Abstract
The diphtheria toxin repressor (DtxR) is a transition metal ion-activated repressor that acts as a global regulatory element in the control of iron-sensitive genes in Corynebacterium diphtheriae. We recently described (L. Sun, J. C. vanderSpek, and J. R. Murphy, Proc. Natl. Acad. Sci. USA 95:14985-14990, 1998) the isolation and in vivo characterization of a hyperactive mutant of DtxR, DtxR(E175K), that appeared to be constitutively active. We demonstrate here that while DtxR(E175K) remains active in vivo in the presence of 300 μM 2,2′dipyridyl, the purified repressor is, in fact, dependent upon low levels of transition metal ion to transit from the inactive apo form to the active metal ion-bound form of the repressor. Binding studies using 8-anilino-1-naphthalenesulfonic acid suggest that the E175K mutation stabilizes an intermediate of the molten-globule form of the repressor, increasing exposure of hydrophobic residues to solvent. We demonstrate that the hyperactive DtxR(E175K) phenotype is dependent upon an intact ancillary metal ion-binding site (site 1) of the repressor. These observations support the hypothesis that metal ion binding in the ancillary site facilitates the conversion of the inactive apo-repressor to its active, operator-binding conformation. Furthermore, these results support the hypothesis that the C-terminal src homology 3-like domain of DtxR plays an active role in the modulation of repressor activity.
The diphtheria toxin repressor (DtxR) is a 226-amino-acid protein encoded by the genome of Corynebacterium diphtheriae (1). First identified for its regulation of the diphtheria toxin structural gene (tox) carried by corynebacteriophage β, DtxR has since been found to regulate the transcription of corynebacterial genes putatively involved in iron homeostasis and host colonization (6, 17, 18). In vivo, the repressor activity of DtxR is modulated by the local concentrations of Fe2+, whereas in vitro-activating divalent transition-metal cations include Ni2+, Co2+, Cd2+, and Mn2+ (21, 22). In the absence of these cations, apo-DtxR is inactive and unable to recognize its target operators. Homologues of DtxR have been identified in a number of microorganisms, including Staphylococcus aureus (5), Mycobacterium tuberculosis (19), Treponema pallidum (13), and Streptococcus mutans (20). Although different homologues display different cation selectivities, all members of this protein family appear to regulate genes responsible for transition metal cation homeostasis and virulence.
The mechanism by which DtxR transits from the inactive apo-protein to a fully active repressor has been a topic of ongoing investigation. Upon binding ferrous cation (or other activating cations), DtxR undergoes significant structural organization, culminating in homodimerization and its ability to recognize and bind to target operator sequences (16, 24). In the case of the tox promoter-operator sequence (toxPO), two DtxR dimers have been shown to bind to opposite faces of the DNA helix, covering the −10 sequence of the promoter (27). X-ray crystal structures of DtxR have demonstrated two separate cation-binding sites (14-16). Previous work in this laboratory has suggested that only one of these sites (site 2, or the primary site) is essential for DtxR activation, whereas the other (site 1, or the ancillary site) is not required for metal ion activation of DtxR (3).
DtxR is comprised of two domains, linked by a flexible tether. The N-terminal domain (residues 1 to 136) contains residues involved in cation binding, dimerization, and DNA recognition (14, 16). This domain appears to undergo the more significant structural change during metal ion-induced activation, changing from a molten globule in the apo form (25) to an organized structure upon cation binding. In contrast, the C-terminal domain (approximately residues 148 to 226) folds into an src homology 3 (SH3)-like structure (14, 26). Although in vitro experiments have demonstrated a weak interaction between the purified C-terminal domain and a proline-rich peptide identical in sequence to residues 125 to 139 of DtxR, the biological function of this domain remains unclear (26). Some crystallographic reports have suggested that the C-terminal domain donates two ligands to the ancillary cation-binding site, but no biological function of these residues has been reported to date (12).
To better understand the activation of DtxR, constitutively active mutants of DtxR were previously identified by using an in vivo genetic screen (21). A number of these gain-of-function mutants, designated self-activating DtxRs, were identified. In vivo studies of these DtxR mutants suggested that their activity was iron independent. In this report, we describe the characterization, both in vivo and in vitro, of DtxR(E175K). The data demonstrate that DtxR(E175K) is, in fact, a cation-dependent repressor. However, the E175K mutation in the SH3-like domain of the repressor results in a significant reduction in the concentration of cation necessary for the complete activation of the apo-repressor. We demonstrate that an intact ancillary metal ion-binding site is required for the hyperactive DtxR(E175K) phenotype. These results support the hypothesis that the DtxR SH3-like domain is able to modulate repressor cation sensitivity through the ancillary cation-binding site.
