Abstract
YqjH and YqjW are Bacillus subtilis homologs of the UmuC/DinB or Y superfamily of DNA polymerases that are involved in SOS-induced mutagenesis in Escherichia coli. While the functions of YqjH and YqjW in B. subtilis are still unclear, the comparisons of protein structures demonstrate that YqjH has 36% identity to E. coli DNA polymerase IV (DinB protein), and YqjW has 26% identity to E. coli DNA polymerase V (UmuC protein). In this report, we demonstrate that both YqjH and the products of the yqjW operon are involved in UV-induced mutagenesis in this bacterium. Furthermore, resistance to UV-induced damage is significantly reduced in cells lacking a functional YqjH protein. Analysis of stationary-phase mutagenesis indicates that absences of YqjH, but not that of YqjW, decreases the ability of B. subtilis to generate revertants at the hisC952 allele via this system. These data suggest a role for YqjH in the generation of at least some types of stationary-phase-induced mutagenesis.
In Escherichia coli, the dinB gene is required for bacteriophage λ untargeted mutagenesis (UTM), an error-prone pathway observed when undamaged λ DNA infects SOS-induced E. coli cells (4, 55). Overexpression of the dinB gene confers a mutator phenotype on the cells (22). However, mutations in the dinB gene only caused a modest UV sensitivity phenotype, indicating that this gene product might not play a major role in the tolerance of DNA lesions introduced by UV irradiation into E. coli (22). The genetic requirements for λ UTM include the recA, uvrA, uvrB, uvrC, and polA genes, as well as DNA polymerase III (DNA Pol III), in addition to dinB (22, 26). However, when the dinB gene is overexpressed on a multicopy plasmid, these requirements for genes besides dinB for λ UTM are bypassed (22). In 1999, it was discovered that the purified DinB protein has a template-directed, DNA-dependent DNA polymerase activity and it was designated the fourth DNA polymerase in E. coli (DNA Pol IV) (51).
The DNA damage-inducible UmuD′ and UmuC proteins are required for another type of SOS mutagenesis in E. coli (40). UmuCD-dependent translesion DNA synthesis allows cells to replicate past DNA damage-induced lesions that would normally block the continuing polymerization by the major replication DNA polymerase (DNA Pol III) in E. coli. This translesion synthesis results in an increased mutation rate (21, 42). The translesion DNA synthesis process requires the products of the SOS-regulated recA gene and the umuDC operon, which was originally identified by screening for E. coli mutants that were not mutable by UV light and other agents (21, 42). The umuDC gene products are also known to be essential components of chromosomal UTM (9, 27), a transient increase in the mutation frequency of chromosomal genes following induction of the SOS response (9, 27, 30). In 1999, UmuC or UmuD′2C was discovered to be a template-directed, DNA-dependent DNA polymerase that was designated the fifth DNA polymerase in E. coli (DNA Pol V) (34, 49).
It has very recently become apparent that UmuC is the founding member of a superfamily of novel DNA polymerases that can replicate over lesions or operate on particular classes of imperfect DNA templates (47). Both DinB and UmuC belong to this superfamily, which has been designated the Y family of DNA polymerases (32). To date, the UmuC protein of E. coli is unique within the Y family in that its polymerase activity is managed and/or activated by the products of the umuD gene (27, 46); whether or not the activities of the other family members are similarly regulated is unknown. UmuC, when complexed with UmuD′2 (the active form of UmuD), can replicate over abasic sites (34, 49), thymine-thymine cyclobutane dimers, and [6-4] photoproducts (48). DinB protein can replicate templates possessing AP (apurinic-apyrimidinic) lesions in the template strand, often causing −1 frameshift mutations (51).
Approximately 10 years ago, a series of experiments were initiated whose results have argued for the existence of adaptive or stationary-phase mutagenesis (6). These observations of adaptive or stationary-phase mutagenesis in this particular E. coli FC40 system were the result of a specific frameshift mutation (5, 38). This type of adaptive mutagenesis was dependent upon recombination functions (13, 18, 19, 37), F′ transfer functions (12, 15), and a component of the SOS system (28). Genetic evidence suggests that DNA Pol III (11, 17) and DNA Pol IV (28, 29) are responsible for the synthesis of errors that lead to these mutations. Recently, results have been presented that indicate that gene amplification, followed by mutations in transiently growing cells, is responsible for this specific type of stationary-phase mutagenesis (20, 39).
Our laboratory has demonstrated the existence of stationary-phase mutagenesis in B. subtilis that does not require a functional RecA protein (44). Furthermore, significant differences have been identified between E. coli and B. subtilis with respect to their SOS systems (54, 56), types and activities of DNA polymerases (3), and the parameters associated with error-prone repair capacities (58).
Since DinB (DNA Pol IV) has been shown to be involved in at least one aspect of stationary-phase mutagenesis in E. coli (29), we investigated whether B. subtilis possesses any members of the Y family and whether or not these proteins are involved in stationary-phase-induced mutagenesis in this bacterium. By using the DinB and UmuC protein sequences of E. coli to do a BLAST search (1) against the Bacillus Database (http://genolist.pasteur.fr/SubtiList/), three genes were identified (uvrX, yqjH, and yqjW) (32). UvrX is a UV damage repair protein that is believed to be part of the endogenous bacteriophage SPβ (24), and it has 25% identity to the E. coli DinB protein. YqjH has 36% identity to the E. coli DinB protein, and its function is unknown. YqjW has 26% identity to the E. coli UmuC protein, and its function is also undetermined. Other recent analyses of the Y family of polymerases in B. subtilis also concluded that UvrX, YqjW, and YqjH are potential Y polymerases (33) and are members of the UmuC gram-positive subfamily (32). The results presented here demonstrate that inactivation of the yqjH gene leads to a significant increase in UV sensitivity, a decrease in UV-induced mutagenesis, and a decrease in at least one aspect of stationary-phase mutagenesis while inactivation of the yqjW operon leads to a decrease in UV-induced mutagenesis.
