Abstract
Identification of biomarkers to recognize individuals with Barrett's esophagus (BE) predisposed to develop malignancy is currently a pressing issue. We utilized gene expression profiling to compare molecular signatures of normal esophagus and stomach, BE, and adenocarcinoma (AC) to identify such potential biomarkers. Over 22,000 genes were analyzed by oligonucleotide microarrays on 38 unique RNA. Unsupervised and supervised clusterings were performed on a subset of 2849 genes that varied most significantly across the specimens. Unsupervised clustering identified two discernable molecular BE profiles, one of which was similar to normal gastric tissue (“BE1”), and another that was shared by several of the AC specimens (“BE2”). The BE1 profile included expression of several genes that have been described as tumor-suppressor genes, most notably trefoil factor 1 (TFF-1). The BE2 profile included expression of genes previously found overexpressed in cancers, such as carboxylesterase-2 (CES-2). IHC demonstrated the loss of TFF-1 late in the progression of BE to AC. It also revealed CES-2 as being upregulated in AC documented to have arisen in the presence of BE. These potential biomarkers, as well as the relative expression of genes from BE1 versus those from BE2, may be validated in the future to aid in risk stratification and guide treatment protocols in patients with BE and associated AC.
Keywords: Trefoil Factor 1, Carboxylesterase 2, Barrett's esophagus, esophageal or gastric adenocarcinoma, tissue microarry
Introduction
Adenocarcinoma (AC) of the esophagus or esophagogastric junction (EGJ) has the most rapidly rising incidence of all malignancies in Western nations [1,2]. In the United States and United Kingdom, these carcinomas combine to rank as the fifth most prevalent cancer [3]. This phenomenon has been shown to correspond with an increase in the incidence of specialized intestinal cell metaplasia of the distal esophagus, or Barrett's esophagus (BE) [4]. BE is recognized as the precursor lesion to AC of esophageal or EGJ origin. Approximately 12% to 18% of patients who undergo upper endoscopy for symptoms of gastroesophageal reflux disease are observed to have BE, and it is estimated that between 0.5% and 2.0% of adults in the United States and Western Europe have BE [5–7]. Survival rates from BE-associated AC are poor (<10% at 5 years), with the mortality rate often matching the incidence rate [8]. Only early diagnosis and aggressive treatment strategies, such as esophagectomy, have been shown to alter the course of this disease. Thus, a better understanding of the genetic changes that accompany the evolution of normal mucosa to AC, through BE, is desired in order to gain insight into this complex disorder and achieve improved outcomes.
Barrett's metaplasia can progress to low-grade dysplasia (LGD), high-grade dysplasia (HGD), and, eventually, AC. Dysplastic changes occur in a minority of cases of BE, and AC develops in only 0.2% to 2.0% of patients. However, with the use of current clinical tools, it is difficult to predict the populations in which metaplasia will advance to neoplasia. Characterization of biomarkers that identify a predisposition of certain individuals with BE to the development of dysplasia and/or malignancy is eagerly awaited to advance the management of patients with this lesion.
Expression profiling using microarray technology is anticipated to aid in the discovery of expression signatures that may have clinical utility applicable to BE-associated AC. Microarray analysis has been utilized in several recent reports from Asia to examine gene expression profiles in gastric adenocarcinoma (GC) [9–11]. These have been promising in that they have led to further understanding of the molecular basis of GC, and the discovery of expression profiles and candidate biomarkers that may have practical value. GC, particularly of the intestinal type, commonly arises from gastric intestinal metaplasia and is regarded by many as having biologic properties similar to BE-associated AC. Microarray technology has been utilized to explore global expression profiles of BE and its associated malignancies in two previous publications [12,13]. These showed some promise in the classification of esophageal AC and premalignant disease; however, they were limited by relatively small sample size, lack of inclusion of expression results for normal tissue, and incomplete exploration of the genome. Two additional recent studies utilized microarray technology to compare gene expression profiles of BE to normal tissues [14], and BE with and without exposure to acid reflux [15], but neither included malignant tissues in their analyses.
Herein, we performed a comprehensive comparison of the gene expression profiles of all relevant tissue subtypes, including normal esophagus and stomach; BE; and AC of the esophagus, EGJ, and stomach. Moreover, we used xenografted human tumors to profile optimally enriched samples for neoplastic cells. These xenografted tumors are genetically stable and are a good representation of the primary tumors from which they are derived [16,17]. Moreover, homogeneous human epithelial neoplastic cells in the xenografted tumors supported by the mouse stroma provide optimal samples for human probes. Confirmation of alterations in gene expression was performed by immunohistochemical staining of a large cohort of tumor specimens assembled on tissue microarrays (TMAs). Our analysis leads to the definition of two unique gene expression profiles within BE, one of which was shared to a significant extent by normal gastric tissue, and another was shared by several of the AC specimens. This study suggests that molecular definition of BE is possible and, with further validation, may have practical utility for the prediction of the aggressive course of this precancerous condition.
Materials and Methods
Tissue Samples
Tissue samples were obtained from patients who had undergone resections for primary esophageal, EGJ, or gastric AC. These samples included fresh tissues for xenografting in nude mice, fresh-frozen tissue stored in liquid nitrogen, and formalin-fixed paraffin-embedded tissue. Tumors were collected from the Department of Pathology at the University of Virginia Health System, Indiana University, Johns Hopkins University, and from Siena, Italy. The University of Virginia Human Investigation Committee (IRB) approved the use of human tissues in this study.
Xenografting
Small pieces of primary human esophageal, EGJ, and gastric tumor tissue were soaked in Matrigel (Collaborative Biomedical Research, Amherst, MA) and then implanted subcutaneously into the flanks of immunodeficient mice (nu/nu from Harlan, Indianapolis, IN or SCID from Charles River, Wilmington, MA) for xenograft growth, as previously reported [18]. First-passage tumors were harvested when their diameter reached ∼ 1 cm.