MATERIALS AND METHODS
β-Galactosidase assays.
The plasmid pROM-dtxR (17) was mutagenized according to the QuikChange mutagenesis protocol (Stratagene). PCRs used Pfu Turbo polymerase and reaction buffer from Stratagene and deoxynucleotides from Perkin-Elmer. Mutagenic oligonucleotide primers were synthesized by Gibco. Mutagenic PCRs were digested with DpnI (Stratagene) prior to transformation into Escherichia coli Top10 (Novagen). The fidelity of the mutated gene was confirmed by automated DNA sequencing (Genetics Core, Boston Medical Center).
Plasmids pROM, pROM-dtxR, and pROM-dtxR(E175K) were transformed into competent E. coli DH5α(λRS45 tacP-toxO-lacZ) and plated on Luria broth (LB) agar plates supplemented with 100 μg of ampicillin (American Bioanalytical) per ml and 50 μg of kanamycin (Sigma) per ml (LB-AMP-KAN). β-Galactosidase assays were performed as described elsewhere (7). Briefly, single colonies of transformants were grown overnight at 37°C in LB-AMP-KAN. Where noted, cultures were grown in the presence of 2,2′-dipyridyl (DP) (Sigma). Solid DP was dissolved in ethanol; all cultures received equivalent total volumes of ethanol. The assay of β-galactosidase activity used 25 μl of cell lysate, following the protocol outlined by Miller (9). For each experiment, all cultures were grown and assayed in quadruplicate.
The introduction of alanine substitutions for residues of the primary and ancillary metal ion-binding sites was achieved with the QuikChange mutagenesis protocol (Stratagene) described above.
The sequences of the oligonucleotides used in this study are available upon request.
Purification of DtxR(E175K).
The plasmid pET-dtxR was mutated to pET-dtxR(E175K) by using the site-directed mutagenic primers and protocol described above for the generation of pROM-dtxR(E175K). Fidelity of the mutation was confirmed by whole-gene sequencing (Genetics Core, Boston Medical Center). The plasmid was transformed into E. coli HMS174(DE3) (Novagen) and plated on LB-AMP. A single colony was picked and grown for 4 h at 37°C in 8 ml of LB-AMP. This culture was used to inoculate 1 liter of M9 medium supplemented with 100 μg of ampicillin per ml and grown overnight at 37°C with shaking. The overnight culture was used to inoculate 10 liters of M9 medium (with 100 μg of ampicillin per ml) in a 15-liter Microferm fermentor (New Brunswick). The fermentor culture was grown at 37°C with aeration (10 liters of air/min) and stirring (500 rpm) until the optical density at 600 nm reached approximately 0.7. Isopropyl-β-d-thiogalactopyranoside (American Bioanalytical) was then added to a final concentration of 1.0 mM. The culture was incubated as described above for 3 h before the bacteria were harvested by centrifugation. Pelleted cells were frozen overnight at −80°C.
Cells were resuspended in 250 ml of 20 mM Tris-Cl (pH 7.5)-5 mM dithiothreitol (DTT) (American Bioanalytical) buffer at 4°C and sonicated to lyse them. Lysate was cleared by centrifugation at 12,500 rpm for 30 min in a JA-20 Beckman rotor. Cleared lysates were pooled and loaded on a ca. 150-ml column of DEAE cellulose (DEAE) DE53 (Whatman) equilibrated with 20 mM Tris-Cl (pH 7.5)-5 mM DTT buffer. The column was washed with two column volumes of 20 mM Tris-Cl (pH 7.5) and eluted with two column volumes of 20 mM Tris-Cl (pH 7.5)-625 mM NaCl in the cold.
The DEAE eluate was loaded onto a 25-ml nickel-nitrilotriacetic acid resin (Qiagen) column pre-equilibrated with 20 mM Tris-Cl (pH 7.5)-625 mM NaCl buffer. The column was washed with six column volumes of the same buffer. Partially purified DtxR(E175K) was then eluted from the column with three column volumes of 20 mM Tris-Cl (pH 7.5)-20 mM imidazole (Sigma) buffer. The nickel column eluate containing partially purified DtxR(E175K) was further purified by anion-exchange high-pressure liquid chromatography (HPLC). The purified DtxR(E175K) was then treated with 10 mM EDTA to remove any contaminating cation and subsequently dialyzed into 20 mM Tris-Cl (pH 7.5)-5 mM DTT buffer and stored at −80°C until used.
