INTRODUCTION
Cell–cell interactions in plants are expected to occur between adjoining cells that share a common developmental history. One exception is the interaction of the pollen or pollen tube with cells of the pistil. As a result, pollen–pistil interactions have emerged as models for the study of cell-to-cell signaling, particularly in the context of genetic self-incompatibility (SI). SI is an intraspecific mating barrier found in a large number of species distributed among many plant families that allows cells of the pistil to recognize and reject self-related pollen (De Nettancourt, 2001). Many SI systems are controlled genetically by a single highly polymorphic locus, the S locus, and pollen inhibition occurs when pollen and pistil are derived from plants that express the same S locus variant(s).
Molecular studies performed during the last two decades have demonstrated that the term “self-incompatibility” does not represent one mechanism of self-recognition and that the S loci of different families are not homologous. Rather, the term encompasses a collection of disparate systems that have distinct evolutionary histories and are based on mechanistically different strategies for the inhibition of self-related pollen. One strategy used by members of the Solanaceae (McClure et al., 1989), Rosaceae (Sassa et al., 1993), Scrophulariaceae (Xue et al., 1996), and Campanulaceae (Stephenson et al., 2000) is directed at inhibiting pollen tubes after they have grown into the style and is based on the cytotoxic activity of stylar-secreted S-RNases, which act inside the pollen tube to inhibit its growth.
Other strategies, such as those used by the Brassicaceae and Papavaraceae, are directed at preventing pollen germination or pollen tube ingress into the pistil; in this strategy, self pollen is inhibited at the stigma surface within minutes of pollen–stigma contact. It is in these two families that SI relies on the perception and transduction of specific signals, albeit using distinct molecular determinants and fundamentally different mechanisms, for the inhibition of self pollen. In the Brassicaceae, a signal is carried by the pollen grain, which is perceived and transduced into a cellular response within the stigma epidermis. In Papaver, the signal is produced by stigmatic cells and perceived by pollen, resulting in a transduction cascade within the pollen tube (Rudd and Franklin-Tong, 2001). Here, we review our understanding of cell-to-cell signaling in the SI system of the Brassicaceae, which is currently the only system for which the stigma and pollen recognition components are known.
SIGNAL PERCEPTION AT THE POLLEN–STIGMA INTERFACE IN THE BRASSICACEAE
The recognition of self-related pollen in the Brassicaceae occurs during the interaction of a pollen grain with an epidermal (papillar) cell of the stigma surface (Figure 1). An incompatible response interrupts the very early events of this interaction, namely, hydration and metabolic activation of the pollen grain, and subsequent elaboration of a pollen tube and its ingress into the papillar cell wall (Heslop-Harrison, 1975). The surface inhibition of self pollen and the rapidity of the response had suggested early on that this SI system likely would be based on the activity of specific cell surface molecules. Indeed, molecular studies performed at several laboratories during the last two decades in Brassica species, as well as more recent studies in Arabidopsis lyrata, have demonstrated that two S locus–encoded and highly polymorphic proteins function as receptor–ligand pairs that determine specificity in the stigma epidermis and pollen.
Figure 1.
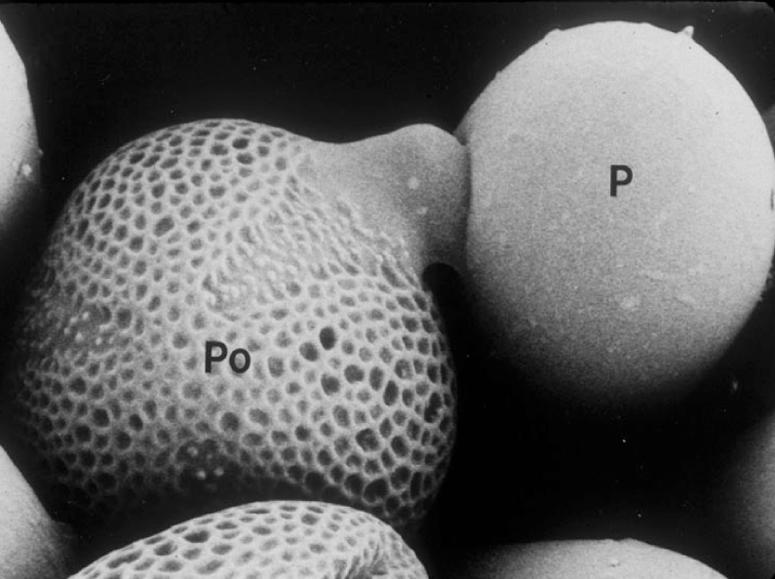
Scanning Electron Micrograph of Pollination in Brassica Showing the Interaction between a Pollen Grain (Po) and a Stigma Papillar Cell (P).
Magnification ×400.
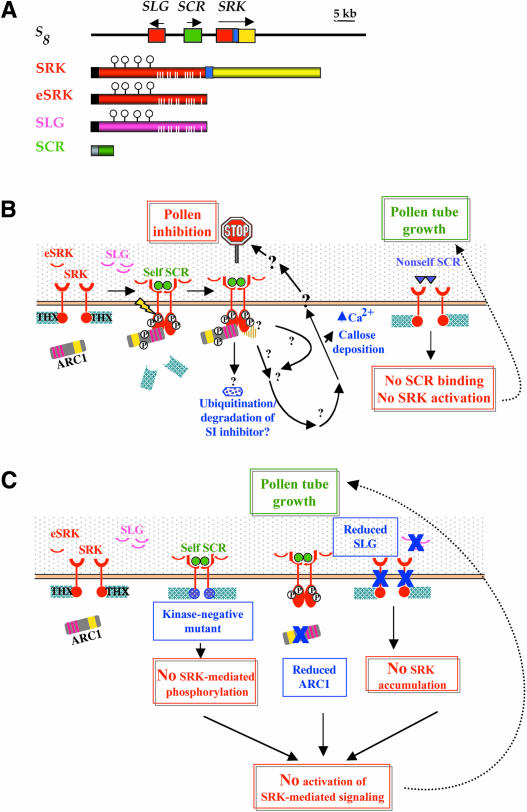
Identification of these receptor–ligand pairs was accomplished in Brassica as a result of molecular cloning, first of S locus–encoded genes whose products are expressed specifically in the stigma epidermis, and then of the SI specificity–determining region of the S locus. This endeavor was both aided and hampered by the highly polymorphic nature of S locus variants or S haplotypes, of which >50 are known in Brassica oleracea (Ockendon, 1974). On the one hand, the sequence polymorphism expected for recognition genes was an invaluable criterion for identifying candidate SI specificity genes. On the other hand, the structural heteromorphism of S haplotypes, manifested by scrambled gene order and variable intergenic distances (Boyes et al., 1997; Suzuki et al., 1999; Cui et al., 2000; Nasrallah, 2000), did not allow straightforward transfer of physical and genetic mapping data between populations segregating for different pairs of S haplotypes. Figure 2A shows the organization of a Brassica S haplotype and the S locus gene products that function in SI.
Figure 2.
The S-Locus Genes of Crucifers, Receptor–Ligand Interactions, and Signal Transduction in the SI Response.
(A) Arrangement of SI genes in the Brassica S8 haplotype and structures of their protein products. Arrows above the genes indicate the 5′→3′ orientations of the genes. The extracellular S domains are shown in red, and the kinase domain of SRK is shown in yellow. White bars in the S domains represent 12 conserved Cys residues, and circles indicate glycosylation sites. Black and gray boxes represent the signal sequences in SRK/SLG and SCR, respectively. The blue box represents the SRK transmembrane domain.
(B) A model of signal perception and response in the SI response. Molecules that have been shown conclusively to function in SI are shown as closed shapes. SRK is shown spanning the plasma membrane (orange bar) of the stigmatic epidermal cell, with its kinase (red circle) in the cytoplasm and its ectodomain (red crescent) extending into the cell wall (stippled area). SLG and eSRK are shown as crescents in the papillar cell wall. SCR molecules are shown as dimeric molecules in the papillar cell wall, to which they are transferred from the pollen coat of pollen. “Self” SCR (green circles) interacts with the SRK ectodomain and triggers a signaling cascade, whereas “nonself” SCR (purple triangles) does not. ARC1 is shown by a gray box, with red bars indicating arm repeats and a yellow bar indicating the U box. Phosphorylation is indicated by “P” enclosed by a circle. Less-well-defined associations are indicated by a question mark and patterned shape. Thioredoxins (THX) are shown by stippled turquoise boxes that are associated with the SRK kinase domain only in the absence of ligand, although the mode of its interaction with SRK activation is not understood. An inhibitor of SI (stippled blue polygon) is drawn as a possible target for ubiquitination. The possible involvement of additional SRK substrates is shown by a brown striped half-circle associated with the activated SRK kinase domain. The black arrows and question marks indicate unknown components of the SI signal transduction pathway, which is likely to terminate with the modification of the papillar cell membrane or wall at the site of contact with self pollen.