(A component of the research presented here represents part of the degree requirements for C.A.R.)
MATERIALS AND METHODS
Strains and genetic manipulations.
The bacterial strains used in these experiments are listed in Table 1. B. subtilis YB955 is a prophage-cured strain that contains the hisC952, metB5, and leuC427 alleles (44, 57, 58).
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| YB955 | hisC952 metB5 leuC427 xin-1 SPβSENS | 58 |
| YB9400 | YB955 carrying ΔyqjH::tet | See Materials and Methods |
| YB9500 | YB955 carrying ΔyqjW::kan | See Materials and Methods |
| YB9600 | YB955 carrying both ΔyqjH::tet and ΔyqjW::kan | See Materials and Methods |
The procedures used for transformation and isolation of chromosomal and plasmid DNAs were described previously (7, 59). B. subtilis strains were maintained on tryptose blood agar base medium (Difco Laboratories, Detroit, Mich.), and liquid cultures were routinely grown in PB medium (antibiotic medium 3; Difco) supplemented with appropriate antibiotics (10 μg of kanamycin per ml and 5 μg of tetracycline per ml). E. coli cultures were grown in LB (2) supplemented with 100 μg of ampicillin per ml as needed.
Bacillus strains YB9400, YB9500, and YB9600 are isogenic derivatives of YB955 (Table 1) in which the genes of interest have been disrupted by the insertion of an antibiotic resistance determinant cassette. Strain YB9400 was constructed by transforming YB955 with a linearized derivative of plasmid pMK3 (43) that carries a defective yqjH gene (http://genolist.pasteur.fr/SubtiList/). The yqjH+ gene was cloned onto the pMK3 plasmid by using primer 5′CGCGGATCCTTATGCCGGGAAAGAGCC3′ for the 5′ end of the gene and primer 5′CGGAATTCAGCTTTTCTTTTCATCTTGA3′ for the 3′ end. The PCR conditions were set as follows: 0.5 μg of genomic DNA in a 100-μl reaction mixture with 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 0.1% Triton X-100, 0.25 mM deoxynucleoside triphosphates, 0.25 μM each primer, and 2.5 U of Taq DNA polymerase (Promega). The PCRs were carried out in an Eppendorf thermocycler (Mastercycler gradient) for 30 cycles. The PCR fragment was then ligated into pMK3 that had been treated with the endonucleases BamHI and EcoRI (Promega; Madison, Wis.; all of the restriction enzymes used were from Promega unless indicated otherwise). The cloned yqjH+ gene on pMK3 was treated with the restriction endonuclease EcoRV, and the tetracycline cassette (cut with EcoRI and PstI from plasmid pDG1515 [16]) was then inserted following use of the Klenow fragment enzyme (Promega) to fill in the single-stranded ends (2). Strain YB9500 was constructed as described above, except that the cloned yqjW+ gene was used (http://genolist.pasteur.fr/SubtiList/). The yqjW+ gene was amplified by PCR and cloned onto plasmid pMK3 by using primer 5′CGCGGATCCGAGGTGTGTATGATGAAAGAA3′ for the 5′ end of the gene and primer 5′CGGAATTCCCACAGCAGGTTGCCCCTT3′ for the 3′ end. The PCR fragment was then ligated into pMK3 opened at the BamHI and EcoRI sites. The cloned yqjW+ gene on the pMK3 vector was treated with the restriction endonuclease SwaI, and the kanamycin cassette (cut with EcoRI from plasmid pDG783 [16]) was then inserted following use of the Klenow fragment enzyme to fill in the single-stranded ends (2). For cloning, E. coli strain DH5α (as described in reference 2) was used. Strain YB9600 was constructed by transforming YB9500 with genomic DNA isolated from strain YB9400 and selecting for resistance to the appropriate antibiotics. Gene disruptions were confirmed by PCR following separation of amplified fragments by gel electrophoresis (data not shown).
Procedure for UV mutagenesis assay.
Twenty milliliters of cells was grown in Spizizen minimal medium (SMM; Spizizen minimal salts and 1× SS [41] supplemented with 0.5% glucose, 50 μg of the auxotrophic required amino acid per ml, and 50 μg (each) of isoleucine and glutamic acid per ml) supplemented with the appropriate antibiotics in a nephloflask at 37°C with aeration (250 rpm) to mid-exponential phase. Bacterial growth was monitored with a Klett-Summerson colorimeter (no. 66 filter; Klett Mfg. Co., Inc., New York, N.Y.; 1 Klett unit ≅ 106 CFU/ml). The doubling time for B. subtilis strains grown under these conditions was approximately 55 min. The cells were harvested at 55 Klett units, pelleted at 10,000 rpm in a Sorvall GSA rotor (DuPont Medical Products, Newtown, Conn.) at 4°C for 10 min, and resuspended in 5 ml of 1× SS. The cells were then transferred into sterile glass petri dishes (100-mm diameter) for UV irradiation. The flux of the UV irradiation was 83 W/m2 (as measured with a Blak-Ray no. J225 UV meter). The cells were exposed to UV light, with stirring, at various fluences in order to generate a survival curve where the killing did not exceed 90%. Following UV irradiation, the cells from each experiment were plated in sextuplicate on SMM that was deficient in histidine (in order to select for His+ revertants). The selecting SMM actually contained two of the auxotrophic required amino acids, leucine and methionine (50 μg/ml), and a subgrowth amount of histidine (200 ng/ml), as well as 0.5% glucose and 50 μg each of glutamic acid and isoleucine per ml (for viability [45]). The total viable cell numbers were determined following titration and then growth on SMM that contained all of the required nutrients. The plates were incubated at 37°C. After 24 h, the viable count was recorded. The His+ revertants were scored and recorded after 48 and 72 h of incubation. The number of revertants recorded after 72 h of incubation was used to determine the UV-induced mutation frequencies.