EMSAs.
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (7, 22). In brief, toxPO DNA was amplified by PCR from the plasmid pRS551-toxPO (1). After cleaning and precipitation, DNA was digested with BamHI and EcoRI (New England Biolabs). Klenow fragment (New England Biolabs) filled in the uneven ends with α-32P-labeled dATP and dTTP (Amersham Bioscience) and unlabeled dGTP and dCTP (Amersham Bioscience). In a 16-μl total volume, 20 fmol of toxPO probe was bound to either DtxR or DtxR(E175K) in 20 mM Tris-Cl (pH 7.5), 40 mM KCl, 2 mM dithiothreitol, 125 μM MnCl2, 10% glycerol, 1 μg of poly(dI-dC), and 5 μg of purified bovine serum albumin. Reaction mixtures were incubated at room temperature for 10 min before loading on a 6% polyacrylamide gel in 40 mM bis-Tris borate (pH 7.5), 125 μM MnCl2, and 2.5% glycerol. Gels were electrophoresed in 40 mM bis-Tris borate (pH 7.5) with 125 μM MnCl2 at a constant 200 V at room temperature. Gels were dried and analyzed by autoradiography.
In vitro transcription-translation assays.
Coupled in vitro transcription-translation assays were performed as described previously (7). Purified DtxR or DtxR(E175K) was added to 0.5 μg of plasmid pJL1 DNA (which carries the luciferase gene, luc, under transcriptional regulation of the tacP-toxO sequence) along with amino acids and premix buffer to S30 extracts of E. coli (Promega). DP was also added to various concentrations ranging from 10 to 50 μM. These components were incubated at room temperature for 10 min prior to the addition of the S30 extracts. Reaction mixtures were incubated at 37°C for 1 h, and luciferase activity was then assayed with a Turner TD-20/20 luminometer.
ANS binding.
The binding of 8-anilino-1-naphthalenesulfonic acid (ANS) (Acros) to purified DtxR and DtxR(E175K) was assayed by published protocols (24). In brief, 5 μM purified protein was mixed with 500 μM ANS in 20 mM Tris-Cl, pH 7.5. ANS binding was measured with a Perkin-Elmer fluorimeter using emission at 500 nm after excitation at 365 nm. With constant stirring, a solution of CoCl2 was used to titrate the purified repressor-ANS reaction mixture. Following the addition of each aliquot, the mixture was allowed to equilibrate without excitation for 10 s before measurement of ANS fluorescence. The end point of the titration was determined by making the final [Co2+] equal to 1 mM.
RESULTS
In vivo activity of DtxR(E175K).
As reported recently (7), we have developed an improved in vivo assay system to measure the repressor activity of DtxR. The E. coli strain DH5α(λRS45 tacP-toxO-lacZ) has an integrated copy of the lacZ gene regulated by a fusion promoter-operator, tacP-toxO. We recently showed (7) that this hybrid promoter-operator lacZ transcriptional fusion increases the level of reporter gene expression ca. 70-fold compared to the native toxPO-lacZ transcriptional fusion in recombinant E. coli. As a result of this increased reporter gene expression, subtle changes in repressor activity can now be measured. We hypothesized that this new reporter system would provide a better understanding of the in vivo activity of DtxR(E175K) than was available at the time of its isolation.
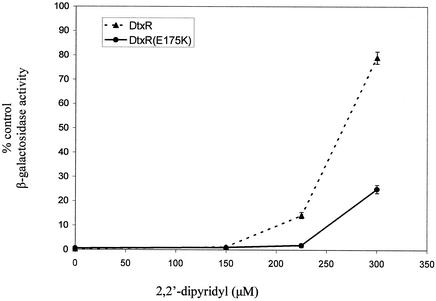
As shown in Fig. 1, both DtxR and DtxR(E175K) are able to repress the expression of lacZ when cultures of transformed DH5α(λRS45 tacP-toxO-lacZ) were grown in LB media. Compared to cultures expressing no DtxR (pROM), DtxR and DtxR(E175K) repressed the expression of β-galactosidase to only 0.3 and 0.5% of control levels of expression, respectively (insignificant difference by paired t test). In contrast, when these cultures were grown in the presence of increasing concentrations of DP, a chelator of divalent transition metal cations, a distinct phenotypic difference between DtxR and DtxR(E175K) was observed. Although wild-type DtxR is nearly completely inactivated by 300 μM DP, DtxR(E175K) retains considerable repressor activity under these conditions (P < 10−6, paired t test).
