Abstract
To counteract fluctuating nutrient environments, plants have evolved high- and low-affinity uptake systems. These two systems were traditionally thought to be genetically distinct, but, recently, two Arabidopsis transporters, AtKUP1 and CHL1, were shown to have dual affinities. However, little is known about how a dual-affinity transporter works and the advantages of having a dual-affinity transporter. This study demonstrates that, in the case of CHL1, switching between the two modes of action is regulated by phosphorylation at threonine residue 101; when phosphorylated, CHL1 functions as a high-affinity nitrate transporter, whereas, when dephosphorylated, it functions as a low-affinity nitrate transporter. This regulatory mechanism allows plants to change rapidly between high- and low-affinity nitrate uptake, which may be critical when competing for limited nitrogen. These results demonstrate yet another regulatory role of phosphorylation in plant physiology.
Keywords: affinity/Arabidopsis/nitrate/phosphorylation/transporter
Introduction
Plants have a remarkable ability to adapt and grow in soils containing widely different levels of ions and nutrients. Since the early studies of Epstein in the 1950s (Epstein, 1972), it has been known that plants have evolved two types of system for nutrient uptake, a high-affinity system and a low-affinity system. The high capacity low-affinity uptake system takes up nutrients when the external substrate concentration is high (e.g. >0.5 mM for nitrate), while the high-affinity system can scavenge substrate at low concentrations (<0.2 mM for nitrate). The saturation kinetics of ion uptake for the two systems have been interpreted as evidence for at least two transporters with distinct affinities, and this idea was further supported by the identification of distinct genes for the respective systems. For example, a model based on the observed biphasic kinetics of potassium influx proposes that high-affinity active uptake is mediated by proton/potassium symporters, while low-affinity passive uptake is mediated by inward-rectifying potassium channels (Kochian and Lucas, 1988; Maathuis et al., 1997). Indeed, both proton-coupled potassium transporter genes (HAK/KUP family) and potassium channel genes (AKT/KAT family) have been isolated in higher plants (Anderson et al., 1992; Sentenac et al., 1992; Santa-Maria et al., 1997). In contrast to potassium uptake, both the high- and low-affinity uptake of negatively charged nitrate are proposed to be mediated by proton-coupled symporters (Glass and Siddiqi, 1995), and, consistent with this, two families of nitrate transporter genes, NRT1 and NRT2, which share no sequence similarity with each other, have been identified in higher plants (Tsay et al., 1993; Trueman et al., 1996) and have been thought for the past decade to be responsible for low- and high-affinity nitrate uptake, respectively.
In contrast to this model in which high- and low-affinity uptake are mediated by different genes, a knock-out mutant of the AKT1 Arabidopsis potassium channel, which was originally thought to be responsible for low-affinity potassium uptake, was also found to be defective in high-affinity potassium uptake (Hirsch et al., 1998). Likewise, the Arabidopsis potassium transporter, AtKUP1, has also been shown to be a dual-affinity transporter (Fu and Luan, 1998; Kim et al., 1998); when expressed in yeast, it exhibits a Km of ∼44 µM for the high-affinity phase of uptake and ∼11 mM for the low-affinity phase (Fu and Luan, 1998). At almost the same time, it was found that the Arabidopsis low-affinity nitrate transporter mutant chl1 (nrt1) is also defective in high-affinity nitrate uptake (Wang et al., 1998; Liu et al., 1999). Using the Xenopus oocyte expression system, it was further demonstrated that CHL1 functions as a dual-affinity nitrate transporter with a Km of ∼50 µM for the high-affinity phase of uptake and ∼4 mM for the low-affinity phase (Liu et al., 1999). These discoveries of dual-affinity uptake by a single transporter raised the question of whether the kinetic properties of other characterized transporters had been examined over a wide enough range of concentrations to rule out the possibility that they also were dual-affinity transporters, especially since chl1 had been considered as a low-affinity nitrate uptake mutant for >20 years (Doddema and Telkamp, 1979; Tsay et al., 1993; Huang et al., 1996; Touraine and Glass, 1997).
Numerous proteins are known to have dual functions. Many use spatially distinct domains for each function. For example, the TRP family uses an N-terminal domain for its channel activity and a C-terminal domain for enzyme activity (kinase or ADP-ribose pyrophosphatase; Cahalan, 2001), the enzyme activity being required for channel activity (Perraud et al., 2001; Runnels et al., 2001). In contrast, in some dual-function proteins, the mode of action is modulated by post-translational modifications or ligand binding. For example, depending on which amino acid residue (threonine 34 or threonine 75) is phosphorylated, DARPP-32, a dopamine- and cyclic AMP-regulated phosphoprotein, functions as either a kinase inhibitor or a phosphatase inhibitor (Bibb et al., 1999). Calmodulin modulation of the P/Q-type (α1A) calcium channel is another prime example, as binding of calcium to the N-terminal or C-terminal lobe of calmodulin results, respectively, in inactivation or activation of the P/Q-type calcium channel (DeMaria et al., 2001).
Dual-function proteins may not have evolved for an economic reason, i.e. to minimize the size of the genome, since gene duplication and functional redundancy are quite common in higher organisms, e.g. 60% of the Arabidopsis genome consists of duplicated segments (The Arabidopsis Genome Initiative, 2000), but rather because of some advantage of a regulatory switch and interplay between two distinctive modes of activities. It is therefore important to study the underlying operating mechanisms of dual-affinity transporters and their physiological significance. In the present study on CHL1, we have elucidated a possible mechanism and identified a novel process of post-transcriptional regulation for plant nutrient uptake.
Results
High- and low-affinity nitrate uptake activities of CHL1 are differentially regulated by PKA activators or inhibitor
Since phosphorylation is known to modulate ion channel activity (Esguerra et al., 1994; Wang et al., 1996; Beguin et al., 1999; Gong et al., 1999; Schiff et al., 2000), we speculated that it might also play a role in regulating CHL1-mediated uptake activity. We therefore examined nitrate uptake by CHL1 cRNA-injected Xenopus oocytes in which cAMP-dependent protein kinase (PKA) activity was either increased or reduced by cAMP agonists or a PKA inhibitor.
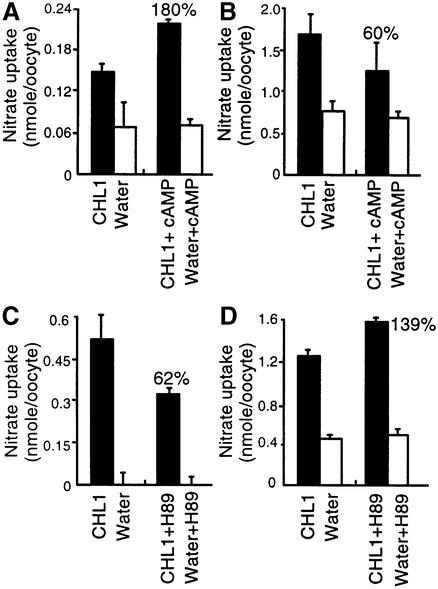
When oocytes were treated with cAMP agonists [a mixture of 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP), forskolin and 3-isobutyl-1-methylxanthine (IBMX)] to enhance PKA activity, the nitrate uptake activity of CHL1-injected oocytes in medium containing 150 µM nitrate (high-affinity concentration) increased to ∼180% of that in the untreated control (Figure 1A), while that measured using 10 mM nitrate (low-affinity concentration) decreased to ∼60% of untreated control levels (Figure 1B). Conversely, when CHL1-injected oocytes were treated with the PKA inhibitor H89, the uptake activity measured using 150 µM nitrate decreased by ∼40% (Figure 1C), while that measured using 10 mM nitrate increased by ∼40% compared with control levels (Figure 1D). Similar results were obtained using another two batches of oocytes isolated from two other toads (Table I). These data suggest that phosphorylation activates the high-affinity nitrate transport activity of CHL1 and inhibits the low-affinity transport activity.