Stationary-phase mutagenesis assay.
Procedures for the stationary-phase mutagenesis assay were as previously described (44). Essentially, 10 ml of cells was grown in PB medium supplemented with appropriate antibiotics in a nephloflask at 37°C, with aeration (250 rpm), to 90 min after the cessation of exponential growth (designated T90). Bacterial growth was monitored with a Klett-Summerson colorimeter (no. 66 filter). The cells were harvested by centrifugation at 10,000 × g for10 min (room temperature) and then resuspended in 10 ml of 1× SS in order to reduce the amount of trace nutrients. The cells were then plated in quintuplicate and incubated at 37°C on SMM (supplemented with 0.5% glucose, either 50 or 200 ng of the required amino acid per ml, and 50 μg (each) of isoleucine and glutamic acid per ml). The experiments were repeated at least three times. In addition, each time an isogenic derivative of strain YB955 was examined for the ability to perform stationary-phase mutagenesis, a YB955 control was tested simultaneously. The concentration of the amino acid used depended upon the reversion that was being selected. For instance, when selecting for His+ revertants, 50 μg (each) of methionine and leucine per ml and 200 ng of histidine per ml were added to the medium. Isoleucine and glutamic acid were added as described previously (45) in order to protect the viability of the cells. Appropriate antibiotic concentrations were maintained throughout the experiments. The number of revertants was scored daily (44). The initial number of bacteria plated for each experiment was determined by titration, and the cells were grown on a minimal medium containing all three essential amino acids.
The survival rates of the bacteria plated on the minimal selective media were determined by removing bacterium-containing media from the selection plates (44). In these experiments, three agar plugs were removed from each selection plate every day. The plugs were removed by using sterile Pasteur pipettes and taken from areas of the plates in which no growth of revertants was observed. The plugs were suspended in 400 μl of 1× SS, mixed, diluted, and plated on SMM containing all of the essential amino acids (50 μg/ml). Again, the numbers of colonies were determined following 48 h of growth at 37°C.
Growth parameters of prototrophic revertants.
About 500 of the revertant (e.g., His+, Leu+, or Met+) CFU per ml (revertants isolated as described above in adaptive mutagenesis experiments) were mixed with 108 CFU of strain YB955 or the strain from which they had been isolated per ml in order to determine the growth parameters of these mutants. Next, 100 μl of these mixed populations of cells were plated on the specific SMMs that are used to select for the revertant types (e.g., His−, Leu−, or Met−, as described above for adaptive mutagenesis experiments) and the cells were incubated at 37°C. The colony number on each plate was checked and recorded after 48 h of growth.
Analysis of mutation rates.
The growth-dependent reversion frequencies for the His+ allele were measured by fluctuation tests as previously described (44). Specifically, in order to determine the mutation rates of various strains, the bacteria were grown to saturation at 37°C, with aeration, in PB medium. The saturated cultures were diluted 10−4-fold into fresh PB medium before being dispensed into 18-mm test tubes (1 ml in each tube). For these experiments, 38 of the 18-mm test tubes, each containing almost the same number of cells, were incubated overnight to saturation (with aeration at 37°C). The saturated cultures were pelleted and resuspended in 100 μl of 1× SS medium, and the cells were spread onto the appropriate selection media. The revertants were scored and recorded in 48 h after plating. The median (ŕ) is the mean of the 19th and 20th values of r (observed number of mutants per culture) when the r values are ranked. The number of mutations per culture (m) was calculated with the Lea-Coulson formula (ŕ/m − ln(m) = 1.24). Three parallel cultures were used to determine the final number of cells in the culture (Nt) by titration. The mutation rates were calculated with the formula m/2Nt, (25, 35, 50).
RESULTS
Absence of the uvrX gene in YB955.
Following a BLAST search (1, 32) for the E. coli DinB and UmuC protein sequences in B. subtilis (http://genolist.pasteur.fr/SubtiList/), three proteins were identified (UvrX, YqjH, and YqjW). UvrX has 25% identity to the E. coli DinB protein. uvrX is a 1,248-bp gene that encodes a UV damage repair protein. The uvrX gene is believed to be part of the genome of the B. subtilis endogenous prophage SPβ (24). This conclusion was supported by our inability to amplify the uvrX gene from B. subtilis strain YB955 by PCR with primer 5′CGGGATCCACATACAATGATTGATTACTCAC3′ for the 5′ end of the gene and primer 5′CGGAATTCATCTTAACTCTTATGCCCACCTG3′ for the 3′ end (data not shown) since this strain is cured of prophage SPβ (57, 58). In order to rule out the possibility that our inability to amplify the uvrX gene from YB955 was the result of a primer design problem, the uvrX gene from B. subtilis strain BR151 (57) was subjected to PCR amplification. The primers described above were able to amplify the uvrX gene from prophage-carrying strain BR151, the parent of strain YB955. Contrary to the results obtained with the uvrX gene, the appropriate primers (see Materials and Methods) were able to amplify the yqjH and yqjW genes following PCR amplification of the DNA isolated from strain YB955 (data not shown).