FIG. 1.
During cation starvation in vivo, DtxR(E175K) retains greater repressor activity than wild-type DtxR. Cultures of DH5α(λRS45 tacP-toxO-lacZ) transformed with pROM, pROM-dtxR, or pROM-dtxR(E175K) were grown overnight in LB-AMP-KAN with various concentrations of DP. The β-galactosidase activity of each of four independent cultures was assayed for each construct at each concentration of DP. Because DP alters the basal expression of lacZ in this strain, data are presented as the percentage of control β-galactosidase activity compared to that in cultures expressing no repressor (pROM). Data are means ± standard deviations.
This observation is comparable to those made at the time of isolation and in vivo characterization of DtxR(E175K). However, the increased sensitivity of the new reporter system showed that, contrary to earlier observations, the activity of DtxR(E175K) is weakly sensitive to the concentration of Fe2+ in the growth medium. In the presence of 300 μM DP, DtxR(E175K) is partially inactivated compared to cultures grown in the absence of the chelator. This observation suggested that, like wild-type DtxR, DtxR(E175K) is a cation-dependent repressor; however, the mutant retains repressor function at Fe2+ concentrations well below the threshold required to activate wild-type DtxR.
In vitro repressor activity of DtxR(E175K) demonstrates decreased metal ion dependence.
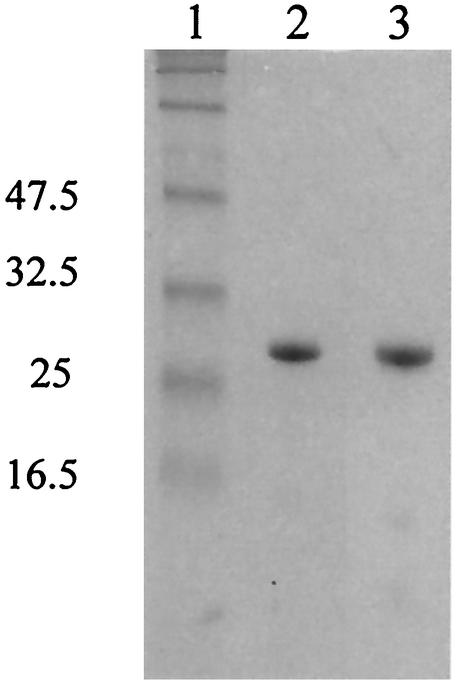
Because in vivo experiments provide many confounding variables, DtxR(E175K) was purified from E. coli HMS174(DE3)/pET-dtxR(E175K). The protocol used for the purification of DtxR(E175K) largely paralleled that used to purify wild-type DtxR, except that fermentation culture growth utilized M9 minimal medium. After a series of chromatographic purification steps, including DEAE ion-exchange chromatography, nickel affinity chromatography, and HPLC anion-exchange chromatography, densitometric analysis of Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels suggested that DtxR(E175K) was approximately 94% pure (Fig. 2).
FIG. 2.
Polyacrylamide gel electrophoresis demonstrating purification of DtxR(E175K). Lane 1, molecular mass standards; lane 2, purified wild-type DtxR; lane 3, HPLC-purified DtxR(E175K). Molecular masses are on the left, in kilodaltons.
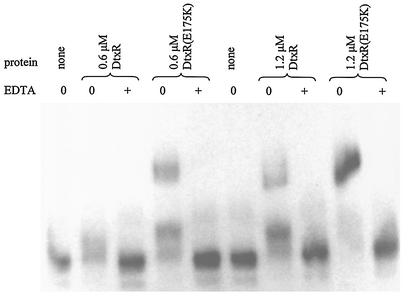
Initial in vitro experiments using purified DtxR(E175K) were EMSAs using a 32P-labeled toxPO probe as previously described (7, 22). As shown in Fig. 3, DtxR(E175K) was found to be cation dependent for binding to the toxPO probe, as the chelator EDTA was able to completely abolish DNA-binding activity. Unfortunately, EMSAs with DtxR must be performed in the presence of activating Mn2+ in the reaction mixture, gel, and buffer; in the absence of these conditions, the protein-DNA complex dissociates (22). The same behavior was observed with purified DtxR(E175K) (data not shown). As a result, EMSA is unable to detect subtle changes in cation dependence between wild-type DtxR and DtxR(E175K).