Fig. 1. Effect of cAMP agonists and a PKA inhibitor on high- and low-affinity nitrate uptake in CHL1 cRNA-injected oocytes. (A and B) Effect of cAMP agonists on the high- (A) and low- (B) affinity nitrate uptake activity of CHL1-expressing oocytes. Water- or CHL1-injected oocytes were pre-incubated for 90 min in ND-96 medium (Roche and Treistman, 1998) alone or in ND-96 medium containing a mixture of 0.5 mM 8-Br-cAMP, 50 µM forskolin and 0.5 mM IBMX, then uptake was measured. (C and D) Effect of the PKA inhibitor H89 on the high- (C) and low- (D) affinity nitrate uptake activity of CHL1-expressing oocytes. As in (A) and (B), except that pre-incubation was in ND-96 medium or in ND-96 medium containing 50 µM H89. High-affinity nitrate uptake was measured using 150 µM NO3– and low-affinity uptake using 10 mM NO3–; oocytes were incubated with the nitrate solution for 90 min, then the amount of nitrate retained in the oocyte (low-affinity uptake) or depleted from the solution (high-affinity uptake) was determined by HPLC (Liu et al., 1999). Every data point represents the mean of three measurements, each based on five oocytes from a single batch; the error bar represents the standard error. Similar results were obtained using another two batches of oocytes isolated from two different toads (see Table I).
Table I. Nitrate uptake activities of CHL1-injected Xenopus oocytes treated with PKA activators or a PKA inhibitor.
| cAMP agonists | Percentage of control activity measured at |
|
|---|---|---|
| 150 µM | 10 mM | |
| Exp. 1 | 180 | 60 |
| Exp. 2 | 206 | 41 |
| Exp. 3 |
223 |
12 |
| H89 | Percentage of control activity measured at |
|
| |
150 µM |
10 mM |
| Exp. 1 | 62 | 139 |
| Exp. 2 | 31 | 158 |
| Exp. 3 | 0 | 185 |
The three experiments were performed in triplicate (samples of five oocytes) on three different batches of oocytes from different toads.
CHL1 protein can be phosphorylated on threonine residues
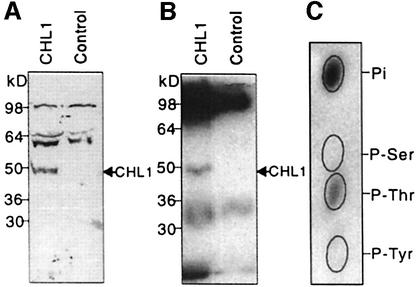
To determine whether the CHL1 protein could be phosphorylated, a plasma membrane-enriched fraction was purified from CHL1- or water-injected oocytes, then part of the sample was subjected to immunoblot analysis using anti-CHL1 antibodies (Figure 2A), while the rest of the sample was incubated with [γ-32P]ATP and cAMP-dependent protein kinase catalytic subunit, immunoprecipitated with anti-CHL1 antibodies, then electrophoresed and an autoradiogram made from the gel (Figure 2B). The results showed that, compared with the water-injected control, CHL1-injected oocytes contained an additional band that could be phosphorylated in vitro (Figure 2B) and had the same mobility as CHL1 detected in immunoblots (Figure 2A).
Fig. 2. Phosphorylation of CHL1 at threonine residues. (A) Immunoblot analysis of Xenopus oocyte membrane proteins probed using anti-CHL1 antibodies. Plasma membrane-enriched fractions, isolated from CHL1- or water-injected oocytes (Maurel et al., 1995), were separated by SDS–PAGE for immunoblot analysis using anti-CHL1 antiserum. (B) Autoradiogram of in vitro phosphorylated oocyte membrane proteins immunoprecipitated using anti-CHL1 antibodies. The same fractions used in (A) were phosphorylated in vitro in the presence of [γ-32P]ATP (NEN Life Science Products) and cAMP-dependent kinase catalytic subunit (New England Biolabs). (C) Phosphoamino acid analysis of in vitro phosphorylated CHL1. The 50 kDa phosphorylated peptide on gel (B) was eluted, hydrolyzed and subjected to thin-layer electrophoresis at pH 3.5 (Xu et al., 1996). The ovals show the positions of the non-radioactive standards, phosphoserine (P-Ser), phosphothreonine (P-Thr) and phosphotyrosine (P-Tyr).
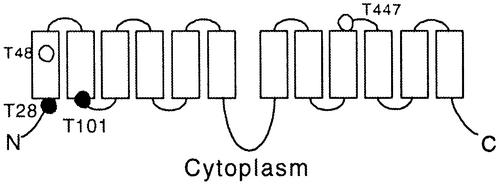
When this 50 kDa phosphorylated protein was eluted and subjected to phosphoamino acid analysis, the results showed that it was phosphorylated on threonine residues (Figure 2C). The CHL1 protein sequence contains four putative phosphorylation sites (RXXT/S), all of which use threonine as the phospho-acceptor (Figure 3). In the widely accepted membrane topology model for CHL1 and related transporters (Hagting et al., 1997; Covitz et al., 1998), two of these four putative phosphorylation sites, T28 and T101, face the cytoplasm and can probably be phosphorylated. These two sites were therefore subjected to site-directed mutagenesis analysis.
Fig. 3. Putative membrane topology and phosphorylation sites of CHL1. The black circles represent the consensus phosphorylation sites facing the cytoplasm and the white circles those buried in the membrane or on the extracellular surface.
Mutation at threonine 101 affects the high- and low-affinity nitrate uptake activities of CHL1
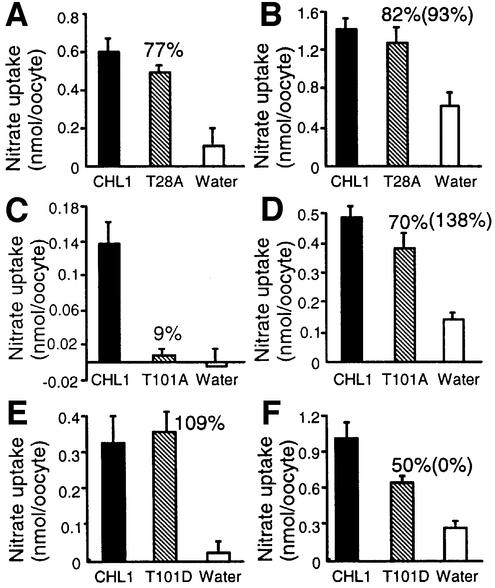
Threonines 28 and 101 were separately mutated to alanine and the mutants tested in nitrate uptake studies. When compared with Xenopus oocytes expressing wild-type CHL1, oocytes expressing the T28A mutant exhibited normal high- and low-affinity nitrate transport activities (Figure 4A and B; Table I), whereas those expressing the T101A mutant had 9 or 70% of the wild-type CHL1 activity when measured using 150 µM or 10 mM nitrate, respectively (Figure 4C and D), showing that high-affinity uptake activity was greatly reduced. Uptake measured using 10 mM nitrate includes contributions from both the low- and high-affinity components; when the contribution of the high-affinity component (Vmax of the high-affinity component) was subtracted, the T101A mutant was found to have 138% of the low-affinity uptake of wild-type CHL1 (Figure 4D). The Vmax values of the high-affinity component were calculated assuming that the Km in the high-affinity phase was 50 µM. Similar results were obtained using another two batches of oocytes isolated from another two toads (Table II). These data suggest that the inability to phosphorylate T101 converts CHL1 into an exclusively low-affinity nitrate transporter.
