Abstract
The aim of this study was to investigate the effect of treatment of experimental ovarian cancers with targeted cytotoxic analogs as single compounds and in combination. Targeted cytotoxic analogs of bombesin (AN-215), somatostatin (AN-238), and luteinizing hormone-releasing hormone (AN-207) consisted of 2-pyrrolinodoxorubicin (AN-201) linked to the respective peptide carrier. AN-238 at 200 nmol/kg significantly inhibited growth of UCI-107, ES-2 and OV-1063 ovarian cancers. AN-215 alone at 200 nmol/kg and its combination with AN-238 at one-half of the dose were also able to inhibit the growth of UCI-107 tumors. A combination of AN-238 with AN-207at 50% of the dose strongly suppressed the proliferation of ES-2 and OV-1063 ovarian tumors. Cytotoxic radical AN-201 was toxic and had no significant effect on tumor growth. In contrast, the toxicity of the conjugated peptide analogs was low. Because ovarian cancers tend to acquire chemoresistance, we used real-time PCR to measure the mRNA expression of multidrug resistance protein 1, multidrug resistance-related protein 1, and breast cancer resistance protein after treatment. Low or no induction of multidrug resistance protein 1, multidrug resistance-related protein, and breast cancer resistance protein occurred after treatment with AN-238, AN-215, and the combination of AN-238 with AN-207 or AN-215. These results demonstrate that a therapy with cytotoxic analogs such as single agents and combinations is effective and nontoxic. Our work suggests that cytotoxic peptide analogs of luteinizing hormone-releasing hormone, somatostatin, and bombesin could be used for the therapy of ovarian cancers, considering the lack of induction of chemoresistance.
Ovarian cancer is the most common cause of death from gynecological malignancies in the United States (1). In more than two-thirds of the cases, patients present with late-stage disease at the time of initial diagnosis. Advanced epithelial ovarian cancer is currently treated by cytoreductive surgery and chemotherapy (2). The preferred systemic treatment for patients with advanced-stage disease is the paclitaxel/carboplatin combination, which produces a significant improvement in both progression-free and overall survival rates (3). However, after an initial response (4), patients eventually experience a recurrence of the disease (5) due to secondary induction of chemoresistance of the cancer cells. This phenomenon is reflected by the 5-year survival rate of 37% for FIGO (International Federation of Gynecology and Obstetrics) stage III disease and 25% for FIGO stage IV disease. In addition, the systemic administration of cytotoxic agents is usually accompanied by toxic side effects (2, 4). Consequently, the therapy of late-stage ovarian cancer remains a challenge, and new treatment approaches are needed.
The elucidation of specific molecular characteristics of tumor cells led to the development of a new treatment strategy known as targeted therapy. Modern targeted anticancer drugs include antibodies against surface structures on malignant cells and conjugates consisting of receptor-specific ligands linked to toxins, radionuclides, or chemotherapeutic agents (6). Thus, the antineoplastic drugs can be delivered directly to cancer cells. Their higher intratumoral concentration is expected to result in a greater antitumor efficacy and reduced systemic toxicity and may overcome chemoresistance of malignant cells. In recent years, several cytotoxic hormone analogs were synthesized in our laboratory, in an endeavor to develop a new class of antineoplastic agents (7). These compounds include the cytotoxic analogs of bombesin (AN-215), somatostatin (AN-238), and luteinizing hormone-releasing hormone (LHRH) (AN-207), which were synthesized essentially by coupling 2-pyrrolinodoxorubicin (2-pyrrolino-DOX) (AN-201) to the respective hormone analogs (8). Because the receptors for LHRH, somatostatin, and bombesin are present in 80%, 65%, and 77% of ovarian cancers, respectively (9–11), these binding sites could be used as targets for the cytotoxic hormone analogs.
Multidrug resistance (MDR) in cancer cells is due to the simultaneous development of resistance to a variety of antitumor agents that appear to be structurally and functionally unrelated. One mechanism of action of MDR is the increased efflux of chemotherapeutic agents mediated by transport proteins. The product of the MDR-1 gene, an ATP-dependent membrane transporter termed P-glycoprotein, and the recently discovered MDR-related protein 1 (MRP-1) use this mechanism of action (12, 13). Breast cancer resistance protein (BCRP) is another overlapping, but distinct type of MDR, based on drug efflux (14). Ovarian cancers show the tendency to acquire chemoresistance throughout therapy, and it was demonstrated in vitro that the MDR-1 gene could induced by chemotherapeutic agents, such as DOX, cisplatin, and paclitaxel (15). MRP expression was shown to be associated with a significantly (P < 0.05) poorer prognosis in patients with ovarian cancer (16).