Microarray Hybridization and Data Analysis
RNA extraction, cRNA synthesis, and GeneChip hybridizations were preformed as described previously [19]. Raw data from the GeneChips were processed using MAS5.0 (Affymetrix, Santa Clara, CA), scaled to an average difference value of 200 and collated in Microsoft EXCEL for further analysis. Agglomerative clustering was performed using Cluster, and the results of clustering were visualized in Treeview [20]. The standard deviation from the mean of each gene's expression value across samples was used as a measure of variability to select the genes for clustering. For the data presented in Figure 1, a value of 250 was used. Supervised learning was performed using GeneCluster2 (http://www.broad.mit.edu/cancer/software.html) [21]. Processed GeneChip hybridization data were imported to GeneCluster2 in .gct format and the class of each sample was specified. For classification, a k-nearest neighbors (k-NN) algorithm was used, specifying k = 3. Class predictions were calculated for variable numbers of genes in increments of 10 (from 10 to 100). Feature summary data (i.e., from the subset of the genes used in class prediction) were exported from GeneCluster2 and visualized in Treeview. A signal-to-noise (S2N) metric was used to identify 20 genes per class for each of the four groups of samples, and also exported and visualized in Treeview. To identify genes specifically expressed in a group of samples, we used EXCEL to perform gene ranking. The difference in expression was first examined using the fold ratios of the average of each gene's expression in each group. In the case of BE, we also specified that the average expression of a gene must be <50 in samples of stomach and esophageal mucosa and >200 in BE. Only those genes with a fold change >2 in BE versus all other samples were selected (n = 21).
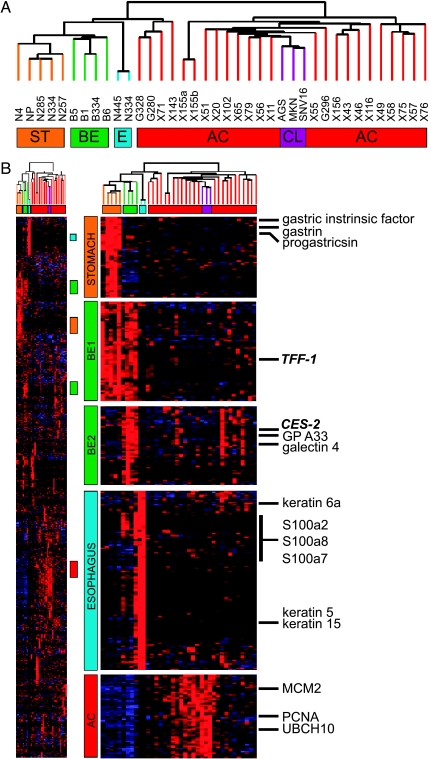
Figure 1.
Agglomerative clustering analysis. Analysis of the molecular profiles generated using agglomerative clustering demonstrating a strong relationship between normal, neoplastic, and BE specimens (panel A). The far right column lists representative genes predominantly expressed in each of the tissue types (panel B). ST = stomach; BE = Barrett's esophagus; E = esophagus; AC = adenocarcinoma; CL = cell line.
TMAs
Targeted tissues (tumor and normal) were marked on H&E-stained slides corresponding to their paraffin blocks. One to four 0.6-mm cores from each tumor were obtained from donor blocks using a manual microarray device (Beecher Instruments, Silver Spring, MD) and inserted into recipient paraffin blocks in gridded arrays. The TMAs were sectioned at 4 µm thickness and placed on charged slides.
Immunohistochemistry (IHC)
TFF-1 Following application of a monoclonal antibody to TFF-1 (Zymed Laboratory, San Francisco, CA), as described previously [18], expression was evaluated by our pathologist (C.A.M.) using a semiquantitative scoring system. As staining intensity for TFF-1 did not greatly vary in cells exhibiting staining, the extent of cell staining was scored on a percentage basis: 0 (<1%, “negative”), 1+ (1–25%, “weak”), 2+ (26–50%, “moderate”), 3+ (51–75%, “strong”), and 4+ (76–100%, “marked”). In evaluating tumors, all cancer cells in the specimen were evaluated; in non-neoplastic gastric mucosa, only foveolar neck and surface mucous epithelial cells were evaluated; in metaplastic Barrett's mucosa, only epithelial cells in the superficial portions of the glands and the surface epithelial cells were evaluated.
CES-2 The primary antibody was generated by Genemed Synthesis, Inc. (South San Francisco, CA), as presented previously [22]. For immunostaining, streptavidin-biotin complex IHC was performed using an i6000 automated staining system (BioGenex, San Ramon, CA) at room temperature without antigen retrieval. The protocol was as follows: dewax, 10 minutes; peroxide block, 20 minutes; biotin block, 20 minutes; power block, 20 minutes; CES-2 (1:150), 1 hour; link, 30 minutes; label, 20 minutes; 3,3′-diaminobenzidine tetrahydrochloride, 5 minutes; and hematoxylin, 1 minute. The negative controls were omission of the primary antibody or antibody preadsorption with its cognate peptide (1 mg/ml) overnight at 4°C.
CES-2 expression was evaluated by two pathologists (H.Z. and W.Z.) using a semiquantitative scoring system. The intensity of staining was scored as 0 (negative), 1 (weak), 2 (medium), or 3 (strong). The extent of staining was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%), according to the percentages of the positive-staining tumor cells within each specimen on the tissue array. The sum of the scores for the intensity and extent of staining was determined. Tissues having a final staining score of less than 2 were considered “negative,” and tissues having a score of greater than 2 were considered “positive.” A sum score of 2 to 3 was considered “weak” (1+); 4 to 5 was “moderate” (2+); and 6 to 7 was “marked” (3+).
CD-13 Prior to avidin-biotin immunoperoxidase, slides were placed in citrate buffer and treated with microwave heat for 20 minutes. The monoclonal antibody to CD-13 (clone 38C12, 1:50 dilution; Novocastra Laboratories, Newcastle upon Tyne, UK) was applied for 1 hour at room temperature. Staining was evaluated by one pathologist (H.F.F.) and scored as 0 (“negative”), 1+ (1–10% positive cells, “weak”), 2+ (11–50%, “moderate”), or 3+ (>50%, “marked”).
Results
Gene Expression Profiles
Total RNA from 38 tissue specimens was hybridized on oligonucleotide microarrays containing probe sets for approximately 22,000 genes. The specimens included biopsies of normal esophagus (n = 2), normal stomach (n = 5, including one set of normal gastric tissue pooled from a total of four subjects), BE (n = 4), primary human esophageal and gastric AC (n = 5), and xenografted human esophageal and gastric AC (n = 20). Clinical and pathologic information on each of the specimens is detailed in Table 1. The primary cancers and xenografts studied were each derived from unique individuals with the exception of G208 and X71, which originated from the same patient. One xenografted specimen (X155) was hybridized in duplicate to determine the reproducibility of the assays. In addition, three separate human gastric AC-derived cell lines were included in the analysis.
Table 1.