UV-induced mutagenesis.
YqjH and YqjW are homologs of the E. coli Y family of DNA polymerases that are responsible for major aspects of SOS-induced mutagenesis in E. coli, especially UTM (23, 27, 48, 52). The structure of the YqjH protein is similar to that of a DNA damage repair protein, and the structure of YqjW is similar to that of ATP/GTP binding proteins (http://genolist.pasteur.fr/SubtiList/). However, both proteins have been placed in the UmuC gram-positive subfamily of the Y superfamily of polymerases (32).
Following irradiation with UV (see Materials and Methods), strains YB955 and YB9500 (yqjW) showed similar survival profiles (D50 [fluence required to kill 50% of the cells] values of 16 to 20 J/m2) while strains YB9400 (yqjH) and YB9600 (yqjH yqjW) demonstrated enhanced susceptibility to irradiation (D50 values of 6 to 9 J/m2) (Fig. 1).
FIG. 1.
Survival curves of B. subtilis strains following UV irradiation. The results presented are averages of six different plates from each experiment for each strain following UV irradiation (see Material and Methods for details). Error bars represent 1 standard error. The results are representative, and the experiments were repeated at least four times.
On the basis of the data obtained from the UV survival studies, the ability of irradiation to induce mutagenesis was calculated. As described in Materials and Methods, the isogenic set of strains was irradiated and then plated on selection medium to quantitate the number of His+ revertants induced (Fig. 2). The results indicated that the absence of functional YqjH or YqjW protein significantly reduced the number of His+ revertants following UV irradiation (Fig. 2). The results demonstrated that loss of a functional yqjH gene had a more pronounced effect on the reduction of UV-induced mutagenesis than did loss of the yqjW gene. For instance, at the 20% survival level, strain YB9500 (yqjW) lost about 50% of the UV-induced revertants while, at that same level of survival, strain YB9400 (yqjH) showed a 70% reduction in the number of UV-induced revertants (Fig. 2). In addition, strain YB9600 (yqjH yqjW) appeared to be as deficient in the UV induction of His+ revertants as strain YB9400 (yqjH). Thus, loss of the yqjW gene does not further reduce the ability of cells lacking the yqjH gene to generate UV-induced mutations.
FIG. 2.
UV mutagenesis of YB955 and isogenic derivatives thereof. The results presented are the average number of His+ revertants from six different plates from each experiment for each strain following UV irradiation (see Material and Methods for details). Error bars represent 1 standard error. The results are representative, and the experiments were repeated at least four times.
Effect of YqjH on stationary-phase mutagenesis.
In one of the E. coli systems used to measure stationary-phase mutagenesis, inactivation of DNA Pol IV has been shown to significantly decrease the appearance of mutations (29). We have previously established the existence of a stationary-phase mutagenesis system in B. subtilis (44). Therefore, we tested the ability of strains YB955, YB9400, YB9500, and YB9600 to generate stationary-phase mutants. As demonstrated by the results shown in Fig. 3, the absence of a functional YqjH protein significantly decreases the number of His+ revertants generated during stationary-phase mutagenesis. However, the absence of a functional YqjW protein had no effect on the number of His+ stationary-phase mutants that were generated. The reduction in the number of His+ stationary-phase mutants generated by strain YB9400, compared to the other members of the isogenic set, cannot be attributed to decreased survival of this strain on the selection medium, as shown by the results in Fig. 4. In contrast to the results obtained for the generation of stationary-phase His+ revertants, neither YqjH nor YqjW had an effect on the number of Leu+ revertants generated via stationary-phase mutagenesis (data not shown).
FIG. 3.
Stationary-phase-induced His+ reversion frequencies of YB955 and isogenic derivatives thereof. The results presented are the average number of revertants on each day from five different selection plates. Error bars represent 1 standard error, as described in Materials and Methods. These results are representative of experiments repeated at least three times.
FIG. 4.
Abilities of YB955 and isogenic derivatives thereof to survive under histidine starvation. Three plugs of bacteria containing agar were taken from selection plates each day for testing of the viability of bacteria on the selecting medium (see Material and Methods for details). The experiments were repeated at least twice.
In order to rule out the possibility that the results described above were due to the slow growth of the His+ revertants generated in the strains carrying the inactivated yqjH and/or yqjW genes, a reconstruction experiment was performed (see Materials and Methods). Six His+ revertants that appeared after 6 days of incubation in the stationary-phase mutagenesis experiments were isolated (two each of YB9400, YB9500, and YB9600). As described in Materials and Methods, approximately 500 CFU of these revertants was mixed with 108 CFU of the strain from which they had been isolated. These mixtures of cells were then plated on the appropriate selection media, and the numbers of His+ colonies that arose after 48 h of incubation were scored. As expected, for each revertant checked, approximately 50 His+ colonies were detected after 48 h of incubation. Thus, the late-arising, stationary-phase-generated His+ revertants did not have any slow-growth physiological defects. Therefore, these results confirmed that the role played by the yqjH gene in stationary-phase mutagenesis was not due to physiological changes in the growth parameters of the revertants.