The aim of this study was to evaluate the antiproliferative activity of the cytotoxic hormone analogs AN-207, AN-215, and AN-238 as single compounds and in some combinations in experimental ovarian cancers. The effect of targeted therapy on the development of chemoresistance through the induction of the MDR-1, MRP-1, and BCRP proteins was also investigated.
Results
Effects of Treatment with Cytotoxic Compounds on Tumor Growth in Vivo.
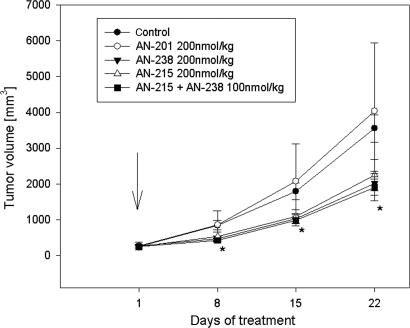
In Experiment 1, cytotoxic analogs AN-238 and AN-215 at a single dose of 200 nmol/kg and a combination of both compounds at 100 mmol/kg of each significantly inhibited the growth of UCI-107 human ovarian cancers. In animals treated with AN-238 and AN-215, tumor volume and weight were significantly reduced by 36.7–43.1% (P < 0.05) (Fig. 1 and Table 1). The combination of AN-238 and AN-215 at doses of 100 nmol/kg resulted in a 46.2% and 44.2% reduction, respectively, in tumor volume and weight (P < 0.01). The tumor doubling time was also significantly prolonged after treatment with AN-238 and the combination therapy (P < 0.05). An equimolar dose of the cytotoxic radical AN-201 had no significant effects on any growth characteristics (Fig. 1 and Table 1).
Fig. 1.
Effects of a single injection of the targeted cytotoxic bombesin analog AN-215, the targeted cytotoxic somatostatin analog AN-238, a combination of AN-215 and AN-238, and the cytotoxic radical AN-201 on the growth of UCI-107 human ovarian carcinoma xenografts. The arrow indicates treatment (∗, P < 0.05; two-tailed Student’s t test).
Table 1.
Effects of therapy with cytotoxic analogs of somatostatin (AN-238), LHRH (AN-207), bombesin (AN-215), and some combinations of these analogs as well as cytotoxic radical AN-201 on the growth of UCI-107, OV-1063, and ES-2 human ovarian carcinomas xenografted into nude mice
| Experiment and cell line | Treatment | Initial tumor volume, mm3 | Final tumor volume, mm3 (% inhibition) | Tumor weight, mg (% inhibition) | Tumor doubling time, days | WBC on day 8, cells per mm3 | Deaths in experiment groups |
|---|---|---|---|---|---|---|---|
| UCI-107 | Control | 254.4 ± 56.0 | 3,350.8 ± 378.1 | 3,779.1 ± 377.0 | 5.9 ± 0.5 | 9,460 ± 1081 | 0 |
| AN-238 | 258.3 ± 43.0 | 2,020.8 ± 332.1 (43.1)** | 2,192.3 ± 345.2 (42.0)** | 8.0 ± 0.9* | 8,332 ± 226 | 0 | |
| AN-215 | 256.4 ± 51.4 | 2,248.1 ± 437.8 (36.7)* | 2,391.1 ± 448.5 (36.7)* | 7.2 ± 0.4 | 8,580 ± 486 | 0 | |
| AN-215 + AN-238 | 246.5 ± 31.7 | 1,908.9 ± 380.7 (46.2)** | 2,108.2 ± 381.5 (44.2) | 9.9 ± 1.5* | 8,415 ± 244 | 0 | |
| AN-201 | 266.1 ± 115.3 | 4,037.2 ± 1,899.6 (+13.7) | 4,471.6 ± 2,127.3 (18.3) | 6.0 ± 0.9 | 5,720 ± 841* | 0 | |
| OV-1063 | Control | 62.1 ± 16.1 | 3,460.0 ± 832 | 4,646.3 ± 1,665.2 | 4.4 ± 0.3 | 7,260 ± 389 | 0 |