Fig. 4. Nitrate uptake activities and kinetic analyses using wild-type CHL1-, T28A-, T101A-, or T101D-injected oocytes. Nitrate uptake in oocytes injected with cRNAs encoding wild-type CHL1 or mutants T28A (A and B), T101A (C and D), or T101D (E and F). (A, C and E) High-affinity nitrate uptake activity determined by incubating oocytes with 150 µM NO3– for 1.5 h; (B, D and F) nitrate uptake activities determined by incubating oocytes with 10 mM NO3– for 1.5 h. The numbers above the striped bars indicate the activity expressed as a percentage of wild-type CHL1 activity after subtraction of background activity (water-injected oocytes), while the numbers in parentheses show the actual low-affinity nitrate uptake activity calculated by subtracting the contribution of the high-affinity component from the 10 mM nitrate uptake activity.
Table II. Nitrate uptake activities of Xenopus oocytes injected with mutated CHL1.
| T28A | Percentage of wild-type CHL1 activity measured at |
Percentage of wild-type CHL1 activity calculated |
||
|---|---|---|---|---|
| 150 µM | 10 mM | High-affinity component | Low-affinity component | |
| Exp. 1 | 77 | 82 | 77 | 93 |
| Exp. 2 | 91 | 92 | 91 | 92 |
| Exp. 3 |
54 |
39 |
54 |
30 |
| T101A | Percentage of wild-type CHL1 activity measured at |
Percentage of wild-type CHL1 activity calculated |
||
| |
150 µM |
10 mM |
High-affinity component |
Low-affinity component |
| Exp. 1 | 9 | 70 | 9 | 138 |
| Exp. 2 | 0 | 67 | 0 | 182 |
| Exp. 3 |
0 |
76 |
0 |
88 |
| T101D | Percentage of wild-type CHL1 activity measured at |
Percentage of wild-type CHL1 activity calculated |
||
| |
150 µM |
10 mM |
High-affinity component |
Low-affinity component |
| Exp. 1 | 109 | 50 | 109 | 0 |
| Exp. 2 | 114 | 58 | 114 | 0 |
| Exp. 3 | 131 | 51 | 131 | 17 |
The low-affinity nitrate uptake activity was calculated by subtracting the contribution of the high-affinity component to uptake at 10 mM nitrate.
To mimic the phosphorylated structure, the threonine residue at 101 was changed to aspartic acid to create the mutant T101D. The uptake activity of T101D-injected oocytes measured using 150 µM or 10 mM nitrate was 109 or 50% of that in wild-type injected oocytes, respectively (Figure 4E and F); when the contribution of the high-affinity component to the activity measured at 10 mM nitrate was taken into account, the low-affinity uptake of the T101D mutant was close to 0% compared with wild-type CHL1 (Figure 4F; Table II). Thus, by mimicking a constitutively phosphorylated state, the T101D mutation acted as a pure high-affinity nitrate transporter.
Oocytes expressing T101A or T101D exhibit a monophasic nitrate uptake pattern
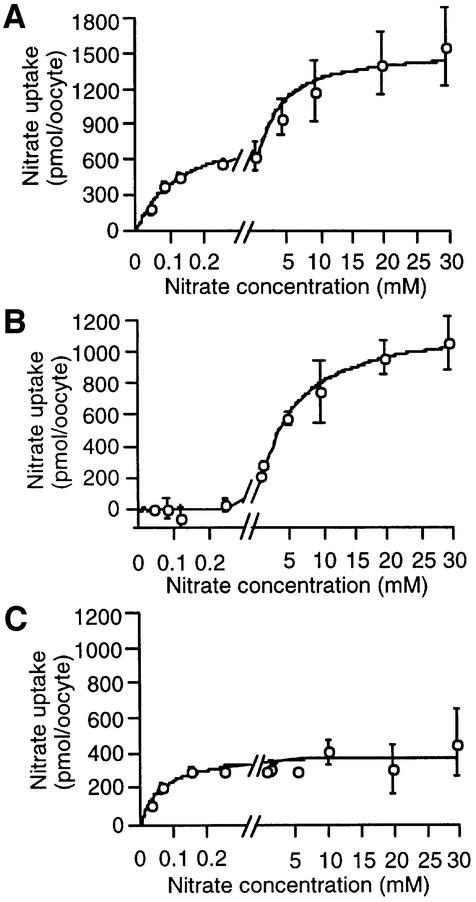
Kinetic studies were performed by measuring the uptake activities of oocytes expressing CHL1, T101A or T101D at nitrate concentrations ranging from 40 µM (high-affinity range) to 30 mM (low-affinity range). As shown in Figure 5A and in our previous study (Liu et al., 1999), wild-type CHL1 exhibited a biphasic pattern, with a Km of 125 ± 35 µM for the high-affinity phase and 2.2 ± 0.7 mM for the low-affinity phase. In contrast, nitrate uptake by both the T101A and T101D mutants was monophasic, high-affinity activity being absent in oocytes expressing the T101A mutant (Figure 5B) and low-affinity activity being absent in oocytes expressing the T101D mutant (Figure 5C). The Km for T101A-injected oocytes was ∼5.1 ± 1.1 mM and that for T101D-injected oocytes was ∼51 ± 19 µM.
Fig. 5. Kinetic analysis of nitrate uptake in oocytes injected with cRNA encoding wild-type CHL1 (A), T101A (B) or T101D (C). Uptake was determined by incubating oocytes for 1.5 h at pH 5.5 with nitrate concentrations ranging from 40 µM to 30 mM; the part of the scale between 0 and 0.3 mM has been enlarged. Each data point is the mean of the results for three duplicate experiments, each consisting of five oocytes.
Rescue of the high- but not the low-affinity uptake defect in chl1 plants expressing the CHL1 T101A mutant
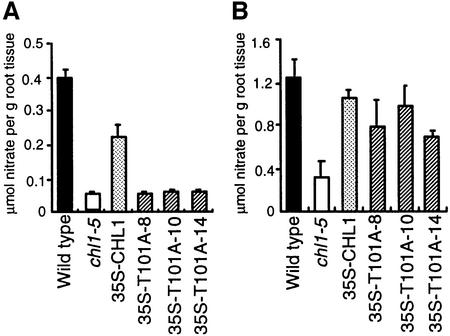
The studies described above were performed using a Xenopus oocyte expression system. To determine whether the phosphorylation regulatory mechanism of CHL1 was also effective in plants, CHL1 cDNA containing the T101A mutation was fused to the cauliflower mosaic virus 35S promoter and introduced into the chl1 deletion mutant, chl1-5 (Tsay et al., 1993). This mutant is defective in both high- and low-affinity nitrate uptake, and transgenic chl1-5 plants expressing wild-type CHL1 cDNA driven by the 35S promoter can rescue the defect in both activities (Figure 4B and C; Huang et al., 1996; Liu et al., 1999). Consistent with the results obtained using the hetero-expressing oocyte system, three independent transgenic chl1-5 plants expressing T101A failed to show recovery of the defect in high-affinity activity (Figure 6A), but did show recovery of the defect in low-affinity activity (Figure 6B).