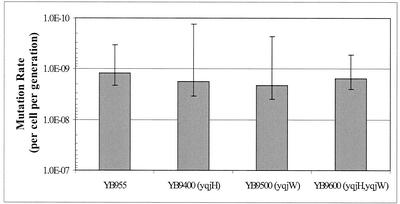
YB9400, YB9500, and YB9600 were also tested for the ability to generate His+ revertants during exponential growth. The data in Fig. 5 demonstrate that the growth-dependent mutation rates of all of these strains are similar. Thus, the presence or absence of YqjH and/or YqjW does not affect the spontaneous mutation rates of these bacteria.
FIG. 5.
Analysis of mutation rates. YB955 and isogenic derivatives of strain YB955 carrying the yqjH and/or yqjW alleles were tested for the ability to produce His+ revertants during exponential growth as described in Materials and Methods. The revertants were scored and recorded in 48 h after plating. The median (ŕ) is the mean of the 19th and 20th values of r (the observed number of mutants per culture in a 38-test-tube fluctuation test) when the r values are ranked. The number of mutations per culture (m) was calculated with the Lea-Coulson formula (ŕ/m − ln(m) = 1.24). Three parallel cultures were used to determine Nt by titration. The mutation rates were calculated with the formula m/2Nt (25, 35, 50). The results presented are the average mutation rates from three individual fluctuation tests. Error bars represent 1 standard error.
DISCUSSION
For more than a decade, there has been considerable interest in a phenomenon that has been called adaptive or stationary-phase mutagenesis. The result of the mechanism(s) responsible for this phenomenon is the production of mutations that arise in nondividing or stationary-phase bacteria when the cells are subjected to nonlethal selective pressure, such as nutrient-limited environments (6, 10, 20, 36). We have previously demonstrated the existence of stationary-phase mutagenesis in B. subtilis (44). The system that we characterized by using strainYB955 and isogenic derivatives was not dependent upon a functional RecA protein or on the presence of the σB form of the RNA polymerase. However, at least two transcription factors that are involved in the regulation of differentiation and development in this bacterium (ComA and ComK) did influence the eventual production of stationary-phase-induced mutations. Furthermore, the three auxotrophic alleles that are present in strain YB955 did not appear to be reverted during stationary-phase mutagenesis by the same molecular mechanisms. On the basis of those results, we have advanced the hypothesis that during periods of environmental stress, such as those found in nutrient-limited stationary-phase cells, subpopulations are differentiated within a culture in order to generate genetic diversity (44). We proposed that within these subpopulations, mutation frequencies are increased (hypermutability) by the suppression of DNA repair systems and/or the activation of mechanisms that would increase the introduction of DNA damage into the genome. In order to test this hypothesis, we have continued to elucidate the genes that control the differentiation of these subpopulations and we have begun to analyze which aspects of DNA metabolism might be involved in the generation of hypermutability.
While it is always tempting to extrapolate back to results obtained with the E. coli model system, in the case of DNA polymerases, there are clear and important differences between the types of polymerases found in the low-GC gram-positive bacteria and what is known about E. coli (3). However, the UmuC/DinB or Y superfamily of DNA polymerases still appeared to be a logical starting point for examination of the hypermutability putatively associated with stationary-phase mutagenesis. These polymerases have been associated with error-prone repair and UTM (4, 14, 34, 49, 55). We first sought to define whether or not representatives of the Y family of proteins are present in B. subtilis by performing the BLAST search described above. Our results, along with those of Ohmori et al. (32) and Permina et al. (33), demonstrated that three potential members of that family could be identified in the genome of this bacterium: uvrX, yqjH, and yqjW (http://genolist.pasteur.fr/SubtiList/) (24). Actually, Permina et al. (33) suggested that a fourth gene, yozK, may also code for a member of this family of polymerases and that this gene is associated with an endogenous prophage. PCR analysis of chromosomal DNA isolated from strain YB955 demonstrated that this strain contained yqjH and yqjW but did not contain uvrX (data not shown). These data are in agreement with the conclusion that the uvrX gene is part of the genome of the endogenous prophage SPβ and that strain YB955 is cured of this prophage (24, 57, 58).
Next, the yqjH and yqjW genes were cloned via PCR and inactivated in the bacterial chromosome by insertion of antibiotic resistance determinants as described in Materials and Methods (Table 1). Strains carrying one or both of these inactivated genes were then examined for sensitivity to UV irradiation, the ability to generate UV-induced mutations, and the ability to generate stationary-phase-induced mutations. The absence of the YqjH protein (a protein that has 36% identity to the DinB protein [DNA Pol IV] of E. coli) rendered the bacteria significantly more sensitive to UV irradiation (Fig. 1), severely reduced their ability to generate UV-induced revertants to the hisC952 allele (Fig. 2), and reduced their ability to generate stationary-phase revertants of the hisC952 allele (Fig. 3 and 4). On the other hand, the absence of the YqjW protein (a protein that has 26% identity to the UmuC protein [DNA Pol V] of E. coli) did not appear to increase the sensitivity of B. subtilis to UV irradiation (Fig. 1). While loss of the YqjW protein did decrease the ability of the bacteria to generate UV-induced reversions of the hisC952 allele, it had no effect on the induction, by stationary-phase mutagenesis, of the reversion of that allele (Fig. 2 and 3). Furthermore, loss of YqjH, YqjW, or both proteins had no effect on the induction, by stationary-phase mutagenesis, of reversion of the leuC427 allele (data not shown). These results again suggest that more than one molecular mechanism is involved in the generation of stationary-phase mutations in B. subtilis (44).
