Abstract
The contribution of neuropeptide Y (NPY), deriving from adrenal medulla, to the adrenosympathetic tone is unknown. We found that in response to NPY, primary cultures of mouse adrenal chromaffin cells secreted catecholamine, and that this effect was abolished in cultures from NPY Y1 receptor knockout mice (Y1−/−). Compared with wild-type mice (Y1+/+), the adrenal content and constitutive release of catecholamine were increased in chromaffin cells from Y1−/− mice. In resting animals, catecholamine plasma concentrations were higher in Y1−/− mice. Comparing the adrenal glands of both genotypes, no differences were observed in the area of the medulla, cortex, and X zone. The high turnover of adrenal catecholamine in Y1−/− mice was explained by the enhancement of tyrosine hydroxylase (TH) activity, although no change in the affinity of the enzyme was observed. The molecular interaction between the Y1 receptor and TH was demonstrated by the fact that NPY markedly inhibited the forskolin-induced luciferin activity in Y1 receptor-expressing SK-N-MC cells transfected with a TH promoter sequence. We propose that NPY controls the release and synthesis of catecholamine from the adrenal medulla and consequently contributes to the sympathoadrenal tone.
Keywords: adrenal gland, tyrosine hydroxylase, Y1 knockout mice
Neuropeptide Y (NPY) is a 36-aa peptide coreleased with norepinephrine (NE) during sympathetic nerve activation (1, 2). NPY acts through different G protein-coupled receptors termed Y1, Y2, Y3, Y4, and Y5 (3, 4). NPY is an important neurotransmitter of the sympathetic function that potentiates the catecholamine vasoconstrictor activity through the Y1 receptor and exerts prejunctional inhibitory effects on NE release from the sympathetic nerve endings of the heart through the Y2 receptor (5). In addition, the nerve terminals of parasympathetic neurons in the mouse heart possess Y2 receptors, which, when activated, reduce acetylcholine release, also causing an inhibition of the parasympathetic nervous system (6). We have shown that NPY Y1 knockout mice (Y1−/−) lose their ability to potentiate NE-induced vasoconstriction and have normal blood pressure, probably indicating a minor role of NPY in the maintenance of blood pressure homeostasis (7). Recently, these mice were investigated for their cardiac sympathovagal balance in baseline conditions and during an acute social challenge. Reduced somatomotor activity during nonsocial challenges, lower heart rate in baseline conditions, and larger heart rate responsiveness during social defeat were reported (8). Besides its presence in nerve endings, NPY is produced by chromaffin cells of adrenal medulla of different species, including human (9). The mouse has higher adrenal NPY content than rat, pig, or humans (10, 11). The effect of NPY on the adrenal medulla is controversial. NPY stimulated catecholamine release from intact rat adrenal capsular tissue (12), although an inhibitory effect of NPY on catecholamine secretion in rat adrenomedullary primary cell cultures was also observed (13). Moreover, there is a weak inhibitory effect of NPY on NE and epinephrine (EP) release from bovine chromaffin cells, evoked by addition of a cholinergic agonist (14, 15). However, depending on the experimental conditions, conflicting results were obtained. In perfused bovine adrenal gland, NPY stimulated the secretion of catecholamine in the presence of cholinergic agonists (14, 15). The exact subtype(s) of the NPY receptor(s) involved in the modulation of bovine catecholamine secretion remain(s) undefined, although the presence of NPY Y1 and the Y3 receptors has been reported (16–18). In human chromaffin cells, we demonstrated that NPY stimulates basal release of catecholamines through a receptor that exhibits a Y3 pharmacological profile (9).
In the present study, we investigated the effect of NPY on catecholamine release from mouse chromaffin cells. Using Y1−/− mice, we studied the changes resulting from the lack of the Y1 receptor on catecholamine release and synthesis from the mouse adrenal gland. In addition, we compared baseline plasma NE and EP concentrations in Y1−/− mice with those in Y1+/+ animals to establish the contribution of the Y1 receptor on adrenal medulla catecholamine synthesis in vivo.
Results
Plasma Catecholamine Concentrations.
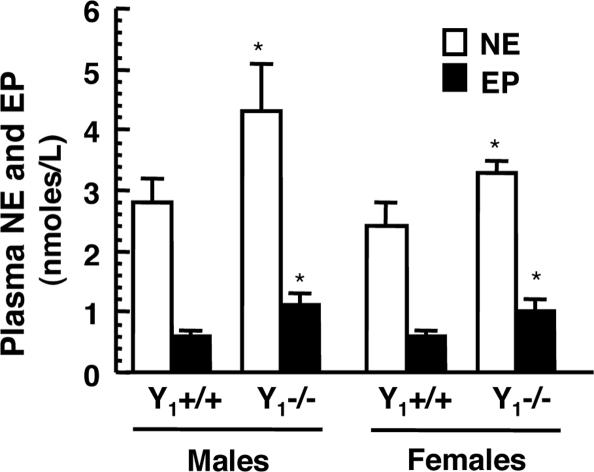
The baseline plasma NE and EP concentrations were increased in Y1−/− compared with Y1+/+ mice (Fig. 1). In Y1−/− mice, plasma NE was 4.3 ± 0.9 nM in male and 3.3 ± 0.3 nM in female and in Y1+/+ mice, it was 2.8 ± 0.4 nM in male and 2.4 ± 0.7 nM in female (P < 0.05 between Y1−/− and Y1+/+). In Y1−/− mice, plasma EP was 1.1 ± 0.2 nM in male and 1.4 ± 0.6 nM in female, whereas in Y1+/+ mice, it was 0.6 ± 0.1 nM in male and 0.8 ± 0.5 nM in female (P < 0.05 between Y1 −/− and Y1+/+). There was no difference between male and female NE and EP plasma concentrations.
Fig. 1.
Plasma NE and EP concentrations are higher in Y1−/− compared with Y1+/+ mice. Plasma catecholamine was measured in seven or eight mice of each gender and each genotype. ∗, P < 0.05 compared with Y1+/+ mice.
