Abstract
Many photosynthetic microorganisms acclimate to CO2 limited environments by induction and operation of CO2-concentrating mechanisms (CCMs). Despite their central role in CCM function, inorganic carbon (Ci) transport systems never have been identified in eukaryotic photosynthetic organisms. In the green alga Chlamydomonas reinhardtii, a mutant, pmp1, was described in 1983 with deficiencies in Ci transport, and a Pmp1 protein-associated Ci uptake system has been proposed to be responsible for Ci uptake in low CO2 (air level)-acclimated cells. However, even though pmp1 represents the only clear genetic link to Ci transport in microalgae and is one of only a very few mutants directly affecting the CCM itself, the identity of Pmp1 has remained unknown. Physiological analyses indicate that C. reinhardtii possesses multiple Ci transport systems responsible for acclimation to different levels of limiting CO2 and that the Pmp1-associated transport system is required specifically for low (air level) CO2 acclimation. In the current study, we identified and characterized a pmp1 allelic mutant, air dier 1 (ad1) that, like pmp1, cannot grow in low CO2 (350 ppm) but can grow either in high CO2 (5% CO2) or in very low CO2 (<200 ppm). Molecular analyses revealed that the Ad1/Pmp1 protein is encoded by LciB, a gene previously identified as a CO2-responsive gene. LciB and three related genes in C. reinhardtii compose a unique gene family that encode four closely related, apparently soluble plastid proteins with no clearly identifiable conserved motifs.
Keywords: bicarbonate, CO2-concentrating mechanism, microalgae, photosynthesis
Although present in small quantities in the air, carbon dioxide (CO2) has profound influences on the living environments by serving as the major substrate for photosynthesis. In nature, the ambient CO2 concentrations for photosynthetic organisms can vary across orders of magnitude and often become the limiting factor for carbon acquisition. Many aquatic photosynthetic microorganisms use a CO2-concentrating mechanism (CCM) to maximize photosynthesis under limiting CO2 conditions. Photosynthesis of these microorganisms grown in limited CO2 environments displays a characteristic similar to that in C4 photosynthesis, with much higher apparent affinity for CO2 (1, 2). However, unlike the CO2 enrichment in C4 plants, CCMs in aquatic photosynthetic microorganisms operate by accumulating a large amount of dissolved inorganic carbon (Ci; CO2 and/or bicarbonate) intracellularly, the uptake of which is driven by energy-coupled Ci transport systems.
As vital components of CCMs, Ci transport systems have been extensively studied in the prokaryotic model organisms, cyanobacteria. With the aid of mutant studies and the recent availability of several genomes, at least four transport modes involved in Ci uptake have been identified and characterized in cyanobacteria, including two bicarbonate transporters and two CO2 uptake systems associated with the operation of specialized NDH-1 complexes (3). However, little information other than its physiological demonstration is available regarding Ci transport in eukaryotic photosynthetic microorganisms. The unicellular green alga Chlamydomonas reinhardtii has served as a key model system to study CCMs for many years, and several genes required for acclimation to limiting CO2 have been cloned and characterized in this organism (4–10), but no transport system for Ci uptake has been definitively identified and characterized.
Even though specific defects in several mutants requiring elevated CO2 for survival have been identified, only three characterized mutants can be argued as having defects in genes required unambiguously for operation of the CCM, and one of these mutants, cia5 (and various alleles, including ccm1), appears to be defective in a master regulator (Cia5 or Ccm1) for induction of the CCM and other proteins required for limiting CO2 acclimation (4, 6, 11), rather than a functional component of the CCM. Another key mutant, ca1 (and various alleles, including cia3), corresponds to a thylakoid lumen carbonic anhydrase (Cah3) apparently required for the rapid dehydration of intracellular bicarbonate accumulated by active Ci transport (5, 7, 12). The third of these key mutants, pmp1, was characterized more than two decades ago as being impaired in Ci transport (13) and, thus, far represents the only mutant identified with a specific defect in Ci transport in a eukaryotic photosynthetic organism. These mutants form the foundation for our understanding of the C. reinhardtii CCM, demonstrating the requirement for active Ci transport (pmp1) to accumulate intracellular Ci and a thylakoid lumen CA (ca1) for dehydration of the intracellular Ci accumulated as a bicarbonate.
