INTRODUCTION
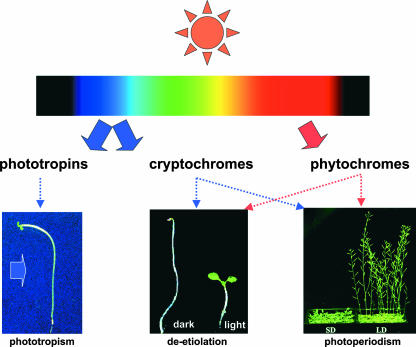
A plant blue light response was documented as early as 1881 by Darwin when he discovered what is now known as the blue light–induced phototropic response (Darwin, 1881). However, blue light receptors mediating phototropism and other photoresponses in plants have remained elusive until recently. On the basis of molecular genetic studies in Arabidopsis, it is clear now that there are two types of blue light receptors in plants: cryptochromes and phototropins. Cryptochromes are found not only in plants but also in animals, including humans, making them ubiquitous photoreceptors throughout higher eukaryotes. Proteins related to phototropins are also found in different organisms and regulate responses to environmental stimuli, such as light and oxygen. Cryptochromes work together with phytochromes to regulate photomorphogenic responses, including the regulation of cell elongation and photoperiodic flowering; phototropins, on the other hand, mediate movement responses including the phototropic curvature that attracted Darwin's attention more than a century ago (Figure 1). The combined absorption spectra of the red/far-red light receptors (phytochromes) and the blue light receptors (cryptochromes and phototropins) overlap with those of the photosynthetic pigments, allowing coordinated control of development and energy production in plants. Although detailed signal transduction mechanisms of neither cryptochromes nor phototropins are well understood, significant progress has been made in recent years. This article will focus on advances in our understanding of the functions and signal transductions of blue light receptors. It is not intended to cover every aspect of the field; readers are referred to other review articles for historical perspectives and a more comprehensive understanding of these photoreceptors (Briggs and Huala, 1999; Cashmore et al., 1999; Lin, 2000b; Sancar, 2000).
Figure 1.
Functions of Blue Light Receptors in Phototropism, Photomorphogenesis, and Photoperiodic Flowering.
Solid arrows depict light, and dashed arrows depict signal transduction of photoreceptors.
CRYPTOCHROMES AND PHOTOMORPHOGENESIS
Cryptochromes share sequence similarity to the DNA repair enzyme photolyase but have no DNA repair activity. Cryptochromes and DNA photolyases share similarities not only in amino acid sequences but also in chromophore composition and in the light-dependent nature of their respective biochemical activities. Cryptochromes appear to be evolutionarily derived from gene duplication events of ancestral photolyase genes, because many organisms, including Arabidopsis and Drosophila, are known to have both cryptochromes and photolyases, functioning as photoreceptors and DNA repair enzymes, respectively (Cashmore et al., 1999).
Cryptochrome Genes and Proteins
Cryptochromes, which were historically defined by their action spectra, are photolyase-like blue light receptors (Gressel, 1979; Briggs and Huala, 1999; Cashmore et al., 1999; Lin, 2000b). Most organisms examined to date have more than one cryptochrome, and different cryptochromes of the same organism often mediate related light responses. No biochemical activity has been demonstrated for a cryptochrome, but the expression of a cryptochrome gene may be regulated by light via different mechanisms from transcription to degradation.
Cryptochromes Are Photolyase-Like Flavoproteins
DNA photolyases are ∼55 to 65 kD flavoproteins widely found in microbes including bacteria, Archaea, and yeast (Sancar, 1994). Photolyases catalyze blue/UV-A light–dependent repair of DNA damage resulting from exposure to high-energy short-wavelength (<350 nm) UV light (UV-B and UV-C). There are two types of structurally related DNA photolyases, one (called photolyase) that repairs cyclobutane pyrimidine dimers and another (called 6-4 photolyase) that repairs pyrimidine-pyrimidine 6-4 photoproducts (Sancar, 1994, 2000). A phylogenetic analysis indicated that plant cryptochromes are more closely related to the photolyases than to the 6-4 photolyases, whereas animal cryptochromes are more closely related to 6-4 photolyases than to the photolyase (Cashmore et al., 1999). Therefore, it appears that plant cryptochromes and animal cryptochromes arose from independent gene duplications of their respective ancestral photolyase genes.
Most plant cryptochromes are 70- to 80-kD proteins with two recognizable domains, an amino terminal PHR (for photolyase-related) domain that shares sequence homology with photolyases and a carboxyl terminal domain that has no strong sequence similarity to known protein domains (Cashmore et al., 1999; Mockler and Lin, unpublished data). Photolyase contains two chromophores, a light-harvesting chromophore, which is either a folate (methenyltetrahydrofolate) or a deazaflavin, and a catalytic chromophore that is flavin adenine dinucleotide (FAD). Almost all residues known to be important for chromophore binding in photolyase are conserved in cryptochromes, whereas residues of photolyases that are critical for the binding of DNA lesions and the catalysis of DNA repair are not equally conserved in cryptochromes (Ahmad and Cashmore, 1993; Todo et al., 1996; Imaizumi et al., 2000). The chromophore composition of cryptochromes has been investigated using recombinant cryptochrome purified from heterologous expression systems. The full-length Arabidopsis CRY1 expressed and purified from insect cells non-covalently binds a stoichiometric amount of FAD (Lin et al., 1995b). The PHR domain of Arabidopsis CRY1 expressed as a fusion protein to maltose binding protein (CRY1N-MBP) and purified from Escherichia coli also contained non-covalently bound FAD, indicating that the PHR domain is indeed the chromophore binding domain of cryptochrome. In addition, the CRY1N-MBP recombinant protein purified from E. coli contains methenyltetrahydrofolate, the light-harvesting chromophore found in many photolyases (Sancar, 1994; Malhotra et al., 1995). Consistent with the notion that cryptochromes are photosensory receptors, they showed no photolyase activity in different in vitro assays, and expression of cryptochromes could not rescue the photolyase-deficient E. coli mutant (Lin et al., 1995b; Malhotra et al., 1995; Hoffman et al., 1996; Imaizumi et al., 2000; Perrotta et al., 2000).
Cryptochromes Are Found Throughout the Plant Kingdom
Cryptochromes have been found in dicots (Arabidopsis, tomato, etc.; Ahmad and Cashmore, 1993; Batschauer, 1993; Guo et al., 1998; Ninu et al., 1999), monocots (rice, barley, etc.; Lin and Cashmore, 1996; Imaizumi et al., 2000; Perrotta et al., 2001), fern (Adiantum capillus-veneris; Kanegae and Wada, 1998; Imaizumi et al., 2000), moss (Physcomitrella patens; Imaizumi et al., 2001), and algae (Chlamydomonas reinhardtii; Small et al., 1995). Most plant species studied contain multiple cryptochromes. For example, Arabidopsis has two cryptochrome genes, CRY1 and CRY2 (Ahmad and Cashmore, 1993; Hoffman et al., 1996; Lin et al., 1996b); and tomato and barley each have at least 3 cryptochrome genes, CRY1a, CRY1b, and CRY2 (Perrotta et al., 2000, 2001); fern and moss have five and at least two cryptochrome genes, respectively (Kanegae and Wada, 1998; Imaizumi et al., 2000, 2001). The amino acid sequences of tomato CRY1 (CRY1a or CRY1b) and CRY2 are more similar to their Arabidopsis counterparts than to each other, suggesting that the gene duplication event resulting in CRY1 and CRY2 occurred over 100 million years ago, before the divergence of Brassicaceae (e.g., Arabidopsis) and Solanaceae (e.g., tomato) (Ku et al., 2000; Perrotta et al., 2000).
Most plant cryptochromes identified contain a C-terminal extension in addition to the N-terminal PHR domain, and the C-terminal domain has been found to be critical to the function of Arabidopsis cry1 and cry2 (Ahmad et al., 1995; Yang et al., 2000; Guo and Lin, unpublished data). However, the C-terminal domains of different cryptochromes vary significantly in length, from ∼380 amino acids long in algae (C. reinhardtii), ∼190 amino acids and ∼120 amino acids in Arabidopsis CRY1 and CRY2, respectively, to almost no C-terminal extension in the SH-PHR of white mustard and AcCRY5 of the fern A. capillus-veneris. The white mustard (Sinapis alba) PHR gene (SaPHR) was initially thought to encode a DNA photolyase (Batschauer, 1993), but it was later found to have no DNA repair activity and therefore is likely to be a cryptochrome (Malhotra et al., 1995). Physiological functions have not been reported for SaPHR or Adiantum CRY5. It is interesting to note that although SaPHR contains no C-terminal extension, its amino acid sequence is over 95% identical to the PHR domain of the Arabidopsis CRY2, which is much higher than that between the two Arabidopsis cryptochromes. A study of physiological activity of SaPHR of white mustard or AcCRY5 of Adiantum would likely tell us more about the structure–function relationships of the C-terminal extension of the cryptochrome.
Cryptochromes show much higher sequence similarity in the PHR domain than in the C-terminal domain (Lin et al., 1998; Imaizumi et al., 2000; Perrotta et al., 2000). For example, Arabidopsis CRY1 and CRY2 are 58% identical in the PHR domain, but the sequence similarity of their C-terminal domains (14% identical) is lower than that between the PHR domain of either of them compared with the E. coli photolyase (∼30% identical; Hoffman et al., 1996; Lin et al., 1996b, 1998). Despite the lack of overall sequence similarity in the C-terminal domains of different cryptochromes, three recognizable motifs can be found in the C-terminal domain of cryptochromes from most plants examined (Figure 2). These three motifs are the DQXVP, an acidic motif containing a short stretch of 3-5 acidic residues (E or D), and STAES. Because these three motifs and their linear order are well conserved in cryptochromes from Arabidopsis to Physcomitrella, the region of cryptochrome C-terminal extension containing these three motifs is referred to as the DAS domain (for DQXVP-acidic-STAES; Figure 2). The presence of these three motifs in an orderly arrangement in cryptochromes from moss to angiosperm suggests that the DAS domain may have existed in the ancestral cryptochrome, and that the evolutionary history of cryptochromes is likely to be over 400 million years, before the wide spread of vascular plants on the earth (Kenrick and Crane, 1997). The role of the DAS domain in cryptochrome function is not clear, although STAES may represent a protein phosphorylation site (Shalitin and Lin, unpublished data). The functional significance of at least one of the three motifs of the DAS domain, DQXVP, has been shown for Arabidopsis cry1. A mutation (P549-L) in the DQXVP motif of Arabidopsis CRY1 almost completely eliminated its activity in mediating hypocotyl inhibition in the hy4-9 mutant allele (Ahmad et al., 1995).
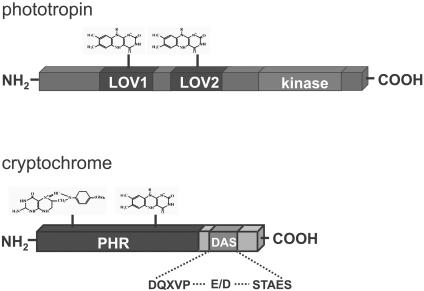
Figure 2.
Domain Organization of Blue Light Receptors.
LOV (light, oxygen, voltage) domains of phototropin, PHR (photolyase related), and DAS (DQXVP-acidic-STAES) domains of cryptochrome, flavin, and folate chromophores are shown. The scale of individual domains is not drawn to reflect the actual size in the protein.
