2+ influx, effluxes of K+ and Cl−, extracellular alkalinization, production of reactive oxygen species (ROS), and post-translational activation of a MAP kinase (Nürnberger et al., 1994; Jabs et al., 1997; Ligterink et al., 1997). Extracellular Ca2+ was found to be indispensable for activation of all these plant responses (Nürnberger et al., 1994; Ligterink et al., 1997). Use of peptides structurally related to Pep-13 revealed a functional link between elicitor perception and activation of Ca2+ influx as a requirement for subsequent production of superoxide anions. ROS themselves are both necessary and sufficient to trigger pathogen defense responses in parsley (Nürnberger et al., 1994; Jabs et al., 1997). Patch-clamp analyses demonstrated the receptor-mediated activation of a Pep-13–responsive Ca2+-permeable plasma membrane ion channel (Zimmermann et al., 1997). We now show that parsley cells stably expressing the apoaequorin gene respond to elicitor treatment with a characteristic biphasic [Ca2+]cyt signature consisting of a rapidly induced [Ca2+]cyt transient peak followed by sustained concentrations of [Ca2+]cyt. Intriguingly, although both phases of the Pep-13–induced [Ca2+]cyt response in parsley cells are due to Pep-13 receptor activation, only sustained increases in [Ca2+]cyt constitute an element of the signaling cascade triggering pathogen defense in parsley.
RESULTS
Establishment of Parsley Cell Lines Stably Expressing Cytoplasmic Apoaequorin
Particle bombardment technology was used to introduce a DNA cassette encoding apoaequorin into suspension-cultured parsley cells (Finer et al., 1992; Frohnmeyer et al., 1999). When holoaequorin was reconstituted with coelenterazine, 27 of 37 hygromycin-resistent calli exhibited Ca2+-dependent bioluminescence. Cell suspensions were established from those calli showing the greatest amounts of reconstituted bioluminescent aequorin (Table 1).
Table 1.
Characterization of Transgenic Parsley Cell Lines Expressing Apoaequorin
| Line | Number of Transgene Insertionsa |
Bioluminescence of Reconstituted Aequorinb(RLU/sec/mg FW) | Oxidative Burstc(Percentage of Untransformed Cells) | Phytoalexin Productiond(Percentage of Untransformed Cells) |
|---|---|---|---|---|
| 1/10 | 1 | 1.87 × 105 | 146 × 2 | 68 × 12 |
| 1/14 | 4–6 | 1.98 × 105 | 127 × 12 | 54 × 29 |
| 1/17 | 1 | 2.06 × 105 | NDe | 40 × 17 |
| 1/18 | 1 | 1.41 × 105 | 125 × 10 | 61 × 28 |
| 1/19 | 3 | 1.27 × 105 | 72 × 27 | 57 × 32 |
| 2/2 | 2 | 2.66 × 105 | 95 × 32 | 112 × 9 |
| 2/5 | NDe | 0.33 × 105 | 219 × 20 | 69 × 17 |
| 2/12 | 2 | 6.25 × 105 | 114 × 7 | 78 × 26 |
Integration of the aequorin construct was investigated by genomic DNA gel blot analysis.
Determined by complete discharge of aequorin with excess Ca2+.
Oxidative burst measurements were performed in triplicate.
Phytoalexin production was quantified whenever cell lines were used for bioluminescence experiments.
Not determined.
FW, fresh weight; RLU, relative light units.
DNA gel blot analysis confirmed integration of one to six copies of the apoaequorin-encoding DNA into the parsley genome. Maintenance of the Ca2+-dependent bioluminescence in parsley cell lines cultivated for >12 months in the absence of selecting antibiotic proved that the integration of the transgene was stable. Phenotypic appearance and growth behavior of transgenic parsley cell lines were indistinguishable from those of nontransformed cell lines. Most importantly, when treated with the P. sojae–derived oligopeptide elicitor Pep-13, apoaequorin-transgenic parsley cell lines produced ROS and furanocoumarin phytoalexins in quantities comparable to those produced by nontransformed cell lines (Table 1). Nevertheless, to avoid misinterpretation of data in consideration of adverse effects of transgene integration on cellular responsiveness, all bioluminometric experiments were routinely performed in triplicate with at least two transgenic cell lines.
Because of the double logarithmic relationship between aequorin bioluminescence and [Ca2+] (Blinks et al., 1978), relative changes in light emission do not precisely reflect the extent of any change in actual [Ca2+]. Moreover, because aequorin is consumed during the experiment and because the amount of reconstituted aequorin varies between individual experiments, meaningful analysis of recorded relative light units (RLUs) required conversion into [Ca2+] by using a calibration curve established in vitro. All relative changes in bioluminescence monitored were therefore transformed into absolute values for [Ca2+]cyt.
Increased [Ca2+]cyt in Elicitor-Treated Parsley Cells
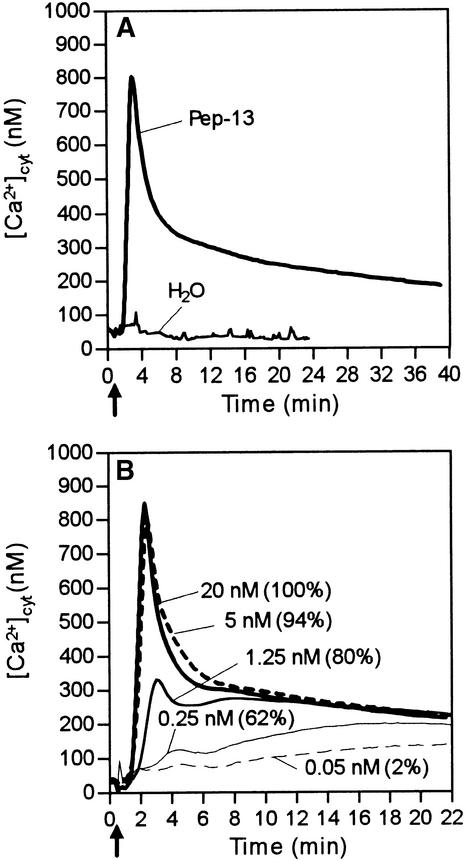
In transgenic parsley lines reconstituted aequorin reported a basal [Ca2+]cyt level of 40 to 110 nM. Treating the parsley cells with the oligopeptide elicitor Pep-13 produced characteristic changes in [Ca2+]cyt (Figure 1), which were similar in all transgenic cell lines listed in Table 1. After a delay of 30 to 40 sec, [Ca2+]cyt increased rapidly, peaked at ∼1 μM after 2 min, and subsequently decreased to a slowly declining plateau of ∼300 nM during the next 10 to 40 min (Figure 1A). This biphasic response differed greatly from the immediate, transient, small peak detectable after addition of water or organic solvents. In contrast to the peak maximum (phase 1), which varied between 600 and 1100 nM among individual experiments performed with independent transgenic lines, the magnitude of the plateau value (phase 2) and the kinetics of the total response were consistently found to be identical across all lines.
Figure 1.
Elicitor-Induced Changes in [Ca2+]cyt in Apoaequorin-Transformed Parsley Cells.
Bioluminescence of reconstituted aequorin was monitored in parsley cells treated with water or Pep-13 at the time (arrow) indicated.
(A) Addition of water or 100 nM Pep-13, respectively.
(B) Addition of Pep-13 at the concentrations indicated. Numbers in parentheses represent amounts of phytoalexins produced by parsley cells treated with the given Pep-13 concentrations relative to those produced by cells treated with 100 nM Pep-13.
The extent of the [Ca2+]cyt response depended on the concentration of elicitor used, becoming saturated at Pep-13 concentrations >5 nM (Figure 1B). Decreasing the elicitor concentration preferentially reduced the transient [Ca2+]cyt peak, whereas sustained increases of [Ca2+]cyt could be elicited at Pep-13 concentrations as low as 0.25 nM. Lowering the Pep-13 concentration further also diminished the activation of the second phase of the [Ca2+]cyt response. Importantly, we found a close quantitative correlation between the elicitor concentrations required to efficiently stimulate increased [Ca2+]cyt and phytoalexin formation (Figure 1B). Maximum phytoalexin production was observed only at Pep-13 concentrations that elicited both phases of the [Ca2+]cyt response. However, large amounts of phytoalexins (80% of maximum) were also produced in response to Pep-13 concentrations that stimulated only phase 2 of the [Ca2+]cyt response. The EC50 value for Pep-13 to induce the sustained [Ca2+]cyt plateau (0.2 nM) closely corresponded to the elicitor concentrations required to half-maximally elicit Ca2+ influx, K+ and Cl− efflux, medium alkalinization, oxidative burst, and phytoalexin formation (Table 2; Nürnberger et al., 1994).
Table 2.