Among these three classic mutants, the defective gene in pmp1 and the identity of Pmp1 protein, thus far, have resisted identification. Although initially identified as a probable Ci transport mutant, a recent study reported that the expression profiles of several CO2 responsive genes in pmp1 differ from those in wild type and suggested that the Pmp1 gene product might regulate the expression of Ci transporter genes (14). Another recent observation regarding pmp1 is its unusual, air-dieing phenotype (15, 16): it grows well in either high (5%) or very low (<200 ppm) CO2, but dies in low (air-level) CO2 (350–450 ppm). This conspicuous phenotype distinguishes pmp1 from most other high CO2-requiring mutants and indicates the existence of multiple Ci transport systems in C. reinhardtii corresponding to multiple, CO2 level-dependent acclimation states. Indeed, at least three distinct CO2 acclimation states have been demonstrated in C. reinhardtii, corresponding to: high CO2, ≥0.5% CO2; low CO2, 0.4–0.03% CO2; and very-low CO2, ≤0.01% CO2 (17). Therefore, the Pmp1 protein must play either a functional or a regulatory role in a Ci transport system specific for the low (air-level) CO2 acclimation state.
To understand the mechanism of limiting CO2 acclimation in eukaryotic photosynthetic organisms, we have taken an insertional mutagenesis approach to identify functional components involved in limiting CO2 acclimation in C. reinhardtii. Here we describe the identification and characterization of a mutant that displays an air-dieing phenotype, air dier1 (ad1). Our results demonstrate that the defective gene in ad1 is allelic to pmp1 and that it belongs to a small family of genes encoding an apparently unique group of proteins in C. reinhardtii.
Results
Identification of the air dier 1 (ad1) Mutant.
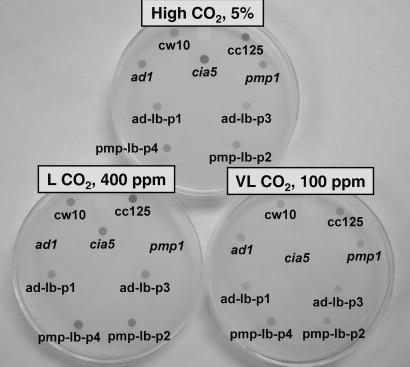
To isolate and identify C. reinhardtii mutants unable to acclimate to air levels of CO2, we performed insertional mutagenesis by using a BleR-containing plasmid (pSP24s) (18) to transform a wallless, wild-type strain, cw10. The air dieing phenotype was evaluated in spot tests, based on the ability to grow in high, low (air-level), or very low CO2 concentrations. From ≈2,500 transformants, two mutants displayed the “air dier” (ad) phenotype, and one, ad1, was selected for further investigation. As with pmp1, ad1 and wild type grow in either high CO2 or very low CO2 but dies in low (air-level) CO2 (Fig. 1). In contrast, another classic mutant, cia5, previously identified as defective in acclimation responses to limiting CO2, grows similar to wild type in high CO2, somewhat more slowly in low (air-level) CO2, but dies in very low CO2.
Fig. 1.
Spot test for growth of C. reinhardtii strains in high CO2 [5% (vol/vol)], low CO2 (400 ppm), and very low CO2 (100 ppm). The strains include wild-type (cw10 and CC125), mutants cia5, pmp1, and ad1, and the LciB-complemented ad1 (ad-lb-p1 and ad-lb-p3) and pmp1 (pmp-lb-p2 and pmp-lb-p4) cell lines.
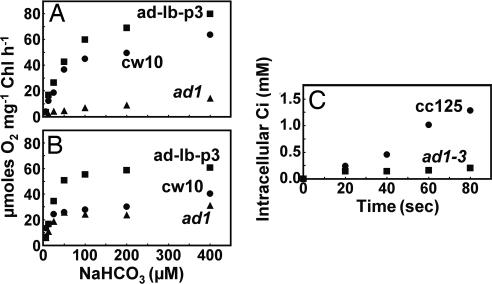
Photosynthetic O2 evolution in response to Ci concentrations for low CO2 acclimated and very low CO2 acclimated wild-type and ad1 cells was compared (Fig. 2 A and B). The ad1 mutant cells acclimated in low CO2 showed dramatically decreased photosynthetic Ci affinity compared with wild-type cells grown under the same conditions. In contrast, when acclimated to very low CO2, the photosynthetic O2 evolution of ad1 and wild type exhibited similar responses to external Ci concentrations. Furthermore, ad1 acclimated to low CO2 exhibited dramatically reduced Ci accumulation compared with wild type (Fig. 2C), similar to that observed with pmp1 (13). These results demonstrate that the air dier phenotype in ad1 is due to an impaired photosynthetic affinity in low (air-level) CO2, which is caused by a deficiency in Ci transport in this CO2 concentration.
Fig. 2.
Inorganic carbon (Ci)-dependent photosynthetic O2 evolution and internal Ci accumulation in wild-type C. reinhardtii and the ad1 mutant. (A and B) Response of Ci-dependent photosynthetic O2 evolution (measured at pH 7.3 and a chlorophyll concentration of 20 μg/ml) to NaHCO3 concentrations in wild-type (cw10; ●), ad1 (▴), and LciB-complemented ad1 (ad-lb-p3; ■) cells acclimated to low CO2 (A) or very low CO2 (B). (C) acid-labile intracellular Ci accumulation by wild-type (CC125; ●) and ad1-3 (a walled progeny of ad1; ■) cells (25 μg/ml chlorophyll) acclimated in low CO2 for 24 h. 14C-uptake was measured at pH 7.3 and an initial added Ci concentration of 50 μM (NaH14CO3).