FIG. 3.
EMSA demonstrates the cation dependence of DNA binding by both wild-type DtxR and DtxR(E175K). All reactions were performed with 1.25 nM 32P-labeled toxPO probe, and 125 μM Mn2+ was present in reaction mixtures, gel, and buffer. The final concentration of EDTA was 1 mM.
We have described a coupled in vitro E. coli S30 extract transcription-translation assay for investigating the in vitro repressor activity of DtxR and DtxR mutants (7). In this system, S30 extracts of E. coli are programmed with purified pJL1 plasmid DNA, which carries the luciferase gene (luc) regulated by the tacP-toxO hybrid promoter-operator. The in vitro production of luciferase can then be readily measured as a function of DtxR repressor activity. Although limited by conditions necessary for transcription and translation, this assay system allows quantitative comparison of wild-type and mutant DtxR under carefully controlled levels of DP, transition metal ions, and repressor concentrations.
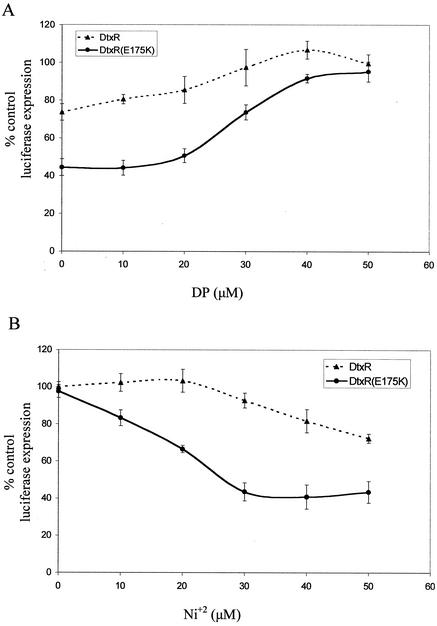
The in vitro activities of DtxR and DtxR(E175K) were measured at different DP concentrations by using the in vitro coupled transcription-translation assay. In reaction mixtures containing wild-type DtxR, luciferase expression was found to increase at lower DP concentrations than in parallel reactions conducted with DtxR(E175K) (Fig. 4A). To confirm this dependence, the inverse experiment was performed: the repressor activities of both DtxR and DtxR(E175K) were assayed at an equivalent, inactivating concentration of DP (55 μM) in the absence and presence of increasing concentrations of Ni2+. As expected, DtxR(E175K) was found to repress luciferase expression at significantly lower Ni2+ concentrations than DtxR (Fig. 4B). One possibility that we cannot eliminate is that the exogenously added Ni2+ did not itself activate DtxR in these assays but rather liberated Fe2+ from DP chelation. This would explain the variability in total DtxR activation we have observed between different lots of S30 extracts. Similar experiments, with identical outcomes, were performed with Co2+ and Mn2+ (data not shown). These results are consistent with the hypothesis that the E175K mutation in DtxR results in a decrease in the threshold concentration of transition metal ion necessary for activation of the apo-repressor.
FIG. 4.
In vitro, DtxR(E175K) is active at lower metal ion concentrations that wild-type DtxR. Coupled in vitro transcription-translation reactions using pJL1 and 1 μg of either DtxR or DtxR(E175K) were performed. (A) Increasing concentrations of DP were added to the reaction mixtures. (B) All reactions took place in the presence of 55 μM DP, and various concentrations of NiCl2 were added. Data are percent luciferase expression in S30 extracts in the presence of equivalent DP or DP-Ni2+ in the absence of repressor. Data are the means of four independent reactions ± standard deviations.
ANS binding: protein conformation as a function of metal ion concentration.
Recent studies of DtxR have suggested that the N-terminal domain of the apo-repressor possesses the characteristics of a molten-globule structure (25). ANS is an intrinsically fluorescent molecule whose fluorescence increases when it interacts nonspecifically with hydrophobic domains of proteins. Because molten globules expose additional hydrophobic faces compared to their fully folded structures, the fluorescence of ANS has been used to probe protein conformation (25). The activation of apo-DtxR upon metal ion binding has been shown to decrease significantly the fluorescence of ANS, suggesting an overall gain of protein structure (25).