(C) Mutations that lead to loss of the SI response in stigmas. The diagram shows mutations in SRK, ARC1, and SLG and their consequences.
eSRK, soluble form of the SRK ectodomain; SCR, S-locus Cys-rich protein; SLG, S-locus glycoprotein; SRK, S-locus receptor protein kinase.
Stigma Receptors and Pollen Ligands for SI Specificity
The determinant of SI specificity in the stigma epidermis is the S locus receptor protein kinase (SRK) gene. The SRK nucleotide sequence predicts a protein with an extracellular domain, a single-pass transmembrane domain, and a cytoplasmic kinase domain (Figure 2A) (Stein et al., 1991) that exhibits Ser/Thr kinase activity (Goring and Rothstein, 1992; Stein and Nasrallah, 1993). SRK is the prototype of a ubiquitous class of plant receptor-like protein kinases (Shiu and Bleecker, 2001). These proteins share similar predicted extracellular domains, referred to as S domains, because they exhibit sequence similarity to the S locus glycoprotein (SLG), the first S locus–encoded gene isolated (Figure 2A) by Nasrallah et al. (1985). As expected for a receptor protein, SRK is an integral component of the plasma membrane (Stein et al., 1996; Dixit et al., 2000; Giranton et al., 2000), where it is displayed with its S domain on the outside of the cell and its kinase domain within the cytoplasm (Letham et al., 1999). At least one SRK allele (and possibly others) also produces soluble truncated forms of the SRK ectodomain, designated eSRKs (Giranton et al., 1995) (Figure 2A), apparently encoded by short transcripts that terminate within the first intron of the gene (Stein et al., 1991).
The SRK protein is expressed specifically in the stigma epidermis, SRK transcripts accumulate concomitantly with the attainment of SI by mature stigmas (Stein et al., 1991, 1996), and SRK alleles can exhibit as much as 35% overall amino acid sequence divergence (Stein et al., 1991; Nishio and Kusaba, 2000; Kusaba et al., 2001a). Moreover, transformation with an SRK transgene resulted in the acquisition by transgenic stigmas of the corresponding SI specificity and the ability to reject pollen expressing the same S haplotype as that of the transgene (Takasaki et al., 2000). Significantly, the pollen of these transgenic plants did not acquire new SI specificity. This result confirmed previous data that suggested the existence of distinct S locus genes for pollen and stigma specificity. Self-compatible mutants of Brassica were known that exhibited defects in stigma SI function alone (Nasrallah, 1974; Hinata and Okazaki, 1986; Nasrallah et al., 1992, 1994) or in pollen SI function alone (Nasrallah et al., 2000). Moreover, both homology-dependent silencing of SRK (Conner et al., 1997) and expression of a dominant-negative form of SRK in transgenic plants (Stahl et al., 1998) had led to the stigma-specific breakdown of SI.
The molecular cloning of SRK was facilitated by the previous isolation of SLG (Nasrallah et al., 1985), another highly polymorphic S locus gene that shares a high degree of sequence identity with the SRK ectodomain (Figure 2A) and is expressed at much higher levels than SRK. The SLG glycoprotein is an abundant component of the papillar cell wall (Kandasamy et al., 1989), which does not determine specificity in the SI response but appears to have an accessory role in SI. SLG28 enhanced the SI phenotype determined by the SRK28 transgene (Takasaki et al., 2000), and SLG was shown to facilitate the proper maturation of SRK and its accumulation to physiologically relevant levels in the stigma epidermis (Dixit et al., 2000). However, SLG is not absolutely required for a robust SI response. SLG910 did not enhance the strength of the SI response in transgenic plants expressing the SRK910 transgene (Silva et al., 2001). Furthermore, at least two Brassica S haplotypes (Suzuki et al., 2000) and the two A. lyrata S haplotypes analyzed to date (Kusaba et al., 2001a) do not contain an SLG gene. In these cases, the SRK isoforms might be inherently stable or might be stabilized by the corresponding eSRK molecules (Figure 2A).
SRK's partner in recognition is the S locus Cys-rich protein SCR (Schopfer et al., 1999; Schopfer and Nasrallah, 2000), also designated SP-11 (Suzuki et al., 1999; Takayama et al., 2000; Shiba et al., 2001). The SCR gene is maintained in tight genetic linkage with SRK, probably as a result of the structural heteromorphism of S haplotypes and a reduced frequency of recombination at the S locus (Casselman et al., 2000). The SCR gene exhibits extensive S haplotype–associated polymorphism, is expressed exclusively in anthers, and encodes a small basic peptide of 50 to 59 amino acids.
The SCR gene is necessary and sufficient for SI in pollen. SCR transcripts are undetectable in a self-compatible mutant of Brassica that exhibits a pollen-specific breakdown of SI (Schopfer et al., 1999). Transformation of Brassica plants homozygous for one S haplotype with an SCR allele derived from another haplotype results in the acquisition by transgenic pollen of the SI specificity encoded by the transgene (Schopfer et al., 1999; Shiba et al., 2001). Furthermore, pretreatment of stigma papillar cells with recombinant bacterially expressed “self” SCR inhibits the hydration of cross-pollen in pollination bioassays (Takayama et al., 2000, 2001; Kachroo et al., 2001).
The SCR protein has been shown to be localized to the pollen coat by immunocytochemistry (Shiba et al., 2001) and by biochemical fractionation (Kachroo et al., 2001). Such a localization had been predicted previously based on the SCR expression pattern (Schopfer et al., 1999; Schopfer and Nasrallah, 2000; Takayama et al., 2000) and on a pollination bioassay that determined the pollen SI factor to be a component of the pollen coat (Stephenson et al., 1997). The site of SCR synthesis has not been resolved conclusively, however. Pollen coat proteins may be synthesized in the tapetum (i.e., sporophytically) or in microspores (i.e., gametophytically). All Brassica SCR alleles reported to date exhibit sporophytic and gametophytic expression, as evident from the accumulation of SCR transcripts in the anther tapetum and in microspores, respectively (Schopfer et al., 1999; Schopfer and Nasrallah, 2000; Takayama et al., 2000; Shiba et al., 2001). In A. lyrata, the SCRb allele is expressed in tapetum and microspores, but the SCRa allele is expressed only in the tapetum of Sa homozygotes (Kusaba et al., 2001b). Therefore, sporophytic expression of SCR in the tapetum is sufficient for SI, and expression of two SCR alleles in the tapetum is an adequate explanation for the sporophytic control of pollen SI specificity in crucifers. The additional gametophytic expression exhibited by another A. lyrata allele, SCRb, and all reported Brassica SCR alleles might be redundant or serve to increase SCR levels in individual pollen grains.
Like SRK, SCR is a member of a large gene family that includes some 28 members in Arabidopsis thaliana (Vanoosthuyse et al., 2001). A distantly related family is the PCP-A1 gene family, which includes genes encoding pollen coat proteins of unknown biological function (Doughty et al., 1998). SCRs exhibit some resemblance, but not sequence similarity, to defensins, which are small Cys-rich peptides that are expressed in mammals, insects, and plants (Brockaert et al., 1995; Salzet, 2001). In particular, they appear to have the same arrangement of four disulfide bonds as the Triticum defensin γ1-P (Takayama et al., 2001). Some SCR alleles lack the C-terminal Cys residue (Watanabe et al., 2000), however, and these SCRs may form only three disulfide bonds.