Fig. 6. Rescue of the low-affinity, but not the high-affinity, uptake defect in chl1-5 plants expressing T101A. (A) High-affinity nitrate uptake activity of wild-type plants, chl1-5 plants or transgenic chl1-5 plants containing 35S-CHL1 or 35S-T101A; 35S-T101A-8, 35S-T101A-10 and 35S-T101A-14 are three independent transgenic T101A-expressing chl1-5 plants. Uptake activities were measured by incubating 8-day-old ammonium-grown plants in 250 µM KNO3 for 1 h. (B) Low-affinity nitrate uptake activity of the same batches of plants measured using 5 mM KNO3.
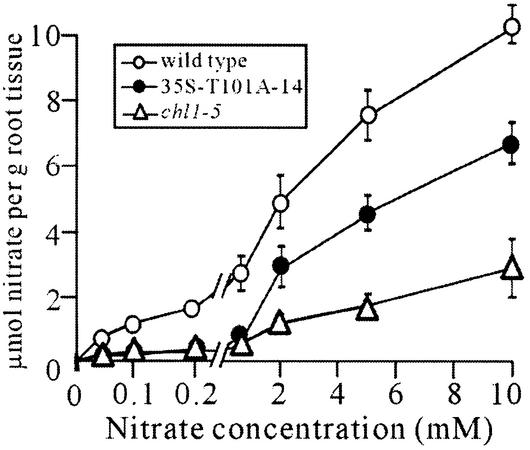
To confirm that the T101A mutant functions as a low-affinity nitrate transporter, but not as a high-affinity transporter, a kinetic analysis of nitrate uptake activity was performed on transgenic line 35S-T101A-14, nitrate uptake activity being measured at seven different nitrate concentrations ranging from 40 µM to 10 mM to cover both the high- and low-affinity phases of uptake. As shown in Figure 7, in wild-type plants, nitrate uptake was concentration-dependent and showed a biphasic pattern. Consistent with previous data (Wang et al., 1998; Liu et al., 1999), the deletion mutant, chl1-5, showed reduced high- and low-affinity phase uptake activity. Expression of T101A in the chl1-5 mutant (35S-T101A-14) resulted in recovery of the low-affinity, but not the high-affinity, uptake activity. The Km values for the low-affinity phase in the wild-type and 35S-T101A-14 plants were, respectively, 3.7 ± 0.6 and 5.2 ± 1.7 mM, while the corres ponding Vmax values were 13.7 ± 0.9 and 10.1 ± 1.5 µmol/g root tissue; in contrast, the high-affinity phase activity in the 35S-T101A-14 plant was as low as that in the chl1-5 mutant. These results show that, in plants, T101A functions exclusively as a low-affinity transporter and that the T101 phosphorylation site is required for CHL1 to function as a dual-affinity transporter.
Fig. 7. Kinetic analysis of nitrate uptake in transgenic chl1-5 plants expressing 35S-T101A. Uptake activities of wild-type plants (white circles), the chl1-5 deletion mutant (white triangles) and the transgenic plant 35S-T101A-14 (chl1-5 expressing T101A; black circles), measured by incubating 8-day-old ammonium-grown plants in 0.04, 0.1, 0.2, 0.65, 2, 5 or 10 mM KNO3 for 40 min. The scale between 0 and 0.2 mM is enlarged. Each data point is the mean of three duplicate experiments.
CHL1 phosphorylation in plants is regulated by the nitrogen concentration
To directly demonstrate that CHL1 protein can be phosphorylated in vivo, metabolic labeling with [32P]orthophosphate was performed, followed by immunoprecipitation. Immunoblot analysis using anti-CHL1 antibodies raised against the central hydrophobic loop of CHL1 showed that a single protein with a predicted molecular mass of ∼50 kDa on the SDS gel was detected in wild-type plants, but not in the chl1-5 mutant, showing that the protein detected was the endogenous CHL1 protein and confirming the specificity of the antiserum (Figure 8A). In Figure 8B, autoradiography of 32P-labeled membrane proteins immunoprecipitated with anti-CHL1 antibodies revealed a protein with the same mobility as the CHL1 protein detected in immunoblots, indicating that CHL1 is phosphorylated in plants.
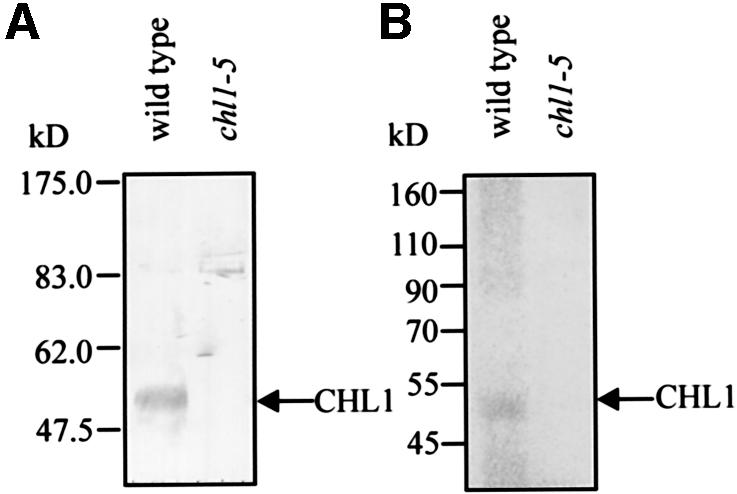
Fig. 8. Immunoprecipitation of 32P-labeled CHL1 synthesized in vivo in plants. (A) Immunoblot analysis of plant membrane proteins probed with anti-CHL1 antibody. Membrane proteins were isolated from root tissue of 10-day-old wild-type or chl1-5 mutant plants and 20 µg of proteins loaded in each lane. (B) Autoradiogram of 32P-labeled plant membrane proteins immunoprecipitated with anti-CHL1 antibodies. Ten-day-old wild-type and chl1-5 plants were incubated with [32P]orthophosphate, then membrane proteins were extracted from the root tissue, immunoprecipitated using anti-CHL1 antibodies, and the precipitated proteins separated by SDS–PAGE. Molecular mass markers are indicated on the left. The arrows indicate CHL1.
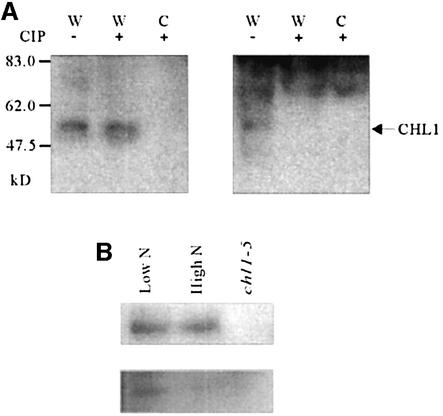
To detect the status of CHL1 phosphorylation at different nitrogen concentrations, anti-P-T101 antibodies were raised against phosphorylated synthetic peptide IADTFLGRYLT[p]IAIF, corresponding to residues 91– 105 of CHL1, and pre-cleaned by affinity chromatography on the non-phosphorylated peptide. As shown in the right panel of Figure 9A, the pre-cleaned anti-P-T101 antibodies also detected an ∼50 kDa protein in wild-type plants, but not in the chl1-5 mutant. The specificity of the anti-P-T101 antibodies was confirmed by calf alkaline intestinal phosphatase (CIP) treatment of the membrane proteins, which resulted in elimination of the anti-P-T101 antibody signal (Figure 9A, right panel, lane 2), but not the anti-CHL1 antibody signal (left panel, lane 2), confirming that the anti-P-T101 antibodies recognize phosphorylated CHL1, but not dephosphorylated CHL1.