Characterization of Cases Analyzed by Oligonucleotide Microarray Hybridization.
| Case | Age | Site | Histology | TNM | Stage | Grade | Barrett's |
| Normals | |||||||
| N334 | 59 | E | N | ||||
| N445 | 74 | E | N | ||||
| N4 | 57 | S | N | ||||
| N257 | 68 | S | N | ||||
| N285 | 68 | S | N | ||||
| N334 | 59 | S | N | ||||
| NP | 50 | S | N | ||||
| Barrett's esophagus | |||||||
| B1 | 69 | E | Y | ||||
| 334 | 59 | E | Y | ||||
| B5 | 66 | E | Y | ||||
| B6 | 60 | E | Y | ||||
| Xenografts | |||||||
| X11 | 67 | EGJ | D | II | PD | N | |
| X20 | 66 | EGJ | I | T1N1M0 | IIB | MD | Y |
| X43 | 78 | MID | I | T4N1M0 | IV | PD | N |
| X46 | 70 | EGJ | I | T4N1M0 | IV | MD | Y |
| X49 | 64 | EGJ | D | T3N1M0 | III | PD | Y |
| X51 | 44 | EGJ | I | T4N1M1 | IV | MD | N |
| X55 | 62 | EGJ | I | T2N1M0 | IIB | MD | Y |
| X56 | 70 | EGJ | I | T3N1M0 | IIIA | MD | Y |
| X57 | 64 | EGJ | I | T3N1M1 | IV | MD | N |
| X58 | 64 | CARD | I | T4N0M0 | IIIA | PD | N |
| X65 | 46 | DIST | I | T3N1M0 | IIIA | PD | N |
| X71 | 64 | MID | M | T2N1M0 | IIB | PD | N |
| X75 | 60 | E | I | T3N1M0 | III | MD | Y |
| X76 | 60 | CARD | I | T3N1M0 | IIIA | WD | N |
| X79 | 72 | E | I | T2N0M0 | IIA | MD | Y |
| X102 | 72 | DIST | I | T3N1M0 | IIIA | UD | N |
| X116 | 59 | EGJ | I | T3N1M0 | MD | ND | |
| X143 | 48 | E | I | T3N1M0 | III | PD | Y |
| X155 | 62 | MID | D | T2N2 | IIIA | MD | ND |
| X156 | 77 | EGJ | I | T2N0M0 | IB | MD | ND |
| Primary tumors | |||||||
| G208 | 60 | CARD | I | T3N1M0 | IIIA | WD | N |
| G234 | 58 | E | I | T4N0M0 | III | MD | N |
| G280 | 68 | CARD | D | T3N2M0 | IIIB | PD | N |
| G296 | 42 | CARD | D | T3N1M0 | IIIA | PD | Y |
| G328 | 84 | EGJ | M | T3N1M0 | IIIA | PD | Y |
E = esophagus; S = stomach; EGJ = gastroesophageal junction; CARD = gastric cardia; MID = midstomach; DIST = distal stomach; WD = well-differentiated; MD = moderately differentiated; PD = poorly differentiated; UD = undifferentiated; ND = not determined.
Agglomerative Clustering Analysis
To reveal distinctions between individual samples, agglomerative clustering was performed on a subset of 2849 genes that varied most significantly across the specimens. Clustering demonstrated a strong relationship between the normal specimens, and a similarly strong relationship among the AC specimens. The BE specimens were also highly associated with one another (Figure 1). The reproducibility of this analysis was underscored by the significant coclustering of duplicate samples studied from the same patient (X155a and X155b), as well as the almost identical coclustering of a xenograft (X71) and primary tumor sample (G208) from the same patient.
As expected, normal esophageal specimens exhibited expression of genes specific to stratified squamous epithelial cells. These included multiple cytokeratins, specifically CK-1, CK-4, CK-5, CK-6a, CK-6b, and CK-13 to CK-17 (Figure 1) [23,24]. Other than CK-17, which was expressed in some of the AC specimens, none of these cytokeratin genes was expressed in any of the normal stomach or AC specimens. Additionally, several members of the EF hand calcium binding proteins, notably S100A2, S100A7 to S100A9, S100A11, and S100A14, were found to be highly expressed in normal esophageal specimens. Again, these genes were not expressed in any of the normal stomach or AC samples in our analysis. Similarly, specimens derived from normal stomach expressed genes known to be specific to this tissue, including gastric intrinsic factor, gastric lipase, gastrin, and progastricin (Figure 1) [25–27].
Three types of esophageal and gastric AC specimens were studied: primary human tumors, xenografted human tumors, and human gastric cancer cell lines. These were found to cluster together, separate from normal and BE tissue samples. Primary tumors and xenografts were either clustered on a subbranch that also subtended the normal and BE cases, or entirely within a separate branch. Of the specimens in the latter branch, a subset was shown to cluster directly with the cell lines. This tight association appeared to be driven by the high relative expression of genes involved in cell cycle progression and cellular replication, including MCM2, PCNA, and UBCH10, each of which has been previously described as a marker of aggressive tumor invasion and poor prognosis in various malignancies [28–36]. Statistical comparison of the clinical features of the tumors that clustered in each of the subbranches, such as TNM stage, pathologic grade, or mortality, did not reveal any distinct differences among these groups.
BE specimens expressed two clearly discernable profiles, one of which was shared to a significant extent by the normal stomach mucosal biopsy samples (termed “BE1”; Figure 1) and another that was shared by a subgroup of AC samples (termed “BE2”; Figure 1). BE1 included several genes that have been implicated as tumor-suppressor genes, most notably trefoil factor 1 (TFF-1) [18,37–46]. BE2 included several genes that have been previously shown to be overexpressed in cancer such as carboxylesterase 2 (CES-2), galectin-4, glycoprotein A33, and liver-intestine cadherin (LI cadherin) [13,22,47–49]. Interestingly, this subgroup of cancers that shared the BE2 profile also expressed intestinal-like genes such as A33 and LI cadherin, and are unique from the highly proliferative subset. We also identified genes that were coexpressed between BE and normal esophagus, notably CK-4, CK-5, CK-6a, CK-6b, and CK-13 to CK-16, as well as S100A2 and S100A7 to S100A9. Thus, BE is molecularly similar to stomach and, in part, esophagus, as well as a small subgroup of the carcinomas.
Subgroup analysis was performed to compare the expression profiles of all BE and AC specimens combined with that of all normal esophageal and stomach tissues. Genes were manually ranked by both foldchange and t-test. Several genes emerged as being highly expressed in the BE and AC specimens, but showed a low level of expression in normal esophagus and stomach. These included claudin 3, RhoGTPase 8, highly expressed in cancer/rich in heptad repeats (HEC), and E2F-3.