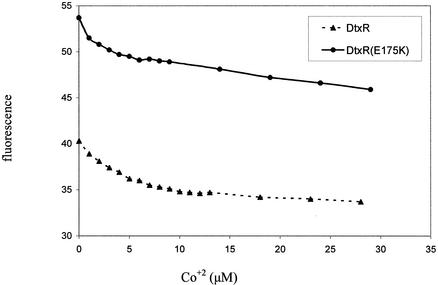
We hypothesized that apo-DtxR and apo-DtxR(E175K) would bind ANS differently, especially in response to small changes in metal ion concentrations. To compare the effect of metal ion on the structures of apo-DtxR and apo-DtxR(E175K), ANS fluorescence as a function of Co2+ concentration was measured. As shown in Fig. 5, in the absence of transition metal ions, identical concentrations of apo-DtxR and apo-DtxR(E175K) bound different amounts of ANS. The hyperactive mutant form of the repressor demonstrated increased binding of ANS compared to wild-type DtxR, suggesting that DtxR(E175K) has an increased number of hydrophobic surfaces exposed to solvent. The E175K mutation may partially stabilize an intermediate in the molten-globule form of the apo-repressor such that hydrophobic faces are less transient. Alternatively, the E175K mutation may instead produce an organization of the dimerization domain, a large hydrophobic surface found at the interface of two activated monomers (16). Importantly, both DtxR and DtxR(E175K) demonstrated parallel decreases in ANS binding upon the addition of increasing concentrations of the activating transition metal ion Co2+. Similar results were found when Ni2+ was added as the activating metal ion (data not shown).
FIG. 5.
ANS binding. The ordered structure of DtxR and DtxR(E175K) was studied by using the intrinsic fluorescence of ANS. With increasing concentrations of Co2+, both proteins underwent a decrease in ANS binding, demonstrated by the decrease in fluorescence.
The role of the primary and ancillary metal ion-binding sites in the activity of DtxR(E175K).
Both biophysical and biochemical data have suggested that DtxR has two separate metal ion-binding sites (3, 15, 16). The primary site (site 2) is comprised of the side chains of M10, C102, E105, and H106, the carbonyl group of C102, and a structural water molecule. All of the X-ray crystal structures that have been described to date agree that the ancillary cation-binding site (site 1) is composed of the side chains of H79, E83, and H98. Although some structures have suggested a role for the C-terminal domain residues E170 and Q173, confirming biologic data on these sites have yet to be published. Previous work in this laboratory has proposed that the primary metal ion-binding site is essential to the activation of DtxR, hypothesizing that the structural changes necessary for DNA binding accompany cation occupancy of this site (3). That work, however, could not exclude a facilitating role for the ancillary site due to a hydrogen bond network between the two sites.
Given the hypothesis that the E175K mutation in DtxR lowers the threshold of transition metal ion concentration necessary to bring about repressor activation, we reasoned that mutations in the primary and ancillary cation-binding sites might help explain the E175K phenotype. Each residue of the primary and ancillary metal ion-binding sites was individually mutated to alanine in both DtxR and DtxR(E175K) backgrounds. The in vivo activity of the resultant mutant repressors in DH5α(λRS45 tacP-toxO-lacZ) was then measured in the presence and absence of the chelator DP. Not surprisingly, mutations in the primary metal ion-binding site (M10A, C102A, E105A, and H106A) completely abolished the in vivo activity of both DtxR and DtxR(E175K) (data not shown). These observations are consistent with the hypothesis that the primary site is essential for the structural reorganization of the N-terminal domain that is necessary for operator DNA recognition and binding.