Fig. 9. In vivo CHL1 phosphorylation status under different nitrogen conditions. (A) Specificity of anti-P-T101 antibodies for phosphorylated CHL1 protein. Membrane proteins were isolated from the root tissue of wild-type (W) or chl1-5 (C) plants and treated with (+) or without (–) CIP for 60 min, then 20 µg of protein was loaded in each lane; after electrophoresis and transfer, the blots were probed with anti-CHL1 antibodies (left) or anti-P-T101 antibodies (right). (B) Regulation of CHL1 phosphorylation by nitrogen conditions. Ten-day-old wild-type plants were shifted to 150 µM NH4NO3 (Low N) or 12.5 mM NH4NO3 (High N) medium for 2.5 h, then membrane proteins were isolated. The upper panel shows western blots probed with anti-CHL1 antibodies and the lower panel those probed with anti-P-T101 antibodies. Membrane protein from the chl1-5 mutant was loaded in the right-hand lane as a negative control.
To determine whether CHL1 phosphorylation was regulated by the nitrogen status, 10-day-old plants were shifted for 2.5 h to low-nitrogen medium (150 µM NH4NO3) or high-nitrogen medium (12.5 mM NH4NO3), then membrane proteins were isolated and subjected to immunoblot analysis using anti-CHL1 antibodies (Figure 9B, top panel) or anti-P-T101 antibodies (Figure 9B, bottom panel). As shown, similar amounts of CHL1 were detected in plants grown under these two conditions, but the phosphorylation levels were different, CHL1 from plants grown under low-nitrogen conditions being highly phosphorylated, while that from plants grown under high-nitrogen conditions was much less phosphorylated. This result shows that a low nitrogen concentration in the medium stimulates CHL1 phosphorylation in plants.
Discussion
Regulation of membrane transport by phosphorylation
The transport of ions and substrates across cell membranes by pumps, channels and transporters is critical to the living cell. The activities of certain pumps, channels and transporters are known to be regulated by protein phosphorylation. In the case of pumps, phosphorylation of a threonine residue in plant H+-ATPase creates a binding site for regulatory 14-3-3 protein (Fuglsang et al., 1999; Svennelid et al., 1999), while channel activities are often regulated through such processes as phosphorylation modulation of voltage sensitivity (Esguerra et al., 1994; Kwak et al., 1999), open probability (Wang et al., 1996; Beguin et al., 1999), burst duration (Beguin et al., 1999) and channel complex assembly (Schiff et al., 2000).
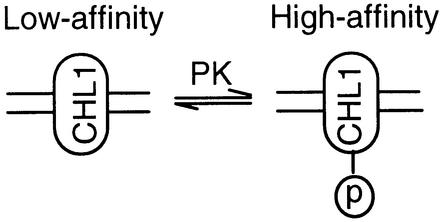
In contrast to pumps and channels, except for its effect on the trafficking of transporters to the cell surface, little is known about the detailed mechanism by which phosphorylation affects transporter activity (Blakely and Bauman, 2000). In this study, we provide both in vitro and in vivo evidence that the two action modes of the dual-affinity nitrate transporter, CHL1, are switched by phosphorylation and dephosphorylation at threonine 101. Namely, CHL1 functions as a high-affinity nitrate transporter when phosphorylated, and functions as a low-affinity nitrate transporter when dephosphorylated (Figure 10). Although the only two dual-affinity transporters so far identified are both plant transporters, the regulatory mechanism discovered here might be quite general and extend beyond plants. Supporting this suggestion is the report of Mehrens et al. (2000), which showed that the IC50 values of the rat organic cation transporter, rOCT1, for transported and non-transported inhibitors decrease after stimulation of protein kinase C by sn-1,2-dioctanoyl glycerol.
Fig. 10. Model for phosphorylation modulation of CHL1 nitrate uptake affinity.
Corresponding phosphorylation sites in other CHL1 homologs
Not every nitrate transporter in the NRT1 family exhibits dual-affinity uptake activity. For example, AtNRT1:2, a CHL1 homolog constitutively expressed in Arabidopsis, is a mono-affinity (low-affinity) nitrate transporter (Huang et al., 1999). Consistent with the present finding, AtNRT1:2 lacks the phosphorylation sequence motif (RXXT/S) at the site corresponding to T101 of CHL1. Of the 52 CHL1 homologs in Arabidopsis (The Arabidopsis Genome Initiative, 2000), 36 contain this phosphorylation consensus site, but it remains to be determined whether the uptake activity of any of these is regulated by phosphorylation.
Nitrate transporters in the NRT1 family share sequence similarity with a large family known as proton-coupled oligopeptide transporters (POT) (Paulsen and Skurray, 1994), found in animals, plants, fungi, and bacteria. The human transporters, PepT1 and PepT2, two of the best characterized POT proteins, are responsible for peptide absorption in the intestine or kidney, respectively (Fei et al., 1994; Liu et al., 1995; Boll et al., 1996; Saito et al., 1996). PepT1 and PepT2 have very similar substrate specificities, recognizing a wide variety of di- and tri-peptides, but differ in their affinities for peptides, PepT1 being a low-affinity transporter and PepT2 a high-affinity transporter (Leibach and Ganapathy, 1996). In contrast to the Arabidopsis CHL1 homologs, all of the POT transporters so far identified in animals and bacteria lack the phosphorylation consensus motif (RXXT/S) at the site corresponding to T101 of CHL1 (K.-H.Liu and Y.-F.Tsay, unpublished observation). This may indicate that, despite significant homology in the protein sequences, high-affinity nitrate uptake and high-affinity peptide uptake by the POT family have evolved by different mechanisms.
Physiological role of the phosphorylation switch mechanism
In higher plants, in addition to CHL1 (AtNRT1), the NRT2 protein family also mediates high-affinity nitrate uptake (Williams and Miller, 2001), raising the question of why CHL1 should have evolved dual-uptake activity, given the presence of NRT2. The answer may lie in the regulatory mechanism uncovered in this study. Once seeded, plants cannot move and therefore, in order to survive and sustain growth, must respond rapidly to changes in the environment. In contrast to the turning on and off of the expression of different mono-affinity transporters, which takes time, the phosphorylation and dephosphorylation of a protein already present in the membrane is a much more efficient way of adjusting high- and low-affinity uptake capacity in response to environmental changes. One particular example is when nutrient levels in the soil are low and diffusion limits nutrient movement to the root surface, resulting in a nutrient-depleted zone adjacent to the root surface (Mengel and Kirkby, 1987). In this situation, nutrient absorption relies on the high-affinity uptake system. In order to find a new nutrient source, the roots grow out into fresh soil and, when nutrients are found, the plant can then take advantage of the higher capacity of the low-affinity uptake system. Having the ability to switch between high- and low-affinity modes simply by phosphorylation/dephosphorylation, CHL1 can rapidly change its affinity as the root grows. In support of this idea are the following observations: (i) CHL1 phosphorylation was high when plants were grown under nitrogen-limited conditions, but undetectable when plants were exposed to a high nitrogen concentration (Figure 9); (ii) the CHL1 gene is abundantly expressed in the root tip (Huang et al., 1996; Guo et al., 2001); (iii) CHL1 is localized in the plasma membrane (S.-C.Hou and Y.-F.Tsay, unpublished data); and (iv) the chl1 mutant exhibits a root growth defect (Guo et al., 2001). Taken together, the dynamic regulatory mechanism described in this study could be a key player in the plant’s fight for limited nitrogen.