Correlation between Elicitor-Specific Responses in Parsley Challenged with Pep-13 and Structural Analogs
| Activity Index (Pep-X/Pep-13)a
|
|||||
|---|---|---|---|---|---|
| Elicitor Activity
|
|||||
| Peptide Sequence | Competitor Activity | Ca2+ Influx | Δ pCacyt (Aequorin) | H2O2 Formation | Phytoalexin Formation |
| Pep-13 | 1 | 1 | 1 | 1 | 1 |
| VWNQPVRGFKVYE | (0.2 nM)b | (0.19 nM) | (0.4 nM) | ||
| Pep-13/A12 | 1.3 | 1.7 | 1.6 | 1.3 | 1.6 |
| VWNQPVRGFKVAEc | |||||
| Pep-13/A2 | 1,500 | 390 | 280 | 70 | 230 |
| VANQPVRGFKVYE | |||||
| Pep-10 | 7,000 | — | 15,500 | 3,400 | 1,100 |
| NQPVRGFKVY | |||||
The activity index (Pep-X/Pep-13) represents the quotient of the half-maximal concentration of the respective Pep-13 structural derivative (Pep-X) required to stimulate the particular plant response and the half-maximal effector concentration of Pep-13. The difference in pCacyt between the unstimulated basal level and the sustained level obtained 10 min after addition of peptides was taken to compare the calcium response elicited by Pep-13 and structural analogs in aequorin-transformed parsley cells. To enable better comparison, we include previously determined indices for other elicitor-specific responses (Nürnberger et al., 1994).
Numbers in parentheses represent the absolute EC50 values derived from dose–response curves using aequorin-transformed as well as untransformed parsley cells.
Bold letters represent alanine substitution sites within Pep-13.
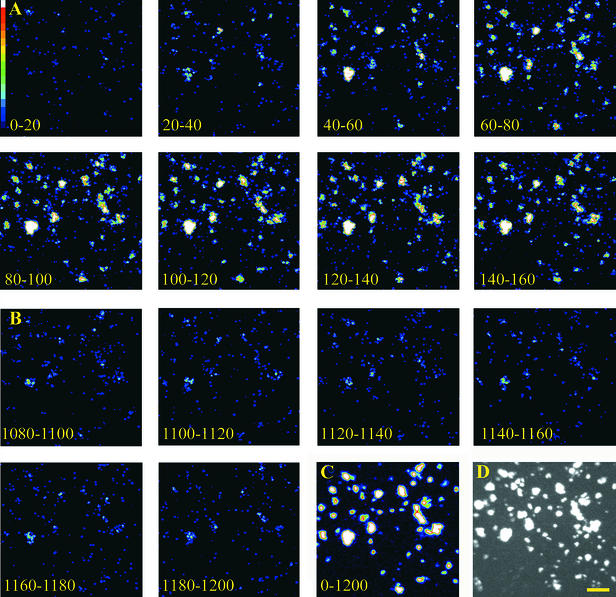
In contrast to luminometric analyses of large cell populations (5 × 103 cells per experiment), in vivo imaging of aequorin activity using photon-counting video equipment permitted analysis of small cell clusters (10 to 20 cells). These experiments were performed to investigate whether the elicitor-induced [Ca2+]cyt signature might be the result of cumulative light emission from cell populations responding asynchronously to elicitor with differing lag phases, and whether the [Ca2+]cyt plateau might be brought about by alternately oscillating [Ca2+]cyt spikes. As shown in Figure 2, the parsley cell clusters responded rather synchronously to elicitor treatment. Maximum light emission was observed between 60 and 160 sec after addition of elicitor (Figure 2A), corresponding precisely to the time of the large [Ca2+]cyt spike seen in luminometric assays (Figure 1A). Subsequently, light emission declined (Figure 2B) but remained constantly higher than that observed in untreated cells (not shown) or in cells immediately after the administration of elicitor (first image, Figure 2A). This is reminiscent of the plateau phase monitored in luminometric measurements (Figure 1A). In all experiments performed, the majority of cell clusters responded to elicitor treatment (Figures 2C and 2D). Because the light emission of elicitor-treated parsley cells was synchronous and continuous rather than asynchronous or oscillating, the [Ca2+]cyt signature observed in luminometric assays (Figure 1) is unlikely to result from cell populations responding substantially differently from or later than the majority of cells.
Figure 2.
In Vivo Imaging of Pep-13–Induced Changes in [Ca2+]cyt in Parsley Cells.
Bioluminescence of reconstituted aequorin in elicitor-treated parsley cells was monitored with a photon-counting video system. Individual images show light emission of parsley cells integrated for the times indicated. Pep-13 (100 nM) was added 30 sec before the start of measurements (i.e., before time zero).
(A) Image series (0 to 160 sec) corresponding to the peak phase observed in luminometric analyses (Figure 1).
(B) Image series (1080 to 1200 sec) corresponding to the plateau phase observed in luminometric analyses (Figure 1).
(C) Integration of light emission over the time course of the experiment (0 to 1200 sec).
(D) Bright-field image of parsley cells used in this experiment. 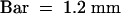 .
.
Elicitor Specificity of the [Ca2+]cyt Response
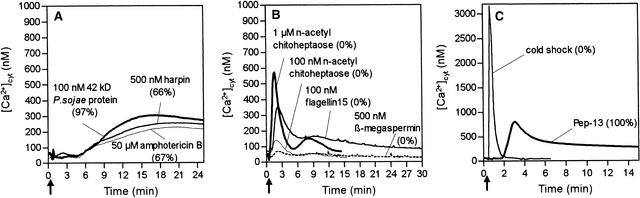
The 42-kD P. sojae cell wall glycoprotein harboring Pep-13 was tested for its ability to elicit the [Ca2+]cyt response in parsley cells. Somewhat unexpectedly, this glycoprotein elicitor did not trigger the large [Ca2+]cyt transient peak in the way Pep-13 did. Instead, a lag phase of ∼4 min preceded the increase in [Ca2+]cyt, which reached a sustained value (phase 2) of 300 nM, the same as that of the Pep-13–treated cells, 12 to 15 min after the glycoprotein was added (Figure 3A). Because this elicitor stimulated phytoalexin production in parsley cells as efficiently as Pep-13 did, the characteristic transient [Ca2+]cyt spike triggered by Pep-13 appears to be dispensable for activation of pathogen defense–associated responses in parsley.
Figure 3.
Effect of Abiotic and Biotic Stimuli on [Ca2+]cyt Concentrations in Parsley Cells.
Bioluminescence of reconstituted aequorin was monitored in parsley cells treated with various stimuli at the times indicated (arrows). Numbers in parentheses represent amounts of phytoalexins produced by parsley cells treated as indicated relative to those produced by cells treated with 100 nM Pep-13.
(A) Addition of elicitors stimulating phytoalexin production in parsley cells. The P. sojae–derived 42-kD cell wall glycoprotein, recombinant P. syringae pv phaseolicola–derived harpin, and the polyene antibiotic amphotericin B were added at concentrations sufficient to stimulate elicitor-specific maximum phytoalexin production in parsley cells.
(B) Addition of elicitors of plant defense-associated responses incapable of stimulating phytoalexin production in parsley cells. Synthetic N-acetylchitoheptaose, a synthetic 15-mer fragment of bacterial flagellin, and purified β-megaspermin from P. megasperma were added at concentrations at least 10-fold greater than those reported to induce defense-associated responses in various plants.
(C) Cold shock was applied by adding an equal volume of ice-cold culture medium to parsley cells.
The impact on parsley [Ca2+]cyt of a series of plant defense elicitors was investigated. Interestingly, elicitors that induced phytoalexin production in parsley cells stimulated only phase 2 of the [Ca2+]cyt response (Figure 3A). At concentrations sufficient to activate phytoalexin production in parsley cells, harpin, the product of the HRPZ gene from Pseudomonas syringae pv phaseolicola, yielded increased [Ca2+]cyt with kinetics and magnitude (∼250 nM) comparable to those of the P. sojae glycoprotein elicitor. Furthermore, the polyene antibiotic amphotericin B, previously shown to trigger sustained ion fluxes across the plasma membrane as well as phytoalexin production in parsley cells in the absence of elicitor (Jabs et al., 1997), increased the [Ca2+]cyt with kinetics and magnitude (∼220 nM) very similar to those shown by harpin and the P. sojae glycoprotein elicitor.
In contrast, elicitors incapable of inducing phytoalexin production in parsley cells were either ineffective with respect to increasing [Ca2+]cyt (Phytophthora megasperma β-elicitin, β-megaspermin) or induced only a rapid, transient increase in [Ca2+]cyt even at concentrations 10-fold greater than those reported from other plant systems to be sufficient to trigger pathogen defense reactions (Figure 3B). The [Ca2+]cyt signature induced by fungal N-acetylchitoheptaose was reminiscent of phase 1 of the Pep-13–induced [Ca2+]cyt response. Using less-polymerized chitooligosaccharides resulted in concomitant reduction (chitopentaose, not shown) or complete loss (chitotriose, not shown) of their [Ca2+]cyt-increasing activity. A 15-mer peptide derived from a conserved domain of bacterial flagellin, which was shown to elicit plant defense-associated responses in various dicot plant cells (Felix et al., 1999), increased [Ca2+]cyt in parsley cells transiently up to 350 nM.
Among other abiotic stimuli, cold shock reportedly triggers an increase in [Ca2+]cyt in apoaequorin-transgenic tobacco plants and cultured cells (Knight et al., 1991, 1996; Chandra and Low, 1997). As shown in Figure 3C, addition of ice-cold medium to transgenic parsley cells precipitously induced a [Ca2+]cyt spike, which reached a maximum after 20 sec at concentrations >3 μM, subsequently declining to basal values within 150 sec. However, this cold shock could neither increase the [Ca2+]cyt to sustained values nor stimulate phytoalexin production in parsley cells.