| AN-238 | 54.8 ± 18.9 | 1,130.1 ± 427.7 (67.3)* | 1,125.0 ± 305.9 (75.8) | 9.5 ± 0.7 | 6,490 ± 444 | 2/9 | |
| AN-238 + AN-207 | 51.7 ± 17.2 | 1,449.8 ± 526.1 (58.10)* | 1,433.8 ± 489.0 (69.1)* | 4.9 ± 0.3 | 6,682 ± 566 | 0 | |
| AN-201 | 55.3 ± 15.1 | 1,979.5 ± 735.5 (42.8) | 2,636.7 ± 1,148.8 (43.2) | 5.0 ± 0.6 | 5,527 ± 197* | 1/9 | |
| ES-2 | Control | 76.1 ± 16.1 | 316.6 ± 52.8 | 316.7 ± 51.2 | 11.9 ± 1.6 | 8,910 ± 548 | 0 |
| AN-238 | 72.1 ± 18.6 | 157.8 ± 30.2 (50.2)* | 211.2 ± 38.5 (33.3) | 26.6 ± 8.4* | 7,122.5 ± 960 | 2/9 | |
| AN-238 + AN-207 | 63 ± 14.4 | 147.4 ± 27.3 (53.5)** | 235 ± 45.4 (25.79) | 27.5 ± 7.8* | 7205 ± 484 | 0 | |
| AN-201 | 80.3 ± 14.0 | 291.8 ± 50.6 (7.9) | 308.6 ± 44.3 (2.56) | 13.8 ± 2.4 | 4372 ± 549** | 1/9 |
WBC levels and animal deaths are shown in the last two columns.
*, P < 0.05;
**, P < 0.01; two-tailed Student’s t test).
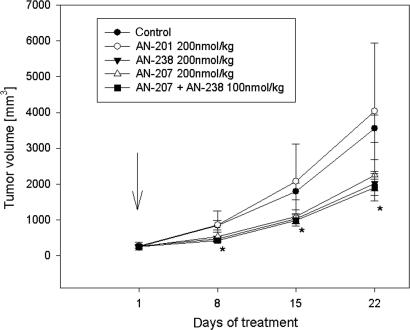
In Experiment 2, animals bearing OV-1063 tumors were injected three times (on days 1, 8, and 15) with 200 nmol/kg of cytotoxic analog AN-238 alone or with a combination of AN-238 with AN-207 at the doses of 100 nmol/kg of each. In animals treated with AN-238, the mean tumor volume was significantly reduced by 67.3% (P < 0.05), and the weight was reduced by 75.8% (Fig. 2 and Table 1). The simultaneous injection of 100 nmol/kg AN-207 and AN-238 significantly reduced tumor volume and weight by 58.1% and 69.1%, respectively (P < 0.05) (Fig. 2 and Table 1). The tumor doubling time was significantly prolonged after treatment with AN-238 (P < 0.05) (Table 1). An equimolar dose of the cytotoxic radical AN-201 had no significant effects on tumors (Fig. 2 and Table 1).
Fig. 2.
Effects of three injections of the targeted cytotoxic somatostatin analog AN-238, a combination of AN-238 and the targeted cytotoxic LHRH analog AN-207, and the cytotoxic radical AN-201 on the growth of OV-1063 human ovarian carcinoma xenografts. The arrow indicates treatment (∗, P < 0.05; two-tailed Student’s t test).
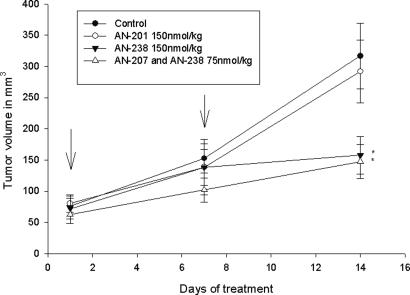
In Experiment 3, an injection of 150 nmol/kg AN-238 administered on days 1 and 8 significantly inhibited the growth of ES-2 human ovarian cancers, tumor volumes being >50% smaller than in controls (P < 0.05). The combination of 75 nmol/kg AN-238 and AN-207 on day 1 and 8 resulted in a 53.5% reduction of tumor volume (P < 0.01). Equimolar doses of the radical AN-201 had no significant effects on tumor growth (Fig. 3 and Table 1).
Fig. 3.
Effects of two injections of the targeted cytotoxic somatostatin analog AN-238, a combination of AN-238 and the targeted cytotoxic LHRH analog AN-207, and the cytotoxic radical AN-201 on the growth of ES-2 human ovarian carcinoma xenografts. The arrows indicate treatment (∗, P < 0.05; two-tailed Student’s t test).