Materials and methods
Plasmid construction and site-directed mutagenesis
Point mutations T101A, T101D, and T28A were introduced using the recombinant PCR technique described previously (Higuchi, 1990). Wild-type CHL1 cDNA was cloned into the pGEMHE vector (Liman et al., 1992) as a template with BamHI, EcoRI and XhoI cloning sites on the 5′ end of the CHL1 cDNA, and HindIII, XbaI, and EcoRI cloning sites on the 3′ end. PCR amplifications were performed using VioTaq DNA polymerase (Viogene, Taiwan). T101A and T101D cDNAs were first prepared by PCR amplification using a T101A primer or T101D primer, respectively, in conjunction with a CHL1C primer, then the amplified DNA fragments were gel purified, digested with KpnI and HindIII, and used to replace the corresponding fragment in wild-type pGEMHE-CHL1 to generate the plasmids, pGEMHE-T101A and pGEMHE-T101D.
To generate T28A cDNA, two primers, the T28A forward primer and T28A reverse primer, were synthesized. In these, the CHL1 ATCGCT close to the sequence coding for threonine 28 was replaced with ATCGAT to create a ClaI cloning site without changing the amino acid sequence (both CGC and CGA code for arginine). In addition to this newly generated ClaI site, wild-type CHL1 cDNA has an endogenous ClaI site located 600 bp upstream of its stop codon. Two steps were needed to generate the plasmid pGEMHE-T28A. In the first, pGEMHE-CHL1 was digested with BamHI and ClaI, and the 3.6 kb fragment containing the pGEMHE vector and the 0.6 kb 3′ portion of CHL1 cDNA gel were purified. The 109 bp DNA fragment amplified with the CHL1N primer and T28A reverse primer was digested with BamHI and ClaI, and subcloned into the 3.6 kb fragment described above to generate plasmid pGEMHE-CHL1-109. In the second step, a 1.1 kb DNA fragment amplified with the T28A forward primer and CHL1C primer was produced by ClaI digestion and cloned into the ClaI site of pGEMHE-CHL1-109 to generate plasmid pGEMHE-T28A.
The sequences of the primers used were: T101A primer: 5′-GGGCAGGTACCTAGCGATTGC-3′ (sense, KpnI), T101D primer: 5′-GGGCAGGTACCTAGACATTGC-3′ (sense, KpnI), CHL1C primer: 5′-CCC AAGCTTCATGATCAATGACCCATTGG-3′ (antisense, HindIII), CHL1N primer: 5′-CCCGGATCCGATCATTAATCCATCTTCAAG-3′ (sense, BamHI), T28A reverse primer: 5′-TTGATCGATCGGCGGG-3′ (antisense, ClaI) and T28A forward primer: 5′-CCGATCGATCAAAAGCCG-3′ (sense, ClaI). The substitution sites for the threonine 101 residue (ACG) and threonine 28 residue (ACC) are underlined; restriction sites are shown in bold.
Xenopus oocyte nitrate uptake assay
Oocytes were isolated and injected with 50 ng of in vitro synthesized CHL1 cRNA, T28A cRNA, T101A cRNA, or T101D cRNA as described previously (Tsay et al., 1993). In PKA activation experiments, oocytes were pre-incubated for 90 min in ND-96 medium (Roche and Treistman, 1998), containing 0.5 mM 8-Br-cAMP (a cAMP analog), 50 µM forskolin (an activator of adenylate cyclase) and 0.5 mM IBMX (an inhibitor of the cAMP degrading enzyme, phosphodiesterase), in order to increase the cytosolic cAMP concentration (Maurel et al., 1995). In PKA inhibition experiments, oocytes were pre-incubated for 90 min in ND-96 medium containing 50 µM H89. In nitrate uptake studies, oocytes were incubated for 90 min in medium containing the indicated nitrate concentration, and the amount of nitrate retained in the oocyte (low-affinity experiments) or depleted from the solution (high-affinity experiments) was determined by HPLC (Liu et al., 1999).
In vitro phosphorylation and immunoprecipitation of oocyte membranes
Anti-CHL1 antibody was raised in rabbits against a purified recombinant protein corresponding to the 94 amino acid central loop of CHL1, which shares least homology with other Arabidopsis CHL1 homologs. Two hundred CHL1- or water-injected oocytes were homogenized in 5 ml of 30 mM Tris–HCl pH 7.6, 100 mM NaCl, 5 mM EDTA, 5 mM EGTA and 1 mM phenylmethylsulfonyl fluoride (PMSF), then membranes were collected as described by Maurel et al. (1995). For immunoblot analysis, membrane protein from 50 oocytes was loaded in each lane. For in vitro phosphorylation, the same amount of protein was incubated for 15 min at 30°C in 100 µl of 500 mM HEPES pH 7.5, 10 mM MgCl2, 0.2% Triton X-100, 1 mM dithiothreitol and 25 µCi [γ-32P]ATP (NEN Life Science Products, MA), containing 2.5 U of cAMP-dependent kinase catalytic subunit (New England Biolabs, MA), then the reaction was stopped by placing the tubes at 0°C and adding a final concentration of 7.2% TCA. The labeled membrane proteins were resuspended in buffer I (50 mM Tris–HCl pH 7.6, 200 mM NaCl, 50 mM NaF, 50 mM EDTA, 5 mM EGTA, 5 mM β-glycerophosphate, 1 mM PMSF) containing 1.5% SDS, then the solubilized membrane proteins were incubated overnight at 4°C with anti-CHL1 antiserum in buffer I containing 1% Triton X-100 and 0.2% SDS. Immune complexes were absorbed onto protein A–Sepharose CL-4B (Sigma), which was washed as described by Maurel et al. (1995), then the bound proteins were eluted at 100°C for 5 min with 50 µl of SDS sample buffer (12% SDS, 6% mercaptoethanol, 30% glycerol, 0.05% Serva blue G, 150 mM Tris–HCl pH 7) and separated on a 10% SDS–PAGE gel.
Phosphoamino acid analysis
Phosphoamino acid analysis of in vitro phosphorylated CHL1 protein was performed as described by Xu et al. (1996).