Identification of the Ad1 Gene.
The ad1 strain was crossed with wild-type strain CC620 to determine whether the air dier phenotype in the ad1 mutant cosegregated with the inserted BleR gene. More than 100 random progeny were tested for their growth in different levels of CO2 and their resistance to zeocin, which indicates the presence of the BleR insert. Although 50 random progeny with the air dier phenotype all exhibited zeocin resistance, all zeocin-sensitive progeny showed wild-type growth in low CO2, indicating cosegregation of the air dier phenotype with the BleR insert. DNA gel blot analysis with probes specific for the BleR gene and pBluescript sequences indicated a single insert present in ad1 (data not shown).
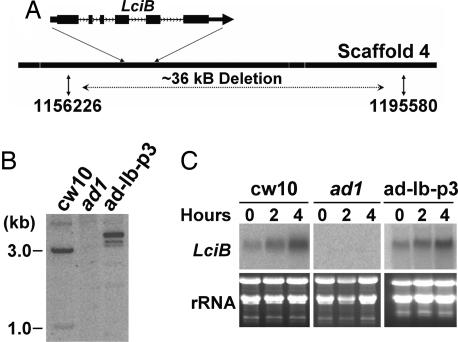
DNA flanking the BleR gene in ad1 was cloned from ad1 genomic DNA by inverse PCR. This sequence was used in a blast search against the C. reinhardtii genome, and the insertion site was shown to be located on scaffold 4 of the genome draft (version 3.0 of the C. reinhardtii genome, http://genome.jgi-psf.org/Chlre3/Chlre3.home.html). Further PCR and DNA gel blot analyses revealed a large deletion of a large segment of the genomic DNA sequence in ad1 at the site of insertion, presumably caused by the integration of the BleR insert (Fig. 3A). Based on the C. reinhardtii genome sequence and PCR analysis of the deleted region, we used PCR to recover the sequence flanking the opposite end of the BleR insert and found the deletion to encompass a region of ≈36 kb containing several predicted ORFs (Fig. 3A) (scaffold_4:1156226–1195580; http://genome.jgi-psf.org/Chlre3/Chlre3.home.html).
Fig. 3.
Cloning of Ad1 and complementation of ad1 and pmp1 by LciB. A. Integration of pSP124s resulted in a deletion of −36 kb of genomic DNA. A schematic presentation of the exon-intron structure of LciB is shown with exons of the coding region (filled boxes) and introns (black lines) indicated. (B) Southern blot analysis indicating the presence of the LciB gene in wild-type cw10 and one of the ad1 strains (ad-lb-p3) complemented with a PCR-amplified genomic DNA fragment containing the LciB gene, but not in ad1. Genomic DNA was digested with SalI, separated by agarose gel electrophoresis and hybridized against an LciB gene specific probe. (C) Northern blot analysis indicates the expression of LciB recovered in one of the complemented ad1 strains (ad-lb-p3). Total RNA (10 μg per lane) was isolated after high CO2 grown cells (0 h) were transferred into low CO2 for 2 and 4 h. Hybridization was performed with an LciB gene-specific probe.
A sequence flanking the BleR insert was used as probe to identify C. reinhardtii bacterial artificial chromosome (BAC) clones containing wild-type genomic DNA overlapping the site of the insertion, and DNA from two identified clones was demonstrated to complement the air dier phenotype of ad1. Using Southern and PCR analyses to determine whether any of the predicted genes located within the deleted region were present in complemented lines, only one gene, identical in sequence to a previously reported CO2 responsive gene, LciB (14), was found to be present in all BAC complemented ad1 lines, and other predicted genes either were not present at all or were present only in some complemented lines (data not shown).
To confirm whether the LciB gene is the Ad1 gene, we PCR-amplified a genomic DNA fragment containing LciB from a wild-type BAC and found the DNA fragment could complement ad1. Complemented ad1 lines and wild type grew in both low and very low CO2 (Fig. 1), and Southern analysis indicated that all putative complemented lines carried the genomic DNA of the LciB gene, which is absent from the ad1 mutant (Fig. 3B). In addition, RNA gel blot analysis showed that all complemented lines recovered the expression of LciB (Fig. 3C). Complementation of ad1 also was achieved by expressing an LciB cDNA under control of the constitutive PsaD promoter and terminator (data not shown).
ad1 Is a pmp1 Allele.