Evaluation of MDR-1, MRP-1, and BCRP mRNA Expression by Real-Time PCR.
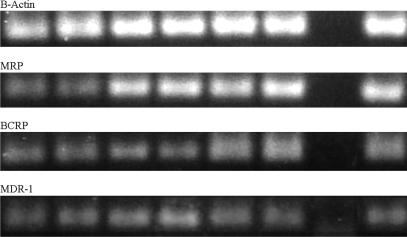
mRNA for MDR-1, MRP-1, and BCRP was detected in UCI-107, ES-2, and OV-1063 ovarian cancers. The PCR products were of the expected sizes of 95 bp for MDR-1, 127 bp for MRP-1, 140 bp for BCRP, and 140 bp for β-actin (data not shown). The efficiencies, E, were 1.989 for MDR-1, 1.999 for MRP-1, 1.987 for BCRP, and 1.997 for β-actin.
In OV-1063 tumors, administration of AN-201 caused a 2-fold induction of MDR-1 after three treatment cycles. In groups treated with AN-238 alone or combined with AN-207, no significant changes of MDR-1, MRP-1, and BCRP could be detected. In ES-2 tumors, therapy with AN-201 was associated with an 3.9-, 3.6-, and 2.2-fold induction of mRNA for MDR-1, MRP-1, and BCRP, respectively, after two treatment cycles. AN-238 alone or AN-238 in combination with AN-207 did not cause any up-regulation in ES-2 cells. In UCI-107 tumors, no induction of MDR-1, MRP-1, and BCRP was observed in any of the experimental groups (Fig. 4). In UCI-107 tumors, after two treatment cycles AN-201 did not induce MDR-1, MRP-1, or BCRP to a major extent. Similarly, AN-215, AN-238, and the combination of both in UCI-107 tumors or the combination of AN-238 with AN-207 in ES-2 tumors did not cause a major induction of MRD-1, MRP-1, and BCRP in any of the experiments (Fig. 4).
Fig. 4.
Expression for mRNA for the MDR-1, MRP-1, and BCRP in human ovarian cancer cell lines as revealed by real-time PCR. PCR products were separated by 1.8% agarose gel and stained with ethidium bromide. Lanes 1 and 2, OV-1063; lanes 3 and 4, ES-2; lanes 5 and 6, UCI-107; lane 7, negative control; lane 8, positive control.
Side Effects and Toxicity.
AN-238 and AN-215, as well as a mixture of AN-215 and AN-238 at a single dose of 200 nmol/kg, caused no significant decrease in the WBC in Experiment 1. In Experiments 2 and 3, AN-238 alone and in combination with AN-207 administered three times and twice, respectively, caused no significant decrease in WBC. AN-201, given at equimolar doses, significantly (P < 0.05) suppressed the WBC 8 days after the administration in all experiments (Table 1). Minor body weight loss compared with controls was observed on treatment day 8 after the injection of AN-238, AN-207, AN-215, and AN-201 in all experimental groups. However, on day 15, the body weights of the treated animals of all groups were no longer different from the control groups. No deaths because of drug-related toxicity occurred in Experiment 1. In both Experiments 2 and 3, two animals died in the groups treated with AN-238, and one animal died in each AN-201 group.
Discussion
The therapy of advanced ovarian cancer remains a challenge because throughout the treatment a high percentage of the tumors acquires resistance to chemotherapy (4). Drug resistance proteins such as MDR-1 and MRP-1 appear to be involved in this process (15, 16). Targeted chemotherapy was developed to selectively deliver cytotoxic radicals to malignant cells and, thus, to achieve a higher intratumoral concentration of cytotoxic drugs. This innovative treatment approach results in a higher antitumor activity and can overcome chemoresistance of some neoplastic cells. Accordingly, in previous studies with OV-1063 and ES-2 human ovarian carcinomas, we showed a strong inhibition of tumor growth after treatment with the targeted cytotoxic LHRH analogs AN-152 and AN-207, which consist of DOX or 2-pyrrolino-DOX linked to [d-Lys-6]-LHRH, respectively (17–20). The cytotoxic analogs of bombesin and somatostatin, AN-215 and AN-238, also caused a strong inhibition of growth of experimental ovarian cancers (21), whereas the nontargeted radicals DOX and 2-pyrrolino-DOX had only minor effects (17–21)
In the current study, the antitumor activity of our cytotoxic analogs AN-238, AN-215, and AN-207 given as single agents or in different combinations was investigated in three human ovarian cancer cell lines, which were shown to be positive for the respective receptors in our previous work (8). AN-215, AN-238, or their combination significantly inhibited the growth of UCI-107 tumors. AN-238 alone and in combination with AN-207 at different doses significantly inhibited the growth of OV-1063 and ES-2 human ovarian cancer xenografts. Previous work showed that AN-207, AN-215, and AN-238 given as single agents suppressed growth of experimental human ovarian cancers (7, 22). In agreement with previous studies, the cytotoxic radical AN-201 did not exert a significant tumor inhibition in any of the three experiments (7).