Arabidopsis nitrate uptake assay
The T101A CHL1 mutant cDNA was fused to the cauliflower mosaic virus 35S promoter, then cloned into the binary vector, pBIN19, to produce plasmid p35S-T101A, which was introduced into the CHL1 deletion mutant, chl1-5, using the in planta vacuum infiltration procedure (Bechtold et al., 1993). Three independent transgenic plants, 35S-T101A-8, 35S-T101A-10 and 35S-T101A-14, were isolated and their inserts confirmed by kanamycin resistance and genomic DNA gel blot analysis; 35S-T101A-8 and 35S-T101A-14 contained a single copy of the insert, whereas 35S-T101A-10 contained two copies. Plants were grown in 50 ml flasks containing 10 ml of liquid medium with 12.5 mM (NH4)2 succinate as the sole nitrogen source at pH 6.5. Nitrate uptake activities of 8-day-old plants were determined over a 30 min period in 4 ml of medium containing 250 µM KNO3 for high-affinity measurements and in 2.5 ml of medium containing 5 mM KNO3 for low-affinity measurements. For kinetic analysis, nitrate uptake activities of 8-day-old plants were determined over a 40 min period in 2–4 ml of medium containing the indicated KNO3 concentration. The kinetic parameters, presented as estimated Km or Vmax ± error, were calculated by fitting the data to the Michaelis–Menten equation using a non-linear least-squares method in the program Origin 5.0 (Microcal Software Inc., Northampton, MA).
In vivo phosphorylation study in Arabidopsis
Unless specified, the plants used for immunoblot analysis and in vivo labeling were grown hydroponically for 10 days on mesh in Magenta vessels (Sigma) containing 50 ml of liquid medium with 12.5 mM NH4NO3 as the sole nitrogen source at pH 5.5.
For in vivo labeling experiments, the seedlings were washed three times with phosphate-free medium containing 12.5 mM NH4NO3, and incubated for 2 h in phosphate-free medium, then for 3 h in phosphate-free medium containing 5 mCi [32P]orthophosphate. The roots were homogenized in homogenization buffer [15 mM Tris–HCl pH 7.8, 250 mM sucrose, 1 mM EDTA, 2 mM DTT, 1 mM PMSF, 0.6% (w/v) polyvinylpyrrolidone and 50 mM β-glycerophosphate] and membranes collected as described by Guo et al. (2001). They were then resuspended in homogenization buffer and diluted 100-fold in 20 mM Na2HPO4· NaH2PO4 buffer pH 7.0, containing 250 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM PMSF and 50 mM β-glycerophosphate. The diluted samples were pre-cleared by incubation for 15 min with protein A– Sepharose CL-4B beads and removal of the protein A–Sepharose CL-4B beads by centrifugation, then anti-CHL1 antibodies pre-incubated for 30 min at 4°C with membrane proteins from chl1-5 to remove non-specific binding were added and the mixture incubated for 3 h at 4°C. The immune complexes formed were absorbed onto protein A–Sepharose CL-4B (Sigma), which was then washed four times with wash buffer (20 mM Na2HPO4·NaH2PO4 buffer pH 7.4, 150 mM NaCl, 1% Triton X-100) and twice with wash buffer without 1% Triton X-100, as described by Adan et al. (2000) and eluted for 5 min at 100°C with 50 µl of SDS sample buffer. The eluted proteins were then separated on an 8% SDS–PAGE gel.
To determine the phosphorylation status of CHL1, a rabbit antiserum against CHL1 phosphorylated at T101 (anti-P-T101 antibodies) was raised by Pro-e Biotech (Taiwan) using the synthetic peptide, IADTFLGRYLT[p]IAIF (residues 91–105 of T101-phosphorylated CHL1) as antigen. The antiserum was pre-cleaned by affinity chromatography using the corresponding non-phosphorylated CHL1 peptide (IADTFLGRYLTIAIF) coupled to agarose (Pro-e Biotech). To check the specificity of the antibodies for T101-phosphorylated CHL1, membrane protein extracts from wild-type plants and the chl1-5 mutant were prepared in the absence of 50 mM β-glycerophosphate, then diluted ∼1:5 in 1× reaction buffer 3 (New England BioLabs), treated for 60 min at 37°C with buffer or CIP (Promega; 20 U of CIP and 40 µg of membrane protein in a volume of 80 µl), then tested for antibody binding on western blots. To test the phosphorylation status of CHL1, seedlings were washed three times and shifted to 150 µM NH4NO3 or 12.5 mM NH4NO3 medium for 2.5 h, then root tissue membrane proteins were extracted (Guo et al., 2001) and 20 µg of the sample separated on SDS–PAGE gels, transferred to PVDF membranes and tested for binding of anti-CHL1 and anti-P-T101 antibodies.
Acknowledgments
Acknowledgements
We are grateful to Drs Heven Sze, Tuan-Hua David Ho and Ming-Jing Hwang for their comments on this manuscript. This work was supported by the Institute of Molecular Biology, Academia Sinica, Taiwan and by NSC grants (NSC-90-2311-B001-060 and NSC-91-2321-B001-007) to Y.-F.T.
References
- Adan C.-C., Chen,D.L., Yeh,K.-C. and Abel,S. (2000) Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol., 124, 1728–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.A., Huprikar,S.S., Kochian,L.V., Lucas,W.J. and Gaber,R.F. (1992) Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA, 89, 3736–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N., Ellis,J. and Pelletier,G.C.R. (1993) In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris, 316, 1194–1199. [Google Scholar]
- Beguin P., Nagashima,K., Nishimura,M., Gonoi,T. and Seino,S. (1999) PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J., 18, 4722–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J.A. et al. (1999) Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signaling in neurons. Nature, 402, 669–671. [DOI] [PubMed] [Google Scholar]
- Blakely R.D. and Bauman,A.L. (2000) Biogenic amine transporters: regulation in flux. Curr. Opin. Neurobiol., 10, 328–336. [DOI] [PubMed] [Google Scholar]
- Boll M., Herget,M., Wagener,M., Weber,W.M., Markovich,D., Biber,J. and Clauss,W. (1996) Expression cloning and functional characterization of the kidney cortex high-affinity proton-coupled peptide transporter. Proc. Natl Acad. Sci. USA, 93, 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M.D. (2001) Cell biology. Channels as enzymes. Nature, 411, 542–543. [DOI] [PubMed] [Google Scholar]
- Covitz K.M., Amidon,G.L. and Sadee,W. (1998) Membrane topology of the human dipeptide transporter, hPEPT1, determined by epitope insertions. Biochemistry, 37, 15214–15221. [DOI] [PubMed] [Google Scholar]
- DeMaria C.D., Soong,T.W., Alseikhan,B.A., Alvania,R.S. and Yue,D.T. (2001) Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature, 411, 484–489. [DOI] [PubMed] [Google Scholar]
- Doddema H. and Telkamp,G.P. (1979) Uptake of nitrate by mutants of Arabidopsis thaliana, disturbed in uptake or reduction of nitrate. Physiol. Plant, 45, 332–338. [Google Scholar]
- Epstein E. (1972) Mineral Nutrition of Plants: Principles and Perspectives. Wiley, New York, NY.