The ad1 mutant appears very similar to pmp1 in its air dier phenotype and impaired photosynthesis and Ci transport. Miura et al. (14) reported that induction of three CO2-responsive genes by limiting CO2 was abolished in pmp1. These genes include two putative Ci transporter genes, LciA and Mrp1, and LciB, which also was suggested by Miura et al. (14) to be a putative Ci transporter gene. When we compared the low CO2-induced expression of LciA and Mrp1 in ad1 and pmp1 with that in wild type (Fig. 4), we found that expression of both LciA and Mrp1 was induced in all three strains upon exposure of high CO2-grown cells to either low (air-level) CO2 or very low CO2. Although we did observe a slightly reduced expression of LciA and Mrp1 in ad1 and pmp1 to a variable extent, it seems unlikely that this is a direct result of the lesion in LciB. The failure of Miura et al. (14) to observe the induction of LciA and Mrp1 gene expression in pmp1 might be explained by their short induction time (2 h) and different growth conditions. Nevertheless, our results demonstrate ad1 is very similar to pmp1 in its expression of these putative Ci transporter genes and that any reduced induction of LciA and Mrp1 in pmp1 or ad1 is relatively minor and likely to be pleiotropic.
Fig. 4.
Mrp1 and LciA transcript accumulation in wild-type (cw10), ad1, and pmp1 cells. Total RNA (10 μg per lane) was isolated from high (5%) CO2-grown cells and from cells acclimated to low (air-level) CO2 or very low (100 ppm) CO2 for time durations as indicated and probed with fragments corresponding to Mrp1-and LciA-coding regions.
Crosses between ad1 and pmp1 failed to produce recombinants or diploids with a wild-type growth phenotype, suggesting that ad1 is likely to be a pmp1 allele. This conclusion was confirmed by the complementation of pmp1 with LciB. Both the genomic and cDNA forms of LciB that complemented ad1 also successfully complemented pmp1 (Fig. 1). Comparison of the DNA sequence of LciB from pmp1 and that from wild type revealed a point mutation (C > A) at nucleotide position 105 in pmp1. This mutation would result in a stop codon in place of tyrosine at amino acid 35 of the wild-type gene product and, therefore, result in an extremely truncated LciB gene product in pmp1 (Fig. 5).
Fig. 5.
Sequence similarity among the deduced proteins of the LciB family. Amino acids in a black background represent identical residues, and a gray background represents conserved residues for the four sequences. The arrow indicates the site of mutation in LciB of pmp1-converting tyrosine codon to a stop codon.
LciB Gene Family.
blast searches and domain searches of several databases with LciB revealed no significant recognizable domains nor significant homologies, except for three additional genes in the C. reinhardtii genome (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html; Fig. 5): a similar CO2 responsive gene, LciC (14), on scaffold 12, and two previously unreported genes, LciD and LciE, both on scaffold 15. As noted by Miura et al. (14), the LciB and LciC gene products are predicted to be soluble proteins, probably targeted to the plastid. This situation also is the case for LciD and LciE. However, considering the low sensitivity of currently available prediction tools for discriminating subcellular targeting for C. reinhardtii proteins, especially for differentiating between plastid and mitochondrion proteins, one cannot exclude the possibility that the products of LciB gene family are mitochondrion localized.
The LciB and LciC gene products are quite similar in their predicted amino acid sequence (57% identity; 73% similarity), as are LciD and LciE (71% identity; 78% similarity), with these two protein pairs also sharing substantial similarity with each other (40–44% identity; 62–65% similarity), thus constituting an LciB protein family. LciD and LciE are aligned head to head in the genome with another pair of CO2 responsive genes, Ccp2 (LIP36 G2) and Ccp1 (LIP36 G1) (1, 19), respectively, in an apparent inverted repeat. The inverted regions flank another set of CO2 responsive genes, Cah1 and Cah2 (20), forming a cluster of six CO2 responsive genes within a 75-kb region on scaffold 15. The significance of this arrangement, if any, is not clear.
Genes of the LciB Family as CO2 Responsive Genes.
Miura et al. (14) demonstrated that LciB and LciC were up-regulated by limiting CO2. Because the two genes share high similarity in their coding sequence, we used gene-specific 3′ UTR probes for LciB and LciC in Northern blot analyses and showed that the LciB and LciC genes had very similar patterns of limiting CO2-induced expression (Fig. 6). In wild type, both genes showed constitutive expression with low mRNA abundance under high CO2 conditions, whereas both mRNA levels increased dramatically when cells were transferred into either low CO2 (400 ppm) or very low CO2 (100 ppm; Fig. 6), or into a level of CO2 (1,500 ppm) intermediate between low and high CO2 (data not shown), indicating that up-regulation of LciB and LciC expression was not confined to a specific level of limiting CO2.
Fig. 6.