We compared toxicity of the cytotoxic analogs and their radicals in terms of the incidence of deaths and levels of WBC. Only six deaths occurred in three experiments, with four animals dying after treatment with AN-238 and two after AN-201. However, none of the deaths could be related to toxicity. Haematotoxicity is the most common syndrome and often dose-limiting effect of chemotherapy. Accordingly, in all experiments, cytotoxic radical AN-201 caused a significantly stronger suppression of the WBC than the targeted analogs.
We investigated different dosing schedules of the compounds and treatment combinations to establish the most favorable treatment protocol. With respect to efficacy, however, no major difference was observed between single and multiple dosing schedules. Surprisingly, AN-215 and AN-238 given as single agents or equimolar amount of a combination of AN-238 and AN-215, as well as AN-207 coadministered with AN-238, were all essentially equally potent in suppressing tumor growth. Our finding can be explained by the fact that all three of our animal models of human tumor cell lines strongly overexpressed the peptide receptors for the respective analogs. Tumor cell lines tend to have a more homogenous pattern of receptor expression than primary tumors. Thus, the intratumoral level of cytotoxic radical, after single agent or combination therapy, may have been similar in the most of the cells and resulted in a similar inhibition of growth. However, most human solid tumors show a varying intensity of receptor expression on the neoplastic tissue, and combination therapy should be advantageous in this situation. Because toxicity after combination treatment was low, further dose escalation should be possible. Thus, combination therapy with cytotoxic peptide analogs could be a favorable treatment approach, which should be investigated in further studies.
In this trial, no major induction of the mRNA levels of MDR proteins MDR-1, MRP-1, and BCRP after therapy with AN-238 and AN-215 alone and in combination, as well as after AN-238 with AN-207, could be demonstrated in ovarian cancer cell lines investigated. AN-201 caused an increase of the mRNA levels for the MDR-1 in OV-1063 ovarian cancer and an induction of MDR-1, MRP-1, and BCRP in ES-2 ovarian cancer. Similar results were observed in vitro in the HEC-1A endometrial cancer line after treatment with the targeted cytotoxic LHRH analog AN-152 (23). Both AN-152 and its radical DOX induced surface expression of MDR-1 gene product P-glycoprotein, but the effect of AN-152 was smaller than that of DOX. Thus, the development of chemoresistance may be delayed by targeted chemotherapy.
In conclusion, this study demonstrates that a combination therapy with cytotoxic peptide analogs of somatostatin, bombesin, and LHRH is feasible, effective, and associated with low toxicity. In addition, our work shows that drug resistance proteins such as MDR-1, MRP-1, and BCRP are not induced by our cytotoxic peptide analogs.
Materials and Methods
Peptides and Cytotoxic Radical.
Cytotoxic radical 2-pyrrolino-DOX (AN-201), the bombesin-like carrier analog Gln-Trp-Ala-Val-Gly-His-Leu-ψ-(CH2–NH)-Leu-NH2 (RC-3094) and the cytotoxic bombesin analog 2-pyrrolino-DOX-14-O-hemiglutarate linked to the amino terminal of Gln-Trp-Ala-Val-Gly-His-Leu-ψ-(CH2–NH)-Leu-NH2 (AN-215) were synthesized in our laboratory, as described in ref. 24. The carrier somatostatin octapeptide RC-121 (d-Phe-Cys-Tyr-d-Trp-Lys-Val-Cys-Thr-NH2) was also synthesized in our laboratory as described in refs. 25 and 26. AN-238 was made by coupling AN-201–14-O-hemiglutarate to the amino terminus of [Lys-(N-(9-fluorenyl/methoxycarbonyl)5]RC-121, followed by deprotection and purification (27). Cytotoxic LHRH-conjugate AN-207 was synthesized in our laboratory by coupling one molecule of AN-201–14-O-hemiglutarate to the ε-amino group of the d-Lys side chain of the carrier peptide [d-Lys-6]LHRH, as described in ref. 28. Before i.v. injection, the compounds were dissolved in 5% (wt/vol) aqueous d-mannitol solution (Sigma).