Defensins function primarily in innate immunity, although they also have additional, less-well-defined roles. As antibacterial and antifungal peptides, the positively charged defensins present a first line of defense against microbial challenge. They bind to microbes, not through interaction with specific receptors but rather by recognizing surface patterns, and form pores in microbial membranes. Although SCR is a signaling molecule with a very different biological function and mode of action than defensins, it is tempting to speculate that a function directed at recognizing nonself patterns in microbial pathogens was preempted for self/nonself recognition in the SI response. It should be noted, however, that attempts to resolve the evolutionary relationships have been thwarted even in comparisons of different classes of mammalian defensins (Hughes, 1999). It will be even more difficult to retrace the evolutionary history of SCR because of the rapid evolution of the SCR gene. Indeed, only seven Cys residues and one Gly residue are conserved among the 22 SCR sequences identified to date in Brassica and A. lyrata (Schopfer and Nasrallah, 2000; Watanabe et al., 2000; Kusaba et al., 2001a). In addition, SCR sequences cannot be aligned unambiguously because of the variable numbers of residues between the Cys residues.
Receptor–Ligand Interactions
The sequences and localization of SRK and SCR, as well as the transgenic experiments summarized above, support the conclusion that they act as a receptor–ligand pair in the SI response. The current model of SI is that SCR is a diffusible signal carried in the pollen coat that functions as a ligand for the plasma membrane–localized SRK. Upon self-pollination, the SCR protein would be delivered to the stigmatic surface via the adhesion zone formed by the pollen coat. The papillar cell wall allows the diffusion of molecules of >60 kD, such as SLG (Kandasamy et al., 1989). Therefore, it is expected that the translocation of SCR molecules through this cell wall occurs within minutes of pollen–stigma contact. The subsequent interaction of SCR with the SRK ectodomain then would trigger a signaling cascade that culminates in the inhibition of self pollen tube development. Figure 2B diagrams the known elements of this signal transduction model, and Figure 2C shows mutations that support the involvement of specific molecules in SRK-mediated signaling.
Two major predictions of this model are that SCR is the ligand for SRK and that specificity in the SI response derives from allelic specificity in SRK–SCR binding or the activation of SRK. Indeed, the S haplotype–specific binding of SCR to the SRK ectodomain has been demonstrated (Kachroo et al., 2001). For this study, the ectodomain of B. oleracea SRK6 was expressed as a FLAG fusion in Nicotiana benthamania leaves. The SCR6 and SCR13 proteins were expressed in the bacterial periplasm as myc-His–tagged recombinant proteins that were active biologically in pollination bioassays. Recombinant SRK6 ectodomain had a high affinity for SCR6, but it did not bind SCR13. The SRK6–SCR6 in vitro interaction was saturable and did not require additional molecules specific to the stigma or pollen surfaces. It is noteworthy that this binding occurred in the absence of the S locus–related SLR1 (Lalonde et al., 1989) and SLR2 (Boyes et al., 1991) glycoproteins, two cell wall components of the stigma epidermis that exhibit sequence similarity to SLG and the SRK ectodomain. SRK–SCR binding also did not require the pollen coat protein defensin-like PCP-A1, which was shown previously to bind SLG in vitro (Doughty et al., 1998).
Recombinant SRK6 and SCR6 proteins also bound specifically to native SCR6 from pollen coat extracts and to SRK6 from stigmas, respectively. Interestingly, SCR6 occurred largely as an ∼16-kD dimer (rather than the expected 8-kD monomer) in pollen coat preparations and in bacterial extracts, raising the possibility that SCR binds its cognate SRK as a homodimer in vivo. For its part, recombinant SCR6 bound SRK6 and not SRK13 from stigma microsome preparations. SCR6 also exhibited modest binding to soluble stigma ∼60-kD proteins that likely represent SLG6 but also could include eSRK6.
The binding of stigma SRK to its ligand also was demonstrated independently in experiments that used proteins encoded by the S8 haplotype of Brassica campestris (Takayama et al., 2001). In this study, binding assays and cross-linking experiments demonstrated the binding of biologically active 125I-labeled synthetic ligand to stigma microsomal membrane preparations. The labeled ligand was cross-linked to the 120-kD SRK8 protein and to a 65-kD protein that might correspond to eSRK8 or SLG8, possibly the fraction of SLG shown previously to be membrane associated (Dixit et al., 2000). In contrast to findings in the study by Kachroo et al. (2001), however, the ligand was detected in monomeric form and the synthetic ligand did not bind to a recombinant form of the SRK S domain that was expressed in insect cells as a fusion with glutathione-S-transferase. Although the latter result may be explained by aberrant folding or modification of the recombinant protein in the heterologous insect expression system, further studies are required to determine if the ligand occurs in monomeric or dimeric form and which of these forms is active in receptor binding.
It is noteworthy that the two studies of SRK–SCR interaction described above both indicated an association of SLG or eSRK with the receptor and ligand. The physiological significance of this binding is not known. In the stigma, SLG levels exceed those of SRK by 2 orders of magnitude (Stein et al., 1991), and even low affinities for SCR might be significant. Interestingly, and despite this large excess of SLG over SRK, these two proteins apparently occurred in an equal local molar concentration at the papillar cell surface, when differences in the volumes of the cell wall and plasma membrane were considered (Stein et al., 1996). Both SLG and eSRK may play regulatory roles, possibly by facilitating the interaction of SCR with SRK, as has been shown for “soluble” receptors in animal models (Flickinger et al., 1992).
MECHANISM OF RECEPTOR ACTIVATION
The SRK protein is one of only four plant single-pass receptor-like kinases whose ligands have been identified. The three other receptor–ligand systems belong to the Leu-rich repeat class of receptor-like kinases. These include the brassinolide-insensitive 1 (BRI1) protein and its ligand brassinolide (Wang et al., 2001); the CLAVATA1 (CLV1) protein, which, together with its ligand, the CLAVATA3 (CLV3) peptide (Trotochaud et al., 2000), is required for the regulation of meristem development in A. thaliana (Clark et al., 1996); and the flagellin receptor FLS2, which binds the bacterial elicitor flagellin (Gómez-Gómez and Boller, 2000). Biochemical studies of these receptor-ligand systems have been initiated only recently, and mechanisms of plant receptor activation are still poorly understood.
Lessons from Other Receptor–Ligand Systems
Two major issues in receptor activation relate to the mechanisms of ligand binding and receptor activation. In mammalian systems, ligand binding is known to induce the dimerization of most receptors, resulting in transphosphorylation of the kinase domains (Heldin, 1995). Phosphorylated residues then act as binding sites for specific substrates. Different ligands use different strategies to induce dimerization of the receptor. For example, cytokines, such as growth hormone and erythropoietin, are bivalent, and one ligand binds two receptor molecules (Kossiakoff and De Vos, 1998; Jiang and Hunter, 1999). Several other growth factors, such as vascular endothelial growth factor (Wiesmann et al., 1997) and platelet-derived growth factor (Heldin et al., 1989), are homodimers themselves and induce dimerization of their receptors.
Mechanisms of receptor activation in plants are likely to be as varied as those in mammalian systems. Comparisons between the SRK–SCR and CLV1–CLV3 receptor–ligand pairs reveal similarities but also some differences. Just as the accumulation and maturation of some SRK isoforms require SLG, the proper accumulation of the CLV1 receptor complex requires CLV2, a receptor-like protein that, like CLV1, contains a Leu-rich repeat extracellular domain and a transmembrane domain but, unlike CLV1, lacks a kinase domain (Jeong et al., 1999). Furthermore, both SCR and CLV3 occur in higher order complexes in planta. The functional CLV3 ligand forms a multimeric complex in vivo (Trotochaud et al., 1999). SCR was detected as an ∼16-kD dimer in pollen coat preparations (Kachroo et al., 2001), although it is not known if these dimers represent the native state of SCR and if this state is necessary for binding to SRK. An important difference, however, is that CLV3 binds only the active CLV1 complex, whereas SCR binds the purified extracellular domain of SRK lacking a functional kinase domain (Kachroo et al., 2001). In this respect, the SRK–SCR interaction is more similar to the binding of Brassinolide to its BRI1 receptor, which does not require a functional kinase domain (He et al., 2000), whereas both CLV1 and FLS2 do (Trotochaud et al., 2000; Gómez-Gómez et al., 2001).
Ligand-Induced Phosphorylation of SRK
Does SCR binding to the SRK ectodomain translate into oligomerization of the receptor, and does this binding result in transphosphorylation of the SRK kinase? The addition of a synthetic form of the ligand to stigma membrane fractions resulted in the autophosphorylation of SRK (Takayama et al., 2000). Furthermore, SRK was shown to autophosphorylate within 60 min after self-pollination (Cabrillac et al., 2001), apparently by transphosphorylation of the kinase chains (Giranton et al., 2000). However, the data are ambiguous regarding how SRK is activated upon ligand binding and how it is maintained in an inactive state in the absence of ligand. The activation of SRK has been suggested to involve oligomerization, because recombinant SRK was phosphorylated in a dose-dependent and saturable manner by antibodies to the N terminus of the SRK protein (Cabrillac et al., 2001). It also has been suggested, however, that SRK signaling may be initiated by the binding of SCR to preformed SRK oligomers and interaction with a coreceptor, because SRK oligomers were detected in unpollinated stigmas (i.e., in the absence of SCR) (Giranton et al., 2000).