As mentioned above, there is evidence that, in the E. coli FC40 system for measuring stationary-phase mutagenesis, gene amplification of leaky alleles, followed by mutations in the transiently growing cells, is responsible for the observed revertant colonies (20, 39). Previous analysis (44) demonstrated that the revertants generated via the characterized B. subtilis stationary-phase mutagenesis system(s) were not dependent upon the presence of a functional recombination system, nor were they phenocopies (i.e., accumulation of leaky mutations). DNA sequence determinations showed that the hisC952 allele was the result of a transition (C→T at position 952 bp) in the gene that encodes the histidinol-phosphate aminotransferase. This transition resulted in Gln being mutated to an amber codon. Additional data obtained revealed that both transitions and transversions at the three base pairs within this codon (bp 952 to 954) could yield functional His+ revertants. Mutations that caused the amino acid Gln, Lys, Trp, or Tyr to be placed in the protein (at the site of termination caused by the amber mutation) could restore the hisC gene to functionality (44). In addition, approximately 20% of the His+ revertants generated during exponential growth and 6% of those generated during stationary-phase mutagenesis were nonsense suppressor mutations. Thus, there is a considerably wide spectrum of mutations, in at least two genes (the hisC gene and the gene[s] responsible for extrachromosomal suppression of the amber mutation), that can result in the generation of His+ mutants. Also, neither loss of the YqjH protein nor loss of the protein(s) made by the yqjW operon affected the spontaneous His+ mutation frequency (Fig. 5), and the His+ revertants that were generated in all of the isogenic strains demonstrated normal growth characteristics (see Results). Therefore, we conclude that the decrease in His+ mutations observed following stationary-phase mutagenesis and/or UV-induced mutagenesis in yqjH and yqjW mutant strains is due to the direct effects of these proteins on the mutagenesis processes.
It is important to note that both the yqjH and yqjW genes were inactivated by insertion of an antibiotic resistance determinant into the middle of each gene (see Materials and Methods). Accordingly, a knockout of each gene could actually lead to the loss of any other gene downstream within the same operon. In the case of yqjH, the only other gene that might be affected is yqjI, a gene of unknown function that is similar to that which encodes 6-phosphogluconate dehydrogenase (http://genolist.pasteur.fr/SubtiList/). Northern hybridizations demonstrated that the expression of yqjI was not affected by knockout of the yqjH gene in strain YB9600 (data not shown). Furthermore, DNA sequence analysis strongly suggests that yqjH and yqjI are not in the same operon (24). The yqjW gene appears to be part of an operon that is a member of the DNA damage-inducible SOS regulon of B. subtilis (8, 33). Northern hybridizations suggest that very little transcription of yqjW occurs without DNA damage (data not shown). Furthermore, it has been suggested that the yqjW operon is somewhat homologous to the umuDC operon of E. coli and that the other gene(s) in this operon is functionally equivalent to the UmuD subunit of DNA Pol V from this gram-negative bacterium (33). We are now in the process of sequentially testing the other potential gene(s) within the yqjW operon to determine if it is responsible for the decreased UV-induced mutagenesis phenotype that we have described.
As previously mentioned, the role of DNA Pol IV in a type of stationary-phase mutagenesis in E. coli has already been described (29). The gene that encodes this polymerase, dinB, is a member of the SOS regulon (14). In the B. subtilis system that we have described, the presence or absence of the RecA protein, a protein essential for the induction of most of the phenomena associated with the SOS regulon of this bacterium (56), has no discernible effect on stationary-phase-induced mutagenesis (44). Sequence analysis of the promoter-operator region of the yqjH gene, whose product has similarities to E. coli DNA Pol IV and is involved in stationary-phase-induced mutagenesis in this bacterium (Fig. 3), shows no obvious DinR (B. subtilis homolog of LexA) or SOS regulatory binding site (8, 53). However, it is possible that this gene is still part of the SOS regulon since in B. subtilis, type II and III DNA damage-induced SOS phenomena can sometimes be induced in the absence of a functional RecA protein (56). Thus, we cannot eliminate the possibility that the SOS regulon is a contributor to stationary-phase mutagenesis in B. subtilis. However, if the SOS system is involved in the generation of mutations via the stationary-phase mechanism(s), then it must affect phenomena that are not directly controlled by the RecA apoprotease activity on the DinR SOS repressor (31, 53, 54). On the other hand, the yqjW operon (the gene[s] that encodes the putative homolog of E. coli DNA Pol V) is involved in the generation of UV-induced mutations and does appear to have a functional DinR-SOS binding site in its promoter-operator region (8, 33, 53) but has not been shown to play any role in B. subtilis stationary-phase-induced mutagenesis.
The results presented in this report again demonstrate important differences in DNA metabolism between gram-negative bacteria and low-GC gram-positive bacteria. For instance, the apparent B. subtilis DNA Pol IV homolog (YqjH) is significantly involved in the survival of the bacteria following UV irradiation (Fig. 1), while this is not the case for DinB in E. coli (22, 29). Also, it is possible that both the yqjH gene product and the product(s) associated with the yqjW operon are involved in the bypassing of similar types of lesions in B. subtilis DNA since loss of these two genes does not reduce the number of UV-induced mutants below the level seen in strains carrying the single yqjH knockout (Fig. 2).