- Esguerra M., Wang,J., Foster,C.D., Adelman,J.P., North,R.A. and Levitan,I.B. (1994) Cloned Ca2+-dependent K+ channel modulated by a functionally associated protein kinase. Nature, 369, 563–565. [DOI] [PubMed] [Google Scholar]
- Fei Y.-J., Kanai,Y., Nussberger,S., Ganapathy,V., Leibach,F.H., Romero,M.F., Singh,S.K., Boron,W.F. and Hediger,M.A. (1994) Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature, 368, 563–566. [DOI] [PubMed] [Google Scholar]
- Fu H.-H. and Luan,S. (1998) AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell, 10, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglsang A.T., Visconti,S., Drumm,K., Jahn,T., Stensballe,A., Mattei,B., Jensen,O.N., Aducci,P. and Palmgren,M.G. (1999) Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946-Thr-Val and requires phosphorylation of Thr947. J. Biol. Chem., 274, 36774–36780. [DOI] [PubMed] [Google Scholar]
- Glass A.D.M. and Siddiqi,M.Y. (1995) Nitrogen absorption by plant roots. In Srivastava,H.S. and Singh,R.P. (eds), Nitrogen Nutrition in Higher Plants. Associated Publishing Co., New Delhi, India, pp. 21–56.
- Gong J., Xu,J., Bezanila,M., van Huizen,R., Derin,R. and Li,M. (1999) Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science, 285, 1565–1569. [DOI] [PubMed] [Google Scholar]
- Guo F.Q., Wang,R., Chen,M. and Crawford,N.M. (2001) The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell, 13, 1761–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagting A., vd Velde,J., Poolman,B. and Konings,W.N. (1997) Membrane topology of the di- and tripeptide transport protein of Lactococcus lactis. Biochemistry, 36, 6777–6785. [DOI] [PubMed] [Google Scholar]
- Higuchi R. (1990) Recombinant PCR. In Innis,M.A., Gelfand,D.H., Sninsky,J.J. and White,T.J. (eds), PCR protocols. Academic Press, San Diego, CA, pp. 177–183.
- Hirsch R.E., Lewis,B.D., Spalding,E.P. and Sussman,M.R. (1998) A role for the AKT1 potassium channel in plant nutrition. Science, 280, 918–921. [DOI] [PubMed] [Google Scholar]
- Huang N.-C., Chiang,C.-S., Crawford,N.M. and Tsay,Y.-F. (1996) CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. Plant Cell, 8, 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N.-C., Liu,K.-H., Lo,H.-J. and Tsay,Y.-F. (1999) Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. Plant Cell, 11, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.J., Kwak,J.M., Uozumi,N. and Schroeder,J.I. (1998) AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell, 10, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian L.V. and Lucas,W.J. (1988) Potassium transport in roots. Adv. Bot. Res., 15, 93–178. [Google Scholar]
- Kwak Y.-G., Navarro-Polanco,R.A., Grobaski,T., Gallagher,D.J. and Tamkun,M.M. (1999) Phosphorylation is required for alteration of Kv1.5K+ channel function by the Kvb1.3 subunit. J. Biol. Chem., 274, 25355–25361. [DOI] [PubMed] [Google Scholar]
- Leibach F.H. and Ganapathy,V. (1996) Peptide transporters in the intestine and the kidney. Annu. Rev. Nutr., 16, 99–119. [DOI] [PubMed] [Google Scholar]
- Liman E.R., Tytgat,J. and Hess,P. (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron, 9, 861–871. [DOI] [PubMed] [Google Scholar]
- Liu K.-H., Huang,C.-Y. and Tsay,Y.-F. (1999) CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell, 11, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Liang,R., Ramamoorthy,S., Fei,Y.J. and Ganapathy,M.E. (1995) Molecular cloning of PepT2, a new member of the H+/peptide cotransporter family from human kidney. Biochim. Biophys. Acta, 1235, 461–466. [DOI] [PubMed] [Google Scholar]
- Maathuis F.J., Ichida,A.M., Sanders,D. and Schroeder,J.I. (1997) Roles of higher plant K+ channels. Plant Physiol., 114, 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C., Kado,R.T., Guern,J. and Chrispeels,M.J. (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. EMBO J., 14, 3028–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrens T., Lelleck,S., Cetinkaya,I., Knollmann,M., Hohage,H., Gorboulev,V., Boknik,P., Koepsell,H. and Schlatter,E. (2000) The affinity of the organic cation transporter rOCT1 is increased by protein kinase C-dependent phosphorylation. J. Am. Soc. Nephrol., 11, 1216–1224. [DOI] [PubMed] [Google Scholar]
- Mengel K. and Kirkby,E.A. (1987) Principles of Plant Nutrition. International Potash Institute, Bern, Switzerland.
- Paulsen I.T. and Skurray,R.A. (1994) The POT family of transport proteins. Trends Biochem. Sci., 19, 404. [DOI] [PubMed] [Google Scholar]
- Perraud A.L. et al. (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature, 411, 595–599. [DOI] [PubMed] [Google Scholar]
- Roche J.P. and Treistman,S.N. (1998) Ca2+ channel β3 subunit enhances voltage-dependent relief of G-protein inhibition induced by muscarinic receptor activation and Gβγ. J. Neurosci., 18, 4883–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels L.W., Yue,L. and Clapham,D.E. (2001) TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science, 291, 1043–1047. [DOI] [PubMed] [Google Scholar]
- Saito H., Terada,T., Okuda,M., Sasaki,S. and Inui,K. (1996) Molecular cloning and tissue distribution of rat peptide transporter PEPT2. Biochim. Biophys. Acta, 1280, 173–177. [DOI] [PubMed] [Google Scholar]
- Santa-Maria G.E., Rubio,F., Dubcovsky,J. and Rodriguez-Navarro,A. (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell, 9, 2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M.L., Siderovski,D.P., Jordan,J.D., Brothers,G., Snow,B., De Vries,L., Ortiz,D.F. and Diverse-Pierluissi,M. (2000) Tyrosine kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature, 408, 723–727. [DOI] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud,N., Minet,M., Lacroute,F., Salmon,J.-M., Gaymard,F. and Grignon,C. (1992) Cloning and expression in yeast of a plant potassium ion transport system. Science, 256, 663–665. [DOI] [PubMed] [Google Scholar]
- Svennelid F., Olsson,A., Piotrowski,M., Rosenquist,M., Ottman,C., Larsson,C., Oecking,C. and Sommarin,M. (1999) Phosphorylation of Thr-948 at the C terminus of the plasma membrane H+-ATPase creates a binding site for the regulatory 14-3-3 protein. Plant Cell, 11, 2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Touraine B. and Glass,A.D.M. (1997) NO3– and ClO3– fluxes in the chl1-5 mutant of Arabidopsis thaliana. Plant Physiol., 114, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman L.J., Richardson,A. and Forde,B.G. (1996) Molecular cloning of higher plant homologues of the high-affinity nitrate transporters of Chlamydomonas reinhardtii and Aspergillus nidulans. Gene, 175, 223–231. [DOI] [PubMed] [Google Scholar]
- Tsay Y.-F., Schroeder,J.I., Feldmann,K.A. and Crawford,N.M. (1993) The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell, 72, 705–713. [DOI] [PubMed] [Google Scholar]
- Wang R., Liu,D. and Crawford,N.M. (1998) The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc. Natl Acad. Sci. USA, 95, 15134–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.-T., Yu,X.-M. and Salter,M.W. (1996) Ca2+-independent reduction of N-methyl-d-aspartate channel activity by protein tyrosin phosphatase. Proc. Natl Acad. Sci. USA, 93, 1721–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. and Miller,A. (2001) Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol., 52, 659–688. [DOI] [PubMed] [Google Scholar]
- Xu Z.C., Yang,Y. and Hebert,S.C. (1996) Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J. Biol. Chem., 271, 9313–9319. [DOI] [PubMed] [Google Scholar]