Cryptochromes Are Mostly Nuclear Proteins
Unlike Arabidopsis phytochromes that are imported to the nucleus upon exposure to light (Kircher et al., 1999; Yamaguchi et al., 1999; Hisada et al., 2000), Arabidopsis cryptochromes seem to accumulate in the nucleus either constitutively (cry2) or primarily in the dark (cry1). Arabidopsis cry2 was shown to be a nuclear protein by both cell fractionation analysis and fusion protein studies (Guo et al., 1999; Kleiner et al., 1999; Mas et al., 2000). Fusion proteins of CRY2-GUS and CRY2-GFP were found to accumulate in the nucleus of the transgenic plants grown in dark or in light, suggesting that Arabidopsis cry2 is a constitutive nuclear protein. A recent report showed that Arabidopsis cry2 may be associated with chromosomes (Cutler et al., 2000). When transgenic Arabidopsis plants expressing random GFP-cDNA fusions were examined for the subcellular localization of individual fusion proteins, a line that expressed GFP-CRY2C was identified. Interestingly, the GFP-CRY2C fusion protein was found to bind to all chromosomes (Cutler et al., 2000) (http://deepgreen.stanford.edu/). In another study, CRY2-GFP fusion protein was found to accumulate homogeneously in the nucleus of transfected tobacco BY-2 protoplasts kept in the dark (Mas et al., 2000). Blue light induces the formation of nuclear speckles of the CRY2-GFP fusion protein, which co-localized with the nuclear speckles formed by PHYB-GFP fusion protein when they were co-expressed (Mas et al., 2000).
Onion epidermal cells transfected with a gene encoding Arabidopsis CRY1 and GFP fusion protein were shown to accumulate CRY1-GFP protein in the nucleus (Cashmore et al., 1999). However, a fusion protein of β-glucuronidase (GUS) and the CRY1 C-terminal domain (GUS-CCT1) was found in the nucleus in dark-grown Arabidopsis transgenic plants, but in the cytosol in light-grown plants (Yang et al., 2000). Therefore, Arabidopsis cry1 appears to be enriched in the nucleus or cytosol in dark or light, respectively.
The subcellular localization of all five cryptochromes in the fern A. cappillus-veneris has been systematically studied. It was shown that Adiantum CRY1, CRY2, and CRY5 are cytosolic proteins, whereas Adiantum CRY3 and CRY4 are nuclear proteins (Imaizumi et al., 2000). Adiantum CRY3 is imported to the nucleus in dark (or in red light), but not in blue light, whereas Adiantum CRY4 accumulated constitutively in the nucleus (Imaizumi et al., 2000). Although the PHR domain of a cryptochrome may be expected to possess the sequence necessary for nuclear importing because photolyases are nuclear proteins in eukaryotes, the nuclear localization signals of many cryptochromes are actually found in their C-terminal extension. Moreover, the intracellular localization of chromophoreless fusion proteins of GUS-CCT1 (Arabidopsis) and GUS-CRY3C (Adiantum) retained their ability to be regulated by light, possibly through the action of the endogenous cryptochromes or phytochromes in the transgenic plants. These observations suggest that the C-terminal domains of Arabidopsis CRY1 and Adiantum CRY3 contain both nuclear import and export signals.
Light Regulation of Cryptochrome Expression
Arabidopsis CRY1 and CRY2 mRNA appears to be ubiquitously expressed throughout the plant with no significant differences found in different tissues or in plants treated with different light conditions (Ahmad and Cashmore, 1993; Lin et al., 1998). But CRY1 and CRY2 mRNA levels have been recently shown by a DNA microarray analysis to oscillate with a circadian rhythm of relatively low amplitudes (Harmer et al., 2000). Tomato CRY1 and CRY2, and Chlamydomonas CPH1 gene showed no obvious light regulation for their mRNA expression (Small et al., 1995; Perrotta et al., 2000). On the other hand, white mustard SaPHR gene expression is light induced, and both developmental regulation and light regulation of mRNA expression have been reported for some fern cryptochrome genes (Batschauer, 1993; Imaizumi et al., 2000). The mRNA level of the fern CRY5 gene in germinating spores can increase up to 300- to 400-fold within 12 hr after red or blue light treatment (Imaizumi et al., 2000).
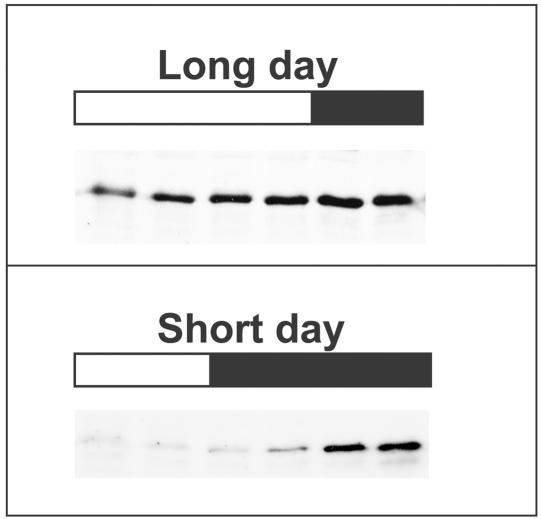
At the protein level, Arabidopsis CRY1 expression is not obviously affected by light, whereas CRY2 is negatively regulated by blue light (Ahmad et al., 1998a; Lin et al., 1998). When etiolated Arabidopsis seedlings were exposed to 20 to 30 μmol m−2 sec−1 blue light, CRY2 protein level declined more than 10-fold within one hour (Ahmad et al., 1998a; Lin et al., 1998). It has been demonstrated that a protein degradation mechanism is responsible for the blue light–dependent regulation of the CRY2 protein abundance (Ahmad et al., 1998a; Lin et al., 1998). The cry2 protein is likely the photoreceptor mediating its own degradation, because its turnover is not affected by phytochrome or cry1 mutations (Lin et al., 1998; Yang and Lin, unpublished data). The blue light regulation of CRY2 protein level has been correlated with its functions in both de-etiolation and photoperiodic flowering. The function of cry2 in de-etiolation is largely limited to relatively low light, and this is interpreted as the consequence of a relatively low level of CRY2 protein in plants exposed to high light (Lin et al., 1998). The CRY2 protein level shows a photoperiod-dependent diurnal cycle (El-Din El-Assal et al., 2001; Figure 3). For plants grown in short-day photoperiods, CRY2 protein level is lower during the day but higher in the night (Figure 3). The diurnal cycle of the CRY2 protein level is less apparent in plants grown in long-day photoperiods (Figure 3). Such a photoperiod-dependent differential expression of cry2 protein may provide a mechanism allowing plants to sense different photoperiods, which is consistent with the known function of cry2 in photoperiodic flowering. Indeed, the physiological significance of such a photoperiod-dependent expression pattern of CRY2 protein has been directly demonstrated recently (El-Din El-Assal et al., 2001; see below).
Figure 3.
Immunoblot Showing CRY2 Protein Levels at Different Times of a Day in 7-Day-Old Arabidopsis Seedlings Grown in Long Day (16-Hr-Light/8-Hr-Dark) or Short Day (8-Hr-Light/16-Hr-Dark).
Open bars represent light periods, and solid bars depict dark periods. The absolute levels of CRY2 in two immunoblots are not directly comparable.
Functions of Cryptochromes in Plant Photomorphogenesis
It seems clear now that, at least in Arabidopsis, the function of cryptochromes in plant photomorphogenesis overlaps almost entirely with the function of phytochromes. For example, the roles of Arabidopsis cryptochromes in mediating de-etiolation, gene expression, and photoperiodic flowering are performed by both cryptochromes and phytochromes, acting primarily in response to blue/UV-A and red/far-red spectra of light, respectively.
Function of Cryptochromes in De-Etiolation
A dicot plant germinated in dark develops an etiolated seedling with a rapidly elongating hypocotyl that allows the unopened cotyledons containing no photosynthetically competent chloroplasts to emerge rapidly from soil. Exposure to light results in de-etiolation or photomorphogenesis. The de-etiolation responses include inhibition of hypocotyl elongation, stimulation of cotyledon opening, change of gene expression, and induction of chloroplast development. Because the hypocotyl inhibition response is easy to monitor and measure, it has become the most widely used assay in the study of cryptochromes.
Arabidopsis CRY1 was identified on the basis of the study of the hy4 mutant impaired in blue light inhibition of hypocotyl elongation (Koornneef et al., 1980; Ahmad and Cashmore, 1993). When grown in blue light, hy4 mutants showed a significantly reduced blue light inhibition of hypocotyl elongation, resulting from mutations of the CRY1 gene. Transgenic tobacco or Arabidopsis seedlings overexpressing Arabidopsis CRY1 had hypocotyls shorter than those of the wild type when grown in blue light (Lin et al., 1995a, 1998), indicating that the amount of cry1 is rate limiting in the process of blue light inhibition of hypocotyl elongation. The fact that overexpression of Arabidopsis CRY1 in transgenic tobacco also resulted in a short hypocotyl phenotype suggests that the cry1 signaling process is conserved in different plant species. Complex functional interactions of cry1 with phytochromes have been analyzed in a number of studies using Arabidopsis or tomato mutant lines impaired in both types of photoreceptors (Casal and Boccalandro, 1995; Ahmad and Cashmore, 1997; Ahmad et al., 1998b; Casal and Mazzella, 1998; Neff and Chory, 1998; Wang and Iino, 1998; Hennig et al., 1999a, 1999b; Folta and Spalding, 2001a; Weller et al., 2001).
Transgenic Arabidopsis plants overexpressing CRY2 also showed a short hypocotyl phenotype in blue light, which led to the hypothesis that cry2 is also involved in hypocotyl inhibition. Indeed, cry2 mutants were isolated by screening for long-hypocotyl seedlings in blue light (Guo et al., 1998). When grown in continuous blue light, cry2 mutant seedlings developed long hypocotyls (Lin et al., 1998). The long-hypocotyl phenotype of cry2 mutants is relatively more pronounced in low light than in high light, which is interpreted as the consequence of CRY2 protein degradation in blue light (Lin et al., 1998). There is an apparent functional redundancy between cry1 and cry2, because the cry1cry2 double mutant has more severe phenotypic defect in various aspects of de-etiolation than does either the cry1 or cry2 monogenic mutant (Mockler et al., 1999; Mazzella et al., 2001). A functional interaction of cry2 and phytochromes in de-etiolation is also suggested by the observation that cry2 mutant seedlings exhibit long hypocotyl when grown in light enriched in far-red spectrum (Mas et al., 2000).
In addition to hypocotyl inhibition, cryptochromes have also been shown to mediate blue light regulation of other aspects of de-etiolation. The Arabidopsis cry1 (hy4) mutant is defective in light-dependent anthocyanin accumulation, indicating its function in this blue light response (Ahmad et al., 1995; Lin et al., 1996a; Jenkins, 1997). The cry2 mutant showed reduced cotyledon opening in low-irradiance blue light, suggesting a role of cry2 in this response (Lin et al., 1998). The activity of cry1 and cry2 in de-etiolation for seedlings grown in white light has also been shown (Koornneef et al., 1980; Mazzella et al., 2001).
Consistent with the finding that cryptochrome signal transduction is conserved in different plants, a similar function of cryptochromes in de-etiolation has also been shown in tomato. Transgenic tomato plants expressing a CRY1a antisense transgene contained reduced CRY1a protein and showed long hypocotyl in blue light but not in red light (Ninu et al., 1999). Recently, a loss-of-function tomato cry1a mutant has been isolated (Weller et al., 2001). Study of the tomato cry1a mutant further confirmed that cry1a mediates blue light inhibition of hypocotyl inhibition in tomato (Weller et al., 2001). Similar to the Arabidopsis cry1 mutant, the tomato cry1a mutant is also impaired in anthocyanin accumulation and cotyledon development in blue light, indicating again the similar functions of Arabidopsis and tomato cryptochromes in de-etiolation (Weller et al., 2001).