Requirement of Extracellular Ca2+ for Elicitor-Induced Increase of [Ca2+]cyt
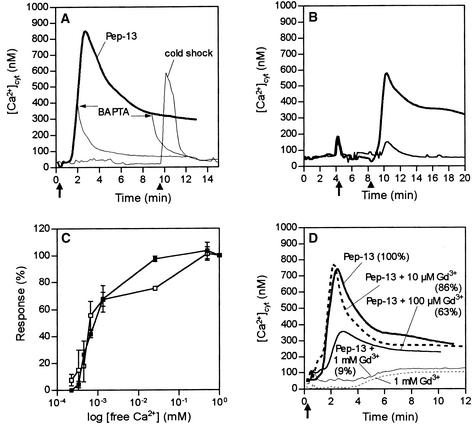
Previous experiments proved that extracellullar Ca2+ is essential for activation of the multifaceted defense response in elicitor-treated parsley cells (Nürnberger et al., 1994; Jabs et al., 1997; Ligterink et al., 1997). In addition, Pep-13 was shown to initiate influx of 45Ca2+ into parsley cells and to activate a plasma membrane Ca2+-permeable ion channel (Nürnberger et al., 1994; Zimmermann et al., 1997). Thus, an elicitor-induced increase in [Ca2+]cyt may be a direct consequence of Ca2+ influx through plasma membrane ion channels. Alternatively, generation of inositol trisphosphate (IP3) or cyclic ADP-ribose and activation of their corresponding receptors could mobilize Ca2+ from internal stores, such as the vacuole. As shown in Figure 4A, chelation of extracellular Ca2+ by the chelating agent 1,2-bis(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) abolished both phases of the Pep-13–induced increase in [Ca2+]cyt. Similarly, EGTA inhibited the [Ca2+]cyt response in elicitor-treated parsley cells (not shown). Addition of BAPTA to parsley cells after onset of elicitor treatment immediately abrogated both the transient [Ca2+]cyt peak and the plateau phase of the [Ca2+]cyt response. In contrast, cold shock, a stimulus known to trigger Ca2+ release from vacuolar stores in addition to influx of external Ca2+ (Knight et al., 1996), evoked a [Ca2+]cyt transient peak in parsley cells in the presence of BAPTA (Figure 4A). Addition of excess Ca2+ to parsley cells preincubated with BAPTA fully restored the [Ca2+]cyt response (Figure 4B) and the ability of elicitor-treated parsley cells to produce ROS and phytoalexins (not shown). Thus, irreversible damage of parsley plasma membranes caused by BAPTA appears unlikely. Moreover, the Pep-13–induced [Ca2+]cyt response of parsley cells treated simultaneously with BAPTA and excess CaCl2 was indistinguishable from that of parsley cells treated with Pep-13 alone. Modulation of the extracellular free Ca2+ concentration by BAPTA resulted in a concomitant decrease in the ability of elicited parsley cells to mount a [Ca2+]cyt response and an oxidative burst (Figure 4C). This suggests that induction of both cellular responses is similarly dependent on external Ca2+. Strikingly, as long as the extracellular free [Ca2+] exceeded the [Ca2+]cyt, parsley cells remained responsive to Pep-13.
Figure 4.
Inhibition of Pep-13–Induced Cellular Responses in Parsley Cells by Modulation of Ca2+ Influx across the Plasma Membrane.
(A) Parsley cells incubated in culture medium (containing 1 mM CaCl2) were challenged with 100 nM Pep-13 at the time point indicated (thick arrow), and changes in [Ca2+]cyt were recorded. The heavy line represents recordings obtained from parsley cells treated with Pep-13 only. The thin line represents recordings obtained from parsley cells treated first with the Ca2+ chelator BAPTA (4 mM) 5 min before the addition of Pep-13 and then with an equal volume of ice-cold culture medium (cold shock) as indicated (arrowhead). The thin lines descending from the Pep-13 line represent recordings obtained from parsley cells treated with 4 mM BAPTA at the times designated by thin arrows.
(B) Recording of [Ca2+]cyt concentrations in parsley cells treated with BAPTA (4 mM) 8 min before the addition of 10 mM CaCl2 (arrow). The cells were subsequently treated with 100 nM Pep-13 (heavy line) or water (thin line) at the time indicated (arrowhead).
(C) Dependence of the Pep-13–induced increase in [Ca2+]cyt (filled squares) and oxidative burst (open squares) on extracellular [Ca2+]. To progressively decrease the amount of extracellular free [Ca2+], parsley cells were incubated with increasing amounts of BAPTA (0 to 4 mM). Extracellular free [Ca2+] was calculated by using MaxChelator software (version 2.5 for Windows; http://www.stanford.edu/~cpatton/) (Bers et al., 1994). Data points represent means of four independent experiments. Error bars indicate sd.
(D) Changes in [Ca2+]cyt were recorded from parsley cells treated with the Ca2+ channel inhibitor GdCl3 at the concentrations indicated 5 min before the addition of water (dotted line) or 100 nM Pep-13 (as indicated) at the time noted (arrow). The solid line represents recordings obtained from parsley cells treated with Pep-13 only. Numbers in parentheses indicate the amounts of phytoalexins produced by parsley cells treated as indicated relative to those produced by cells treated with 100 nM Pep-13.
Lanthanides (Gd3+, La3+) are frequently used to inhibit Ca2+ importation across the plant plasma membrane. When added to parsley cells either before or together with Pep-13, 1 mM Gd3+ abolished the Pep-13–induced [Ca2+]cyt signature (Figure 4D) and reduced phytoalexin production in elicitor-treated parsley cells by 91%. Use of 1 mM La3+ gave similar results (not shown). However, lanthanides are reported both to enter cells at millimolar external concentrations (Shimizu et al., 1997) and to affect plasma membrane Ca2+-ATPase (Quiquampoix et al., 1990) and K+ channels (Lewis and Spalding, 1998). Thus, our findings lend further support to the prime role of extracellular Ca2+ for activating plant defense in parsley but do not provide evidence for specific plasma membrane Ca2+ channels to mediate an elicitor-induced Ca2+ influx.
In a series of previous experiments, verapamil, nifedipine, and flunarizine, nonpermeable inhibitors of Ca2+ channels, failed to inhibit elicitor-induced alkalinization of the extracellular medium, Ca2+ influx, K+ and Cl− effluxes, oxidative burst, and phytoalexin production in parsley cells (Jabs et al., 1997). These compounds were now found to be also incapable of blocking the elicitor-induced increase in [Ca2+]cyt after 15 min of preincubation with inhibitor (not shown).
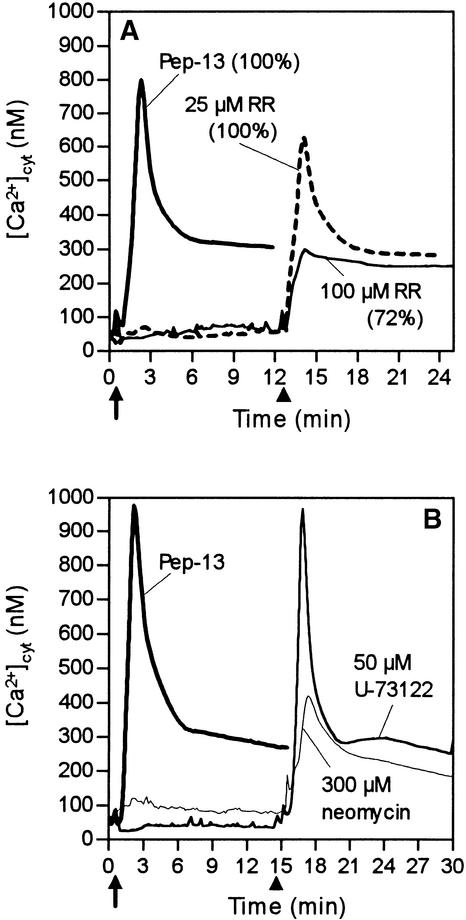
Ruthenium Red (RR), a membrane-permeable Ca2+ channel blocker that predominantly inhibits Ca2+ release from intracellular compartments, was used to elucidate the contribution of internal Ca2+ stores to the elicitor-induced [Ca2+]cyt signature response. At 100 μM, RR markedly inhibited phase 1 but left phase 2 virtually unaffected (Figure 5A). Importantly, in the presence of RR, elicitor-treated parsley cells maintained the ability to produce phytoalexins, thus providing further evidence that phase 2 but not phase 1 of the [Ca2+]cyt response is essential for activating the defense-associated reactions. This also suggests that Pep-13–induced sustained [Ca2+]cyt increases may not be due to release from internal RR-sensitive stores.
Figure 5.
Inhibitors of Ca2+ Release or IP3 Supply Affect the Pep-13–Induced Transient Increase but Not the Sustained Increase in [Ca2+]cyt.
Changes in bioluminescence were recorded from parsley cells treated with different inhibitors at times indicated (arrows) before the addition of 100 nM Pep-13. Arrowheads mark the times at which 100 nM Pep-13 was added to parsley cells. Numbers in parentheses indicated amounts of phytoalexins produced by parsley cells treated as indicated relative to those produced by cells treated with 100 nM Pep-13.
(A) Addition of the Ca2+ channel inhibitor RR (25 or 100 μM).
(B) Addition of phospholipase C inhibitors neomycin (300 μM) or U-73122 (50 μM).