Expression of genes of the LciB family under different CO2 conditions. RNA gel-blot analysis of LciB, LciC, and LciD expression (probed with PCR fragments specifically corresponding to LciB, LciC, and LciD 3′ UTR regions, respectively) was performed in wild-type (cw10), ad1, and cia5 mutants. Total RNA (10 μg per lane) was isolated from high CO2-grown cells or from cells acclimated to low CO2 (air) or very low CO2 (50 ppm) for the time durations indicated.
There were no ESTs available for LciD and LciE, suggesting that they either are genes with low expression or are genes expressed under conditions different from those used for EST identification and from those inducing LciB/LciC expression. We amplified predicted 3′ UTR sequences of LciD and LciE from a cDNA library based on their predicted genomic sequences and used these specific, amplified probes to analyze the expression of these genes. On RNA gel blots, the LciD gene showed two bands hybridizing to the LciD probe (Fig. 6) and a limiting CO2-inducible expression pattern similar to those of LciB and LciC but with relatively lower mRNA abundance. LciE expression was not detectable on RNA gel blots by using a predicted LciE-specific probe, although we did successfully verify this 3′UTR and the expression of LciE by amplifying a partial LciE cDNA from a cDNA library and subsequently sequencing the PCR product. Attempts to identify full-length LciD and LciE cDNAs by screening a cDNA library with predicted LciD and LciE coding region probes yielded five cDNA clones, all of which were determined by sequencing to be from LciD. Comparison of 3′ UTR sequences of the LciD cDNA clones revealed two 3′ UTR sequences with different lengths, indicating that alternative termination occurred, possibly explaining the two LciD-hybridizing bands on gel blots. Because LciE expression was undetectable in Northern analyses and attempts to identify a full-length LciE cDNA from the cDNA library were not successful, it appears that the expression of LciE may be relatively low compared with other genes in this family, at least under the conditions explored in this work and under conditions used for construction of the cDNA library.
Discussion
Ad1/Pmp1: Transporter or Regulator?
Despite being major components in the CCM, Ci transport systems in eukaryotic photosynthetic organisms remain largely unknown. Since its identification more than two decades ago, the pmp1 mutant has been touted as demonstrating a Ci transport requirement in the CCM (1, 13). In this work, we generated a new mutant allele of pmp1, ad1, by insertional mutagenesis, and identified the Ad1/Pmp1 gene. We demonstrated that a lesion in LciB (Ad1/Pmp1) caused the air dier phenotype and greatly decreased Ci transport and photosynthetic activity in ad1 and pmp1, presumably only in low CO2.
Physiological and biochemical characterization of pmp1 suggests that Pmp1 is a functional component involved in Ci transport. Although both pmp1 and ad1 also have reduced CO2 assimilation in low CO2, the dramatically decreased internal Ci accumulation in low CO2 argues strongly for a defect in Ci transport rather than internal Ci utilization. However, being predicted to be a soluble protein with no obvious transmembrane regions, LciB (Pmp1/Ad1) seems unlikely to perform as an intact active Ci transporter by itself, because hydrophobic transmembrane domains are signatures for almost all identified transporters. However, it is possible that LciB interacts with transmembrane proteins as a functional component of a transporter complex. In this case, LciB could either play a regulatory role, or be directly involved in transporting Ci with other components in the complex.
Alternatively, Pmp1 as a general regulator for multiple Ci uptake systems also has been suggested. Miura et al. (14) reported the lack of induction or up-regulation of several putative transporter genes in pmp1, including LciB itself. These authors therefore proposed that Pmp1 is involved in regulation of multiple Ci transporters. In the current study, we have shown that transcripts of these putative transporters still were present in pmp1 and ad1 (except LciB in ad1), although we did observe that their mRNA abundance in low CO2 often was reduced in both the ad1 and pmp1 mutants, but to a variable extent. Because LciB is predicted to localize in plastids (or possibly mitochondria), this protein is not expected to be a transcription factor and directly involved in transcription regulation, like Cia5. If LciB affects the synthesis of new transcripts or the stability of these putative transporter transcripts, it is more likely that it affects these processes in an indirect way.
However, given the probable plastid localization of LciB and the nonreproducibility of the decreased expression of putative Ci transporters in pmp1/ad1, the direct involvement of LciB in Ci uptake seems more plausible than the regulation by LciB of the expression of other Ci transporter genes. In fact, the physiological evidence from pmp1/ad1 for nearly a complete lack of Ci transport, even though the expression of Mrp1 and LciA still is present, argues for a direct involvement of LciB with Ci transport.
It is not clear why a defect in only one gene (LciB itself) from the LciB gene family causes the air dier phenotype in ad1 and pmp1 despite the high sequence similarity and similar limiting CO2-inducible expression patterns among the genes in the LciB family, especially the strong similarities between LciB and LciC. It is possible that interaction of LciC and LciB is required for a functional transporter complex, or a regulator complex for a Ci transporter(s), and this possibility should be investigated.