Taking advantage of the homologous recombination gene disruption technique available in Physcomitrella, the moss cry1a and cry1b loss-of-function mutants have been prepared (Imaizumi et al., 2001). Analysis of these mutants, especially the cry1a cry1b double mutant, demonstrated that moss cry1a and cry1b act, in a largely redundant manner, to mediate blue light induction of side branching of protonema, blue light stimulation of gametophore emergence, and blue light inhibition of gametophore stem elongation (Imaizumi et al., 2001).
Function of Cryptochromes in the Control of Flowering Time
Plant flowering time is controlled by a network of signal transduction cascades that connects various environmental signals to developmental programs. One of the most important environmental signals affecting flowering time is daylength, or the photoperiod. Although it is well known that phytochromes are major photoreceptors regulating flowering time, a role for cryptochrome in photoperiodic response has been shown recently. The function of cryptochrome in the control of flowering time has been investigated by studies of Arabidopsis photoreceptor mutants under photoperiodic conditions with either white light illumination or light of specific wavelengths. The role of Arabidopsis cry1 in promoting floral initiation has been demonstrated by studies showing cry1 mutants flowered later than the wild type in various light conditions (Mozley and Thomas, 1995; Bagnall et al., 1996).
The function of Arabidopsis cry2 in flowering-time control has also been studied using the cry2 mutant. As described above, the Arabidopsis cry2 mutant was isolated on the basis of its defect in de-etiolation (Guo et al., 1998). However, it turned out that the cry2 mutant is late flowering and insensitive to photoperiods, and it is allelic to a previously isolated photoperiod-insensitive late-flowering mutant fha (Koornneef et al., 1991; Guo et al., 1998). The cry2/fha mutant flowers later than the wild type in long day but not in short day, whereas transgenic plants overexpressing cry2 flowered slightly early in short day but not in long day. Therefore, either a mutation or an overexpression of the CRY2 gene resulted in the reduced sensitivity to photoperiods.
Interestingly, although blue light is known to promote flowering of Arabidopsis, the cry2 mutant was found to flower at the same time as the wild type in continuous blue light or red light (Guo et al., 1998). The late-flowering phenotype of cry2 in white light could be phenocopied in blue-plus-red light (Guo et al., 1998; Mockler et al., 1999). Therefore, the flowering-promotion function of cry2 is dependent on both blue light and red light. A study of the genetic interaction between cry2 and phyB mutants provides a possible explanation of why the function of the blue light receptor cry2 is dependent on both blue light and red light with respect to the regulation of flowering-time. It was proposed that phyB mediates a red light–dependent suppression of floral initiation, whereas cry2 mediates a blue light–dependent inhibition of the phyB function (Guo et al., 1998; Mockler et al., 1999).
In addition to phyB, the action of phyA also affects the function of cry2 in the regulation of flowering time. It was found that the cry2 mutant flowered at about the same time as the wild type when plants were grown in white-plus-far-red light (Mas et al., 2000). One interpretation of this observation is that the enrichment in far-red light may stimulate phyA activity in promoting flowering, which compensates (or overrides) the effect of the loss of the CRY2 gene (Mas et al., 2000). Consistent with the hypothesis that phyA may mediate far-red light–dependent promotion of flowering, it was found that the phyA mutant failed to flower when grown in the tissue culture medium illuminated with continuous far-red light (Mockler and Lin, unpublished data). The complicated interactions between cryptochromes and phytochromes indicate that the flowering time of wild-type plants grown in natural light condition is determined, in part, by the balanced action of different photoreceptors exerting antagonistic or redundant effects on the developmental program (Lin, 2000a).
The function of cry2 in photoperiodic flowering is further demonstrated by a recent study of a quantitative trait locus that determines the natural variation of flowering time in Arabidopsis (El-Din El-Assal et al., 2001). In this study, the EDI (early daylength insensitive) quantitative trait locus of the Cvi accession (ecotype), collected in the tropical Cape Verde Islands, was mapped and cloned. The EDI locus, which is largely responsible for the dominant photoperiod insensitive and early-flowering traits of the Cvi accession, was identified to be the CRY2 gene. CRY2-Cvi encodes CRY2 protein with a methionine substitution for the valine at position 376 (V367M). Val376 was completely conserved among 8 different cryptochrome genes compared, except for CRY2-Cvi. Transgenic plants expressing the mutated CRY2-Ler (CRY2 gene of Ler accession) with the V367M substitution flowered similar to Cvi, whereas plants expressing the mutated CRY2-Cvi with a M367V substitution flowered just like the Ler wild type. This experiment confirmed that the single V367M substitution in the CRY2-Cvi protein is indeed the cause of the photoperiodic-insensitive early flowering of the Cvi ecotype. Moreover, it was found that the V367M substitution of the CRY2 protein resulted in a change in the photoperiod-dependent diurnal cycling of CRY2 expression. The CRY2-Cvi type protein with the V367M substitution showed a reduced amplitude of its diurnal rhythm of protein expression level in short day, suggesting that the reduced expression change of CRY2-Cvi protein level in response to photoperiods results in the reduced daylength insensitivity and early flowering in Cvi plants.
Function of Cryptochromes in the Circadian Clock and Light Regulation of Gene Expression
Regulation of gene expression is intuitively a major mechanism through which photoreceptors exert roles in plant development such as control of photoperiodic flowering. There are two mechanisms by which light may affect transcription of a gene: light may affect transcription via direct signal transduction from a photoreceptor to transcriptional regulators (Ni et al., 1998; Martinez-Garcia et al., 2000); or light may affect gene expression through the action of the circadian clock (McClung and Kay, 1994; Terzaghi and Cashmore, 1995). The circadian clock is composed of input components, output components, and a central oscillator that is a transcription complex for which activity and turnover are regulated by light in a negative feedback loop (Dunlap, 1999; Wager-Smith and Kay, 2000). Cryptochromes have been demonstrated to be photoreceptors mediating light regulation of the circadian clock in Drosophila, mice, and Arabidopsis. However, although cryptochrome acts as an integral part of the central oscillator in animals, a similar configuration has yet to be directly demonstrated for plant cryptochromes.
Functions of Cryptochromes in the Circadian Clock of Animals
Cryptochrome as a photoreceptor functioning in the entrainment of the circadian clock has been well established in Drosophila. A Drosophila cry mutant, cryb, was identified on the basis of its defect in regulating the circadian rhythm of activity of the PER promoter (Emery et al., 1998; Stanewsky et al., 1998). The cryb mutation abolished cycling of PER (period) and TIM (timeless) expression (Stanewsky et al., 1998), reduced sensitivity to blue light entrainment of the circadian clock (Egan et al., 1999), and abrogated the effect of constant illumination on the circadian behavior (Emery et al., 2000a). Transgenic flies overexpressing cryptochrome showed increased circadian photosensitivity (Emery et al., 1998, 2000b). However, cryptochrome is apparently not the only photoreceptor that entrains the circadian clock in Drosophila. The cryb mutant fly still allows an entrainment of the behavior rhythmicity in blue light unless the signal transduction for the visual pigment is also eliminated (Stanewsky et al., 1998). Therefore, as we find in other systems (see below) there is usually a functional redundancy between cryptochromes and other photoreceptor systems (e.g., phytochromes in plant and rhodopsins in animals).
The detailed molecular mechanism of cryptochrome function is not well understood in any organism, but in Drosophila it has been shown that cryptochrome exerts its function on the circadian clock by physical interaction with central oscillator components. The central oscillator components of Drosophila include PER, TIM, CLK (clock), and CYC (cycle) (Dunlap, 1999). CLK/CYC and TIM/PER are positive regulators and negative regulators, respectively, for the transcription of clock genes. CLK and CYC are basic helix-loop-helix-PAS proteins that act together to activate transcription of clock-regulated genes such as TIM and PER. The transcription of the clock genes PER and TIM is negatively controlled by their own gene products. PER and TIM form heterodimers in the cytosol and then enter the nucleus to suppress their own transcription. It was found that cryptochrome interacts with TIM in a light-dependent manner, and the CRY–TIM interaction results in sequestration of TIM and suppression of TIM-dependent inhibition of transcription (Ceriani et al., 1999).
Recently, mammalian cryptochromes have also been shown to act as photoreceptors for the regulation of the circadian clock and light-induced gene expression, although the role of mammalian cryptochromes as photoreceptors entraining the circadian clock had been an issue of debate (Miyamoto and Sancar, 1998; Thresher et al., 1998; Griffin et al., 1999; Okamura et al., 1999; van der Horst et al., 1999; Vitaterna et al., 1999; Selby et al., 2000). Mice and human each have two cryptochromes (Hsu et al., 1996; Todo et al., 1996). The knock-out mice missing both mCRY1 and mCRY2 retained near-normal behavioral rhythmicity in light/dark cycles, but showed an instantaneous and complete loss of rhythmicity in free-running conditions, suggesting that mCRYs are essential components of the mammalian oscillator (van der Horst et al., 1999).
The fact that cryptochromes are integral parts of the mice central oscillator makes it almost impossible to directly test their role in the light entrainment of the clock. Nevertheless, it was found that somewhat analogous to Drosophila (described above), the mouse cry double mutant retained its ability to mediate light input unless the signal transduction of visual pigments was also disrupted (Selby et al., 2000). Triple-mutant mice carrying both cryptochrome mutations and a retinal degenerative mutation were nearly arrhythmic under light/dark cycling conditions and showed a marked reduction in light-induced gene expression (Vitaterna et al., 1999). Physical interactions of mammalian cryptochromes with other clock proteins have also been reported. It has been shown that cryptochromes interact with mammalian versions of PER, TIM, CLK, or CYC proteins to affect transcription of clock genes in mice (Griffin et al., 1999; Kume et al., 1999; Shearman et al., 2000; Lee et al., 2001).
Functions of Arabidopsis Cryptochromes in Regulating the Circadian Clock and Light-Dependent Gene Expression
One reason most organisms possess a circadian clock may be that it allows an organism to “anticipate” and therefore be prepared for an upcoming change in the environment. For example, a plant starts to synthesize proteins needed for photosynthesis before sunrise, and stops making these proteins in dusk with an “anticipation” of the coming nightfall. It is not surprising that light is the most important regulatory cue for the entrainment and activity of the circadian clock, because in addition to providing energy for photosynthesis, light also affects many other environmental conditions such as temperature and water availability (McClung, 2001). The role of cryptochromes in regulation of the plant circadian clock was indicated by the observation that the Arabidopsis hy1 mutant impaired in the synthesis of phytochrome chromophore showed little effect in the blue light regulation of circadian rhythms of CAB promoter activity (Millar et al., 1995), and that the Arabidopsis cry1 mutant significantly affected the circadian rhythm of the expression of a catalase gene (Zhong et al., 1997).
The function of cryptochromes in regulating the period lengths of the circadian clock was systematically studied recently using Arabidopsis mutants impaired in either the CRY1 or the CRY2 gene (Somers et al., 1998). In this study, period lengths of the circadian rhythm of the CAB promoter activity were analyzed under various light intensities in the photoreceptor mutant lines. It was shown that the cry1 mutant had period lengths longer than those of the wild type in both high and low intensities of blue light, indicating the function of cry1 in the regulation of the circadian clock over a wide range of light intensities. On the other hand, the cry2 mutant showed a slight change in period length only in relatively low intensities of blue light, suggesting that cry2 was not the major blue light receptor setting the clock.
However, the role of cry2 in the regulation of the circadian clock was clearly demonstrated when the cry1 cry2 double mutant was found to have much longer period lengths than either the cry1 or cry2 monogenic mutants in both low and high intensities of blue light (Devlin and Kay, 2000). Clearly, cry1 and cry2 act redundantly in the regulation of the circadian clock. Furthermore, cryptochromes may participate in phytochrome regulation of the circadian clock, because the cry1 cry2 double mutant showed, surprisingly, a very dramatic lengthening of the period length in relatively low intensities of red light (Devlin and Kay, 2000). In contrast to the “dead” clock situation found in cry1 cry2 double mutant mice under “free-running” conditions (van der Horst et al., 1999), Arabidopsis cry1 cry2 double mutant plants still retained robust free-running rhythmicity (Devlin and Kay, 2000), indicating that cryptochromes may not act as integral components of the central oscillator in plants.