Like RR, neomycin, an inhibitor of IP3-releasing phospholipase C (Gabev et al., 1989), partially blocked phase 1 but not phase 2 of the Pep-13–induced [Ca2+]cyt response (Figure 5B). Ca2+ release through IP3-gated Ca2+ channels is therefore also unlikely to contribute to the elicitor-induced sustained increases of [Ca2+]cyt associated with activation of pathogen defense. As shown in Figure 5B, another phospholipase C antagonist, U-73122 (Smallridge et al., 1992), did not inhibit the elicitor-induced [Ca2+]cyt response. Both neomycin and RR strongly inhibited a mastoparan-induced [Ca2+]cyt response (not shown), which is assumed to reflect release from internal stores. We therefore conclude that this experiment probably probed the actual intracellular pathways for Ca2+ release. Similarly, neomycin blocked a cold shock–induced [Ca2+]cyt response, which is believed to result partially from the release of intracellular Ca2+.
In summary, our findings strongly suggest that sustained increases of [Ca2+]cyt in elicitor-treated parsley cells are predominantly the result of continuous Ca2+ influx through plasma membrane Ca2+ channels.
[Ca2+]cyt Constitutes an Early Element of the Signaling Cascade Triggering Pathogen Defense in Parsley
A variety of pharmacological effectors of signal transduction pathways in eukaryotic cells were used to dissect the Pep-13–activated sequence of cellular responses in parsley. Major emphasis was put on elucidating the role of the elicitor-induced increase in [Ca2+]cyt during activation of defense-associated responses, such as production of ROS and phytoalexins.
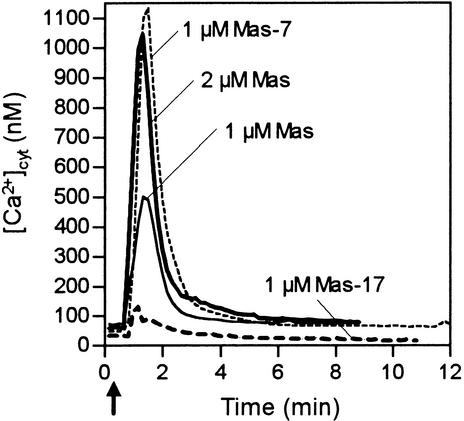
Heterotrimeric GTP binding proteins (G-proteins) are assumed to link cell surface receptors with intracellular effectors (Bischoff et al., 1999). Administration of the G-protein activator mastoparan to parsley cells yielded concentration-dependent large, rapid, and transient [Ca2+]cyt responses (Figure 6). Mas-7, a mastoparan analog with fivefold more biological activity than mastoparan (Higashijima et al., 1990), evoked a substantially larger [Ca2+]cyt transient peak than did mastoparan, whereas the inactive mastoparan analog Mas-17 stimulated only a small increase in [Ca2+]cyt. Our findings suggest that G-protein–activated signaling pathways may function in parsley. However, the [Ca2+]cyt signature response stimulated by mastoparan greatly differed from that induced by the elicitors Pep-13, P. sojae glycoprotein, and harpin (Figures 1A and 3A). Consistently, moreover, unlike these elicitors, mastoparan was incapable of activating ROS and phytoalexin production and did not interfere with Pep-13–induced pathogen defense responses. The G-protein activator, cholera toxin, failed to induce any Pep-13–specific response, including the [Ca2+]cyt response (not shown). Moreover, cAMP, an element of a G-protein–activated signaling cascade, or cGMP, a product of receptor guanylyl cyclase activity, which is known to activate cyclic nucleotide–gated ion channels (both compounds tested as membrane-permeable dibutyryl derivatives), did not activate a [Ca2+]cyt response. In summary, although involvement of G-proteins in pathogen defense signaling has been reported for tomato cells treated with a Cladosporium fulvum–derived elicitor (Blumwald et al., 1998, and references therein), our experiments do not support a role of G-protein–activated signaling pathways in transmitting the elicitor signal in parsley.
Figure 6.
Mastoparan Evokes Changes in [Ca2+]cyt in Parsley Cells.
Bioluminescence recordings were obtained from parsley cells treated with the G-protein activator mastoparan (Mas) or with mastoparan structural derivatives (Mas-7, Mas-17) at the time (arrow) and concentrations designated.
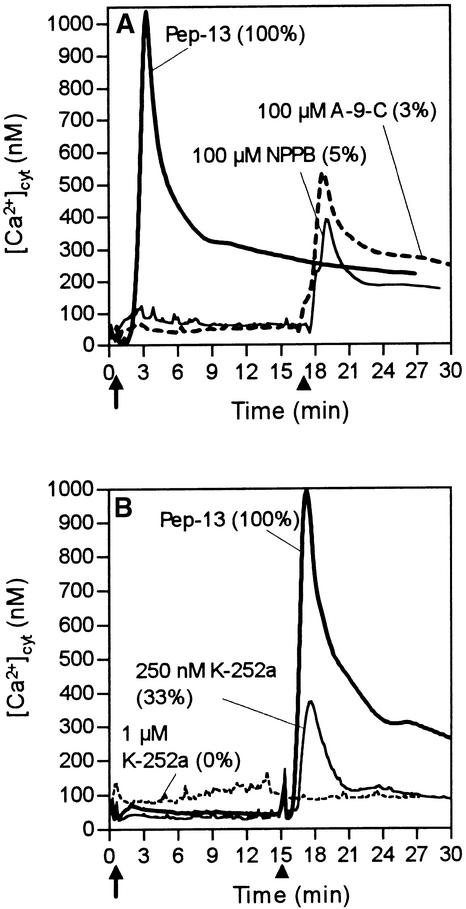
At concentrations previously reported to block chloride efflux, oxidative burst, MAP kinase activation, and phytoalexin production in parsley cells (Jabs et al., 1997; Ligterink et al., 1997) (Figure 7A), use of the chloride channel inhibitors anthracene-9-carboxylate (A-9-C) and 5-nitro-2-(3-phenylpropylamino)benzoate (NPPB) inhibited phase 1 but not phase 2 of the Pep-13–induced [Ca2+]cyt response. The elicitor-induced Ca2+ influx giving rise to sustained increases in [Ca2+]cyt may therefore act upstream of elicitor-induced Cl− channels, which themselves have been shown to be essential for activation of pathogen defense by Pep-13 (Jabs et al., 1997).
Figure 7.
Effect of Chloride Channel and Protein Kinase Antagonists on Elicitor-Induced Changes in [Ca2+]cyt.
(A) and (B) Changes in bioluminescence were recorded from parsley cells treated with 100 nM Pep-13 (solid line) and either the chloride channel inhibitors A-9-C or NPPB (A) or protein kinase inhibitor K-252a (B) at the time (arrows) and concentrations designated. All compounds were applied from stock solutions made up in DMSO (final solvent concentration 0.1%). Arrowheads mark the time of addition of 100 nM Pep-13 to parsley cells pretreated with either inhibitor. Numbers in parentheses indicate the amount of phytoalexins (A) or hydrogen peroxide (B) produced by parsley cells treated as indicated relative to that produced by cells treated with 100 nM Pep-13.
The serine/threonine protein kinase inhibitors K-252a (Figure 7B) and staurosporine (not shown) completely blocked both phases of the Pep-13–induced [Ca2+]cyt response and ROS production in parsley cells. In contrast, the tyrosine kinase inhibitors genistein and lavendustin did not affect either response (not shown), suggesting that the Pep-13 receptor is unlikely to belong to the tyrosine kinase family of plasma membrane receptors. Inhibitors of protein phosphatase 1 (calyculin A, tautomycin) and 2A (okadaic acid, cantharidin) blocked neither the [Ca2+]cyt response nor ROS production in elicited parsley cells (not shown) at concentrations (1 μM) reported to abolish protein phosphatase activity in plant cells (Sopory and Munshi, 1998).