RT-PCR.
We analyzed RNA isolated from mouse adrenal gland obtained from three different mice for the presence of Y1, Y2, and Y5 mRNAs by RT-PCR, and we detected mRNA for Y1, Y2, and Y5 receptors in all samples (Fig. 2). In addition, we found that adrenal glands from Y1−/− mice express Y2 and Y5 receptors (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 2.

Y1, Y2, and Y5 receptor mRNAs are present in a mouse adrenal gland by RT-PCR. Similar findings were obtained in two additional adrenal glands. Samples without RNA were included as negative controls (not shown).
NE, EP, and NPY Contents of Mouse Adrenal Glands.
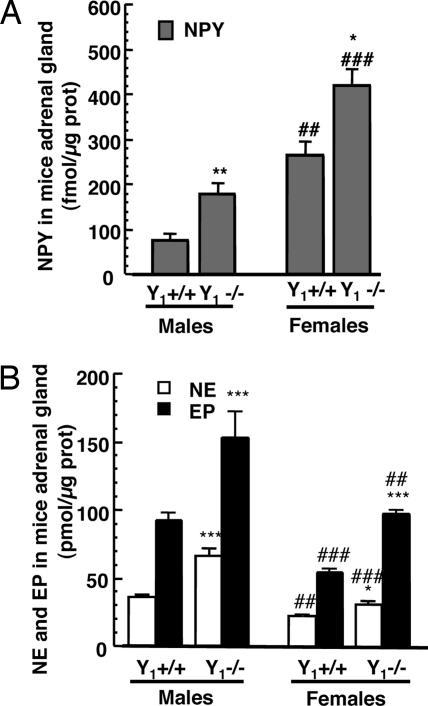
The amount of catecholamines (NE and EP) and NPY was measured in the adrenal glands from female and male mice lacking the Y1 receptor (Y1−/−) compared with age-matched wild-type animals (Y1+/+; see Fig. 3). Adrenal glands from Y1+/+ females contained 265 ± 30 fmol NPY per microgram of protein, 23 ± 1 pmol NE per microgram of protein, 55 ± 3 pmol EP per microgram of protein, and adrenals from Y1−/− female mice contained 422 ± 37 fmol NPY per microgram of protein, 31 ± 3 pmol NE per microgram of protein, and 98 ± 3 pmol EP per microgram of protein. In males, the adrenals from Y1+/+ contained 77 ± 15 fmol NPY per microgram of protein, 36 ± 1 pmol NE per microgram protein, and 93 ± 5 pmol EP per microgram of protein, whereas adrenals from Y1−/− mice contained 180 ± 22 fmol NPY per microgram of protein, 66 ± 5 pmol NE per microgram of protein, and 154 ± 19 pmol EP per microgram of protein. Thus, adrenal contents in catecholamines and NPY are always higher in Y1−/− mice than in Y1+/+ animals. The adrenal glands from males contain more catecholamines but less NPY compared with adrenals from female mice when compared with their homologous genotype (Fig. 3). On a molar basis, in control animals, adrenals from males contain 1,675 times more catecholamine than NPY, whereas adrenals from females contain 290 times more. In Y1−/− mice, adrenals from males contain 1,222 times more catecholamine than NPY, whereas adrenals from females contain 307 times more. The ratio of NE reported to total catecholamine (NE + EP) in the adrenal glands from males and females Y1+/+ mice was similar at 29 ± 2% and 27 ± 1%, respectively. The same ratio was observed in Y1−/− male and female mice at 30 ± 3% and 24 ± 3%, respectively.
Fig. 3.
NPY (A) and NE and EP (B) contents in adrenal gland of control (Y1+/+) and Y1−/− mice. Results (mean ± SEM; n = 6–8) are expressed as pmol NE or EP (B) or fmol NPY (A) per microgram of protein. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared with adrenals of Y1+/+ mice; ##, P < 0.01; ###, P < 0.001 compared with adrenals from males.
Constitutive and Regulated Release of NE and EP from Chromaffin Cells from Y1+/+ and Y1−/− Mice.
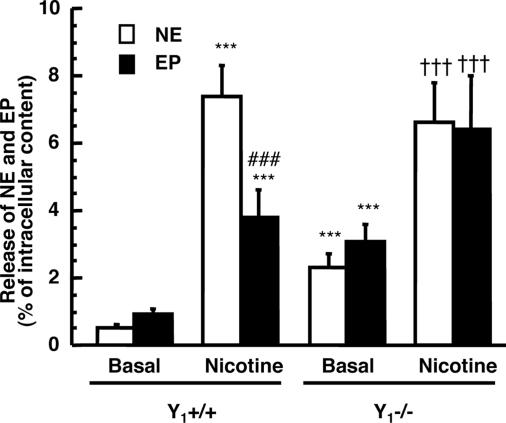
We measured the spontaneous release of NE and EP from chromaffin cells in vitro during 10 min and found that Y1−/− mice secreted a higher amount of catecholamines than Y1+/+ mice (Fig. 4). NE release was 0.52 ± 0.09% of intracellular content in Y1+/+ mice compared with 2.3 ± 0.4% in Y1−/− mice. EP release was 0.93 ± 0.19% of intracellular content in Y1+/+ mice compared with 3.4 ± 0.5% in Y1−/− mice. When chromaffin cells were incubated with nicotine (100 μM) for 10 min (Fig. 4), there was a significant increase in the release of NE and EP (17- and 4-fold increase, respectively). However, nicotine was less potent to stimulate NE and EP secretion from Y1−/− chromaffin cells (3- and 2-fold increase for NE and EP, respectively).
Fig. 4.
Basal and stimulated catecholamine (NE and EP) release from chromaffin cells obtained from adrenals of control mice (Y1+/+) and Y1 knockout mice (Y1−/−). ∗∗∗, P < 0.001 compared with basal release from Y1+/+ chromaffin cells; ###, P < 0.001 compared with NE released with nicotine; †††, P < 0.001 compared with basal release from Y1−/− chromaffin cells.