Acclimation to Multiple Levels of CO2.
The photosynthesis of ad1 was impaired only in low (air-level) CO2-acclimated cells, which apparently is caused by defective Ci transport. In very low CO2-acclimated cells, photosynthesis of ad1 recovered to a level similar to that in wild type. These results confirm the existence of distinct states for very low CO2 acclimation and low (air-level) CO2 acclimation in C. reinhardtii. Therefore, limiting CO2 acclimation in C. reinhardtii must require at least two (probably overlapping) suites of proteins that are differentially expressed or activated in different levels of limiting CO2. LciB obviously is associated with and required for the low (air-level) CO2 acclimation. Photosynthetic measurements also showed that very low CO2-acclimated cells have a relatively higher affinity for Ci but lower photosynthesis at near-saturated Ci concentrations, relative to cells acclimated to low (air-level) CO2. This observation is consistent with a recent report on different physiological states for limiting CO2 acclimation in C. reinhardtii, in which very low CO2-acclimated cells exhibited lower K½(CO2) and Vmax compared with low CO2-acclimated cells (17). The difference in K½(CO2) and Vmax between low CO2-grown cells and very low CO2-grown cells implies that the Ci transport system specific for low (air-level) CO2 has a relatively lower affinity for Ci but higher transport capacity, whereas the system specific for very low CO2 has a higher affinity for Ci but a lower capacity. This acclimation may represent an excellent survival strategy in C. reinhardtii for acclimation to different levels of limiting CO2: In very low CO2, a Ci uptake system with a high affinity and low capacity would allow C. reinhardtii cells to grow at a reasonable rate without depleting all available Ci, whereas in low (air-level) CO2, a high capacity for Ci uptake could maintain optimal growth, and a transporter with relatively low affinity would be sufficient to accommodate the Ci uptake in low CO2.
Identification of the defect responsible for the Ci transport deficiency in pmp1 and ad1 represents a critically important step toward understanding Ci transport, its role in the CCM, and its regulation in eukaryotic microalgae. It clearly will be important to fully understand the role of LciB and the other members of the LciB gene family in limiting CO2 acclimation, including any role they may play in distinguishing the low CO2 and very low CO2 acclimation states. The majority of past research on limiting or low CO2 acclimation in C. reinhardtii and other microalgae has focused mainly on air level CO2 acclimation, whereas targeted research on very low CO2 acclimation has been limited. Future investigation of this distinct state should help fill the gap in our understanding of the multiple levels of CO2 acclimation in C. reinhardtii or other eukaryotic photosynthetic cells.
Materials and Methods
C. reinhardtii Strains, Culture, and Gas Conditions.
C. reinhardtii strains CC849, CC620, and CC125 were obtained from the Chlamydomonas Genetics Center, Duke University, Durham, NC. The pmp1 and cia5 mutants have been described in refs. 11 and 13. Wild-type cells and high CO2-requiring mutants were maintained on agar plates with CO2 minimal medium (21) and kept in Plexiglas chambers at room temperature. Liquid cultures were grown in Erlenmeyer flasks on an orbital shaker at 125 rpm. In both plate and liquid cultures, continuous gas flow was maintained through either the growth chambers or the culture flasks. Three gas conditions used in this study were: high CO2 (5% CO2 in air vol/vol), obtained by mixing compressed CO2 with normal air; low CO2 (normal air, 350–400 ppm); and very low CO2 (50–150 ppm), obtained by mixing normal air with either compressed CO2-free air or CO2-depleted air (air passed through a saturated sodium hydroxide solution).
Isolation of air dier Mutants, Growth Spot Tests, and Genetic Analysis.
C. reinhardtii wall-less strain CC849 (cw10, mt−) was transformed with linearized pSP124s plasmid (ref. 18; a gift from Saul Purton, University of London, London) by the glass bead method (22). Transformed cells were kept in high CO2 and selected on minimal medium plates supplemented with 10 μg/ml zeocin. Zeocin-resistant transformants were transferred to duplicate plates for screening by growth spot tests in high CO2, low CO2, and very low CO2. Mutants exhibiting an air dier phenotype were maintained in the high CO2 chamber.
Spot growth tests were performed by suspending actively growing cells in minimal medium to similar, low-cell densities (<106 cells/ml), then spotting 3 μl of each cell suspension onto agar plates and kept in high CO2, low CO2, or very low CO2 chambers for 8 days.
Genetic crosses and tetrad analyses were performed as described by Harris (23).
Photosynthetic O2 Evolution and Ci Uptake.