Receptor-Mediated Activation of the [Ca2+]cyt Response in Elicitor-Treated Parsley Cells
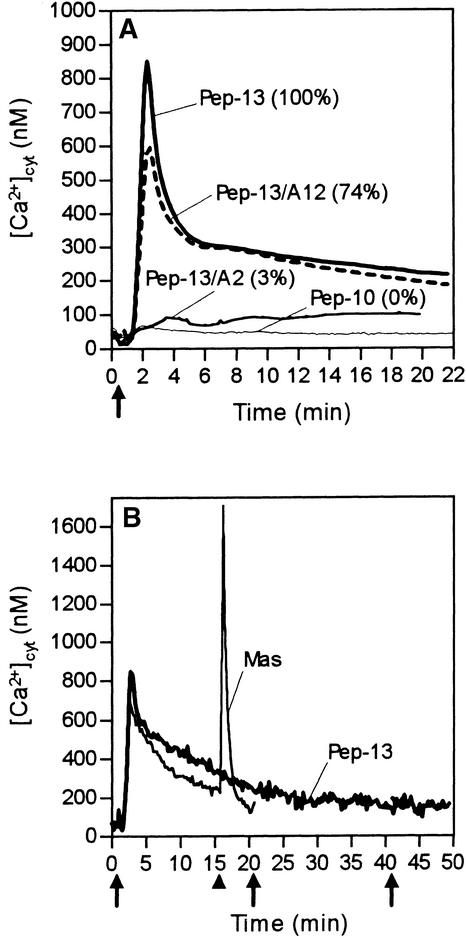
Activation of elicitor-induced reactions in parsley cells is mediated through binding of Pep-13 to a 100-kD plasma membrane receptor protein (Nürnberger et al., 1994, 1995; Ligterink et al., 1997; Zimmermann et al. 1997; Nennstiel et al., 1998). To elucidate a possible functional link between Pep-13 perception, activation of Ca2+ influx, oxidative burst, phytoalexin production, and the Pep-13–induced increase in [Ca2+]cyt, we investigated a series of structural derivatives of Pep-13. As summarized in Figure 8A and Table 2, the [Ca2+]cyt response of elicited parsley cells was activated by those Pep-13 derivatives that efficiently competed for binding of [125I]Pep-13 to its receptor; they also strongly induced all other responses examined. Replacing Y12 (tyrosine at position 12) with alanine (Pep-13/A12) did not affect the ability of this peptide to bind to the Pep-13 receptor and to stimulate all responses in parsley cells. In contrast, replacing W2 with alanine (Pep-13/A2) rendered this derivative largely inactive with respect to increasing [Ca2+]cyt, corresponding to observed losses of competitor activity and the inability to trigger Ca2+ uptake and formation of ROS and phytoalexins. Similarly, deletion of one C-terminal and two N-terminal amino acid residues from Pep-13 (Pep-10) abolished the ability of this derivative to bind to the Pep-13 receptor and to induce increases in [Ca2+]cyt and phytoalexin production. Taken together, our data provide evidence that the Pep-13–stimulated increase in [Ca2+]cyt is a receptor-mediated process.
Figure 8.
Elicitor-Induced Changes of [Ca2+]cyt Are Receptor Mediated and Are Refractory to Consecutive Stimulation by Elicitor.
(A) Changes in bioluminescence recorded from parsley cells treated at the time indicated (arrow) with either 20 nM Pep-13 or 20 nM of the given Pep-13 structural derivatives. Numbers in parentheses indicate amounts of phytoalexins produced by parsley cells treated with each Pep-13 analog relative to those produced by cells treated with 100 nM Pep-13.
(B) Changes in bioluminescence recorded from parsley cells treated repeatedly with 100 nM Pep-13 at the times indicated (arrows). The arrowhead marks the time of addition of 1 μM mastoparan (Mas) to parsley cells pretreated with Pep-13. Measurements were performed in a microplate luminometer with continuous shaking of parsley cells.
Treating apoaequorin-expressing cultured tobacco W38 cells with Pep-13 (0.1 to 1 μM) did not result in increased [Ca2+]cyt (not shown). This is in agreement with the absence of a functional Pep-13 binding site in tobacco membranes (not shown) and corroborates previous observations that Pep-13 recognition and subsequent activation of defense responses is specific to parsley (Nürnberger et al., 1995). We further conclude from these experiments that integration of Pep-13 into plant cell membranes in an ionophore-like manner is unlikely.
Desensitization of plant cells to consecutive treatments with the same stimulus is commonly observed (Boller, 1995). To analyze a refractory state in elicitor-treated parsley cells, Pep-13 was administered at the start of bioluminescence recordings and after the decline of the [Ca2+]cyt transient form. As shown in Figure 8B, no additional [Ca2+]cyt response was evoked in cells that had been pretreated with Pep-13. Thus, addition of elicitor rendered the system refractory to repeated stimulation by the same signal for at least 40 min. Consistently, addition of the Pep-13–harboring P. sojae 42-kD glycoprotein elicitor to parsley cells pretreated with Pep-13 did not induce an additional increase in [Ca2+]cyt. Desensitization was stimulus specific, however, because subsequent addition of mastoparan to Pep-13–treated parsley cells activated a second [Ca2+]cyt spike (Figure 8B). Loss of elicitor responsiveness is therefore assumed to indicate desensitization of the Pep-13 perception system.
DISCUSSION
Stimulus-dependent changes in [Ca2+]cyt control a diverse range of cellular functions, including gene regulation and enzyme activation (Clapham, 1995; Parekh and Penner, 1997). Pulses, repetitive oscillations, and sustained plateaus constitute elements of [Ca2+]cyt signatures (Dolmetsch et al., 1997, 1998; Li et al., 1998), all of which have been detected in plant cells (Thuleau et al., 1998; Rudd and Franklin-Tong, 1999; Trewavas, 1999). In parsley cell lines stably expressing the apoaequorin gene, we have monitored a biphasic [Ca2+]cyt signature in response to elicitor treatment. We provide evidence that receptor-mediated increases in [Ca2+]cyt to sustained higher values are required for activating pathogen defense responses in parsley. Hence, [Ca2+]cyt is assumed to act as an early second messenger in transcriptional activation of an array of defense-related genes and subsequent phytoalexin production.
The Elicitor-Induced Increase in Cytoplasmic Free [Ca2+] in Parsley Cells Is Receptor Mediated
Three lines of experimental evidence indicate that elicitor-induced increase of [Ca2+]cyt is a receptor-mediated process (Table 2, and Figures 2 and 8). (1) Replacement of W2 by alanine or deletion of terminal residues markedly reduced the ability of these Pep-13 derivatives to trigger the [Ca2+]cyt response. These residues were previously reported to be essential for elicitor as well as competitor activity of Pep-13 (Nürnberger et al., 1994, 1995; Zimmermann et al., 1997). Likewise, exchange of alanine for Y12 did not affect the ability of Pep-13 to bind to its receptor and to induce defense responses and left intact the ability of this derivative to trigger the [Ca2+]cyt response. (2) Similar elicitor concentrations were sufficient to saturate the elicitor receptor and to trigger increases of [Ca2+]cyt as well as other plant responses tested (Table 2). (3) Establishment of a refractory state with respect to repeated stimulation by the same signal was observed in parsley cells treated with Pep-13 (Figure 8B). Such a stimulus-specific desensitization of cellular responses is indicative of receptor-mediated activation and has also been reported for cultured tomato cells treated with fungal sterols or chitooligosaccharides (Boller, 1995).
A biphasic [Ca2+]cyt signature of two transient peaks was observed in apoaequorin-transformed soybean cells after treatment with a P. sojae–derived β-glucan fraction or a synthetic hepta-β-glucan (Mithöfer et al., 1999). Although these experiments suggest a functional link between the elicitor-induced [Ca2+]cyt response and phytoalexin production, as is reported here, the investigators could not demonstrate a receptor-mediated transient increase of [Ca2+]cyt as a requirement for activation of pathogen defense. Moreover, hepta-β-glucan concentrations previously reported to saturate the elicitor receptor (Cosio et al., 1990) were insufficient to trigger the [Ca2+]cyt response and phytoalexin production. However, the Mithöfer et al. study does not transform luminescence units into calibrated [Ca2+]cyt, which probably affects the proposed [Ca2+]cyt signature. Thus, evidence for receptor-mediated activation of a defined [Ca2+]cyt response signaling activation of pathogen defense was not given.
When treated with Rhizobium meliloti nodulation factors, alfalfa root hair cells mount a characteristic [Ca2+]cyt signature (Ehrhardt et al., 1996). Image analysis of injected fluorescent Ca2+ indicators reveals oscillating [Ca2+]cyt spikes with a mean oscillation period of 60 sec. Felle et al. (1999) reported a consistent, rapid decrease in extracellular Ca2+ and concomitant increase in [Ca2+]cyt in alfalfa root cells treated with R. meliloti–derived Nod factors. This is remarkably similar to early responses of elicitor-treated parsley cells. Because the structural features of Nod factors required to cause nodulation in alfalfa were also essential for stimulating [Ca2+]cyt spiking, Ehrhardt et al. (1996) concluded that there was a functional link between both responses. In addition, these findings also suggest a plasma membrane receptor–mediated activation of either response. However, although high- and low-affinity Nod factor binding sites were shown to reside in Medicago truncatula root cell plasma membranes (Gressent et al., 1999), genuine Nod factor receptors mediating establishment of symbiosis remain to be uncovered.
Elicitor-Induced Sustained Increase in [Ca2+]cyt Is Causally Involved in Activation of Pathogen Defense Responses in Parsley
Increases in [Ca2+]cyt in parsley cells could be observed as early as 40 sec after addition of Pep-13. A large [Ca2+]cyt transient peak of ∼1 μM declined rapidly, reaching a plateau phase at ∼250 to 350 nM [Ca2+]cyt (Figure 1A). In vivo imaging of aequorin activity revealed steady light emission from small parsley cell clusters during the course of the experiment (Figure 2), suggesting that there is no repeated spiking of [Ca2+]cyt in individual cells. In particular, sustained values for [Ca2+]cyt seem to be maintained continuously in elicitor-treated parsley cells. However, because the minimum interval for light signal integration was 5 sec, asynchronously responding cells of high-frequency [Ca2+]cyt oscillations with mean period times of less than this are possible. On the other hand, studies on stimulus-induced [Ca2+]cyt oscillations in plant cells noted mean period times between 15 sec and several minutes (Campbell et al., 1996; Ehrhardt et al., 1996; Calder et al., 1997; Staxen et al., 1999), which the technology applied would have been sufficient to detect.