Role of the Y1 Receptor in NPY-Induced NE and EP Release from Chromaffin Cells in Culture.
To evaluate the effect of NPY on catecholamine secretion, we incubated chromaffin cells from Y1+/+ mice for 10 min with 100 nM NPY (Fig. 5) and observed an increase on NE (1.3 ± 0.21%) and EP (1.9 ± 0.3%) release compared with basal release (NE, 0.52 ± 0.09; EP, 0.93 ± 0.19%). The stimulatory effect of the Y1/Y5 agonist ([31Leu,34Pro]NPY 100 nM) or PYY (100 nM) on NE and EP release was similar to the stimulatory effect of NPY on chromaffin cells obtained from control animals (Fig. 5). However, 100 nM NPY did not increase NE and EP release from chromaffin cells obtained from Y1−/− mice (Fig. 5), confirming that secretory effect of NPY is mediated by the activation of the Y1 receptor.
Fig. 5.
NPY, PYY, and [31Leu,34Pro]NPY increase NE and EP release from chromaffin cells obtained from control mice (Y1+/+). The release of NE and EP from mice chromaffin cells during 10 min in Krebs buffer (basal) or in the presence of 100 nM NPY or PYY or [31Leu,34Pro]NPY was measured. Mean ± SD of three to four experiments done in triplicate. P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 compared with basal.
Morphometric Analysis of Adrenal Glands from Y1+/+ and Y1−/− Mice.
No significant differences were observed in the area of the adrenal medulla, cortex, and zone X of Y1+/+ and Y1−/− mice (Fig. 6A). Similarly, the number and the size of the cells were not different between Y1+/+ and Y1−/− mice in all areas of the adrenal explored (Fig. 6 B and C). Macroscopically, no lesions were observed in Y1−/− mice. All adrenal glands analyzed were microscopically normal and revealed a normal architecture of the cortex and the medulla. No nodule was observed. Cortical and medullary cells presented no cytonuclear atypia. No necrosis and no image of vascular invasion were observed. Therefore, the increase of catecholamine content and release observed from the adrenal medulla of Y1−/− mice is not associated with hypertrophy or hyperplasia of the gland.
Fig. 6.
Morphometric analyses of adrenal glands from Y1+/+ and Y1−/− mice: area of the medulla, cortex, and X zone (A); cell size (B); and number of cells (C). Six mouse adrenals of each gender and genotype were analyzed. The area of a series of sections performed in each gland was measured in triplicate. The mean area of the largest section was evaluated.
Tyrosine Hydroxylase (TH) Activity in Adrenal Glands from Y1+/+ and Y1−/− Mice.
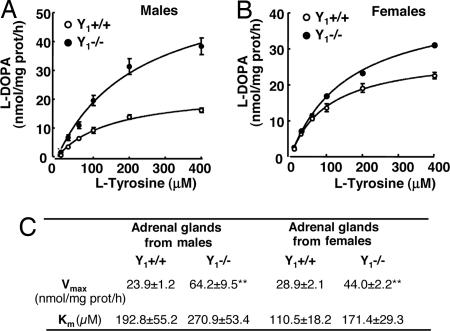
To determine the parameters of TH enzyme kinetics, saturation curves using the substrate [l-3,4-dihydroxyphenylalanine (l-DOPA)] were determined. Incubation of TH assay mixture prepared from adrenals of wild-type or Y1−/− mice using the substrate l-tyrosine (l-Tyr) resulted in a concentration-dependent formation of l-DOPA (Fig. 7). We found that the Vmax values for TH activity were significantly higher in the adrenals of Y1−/− mice compared with wild type, both in males and females (Fig. 7C; P < 0.01); Km values were not significantly different, indicating no differences on TH affinity between the two genotypes. These biochemical differences are consistent with a tight down-regulation of TH synthesis through the activation of the Y1 receptor.
Fig. 7.
TH activity in adrenal glands from wild-type (Y1+/+) and NPY Y1 knockout (Y1−/−) mice. Saturation curves of TH activity obtained with the substrate (l-Tyr) in the adrenals of males (A) and females (B) and Y1+/+ and Y1−/− mice. (C) Kinetic parameters of saturation curves of TH activity in the adrenals of male and female, Y1+/+, and Y1−/− mice. ∗∗, P < 0.01 compared with Y1+/+; n = 5; mean ± SD.
NPY Effect on TH Promoter Activity.
To evaluate at the molecular level the action of NPY on TH gene transcription, we transfected the Y1-receptor-expressing cells SK-N-MC with a plasmid containing a 775-bp stretch of the rat TH promoter (−774 to the CTG initiation site). This stretch contained most transcription factor-binding sites, including the AP1, the SP1, the POU Oct, the GRE, and the two AP2 sites, as well as the cAMP response element sequence (−45 bp) that was shown to be the prime regulator of TH transcription (19). Incubation of transfected cells for 6 h with forskolin increased TH promoter-driven luciferase activity in a concentration-dependent way (Fig. 8A). Maximal luciferase stimulation was achieved with forskolin concentrations >10 μM, and the EC50 value was 1.43 ± 0.089 μM. This effect was concentration-dependently decreased when cells were incubated with NPY (Fig. 8B). Concentrations as low as 10 nM NPY inhibited EC50 forskolin-induced luciferase activity by up to 70%, and IC50 was 1.50 ± 0.47 nM (Fig. 8B). This effect was specific to the Y1 receptor, because the Y1 antagonist BIBP3226 prevented TH promoter inhibition by NPY (Fig. 8C). Whether NPY inhibitory effects were transient or required long-lasting Y1 signaling was investigated by incubating TH-transfected SK-N-MC cells with NPY for different periods before and after forskolin stimulation. As shown in Fig. 8D, a weak but significant inhibition of forskolin-induced TH promoter-driven luciferase activity was achieved when cells were incubated with NPY for up to 16 h before forskolin stimulation. However, maximal inhibition was achieved when NPY was present 1 h before forskolin addition. Surprisingly, significant inhibition was also observed when NPY was added 30 min after forskolin (Fig. 8D).