Photosynthetic O2 evolution was measured at 25°C with a Clark-type O2 oxygen electrode (Rank Brothers, Cambridge, U.K.). Cells from liquid cultures were collected by centrifugation and suspended in N2-saturated Mops-Tris (25 mM, pH 7.3) to a final chlorophyll concentration of 20 μg/ml. Internal and external Ci first were depleted under illumination (500 μmol photons·m−2·s−1) as judged by cessation of O2 evolution before measurements were initiated by addition of various concentrations of NaHCO3.
Ci uptake by C. reinhardtii cells was measured by the silicone oil filtration technique (24, 25) by using one of the walled ad1 progeny from the cross with CC620, because Ci uptake experiments were found to be unreliable with wall-less strains.
DNA and RNA Blot Analysis.
Genomic DNA was isolated and purified in the presence of CTAB as described by Ausubel et al. (26). Total RNA was purified by the acid guanidinium thiocyanate-phenol-chloroform method described by Chomczynski and Sacchi (27).
Southern and Northern analyses were performed by standard procedures (28), and membranes were scanned by using a phosphorimager (Storm).
Isolation of Sequences Flanking the BleR Insert from ad1 by Inverse PCR.
Based on information from Southern blot analysis, BamHI was used to digest the genomic DNA isolated from ad1 to produce a fragment with a size ≈1.5kb, including part of the inserted pSP124s vector and its flanking genomic DNA. The BamHI-digested ad1 genomic DNA (0.2 μg) was circularized with 1 unit of T4 DNA ligase (Invitrogen), precipitated, and the circularized product was used as template for inverse PCR by using standard PCR procedures. Three pairs of primers were designed, with each pair complementing the pSP124s sequence in opposite orientations. All three primer pairs produced PCR products with the correct predicted sizes, and amplified DNA from one primer pair (5′-CTGGACCGCGCTGATGAACA-3′ and 5′-GGAGGTCGTGTCCACGAACT-3′) was sequenced to determine the sequence flanking the insert.
Identification of BAC Clones Containing the Wild-Type Ad1 Gene and Complementation of ad1 and pmp1.
DNA flanking the site of insertion in ad1 was PCR-amplified based on the sequence of the DNA from inverse PCR and the C. reinhardtii genome (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html). Using this amplified DNA as a probe, six BAC clones containing wild-type DNA from the inserted region were identified from a BAC library (Clemson University; www.genome.clemson.edu/groups/bac).
All complementation was performed by the glass bead transformation procedure (22). After transformation, cells were kept in low (air-level) CO2 to observe wild-type growth of complemented mutants. Cells transformed with the empty vector or mock DNA were used as controls. For BAC complementation of ad1, DNA isolated from BAC clones 26B2 and 14L14 were used to transform ad1.
In complementing ad1 and pmp1 with LciB genomic DNA, a 3.6-kb fragment of genomic DNA containing the LciB coding region and putative promoter region was PCR-amplified from a BAC clone (26B2) by using a pair of primers: upper primer, 5′-GAGTAGGCGTCGCGTCGTAA-3′, and lower primer, 5′-CGACACTGACGGCGCAATTA-3′; they were used to transform ad1 and pmp1.
In complementing ad1 and pmp1 with LciB cDNA, LciB cDNA was PCR-amplified from a cDNA library (an expression cDNA library described below) with specific primers that introduced an NdeI site overhanging the start codon ATG at the 5′ end, and an EcoRI site after the stop codon at the 3′ end: upper primer, 5′-ACGCAGCATATGTTCGCTCTGTCTTC-3′; lower primer, 5′-TTGAATTCGTTAGCACGCCAGGAG-3′. The amplified cDNA was digested by EcoRI and NdeI and ligated into EcoRI/NdeI-digested pGenD plasmid (29), which placed the LciB cDNA between the PsaD promoter and terminator. This plasmid was linearized and used to transform ad1 and pmp1.
Construction and Screening of the cDNA Expression Library.
Pooled mRNA (a gift from John Davies, Exelixis Plant Sciences, Portland, OR) isolated from cells grown to mid-log phase in tris-acetate-phosphate (TAP) (acetate-containing) medium in the light, TAP medium in the dark, high salt (HS) (minimal) medium in ambient levels of CO2, and HS medium bubbled with 5% CO2 and identical to that used for constructing the core libraries described by Shrager et al. (30), was used to construct the C. reinhardtii cDNA expression library by using the HybriZAP 2.1 two-hybrid system (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. To reduce secondary structure in the mRNA template, the reverse transcription reaction was performed by using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) at 50°C as suggested by Shrager et al. (30).
For identification of the LciD cDNA, a pair of primers was designed based on the sequence flanking the 3′ end of the predicted LciD coding region (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html): upper primer, 5′-AAGAAAGGCCTCGCTTAACG-3′, and lower primer, 5′-GGTACTGGGTGCAAGCTAAT-3′, and was used to amplify the putative 3′ UTR of LciD from the HybriZaP2.1 library by PCR. The amplified PCR product was used as a probe to screen the HybriZaP2.1 library. Five cDNA clones were identified and sequenced.