Experimental evidence indicates that Pep-13–induced sustained increases in [Ca2+]cyt and activation of pathogen defense in parsley are functionally inseparable. (1) Use of elicitors of pathogen defense in parsley (P. sojae–derived glycoprotein elicitor harboring Pep-13, harpin, amphotericin B) revealed that only the second phase of the [Ca2+]cyt response, the prolonged increase in [Ca2+]cyt, was required for phytoalexin production (Figure 3A). Stimuli triggering only the rapid [Ca2+]cyt transient peaks—chitooligosaccharides, a flagellin fragment, mastoparan, nystatin, cold shock—failed to induce sustained increases in [Ca2+]cyt and phytoalexin production (Figures 3B and 3C). (2) Pep-13 concentrations that were insufficient to trigger the [Ca2+]cyt transient response were sufficient to stimulate both the plateau phase and 80% of the phytoalexins produced at greater Pep-13 concentrations (Figure 1B). (3) The Ca2+ channel inhibitors Gd3+ and RR abolished the Pep-13–induced [Ca2+]cyt spike at 100 μM but only slightly affected the [Ca2+]cyt plateau value and phytoalexin production (Figures 4D and 5A). (4) Reduction of extracellular [Ca2+] by increasing concentrations of BAPTA resulted in gradual inhibition of the ability of Pep-13–treated parsley cells both to mount sustained increases in [Ca2+]cyt and to produce ROS and phytoalexins. Similarly, removal of Ca2+ from the culture medium by several washes of cells with Ca2+-free medium markedly reduced the elicitor-induced [Ca2+]cyt response (not shown), the oxidative burst, the transcriptional activation of defense-related genes, and phytoalexin production in parsley cells (Nürnberger et al., 1994; Jabs et al., 1997). (5) Elicitor treatment of parsley cells in the presence of the Ca2+ channel blockers Gd3+ (Figure 4D) and La3+ or the protein kinase inhibitors (Figure 7B) inhibited the increase in [Ca2+]cyt and production of ROS and phytoalexins. (6) Complementary gain-of-function experiments using amphotericin B revealed that artificially increasing [Ca2+]cyt to sustained values triggered both the [Ca2+]cyt response and the production of ROS and phytoalexins in the absence of elicitor (Figure 3A; Jabs et al., 1997). (7) Identical structural properties of Pep-13 were found to be required for efficient stimulation of the [Ca2+]cyt plateau, of the oxidative burst, and of phytoalexin production (Table 2). (8) Initial changes in [Ca2+]cyt clearly preceded ROS production and phytoalexin formation in elicitor-treated parsley cells (Figure 1A; Nürnberger et al., 1994).
Taken together, these findings indicate that the sustained increase of [Ca2+]cyt rather than a single transient increase in [Ca2+]cyt may encode signal specificity toward activation of pathogen defense. The physiological implication of the large [Ca2+]cyt transient triggered by Pep-13, however, remains elusive.
A similar [Ca2+]cyt response has been implicated in plant adaptation to abiotic stress. Ozone fumigation of Arabidopsis plants elicited a biphasic [Ca2+]cyt response with a prolonged second phase required for transcriptional activation of a glutathione S-transferase gene (Clayton et al., 1999). Similarly, microinjection of cADP ribose into Commelina communis guard cells induced a sustained increase in [Ca2+]cyt and resulted in stomatal closure (Leckie et al., 1998). A biphasic [Ca2+]cyt response involving an initial transient peak followed by a second, prolonged, slow increase was also detected in Arabidopsis roots during cold acclimation (Plieth et al., 1999).
The importance of defined [Ca2+]cyt signals for initiating different response pathways is intriguingly exemplified by activation of the inflammatory response in human B lymphocytes. The proinflammatory transcriptional regulators NF-κB and c-Jun N-terminal kinase (JNK) are activated by a [Ca2+]cyt spike, whereas another essential transcriptional regulator, nuclear factor-activated T cell (NFAT), is activated by a low, sustained [Ca2+]cyt plateau (Dolmetsch et al., 1997). Moreover, the [Ca2+]cyt oscillation frequency was shown to modulate gene expression in these cell lines (Dolmetsch et al., 1998; Li et al., 1998). Apparently, downstream effectors decode complex [Ca2+]cyt signatures, which probably constitute a mechanism by which the multifunctional second messenger [Ca2+]cyt maintains specificity of signaling cascades. This view is supported by De Koninck and Schulman (1998), who reported that Ca2+/calmodulin–dependent protein kinase II can decode the frequency of Ca2+ spikes into distinct amounts of its activity in vitro.
Elicitor-Induced Increase in [Ca2+]cyt and Activation of Pathogen Defense Responses Requires Extracellular Ca2+
Use of pharmacological effectors provided evidence that elicitor-induced sustained concentrations of [Ca2+]cyt may predominantly reflect the influx of extracellular Ca2+. Chelation of extracellular Ca2+ by membrane-impermeable BAPTA (Figures 4A and 4C) or EGTA completely abolished the increase in [Ca2+]cyt and the activation of downstream responses in parsley cells, such as the oxidative burst (Figure 4C). In addition, only under experimental conditions in which an inwardly directed [Ca2+] gradient was maintained were parsley cells sensitive to elicitor treatment. Because BAPTA abrogated the [Ca2+]cyt response also when added after the elicitor, a continuous Ca2+ influx appears to be required for maintaining increased [Ca2+]cyt. To ensure a plateau value for [Ca2+]cyt, extrusion or sequestration of Ca2+ from the cytoplasm may therefore accompany elicitor-induced Ca2+ entry into the cytoplasm. Lanthanide inhibitors of plasma membrane Ca2+ channels blocked the elicitor-induced increase in [Ca2+]cyt and the plant defense responses as well (Figure 4). The same inhibitors had previously also been found to block activation by Pep-13 of a parsley plasma membrane Ca2+-permeable ion channel (Zimmermann et al., 1997). Likewise, treatment of parsley cells with the ionophore amphotericin B resulted in a Ca2+ influx, an increase in [Ca2+]cyt, the transcriptional activation of defense-related genes, and production of ROS and phytoalexins in the absence of elicitor (Jabs et al., 1997; Figure 3A). Furthermore, the attempted modulation of phospholipase C activity by neomycin, with the subsequent Ca2+ release from internal stores, and the inhibition by RR of RR-sensitive intracellular Ca2+ release channels failed to inhibit elicitor-induced sustained increases in [Ca2+]cyt or activation of plant defense responses (Figure 5).
Increases in [Ca2+]cyt in Nod factor–treated alfalfa root hair cells or in elicitor-treated soybean cells were also found to result mainly from a stimulus-induced Ca2+ influx through the plasma membrane (Felle et al., 1999; Mithöfer et al., 1999). In contrast, the increases of [Ca2+]cyt monitored in plant cells in response to anoxia, cold shock, drought, or salinity have been ascribed to both a Ca2+ release from internal stores and an influx of external Ca2+ (Subbaiah et al., 1994; Haley et al., 1995; McAinsh et al., 1995; Knight et al., 1996, 1997). Thus, just as in animal cells, two major pathways for Ca2+ release into the cytoplasm may be operative in plant cells (Clapham, 1995; Parekh and Penner, 1997; Thuleau et al., 1998; Rudd and Franklin-Tong, 1999): Ca2+ influx directly through plasma membrane Ca2+ channels, and Ca2+ entry by second messenger–induced depletion of intracellular stores followed by store replenishment by way of Ca2+ release–activated Ca2+ currents across the plasma membrane.
Numerous Ca2+ channels have been identified in plant cells, but their physiological roles and probable cooperation in response to various stimuli are little understood. Challenging future tasks will be to understand how different Ca2+ channels contribute to signal-specific [Ca2+]cyt signatures and how (spatio)temporal alterations in [Ca2+]cyt can activate downstream effectors, such as Ca2+- or Ca2+/calmodulin–dependent protein kinases (Blumwald et al., 1998; Thuleau et al., 1998; Rudd and Franklin-Tong, 1999; Trewavas, 1999).
METHODS
Materials
The peptides Pep-13, Pep-13/A2, mastoparan, mastoparan-17, and flagellin-15 were synthesized by use of fluorenyl methoxycarbonyl chemistry as described by Nennstiel et al. (1998). Pep-13/A12 and Pep-10 were purchased from Kem-En-Tec A/S (Copenhagen, Denmark), and Mas-7 was from Calbiochem (Bad Soden, Germany). The Phytophthora sojae 42-kD cell wall glycoprotein elicitor was purified according to Nürnberger et al. (1994). Purified recombinant Pseudomonas syringae pv phaseolicola harpin was from Justin Lee (IPB, Halle, Germany). The Phytophthora megasperma elicitin β-megaspermin was provided by Serge Kauffmann (INRA, Strasbourg, France), N-acetylchitoheptaose was from Naoto Shibuya (University of Tsukuba, Tsukaba, Japan), and N-acetylchitopentaose was from Jürg Felix (FMI, Basel, Switzerland). Inhibitors were purchased from Alexis (Grünberg, Germany), anthracene-9-carboxylate (A-9-C) and N-acetylchitotriose from Sigma (Deisenhofen, Germany), amphotericin B from Calbiochem, and EGTA, LaCl3, and GdCl3 as well as high-purity chemicals for in vitro [Ca2+] calibration from Fluka (Deisenhofen, Germany). 1,2-Bis(aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), coelenterazine, and calcium calibration buffers were supplied by Molecular Probes (Leiden, The Netherlands).