Cell Lines.
Human ovarian cancer cell line ES-2 is a poorly differentiated clear cell ovarian carcinoma derived from a tumor of a 47-year-old African-American female (29).
Human ovarian epithelial cancer cell line OV-1063 originated from a metastatic papillary cystadenocarcinoma of the ovary of a 57-year-old woman (30). The two cell lines were obtained from American Type Culture Collection.
The UCI-107 human epithelial ovarian cancer cell line originating from a patient with papillary adenocarcinoma was provided by A. Manetta (University of California, Irvine Medical Center, Orange, CA). UCI-107 cells express receptors for somatostatin and bombesin, and OV-1063 and ES-2 cells carry receptors for LHRH, somatostatin, and bombesin, as determined in our previous work (8, 11, 18, 31). UCI-107 cells are known to have binding sites for somatostatin, bombesin, and express mRNA for all three bombesin receptor subtypes (9).
The cells were grown at 37°C in a humidified atmosphere of 95% air and 5% carbon dioxide, passaged weekly, and routinely monitored for mycoplasma contamination by using a detection kit (Roche Molecular Biochemicals). All culture media were purchased from GIBCO.
Animals.
Five- to 6-week-old female athymic nude mice (Ncr nu/nu) were obtained from National Cancer Institute (Bethesda). The animals were housed in sterile cages under laminar flow hoods in a temperature-controlled room with a 12-h light/12-h dark schedule. They were fed autoclaved chow and water ad libitum. Tumor volume (length × width × height × 0.5236) and body weight were measured weekly. The total leukocyte count (WBC) was determined with the Unopette microcollection kit (Becton Dickinson). At the end of each experiment, mice were killed under anesthesia, tumors were excised and weighed, and necropsy was performed. Tumor specimens were snap frozen and stored at −70°C. The Institutional Animal Care and Use Committee reviewed the protocols of the animal experiments and gave full approval.
Experimental Protocol.
Cells of each cell line growing exponentially were implanted into five female nude mice by s.c. injection of 107 cells in both flanks. Tumors resulting after 4 weeks in donor animals were aseptically dissected and mechanically minced. In all experiments, 3 mm3 pieces of tumor tissue were transplanted s.c. into experimental animals by a trocar needle. Experiments were started when tumors had reached an appropriate size.
In Experiment 1, when UCI-107 tumors had reached a volume of ≈250 mm3, mice were assigned to five experimental groups and received the following treatment as a single injection into the jugular vein: group 1, control, vehicle solution (eight mice); group 2, cytotoxic radical AN-201 at 200 nmol/kg (8 mice); group 3, cytotoxic analog AN-238 at 200 nmol/kg (8 mice); group 4, cytotoxic analog AN-215 at 200 nmol/kg (8 mice); group 5, a combination of the cytotoxic radicals AN-215 and AN-238 at 100 nmol/kg each (8 mice).
In Experiment 2, after OV-1063 tumors reached a mean tumor volume of 80 mm3, the animals were given an i.v. injection of each compound at 200 nmol/kg on day 1, 8, and 15, respectively. Groups 1–3 were treated as in Experiment 1. Group 4 received a combination of the cytotoxic LHRH analog AN-207 and the cytotoxic somatostatin analog AN-238 each at 100 nmol/kg.
In Experiment 3, when ES-2 tumors had reached a mean volume of 50 mm3, the mice were treated on day 1 and 8 with 150 nmol/kg of each compound. Groups 1–3 where treated as in Experiment 1. Group 4 was injected with a mixture of AN-207 and AN-238 at 75 nmol/kg each.
Real-Time PCR for MDR-1, MRP-1, and BCRP mRNA Expression.