Supervised Classification of Tissue Specimens
The results of agglomerative clustering suggested that specimens representing either unique cell types (e.g., normal esophagus versus stomach) or normal versus metaplastic (e.g., BE) versus neoplastic lesions (e.g., AC) could be readily differentiated. To more carefully evaluate the molecular differences between tissues and cell types, a supervised learning analysis was performed.
For these experiments, the group membership of each specimen was defined, and a k-NN algorithm was used to assess the extent to which specimens in the four groups could correctly be classified by leave-out-one cross-validation (LOOCV). Supervised classification with a 100-gene classifier correctly predicted all of the samples according to their specified group membership, confirming and extending the results of unsupervised clustering. Although the k-NN method used different genes to build a predictive model during each successive loop of the LOOCV procedure, the same genes were typically common to each model.
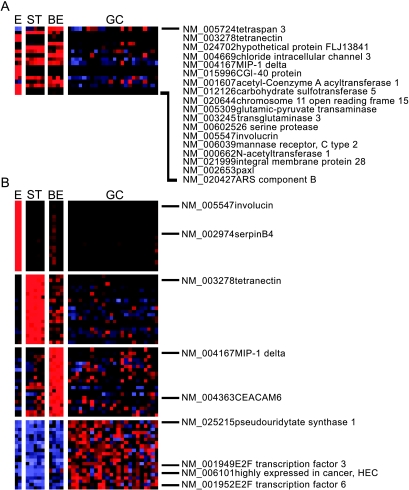
The 18 genes used in all models are depicted in Figure 2A, and the top 20 genes that best discriminated each of the groups by a S2N metric are depicted in Figure 2B. Although we could classify BE from all of the other tissue samples by supervised classification, we found very few instances of genes with BE unique expression. Genes that were strongly expressed in these lesions were also typically expressed to some degree in one or more normal stomach or AC specimens.
Figure 2.
Supervised classification of tissue specimens. Tissue samples were divided into four groups (E = esophagus, ST = stomach, BE = Barrett's esophagus, GC = adenocarcinoma) and subjected to supervised learning using k-NN. The 18 genes in panel A are those that were used in all models to correctly predict all of the tissue samples correctly. Panel B depicts the 20 genes per group best correlated with the distinction between the four types of tissue with a S2N ranking metric.
In an effort to identify sequences that were most predominantly expressed in BE, genes were manually ranked according to their near-uniform expression in BE, selecting against those with expression in normal tissues to the highest extent possible (see Materials and Methods). Expression in AC was also selected against; however, this criterion was less stringent. This analysis yielded 21 genes as highly and near-uniformly positive in all BE specimens, with low expression in most AC specimens (Table 2). Many of these have been previously described as being specific to human intestinal epithelium [50–57]. Genes with high expression in BE that exhibited low or absent expression in 21 of 25 AC specimens included sucrase-isomaltase and microsomal aminopeptidase (CD-13). Other genes, such as fatty acid binding protein 1 (FABP-1) and meprin Aα (PABA peptide hydrolase), exhibited low or absent expression in 17 of 25 AC specimens.
Table 2.
Genes Predominantly Expressed in Barrett's Esophagus.
| Microsomal aminopeptidase |
| Sucrase-isomaltase |
| Hepatocellular carcinoma antigen gene 520 |
| Enterokinase |
| FABP-1 |
| Meprin A (alpha PABA peptide hydrolase) |
| GW112 |
| Carbohydrate (N-acetylglucosamine 6-O) sulfotransferase 5 (CHST5) |
| Hypothetical protein FLJ22800 (FLJ22800) |
| Claudin 15 |
| MIP-1 delta |
| Breast cancer resistance protein (BCRP) |
| FLJ22893 fis, clone KAT04792 |
| MGC:12387 |
| Liver-intestine cadherin |
| Mucin 5, subtype B, tracheobronchial |
| Vanin 1 |
| Cystic fibrosis transmembrane conductance regulator, ATP-binding cassette (subfamily C, member 7) (CFTR) |
| Zinc finger protein (ZNFB7) |
| Caudal type homeobox transcription factor 1 (CDX1) |
| Retinoic acid receptor responder (tazarotene-induced) 1 |
IHC
To extend the results of the expression profiles generated by the microarray analysis, immunohistochemisty was performed. One gene from each of the two sets of molecular profiles previously described as expressed in BE (from BE1, TFF-1; from BE2, CES-2) was selected for further study. An additional gene found in the profile unique to BE (CD-13) was included in this analysis.
TFF-1 Staining with monoclonal antibody to TFF-1 was performed on 15 esophageal biopsy specimens demonstrating a variety of epithelial subtypes, including normal squamous, gastric cardia-like, specialized intestinal metaplasia (BE), and BE with LGD or HGD. In addition, primary esophageal and gastric AC tumor specimens procured from a variety of geographic regions including the University of Virginia, Indiana University, Johns Hopkins University, as well as from Siena, Italy, were examined on TMA analysis. Collectively, these TMAs contained 266 primary tumor specimens and 42 xenografted specimens from 293 individual patients. Among these were 16 tissue specimens included in the oligonucleotide microarray hybridization analysis. Primary tumor sites included the esophagus (n = 30), EGJ (n = 79), and stomach (n = 114), which were subdivided in 46 cases into the proximal stomach (n = 8), midstomach (n = 21), and distal stomach (n = 17).
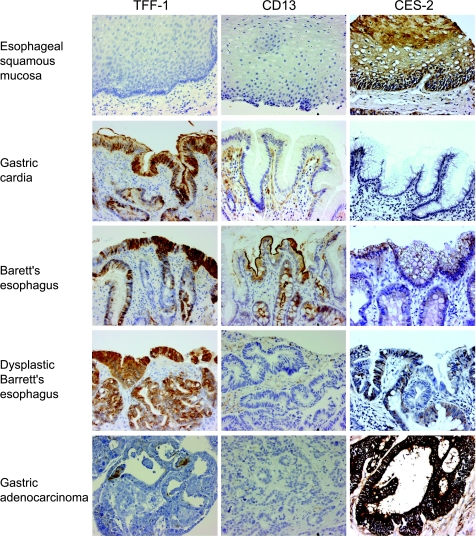
In examining the representative tissue biopsies, gastric cardia-like tissue was visualized in eight specimens and stained markedly positive for the presence of TFF-1 in each. BE without dysplasia stained markedly positive in three of five cases, and each of the remaining two cases stained at least weakly positive. In the five cases of BE with LGD or HGD, four stained markedly positive and one stained strongly positive. Staining intensity did not correlate with degree of dysplasia (Figure 3).
Figure 3.