In addition to their role as photoreceptors regulating the circadian clock, cryptochromes also mediate light regulation of gene expression in general. Cry1 is well known to be the major blue light receptor regulating light induction of expression of flavonoid biosynthesis genes such as CHS (chalcone synthase) in Arabidopsis (Kubasek et al., 1992; Jenkins, 1997). cry1 and cry2 act redundantly in mediating blue light induction of CHS expression, which showed a more pronounced defect in the cry1 cry2 double mutant than in the cry1 or cry2 monogenic mutants (Wade et al., 2001). Cryptochrome regulation of CHS gene expression occurs at the transcription level: transgenic plants expressing PCHS::GUS transgene exhibited lower PCHS::GUS transgene expression in response to blue/UV-A light in the cry1 mutant than in the wild-type background (Fuglevand et al., 1996). In contrast to the blue light regulation of CHS expression that is regulated mainly by cry1, cry2 appears to be the major blue light receptor regulating the activity of the Lhcb1*2 promoter (Mazzella et al., 2001). Transgenic plants expressing the PLhcb1*2::GUS transgene showed significantly lower expression of the transgene in response to white light in the cry2 mutant background than that in the cry1 mutant background (Mazzella et al., 2001).
In addition to their role in regulating nuclear genes, Arabidopsis cry1 and cry2 have been found to be involved in light regulation of chloroplast transcription (Thum et al., 2001). Chloroplasts of cry1 or cry2 mutants showed similarly lower transcription activity than that of the wild type. The blue light–activated chloroplast transcriptional activity decreased by ∼75% in the cry1 cry2 double mutant (Thum et al., 2001). It is not clear how many chloroplast genes may be regulated by cryptochromes. But it has been shown that cry1 and cry2 are both required for blue light–induced transcription of psbD-LRP promoter (Thum et al., 2001).
Cryptochrome regulation of gene expression has been studied in Arabidopsis using DNA microarrays containing 6126 unique expressed sequence tags (Ma et al., 2001). Among these genes, a total of 1712 (∼28% of the 6126) are either up- or downregulated in response to blue light. When the blue light effect on gene expression was investigated using the cry1 cry2 double mutant and the cry1-overexpressing transgenic line, it was found that blue light regulation of 634 genes (∼37% of the 1712) were affected by mutation or overexpression of cryptochromes. It is likely that the remaining two thirds of genes whose expression changes in response to blue light are regulated redundantly by cryptochromes and phytochromes, because phytochromes have been shown to affect both blue light input to the circadian clock and blue light regulation of transcriptional activity of individual genes (Devlin and Kay, 2000; Chun et al., 2001; Thum et al., 2001; Wade et al., 2001).
Signal Transduction of Cryptochromes
The detailed molecular mechanism of cryptochrome signal transduction is unclear. However, results from recent studies indicate that cryptochromes interact with other proteins, suggesting that absorption of a photon may trigger a change of protein–protein interactions of cryptochrome with other proteins. The cryptochrome signaling eventually leads to altered subcellular localization of light-signaling proteins, or changes in ion homeostasis, gene expression, or other cellular activities, resulting in developmental changes of plants in response to initial reaction of cryptochromes.
The Initial Photoreaction of Cryptochrome
The primary photoresponse of cryptochrome has been hypothesized to be a redox reaction involving electron transfers (Cashmore et al., 1999; Sancar, 2000). This model is based largely on the known mechanism of photolyase. The crystal structures of two photolyases (E. coli and Anacystis nidulans) have been solved (Park et al., 1995; Tamada et al., 1997). The polypeptide chain of a photolyase is folded into two major domains, a α/β domain and a helical domain. The light-harvesting chromophore (folate or deazaflavin) is bound to a cleft between the two major domains, and FAD is bound in the center of the helical domain. In the DNA repair reaction, a photolyase binds to the DNA lesion, light energy captured by the light-harvesting chromophore folate (or deazaflavin) is transferred to the catalytic chromophore FAD, a single electron is then transferred from FAD to the cyclobutane ring of pyrimidine dimer to generate two pyrimidines, and a back electron transfer from remaining pyrimidine radical to FAD restores the redox status of the cofactor (Sancar, 1994).
It is remarkable that the 3-D structures of these two photolyases, which share only ∼30% sequence identity and possess different light-harvesting chromophores (folate versus deazaflavin), are almost identical (Park et al., 1995; Tamada et al., 1997). It is therefore likely that the N-terminal PHR domain of a cryptochrome, which, in the case of Arabidopsis CRY1, shares close to 30% sequence identity with E. coli photolyase (Ahmad and Cashmore, 1993), may have a structure similar to that of a photolyase. Assuming this, one may speculate that the initial light reaction of a cryptochrome could be, like that of a photolyase, an electron transfer between flavin of a cryptochrome and a signaling molecule in close proximity. Alternatively, an electron transfer could occur between flavin and the protein moiety of the cryptochrome, resulting in conformational changes in the photoreceptor. In either scenario, an electron transfer to the signaling partner or a conformational change within the photoreceptor may lead to biochemical modifications, such as phosphorylation of the cryptochrome, and alternation of protein–protein interaction between the cryptochrome and signaling proteins it binds to, triggering further signal propagation. Indeed, Arabidopsis cryptochromes have been found to undergo blue light–dependent phosphorylation (Shalitin and Lin, unpublished data).
Cryptochromes Physically Interact with Other Proteins
Blue light signal transduction has been shown to involve direct protein–protein interactions of cryptochromes with other proteins. It was reported that Arabidopsis CRY1 interacts with phyA in yeast two-hybrid experiments and that recombinant oat phyA phosphorylates CRY1 in an in vitro reaction (Ahmad et al., 1998b). Arabidopsis CRY1 was found to be phosphorylated in red light in vivo and the phosphorylation was inhibited in the presence of far-red light (Ahmad et al., 1998b). In addition to phyA, cry1 may also interact with other phytochromes through interactions with a third protein. It was shown that Arabidopsis cry1 and phyB interacted with a PAS/F-box/Kelch domain protein, ADO1/ZTL/LKP1, in yeast two-hybrid assays and in vitro pull-down tests (Kiyosue and Wada, 2000; Somers et al., 2000; Jarillo et al., 2001a).
ADO1/ZTL/LKP1 has been found to play an important role in the regulation of the circadian clock and photoperiodic flowering in Arabidopsis, because mutations in or overexpression of the ADO1/ZTL/LKP1 gene caused a lengthening of the free-running period of clock-controlled transcription, hypocotyl elongation, leaf movement, and altered photoperiodic flowering (Kiyosue and Wada, 2000; Somers et al., 2000; Jarillo et al., 2001a). The demonstration of direct interactions between cry1/phyB and ADO1/ZTL/1LKP1 protein indicates that the input pathway from photoreceptors to the central oscillator may be short even though neither photoreceptor appears to be an integral part of the central oscillator.
Arabidopsis cry2 has been demonstrated to directly interact with phyB (Mas et al., 2000). The cry2–phyB interaction was shown by both yeast two-hybrid assays and coimmunoprecipitation tests. In addition, using fluorescent resonance energy transfer microscopy, an energy transfer was shown to occur between cry2-RFP and phyB-GFP fusion proteins, indicating that these two photoreceptors interact in vivo in a light-dependent manner (Mas et al., 2000). Further evidence that cry2–phyB interaction is essential for the function of cry2 came from a finding that CRY2-RFP, but not CRY1-RFP, was colocalized with phyB in the nuclear speckles (Mas et al., 2000). The recent discovery that phyB could mediate light regulation of transcription via its interaction with the transcription factor PIF3 (Ni et al., 1998; Martinez-Garcia et al., 2000) and the direct interaction between phyB and cry2 (Mas et al., 2000), suggest that alteration of phytochrome-mediated regulation of transcription may be an important mechanism of cryptochrome signal transduction.
Direct protein–protein interactions between cryptochromes and phytochromes also provide a molecular explanation for the coaction of these two types of photoreceptors that has been abundantly documented in physiological studies (Mohr, 1994; Casal, 2000). On the other hand, such interactions cannot fully account for cryptochrome-mediated blue light signal transduction, especially the blue light–specific activity of cryptochromes in de-etiolation. In this regard, the recent discovery of direct interactions between cryptochromes and COP1 protein is particularly interesting because it may provide another mechanism for cryptochrome signal transduction and regulation (Yang et al., 2000; Wang et al., 2001).
It may be expected that if cryptochrome function is dependent on the direct protein–protein interaction, overexpression of its protein–protein interaction domain would confer a dominant phenotypic change in the transgenic plants. Indeed, it was demonstrated that transgenic overexpression of fusion proteins of GUS and cryptochrome C-terminal domains (GUS-CCT) conferred a “dominant positive” phenotype (Yang et al., 2000). Transgenic plants expressing GUS-CCT1 (CRY1 C-terminal domain) or GUS-CCT2 (CRY2 C-terminal domain) fusion proteins showed short hypocotyls, opened cotyledons, and increased anthocyanin accumulation when they were grown in dark or in light, regardless of the wavelength of illumination (Yang et al., 2000). These phenotypes are reminiscent of those found for the cop/det/fus mutants, although cop/det mutants are recessive and often lethal, whereas the GUS-CCT expression caused a dominant but not lethal phenotype (Chory et al., 1989; Deng et al., 1989; Yang et al., 2000). Mutations in the CRY1 C-terminal domain, including E515K, E531K, and R576K, which had previously been shown to affect cry1 activity (Ahmad et al., 1995), eliminated the ability of fusion proteins to confer the cop/det phenotype. Expression of a similar fusion protein, GUS-CRY2C, which is larger by 7 residues at the N terminus of CRY2C (CRY2 C-terminal domain) than GUS-CCT2, did not confer a constitutive photomorphogenic phenotype (Guo et al., 1999). This could be due to the relatively lower level of expression of GUS-CRY2C compared with GUS-CCT2, or because the two different fusion proteins possess significantly different conformations due to the additional 7 residues in GUS-CRY2C. Nevertheless, these results are consistent with a proposition that cryptochromes interact with COP1 or other COP/DET proteins in a light-dependent manner to suppress the activity of COP/DET proteins in wild-type plants exposed to light (Yang et al., 2000). This hypothesis explains the phenotypic changes found in cop/det mutants, cryptochrome mutants, and GUS-CCT transgenic plants (Yang et al., 2000; Wang et al., 2001; Yang et al., 2001). It was shown that full-length CRY1, or CCT1, or GUS-CCT1 fusion proteins interact with COP1 in yeast two-hybrid assays (Wang et al., 2001; Yang et al., 2001). Further evidence of the COP1–CRY1 interaction came from the observation that onion cells coexpressing GFP-CCT1 and COP1 exhibited speckles in the nucleus, which were detected when GFP-COP1 was expressed alone but not when GFP-CCT1 was expressed alone (Wang et al., 2001). The cry1–COP1 interaction does not seem to be dependent on light. cry2 may also interact with COP1. It was found that GUS-CCT2 interacted with COP1 in both yeast two-hybrid and coimmunoprecipitation assays (Wang et al., 2001), although CRY2–COP1 interaction was not detected using a similar yeast two-hybrid assay in another study (Yang et al., 2001). Transgenic plants expressing GUS-CCT1 or GUS-CCT2 fusion proteins accumulated more of the basic leucine zipper transcription factor HY5 in the dark, which is similar to that observed in the cop1 mutant (Osterlund et al., 2000). Moreover, genome-wide gene expression profiles of dark-grown GUS-CCT1 and GUS-CCT2 transgenic seedlings are essentially identical, which was also similar to that of blue light–grown wild-type seedlings or the dark-grown cop1 mutant (Wang et al., 2001).