In one study, recombinant SRK was found to be phosphorylated constitutively in vitro (Giranton et al., 2000). This phosphorylation was inhibited by soluble proteins from stigma extracts or by purified thioredoxin, a protein that interacts with the SRK kinase domain (Bower et al., 1996; Mazzurco et al., 2001) and is known to regulate the activity of several kinases in mammalian systems (Liu et al., 2000). The inhibition of SRK3 phosphorylation by thioredoxin was relieved by the addition of pollen coat proteins from S3 pollen. These results suggest that thioredoxin may bind the SRK kinase domain to prevent the constitutive activation of the SI pathway (Cabrillac et al., 2001), thus allowing cross-pollination to proceed. The relationship of thioredoxins to SRK activation must await further studies, however, in view of other results that did not reveal any involvement of inhibitory factors (Takayama et al., 2001).
Clearly, the study of SRK activation is still in its infancy, and many questions remain unanswered. Data from animal receptor studies demonstrate that ligand binding and receptor oligomerization do not result automatically in receptor activation. In some cases, only those forms of receptor dimers that assume appropriate configurations become autophosphorylated (Lemmon and Schlessinger, 1994; Jiang and Hunter, 1999). In others, the binding of accessory molecules is required for activation. For example, in the case of fibroblast growth factor receptor, the binding of heparin sulfate proteoglycan molecules is required for the stabilization of the receptor dimers induced by ligand binding and for subsequent receptor activation (Spivak-Kroizman et al., 1994). Assuming that the oligomerization of SRK is required for autophosphorylation, is the binding of SCR to SRK sufficient to trigger this phosphorylation and activation of downstream targets? Might the activation of SRK require additional components from the pollen coat or papillar cell wall?
Regulation of SRK Activation
Receptor activation is usually regulated by the stoichiometry of the interacting partners and by the availability of ligand to the receptor. For example, CLV1 activation is restricted to cells within the domain of CLV3 diffusion (Clark, 2001). In the case of SI, SRK activation in the papillar cell is even more restricted and likely to be limited to a small subcellular region subtending the zone of contact with a self pollen grain. It has been shown that the successful germination of a compatible pollen grain is not disrupted by the presence of an incompatible pollen grain on the same papillar cell (Dickinson, 1995). Therefore, the pollen inhibitory reaction stemming from SRK activation is thought to result not from global modifications of the surface of the stigma epidermal cell but from localized alterations restricted to the site of contact with an individual pollen grain.
Within the microscopic zone of pollen–papillar cell contact and SRK activation, the stoichiometry of receptor and ligand appears to be critical for determining the magnitude of the response. We found a positive quantitative correlation between the levels of SCR transcripts (and presumably SCR protein) and the strength of the SI response. In an analysis of Brassica mutant strains generated by γ-irradiation of B. oleracea plants carrying the S13 haplotype, we identified two mutant strains exhibiting pollen-specific defects in SI phenotype (Nasrallah et al., 2000). One strain, m1600, did not produce detectable levels of SCR13 transcripts (Figure 3) (Schopfer et al., 1999), and its pollen was fully compatible with S13S13 stigmas. Another strain, m2134, showed a fourfold reduction in SCR13 transcripts (Figure 3), and its pollen exhibited partial compatibility with S13S13 stigmas.
Figure 3.
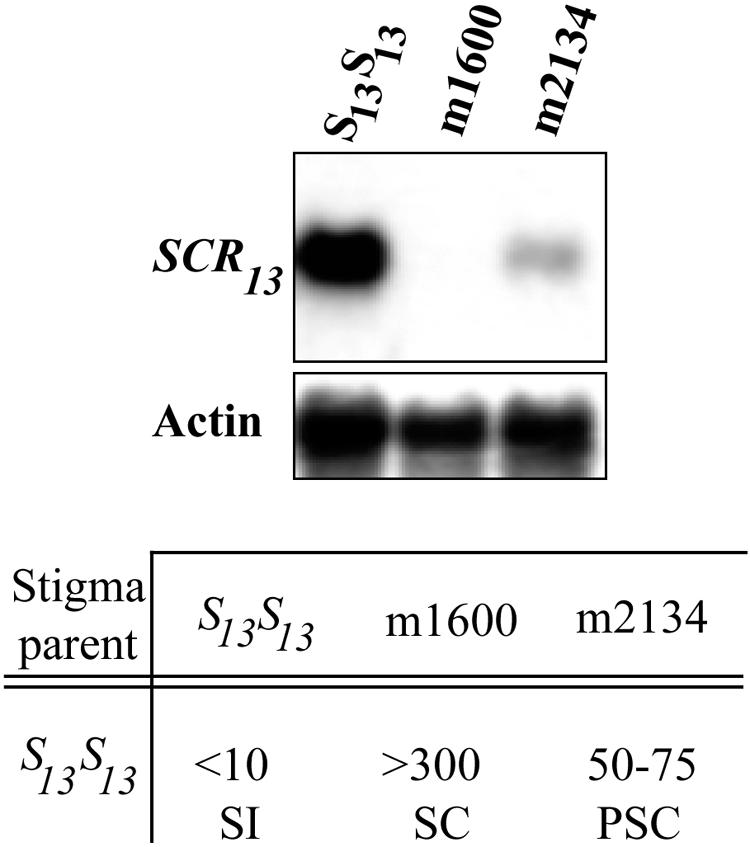
Expression of SCR and Strength of the SI Response.
Gel blot analysis of anther poly(A)+ RNA (2 μg/lane) isolated from a self-incompatible wild-type strain of B. oleracea expressing the S13 haplotype and from the m1600 and m2134 self-compatible mutants. The blot was probed with SCR13 cDNA and with actin as a loading control. The table below the blots shows data from pollinations of S13S13 stigmas with pollen from wild-type S13S13, m1600, and m2134 plants. Wild-type expression of SCR determines a robust self-incompatible (SI) phenotype. The absence of SCR transcripts in m1600 anthers is correlated with full self-compatibility (SC) in pollen (>300 pollen tubes produced per stigma), and a fourfold reduction in SCR mRNA levels in m2134 anthers results in partial self-compatibility (PSC; 50 to 75 pollen tubes produced per stigma).
One interpretation of these results is that the mounting of an effective barrier to self pollen requires the activation of several SRK molecules and the recruitment of their downstream targets at the site of pollen–stigma contact. The magnitude of the response then would depend on the concentration of SCR molecules delivered to this site by a pollen grain. In the m2134 strain, the number of SCR molecules released from the tapetum into the anther locule would be reduced and would be incorporated unevenly into the coat of individual pollen grains. As a result, in self-pollinations, those pollen grains that display adequate levels of SCR would be inhibited and those that carry no or little SCR protein would escape inhibition. Similarly, the inherently “weak” SI response, or pseudocompatibility, specified by some S haplotypes might be associated with a relatively poorly expressed SCR allele.
Dominance–Recessiveness Interactions: Molecular Interference between Allelic SRK–SCR Pairs or Differential Expression of SI Genes?
An intriguing feature of SI in the Brassicaceae is that genetic interactions between S haplotypes influence the expression of SI specificity in stigmas and pollen, a feature that has far-reaching implications for the mechanism of SI as well as for the evolution and maintenance of S haplotypes in a population. Allelic interactions of codominance, dominance, incomplete dominance, or mutual weakening have been reported, and these interactions can differ in stigma and pollen, consistent with the activity of distinct stigma and pollen determinants of SI specificity. These allelic interactions suggest that SRK and SCR allelic pairs or SRK–SCR complexes do not always function independently of other SRK–SCR allelic pairs. Until recently, however, data on the molecular basis for S haplotypic interactions were lacking.