Immunohistochemical analysis. Immunohistochemistry staining of trefoil factor 1 (TFF-1), microsomal aminopeptidase (CD-13), and Carboxylesterase 2 (CES-2) in representative specimens of normal esophagus, normal stomach, Barrett's esophagus, Barrett's esophagus with dysplasia, and adenocarcinoma.
In contrast, staining for TFF-1 was found to be negative or weakly positive in 248 (84.6%) of the 293 AC specimens on the TMAs: 213 demonstrated an IHC score of 0, and 35 had an IHC score of 1 (Table 3). Only 45 (15.4%) of the AC specimens stained at least moderately positive for the presence of TFF-1. This ratio was consistent across all anatomic sites of tumor origin (Table 3). As histopathologic grade worsened, TFF-1 expression decreased somewhat: well-differentiated (n = 12), moderately differentiated (n = 33), and poorly differentiated (n = 88) tumors had negative or weak expression in 75.0%, 81.8%, and 84.1% of specimens, respectively. However, this trend was not shown to be statistically significant (P = .73).
Table 3.
Immunohistochemistry Scores of Tissue Microarrays of Adenocarcinomas by Anatomic Site of Origin.
| IHC Score | “Negative-to-weak” 0 + 1 (%) | “Moderate-to-marked” 2 + 3 (%) |
| TFF-1 staining | ||
| Esophagus (n = 31) | 24 (80.0%) | 6 (20.0%) |
| EG Jxn (n = 72) | 62 (78.5%) | 17 (21.5%) |
| Stomach (n = 103) | 96 (84.2%) | 18 (15.8%) |
| CES-2 staining | ||
| Esophagus (n = 28) | 13 (46.4%) | 15 (53.6%) |
| EG Jxn (n = 80) | 33 (41.3%) | 47 (58.7%) |
| Stomach (n = 115) | 51 (42.2%) | 70 (57.8%) |
Of the 119 combined cases of esophageal, EGJ, and proximal gastric ACs, 13 were known definitively to have arisen in association with biopsy-proven BE. Subgroup analysis of tissue from these cases demonstrated that nine (69.2%) had negative or weak staining for the presence of TFF-1. In the remaining 106 cases, for which association with BE was either not present or unknown, 84 (79.2%) had negative or weak staining. This difference was not shown to be statistically significant (P = .41).
CES-2 TMAs that included 281 primary tumor specimens and 42 xenografted specimens from 306 individual patients were studied with an antibody to CES-2. Among these were 17 tissue specimens included in the oligonucleotide microarray hybridization analysis. Primary tumor sites included the esophagus (n = 28), EGJ (n = 80), and stomach (n = 121), which were subdivided into 54 cases into the proximal stomach (n = 9), midstomach (n = 21), and distal stomach (n = 24).
As expected, CES-2 staining was weak in the representative normal gastric specimens. All of the BE tissues that were examined demonstrated at least weak staining for CES-2 (Figure 3). This was expected, given the high level of expression of CES-2 by the BE specimens in the microarray analysis.
At least moderately positive staining for CES-2 was seen in 176 (57.5%) of all AC specimens combined (Table 3). There was no variation across anatomic sites: 15 (53.6%) esophagus, 47 (58.7%) EGJ, and 70 (57.8%) gastric (Table 3). Well-differentiated (n = 11) and moderately differentiated (n = 34) tumors had moderate to marked expression in 63.6% and 67.6% of specimens, respectively. However, poorly differentiated (n = 86) tumors had moderate to marked expression in only 41.8%. The difference between well- and moderately differentiated tumors, compared to poorly differentiated tumors, was shown to be statistically significant (P = .026). Of 95 Italian specimens, 47 (49.5%) demonstrated at least moderate positive staining, compared with 129 (61.1%) of 211 American specimens. This was not shown to be statistically significant (P = .056).
Of the 114 combined cases of esophageal, EGJ, and proximal gastric ACs, 23 definitively arose in association with biopsy-proven BE. Subgroup analysis of tissue from these cases demonstrated that 18 (78.3%) stained either moderately or markedly for the presence of CES-2 by IHC. In the remaining 91 cases, for which association with BE was either not present or unknown, 42 (46.2%) stained either moderately or markedly positive. This difference was shown to be statistically significant (P = .022).
CD-13 Staining of esophageal and gastric epithelial cells was negative throughout for CD-13, whereas duodenal epithelium and BE stained markedly positive (Figure 3). Histiocytes and stromal cells in each of these types of specimens stained positive as well. Ten (71.4%) of 14 AC specimens with gene expression profile analysis demonstrated either negative (n = 8) or weak (n = 2) staining. Only four (28.6%) stained either moderately or markedly positive (IHC score ≥2) for the presence of CD-13. These results were consistent with those of the oligonucleotide microarray hybridization analysis, which demonstrated that CD-13 expression was high in BE, with decreased expression in both normal and neoplastic specimens.
Discussion
Agglomerative clustering analysis demonstrated a strong relationship between the normal esophageal and gastric specimens, as well as a similarly strong relationship among the AC specimens. Normal esophageal specimens exhibited expression of genes specific to stratified squamous epithelial cells, and specimens derived from normal stomach expressed genes known to be specific to this tissue as expected. Primary tumors and xenografts demonstrated a high relative expression of multiple known oncogenes. Supervised classification of tissue specimens confirmed that the separations observed by agglomerative clustering were primarily driven by the intrinsic transcriptional program of epithelial cells in each specimen, rather than from contamination of specimens by inflammatory or stromal cells.
The BE specimens were highly associated with one another on agglomerative clustering, with a unique gene expression profile distinct from normal and AC specimens. Supervised classification demonstrated these same general features of BE, with shared expression of genes with normal columnar gastric epithelium, and, to a lesser extent, with stratified squamous esophageal epithelium. The 21 sequences that were relatively unique to BE contained several genes known to be expressed in intestinal epithelium.
The presence of two clearly discernable profiles: “BE1,” which was shared by stomach mucosal samples, and “BE2,” which was shared by AC samples, is consistent with BE occurring as an intermediate step in the normal mucosa-to-AC sequence. One could hypothesize that only a fine balance between the expression of tumor-suppressor genes from BE1 and the expression of oncogenes from BE2 limits the malignant potential of any individual BE lesion. In this exploratory study, none of the four BE specimens analyzed with the microarray technique contained any degree of dysplastic epithelium. Further analyses are warranted to examine an increased number of BE specimens with varying degrees of dysplasia, as well as from some individuals who have progressed to AC. The relative expression of BE1 and BE2 sequences in these varying circumstances may eventually prove useful in risk stratification of these patients.