Plant Cell Culture and Elicitor Treatment
Dark-grown cell suspension cultures of Petroselinum crispum were used for elicitor treatment and reconstitution of aequorin 5 days after inoculation. Culture maintenance and quantification of reactive oxygen species (ROS) and furanocoumarin phytoalexin production were performed according to Nürnberger et al. (1994). Cell viability was determined by double-staining with fluorescein diacetate and propidium iodide (Jabs et al., 1997) 30 min or 24 hr after treatment, respectively. Tobacco W38 cell suspensions stably transformed with apoaequorin were obtained from Phil Low (Purdue University, West Lafayette, IN) and maintained as described (Chandra et al., 1997).
Aequorin Constructs and Parsley Cell Transformation
Three-day-old parsley cells were transformed as described (Frohnmeyer et al., 1999) by particle bombardment with a plasmid carrying apoaequorin cDNA inserted between a double cauliflower mosaic virus 35S promoter, a tobacco mosaic virus Ω-element, and a 35S terminator (pAEQ/HYG). This plasmid was derived from pGNAEQ/NEO2 (Mithöfer et al., 1999) by replacing the cassette for the selectable marker NPTII with a hygromycin-resistance gene (HPT) excised from plasmid pGL2 (Mithöfer et al., 1999). Plasmids pGNAEQ/NEO2 and pGL2 were kindly provided by Gunther Neuhaus (University of Freiburg, Germany).
Aequorin Reconstitution and Bioluminescence Measurement
In vivo reconstitution of aequorin was initiated by adding 3 μL of coelenterazine (5 mM stock solution in methanol) to 3 mL of 5-day-old aequorin-transgenic parsley cells. Cells were adjusted to 60 mg (fresh weight) mL−1 culture medium supplemented with 1 mM CaCl2. Subsequently, cells were incubated in the dark under continuous shaking (for 6 hr or overnight, at 26°C and 120 rpm).
Bioluminescence counts from 100-μL cell culture aliquots were recorded at 10-sec intervals (recorded as average relative light units (RLUs)/sec) with a digital luminometer (Lumat LB9501; Berthold, Bad Wildbad, Germany). At the end of each experiment, the remaining aequorin was discharged by adding two volumes of 37.5 mM CaCl2/15% ethanol (v/v). The amount of aequorin consumed at each time point of the experiment never exceeded 5% of the total remaining aequorin. Alternatively, bioluminescence assays were performed with a microplate luminometer (Luminoskan Ascent, version 2.4; Labsystems, Frankfurt/Main, Germany) under continuous shaking of cell suspensions (300-μL aliquots in 48-well plates, measured every 13 sec). No differences in reproducibility were observed between the two bioluminescence monitoring devices used.
In vivo calcium imaging of aequorin activity in parsley cells was performed with a C2400-40H Intensified (CCD) Charge-Coupled Device Camera device (Hamamatsu Photonics GmbH Deutschland, Herrsching, Germany) equipped with a Nikon lens system (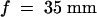 , 40-mm-long extension tube; Nikon Deutschland, Düsseldorf, Germany) and the operating software HiPic 5.1. Parsley cells (5 × 103 in 100 μL) were placed on a microscope slide, treated with elicitor, and subjected to photon counting in the dark. Bioluminescence was recorded in slice mode in dynamic photon-counting format.
, 40-mm-long extension tube; Nikon Deutschland, Düsseldorf, Germany) and the operating software HiPic 5.1. Parsley cells (5 × 103 in 100 μL) were placed on a microscope slide, treated with elicitor, and subjected to photon counting in the dark. Bioluminescence was recorded in slice mode in dynamic photon-counting format.
Calibration of Calcium Measurements
To transform bioluminescence counts into absolute [Ca2+] values, we established a calibration curve from in vitro measurements of protein extracts made from pAEQ/HYG–expressing parsley cells and a series of buffers with various [Ca2+]. Transgenic parsley cells were washed with calcium-free buffer, frozen in liquid nitrogen, and homogenized in reconstitution buffer: 20 mM Tris/HCl, pH 7.4, 150 mM KCl, 5 mM EDTA, 0.1 mM EGTA, 50 mM β-mercaptoethanol, and 1 mM phenylmethylsulfonyl fluoride. After centrifugation (5 min, 4°C, 12,100g) the supernatant was utilized for in vitro reconstitution of aequorin (3 hr, 4°C) in the presence of 0.1% gelatin and 2.5 μM coelenterazine.
Light production was measured in buffers (10 mM Hepes, pH 7.2, 100 mM KCl, 1 mM MgSO4) with known [Ca2+] (Molecular Probes calcium calibration buffer kit). [Ca2+] values were subsequently calculated as rate constants from the total aequorin activity assessed at saturating concentrations of Ca2+ according to Cobbold and Lee (1991). Rate constants represent bioluminescence counts monitored per second divided by the total remaining bioluminescence counts.
All reagents tested were applied to lysates containing recombinant aequorin to examine the direct effects of the reagents on aequorin luminescence. None of these substances interfered with aequorin luminescence.
Extracellular free [Ca2+] in the presence of the chelator BAPTA was calculated using MaxChelator software (version 2.5 for Windows; http://www.stanford.edu/~cpatton/) (Bers et al., 1994).
Acknowledgments
We are indebted to Gunther Neuhaus for critical reading of the manuscript, and to him and Claudia Köhler for assisting in particle bombardment experiments as well as for kindly providing plasmids pGNAEQ/NEO2 and pGL2. We thank Hanns Frohnmeyer and Linda Loyall for helpful discussions on parsley cell transformation, and Hans-Hubert Felle, Birgit Klüsener, Peter Buschmann, and Richard Nuccitelli for assistance in calculating free ion concentrations. Delivery of elicitors by Justin Lee, Serge Kauffmann, Naoto Shibuya, and Jürg Felix and of apoaequorin-transgenic tobacco cells by Phil Low is gratefully acknowledged. We thank Helga Nixdorf and Jutta Elster for peptide syntheses and Annette Romanski for performing Pep-13 binding assays on tobacco membranes. This work was supported by the CAST project of the European Community Biotech program (Grant No. BIO4-CT96-0101) and the Fonds der Chemischen Industrie.
References
- Bers, D.M., Patton, C.W., and Nuccitelli, R. (1994). A practical guide to the preparation of Ca2+ buffers. Methods Cell. Biol. 40, 3–29. [DOI] [PubMed] [Google Scholar]
- Bischoff, F., Molendijk, A., Rajendrakumar, C.S.V., and Palme, K. (1999). GTP-binding proteins in plants. Cell. Mol. Life Sci. 55, 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks, J.R., Mattingly, P.H., Jewell, B.R., van Leeuwen, M., Harrer, G.C., and Allen, D.G. (1978). Practical aspects of the use of aequorin as a calcium indicator: Assay, preparation, microinjection and interpretation of signals. Methods Enzymol. 57, 292–328. [Google Scholar]
- Blumwald, E., Aharon, G.S., and Lam, B.C.-H. (1998). Early signal transduction pathways in plant–pathogen interactions. Trends Plant Sci. 3, 342–346. [Google Scholar]
- Boller, T. (1995). Chemoperception of microbial signals in plant cells. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 189–214. [Google Scholar]
- Calder, G.M., Franklin-Tong, V.E., Shaw, P.J., and Drøbak, B.K. (1997). Ca2+ oscillations in plant cells: Initiation by rapid elevation in cytosolic free Ca2+ levels. Biochem. Biophys. Res. Commun. 234, 690–694. [DOI] [PubMed] [Google Scholar]
- Campbell, A.K., Trewavas, A.J., and Knight, M.R. (1996). Calcium imaging shows differential sensitivity to cooling and communication in luminous transgenic plants. Cell Calcium 19, 211–218. [DOI] [PubMed] [Google Scholar]
- Cessna, S.G., Chandra, S., and Low, P.S. (1998). Hypo-osmotic shock of tobacco cells stimulates Ca2+ fluxes deriving first from external and then internal Ca2+ stores. J. Biol. Chem. 273, 27286–27291. [DOI] [PubMed] [Google Scholar]
- Chandra, S., Stennis, M., and Low, P.S. (1997). Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J. Biol. Chem. 272, 28274–28280. [DOI] [PubMed] [Google Scholar]
- Clapham, D.E. (1995). Calcium signaling. Cell 80, 259–268. [DOI] [PubMed] [Google Scholar]
- Clayton, H., Knight, M.R., Knight, H., McAinsh, M.R., and Hetherington, A.M. (1999). Dissection of the ozone-induced calcium signature. Plant J. 17, 575–579. [DOI] [PubMed] [Google Scholar]
- Cobbold, P.H., and Lee, J.A.C. (1991). Aequorin measurements of cytoplasmic free calcium. In Cellular Calcium: A Practical Approach. J.G. McCormack and P.H. Cobbold, eds (Oxford, UK: Oxford University Press) pp. 55–81.