Recently, Hatakeyama et al. (2001) showed that dominance relationships in the Brassica stigma are a characteristic of the SRK gene and that recessiveness or dominance was not correlated with differences in SRK transcript levels. Similarly, in a study of SI in A. lyrata, the weakening of Sa activity in heterozygous SaSb stigmas was not related to differences in SRK expression levels (Kusaba et al., 2001b). Although these studies did not exclude the possibility that dominance/recessiveness is related to differences in SRK protein levels, it is possible that these genetic interactions reflect interference between receptor or ligand isoforms either in the SRK–SCR interaction or in the recruitment of downstream effectors of the SI response.
More insight has been gained regarding the interactions of S haplotypes in pollen. In A. lyrata, the Sa haplotype is recessive to the Sb haplotype in pollen, and pollen grains from SaSb plants exhibit Sb specificity (Kusaba et al., 2001a). Intriguingly, SCRa transcripts, which are expressed exclusively in the tapetum, were reduced by as much as 80-fold in SaSb heterozygotes relative to Sa homozygotes (Kusaba et al., 2001b). The underlying mechanism of this reduction is not understood. Nevertheless, the recessive–dominant interaction exhibited by the SCRa and SCRb alleles in pollen is explained by monoallelic expression of the dominant SCRb allele in SaSb heterozygotes resulting from severe downregulation of the recessive SCRa allele in the tapetum and the lack of SCRa expression in microspores.
The A. lyrata SCRa allele is the only “pollen-recessive” allele reported to date, and it remains to be determined how general this mechanism of dominance/recessiveness will prove to be. Nevertheless, it is likely that the features of exclusive tapetal expression of the recessive SCR allele and monoallelic expression of the dominant SCR allele will combine to explain many cases of dominant/recessive S haplotypic interactions in the pollen of A. lyrata, Brassica, and other self-incompatible crucifers.
SIGNAL TRANSDUCTION IN THE SI RESPONSE
Molecular Analysis of SRK-Mediated Signal Transduction
How SRK–SCR binding and SRK activation are transduced into the inhibition of self-related pollen is not understood. To date, only the arm repeat–containing protein ARC1 (Gu et al., 1998) (Figure 2B) has been identified conclusively as a component of the SRK signal transduction pathway. Another putative candidate signaling component, the MOD locus–associated aquaporin (MOD-AQP), was identified by virtue of its tight genetic linkage to a modifier of SI and by the fact that it was not expressed in self-compatible plants homozygous for the recessive mod mutation (Ikeda et al., 1997). However, analysis of two additional mod alleles generated by γ-irradiation of self-incompatible plants and of natural MOD-AQP variants subsequently showed that the aquaporin gene was unlikely to represent the MOD locus (Fukai et al., 2001).
ARC1 was identified in a yeast two-hybrid screen as a protein that interacted with the cytoplasmic domain of SRK (Gu et al., 1998). This interaction, which is mediated by the arm repeats in the C terminus of ARC1, requires an active SRK kinase domain and results in the phosphorylation of ARC1 in vitro (Gu et al., 1998). ARC1 transcripts are detected specifically in the stigma, supporting a role for ARC1 in pollination. Significantly, transgenic plants in which ARC1 transcripts were downregulated by the expression of an antisense ARC1 transgene showed a partial breakdown of SI in the stigma and were not affected in pollen SI function (Stone et al., 1999) (Figure 2C). That the breakdown of SI was only partial might be attributable to residual ARC1 expression in transgenic stigmas or might indicate that ARC1 is not the only substrate for SRK. Nevertheless, these results establish a role for ARC1 in SI.
How ARC1 functions is not understood. Some clues may be offered by the recent finding that ARC1 contains a U box (Azevedo et al., 2001), a motif identified initially in the yeast E4 polyubiquitination factor UFD2 (Koegl et al., 1999). Ubiquitination has been shown to play several roles in the regulation of signal transduction pathways, including the modulation of receptor signaling and receptor internalization and degradation. Therefore, the presence of a U box in ARC1 suggests a role for protein ubiquitination in SI. Because ARC1 is a positive effector of SI, it is possible that ARC1 phosphorylation by activated SRK triggers its interaction with components of the ubiquitination machinery and leads to the degradation of an inhibitor of the SI response (Figure 2B). Other scenarios are possible, however, because ubiquitination has been shown to have functions unrelated to protein degradation, such as subcellular targeting of proteins and their recruitment to molecular complexes (Wilkinson, 1999).
Isolation of self-compatible mutants carrying mutations in loci unlinked to the S locus and expressing functional ARC1 protein (Nasrallah et al., 2000) indicates that other as yet unidentified components are involved in the SI signal transduction pathway. The likely complexity of this pathway is underscored by cytological observations of the SI response, which show that the arrest of self pollen can occur at any of several stages, including pollen hydration, germination, or pollen tube ingress into the papillar cell wall (Dickinson, 1995). These observations suggest that the SCR–SRK-triggered signaling pathway has more than one molecular outcome in the papillar cell and may proceed through more than one intermediate.
Clues from the Physiology and Cell Biology of SI
Over the years, clues to the identity of signaling components activated by self-recognition and to the immediate cause of pollen inhibition have been sought through physiological and cell biological studies of pollen tube development in incompatible and compatible pollination. Several factors are known to break down SI in Brassica, including high humidity, high temperature, increased CO2 levels, and treatment of the stigma with sodium chloride, cycloheximide, and tunicamycin. However, none of these factors has provided any significant clues regarding the mechanism of pollen inhibition.
Several studies have focused on the potential role of calcium in SI. Calcium is known to play a crucial role in pollen germination and pollen tube growth (Bednarska and Butowt, 1994; Pierson et al., 1994; Franklin-Tong et al., 1995). Calcium, which probably is released by a regulated Ca2+-ATPase in the papillar cells, is actively taken up by pollen grains (Bednarska, 1993). It may be sensed and sequestered by calcium binding proteins, such as the novel calcium binding protein that is expressed predominantly in the pistil and anthers of Brassica (Furuyama and Dzelzkalns, 1999) or the Brassica napus calcium binding protein 1 (BPC1), which is expressed specifically during pollen maturation (Rozwadowski et al., 1999). BCP1 is of particular interest, because it is localized in the cytosol of mature pollen grains, it leaks into the pollen wall after pollen hydration, and it concentrates near the surface of the elongating pollen tube. Ratio imaging has demonstrated that the elongating pollen tube maintains cytosolic free calcium in a gradient of descending concentration from the tip and that this gradient is established before germination (Bednarska and Butowt, 1994; Pierson et al., 1994; Franklin-Tong et al., 1995).
Germination and elongation of the pollen tube are exquisitely sensitive to changes in this calcium gradient, and it has been suggested that an increase in calcium in the pollen grain may be one of the early events triggered by self-pollination. Indeed, in the SI response of the poppy (Papaver rhoeas), the arrest of pollen tube growth is associated with increased calcium levels and the generation of a calcium wave subapically within the shank of the pollen tube (Franklin-Tong et al., 1995, 1997). In Brassica SI, a role for calcium in pollen arrest was suggested by an early study in which a 65% increase in calcium was observed in pollen during an incompatible pollination (Singh et al., 1989). Whether this result can be repeated using modern ratio imaging methods is not known.
A role for calcium in the stigma SI response also has been suggested. Cytological responses in the stigma include the deposition of callose (β-1,3-glucan) at the site of pollen contact (Dickinson, 1995). This deposition is calcium dependent (Kauss, 1987) and is triggered by the application of pollen grains, pollen coat proteins, and latex beads to the stigma (Dearnaley et al., 1997; Elleman and Dickinson, 1999). However, treatment of stigmas with an inhibitor of callose synthesis prevented callose deposition but did not affect the ability of these stigmas to reject self pollen (Singh and Paollilo, 1990). In addition, the degradation of callose in the papillae of transgenic Brassica expressing β-1,3-glucanase did not affect the SI response (Sulaman et al., 1997). Thus, it is unlikely that callose deposition acts as a physical barrier to pollen tube ingress. Rather, it may represent a secondary effect of increased calcium levels within the papillar cell.
In B. napus, Ca2+ peaks appeared to be required for pollen hydration (Dearnaley et al., 1997). However, no differences between compatible and incompatible pollinations were observed in the magnitude of Ca2+ increase, although the frequency of Ca2+ peaks was higher in papillar cells exposed to compatible pollen relative to those exposed to incompatible pollen. In addition, both compatible and incompatible pollinations were equally effective at inducing increases in average membrane conductance and membrane permeability (Dearnaley et al., 1997).