TFF-1
As was predicted by the results of the microarray analysis, IHC demonstrated absent or minimal expression of the TFF-1 protein product in the vast majority (84.6%) of the 293 combined esophageal and gastric AC specimens. In addition, staining of normal stomach and BE, with and without dysplasia, confirmed an abundant expression of TFF-1 in these tissues. This is the first report of TFF-1 loss being involved in the transformation of BE to AC.
TFF-1 has been extensively characterized as a tumor-suppressor gene in gastric AC, with decreased expression demonstrated at both the mRNA and protein levels [18,37–39,41–43,45]. In addition, TFF-1 knockout mice have been observed to develop gastric lesions [40]. The results of this study indicate that loss of TFF-1 may be involved in the development of BE-associated malignancies. Given its apparent abundant expression in BE with varying degrees of dysplasia, but decreased expression in AC, it appears that loss of TFF-1 expression may be one of the genetic alterations encountered in the transition to invasive AC. This finding has potential important biologic as well as practical implications on the controversial issue of treatment of patients with BE and HGD. Management of such individuals can consist either of aggressive medical therapy and surveillance endoscopy in some centers, or automatic referral for esophagectomy in others. The use of a biomarker such as loss of TFF-1 may aid in the ability to predict the likelihood of progression to malignancy in these patients, and help guide treatment decisions in the future. Validation studies are planned to demonstrate this potential clinical utility.
CES-2
As predicted by the microarray analysis, overexpression of CES-2 protein product in BE was confirmed by IHC. In addition, a major fraction (57.5%) of AC demonstrated significant expression of CES-2. Noteworthy, of the AC specimens that definitively arose in association with BE, 78.3% demonstrated abundant expression of CES-2. This was statistically significantly higher than that seen with AC specimens for which association with BE had not been established (46.2%) (P = .022). These results suggest that the differentiation of normal stratified squamous esophageal mucosa into BE is accompanied by increased expression of CES-2. In addition, it appears that CES-2 continues to be overexpressed in a substantial majority of AC that arise from BE. To our knowledge, this is the first report of CES-2 being preferentially expressed in malignancies known to occur in association with BE.
CES-2 is a hydrolytic enzyme present in a wide variety of organs and tissues. The highest expression is seen in cells of the liver, small intestine, adrenal cortex, and renal proximal tubule [22]. It has also been identified as the key enzyme in the conversion of the chemotherapeutic agent irinotecan—currently utilized in the treatment of malignancies such as colorectal and gastric AC—into its active metabolite. Recent literature suggests that expression of CES-2 in target tumor tissue may correlate with the efficacy of irinotecan therapy [49].
Thus, the presence of CES-2 expression in cancers known to have developed in the setting of BE may ultimately be utilized as a guide for the use of, and predictor of therapeutic response to, irinotecan. This could be particularly applicable in patients with esophageal, EGJ, or proximal gastric AC who are at high risk for complications from surgical intervention, and in whom chemotherapy, external beam radiation, and therapeutic endoscopy are currently the only available treatment options, with limited efficacy. Once validated for clinical utility, CES-2 expression in BE-associated cancers may add to our armamentarium to combat this lethal disease.
Conclusions
This comprehensive utilization of microarray technology to study BE, ACs that arise from this lesion, and normal tissues has facilitated the identification of signature gene expression alterations in these cases for further characterization. The analysis examined the expression of over 22,000 sequences in 38 specimens from all relevant tissue subtypes, including normal epithelium and xenografted tumors, for optimal samples to analyze molecularly. Furthermore, confirmatory analysis was performed, with IHC utilizing TMAs containing nearly 300 tissue specimens of tumors from various sites and pathologic stages from four institutions.
BE-associated AC is a significant source of expense, morbidity, and mortality in developed nations. Further knowledge of the molecular mechanisms responsible for the metaplasia-to-neoplasia sequence may lead to improved understanding of this disease and, eventually, better outcomes. The results of this study reveal novel, enticing findings regarding the unique molecular profile of BE as it compares with normal epithelial tissue and AC of the esophagus, EGJ, and stomach. Future analyses are planned to validate potential biomarkers such as TFF-1 and CES-2 in more expanded studies.
Acknowledgements
The authors would like to acknowledge the technical assistance of Jeffrey C. Harper and Narendra Sankpal.
Footnotes
Support for this project was provided by NIH grant CA67900 (S.M.P.).
References
- 1.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia [comment] JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 2.Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993;104:510–513. doi: 10.1016/0016-5085(93)90420-h. [DOI] [PubMed] [Google Scholar]
- 3.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma [see comment] N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins GJ, Doak SH, Parry JM, D'Souza FR, Griffiths AP, Baxter JN. Genetic pathways involved in the progression of Barrett's metaplasia to adenocarcinoma. Br J Surg. 2002;89:824–837. doi: 10.1046/j.1365-2168.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- 5.Beilstein M, Silberg D. Cellular and molecular mechanisms responsible for progression of Barrett's metaplasia to esophageal carcinoma. Gastroenterol Clin North Am. 2002;31:461–479. doi: 10.1016/s0889-8553(02)00013-4. ix. [DOI] [PubMed] [Google Scholar]