- Cosio, E.G., Frey, T., Verduyn, R., van Boom, J., and Ebel, J. (1990). High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 271, 223–226. [DOI] [PubMed] [Google Scholar]
- De Koninck, P., and Schulman, H. (1998). Sensitivity of CaM Kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230. [DOI] [PubMed] [Google Scholar]
- Delledonne, M., Xia, Y., Dixon, R.A., and Lamb, C. (1998). Nitric oxide functions as a signal in plant disease resistance. Nature 394, 585–588. [DOI] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C., and Healy, J.I. (1997). Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858. [DOI] [PubMed] [Google Scholar]
- Dolmetsch, R.E., Xu, K., and Lewis, R.S. (1998). Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936. [DOI] [PubMed] [Google Scholar]
- Ehrhardt, D.W., Wais, R., and Long, S.R. (1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85, 673–681. [DOI] [PubMed] [Google Scholar]
- Finer, J.J., Vain, P., Jones, M.W., and McMullen, M.D. (1992). Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep. 11, 323–328. [DOI] [PubMed] [Google Scholar]
- Felix, G., Duran, J.D., Volko, S., and Boller, T. (1999). Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 18, 265–276. [DOI] [PubMed] [Google Scholar]
- Felle, H.H., Kondorosi, E., Kondorosi, A., and Schultze, M. (1999). Elevation of the cytosolic free [Ca2+] is indispensible for the transduction of the nod factor signal in alfalfa. Plant Physiol. 121, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer, H., Loyall, L., Blatt, M.R., and Grabov, A. (1999). Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J. 20, 109–117. [DOI] [PubMed] [Google Scholar]
- Gabev, E., Kasianowicz, J., Abbott, T., and McLaughlin, S. (1989). Binding of neomycin to phosphatidylinositol 4,5-bisphosphate (PIP)2. Biochim. Biophys. Acta 979, 105–112. [DOI] [PubMed] [Google Scholar]
- Gelli, A., Higgins, V.J., and Blumwald, E. (1997). Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol. 113, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, M., van der Luit, A.H., Knight, M.R., and Trewavas, A.J. (1998). Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 116, 429–437. [PMC free article] [Google Scholar]
- Gressent, F., Drouillard, S., Mantegazza, N., Samain, E., Geremia, R.A., Canut, H., Niebel, A., Driguez, H., Ranjeva, R., Cullimore, J., and Bono, J.-J. (1999). Ligand specificity of a high-affinity binding site for lipochitooligosaccharide Nod factors in Medicago cell suspension cultures. Proc. Natl. Acad. Sci. USA 96, 4704–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock, K., Scheel, D., Logemann, E., Nürnberger, T., Parniske, M., Reinold, S., Sacks, W.R., and Schmelzer, E. (1995). Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc. Natl. Acad. Sci. USA 92, 4150–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, A., Russel, A.J., Wood, N., Allan, A.C., Knight, M., Campbell, A.K., and Trewavas, A.J. (1995). Effects of mechanical signaling on plant cell cytosolic calcium. Proc. Natl. Acad. Sci. USA 92, 4124–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima, T., Burnier, J., and Ross, E.M. (1990). Regulation of Gi and Go by mastoparan, related amphiphilic peptides, and hydrophobic amines. Mechanisms and structural determinants of activity. J. Biol. Chem. 265, 14176–14186. [PubMed] [Google Scholar]
- Jabs, T., Tschöpe, M., Colling, C., Hahlbrock, K., and Scheel, D. (1997). Elicitor-stimulated ion fluxes and O2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc. Natl. Acad. Sci. USA 94, 4800–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, C.H., Knight, M.R., Kondo, T., Masson, P., Sedbrook, J., Haley, A., and Trewavas, A. (1995). Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science 269, 1863–1865. [DOI] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352, 524–526. [DOI] [PubMed] [Google Scholar]
- Knight, M.R., Smith, S.M., and Trewavas, A.J. (1992). Wind-induced plant motion immediately increases cytosolic calcium. Proc. Natl. Acad. Sci. USA 89, 4967–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1996). Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, H., Trewavas, A.J., and Knight, M.R. (1997). Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 12, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Lamb, C., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275. [DOI] [PubMed] [Google Scholar]
- Leckie, C.P., McAinsh, M.R., Allen, G.J., Sanders, D., and Hetherington, A.M. (1998). Abscisic acid–induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95, 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, B.D., and Spalding, E.P. (1998). Nonselective block by La3+ of Arabidopsis ion channels involved in signal transduction. J. Membr. Biol. 162, 81–90. [DOI] [PubMed] [Google Scholar]
- Lewis, B.D., Karlin-Neumann, G., Davis, R.W., and Spalding, E.P. (1997). Ca2+-activated anion channels and membrane depolarizations induced by blue light and cold in Arabidopsis seedlings. Plant Physiol. 114, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W.-H., Llopis, J., Whitney, M., Zlokarnik, G., and Tsien, R.Y. (1998). Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392, 936–941. [DOI] [PubMed] [Google Scholar]
- Ligterink, W., Kroj, T., zur Nieden, U., Hirt, H., and Scheel, D. (1997). Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276, 2054–2057. [DOI] [PubMed] [Google Scholar]
- McAinsh, M.R., Webb, A.A.R., Taylor, J.E., and Hetherington, A.M. (1995). Stimulus-induced oscillations in the guard cell cytoplasmic free calcium. Plant Cell 7, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer, A., Ebel, J., Bhagwat, A.A., Boller, T., and Neuhaus-Url, G. (1999). Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta 207, 566–574. [Google Scholar]
- Nennstiel, D., Scheel, D., and Nürnberger, T. (1998). Characterization and partial purification of an oligopeptide elicitor receptor from parsley (Petroselinum crispum). FEBS Lett. 431, 405–410. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T. (1999). Signal perception in plant pathogen defense. Cell. Mol. Life Sci. 55, 167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberger, T., Nennstiel, D., Jabs, T., Sacks, W.R., Hahlbrock, K., and Scheel, D. (1994). High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460. [DOI] [PubMed] [Google Scholar]
- Nürnberger, T., Nennstiel, D., Hahlbrock, K., and Scheel, D. (1995). Covalent cross-linking of the Phytophthora megasperma oligopeptide elicitor to its receptor in parsley membranes. Proc. Natl. Acad. Sci. USA 92, 2338–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh, A.B., and Penner, R. (1997). Store depletion and calcium influx. Physiol. Rev. 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Plieth, C., Hansen, U.-P., Knight, H., and Knight, M.R. (1999). Temperature sensing by plants: The primary characteristics of signal perception and calcium response. Plant J. 18, 491–497. [DOI] [PubMed] [Google Scholar]
- Price, A.H., Taylor, A., Ripley, S.J., Griffith, A., Trewavas, A.J., and Knight, M.R. (1994). Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiquampoix, H., Ratcliff, R.G., Ratkovic, S., and Vucinic, Z. (1990). 1H and 31P NMR investigation of gadolinium uptake in maize roots. J. Inorg. Biochem. 38, 265–275. [Google Scholar]
- Rudd, J.J., and Franklin-Tong, V.E. (1999). Calcium signaling in plants. Cell. Mol. Life Sci. 55, 214–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel, D. (1998). Resistance response physiology and signal transduction. Curr. Opin. Plant Biol. 1, 305–310. [DOI] [PubMed] [Google Scholar]
- Sedbrook, J.C., Kronebusch, P.J., Borisy, G.G., Trewavas, A.J., and Masson, P.H. (1996). Transgenic AEQUORIN reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 111, 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, H., Borin, M.L., and Blaustein, M.P. (1997). Use of La3+ to distinguish activity of the plasmalemmal Ca2+ pump from Na+/Ca2+ exchange in arterial myocytes. Cell Calcium 21, 31–41. [DOI] [PubMed] [Google Scholar]
- Smallridge, R.C., Kiang, J.G., Gist, I.D., Fein, H.G., and Galloway, R.J. (1992). U-73122, an aminosteroid phospholipase C antagonist, noncompetetively inhibits thyrotropin-releasing hormone effects in GH3 rat pituitary cells. Endocrinology 131, 1883–1888. [DOI] [PubMed] [Google Scholar]
- Sopory, S.K., and Munshi, M. (1998). Protein kinases and phosphatases and their role in cellular signaling in plants. Crit. Rev. Plant Sci. 17, 245–318. [Google Scholar]
- Staxen, I., Pical, C., Montgomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaiah, C.C., Bush, D.S., and Sachs, M.M. (1994). Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-cultured cells. Plant Cell 6, 1747–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., Isobe, M., and Muto, S.R.A. (1997). An increase in cytosolic calcium ion concentration precedes hypoosmotic shock–induced activation of protein kinases in tobacco suspension culture cells. FEBS Lett. 401, 202–206. [DOI] [PubMed] [Google Scholar]
- Thuleau, P., Schroeder, J.I., and Ranjeva, R. (1998). Recent advances in the regulation of plant calcium channels: Evidence for regulation by G-proteins, the cytoskeleton and second messengers. Curr. Opin. Plant Biol. 1, 424–427. [DOI] [PubMed] [Google Scholar]
- Trewavas, A. (1999). Le calcium, c'est la vie: Calcium makes waves. Plant Physiol. 120, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, M., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean beta glucan elicitor binding protein. Proc. Natl. Acad. Sci. USA 94, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Shah, J., and Klessig, D.F. (1997). Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Zimmermann, S., Nürnberger, T., Frachisse, J.-M., Wirtz, W., Guern, J., Hedrich, R., and Scheel, D. (1997). Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc. Natl. Acad. Sci. USA 94, 2751–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]