Analogies to Other Plant Signal Transduction Pathways
There is often convergence between different signaling pathways, and this convergence often suggests likely signaling intermediates. The inhibition of pollen tube development in SI responses has been compared with the inhibition of fungal pathogens in host disease resistance (Hodgkin et al., 1988; Elleman and Dickinson, 1999). However, there is as yet no evidence for molecular overlap between the SRK-mediated signal pathway and signaling in host–pathogen interactions. Nor is there experimental support for the sharing of signaling intermediates with other plant receptor–ligand signaling systems, the CLV1–CLV3 system in particular. CLV1 associates with other proteins in a 450-kD functional complex that includes its ligand, CLV3 (Trotochaud et al., 2000), CLV2, kinase-associated protein phosphatase (KAPP), and a Rho GTPase-related protein that may be required for downstream signal transduction (Trotochaud et al., 1999). KAPP is a negative regulator of CLV1 (Williams et al., 1997; Stone et al., 1998) and FLS2 (Gómez-Gómez et al., 2001) signal transduction pathways, and the Rho-related protein is thought to function in the relay of signals downstream of receptor activation. SRK is not known to associate with a Rho-related protein, but at least one allele of SRK binds KAPP in vitro (Braun et al., 1997). However, the physiological significance of this binding has not been demonstrated.
FUTURE DIRECTIONS
The last 2 years have seen rapid progress in our understanding of cell-to-cell communication in the SI response and of the specific receptor–ligand interactions responsible for the recognition of self pollen. The foundation has been laid for increasingly detailed studies of pollen–stigma signaling. Analysis of the SRK-SCR signaling system no doubt will be influenced by parallel studies of other plant receptor–ligand systems, such as CLV1–CLV3, BRI1–Brassinolide, and FLS2–flagellin. Another pressing issue is unraveling the evolutionary and functional relationships to members of the large genes families that are related to these prototypic receptors and ligands (Cock and McCormick, 2001; Shiu and Bleecker, 2001; Vanoosthuyse et al., 2001). Elucidating the biological functions of this multitude of genes will be daunting. Even associating a specific receptor with its cognate ligand will be difficult, but this task could be aided by a better understanding of how the polymorphic SRK–SCR pair of genes coevolved. Ultimately, the study of these receptor–ligand systems will identify plant-specific features of signal perception and response as well as commonalities with signaling in animal and yeast models.
Acknowledgments
We thank Muthugapatti K. Kandasamy for the photograph in Figure 1. Research in the authors' laboratory is supported by the National Institutes of Health, the National Science Foundation, and the U.S. Department of Agriculture.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010440.
References
- Azevedo, C., Santos-Rosa, M.J., and Shirasu, K. (2001). The U-box protein family in plants. Trends Plant Sci. 6 354–358. [DOI] [PubMed] [Google Scholar]
- Bednarska, E. (1993). Localization of calcium-dependent ATPase in germinating pollen grain and pollen tube in Vicia faba. Folia Histochem. Cytobiol. 31 147–151. [PubMed] [Google Scholar]
- Bednarska, E., and Butowt, R. (1994). Calcium in pollen-pistil interaction in Petunia hybrida Hort. I. Localization of Ca2+ ions in mature pollen grain using pyroantimonate and autoradiographic methods. Folia Histochem. Cytobiol. 32 265–269. [PubMed] [Google Scholar]
- Bower, M.S., Matias, D.D., Fernandes-Carvalho, E., Mazzurco, M., Gu, T., Rothstein, S.J., and Goring, D.R. (1996). Two members of the thioredoxin-h family interact with the kinase domain of a Brassica S locus receptor kinase. Plant Cell 8 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D.C., Chen, C.H., Tantikanjana, T., Esch, J.J., and Nasrallah, J.B. (1991). Isolation of a second S-locus-related cDNA from Brassica oleracea: Genetic relationships between the S locus and two related loci. Genetics 127 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes, D.C., Nasrallah, M.E., Vrebalov, J., and Nasrallah, J.B. (1997). The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell 9 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, D.M., Stone, J.M., and Walker, J.C. (1997). Interaction of the maize and Arabidopsis kinase interaction domains with a subset of receptor-like protein kinases: Implications for transmembrane signaling in plants. Plant J. 12 83–95. [DOI] [PubMed] [Google Scholar]
- Brockaert, W.F., Terras, F.R., Cammue, B.P., and Osborn, R.W. (1995). Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 108 1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrillac, D., Cock, J.M., Dumas, C., and Gaude, T. (2001). The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 410 220–223. [DOI] [PubMed] [Google Scholar]
- Casselman, A.L., Vrebalov, J., Conner, J.A., Singhal, A., Giovannoni, J., Nasrallah, M.E., and Nasrallah, J.B. (2000). Determining the physical limits of the Brassica S locus by recombinational analysis. Plant Cell 12 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S.E. (2001). Cell signalling at the shoot meristem. Nat. Rev. Mol. Cell Biol. 2 276–284. [DOI] [PubMed] [Google Scholar]
- Clark, S.E., Jacobsen, S.E., Levin, J.Z., and Meyerowitz, E.M. (1996). The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development 122 1567–1575. [DOI] [PubMed] [Google Scholar]
- Cock, J.M., and McCormick, S. (2001). A large family of genes that share homology with CLAVATA3. Plant Physiol. 126 939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner, J.A., Tantikanjana, T., Stein, J.C., Kandasamy, M.K., Nasrallah, J.B., and Nasrallah, M.E. (1997). Transgene-induced silencing of S-locus genes and related genes in Brassica. Plant J. 11 809–823. [Google Scholar]
- Cui, Y., Bi, Y.-M., Brugiere, N., Arnoldo, M., and Rothstein, S.J. (2000). The S locus glycoprotein and the S receptor kinase are sufficient for self-pollen rejection in Brassica. Proc. Natl. Acad. Sci. USA 97 3713–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley, J.D.W., Levina, N.N., Lew, R.R., Heath, B.I., and Goring, D.R. (1997). Interrelationships between cytoplasmic Ca2+ peaks, pollen hydration and plasma membrane conductances during compatible and incompatible pollinations of Brassica napus papillae. Plant Cell Physiol. 38 985–999. [DOI] [PubMed] [Google Scholar]
- De Nettancourt, D. (2001). Incompatibility and Incongruity in Wild and Cultivated Plants. (Berlin: Springer-Verlag).