- 6.Winters C, Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF, III, Johnson DA, Cruess DF, Cotelingam JD, Gurney MS. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 7.Spechler SJ, Zeroogian JM, Antonioli DA, Wang HH, Goyal RK. Prevalence of metaplasia at the gastro-oesophageal junction. Lancet. 1994;344:1533–1536. doi: 10.1016/s0140-6736(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 8.Shahin W, Murray JA. Esophageal cancer and Barrett's esophagus. How to approach surveillance, treatment, and palliation. Postgrad Med. 1999;105:111–114. 119–122, 125–127. doi: 10.3810/pgm.1999.06.619. [DOI] [PubMed] [Google Scholar]
- 9.Kim B, Bang S, Lee S, Kim S, Jung Y, Lee C, Choi K, Lee SG, Lee K, Lee Y, et al. Expression profiling and subtype-specific expression of stomach cancer. Cancer Res. 2003;63:8248–8255. [PubMed] [Google Scholar]
- 10.Tay ST, Leong SH, Yu K, Aggarwal A, Tan SY, Lee CH, Wong K, Visvanathan J, Lim D, Wong WK, et al. A combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypes. Cancer Res. 2003;63:3309–3316. [PubMed] [Google Scholar]
- 11.Hippo Y, Taniguchi H, Tsutsumi S, Machida N, Chong JM, Fukayama M, Kodama T, Aburatani H. Global gene expression analysis of gastric cancer by oligonucleotide microarrays. Cancer Res. 2002;62:233–240. [PubMed] [Google Scholar]
- 12.Selaru FM, Zou T, Xu Y, Shustova V, Yin J, Mori Y, Sato F, Wang S, Olaru A, Shibata D, et al. Global gene expression profiling in Barrett's esophagus and esophageal cancer: a comparative analysis using cDNA microarrays. Oncogene. 2002;21:475–478. doi: 10.1038/sj.onc.1205111. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Selaru FM, Yin J, Zou TT, Shustova V, Mori Y, Sato F, Liu TC, Olaru A, Wang S, et al. Artificial neural networks and gene filtering distinguish between global gene expression profiles of Barrett's esophagus and esophageal cancer. Cancer Res. 2002;62:3493–3497. [PubMed] [Google Scholar]
- 14.Barrett MT, Yeung KY, Ruzzo WL, Hsu L, Blount PL, Sullivan R, Zarbl H, Delrow J, Rabinovitch PS, Reid BJ. Transcriptional analyses of Barrett's metaplasia and normal upper GI mucosae. Neoplasia (New York) 2002;4:121–128. doi: 10.1038/sj.neo.7900221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menges M, Michaeli B, Pueschel W, Zeitz M, Meese E. The influence of the suppression of gastrooesophageal reflux on the gene expression pattern in Barrett's oesophagus. Int J Oncol. 2002;20:1323–1329. [PubMed] [Google Scholar]
- 16.McQueen HA, Wyllie AH, Piris J, Foster E, Bird CC. Stability of critical genetic lesions in human colorectal carcinoma xenografts. Br J Cancer. 1991;63:94–96. doi: 10.1038/bjc.1991.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn SA, Seymour AB, Hoque AT, Schutte M, da Costa LT, Redston MS, Caldas C, Weinstein CL, Fischer A, Yeo CJ, et al. Allelo-type of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995;55:4670–4675. [PubMed] [Google Scholar]
- 18.Beckler AD, Roche JK, Harper JC, Petroni G, Frierson HF, Jr, Moskaluk CA, El-Rifai W, Powell SM. Decreased abundance of trefoil factor 1 transcript in the majority of gastric carcinomas. Cancer. 2003;98:2184–2191. doi: 10.1002/cncr.11789. [DOI] [PubMed] [Google Scholar]
- 19.Welsh JB, Sapinoso LM, Su AI, Kern SG, Wang-Rodriguez J, Moskaluk CA, Frierson HF, Jr, Hampton GM. Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 2001;61:5974–5978. [PubMed] [Google Scholar]
- 20.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, Sallan SE, Lander ES, Golub TR, Korsmeyer SJ. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30:41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Xu G, McLeod HL. Comprehensive evaluation of carboxylesterase-2 expression in normal human tissues using tissue array analysis. Appl Immunohistochem Mol Morphol. 2002;10:374–380. doi: 10.1097/00129039-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Hourihan RN, O'Sullivan GC, Morgan JG. Transcriptional gene expression profiles of oesophageal adenocarcinoma and normal oesophageal tissues. Anticancer Res. 2003;23:161–165. [PubMed] [Google Scholar]
- 24.Viaene AI, Baert JH. Expression of cytokeratin mRNAs in normal human esophageal epithelium. Anat Rec. 1995;241:88–98. doi: 10.1002/ar.1092410112. [DOI] [PubMed] [Google Scholar]
- 25.Abrams CK, Hamosh M, Lee TC, Ansher AF, Collen MJ, Lewis JH, Benjamin SB, Hamosh P. Gastric lipase: localization in the human stomach. Gastroenterology. 1988;95:1460–1464. doi: 10.1016/s0016-5085(88)80063-5. [DOI] [PubMed] [Google Scholar]
- 26.Levine JS, Nakane PK, Allen RH. Human intrinsic factor secretion: immunocytochemical demonstration of membrane-associated vesicular transport in parietal cells. J Cell Biol. 1981;90:644–655. doi: 10.1083/jcb.90.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oien KA, Vass JK, Downie I, Fullarton G, Keith WN. Profiling, comparison and validation of gene expression in gastric carcinoma and normal stomach. Oncogene. 2003;22:4287–4300. doi: 10.1038/sj.onc.1206615. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez MA, Pinder SE, Callagy G, Vowler SL, Morris LS, Bird K, Bell JA, Laskey RA, Coleman N. Minichromosome maintenance protein 2 is a strong independent prognostic marker in breast cancer. J Clin Oncol. 2003;21:4306–4313. doi: 10.1200/JCO.2003.04.121. [DOI] [PubMed] [Google Scholar]
- 29.Isozaki H, Okajima K, Ichinona T, Fujii K, Nomura E, Izumi N, Takeda Y. Significance of proliferating cell nuclear antigen (PCNA) expression in gastric cancer in relation to lymph node metastasis. J Surg Oncol. 1996;61:106–110. doi: 10.1002/(SICI)1096-9098(199602)61:2<106::AID-JSO4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Kato H, Miyazaki T, Fukai Y, Nakajima M, Sohda M, Takita J, Masuda N, Fukuchi M, Manda R, Ojima H, et al. A new proliferation marker, minichromosome maintenance protein 2, is associated with tumor aggressiveness in esophageal squamous cell carcinoma. J Surg Oncol. 2003;84:24–30. doi: 10.1002/jso.10287. [DOI] [PubMed] [Google Scholar]
- 31.Kodani I, Osaki M, Shomori K, Araki K, Goto E, Ryoke K, Ito H. Minichromosome maintenance 2 expression is correlated with mode of invasion and prognosis in oral squamous cell carcinomas. J Oral Pathol Med. 2003;32:468–474. doi: 10.1034/j.1600-0714.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 32.Kruger S, Thorns C, Stocker W, Muller-Kunert E, Bohle A, Feller AC. Prognostic value of MCM2 immunoreactivity in stage T1 transitional cell carcinoma of the bladder. Eur Urol. 2003;43:138–145. doi: 10.1016/s0302-2838(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 33.Luan F, Wang M, You W. The correlation of TGF-alpha, EGFR in precancerous lesions and carcinoma of stomach with PCNA expression. Chung-Hua Ping Li Hsueh Tsa Chih (Chin J Pathol) 1997;26:31–34. [PubMed] [Google Scholar]