Abbreviations
- CCM
CO2 concentrating mechanisms
- Ci
inorganic carbon.
Footnotes
References
- 1.Spalding M. H. In: the Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Rochaix J. D., Goldschmidt-Clermont M., Merchant S., editors. Dordrecht, The Netherlands: Kluwer; 1998. pp. 529–547. [Google Scholar]
- 2.Kaplan A., Reinhold L. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 1999;50:539–559. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 3.Badger M. R., Price G. D. J. Exp. Bot. 2003;54:609–622. doi: 10.1093/jxb/erg076. [DOI] [PubMed] [Google Scholar]
- 4.Xiang Y., Zhang J., Weeks D. P. Proc. Natl. Acad. Sci. USA. 2001;98:5341–5346. doi: 10.1073/pnas.101534498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funke R. P., Kovar J. L., Weeks D. P. Plant Physiol. 1997;114:237–244. doi: 10.1104/pp.114.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuzawa H., Miura K., Ishizaki K., Kucho K., Saito T., Kohinata T., Ohyama K. Proc. Natl. Acad. Sci. USA. 2001;98:5347–5352. doi: 10.1073/pnas.081593498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson J., Clarke A. K., Chen Z. Y., Hugghins S. Y., Park Y. I., Husic H. D., Moroney J. V., Samuelsson G. EMBO J. 1998;17:1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mamedov T. G., Suzuki K., Miura K., Kucho K., Fukuzawa H. J. Biol. Chem. 2001;276:45573–45579. doi: 10.1074/jbc.M103882200. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y., Kanakagiri S., Van K., Spalding M. H. Can. J. Bot. 2005;83:796–809. [Google Scholar]
- 10.Pollock S. V., Colombo S. L., Prout D. L., Jr, Godfrey A. C., Moroney J. V. Plant Physiol. 2003;133:1854–1861. doi: 10.1104/pp.103.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moroney J. V., Husic H. D., Tolbert N. E., Kitayama M., Manuel L. J., Togasaki R. K. Plant Physiol. 1989;89:897–903. doi: 10.1104/pp.89.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spalding M. H., Spreitzer R. J., Ogren W. L. Plant Physiol. 1983;73:268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spalding M. H., Spreitzer R. J., Ogren W. L. Plant Physiol. 1983;73:273–276. doi: 10.1104/pp.73.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura K., Yamano T., Yoshioka S., Kohinata T., Inoue Y., Taniguchi F., Asamizu E., Nakamura Y., Tabata S., Yamato K. T., et al. Plant Physiol. 2004;135:1595–1607. doi: 10.1104/pp.104.041400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van K., Wang Y., Nakamura Y., Spalding M. H. Plant Physiol. 2001;127:607–614. [PMC free article] [PubMed] [Google Scholar]
- 16.Spalding M. H., Van K., Wang Y., Nakamura Y. Funct. Plant Biol. 2002;29:221–230. doi: 10.1071/PP01182. [DOI] [PubMed] [Google Scholar]
- 17.Vance P., Spalding M. H. Can. J. Bot. 2005;83:820–833. [Google Scholar]
- 18.Lumbreras V., Stevens D. R., Purton S. Plant J. 1998;14:441–448. [Google Scholar]
- 19.Chen Z.-Y., Lavigne M. D., Mason C. B., Moroney J. V. Plant Physiol. 1997;114:265–273. doi: 10.1104/pp.114.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujiwara S., Fukuzawa H., Tachiki A., Miyachi S. Proc. Natl. Acad. Sci. USA. 1990;87:9779–9783. doi: 10.1073/pnas.87.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geraghty A. M., Anderson J. C., Spalding M. H. Plant Physiol. 1990;93:116–121. doi: 10.1104/pp.93.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindle K. L. Proc. Natl. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris E. H. The Chlamydomonas Source Book: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic; 1989. [DOI] [PubMed] [Google Scholar]
- 24.Badger M. R., Kaplan A., Berry J. A. Plant Physiol. 1980;66:407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroney J. V., Husic H. D., Tolbert N. E. Plant Physiol. 1985;79:177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Current Protocols in Molecular Biology. New York: Greene & Wiley; 1987. [Google Scholar]
- 27.Chomczynski P., Sacchi N. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J., Fristch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 29.Fischer N., Rochaix J. D. Mol. Genet. Genomics. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- 30.Shrager J., Hauser C., Chang C.-W., Harris E. H., Davies J., McDermott J., Tamse R., Zhang Z., Grossman A. R. Plant Physiol. 2003;131:401–408. doi: 10.1104/pp.016899. [DOI] [PMC free article] [PubMed] [Google Scholar]