COP1 is a zinc-finger and WD40 repeat protein that has a light-regulated nucleocytoplasmic partitioning pattern similar to CRY1; both are enriched in the nucleus in dark, but in the cytosol in light (Von Arnim and Deng, 1994; Yang et al., 2000). COP1 has been proposed to act as a subunit of an E3-ubiquitin ligase complex associated with degradation of the basic leucine zipper transcription factor HY5 in the dark (Osterlund et al., 2000). These observations are consistent with a transcription regulation model for the cryptochrome-mediated de-etiolation response (Yang et al., 2000; Wang et al., 2001). According to this model, COP1 interacts with HY5 in the dark to facilitate its degradation, ensuring the “off” status of light-induced gene expression and thus etiolated development. In light, photoactivated cry1 is excluded, together with COP1, from the nucleus, allowing an accumulation of the transcription factor HY5 and transcription activation of genes required for photomorphogenesis. This model, irrespective of its oversimplification, appears to satisfactorily explain many observations of the genetic studies with respect to cryptochrome-mediated blue light de-etiolation responses.
Ion Homeostasis and Cryptochrome Signal Transduction
In addition to transcription regulation, other cellular processes such as changes of ion homeostasis are also involved in signal transduction, signal propagation, and/or feedback regulation of cryptochromes. Blue light–induced rapid plasma membrane depolarization is one of the early cellular blue light responses discovered (Spalding and Cosgrove, 1988). This membrane depolarization likely results from the opening of ion channels in response to blue light (Cho and Spalding, 1996). Arabidopsis cry1 and cry2 mutants are compromised in both the blue light–induced membrane depolarization and blue light–activated opening of anion channels, indicating that the opening of anion channels plays a critical role in the early signaling process of cryptochromes (Cho and Spalding, 1996; Folta and Spalding, 2001b).
Change of calcium homeostasis has also been shown to be associated with blue light signaling (Christie and Jenkins, 1996; Long and Jenkins, 1998). In these studies, Arabidopsis cell culture was used to monitor blue/UV-A light–induced CHS expression. It was found that blue light promotes calcium efflux in the cytosol, and that compounds that inhibit voltage-gated calcium channels or Ca2+-ATPase significantly altered blue/UV-A light–induced CHS expression (Christie and Jenkins, 1996; Long and Jenkins, 1998).
Although genes encoding the above-mentioned calcium channels and Ca2+-ATPases still remain to be identified, a recent study of the Arabidopsis SUB1 gene has provided genetic evidence supporting a possible involvement of a localized calcium concentration change in cryptochrome signaling (Guo et al., 2001). The sub1 mutant is hypersensitive to blue and far-red light with respect to hypocotyl inhibition and light-induced gene expression changes. Genetic analyses indicated that SUB1 acts downstream from cryptochromes in a fluence-dependent manner, but it is a modulator rather than a signaling protein for phyA. SUB1 protein contains two EF-hand–like calcium binding motifs, and an in vitro calcium binding assay indicated that SUB1 is a low-affinity calcium binding protein (Guo et al., 2001). SUB1 protein is associated with the nuclear envelope (Guo et al., 2001; Guo and Lin, unpublished data). It is likely that blue light may trigger a localized change of the calcium concentration surrounding the nucleus, which in turn affects SUB1 activity and nucleocytoplasmic trafficking of photoreceptors or photoreceptor signaling molecules.
PHOTOTROPINS AND MOVEMENT RESPONSES
Plants are immobile organisms. However, plant organs and organelles do move in response to various environmental stimuli, especially light. For example, hypocotyls bend toward light to maximize photosynthesis in cotyledons, whereas roots curve away from blue light to ensure that they stay in soil for water and nutrient absorption. Chloroplasts move toward relatively weak light for maximum photon capture, but move away from high-intensity light to avoid photodamage. Stomata, pores formed by two surrounding guard cells in epidermis, adjust their aperture in response to light, opening in the daytime to allow gas exchange but closing at night to minimize water loss. For unknown reasons, blue light is usually the wavelength of light most effective in inducing these movement responses (Briggs and Huala, 1999). The blue light receptors mediating plant movement responses have remained elusive until recently. On the basis of genetic studies in Arabidopsis, it has become clear that the phototropin family of flavin-containing blue light receptors regulates all three movement responses mentioned above: phototropism, chloroplast movement, and stomatal opening.
Phototropins and Phototropism
Phototropin was initially identified as a ∼120 kD plasma membrane protein that undergoes blue light–dependent phosphorylation in pea and other plants, including Arabidopsis (Gallagher et al., 1988; Short and Briggs, 1994; Christie and Briggs, 2001). Shortly after, it was found that the light-dependent phosphorylation of this protein occurred at a much lower level in a phototropic-deficient Arabidopsis mutant, JK224, indicating its role in phototropism (Reymond et al., 1992). The gene encoding this 120-kD protein was cloned from another Arabidopsis phototropic-deficient mutant, nph1 (for nonphototropic hypocotyl) that is impaired in both hypocotyl curvature and root curvature in response to light (Liscum and Briggs, 1995; Huala et al., 1997). Mutations in the NPH1 gene were found in different nph1 alleles, confirming its role in phototropism. In keeping with the nomenclature of phytochromes and cryptochromes, the NPH1 gene, nph1 mutant, and the NPH1 apoprotein and holoprotein are now referred to as PHOT1, phot1, PHOT1, and phot1, respectively (Briggs et al., 2001). Arabidopsis PHOT1 encodes a 996-residue polypeptide that contains two PAS domains at the N terminus and a serine/threonine kinase domain at the carboxyl terminus (Figure 2). Because the two PAS domains of PHOT1 are more closely related to those found in a subset of PAS domain–containing proteins that are regulated by light, oxygen, and voltage changes, they were collectively referred to as LOV domains (Huala et al., 1997). Recombinant PHOT1 expressed in insect cells binds noncovalently flavin mononucleotide (FMN), and it undergoes a blue light–dependent autophosphorylation in vitro. The absorption and fluorescence excitation spectra of the recombinant PHOT1 are similar to the action spectrum of phototropism in Arabidopsis. Together with the genetic evidence, these results demonstrated that PHOT1 is a flavin-containing photoreceptor mediating blue light–induced phototropism. The LOV domain fragments expressed and purified from E. coli cells bind FMN stoichiometrically, indicating that LOV domains are the FMN chromophore binding sites of phototropins and that a holophototropin molecule contains two FMNs (Christie et al., 1999).
Genes encoding phototropins have also been found in other plant species, including rice, maize, oat, ice plant, and alga (Kanegae et al., 2000; Briggs et al., 2001; Briggs and Olney, 2001). It is particularly interesting that a gene encoding a phytochrome–phototropin hybrid protein, PHY3, was identified in the fern Adiantum. PHY3 polypeptide consists of an N-terminal domain similar to phytochrome chromophore binding domains and a C-terminal domain that resembles a near full-length phototropin (Nozue et al., 1998). The hypothesis that Adiantum PHY3 is a phytochrome–phototropin hybrid protein is supported by the observation that PHY3 can bind both a red/far-red light–absorbing chromophore precursor, phycocyanobilin, and the blue/UV-A light–absorbing chromophore FMN (Nozue et al., 1998; Christie et al., 1999). Unlike in plants such as Arabidopsis, for which phototropism is largely a blue light response, the phototropism in Adiantum gametophytes and sporophytes can be induced by both blue light and red light, which is consistent with the hypothesis that that PHY3 is a photoreceptor mediating phototropism in Adiantum.
In addition to its role in the phototropic hypocotyl curvature response, phot1 also mediates the phototropic response in roots. In a study of Arabidopsis rpt (root phototropism) mutants impaired in the negative curvature of roots in response to light, it was found that one of the rpt mutants, rpt1, was allelic to nph1/phot1, and both mutants were completely insensitive to both high- and low-intensity blue light with respect to root phototropism. However, it was also found in this study that the hypocotyl curvature, in response to blue light of fluence rates much higher than those previously used, was normal in the phot1 mutants. The fact that the phot1 mutants showed normal hypocotyl curvature in high intensities of blue light indicated the existence of another photoreceptor mediating hypocotyl phototropism (Sakai et al., 2000). Although cryptochromes were implicated in the phototropism, the second photoreceptor for phototropism in Arabidopsis turned out to be another phototropin, NPL1 (NPH1-like), which was later renamed as phot2 (Ahmad et al., 1998c; Lasceve et al., 1999; Sakai et al., 2001).
Phototropins and Chloroplast Movement
The Arabidopsis NPL1 gene was isolated on the basis of its sequence similarity to NPH1/PHOT1 (Jarillo et al., 1998). The amino acid sequence of NPL1 is ∼58% identical to that of PHOT1. A T-DNA insertion mutant, npl1, was isolated and examined for various blue light responses (Jarillo et al., 2001b). Using a leaf light transmittance assay, which measures light transmission changes resulting from chloroplast relocation, npl1 was found to be defective in chloroplast movement in high light but not in low light, suggesting the role of NPL1 in chloroplast avoidance but not in chloroplast accumulation. In a separate study, NPL1 was specifically shown to mediate the chloroplast avoidance response (Kagawa et al., 2001). Using a microbeam irradiation technique, it was shown in Arabidopsis that chloroplasts move toward blue light of relatively low intensity (chloroplast accumulation) but move away from high-intensity blue light that can cause photodamage to the chloroplasts (chloroplast avoidance) (Kagawa and Wada, 1999, 2000). The light sensitivity of chloroplast relocation responses is slightly lower in the phot1 mutant than in the wild type (Kagawa and Wada, 2000). In an elegant genetic screen, in which a mutant impaired in chloroplast avoidance is identified by the lack of change of leaf color after a high-intensity light exposure, an Arabidopsis mutant, cav1 (for chloroplast avoidance), was isolated. cav1 showed defect in chloroplast avoidance in response to high-intensity blue light, and the gene corresponding to the cav1 mutant was found to be NPL1 (Kagawa et al., 2001). It was also discovered in this study that mutation of NPL1 did not affect chloroplast accumulation in low-intensity blue light (Kagawa et al., 2001), suggesting that another blue light receptor was needed to mediate this low-blue-light response. NPL1 is now renamed as PHOT2 (Briggs et al., 2001). Neither the phot1 or phot2 mutant shows other developmental defects, suggesting that the function of phototropins is largely limited to phototropic responses. Like phot1, phot2 is also a flavoprotein that undergoes blue light–induced autophosphorylation (Sakai et al., 2001). The PHOT2 mRNA expression is upregulated by blue light (Jarillo et al., 2001b; Kagawa et al., 2001).
Phototropins Mediate Similar Blue Light Responses with Different Photosensitivities
The question of what photoreceptor mediates hypocotyl phototropism in high light or chloroplast accumulation in low light was answered by a study of the phot1phot2 double mutant (Sakai et al., 2001). It was demonstrated that the phot1phot2 double mutant is completely insensitive to both low and high light in hypocotyl phototropism and chloroplast movement responses. It is clear now that these two phototropins mediate similar blue light responses, but they have different photosensitivities, which is somewhat reminiscent of what was discussed previously for the function of cry1 and cry2 in de-etiolation. In Arabidopsis, phot1 mediates the negative root curvature throughout a wide range of light intensities, and it can act alone to bring about the positive hypocotyl curvature response in low light. In high light, phot1 and phot2 act redundantly in mediating hypocotyl phototropism. On the other hand, phot2 is the major photoreceptor mediating chloroplast avoidance in high light, whereas phot1 and phot2 act redundantly in mediating chloroplast accumulation in low light.