Total RNA was isolated from ≈100 mg of tumor tissue for each sample according to the TRI reagent protocol. One microgram of total RNA was subjected to reverse transcription with the Iscript cDNA synthesis kit (Bio-Rad), following the manufacturer’s protocol. Real-time PCR was used to measure drug resistance gene expression by using the SYBR Green system (Bio-Rad). Primers for MDR-1 (sense, 5′-TCT GGA GGA AGA CAT GAC CAG GTA-3′; antisense, 5′-GGC ACC AAA ATG AAA CCT GAA TGT-3′), MRP-1 (sense, 5′-AGA GAC AGC TCA GCA GCT CCT-3′; antisense, 5′-GCC TTG TCA GCC TCC ATC AG-3′), BCRP (sense, 5′-TAT CAA TGG GAT CAT GAA ACC TGG-3′; antisense, 5′-GCG GTG CTC CAT TTA TCA GAA C-3′), and β-actin (sense, 5′-CTG GAA CGG TGA AGG TGA CA-3′; antisense, 5′-AAG GGA CTT CCT GTA ACA ATG CA-3′) were used to measure gene expression. The thermal cycling conditions comprised an initial denaturation step at 95°C for 3 min and then 40 cycles of two-step PCR including 95°C for 30 s and 60°C for 1 min. Data were collected during the 60°C annealing step and were further analyzed by the icycler iQ Optical system software (Bio-Rad). Real-time PCR efficiencies, E, for MDR-1 (target gene 1), MRP (target gene 2), and β-actin (reference gene) were calculated from the given slopes in the icycler software according to the following equation: E = 10(−1/slope) (32). Three tumor samples from each treatment group were analyzed. For the mathematical model used in this study, it was necessary to determine the crossing points for the transcripts of each sample. Crossing points (CPs) are defined as the number of cycles at which the fluorescence rises appreciably above the background fluorescence. By using CP deviations (ΔCP) for control and treatment (CPcontrols − CPtreatment) of target and reference gene transcripts, quantification of the target genes in treated groups relative to the controls was performed by using the following mathematical model found in the article by Paffl (33): Ratio = (Etarget)ΔCP target (control-treatment)/(Ereference)ΔCP reference (control-treatment).
Statistical Analysis.
Data are expressed as means ± SE. Differences between mean values were evaluated by two-tailed Student’s t test. P < 0.05 was considered significant.
Acknowledgments
This research was supported by the Medical Research Service of the Veterans Affairs Department (A.V.S.) and a grant from ZENTARIS (Frankfurt) to Tulane University (to A.V.S.).
Abbreviations
- LHRH
luteinizing hormone-releasing hormone
- MDR
multidrug resistance
- MRP
MDR-related protein
- BCRP
breast cancer resistance protein
- 2-pyrrolino-DOX
2-pyrrolinodoxorubicin
- CPs
crossing points.
Footnotes
Conflict of interest statement: Tulane University has applied for a patent on the cytotoxic analogs AN-201, AN-207, AN-215, and AN-238 cited in this article. A.V.S. is an inventor on those patents, but this article deals with the effects of these analogs on experimental gynecological oncology.
References
- 1.Jemal A., Murray T., Samuels A., Ghafoor A., Ward E., Thun M. J. CA Cancer J. Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Risch H. A. J. Natl. Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 3.McGuire W. P., Hoskins W. J., Brady M. F., Kucera P. R., Partridge E. E., Look K. Y., Clarke-Pearson D. L., Davidson M. N. Engl. J. Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.McGuire W. P., Ozols R. F. Semin. Oncol. 1998;25:340–348. [PubMed] [Google Scholar]
- 5.Alberts D. S. Semin. Oncol. 1999;26:8–14. [PubMed] [Google Scholar]
- 6.Abou-Jawde R., Choueiri T., Alemany C., Mekhail T. Clin. Ther. 2003;25:2121–2137. doi: 10.1016/s0149-2918(03)80209-6. [DOI] [PubMed] [Google Scholar]
- 7.Schally AV N. A. Trends Endocrinol. Metab. 2004;15:300–310. doi: 10.1016/j.tem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Schally A. V., Comaru-Schally A. M., Nagy A., Kovacs M., Szepeshazi K., Plonowski A., Varga J. L., Halmos G. Front. Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 9.Sun B., Schally A. V., Halmos G. Regul. Pept. 2000;90:77–84. doi: 10.1016/s0167-0115(00)00114-2. [DOI] [PubMed] [Google Scholar]