Taken collectively, the data presented in this report do support the hypothesis that stationary-phase-induced mutagenesis occurs via more than one molecular mechanism in B. subtilis. Specifically, in the stationary-phase-induced mutagenesis system that has been used, the yqjH gene product appears to play an important role in the generation of a majority of the His+ revertants but not in the generation of the Leu+ revertants. Accordingly, our research remains focused on elucidation of the prokaryotic developmental pathway(s) that controls stationary-phase mutagenesis, as well as on the specific mechanism(s) involved in this process(es).
Acknowledgments
We thank Juan González, Mario Pedraza-Reyes, and Roger Woodgate for advice.
This research was supported by MCB-9975140 from the National Science Foundation.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2002. Current protocols in molecular biology (on CD-ROM). John Wiley & Sons, Inc., New York, N.Y.
- 3.Barnes, M. H., S. D. Miller, and N. C. Brown. 2002. DNA polymerases of low-GC gram-positive eubacteria: identification of the replication-specific enzyme encoded by dnaE. J. Bacteriol. 184:3834-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brotcorne-Lannoye, A., and G. Maenhaut-Michel. 1986. Role of RecA protein in untargeted UV mutagenesis of bacteriophage lambda: evidence for the requirement for the dinB gene. Proc. Natl. Acad. Sci. USA 83:3904-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 7.Cheo, D. L., K. W. Bayles, and R. E. Yasbin. 1991. Cloning and characterization of DNA damage-inducible promoter regions from Bacillus subtilis. J. Bacteriol. 173:1696-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubnau, D., and C. M. J. Lovett. 2002. Transformation and recombination, p. 473-482. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 9.Fijalkowska, I. J., R. L. Dunn, and R. M. Schaaper. 1997. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J. Bacteriol. 179:7435-7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, P. L., G. Gudmundsson, J. M. Trimarchi, H. Cai, and M. F. Goodman. 1995. Proofreading-defective DNA polymerase II increases adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 92:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, P. L., and J. M. Trimarchi. 1995. Conjugation is not required for adaptive reversion of an episomal frameshift mutation in Escherichia coli. J. Bacteriol. 177:6670-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, P. L., J. M. Trimarchi, and R. A. Maurer. 1996. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics 142:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedberg, E. C., G. C. Walker, and W. Siede. 1995. DNA repair and mutagenesis. American Society for Microbiology, Washington, D.C.
- 15.Galitski, T., and J. R. Roth. 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268:421-423. [DOI] [PubMed] [Google Scholar]
- 16.Guérout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 17.Harris, R. S., H. J. Bull, and S. M. Rosenberg. 1997. A direct role for DNA polymerase III in adaptive reversion of a frameshift mutation in Escherichia coli. Mutat. Res. 375:19-24. [DOI] [PubMed] [Google Scholar]
- 18.Harris, R. S., S. Longerich, and S. M. Rosenberg. 1994. Recombination in adaptive mutation. Science 264:258-260. [DOI] [PubMed] [Google Scholar]
- 19.Harris, R. S., K. J. Ross, and S. M. Rosenberg. 1996. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics 142:681-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato, T., and Y. Shinoura. 1977. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol. Gen. Genet. 156:121-131. [DOI] [PubMed] [Google Scholar]
- 22.Kim, S. R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui, T. Sofuni, T. Nohmi, and H. Ohmori. 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. USA 94:13792-13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, S. R., K. Matsui, M. Yamada, P. Gruz, and T. Nohmi. 2001. Roles of chromosomal and episomal dinB genes encoding DNA pol IV in targeted and untargeted mutagenesis in Escherichia coli. Mol. Genet. Genomics 266:207-215. [DOI] [PubMed] [Google Scholar]
- 24.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, A. Danchin, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 25.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-285. [DOI] [PubMed] [Google Scholar]
- 26.Maenhaut-Michel, G., and P. Caillet-Fauquet. 1990. Genetic control of the UV-induced SOS mutator effect in single- and double-stranded DNA phages. Mutat. Res. 230:241-254. [DOI] [PubMed] [Google Scholar]
- 27.Maor-Shoshani, A., N. B. Reuven, G. Tomer, and Z. Livneh. 2000. Highly mutagenic replication by DNA polymerase V (UmuC) provides a mechanistic basis for SOS untargeted mutagenesis. Proc. Natl. Acad. Sci. USA 97:565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie, G. J., R. S. Harris, P. L. Lee, and S. M. Rosenberg. 2000. The SOS response regulates adaptive mutation. Proc. Natl. Acad. Sci. USA 97:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie, G. J., P. L. Lee, M. J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 30.Miller, J. H., and K. B. Low. 1984. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell 37:675-682. [DOI] [PubMed] [Google Scholar]
- 31.Miller, M. C., J. B. Resnick, B. T. Smith, and J. C. M. Lovett. 1996. The Bacillus subtilis dinR gene codes for the analogue of Escherichia coli LexA. J. Biol. Chem. 271:33502-33508. [PubMed] [Google Scholar]