- Dickinson, H. (1995). Dry stigmas, water and self-incompatibility. Sex. Plant Reprod. 8 1–10. [Google Scholar]
- Dixit, R., Nasrallah, M.E., and Nasrallah, J.B. (2000). Post-transcriptional maturation of the S receptor kinase of Brassica correlates with co-expression of the S-locus glycoprotein in the stigmas of two Brassica strains and in transgenic tobacco plants. Plant Physiol. 124 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty, J., Dixon, S., Hiscock, S.J., Willis, A.C., Parkin, I.A.P., and Dickinson, H.G. (1998). PCP-A1, a defensin-like Brassica pollen coat protein that binds the S locus glycoprotein, is the product of gametophytic gene expression. Plant Cell 10 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman, C.J., and Dickinson, H.G. (1999). Commonalities between pollen/stigma and host/pathogen interactions: Calcium accumulation during stigmatic penetration by Brassica oleracea pollen tubes. Sex. Plant Reprod. 12 194–202. [Google Scholar]
- Flickinger, T.W., Maihle, N.J., and Kung, H.J. (1992). An alternatively processed mRNA from the avian c-erbB gene encodes a soluble, truncated form of the receptor that can block ligand-dependent transformation. Mol. Cell. Biol. 12 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong, V.E., Ride, J.P., and Franklin, F.C.H. (1995). Recombinant stigmatic self-incompatibility (S-) proteins elicit a Ca+2 transient in pollen of Papaver rhoeas. Plant J. 8 299–307. [Google Scholar]
- Franklin-Tong, V.E., Hackett, G., and Hepler, P.K. (1997). Ratio-imaging of Ca2+ in the self-incompatibility response in pollen tubes of Papaver rhoeas. Plant J. 12 1375–1386. [Google Scholar]
- Fukai, E., Nishio, T., and Nasrallah, M.E. (2001). Molecular genetic analysis of the candidate gene for MOD, a locus required for self-incompatibility in Brassica rapa. Mol. Genet. Genomics 265 519–525. [DOI] [PubMed] [Google Scholar]
- Furuyama, T., and Dzelzkalns, V.A. (1999). A novel calcium-binding protein is expressed in Brassica pistils and anthers late in flower development. Plant Mol. Biol. 39 729–737. [DOI] [PubMed] [Google Scholar]
- Giranton, J.L., Ariza, M.J., Dumas, C., Cock, J.M., and Gaude, T. (1995). The S locus receptor kinase gene encodes a soluble glycoprotein corresponding to the SRK extracellular domain in Brassica oleracea. Plant J. 8 827–834. [DOI] [PubMed] [Google Scholar]
- Giranton, J.L., Dumas, C., Cock, J.M., and Gaude, T. (2000). The integral membrane S-locus receptor kinase of Brassica has serine/threonine kinase activity in a membranous environment and spontaneously forms oligomers in planta. Proc. Natl. Acad. Sci. USA 97 3759–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez, L., and Boller, T. (2000). FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 5 1003–1011. [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez, L., Bauer, Z., and Boller, T. (2001). Both the extracellular leucine-rich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13 1155–1163. [PMC free article] [PubMed] [Google Scholar]
- Goring, D.R., and Rothstein, S.J. (1992). The S-locus receptor kinase gene in a self-incompatible Brassica napus line encodes a functional serine/threonine kinase. Plant Cell 4 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, T., Mazzurco, M., Sulaman, W., Matias, D.D., and Goring, D. (1998). Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc. Natl. Acad. Sci. USA 95 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, K., Takasaki, T., Suzuki, G., Nishio, T., Watanabe, M., Isogai, A., and Hinata, K. (2001). The S receptor kinase gene determines dominance relationships in stigma expression of self-incompatibility in Brassica. Plant J. 26 69–76. [DOI] [PubMed] [Google Scholar]
- He, Z., Wang, Z.Y., Li, J., Zhu, Q., Lamb, C., Ronald, P., and Chory, J. (2000). Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288 2360–2363. [DOI] [PubMed] [Google Scholar]
- Heldin, C.H. (1995). Dimerization of cell surface receptors in signal transduction. Cell 80 213–223. [DOI] [PubMed] [Google Scholar]
- Heldin, C.H., Ernlund, A., Rorsman, C., and Ronnstrand, L. (1989). Dimerization of B-type platelet-derived growth factor receptors occurs after ligand binding and is closely associated with receptor kinase activation. J. Biol. Chem. 264 8905–8912. [PubMed] [Google Scholar]
- Heslop-Harrison, J. (1975). Incompatibility and the pollen-stigma interaction. Annu. Rev. Plant Physiol. 26 403–425. [Google Scholar]
- Hinata, K., and Okazaki, K. (1986). Role of the stigma in the expression of self-incompatibility in crucifers in view of genetic analysis. In Biotechnology and Ecology of Pollen, G.B. Mulcahy, D.L. Mulcahy, and E. Ottaviano, eds (Berlin: Springer-Verlag), pp. 185–190.
- Hodgkin, T., Lyon, G.D., and Dickinson, H.G. (1988). Recognition in flowering plants: A comparison of the Brassica self-incompatibility system and plant pathogen interactions. New Phytol. 110 557–569. [Google Scholar]
- Hughes, A.L. (1999). Evolutionary diversification of the mammalian defensins. Cell. Mol. Life Sci. 56 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, S., Nasrallah, J.B., Dixit, R., Preiss, S., and Nasrallah, M.E. (1997). An aquaporin-like gene in the Brassica self-incompatibility response. Science 276 1564–1566. [DOI] [PubMed] [Google Scholar]
- Jeong, S., Trotochaud, A.E., and Clark, S.E. (1999). The Arabidopsis CLAVATA 2 gene encodes a receptor-like protein required for the stability of the CLAVATA 1 receptor-like kinase. Plant Cell 11 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, G., and Hunter, T. (1999). Receptor activation: When a dimer is not enough. Curr. Biol. 9 R568–R571. [DOI] [PubMed] [Google Scholar]
- Kachroo, A., Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (2001). Allele-specific receptor-ligand interactions in Brassica self-incompatibility. Science 293 1824–1826. [DOI] [PubMed] [Google Scholar]
- Kandasamy, M.K., Paolillo, D.J., Faraday, C.D., Nasrallah, J.B., and Nasrallah, M.E. (1989). The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Dev. Biol. 134 462–472. [DOI] [PubMed] [Google Scholar]
- Kauss, H. (1987). Some aspects of calcium-dependent regulation in plant metabolism. Annu. Rev. Plant Physiol. 38 47–72. [Google Scholar]
- Koegl, M., Hoppe, T., Schlenjer, S., Ulrich, H.D., Mayer, T.U., and Jentsch, S. (1999). A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell 96 635–644. [DOI] [PubMed] [Google Scholar]
- Kossiakoff, A.A., and De Vos, A.M. (1998). Structural basis for cytokine hormone-receptor recognition and receptor activation. Adv. Protein Chem. 52 67–108. [DOI] [PubMed] [Google Scholar]
- Kusaba, M., Dwyer, K., Hendershot, J., Vrebalov, J., Nasrallah, J.B., and Nasrallah, M.E. (2001. a). Self-incompatibility in the genus Arabidopsis: Characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell 13 627–643. [PMC free article] [PubMed] [Google Scholar]
- Kusaba, M., Tung, C.-W., Nasrallah, M.E., and Nasrallah, J.B. (2001. b). Monoallelic expression and dominance interactions in anthers of self-incompatible Arabidopsis lyrata. Plant Physiol., in press. [PMC free article] [PubMed]
- Lalonde, B.A., Nasrallah, M.E., Dwyer, K.G., Chen, C.H., Barlow, B., and Nasrallah, J.B. (1989). A highly conserved Brassica gene with homology to the S-locus-specific glycoprotein structural gene. Plant Cell 1 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon, M.A., and Schlessinger, J. (1994). Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem. Sci. 19 459–463. [DOI] [PubMed] [Google Scholar]
- Letham, D.L.D., Blissard, G.W., and Nasrallah, J.B. (1999). Production and characterization of the Brassica oleracea self-incompatibility locus glycoprotein and receptor kinase in a baculovirus infected insect cell culture system. Sex. Plant Reprod. 12 179–187. [Google Scholar]
- Liu, H., Nishitoh, H., Ichijo, H., and Kyriakis, J.M. (2000). Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 20 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzurco, M., Sulaman, W., Elina, H., Cock, J.M., and Goring, D.R. (2001). Further analysis of interactions between the Brassica S receptor kinase and three interacting proteins (ARC1, THL1 and THL2) in the yeast two-hybrid system. Plant Mol. Biol. 45 365–376. [DOI] [PubMed] [Google Scholar]
- McClure, B.A., Haring, V., Ebert, P.R., Anderson, M.A., Simpson, R.J., Sakiyama, F., and Clarke, A.E. (1989). Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature 342 955–957. [DOI] [PubMed] [Google Scholar]
- Nasrallah, J.B. (2000). Cell-cell signaling in the self-incompatibility response. Curr. Opin. Plant Biol. 3 368–373. [DOI] [PubMed] [Google Scholar]
- Nasrallah, J.B., Kao, T.-H., Goldberg, M.L., and Nasrallah, M.E. (1985). A cDNA clone encoding an S-locus-specific glycoprotein from Brassica oleracea. Nature 318 263–267. [Google Scholar]
- Nasrallah, J.B., Rundle, S.J., and Nasrallah, M.E. (1994). Genetic evidence for the requirement of the Brassica S-locus receptor kinase gene in the self-incompatibility response. Plant J. 5 373–384. [Google Scholar]