- 34.Matturri L, Biondo B, Cazzullo A, Colombo B, Giordano F, Guarino M, Pallotti F, Turconi P, Lavezzi AM. Prognostic significance of different biological markers (DNA index, PCNA index, apoptosis, p53, karyotype) in 126 adenocarcinoma gastric biopsies. Anticancer Res. 1998;18:2819–2825. [PubMed] [Google Scholar]
- 35.Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, Nakagawara A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003;63:4167–4173. [PubMed] [Google Scholar]
- 36.Wagner KW, Sapinoso L, El-Rifai W, Frierson HF, Jr, Butz N, Mestan J, Hampton GM, Deveraux QL, Hampton GM. Overexpression, genomic amplification and therapeutic potential of inhibiting the UbcH10 ubiquitin conjugase in human carcinomas of diverse anatomic origin. Oncogene. 2004;23:6621–6629. doi: 10.1038/sj.onc.1207861. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho R, Kayademir T, Soares P, Canedo P, Sousa S, Oliveira C, Leistenschneider P, Seruca R, Gott P, Blin N, et al. Loss of heterozygosity and promoter methylation, but not mutation, may under-lie loss of TFF1 in gastric carcinoma. Lab Invest. 2002;82:1319–1326. doi: 10.1097/01.lab.0000029205.76632.a8. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto J, Yasui W, Tahara H, Tahara E, Kudo Y, Yokozaki H. DNA hypermethylation at the pS2 promoter region is associated with early stage of stomach carcinogenesis. Cancer Lett. 2000;149:125–134. doi: 10.1016/s0304-3835(99)00349-3. [DOI] [PubMed] [Google Scholar]
- 39.Kirikoshi H, Katoh M. Expression of TFF1, TFF2 and TFF3 in gastric cancer. Int J Oncol. 2002;21:655–659. [PubMed] [Google Scholar]
- 40.Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, LeMeur M, Wendling C, Tomasetto C, Chambon P, Rio MC. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein [see comment] Science. 1996;274:259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 41.Luqmani Y, Bennett C, Paterson I, Corbishley CM, Rio MC, Chambon P, Ryall G. Expression of the pS2 gene in normal, benign and neoplastic human stomach. Int J Cancer. 1989;44:806–812. doi: 10.1002/ijc.2910440510. [DOI] [PubMed] [Google Scholar]
- 42.May FE, Westley BR. Trefoil proteins: their role in normal and malignant cells. J Pathol. 1997;183:4–7. doi: 10.1002/(SICI)1096-9896(199709)183:1<4::AID-PATH1099>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Park WS, Oh RR, Park JY, Lee JH, Shin MS, Kim HS, Lee HK, Kim YS, Kim SY, Lee SH, et al. Somatic mutations of the trefoil factor family 1 gene in gastric cancer. Gastroenterology. 2000;119:691–698. doi: 10.1053/gast.2000.16483. [DOI] [PubMed] [Google Scholar]
- 44.Park WS, Oh RR, Park JY, Yoo NJ, Lee SH, Shin MS, Kim SY, Kim YS, Lee JH, Kim HS, et al. Mapping of a new target region of allelic loss at 21q22 in primary gastric cancers. Cancer Lett. 2000;159:15–21. doi: 10.1016/s0304-3835(00)00525-5. [DOI] [PubMed] [Google Scholar]
- 45.Poulsom R, Hanby AM, Lalani EN, Hauser F, Hoffmann W, Stamp GW. Intestinal trefoil factor (TFF 3) and pS2 (TFF 1), but not spasmolytic polypeptide (TFF 2) mRNAs are co-expressed in normal, hyperplastic, and neoplastic human breast epithelium. J Pathol. 1997;183:30–38. doi: 10.1002/(SICI)1096-9896(199709)183:1<30::AID-PATH1085>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Yamachika T, Werther JL, Bodian C, Babyatsky M, Tatematsu M, Yamamura Y, Chen A, Itzkowitz S. Intestinal trefoil factor: a marker of poor prognosis in gastric carcinoma. Clin Cancer Res. 2002;8:1092–1099. [PubMed] [Google Scholar]
- 47.Hippo Y, Yashiro M, Ishii M, Taniguchi H, Tsutsumi S, Hirakawa K, Kodama T, Aburatani H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001;61:889–895. [PubMed] [Google Scholar]
- 48.Sakamoto J, Kojima H, Kato J, Hamashima H, Suzuki H. Organ-specific expression of the intestinal epithelium-related antigen A33, a cell surface target for antibody-based imaging and treatment in gastrointestinal cancer. Cancer Chemother Pharmacol. 2000;46:S27–S32. doi: 10.1007/pl00014045. [DOI] [PubMed] [Google Scholar]
- 49.Xu G, Zhang W, Ma MK, McLeod HL. Human carboxylesterase 2 is commonly expressed in tumor tissue and is correlated with activation of irinotecan. Clin Cancer Res. 2002;8:2605–2611. [PubMed] [Google Scholar]
- 50.Wu GD, Beer DG, Moore JH, Orringer MB, Appelman HD, Traber PG. Sucrase-isomaltase gene expression in Barrett's esophagus and adenocarcinoma. Gastroenterology. 1993;105:837–844. doi: 10.1016/0016-5085(93)90902-o. [DOI] [PubMed] [Google Scholar]
- 51.Bonner CA, Loftus SK, Wasmuth JJ. Isolation, characterization, and precise physical localization of human CDX1, a caudal-type homeobox gene. Genomics. 1995;28:206–211. doi: 10.1006/geno.1995.1132. [DOI] [PubMed] [Google Scholar]
- 52.Moore JH, Lesser EJ, Erdody DH, Natale RB, Orringer MB, Beer DG. Intestinal differentiation and p53 gene alterations in Barrett's esophagus and esophageal adenocarcinoma. Int J Cancer. 1994;56:487–493. doi: 10.1002/ijc.2910560406. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, Kazenwadel J, James R. Isolation and characterization of the murine homeobox gene Cdx-1. Regulation of expression in intestinal epithelial cells. J Biol Chem. 1993;268:27214–27225. [PubMed] [Google Scholar]
- 54.Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium [see comment] Gastroenterology. 1997;113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 55.Eggermont E, Molla AM, Tytgat G, Rutgeerts L. Distribution of enterokinase activity in the human intestine. Acta Gastroenterol Belg. 1971;34:655–662. [PubMed] [Google Scholar]
- 56.Dumermuth E, Sterchi EE, Jiang WP, Wolz RL, Bond JS, Flannery AV, Beynon RJ. The astacin family of metalloendopeptidases. J Biol Chem. 1991;266:21381–21385. [PubMed] [Google Scholar]
- 57.Gessner R, Tauber R. Intestinal cell adhesion molecules. Liver-intestine cadherin. Ann NY Acad Sci. 2000;915:136–143. doi: 10.1111/j.1749-6632.2000.tb05236.x. [DOI] [PubMed] [Google Scholar]