Fig. 8.
Inhibition of TH promoter activity by NPY. (A) SK-N-MC cells transfected with a plasmid encoding the luciferase gene under the control of the rat TH promoter were incubated for 6 h in the presence of the indicated concentrations of forskolin, and luciferase activity was measured at different times before and after the addition of NPY. A different cellular extract was used at each time point. (B) Transfected SK-N-MC cells were incubated for 10 min with different concentrations of NPY and stimulated for 6 h with 1.25 μM forskolin and luciferase, and activity was measured. (C) Effect of BIBP 3226 (1 μM), a specific Y1 antagonist, on the NPY inhibitory effect of forskolin activation. (D) NPY (1.2 nM) was added before or after forskolin activation, and luciferase activity was measured. SK-N-MC cells were incubated for 10 min with NPY (1.2 nM) with or without H-89, a PKA inhibitor (E), or with or without CalC, a PKC inhibitor (F), and forskolin activation and luciferase activity were measured. Mean ± SD; three to four experiments done in triplicate. ∗∗∗, P < 0.001 compared with control. ###, P < 0.001 compared with NPY; †††, P < 0.001 compared with the respective PK inhibitor.
Whether forskolin-mediated increase in luciferase activity in transfected cells is mediated by PKA or PKC was further investigated by using specific inhibitors. As shown in Fig. 8E, the PKA inhibitor H-89 inhibited luciferase activity >60% at 2 μM, and IC50 was 1.50 ± 0.47 μM. In contrast, the PKC inhibitor calphostin C (CalC) had only marginal effect, even at high concentrations (Fig. 8F). Concentrations >500 nM resulted in significant cell death (results not shown). The additive inhibitory effect of these compounds with NPY was evaluated. No further luciferase inhibition was observed when cells were incubated with NPY + H-89 or NPY + CalC, as compared with NPY alone.
Discussion
This study shows that the NPY-induced release of catecholamine (NE and EP) from adrenal chromaffin cells is mediated by the Y1 receptor, because NPY had no effect on catecholamine release from the adrenal glands of mice lacking NPY Y1 receptor expression.
The effect of NPY is physiologically relevant, because a constitutive release of NPY by the adrenal medulla appears to regulate the secretion of catecholamine by an autocrine mechanism, as shown with human adrenal chromaffin cells (6). Adrenal glands of Y1−/− mice contain higher amounts of NE and EP than Y1+/+ mice. This intracellular accumulation of NE and EP within chromaffin cells could be mediated by the lack of stimulation by NPY on catecholamine secretion. Surprisingly, we observed the opposite, because the accumulation of intracellular catecholamine in Y1−/− mice leads to a significant increase of NE and EP constitutive release relative to their intracellular content. Thus, catecholamine release is positively regulated by NPY, but it is still unclear how Y1−/− mouse chromaffin cells, in addition to doubling their catecholamine concentration, also release in baseline conditions 4-fold more than Y1+/+ chromaffin cells. We hypothesized three possibilities to explain these data: (i) an increase of chromaffin cell number and/or size in the adrenal gland, (ii) a deficiency of catecholamine storage in chromaffin granules, or (iii) an increase of catecholamine turnover rate. The first hypothesis was supported by the increased catecholamine and NPY contents in the adrenals of Y1−/− mice, because a trophic effect of catecholamines, especially NE, on the cardiovascular system has been demonstrated in several studies (20–26). Also, NPY was shown to induce DNA synthesis in a concentration-dependent manner and through the activation of Y1 receptor in vascular smooth muscle cells (27–29), whereas in cardiomyocytes, the trophic effect of NPY is mediated through Y5 receptors (30). Nevertheless, trophic actions of catecholamines and/or NPY do not explain the increased contents observed in the adrenal glands of Y1−/− animals, because we did not observe an increased cell number or size in those adrenals compared with controls. That Y1−/− chromaffin cells in culture are less prone to release catecholamine upon nicotinergic stimulation compared with basal release might indicate a deficiency in vesicle storage or an increased threshold for a depolarization event. However, this hypothesis is unlikely, because the total amount of catecholamine release induced by nicotine is similar in both genotypes. Therefore, we further investigated the possibility of a molecular interaction between NPY and catecholamine synthesis. Indeed, we found an increase of TH activity with no change in the affinity of the enzyme in the adrenals of Y1−/− mice. The effect of NPY on catecholamine synthesis by adrenal medulla has been observed but not explained (31, 32). i.v. administration of NPY or a Y1 agonist induced a marked increase of adrenal TH mRNA and TH protein levels, whereas a Y2 receptor agonist was inactive (31, 32). On the contrary, NPY inhibits depolarization-induced catecholamine synthesis in rat pheochromocytoma cells (33). Interestingly, Bornstein et al. (34) showed a marked increase of NPY mRNA and NPY staining in chromaffin cells of adrenal glands in TH knockout mice. This suggests that the increase in NPY expression compensates for the lack of catecholamine production. Because NPY and catecholamine expression and release are regulated by neural input to the adrenal medulla, the increase in catecholamine as well as NPY production may partially be the consequence of an increased preganglionic stimulation of the chromaffin cells in both Y1 and TH−/− mice.