When phot1 or phot2 monogenic mutants were investigated for blue light responses such as de-etiolation, photoperiodic flowering, and stomatal opening, neither monogenic mutant showed an obvious defect in these responses (Liscum and Briggs, 1995; Lasceve et al., 1999; Jarillo et al., 2001b). The lack of direct function of phototropins in de-etiolation and photoperiodism may not be surprising, because these photomorphogenic responses are known to be controlled by a different type of blue light receptor, cryptochrome, as well as red/far-red light receptor, phytochrome (Lin, 2000b). It remains to be examined whether phot1 and phot2 act redundantly to affect the function of phytochrome and cryptochrome in these light responses.
Phototropins and Stomatal Opening
Stomatal opening is another movement response mediated by phototropins. It has been known for over two decades that the blue light receptor mediating stomatal opening is located in the guard cells (Zeiger and Helper, 1977; Assmann et al., 1985; Zeiger, 2000). It was hypothesized that zeaxanthin might be a candidate chromophore of the photoreceptor (Zeiger, 2000). However, recent genetic evidence indicates that phototropins are photoreceptors mediating stomatal opening. When the pho1phot2 double mutant was examined with an increased fluence rate of blue light in a red light background, no blue light response was detected with respect to stomatal opening (Kinoshita et al., 2001). Because the stomatal opening response was normal in both the phot1 and the phot2 monogenic mutants, phot1 and phot2 apparently act in a functionally redundant manner. It remains to be examined whether there is a separate photoreceptor that contributes to the stomatal opening response and contains zeaxanthin as the chromophore.
The stomatal aperture size is controlled by the volume and shape of guard cells (Christie and Briggs, 2001; Schroeder et al., 2001). In response to light, salt concentration increases in the guard cells, causing an inflow of water, expansion of guard cells, and opening of stomatal aperture. It has been found that blue light induces phosphorylation of a plasma membrane proton ATPase (H+-ATPase; Kinoshita and Shimazaki, 1999). The action of H+-ATPase elevates the inside negative electrical potential gradient across the plasma membrane. This electrical potential gradient drives a voltage-gated K+ channel, resulting in an accumulation of potassium salt inside guard cells and eventually opening of stomata (Schroeder et al., 2001). Since phototropin is a plasma membrane light-dependent protein kinase, it is likely to be responsible for the blue light–induced phosphorylation of the H+-ATPase. Indeed, the blue light–induced increase of H+-ATPase activity was abolished in the phot1phot2 double mutant, presumably because of the lack of phototropin kinases (Kinoshita et al., 2001).
Phototropin Signal Transduction
The initial photochemical reaction of a phototropin has been investigated using recombinant LOV domain proteins expressed and purified from heterologous systems (Salomon et al., 2000; Swartz et al., 2001). It was found that the LOV domains undergo a self-contained photocycle of formation and decay of the FMN-cysteinyl adducts, accompanied by a decreased and recovered blue light absorption. This result is supported by a recent crystal structure study of the LOV domain (Crosson and Moffat, 2001). It is conceivable that such a photochemical reaction may trigger autophosphorylation catalyzed by the kinase domain of phototropin and/or possible intermolecular protein phosphorylation reactions. One possible phototropin substrate may be NPH3. The nph3 mutant was identified in the same genetic screen that identified the nph1/phot1 mutant, and NPH3 was found to act in the same genetic pathway as phot1 (Liscum and Briggs, 1995, 1996). NPH3 encodes a protein possessing BTB/POZ and coil-coil protein–protein interaction domains. It was found that NPH3 physically interacts with phot1 in a yeast two-hybrid study and a pull-down assay, and the LOV domain of phot1 and the BTB/POZ/coil-coil domain of NPH3 are the protein–protein interaction domains of the respective proteins (Motchoulski and Liscum, 1999). NPH3 protein showed a blue light–induced migration mobility shift, implying that phot1 may catalyze a blue light–dependent phosphorylation of NPH3. Phot1 and NPH3 are both plasma membrane proteins, but neither of them contains a membrane spanning sequence. Therefore, a post-translational lipid modification of either protein may be required for their colocalization at the plasma membrane.
Another protein important for phototropin signal transduction is the recently isolated RPT2 (Sakai et al., 2000). The rpt2 mutant was identified because of its defect in root phototropism, but it also showed a defect in hypocotyl phototropism. The RPT2 gene encodes an NPH3-like protein, containing a BTB/POZ domain at the N terminal and a coil-coil domain at the C terminal, but it is not known whether RPT2 may interact with and be modified by PHOT1. Because RPT2 also contains a nuclear localization signal and many BTB/POZ domain proteins are known to interact with transcription factors, it is possible that RPT2, NPH3, or other BTB/POZ domain proteins may connect the signal from phototropin at the plasma membrane to transcription factors in the nucleus.
One transcription factor acting downstream from phototropins has been shown to be an auxin-response factor, NPH4/ARF7. The nph4 mutant was originally isolated as a phototropic-deficient mutant, which, like nph1/phot1, showed defect in blue light–induced phototropic response (Liscum and Briggs, 1995). However, it turned out that the nph4 mutation affects not only phototropism but also gravitropism, auxin-resistant growth, and auxin-regulated gene expression (Stowe-Evans et al., 1998). Moreover, unlike nph1, the aphototropic phenotype of nph4 can be suppressed by ethylene, suggesting a role of the NPH4 gene in hormonal interaction (Harper et al., 2000). The NPH4 gene encodes an auxin response factor, ARF7 (Harper et al., 2000), belonging to the ARF-type transcription factor family that is involved in auxin responses and is regulated by auxin (Guilfoyle et al., 1998). This finding is particularly interesting with respect to the signaling process of phototropins, because auxin is well known for its involvement in phototropism (Briggs et al., 1957; Briggs, 1963). It was proposed that NPH4/ARF7 acts as a transcription activator mediating differential growth in response to light and other environmental stimuli (Harper et al., 2000).
Phototropin may also confer its effect through the change of ion homeostasis. A transient blue light–induced increase of cytosolic calcium was shown in transgenic plants expressing the recombinant calcium-binding fluorescent protein aequorin (Baum et al., 1999). This transient change in calcium homeostasis is specifically relevant to phototropic responses because it was attenuated in the nph1 mutant but not in the cry1 or cry2 mutants (Baum et al., 1999). It is conceivable that phot1 may catalyze phosphorylation of calcium transporters at the plasma membrane, triggering other changes in the cell and consequently the differential growth of hypocotyls.
It is interesting to note that the nph1/phot1 mutant, which is impaired in phototropism but not in hypocotyl inhibition (Liscum and Briggs, 1995), showed defect in blue light–induced plasma membrane depolarization and blue light–induced rapid inhibition of cell growth; both were previously reported in the cry1 and cry2 mutants (Folta and Spalding, 2001b). It was suggested that blue light activation of phototropins may influence cryptochrome signaling leading to hypocotyl inhibition (Folta and Spalding, 2001b). Alternatively, membrane depolarization and rapid growth inhibition may be independently associated with differential growth response of phototropism and long-term growth inhibition response of de-etiolation.
Although stomatal opening is the latest movement response for which the identity of the responsible photoreceptors has became known, we seem to know much more about the downstream molecular mechanism associated with stomatal opening than that associated with either phototropic curvature or chloroplast relocation (Zeiger, 2000; Christie and Briggs, 2001; Schroeder et al., 2001). It is likely that our knowledge of blue light–induced stomatal opening may provide additional clues regarding where to look for the signaling components downstream from phototropins associated with phototropic and chloroplast movement responses. For example, phototropins may regulate ion transporters at the plasma membrane in leaf cells. This could change ion homeostasis in leaf cells, which could alter the network of the cytoskeleton, change the status of the cytoplasmic stream, and eventually change the location of chloroplasts. It is also conceivable that phototropins may interact with and phosphorylate auxin transporters at the plasma membrane to alter the signaling process of this phytohormone, resulting in differential growth.
PROSPECTS
Our understanding of blue light photoreceptors, like that of many other aspects of signal transduction in plants, has been greatly facilitated by Arabidopsis genetic studies. However, detailed molecular mechanisms of photoreceptor signal perception, signal transduction, and desensitization remain to be elucidated. For example, the biochemical nature of the initial photoreaction of cryptochromes is not clear. Protein phosphorylation or other types of protein modification have been found for almost every well-characterized photoreceptor; cryptochrome is unlikely to be the exception. It is important to examine blue light–dependent biochemical modification of cryptochromes and the manner in which such modifications are associated with cryptochrome activity and regulation. With respect to the initial photoreaction, we currently know more about phototropins than about cryptochromes. It is likely that phototropins catalyze blue light–dependent phosphorylation of different plasma membrane proteins in different tissues—H+-ATPase in guard cells, ion transporters or channel proteins in other leaf cells, and/or auxin transporters in stem cells, resulting in different movement responses in different parts of a plant.
Cryptochrome and phototropin signal transductions are integral parts of plant growth and developmental programs, and our immediate challenge is to identify all the genes associated with photoreceptor function and regulation. In this respect, protein interaction analyses and genetics studies, which have provided most of our current advances in the field of photoreceptor signal transduction, will continue to serve as two powerful approaches. It is also expected that the recently available bioinformatics and genomics tools in Arabidopsis will further enhance our ability not only to identify all the genes involved in the signal transduction of blue light receptors but also to understand the more-complex problems of how blue light receptor signal transduction interacts with other signaling pathways and cellular activities to eventually bring about growth and developmental changes in plants.
Acknowledgments
The author thanks Todd Mockler and Dr. May Santiago-Ong for critical readings of the manuscript, and Hongyun Yang for preparation of figures. The work in the author's laboratory is supported in part by NIH Grant No. GM56265 and NSF Grant No. MCB-0091391.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.000646.
References
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., and Cashmore, A.R. (1997). The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 11 421–427. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., and Cashmore, A.R. (1998. a). Chimeric proteins between cry1 and cry2 Arabidopsis blue light photoreceptors indicate overlapping functions and varying protein stability. Plant Cell 10 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998. b). The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1 939–948. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998. c). Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392 720–723. [DOI] [PubMed] [Google Scholar]
- Ahmad, M., Lin, C., and Cashmore, A.R. (1995). Mutations throughout an Arabidopsis blue-light photoreceptor impair blue-light-responsive anthocyanin accumulation and inhibition of hypocotyl elongation. Plant J. 8 653–658. [DOI] [PubMed] [Google Scholar]
- Assmann, S.M., Simoncini, L., and Schroeder, J.I. (1985). Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318 285–287. [Google Scholar]
- Bagnall, D.J., King, R.W., and Hangarter, R.P. (1996). Blue-light promotion of flowering is absent in hy4 mutants of Arabidopsis. Planta 200 278–280. [DOI] [PubMed] [Google Scholar]
- Batschauer, A. (1993). A plant gene for photolyase: An enzyme catalyzing the repair of UV-light-induced DNA damage. Plant J. 4 705–709. [DOI] [PubMed] [Google Scholar]
- Baum, G., Long, J.C., Jenkins, G.I., and Trewavas, A.J. (1999). Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca(2+). Proc. Natl. Acad. Sci. USA 96 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R. (1963). The phototropic responses of higher plants. Annu. Rev. Plant Phy. 14 311–352. [Google Scholar]
- Briggs, W.R., and Huala, E. (1999). Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol. 15 33–62. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R., and Olney, M.A. (2001). Photoreceptors in plant photomorphogenesis to date. five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 125 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., Tocher, R.D., and Wilson, J.F. (1957). Phototropic auxin redistribution in corn coleoptiles. Science 126 210–212. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R, et al. (2001). The phototropin family of photoreceptors. Plant Cell 13 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal, J.J. (2000). Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 71 1–11. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., and Boccalandro, H. (1995). Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta 197 213–218. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., and Mazzella, M.A. (1998). Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 118 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore, A.R., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284 760–765. [DOI] [PubMed] [Google Scholar]
- Ceriani, M.F., Darlington, T.K., Staknis, D., Mas, P., Petti, A.A., Weitz, C.J., and Kay, S.A. (1999). Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285 553–556. [DOI] [PubMed] [Google Scholar]
- Cho, M.H., and Spalding, E.P. (1996). An anion channel on Arabidopsis hypocotyls activated by blue light. Proc. Natl. Acad. Sci. USA 93 8134–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory, J., Peto, C., Feinbaum, R., Pratt, L., and Ausubel, F. (1989). Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell 58 991–999. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., and Briggs, W.R. (2001). Blue light sensing in higher plants. J. Biol. Chem. 276 11457–11460. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., and Jenkins, G.I. (1996). Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8 1555–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, J.M., Salomon, M., Nozue, K., Wada, M., and Briggs, W.R. (1999). LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA 96 8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun, L., Kawakami, A., and Christopher, D.A. (2001). Phytochrome A mediates blue light and UV-A-dependent chloroplast gene transcription in green leaves. Plant Physiol. 125 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, S., and Moffat, K. (2001). Structure of a flavin-binding plant photoreceptor domain: Insights into light-mediated signal transduction. Proc. Natl. Acad. Sci. USA 98 2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, S.R., Ehrhardt, D.W., Griffitts, J.S., and Somerville, C.R. (2000). Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA 97 3718–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin, C. (1881). The Power of Movement in Plants. (New York: D. Appleton and Company).