- 32.Ohmori, H., E. C. Friedberg, R. P. Fuchs, M. F. Goodman, F. Hanaoka, D. Hinkle, T. A. Kunkel, C. W. Lawrence, Z. Livneh, T. Nohmi, L. Prakash, S. Prakash, T. Todo, G. C. Walker, Z. Wang, and R. Woodgate. 2001. The Y family of DNA polymerases. Mol. Cell 8:7-8. [DOI] [PubMed] [Google Scholar]
- 33.Permina, E. A., A. A. Mironov, and M. S. Gelfand. 2002. Damage-repair error-prone polymerases of eubacteria: association with mobile genome elements. Gene 293:133-140. [DOI] [PubMed] [Google Scholar]
- 34.Reuven, N. B., G. Arad, A. Maor-Shoshani, and Z. Livneh. 1999. The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA, and SSB and is specialized for translesion replication. J. Biol. Chem. 274:31763-31766. [DOI] [PubMed] [Google Scholar]
- 35.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg, S. M. 2001. Evolving responsively: adaptive mutation. Nat. Rev. Genet. 2:504-515. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg, S. M., R. S. Harris, S. Longerich, and A. M. Galloway. 1996. Recombination-dependent mutation in non-dividing cells. Mutat. Res. 350:69-76. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg, S. M., R. S. Harris, and J. Torkelson. 1995. Molecular handles on adaptive mutation. Mol. Microbiol. 18:185-189. [DOI] [PubMed] [Google Scholar]
- 39.Slechta, E. S., J. Harold, D. I. Andersson, and J. R. Roth. 2002. The effect of genomic position on reversion of a lac frameshift mutation (lacIZ33) during non-lethal selection (adaptive mutation). Mol. Microbiol. 44:1017-1032. [DOI] [PubMed] [Google Scholar]
- 40.Sommer, S., J. Knezevic, A. Bailone, and R. Devoret. 1993. Induction of only one SOS operon. umuDC, is required for SOS mutagenesis in Escherichia coli. Mol. Gen. Genet. 239:137-144. [DOI] [PubMed] [Google Scholar]
- 41.Spizizen, J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. USA 44:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinborn, G. 1978. uvm mutants of Escherichia coli K-12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol. Gen. Genet. 165:87-93. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan, M. A., R. E. Yasbin, and F. E. Young. 1984. New shuttle vectors for Bacillus subtilis and Escherichia coli which allow rapid detection of inserted fragments. Gene 29:21-26. [DOI] [PubMed] [Google Scholar]
- 44.Sung, H.-M., and R. E. Yasbin. 2002. Adaptive, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis. J. Bacteriol. 184:5641-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sung, H.-M., and R. E. Yasbin. 2000. Transient growth requirement in Bacillus subtilis following the cessation of exponential growth. Appl. Environ. Microbiol. 66:1220-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutton, M. D., T. Opperman, and G. C. Walker. 1999. The Escherichia coli SOS mutagenesis proteins UmuD and UmuD′ interact physically with the replicative DNA polymerase. Proc. Natl. Acad. Sci. USA 96:12373-12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton, M. D., B. T. Smith, V. G. Godoy, and G. C. Walker. 2000. The SOS response: recent insights into umuDC-dependent mutagenesis and DNA damage tolerance. Annu. Rev. Genet. 34:479-497. [DOI] [PubMed] [Google Scholar]
- 48.Tang, M., P. Pham, X. Shen, J. S. Taylor, M. O'Donnell, R. Woodgate, and M. F. Goodman. 2000. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404:1014-1418. [DOI] [PubMed] [Google Scholar]
- 49.Tang, M., X. Shen, E. G. Frank, M. O'Donnell, R. Woodgate, and M. F. Goodman. 1999. UmuD′(2)C is an error-prone DNA polymerase, Escherichia coli pol V. Proc. Natl. Acad. Sci. USA 96:8919-8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Borstel, R. C. 1978. Measuring spontaneous mutation rates in yeast. Methods Cell Biol. 20:1-24. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, J., P. Gruz, S. R. Kim, M. Yamada, K. Matsui, R. P. Fuchs, and T. Nohmi. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell 4:281-286. [DOI] [PubMed] [Google Scholar]
- 52.Wagner, J., and T. Nohmi. 2000. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J. Bacteriol. 182:4587-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winterling, K. W., D. Chafin, J. J. Hayes, J. Sun, A. S. Levine, R. E. Yasbin, and R. Woodgate. 1998. The Bacillus subtilis DinR binding site: redefinition of the consensus sequence. J. Bacteriol. 180:2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterling, K. W., A. S. Levine, R. E. Yasbin, and R. Woodgate. 1997. Characterization of DinR, the Bacillus subtilis SOS repressor. J. Bacteriol. 179:1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wood, R. D., and F. Hutchinson. 1984. Non-targeted mutagenesis of unirradiated lambda phage in Escherichia coli host cells irradiated with ultraviolet light. J. Mol. Biol. 173:293-305. [DOI] [PubMed] [Google Scholar]
- 56.Yasbin, R. E., D. Cheo, and D. Bol. 1993. DNA repair systems, p. 529-537. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 57.Yasbin, R. E., P. I. Fields, and B. J. Andersen. 1980. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12:155-159. [DOI] [PubMed] [Google Scholar]
- 58.Yasbin, R. E., R. Miehl-Lester, and P. E. Love. 1987. Mutagenesis in Bacillus subtilis, p. 73-84. In M. Alacevic, D. Hranueli, and Z. Tomen (ed.), Genetics of industrial microorganisms. GIM-86, Split, Yugoslavia.
- 59.Yasbin, R. E., G. A. Wilson, and F. E. Young. 1973. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J. Bacteriol. 113:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]