In the present study, we also show that NPY decreases the TH promoter activity in Y1 receptor-expressing cells, SK-N-MC. The Y1 antagonist, BIBP3226, prevents this effect. These results indicate that NPY, in addition to controlling catecholamine release, may also regulate NE and EP synthesis by acting directly on TH expression. Indeed, activation of PKA with either forskolin or cAMP analogs leads to a dramatic induction of TH mRNA and TH protein in many different cell types, including SK-N-MC cells (35–40). This PKA-mediated response depends on the TH cAMP response element (CRE; see refs. 35 and 41–44). The CRE (position −45–38) site within the TH promoter is present in our plasmid and may mediate, at least in part, the forskolin-triggered TH promoter activation. In addition, both the CRE and AP1 (−201–195) sites appear to mediate PKC-mediated TH promoter activation (35, 45–49). Our observed impairment of forskolin-induced TH promoter activation by the PKA inhibitor, CalC, further confirms the reported role of PKA-mediated cAMP-dependent signaling in this process. In this context, the absence of additive inhibitory effects of CalC and NPY may be explained by the fact that the CRE-binding protein CREB was also shown to be essential for the response of the TH promoter to PKA or PKC activation (45, 46).
Phenylethanolamine-N-methyl transferase, the enzyme involved in the methylation of NE into EP, is still able to ensure the conversion of NE into EP proportionally to the increase in substrate observed in Y1−/− mice, because we found a similar ratio of NE reported to total catecholamine (NE + EP) in the adrenal glands of males and females of both genotypes. This resulted in a higher EP content and release from chromaffin cells in culture, contributing to an increase in the EP plasma levels in those mice. Because EP is produced exclusively by the adrenal medulla, and a 40–50% increase in plasma circulation of EP is observed in Y1−/− compared with Y1+/+ mice, we estimated that NPY contributed to half of the baseline catecholamine secretory activity of the mouse adrenal medulla.
We observed differences between males and females in catecholamine adrenal content. Adrenal glands from female mice contain less catecholamine than those from males, as described in rats (50). One possible explanation is that the adrenal medullary content of NE and EP in female rats is under the control of estrogen (51). The paradoxical fact that catecholamine concentrations in plasma are similar in males and females reflects differences in the level of sympathetic activation, rate of synthesis, release, uptake, and metabolism. Indeed, the degradation of adrenal catecholamine is also affected by sex hormones, and the levels of monoamine oxidase and catechol-O-methyl transferase activity vary during the oestral cycle (52). Those gender differences may also be a result of differences in the clearance of released NE (i.e., neuronal and extraneuronal uptake). An increase in adrenaline clearance in women is suggested by a study that compared the effects of exogenous EP in men and women (53). Interestingly, adrenal glands from female mice contain more NPY than those from males. It is tempting to hypothesize that the higher NPY content of female adrenal glands is due to a compensation of lower catecholamine concentration.
Our results shed light on an important role of NPY that controls catecholamine synthesis and secretion in the adrenal gland of mice, by directly acting on TH expression. In conclusion, not only is NPY a neurotransmitter, but it also may act as a hormone to finely tune the adrenosympathetic tone.
Materials and Methods
Peptides and Chemicals.
NPY1–36, [31Leu,34Pro]NPY and PYY were purchased from Nova Biochem. The stock solution of forskolin (Sigma) was 100 mg/ml in ethanol. The NPY stock solution was 200 μM in H2O. H-89 (a PKA inhibitor) and CalC (a PKC inhibitor) were from Calbiochem. Stock solutions (25 mM for H-89 and 0.8 mM for CalC) were prepared in dimethyl sulfoxide. FCS was obtained from Seromed Biochrom, Berlin; and collagenase type I, nicotine, and DNase were from Sigma. The Y1 antagonist, BIBP3226 was a kind gift from K. Hofbauer (Novartis, Basel).
Animals.
We used 3-mo-old male and female Y1−/− mice (7) and their corresponding Y1+/+ mice (C57BL/6). They were backcrossed for eight generations on a C57BL/6 background (Iffa Credo) by crossing homozygous Y1−/− with C57BL/6 Y1+/+ mice. Heterozygous mice were then crossed together to produce homozygous mutants (Y1−/−) and wild-type control littermates (Y1+/+).
Adrenal Gland and Chromaffin Cell Culture.
Mice were manipulated during 3 weeks and were killed by decapitation. Adrenal glands were rapidly dissected out and flash-frozen in liquid nitrogen before catecholamine and NPY assays. The cell culture was performed as described (54). Briefly, freshly collected adrenal glands were cleaned of fat tissue and digested in 1 ml of a 0.3% collagenase type I solution containing 0.02% DNase. The cells were cultured in 48 multiwell plates (75,000 chromaffin cells per well) and maintained during 2–4 days at 37°C with 5% CO2. Cell viability, as determined by trypan blue dye exclusion, was generally >95%.
RT-PCR.
To determine the presence of NPY Y1, Y2, and Y5 receptors, total RNA was extracted from frozen adrenal glands, and RT-PCR was carried out as described (30).
NE, EP, and NPY Quantification in Adrenal Glands.
Adrenal glands were sonicated in 0.4 M perchloric acid, and catecholamine was extracted from the homogenate by adsorption on alumina, eluted and separated by HPLC, and quantified by electrochemical detection (55). The NPY content in adrenal glands was determined after sonication of the glands in Krebs buffer (111 mM NaCl/2.5 mM CaCl2/4.7 mM KCl/1.2 mM MgSO4/1.2 mM KH2PO4/24.8 mM NaHCO3/11.1 mM glucose/15 mM Hepes, pH 7.4) with 0.08% Tween 20 (Pierce) followed by centrifugation. NPY was measured by ELISA (56). Results were expressed in fmol NPY or pmol NE or EP per microgram of protein.
NE and EP Determination in Mouse Plasma.
Mice were anesthetized with halothane and underwent catheterization of the carotid artery for blood sampling, heart rate, and blood pressure monitoring. Mice were allowed to recover from anesthesia for 4 h, and 0.2 ml of blood was collected and centrifuged immediately at 2,000 × g (57); this method of blood collection is one of the less stressful for measuring catecholamine in mice (57). Plasma was separated and frozen at −80°C until assayed. NE and EP in plasma were extracted on alumina and measured by HPLC with electrochemical detection (ED) (55).
Catecholamine Release Experiments in Mouse Chromaffin Cells.