- Nasrallah, M.E. (1974). Genetic control of quantitative variation in self-incompatibility proteins detected by immunodiffusion. Genetics 76 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah, M.E., Kandasamy, M.K., and Nasrallah, J.B. (1992). A genetically defined trans-acting locus regulates S-locus function in Brassica. Plant J. 2 497–506. [Google Scholar]
- Nasrallah, M.E., Kandasamy, M.K., Chang, M.-C., Stadler, Z., Lim, S., and Nasrallah, J.B. (2000). Identifying genes for pollen-stigma recognition in crucifers. Ann. Bot. 85 (suppl. A), 125–132. [Google Scholar]
- Nishio, T., and Kusaba, M. (2000). Sequence diversity of SLG and SRK in Brassica oleracea L. Ann. Bot. 85 (suppl. A), 141–146. [Google Scholar]
- Ockendon, D.J. (1974). Distribution of self-incompatibility alleles and breeding structure of open-pollinated cultivars of brussel sprouts. Heredity 33 159–171. [Google Scholar]
- Pierson, E.S., Miller, D.D., Callaham, D.A., Shipley, A.M., Rivers, B.A., Cresti, M., and Hepler, P.K. (1994). Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 6 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozwadowski, K., Zhao, R., Jackman, L., Huebert, T., Burkhart, W.E., Hemmingsen, S.M., Greenwood, J., and Rothstein, S.J. (1999). Characterization and immunolocalization of a cytosolic calcium-binding protein from Brassica napus and Arabidopsis pollen. Plant Physiol. 120 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd, J.J., and Franklin-Tong, V.E. (2001). Unravelling response-specificity in Ca2+ signalling pathways in plant cells. New Phytol. 151 7–33. [DOI] [PubMed] [Google Scholar]
- Salzet, M. (2001). Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 22 285–288. [DOI] [PubMed] [Google Scholar]
- Sassa, H., Hirano, H., and Ikehashi, H. (1993). Identification and characterization of stylar glycoproteins associated with self-incompatibility genes of Japanese pear, Pyrus serotina Rehd. Mol. Gen. Genet. 241 17–25. [DOI] [PubMed] [Google Scholar]
- Schopfer, C.R., and Nasrallah, J.B. (2000). Self-incompatibility: Prospects for a novel putative peptide-signaling molecule. Plant Physiol. 124 935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer, C.R., Nasrallah, M.E., and Nasrallah, J.B. (1999). The male determinant of self-incompatibility in Brassica. Science 286 1697–1700. [DOI] [PubMed] [Google Scholar]
- Shiba, H., Takayama, S., Iwano, M., Shimosato, H., Funato, M., Nakagawa, T., Che, F.S., Suzuki, G., Watanabe, M., Hinata, K., and Isogai, A. (2001). A pollen coat protein, SP11/SCR, determines the pollen S-specificity in the self-incompatibility of Brassica species. Plant Physiol. 125 2095–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu, S.H., and Bleecker, A.B. (2001). Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 98 10763–10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, N.F., Stone, S.L., Christie, L.N., Sulaman, W., Nazarian, K.A., Burnett, L.A., Arnoldo, M.A., Rothstein, S.J., and Goring, D.R. (2001). Expression of the S receptor kinase in self-compatible Brassica napus cv. Westar leads to the allele-specific rejection of self-incompatible Brassica napus pollen. Mol. Genet. Genomics 265 552–559. [DOI] [PubMed] [Google Scholar]
- Singh, A., and Paollilo, D.J. (1990). Role of calcium in the callose re-sponse of self-pollinated Brassica stigmas. Am. J. Bot. 77 128–133. [Google Scholar]
- Singh, A., Perdue, T.D., and Paollilo, D.J. (1989). Pollen-pistil interactions in Brassica oleracea: Cell calcium in self and cross pollen grains. Protoplasma 151 57–61. [Google Scholar]
- Spivak-Kroizman, T., Lemmon, M.A., Dikic, I., Ladbury, J.E., Pinchasi, D., Huang, J., Jaye, M., Crumley, G., Schlessinger, J., and Lax, I. (1994). Heparin-induced oligomerization of FGF molecules is responsible for FGF receptor dimerization, activation, and cell proliferation. Cell 79 1015–1024. [DOI] [PubMed] [Google Scholar]
- Stahl, R.J., Arnoldo, M., Glavin, T.L., Goring, D.R., and Rothstein, S.J. (1998). The self-incompatibility phenotype in Brassica is altered by the transformation of a mutant S-locus receptor kinase. Plant Cell 10 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J.C., and Nasrallah, J.B. (1993). A plant receptor-like gene, the S-locus receptor kinase of Brassica oleracea L., encodes a functional serine/threonine kinase. Plant Physiol. 101 1103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J.C., Howlett, B., Boyes, D.C., Nasrallah, M.E., and Nasrallah, J.B. (1991). Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl. Acad. Sci. USA 88 8816–8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, J.C., Dixit, R., Nasrallah, M.E., and Nasrallah, J.B. (1996). SRK, the stigma-specific S locus receptor kinase of Brassica, is targeted to the plasma membrane in transgenic tobacco. Plant Cell 8 429–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, A.G., Doughty, J., Dixon, S., Elleman, C., Hiscock, S., and Dickinson, H.G. (1997). The male determinant of self-incompatibility in Brassica oleracea is located in the pollen coating. Plant J. 12 1351–1359. [Google Scholar]
- Stephenson, A.G., Good, S.V., and Vogler, D.W. (2000). Interrelationships among inbreeding depression, plasticity in the self-incompatibility system, and the breeding system of Campanula rapunculoides L-(Campanulaceae). Ann. Bot. 85 (suppl. A), 211–219. [Google Scholar]
- Stone, J.M., Trotochaud, A.E., Walker, J.C., and Clark, S.E. (1998). Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol. 117 1217–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, S.L., Arnoldo, M., and Goring, D.R. (1999). A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science 26 1729–1731. [DOI] [PubMed] [Google Scholar]
- Sulaman, W., Arnoldo, M., Yu, K., Tulsieram, L., Rothstein, S.J., and Goring, D.R. (1997). Loss of callose in the stigma papillae does not affect the Brassica self-incompatibility phenotype. Planta 203 327–331. [Google Scholar]
- Suzuki, G., Kai, N., Hirose, T., Fukui, T., Nishio, T., Takayama, S., Isogai, A., Watanabe, M., and Hinata, K. (1999). Genomic organization of the S locus: Identification and characterization of genes in SLG/SRK region of S(9) haplotype of Brassica campestris (syn. rapa). Genetics 153 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Kusaba, M., Matsushita, M., Okazaki, K., and Nishio, T. (2000). Characterization of Brassica S-haplotypes lacking S-locus glycoprotein. FEBS Lett. 482 102–108. [DOI] [PubMed] [Google Scholar]
- Takasaki, T., Hatakeyama, K., Suzuki, G., Watanabe, M., Isogai, A., and Hinata, K. (2000). The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 403 913–916. [DOI] [PubMed] [Google Scholar]
- Takayama, S., Shiba, H., Iwano, M., Shimosato, H., Che, F.S., Kai, N., Watanabe, M., Suzuki, G., Hinata, K., and Isogai, A. (2000). The pollen determinant of self-incompatibility in Brassica campestris. Proc. Natl. Acad. Sci. USA 97 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama, S., Shimosato, H., Shiba, H., Funato, M., Che, F.S., Watanabe, M., Iwano, M., and Isogai, A. (2001). Direct ligand-receptor complex interaction controls Brassica self-incompatibility. Nature 413 534–538. [DOI] [PubMed] [Google Scholar]
- Trotochaud, A.E., Hao, T., Wu, G., Yang, Z., and Clark, S.E. (1999). The CLAVATA1 receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein. Plant Cell 11 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotochaud, A.E., Jeong, S., and Clark, S.E. (2000). CLAVATA3, a multimeric ligand for the CLAVATA1 receptor-kinase. Science 289 613–617. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse, V., Miege, C., Dumas, C., and Cock, J.M. (2001). Two large Arabidopsis thaliana gene families are homologous to the Brassica gene superfamily that encodes pollen coat proteins and the male component of the self-incompatibility response. Plant Mol. Biol. 46 17–34. [DOI] [PubMed] [Google Scholar]
- Wang, Z.-Y., Seto, H., Fujioka, S., Yoshida, S., and Chory, J. (2001). BRl 1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410 380–383. [DOI] [PubMed] [Google Scholar]
- Watanabe, M.A., et al. (2000). Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett. 473 139–144. [DOI] [PubMed] [Google Scholar]
- Wiesmann, C., Fuh, G., Christinger, H.W., Eigenbrof, C., Wells, J.A., and de Vos, A.M. (1997). Crystal structure at 1.7Å resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 91 695–704. [DOI] [PubMed] [Google Scholar]
- Wilkinson, K.D. (1999). Ubiquitin-dependent signaling: The role of ubiquitination in the response of cells to their environment. J. Nutr. 129 1933–1936. [DOI] [PubMed] [Google Scholar]
- Williams, R.W., Wilson, J.M., and Meyerowitz, E.M. (1997). A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway. Proc. Natl. Acad. Sci. USA 94 10467–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Y., Carpenter, R., Dickinson, H.G., and Coen, E.S. (1996). Origin of allelic diversity in Antirrhinum S locus RNases. Plant Cell 8 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]