- 10.Srkalovic G., Schally A. V., Wittliff J. L., Day T. G., Jr., Jenison E. L. Int. J. Oncol. 1998;12:489–498. doi: 10.3892/ijo.12.3.489. [DOI] [PubMed] [Google Scholar]
- 11.Halmos G., Sun B., Schally A. V., Hebert F., Nagy A. J. Clin. Endocrinol. Metab. 2000;85:3509–3512. doi: 10.1210/jcem.85.10.3509. [DOI] [PubMed] [Google Scholar]
- 12.Juliano R. L., Ling V. Biochim. Biophys. Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 13.Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 14.Ross D. D., Yang W., Abruzzo L. V., Dalton W. S., Schneider E., Lage H., Dietel M., Greenberger L., Cole S. P., Doyle L. A. J. Natl. Cancer Inst. 1999;91:429–433. doi: 10.1093/jnci/91.5.429. [DOI] [PubMed] [Google Scholar]
- 15.Schondorf T., Kurbacher C. M., Gohring U. J., Benz C., Becker M., Sartorius J., Kolhagen H., Mallman P., Neumann R. Anticancer Res. 2002;22:2199–2203. [PubMed] [Google Scholar]
- 16.Yokoyama Y., Sato S., Fukushi Y., Sakamoto T., Futagami M., Saito Y. J. Obstet. Gynaecol. Res. 1999;25:387–394. doi: 10.1111/j.1447-0756.1999.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 17.Arencibia J. M., Schally A. V., Krupa M., Bajo A. M., Nagy A., Szepeshazi K., Plonowski A. Int. J. Oncol. 2001;19:571–577. doi: 10.3892/ijo.19.3.571. [DOI] [PubMed] [Google Scholar]
- 18.Arencibia J. M., Schally A. V., Halmos G., Nagy A., Kiaris H. Anticancer Drugs. 2001;12:71–78. doi: 10.1097/00001813-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Arencibia J. M., Bajo A. M., Schally A. V., Krupa M., Chatzistamou I., Nagy A. Anticancer Drugs. 2002;13:949–956. doi: 10.1097/00001813-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M., Schally A. V., Nagy A., Lamharzi N., Halmos G., Szepeshazi K., Armatis P. Am. J. Obstet. Gynecol. 1999;180:1095–1103. doi: 10.1016/s0002-9378(99)70600-9. [DOI] [PubMed] [Google Scholar]
- 21.Plonowski A., Schally A. V., Koppan M., Nagy A., Arencibia J. M., Csernus B., Halmos G. Cancer. 2001;92:1168–1176. doi: 10.1002/1097-0142(20010901)92:5<1168::aid-cncr1435>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Engel J. B., Keller G., Schally A. V., Halmos G., Hammann B., Nagy A. Clin. Cancer Res. 2005;11:2408–2415. doi: 10.1158/1078-0432.CCR-04-1670. [DOI] [PubMed] [Google Scholar]
- 23.Gunthert A. R., Grundker C., Bongertz T., Nagy A., Schally A. V., Emons G. Breast Cancer Res. Treat. 2004;87:255–264. doi: 10.1007/s10549-004-8806-8. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A., Armatis P., Cai R. Z., Szepeshazi K., Halmos G., Schally A. V. Proc. Natl. Acad. Sci. USA. 1997;94:652–656. doi: 10.1073/pnas.94.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Proc. Natl. Acad. Sci. USA. 1986;83:1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy A., Armatis P., Schally A. V. Proc. Natl. Acad. Sci. USA. 1996;93:2464–2469. doi: 10.1073/pnas.93.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagy A., Schally A. V., Halmos G., Armatis P., Cai R. Z., Csernus V., Kovacs M., Koppan M., Szepeshazi K., Kahan Z. Proc. Natl. Acad. Sci. USA. 1998;95:1794–1799. doi: 10.1073/pnas.95.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy A., Schally A. V., Armatis P., Szepeshazi K., Halmos G., Kovacs M., Zarandi M., Groot K., Miyazaki M., Jungwirth A., Horvath J. Proc. Natl. Acad. Sci. USA. 1996;93:7269–7273. doi: 10.1073/pnas.93.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau D. H., Lewis A. D., Ehsan M. N., Sikic B. I. Cancer Res. 1991;51:5181–5187. [PubMed] [Google Scholar]
- 30.Horowitz A. T., Treves A. J., Voss R., Okon E., Fuks Z., Davidson L., Biran S. Oncology. 1985;42:332–337. doi: 10.1159/000226056. [DOI] [PubMed] [Google Scholar]
- 31.Yano T., Pinski J., Radulovic S., Schally A. V. Proc. Natl. Acad. Sci. USA. 1994;91:1701–1705. doi: 10.1073/pnas.91.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen . Rapid Cycle Real-Time PCR: Methods and Applications. In: Meuer S., Wittwer C., Nakagawara K., editors. Heidelberg: Springer; 2001. pp. 21–34. [Google Scholar]
- 33.Pfaffl M. W. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]