The release experiments were performed as described (9). Briefly, chromaffin cells were incubated for 10 min with Krebs buffer (basal) or Krebs buffer with drugs. The released catecholamine in supernatants was measured by HPLC-ED and ELISA, respectively. Catecholamine release was expressed as the percent of total catecholamine content in chromaffin cells.
Morphometric Analyses of Mouse Adrenal Glands.
Adrenal glands were carefully dissected, measured, weighed, and immersed in 4.5% phosphate-buffered formalin (pH 7) and rinsed in PBS. During the cutting step of the adrenals, all macroscopic lesions such as nodules, areas of necrosis, hemorrhage, or cyst formation were analyzed. If no macroscopic lesions were found, the gland was processed. All samples were embedded in paraffin wax by using standard methods. Four-micrometer sections of the whole adrenals were made and stained with hematoxylin/eosin. Morphometric analysis was performed, and the area of a series of sections was measured in triplicate for each section. The mean area of the largest section was evaluated. The architecture of the adrenal cortex and medulla was analyzed.
Determination of TH Activity.
Twelve-week-old wild-type and Y1−/− mice were anesthetized with 60 mg/kg pentobarbital. Left and right adrenal glands were removed, fat was discarded, and they were rapidly frozen at −80°C. TH activity was measured as described (58). In brief, the adrenal gland was homogenized in 1.5 ml of a 0.25 M sucrose solution by using a glass homogenizer, and an aliquot of 40 μl was used for each TH assay. The reaction mixture for the TH assay contained 0.1 M sodium acetate buffer (pH 6.0) and 1 M 6-methyl-5, 6, 7, 8-tetrahydropterine (BH4) in 1 M 2-mercaptoethanol/0.50 mM ferrous sulfate heptahydrate. In the standard assay, 10-min incubation at 37°C was performed with a saturating concentration of substrate l-Tyr (100 μM), and the reaction was stopped with 300 μl of 0.5 M perchloric acid. In the blank incubation, l-Tyr was replaced by 100 μM d-Tyr, and 100 μM 3-iodo- l-Tyr, a TH inhibitor, was present. For TH kinetic analysis, the same experimental conditions were used except for increasing concentrations of either l-Tyr (10–400 μM) or 6-methyl-5,6,7,8-tetrahydropterine (50–1,600 μM). The [l-3,4-dihydroxyphenylalanine (l-DOPA) formed in the reaction was measured by HPLC-ED after the alumina adsorption method, as described (59). TH activity was expressed as the amount of l-DOPA formed per milligram of protein per hour.
Study of TH Promoter Activity.
SK-N-MC cells (that express the Y1 receptor) were transfected with a plasmid encoding the firefly luciferase gene under the control of a 775-bp stretch of the human TH gene (−774 to the CTG initiation site), a kind gift of Faucon Biguet (Centre National de la Recherche Scientifique, Hôpital Pitié-Salpêtrière, Paris). Transfection was carried out by electroporation (10 μg of plasmid for 5 × 106 cells) at 960 μF and 280 V in complete culture medium (DMEM supplemented with 10% FCS/10 mM Hepes/1% nonessential amino acids) by using a Bio-Rad GenePulser apparatus. This routinely resulted in 50–70% transfected cells, as assessed by fluorescence (FACS) analysis of cells electroporated under the same conditions with an EGFP-encoding plasmid (not shown). Cells were washed and allowed to recover for 24 h, then seeded (105 cells per well) in 48-well plates in DMEM supplemented with 2% FCS. After 24 h, cells were incubated with the test drugs. To prevent NPY absorption to plastic, NPY solutions were prepared in DMEM supplemented with 0.2% FCS and 0.0001% Tween-20. This medium only marginally (4–8%) inhibited forskolin-induced TH luciferase activity (data not shown). Determination of luciferase activity was performed by using the luciferase kit (Promega) according to the manufacturer’s recommendations.
Statistical Analysis.
Statistical comparisons were performed by ANOVA with the Bonferroni multiple comparison test.
Supplementary Material
Acknowledgments
We thank Dr. E. Potter (Prince of Wales Medical Research Institute, Sydney) for the careful reading of the manuscript. This work was supported by Fundação para a Ciência e a Tecnologia (Portugal) Grants SFRH/BD/10394/2002 and POCI/SAU-FCF/60399/2004, Fundo Europeu de Desenvolvimento Regional (Portugal), and Fonds National Suisse pour la Recherche Grant 3100AO-101999.