- Deng, X.-W., Caspar, T., and Quail, P.H. (1989). COP1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 5 1172–1182. [DOI] [PubMed] [Google Scholar]
- Devlin, P.F., and Kay, S.A. (2000). Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 12 2499–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap, J.C. (1999). Molecular bases for circadian clocks. Cell 96 271–290. [DOI] [PubMed] [Google Scholar]
- Egan, E.S., Franklin, T.M., Hilderbrand-Chae, M.J., McNeil, G.P., Roberts, M.A., Schroeder, A.J., Zhang, X., and Jackson, F.R. (1999). An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J. Neurosci. 19 3665–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din El-Assal, S., Alonso-Blanco, C., Peeters, A.J., Raz, V., and Koornneef, M. (2001). A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Natl. Genet 29 435–440. [DOI] [PubMed] [Google Scholar]
- Emery, P., So, W.V., Kaneko, M., Hall, J.C., and Rosbash, M. (1998). CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95 669–679. [DOI] [PubMed] [Google Scholar]
- Emery, P., Stanewsky, R., Hall, J.C., and Rosbash, M. (2000. a). A unique circadian-rhythm photoreceptor. Nature 404 456–457. [DOI] [PubMed] [Google Scholar]
- Emery, P., Stanewsky, R., Helfrich-Forster, C., Emery-Le, M., Hall, J.C., and Rosbash, M. (2000. b). Drosophila CRY is a deep brain circadian photoreceptor. Cell 26 493–504. [DOI] [PubMed] [Google Scholar]
- Folta, K.M., and Spalding, E.P. (2001. a). Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J. 28 333–340. [DOI] [PubMed] [Google Scholar]
- Folta, K.M., and Spalding, E.P. (2001. b). Unexpected roles for cryptochrome 2 and phototropin revealed by high- resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26 471–478. [DOI] [PubMed] [Google Scholar]
- Fuglevand, G., Jackson, J.A., and Jenkins, G.I. (1996). UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8 2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, S., Short, T.W., Ray, P.M., Pratt, L.H., and Briggs, W.R. (1988). Light-mediated changes in two proteins found associated with plasma membrane fractions from pea stem sections. Proc. Natl. Acad. Sci. USA 85 8003–8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel, J. (1979). Blue light photoreception. Photochem. Photobiol. 30 749–754. [Google Scholar]
- Griffin, E.A., Jr., Staknis, D., and Weitz, C.J. (1999). Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286 768–771. [DOI] [PubMed] [Google Scholar]
- Guilfoyle, T., Hagen, G., Ulmasov, T., and Murfett, J. (1998). How does auxin turn on genes? Plant Physiol. 118 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, H., Duong, H., Ma, N., and Lin, C. (1999). The Arabidopsis blue light receptor cryptochrome 2 is a nuclear protein regulated by a blue light-dependent post-transcriptional mechanism. Plant J. 19 279–287. [DOI] [PubMed] [Google Scholar]
- Guo, H., Mockler, T.C., Duong, H., and Lin, C. (2001). SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291 487–490. [DOI] [PubMed] [Google Scholar]
- Guo, H., Yang, H., Mockler, T.C., and Lin, C. (1998). Regulation of flowering time by Arabidopsis photoreceptors. Science 279 1360–1363. [DOI] [PubMed] [Google Scholar]
- Harmer, S.L., Hogenesch, J.B., Straume, M., Chang, H.S., Han, B., Zhu, T., Wang, X., Kreps, J.A., and Kay, S.A. (2000). Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290 2110–2113. [DOI] [PubMed] [Google Scholar]
- Harper, R.M., Stowe-Evans, E.L., Luesse, D.R., Muto, H., Tatematsu, K., Watahiki, M.K., Yamamoto, K., and Liscum, E. (2000). The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig, L., Funk, M., Whitelam, G.C., and Schafer, E. (1999. a). Functional interaction of cryptochrome 1 and phytochrome D. Plant J. 20 289–294. [DOI] [PubMed] [Google Scholar]
- Hennig, L., Poppe, C., Unger, S., and Schafer, E. (1999. b). Control of hypocotyl elongation in Arabidopsis thaliana by photoreceptor interaction. Planta 208 257–263. [DOI] [PubMed] [Google Scholar]
- Hisada, A., Hanzawa, H., Weller, J., Nagatani, A., Reid, J., and Furuya, M. (2000). Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 12 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, P.D., Batschauer, A., and Hays, J.B. (1996). PHH1, a novel gene from Arabidopsis thaliana that encodes a protein similar to plant blue-light photoreceptors and microbial photolyases. Mol. Gen. Genet. 253 259–265. [DOI] [PubMed] [Google Scholar]
- Hsu, D.S., Zhao, X., Zhao, S., Kazantsev, A., Wang, R.P., Todo, T., Wei, Y.F., and Sancar, A. (1996). Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry 35 13871–13877. [DOI] [PubMed] [Google Scholar]
- Huala, E., Oeller, P.W., Liscum, E., Han, I.S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278 2120–2123. [DOI] [PubMed] [Google Scholar]
- Imaizumi, T., Kadota, A., Hasebe, M., and Wada, M. (2002). Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell 14 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi, T., Kanegae, T., and Wada, M. (2000). Cryptochrome nucleocytoplasmic distribution and gene expression are regulated by light quality in the fern Adiantum capillus-veneris. Plant Cell 12 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo, J., Ahmad, M., and Cashmore, A.R. (1998). NPL1: A second member of the NPH serine/threonine kinase family of Arabidopsis. Plant Physiol. 117 719. [Google Scholar]
- Jarillo, J.A., Capel, J., Tang, R.H., Yang, H.Q., Alonso, J.M., Ecker, J.R., and Cashmore, A.R. (2001. a). An Arabidopsis circadian clock component interacts with both CRY1 and phyB. Nature 410 487–490. [DOI] [PubMed] [Google Scholar]
- Jarillo, J.A., Gabrys, H., Capel, J., Alonso, J.M., Ecker, J.R., and Cashmore, A.R. (2001. b). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410 952–954. [DOI] [PubMed] [Google Scholar]
- Jenkins, G.I. (1997). UV-A and blue light signal transduction in Arabidopsis. Plant Cell Environ. 20 773–778. [DOI] [PubMed] [Google Scholar]
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K., and Wada, M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291 2138–2141. [DOI] [PubMed] [Google Scholar]
- Kagawa, T., and Wada, M. (1999). Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol. 119 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa, T., and Wada, M. (2000). Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol. 41 84–93. [DOI] [PubMed] [Google Scholar]
- Kanegae, H., Tahir, M., Savazzini, F., Yamamoto, K., Yano, M., Sasaki, T., Kanegae, T., Wada, M., and Takano, M. (2000). Rice NPH1 homologues, OsNPH1a and OsNPH1b, are differently photoregulated. Plant Cell Physiol. 41 415–423. [DOI] [PubMed] [Google Scholar]
- Kanegae, T., and Wada, M. (1998). Isolation and characterization of homologues of plant blue-light photoreceptor (cryptochrome) genes from the fern Adiantum capillus-veneris. Mol. Gen. Genet. 259 345–353. [DOI] [PubMed] [Google Scholar]
- Kenrick, P., and Crane, R. (1997). The origin and early evolution of plants on land. Nature 389 33–39. [Google Scholar]
- Kinoshita, T., Michio Doi, M., Suetsugu, N., Kagawa, T., Wada, M., and Shimazaki, K.-I. (2001). phot1 and phot2 mediate blue light regulation of stomatal opening . Nature 414 656–660. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Shimazaki, K. (1999). Blue light activates the plasma membrane H(+)-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18 5548–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Kozma-Bognar, L., Kim, L., Adam, E., Harter, K., Schafer, E., and Nagy, F. (1999). Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyosue, T., and Wada, M. (2000). LKP1 (LOV Kelch protein 1): A factor involved in the regulation of flowering time in arabidopsis. Plant J. 23 807–815. [DOI] [PubMed] [Google Scholar]
- Kleiner, O., Kircher, S., Harter, K., and Batschauer, A. (1999). Nuclear localization of the Arabidopsis blue light receptor cryptochrome 2. Plant J. 19 289–296. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Hanhart, C.J., and van der Veen, J.H. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229 57–66. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Rolff, E., and Spruit, C.J.P. (1980). Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z. Pflanzenphysiol. Bd. 100 147–160. [Google Scholar]
- Ku, H.M., Vision, T., Liu, J., and Tanksley, S.D. (2000). Comparing sequenced segments of the tomato and Arabidopsis genomes: Large-scale duplication followed by selective gene loss creates a network of synteny. Proc. Natl. Acad. Sci. USA 97 9121–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasek, W.L., Shirley, B.W., McKillop, A., Goodman, H.M., Briggs, W., and Ausubel, F.M. (1992). Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 4 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume, K., Zylka, M.J., Sriram, S., Shearman, L.P., Weaver, D.R., Jin, X., Maywood, E.S., Hastings, M.H., and Reppert, S.M. (1999). mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98 193–205. [DOI] [PubMed] [Google Scholar]
- Lasceve, G., Leymarie, J., Olney, M.A., Liscum, E., Christie, J.M., Vavasseur, A., and Briggs, W.R. (1999). Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol. 120 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C., Etchegaray, J.P., Cagampang, F.R., Loudon, A.S., and Reppert, S.M. (2001). Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107 855–867. [DOI] [PubMed] [Google Scholar]
- Lin, C. (2000. a). Photoreceptors and regulation of flowering time. Plant Physiol. 123 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. (2000. b). Plant blue-light receptors. Trends Plant Sci. 5 337–342. [DOI] [PubMed] [Google Scholar]
- Lin, C., Ahmad, M., and Cashmore, A.R. (1996. a). Arabidopsis cryptochrome 1 is a soluble protein mediating blue light-dependent regulation of plant growth and development. Plant J. 10 893–902. [DOI] [PubMed] [Google Scholar]
- Lin, C., Ahmad, M., Chan, J., and Cashmore, A.R. (1996. b). CRY2, a second member of the Arabidopsis cryptochrome gene family. Plant Physiol. 110 1047.8819875 [Google Scholar]
- Lin, C., Ahmad, M., Gordon, D., and Cashmore, A.R. (1995. a). Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc. Natl. Acad. Sci. USA 92 8423–8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C., and Cashmore, A.R. (1996). Cryptochrome and plant photomorphogenesis. In Regulation of Plant Growth and Development by Light, W.R. Briggs, R.L. Heath, and E.M. Tobin, eds (Rockville, MD: American Society of Plant Physiologists), pp. 30–39.