Abbreviations
- NPY
neuropeptide Y
- NE
norepinephrine
- EP
epinephrine
- Y1−/−
NPY Y1 knockout mice
- Y1+/+
wild-type mice
- TH
tyrosine hydroxylase
- CalC
calphostin C
- l-Tyr
l-tyrosine.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lundberg J. M., Torssell L., Sollevi A., Pernow J., Theodorsson Norheim E., Anggard A., Hamberger B. Regul. Pept. 1985;13:41–52. doi: 10.1016/0167-0115(85)90085-0. [DOI] [PubMed] [Google Scholar]
- 2.Takiyyuddin M. A., Brown M. R., Dinh T. Q., Cervenka J. H., Braun S. D., Parmer R. J., Kennedy B., O’Connor D. T. J. Auton. Pharmacol. 1994;14:187–200. doi: 10.1111/j.1474-8673.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Michel M. C., Beck-Sickinger A., Cox H., Doods H. N., Herzog H., Larhammar D., Quirion R., Schwartz T., Westfall T. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 4.Silva A. P., Cavadas C., Grouzmann E. Clin. Chim. Acta. 2002;326:3–25. doi: 10.1016/s0009-8981(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 5.Walker P., Grouzmann E., Burnier M., Waeber B. Trends Pharmacol. Sci. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- 6.Smith-White M. A., Iismaa T. P., Potter E. K. Br. J. Pharmacol. 2003;140:170–178. doi: 10.1038/sj.bjp.0705404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedrazzini T., Seydoux J., Kunstner P., Aubert J. F., Grouzmann E., Beermann F., Brunner H. R. Nat. Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- 8.Costoli T., Sgoifo A., Stilli D., Flugge G., Adriani W., Laviola G., Fuchs E., Pedrazzini T., Musso E. Neurosci. Biobehav. Rev. 2005;29:113–123. doi: 10.1016/j.neubiorev.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Cavadas C., Silva A. P., Mosimann F., Cotrim M. D., Ribeiro C. A., Brunner H. R., Grouzmann E. J. Clin. Endocrinol. Metab. 2001;86:5956–5963. doi: 10.1210/jcem.86.12.8091. [DOI] [PubMed] [Google Scholar]
- 10.Allen J. M., Adrian T. E., Polak J. M., Bloom S. R. J. Auton. Nerv. Syst. 1983;9:559–563. doi: 10.1016/0165-1838(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg J. M., Hokfelt T., Hemsen A., Theodorsson-Norheim E., Pernow J., Hamberger B., Goldstein M. Regul. Pept. 1986;13:169–182. doi: 10.1016/0167-0115(86)90224-7. [DOI] [PubMed] [Google Scholar]
- 12.Renshaw D., Thomson L. M., Carroll M., Kapas S., Hinson J. P. Endocrinology. 2000;141:169–173. doi: 10.1210/endo.141.1.7251. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda K., Shen G. H., Pfeiffer R. F., McComb R. D., Yang H. Y. Neurochem. Int. 1993;23:71–77. doi: 10.1016/0197-0186(93)90145-u. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi H., Costa E., Yang H. Y. J. Pharmacol. Exp. Ther. 1988;244:468–474. [PubMed] [Google Scholar]
- 15.Hexum T. D., Russett L. R. Neuropeptides. 1989;13:35–41. doi: 10.1016/0143-4179(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 16.Norenberg W., Bek M., Limberger N., Takeda K., Illes P. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995;351:337–347. doi: 10.1007/BF00169073. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J., Zhang P., Hexum T. D. Mol. Pharmacol. 1997;52:1027–1033. doi: 10.1124/mol.52.6.1027. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P., Zheng J., Vorce R. L., Hexum T. D. Regul. Pept. 2000;87:9–13. doi: 10.1016/s0167-0115(99)00093-2. [DOI] [PubMed] [Google Scholar]
- 19.Lewis-Tuffin L. J., Quinn P. G., Chikaraishi D. M. Mol. Cell Neurosci. 2004;25:536–547. doi: 10.1016/j.mcn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Abel P. W., Trapani A., Aprigliano O., Hermsmeyer K. Circ. Res. 1980;47:770–775. doi: 10.1161/01.res.47.5.770. [DOI] [PubMed] [Google Scholar]
- 21.Chen L., Xin X., Eckhart A. D., Yang N., Faber J. E. J. Biol. Chem. 1995;270:30980–30988. doi: 10.1074/jbc.270.52.30980. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H., Facemire C. S., Banes A. J., Faber J. E. Am. J. Physiol. 2002;282:H2364–H2370. doi: 10.1152/ajpheart.00858.2001. [DOI] [PubMed] [Google Scholar]
- 23.Jackson C. L., Schwartz S. M. Hypertension. 1992;20:713–736. doi: 10.1161/01.hyp.20.6.713. [DOI] [PubMed] [Google Scholar]
- 24.Erami C., Zhang H., Ho J. G., French D. M., Faber J. E. Am. J. Physiol. 2002;283:H1577–H1587. doi: 10.1152/ajpheart.00218.2002. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M. D., Grignolo A., Kuhn C. M., Schanberg S. M. Life Sci. 1983;33:169–180. doi: 10.1016/0024-3205(83)90410-1. [DOI] [PubMed] [Google Scholar]
- 26.van Kleef E. M., Smits J. F., De Mey J. G., Cleutjens J. P., Lombardi D. M., Schwartz S. M., Daemen M. J. Circ. Res. 1992;70:1122–1127. doi: 10.1161/01.res.70.6.1122. [DOI] [PubMed] [Google Scholar]
- 27.Shigeri Y., Fujimoto M. Neurosci. Lett. 1993;149:19–22. doi: 10.1016/0304-3940(93)90337-k. [DOI] [PubMed] [Google Scholar]
- 28.Zukowska-Grojec Z., Pruszczyk P., Colton C., Yao J., Shen G. H., Myers A. K., Wahlestedt C. Peptides. 1993;14:263–268. doi: 10.1016/0196-9781(93)90040-n. [DOI] [PubMed] [Google Scholar]
- 29.Erlinge D., Brunkwall J., Edvinsson L. Regul. Pept. 1994;50:259–265. doi: 10.1016/0167-0115(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 30.Pellieux C., Sauthier T., Domenighetti A., Marsh D. J., Palmiter R. D., Brunner H. R., Pedrazzini T. Proc. Natl. Acad. Sci. USA. 2000;97:1595–1600. doi: 10.1073/pnas.030533197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong M., Li S., Fournier A., St-Pierre S., Pelletier G. Neuroendocrinology. 1995;61:85–88. doi: 10.1159/000126816. [DOI] [PubMed] [Google Scholar]
- 32.Erdem S. R., Broxson C. S., Erdem A., Spar D. S., Williams R. T., Tumer N. Neuropharmacology. 2002;43:1280–1288. doi: 10.1016/s0028-3908(02)00293-9. [DOI] [PubMed] [Google Scholar]
- 33.McCullough L. A., Westfall T. C. Eur. J. Pharmacol. 1995;287:271–277. doi: 10.1016/0014-2999(95)00496-3. [DOI] [PubMed] [Google Scholar]
- 34.Bornstein S. R., Tian H., Haidan A., Bottner A., Hiroi N., Eisenhofer G., McCann S. M., Chrousos G. P., Roffler-Tarlov S. Proc. Natl. Acad. Sci. USA. 2000;97:14742–14747. doi: 10.1073/pnas.97.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim K. S., Park D. H., Wessel T. C., Song B., Wagner J. A., Joh T. H. Proc. Natl. Acad. Sci. USA. 1993;90:3471–3475. doi: 10.1073/pnas.90.8.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waymire J. C., Weiner N., Prasad K. N. Proc. Natl. Acad. Sci. USA. 1972;69:2241–2245. doi: 10.1073/pnas.69.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis E. J., Tank A. W., Weiner N., Chikaraishi D. M. J. Biol. Chem. 1983;258:14632–14637. [PubMed] [Google Scholar]
- 38.Tank A. W., Curella P., Ham L. Mol. Pharmacol. 1986;30:497–503. [PubMed] [Google Scholar]
- 39.Fossom L. H., Sterling C. R., Tank A. W. Mol. Pharmacol. 1992;42:898–908. [PubMed] [Google Scholar]
- 40.Kim K. S., Tinti C., Song B., Cubells J. F., Joh T. H. J. Neurochem. 1994;63:834–842. doi: 10.1046/j.1471-4159.1994.63030834.x. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z. M., Thewke D., Gong Q. Q., Schlichter D., Wicks W. D. Brain Res. Mol. Brain Res. 1991;11:309–319. doi: 10.1016/0169-328x(91)90040-5. [DOI] [PubMed] [Google Scholar]
- 42.Fung B. P., Yoon S. O., Chikaraishi D. M. J. Neurochem. 1992;58:2044–2052. doi: 10.1111/j.1471-4159.1992.tb10945.x. [DOI] [PubMed] [Google Scholar]
- 43.Best J. A., Chen Y., Piech K. M., Tank A. W. J. Neurochem. 1995;65:1934–1943. doi: 10.1046/j.1471-4159.1995.65051934.x. [DOI] [PubMed] [Google Scholar]
- 44.Tinti C., Yang C., Seo H., Conti B., Kim C., Joh T. H., Kim K. S. J. Biol. Chem. 1997;272:19158–19164. doi: 10.1074/jbc.272.31.19158. [DOI] [PubMed] [Google Scholar]
- 45.Piech-Dumas K. M., Best J. A., Chen Y., Nagamoto-Combs K., Osterhout C. A., Tank A. W. J. Neurochem. 2001;76:1376–1385. doi: 10.1046/j.1471-4159.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 46.Piech-Dumas K. M., Tank A. W. Brain Res. Mol. Brain Res. 1999;70:219–230. doi: 10.1016/s0169-328x(99)00149-7. [DOI] [PubMed] [Google Scholar]
- 47.Icard-Liepkalns C., Biguet N. F., Vyas S., Robert J. J., Sassone-Corsi P., Mallet J. J. Neurosci. Res. 1992;32:290–298. doi: 10.1002/jnr.490320219. [DOI] [PubMed] [Google Scholar]
- 48.Nankova B., Hiremagalur B., Menezes A., Zeman R., Sabban E. Brain Res. Mol. Brain Res. 1996;35:164–172. doi: 10.1016/0169-328x(95)00201-3. [DOI] [PubMed] [Google Scholar]
- 49.Nagamoto-Combs K., Piech K. M., Best J. A., Sun B., Tank A. W. J. Biol. Chem. 1997;272:6051–6058. doi: 10.1074/jbc.272.9.6051. [DOI] [PubMed] [Google Scholar]
- 50.Lange D. L., Haywood J. R., Hinojosa-Laborde C. Hypertension. 1998;31:403–408. doi: 10.1161/01.hyp.31.1.403. [DOI] [PubMed] [Google Scholar]
- 51.de Miguel R., Fernandez-Ruiz J. J., Hernandez M. L., Ramos J. A. Life Sci. 1989;44:1979–1986. doi: 10.1016/0024-3205(89)90411-6. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Ruiz J. J., Bukhari A. R., Martinez-Arrieta R., Tresguerres J. A., Ramos J. A. Life Sci. 1988;42:1019–1028. doi: 10.1016/0024-3205(88)90432-8. [DOI] [PubMed] [Google Scholar]
- 53.Lenders J. W., De Boo T., Lemmens W. A., Reijenga J., Willemsen J. J., Thien T. Am. J. Cardiol. 1988;61:1288–1291. doi: 10.1016/0002-9149(88)91171-x. [DOI] [PubMed] [Google Scholar]
- 54.Cavadas C., Silva A. P., Cotrim M. D., Ribeiro C. A., Brunner H. R., Grouzmann E. Ann. N.Y. Acad. Sci. 2002;971:335–337. doi: 10.1111/j.1749-6632.2002.tb04489.x. [DOI] [PubMed] [Google Scholar]
- 55.Grouzmann E., Werffeli-George P., Fathi M., Burnier M., Waeber B., Waeber G. J. Clin. Endocrinol. Metab. 1994;79:1852–1856. doi: 10.1210/jcem.79.6.7989494. [DOI] [PubMed] [Google Scholar]
- 56.Grouzmann E., Aubert J. F., Waeber B., Brunner H. R. Peptides. 1992;13:1049–1054. doi: 10.1016/0196-9781(92)90004-m. [DOI] [PubMed] [Google Scholar]
- 57.Grouzmann E., Cavadas C., Grand D., Moratel M., Aubert J. F., Brunner H. R., Mazzolai L. Pflügers Arch. 2003;447:254–258. doi: 10.1007/s00424-003-1140-x. [DOI] [PubMed] [Google Scholar]
- 58.Moura E., Pinho Costa P. M., Moura D., Guimaraes S., Vieira-Coelho M. A. Life Sci. 2005;76:2953–2964. doi: 10.1016/j.lfs.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 59.Soares-da-Silva P., Pestana M., Vieira-Coelho M. A., Fernandes M. H., Albino-Teixeira A. Br. J. Pharmacol. 1995;114:1403–1413. doi: 10.1111/j.1476-5381.1995.tb13362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.