- Lin, C., Robertson, D.E., Ahmad, M., Raibekas, A.A., Jorns, M.S., Dutton, P.L., and Cashmore, A.R. (1995. b). Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science 269 968–970. [DOI] [PubMed] [Google Scholar]
- Lin, C., Yang, H., Guo, H., Mockler, T., Chen, J., and Cashmore, A.R. (1998). Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 95 2686–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum, E., and Briggs, W. (1995). Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum, E., and Briggs, W.R. (1996). Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol. 112 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, J.C., and Jenkins, G.I. (1998). Involvement of plasma membrane redox activity and calcium homeostasis in the UV-B and UV-A/blue light induction of gene expression in Arabidopsis. Plant Cell 10 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, L., Li, J., Qu, L., Hager, J., Chen, Z., Zhao, H., and Deng, X.W. (2001). Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13 2589–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, K., Kim, S.T., Batschauer, A., Dawut, L., and Sancar, A. (1995). Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homology to DNA photolyase contain the two photolyase cofactors but lack DNA repair activity. Biochemistry 34 6892–6899. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia, J.F., Huq, E., and Quail, P.H. (2000). Direct targeting of light signals to a promoter element-bound transcription factor. Science 288 859–863. [DOI] [PubMed] [Google Scholar]
- Mas, P., Devlin, P.F., Panda, S., and Kay, S.A. (2000). Functional interaction of phytochrome B and cryptochrome 2. Nature 408 207–211. [DOI] [PubMed] [Google Scholar]
- Mazzella, M.A., Cerdan, P.D., Staneloni, R.J., and Casal, J.J. (2001). Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development 128 2291–2299. [DOI] [PubMed] [Google Scholar]
- McClung, C.R. (2001). Circadian rhythms in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 139–162. [DOI] [PubMed] [Google Scholar]
- McClung, C.R., and Kay, S. (1994). Circadian rhythms in Arabidopsis thaliana. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 615–637.
- Millar, A.J., Straume, M., Chory, J., Chua, N.H., and Kay, S.A. (1995). The regulation of circadian period by phototransduction pathways in Arabidopsis. Science 267 1163–1166. [DOI] [PubMed] [Google Scholar]
- Miyamoto, Y., and Sancar, A. (1998). Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in man and mouse. Proc. Natl. Acad. Sci. USA 95 6097–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler, T.C., Guo, H., Yang, H., Duong, H., and Lin, C. (1999). Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 126 2073–2082. [DOI] [PubMed] [Google Scholar]
- Mohr, H. (1994). Coaction between pigment systems. In Photomorphogenesis in Plants, 2nd ed. R.E. Kendrick and G.H.M. Kronenberg, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers).
- Motchoulski, A., and Liscum, E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286 961–964. [DOI] [PubMed] [Google Scholar]
- Mozley, D., and Thomas, B. (1995). Developmental and photobiological factors affecting photoperiodic induction in Arabidopsis thaliana Heynh. Landsberg erecta. J. Exp. Bot. 46 173–179. [Google Scholar]
- Neff, M.M., and Chory, J. (1998). Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 118 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, M., Tepperman, J.M., and Quail, P.H. (1998). PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 657–667. [DOI] [PubMed] [Google Scholar]
- Ninu, L., Ahmad, M., Miarelli, C., Cashmore, A.R., and Giuliano, G. (1999). Cryptochrome 1 controls tomato development in response to blue light. Plant J. 18 551–556. [DOI] [PubMed] [Google Scholar]
- Nozue, K., Kanegae, T., Imaizumi, T., Fukuda, S., Okamoto, H., Yeh, K.C., Lagarias, J.C., and Wada, M. (1998). A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. USA 95 15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura, H., Miyake, S., Sumi, Y., Yamaguchi, S., Yasui, A., Muijtjens, M., Hoeijmakers, J.H., and van der Horst, G.T. (1999). Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science 286 2531–2534. [DOI] [PubMed] [Google Scholar]
- Osterlund, M.T., Hardtke, C.S., Wei, N., and Deng, X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405 462–466. [DOI] [PubMed] [Google Scholar]
- Park, H.W., Kim, S.T., Sancar, A., and Deisenhofer, J. (1995). Crystal structure of DNA photolyase from Escherichia coli. Science 268 1866–1872. [DOI] [PubMed] [Google Scholar]
- Perrotta, G., Ninu, L., Flamma, F., Weller, J.L., Kendrick, R.E., Nebuloso, E., and Giuliano, G. (2000). Tomato contains homologues of Arabidopsis cryptochromes 1 and 2. Plant Mol. Biol. 42 765–773. [DOI] [PubMed] [Google Scholar]
- Perrotta, G., Yahoubyan, G., Nebuloso, E., Renzi, L., and Giuliano, G. (2001). Tomato and barley contain duplicated copies of cryptochrome 1. Plant Cell Environ. 24 991–997. [Google Scholar]
- Reymond, P., Short, T.W., Briggs, W.R., and Poff, K.L. (1992). Light-induced phosphorylation of a membrane protein plays an early role in signal transduction for phototropism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89 4718–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T.E., Christie, J.M., Briggs, W.R., Wada, M., and Okada, K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T., Wada, T., Ishiguro, S., and Okada, K. (2000). RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Christie, J.M., Knieb, E., Lempert, U., and Briggs, W.R. (2000). Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39 9401–9410. [DOI] [PubMed] [Google Scholar]
- Sancar, A. (1994). Structure and function of DNA photolyase. Biochemistry 33 2–9. [DOI] [PubMed] [Google Scholar]
- Sancar, A. (2000). Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu. Rev. Biochem. 69 31–67. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52 627–658. [DOI] [PubMed] [Google Scholar]
- Selby, C.P., Thompson, C., Schmitz, T.M., Van Gelder, R.N., and Sancar, A. (2000). Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc. Natl. Acad. Sci. USA 97 14697–14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman, L.P., Sriram, S., Weaver, D.R., Maywood, E.S., Chaves, I., Zheng, B., Kume, K., Lee, C.C., van der Horst, G.T., Hastings, M.H., and Reppert, S.M. (2000). Interacting molecular loops in the mammalian circadian clock. Science 288 1013–1019. [DOI] [PubMed] [Google Scholar]
- Short, T.W., and Briggs, W.R. (1994). The transduction of blue light signals in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45 143–171. [Google Scholar]
- Small, D.G., Min, B., and Lefebvre, P.A. (1995). Characterization of a Chlamydomonas reinhardtii gene encoding a protein of the DNA photolyase/blue light photoreceptor family. Plant Mol. Biol. 28 443–454. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Devlin, P.F., and Kay, S.A. (1998). Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282 1488–1490. [DOI] [PubMed] [Google Scholar]
- Somers, D.E., Schultz, T.F., Milnamow, M., and Kay, S.A. (2000). ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101 319–329. [DOI] [PubMed] [Google Scholar]
- Spalding, E., and Cosgrove, D. (1988). Large plasma-membrane depolarization precedes rapid blue-light-induced growth inhibition in cucumber. Planta 178 407–410. [PubMed] [Google Scholar]
- Stanewsky, R., Kaneko, M., Emery, P., Beretta, B., Wager-Smith, K., Kay, S.A., Rosbash, M., and Hall, J.C. (1998). The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95 681–692. [DOI] [PubMed] [Google Scholar]
- Stowe-Evans, E.L., Harper, R.M., Motchoulski, A.V., and Liscum, E. (1998). NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 118 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, T.E., Corchnoy, S.B., Christie, J.M., Lewis, J.W., Szundi, I., Briggs, W.R., and Bogomolni, R.A. (2001). The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 276 36493–36500. [DOI] [PubMed] [Google Scholar]
- Tamada, T., Kitadokoro, K., Higuchi, Y., Inaka, K., Yasui, A., de Ruiter, P.E., Eker, A.P., and Miki, K. (1997). Crystal structure of DNA photolyase from Anacystis nidulans. Natl. Struct Biol 4 887–891. [DOI] [PubMed] [Google Scholar]
- Terzaghi, W.B., and Cashmore, A.R. (1995). Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 419–444. [Google Scholar]
- Thresher, R.J., Vitaterna, M.H., Miyamoto, Y., Kazantsev, A., Hsu, D.S., Petit, C., Selby, C.P., Dawut, L., Smithies, O., Takahashi, J.S., and Sancar, A. (1998). Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science 282 1490–1494. [DOI] [PubMed] [Google Scholar]
- Thum, K.E., Kim, M., Christopher, D.A., and Mullet, J.E. (2001). Cryptochrome 1, Cryptochrome 2, and Phytochrome A co-activate the chloroplast psbD blue light-responsive promoter. Plant Cell 13 2747–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo, T., Ryo, H., Yamamoto, K., Toh, H., Inui, T., Ayaki, H., Nomura, T., and Ikenaga, M. (1996). Similarity among the Drosophila (6–4)photolyase, a human photolyase homolog, and the DNA photolyase-blue-light photoreceptor family. Science 272 109–112. [DOI] [PubMed] [Google Scholar]
- van der Horst, G.T., et al. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398 627–630. [DOI] [PubMed] [Google Scholar]
- Vitaterna, M.H., Selby, C.P., Todo, T., Niwa, H., Thompson, C., Fruechte, E.M., Hitomi, K., Thresher, R.J., Ishikawa, T., Miyazaki, J., Takahashi, J.S., and Sancar, A. (1999). Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. USA 96 12114–12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Arnim, A.G., and Deng, X.-W. (1994). Light inactivation of Arabidopsis photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning. Cell 79 1035–1045. [DOI] [PubMed] [Google Scholar]
- Wade, H.K., Bibikova, T.N., Valentine, W.J., and Jenkins, G.I. (2001). Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J. 25 675–685. [DOI] [PubMed] [Google Scholar]
- Wager-Smith, K., and Kay, S.A. (2000). Circadian rhythm genetics: From flies to mice to humans. Natl. Genet 26 23–27. [DOI] [PubMed] [Google Scholar]
- Wang, H., Ma, L.G., Li, J.M., Zhao, H.Y., and Deng, X.W. (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294 154–158. [DOI] [PubMed] [Google Scholar]
- Wang, X., and Iino, M. (1998). Interaction of cryptochrome 1, phytochrome, and ion fluxes in blue- light-induced shrinking of Arabidopsis hypocotyl protoplasts. Plant Physiol. 117 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, J.L., Perrotta, G., Schreuder, M.E., Van Tuinen, A., Koornneef, M., Giuliano, G., and Kendrick, R.E. (2001). Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J. 25 427–440. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.Q., Tang, R.H., and Cashmore, A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13 2573–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.-Q., Wu, Y.-J., Tang, R.-H., Liu, D., Liu, Y., and Cashmore, A.R. (2000). The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103 815–827. [DOI] [PubMed] [Google Scholar]
- Zeiger, E. (2000). Sensory transduction of blue light in guard cells. Trends Plant Sci. 5 183–185. [DOI] [PubMed] [Google Scholar]
- Zeiger, E., and Helper, P. (1977). Light and stomatal function: Blue light stimulates swelling of guard cell protoplasts. Science 196 887–889. [DOI] [PubMed] [Google Scholar]
- Zhong, H.H., Resnick, A.S., Straume, M., and McClung, C.R. (1997). Effects of synergistic signaling by phytochrome A and cryptochrome 1 on circadian clock-regulated catalase expression. Plant Cell 9 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]