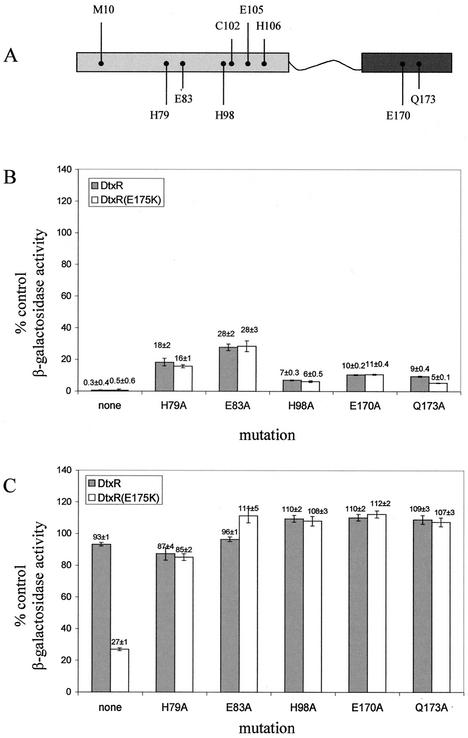
Alanine substitution in each of the ancillary-site residues displayed various effects on the in vivo repressor activity. As shown in Fig. 6, in the absence of DP, the E83A mutation had the largest effect on repressor activity in both DtxR and DtxR(E175K) backgrounds, while the H98A mutation only minimally affected activity. Interestingly, the two proposed C-terminal SH3-like domain ligands, E170 and Q173, demonstrated an intermediate effect. Although the activities of DtxR and DtxR(E175K) were equally affected by each of these five mutations, DtxR(E175K) consistently displayed increased repressor function in these mutations. This hierarchy was observed in the DtxR(E175K) background as well, although the effect of each mutation was somewhat diminished in the DtxR(E175K) background compared to the wild-type background. Only the difference between DtxR(Q173A) and DtxR(Q173A,E175K) achieved statistical significance (P < 0.05, paired t test).
FIG. 6.
Mutations in the ancillary cation-binding site affect DtxR and DtxR(E175K) similarly. (A) Schematic representation of DtxR, showing the larger N-terminal domain (light grey) attached to the C-terminal domain (dark grey) via the flexible tether (line). Residues comprising the primary metal ion-binding site (site 2) are labeled above the diagram, while residues making up the ancillary metal ion-binding site (site 1) are labeled below the diagram. Each of these labeled residues was mutated to alanine in both wild-type DtxR and DtxR(E175K) backgrounds. (B and C) Cultures of DH5α(λRS45 tacP-toxO-lacZ) transformed with plasmids derived from pROM-dtxR or pROM-dtxR(E175K) were grown overnight in LB-AMP-KAN either without (B) or with (C) 300 μM DP and assayed for β-galactosidase activity. pROM, which does not encode DtxR, was used as a control. Data are means ± standard deviations for four cultures.
In contrast, when these ancillary-site mutations in DtxR and DtxR(E175K) were grown in the presence of the transition metal ion chelator DP, the importance of the ancillary site for the hyperactive E175K phenotype was demonstrated. As shown in Fig. 6, a substitution of any of the ancillary-site residues with alanine abolishes the ability of DtxR(E175K) to bind to the toxO and repress lacZ expression in the presence of DP. As a result, the gain-of-function phenotype of DtxR(E175K) is lost, and the double mutants display the native DtxR phenotype in iron-limited media. A functional ancillary cation-binding site, therefore, is essential for the observed in vivo phenotypic difference between DtxR and DtxR(E175K).
DISCUSSION
The experiments described here were designed to probe the phenotypic differences between DtxR and the hyperactive mutant DtxR(E175K). The E175K mutation was originally isolated from an in vivo genetic screening system, PSDT (for “positive selection of DtxR alleles and targets”), designed to identify mutations in DtxR that led to an iron-insensitive phenotype (21). In vivo experiments originally suggested that DtxR(E175K) was constitutively active and insensitive to the presence of 300 μM DP in the growth medium. In E. coli, the corynebacterial promoter toxPO drives only modest levels of reporter gene expression. Therefore, strains carrying the toxPO-lacZ transcriptional fusion are relatively insensitive and provide only limited information about subtle changes in repressor activity. With the recent development of reporter strains that carry the tacP-toxO hybrid promoter-operator and express 70-fold-higher levels of reporter β-galactosidase activity, we have been able to detect and measure subtle functional changes accompanying some mutations in DtxR (7). For example, only with the use of E. coli DH5α(λRS45 tacP-toxO-lacZ) did the small but detectable transition metal ion dependence of DtxR(E175K) first become noticeable.
As described in this work, in vitro experiments using purified DtxR(E175K) have provided data consistent with the hypothesis that the E175K mutation lowers the threshold of transition metal ion concentration that is required for the conversion of the apo-repressor to its fully activated state. Through the use of a coupled in vitro transcription-translation assay system, we have now examined the relationship between transition metal ion concentration and activation of repressor function. These experiments clearly demonstrate the conversion of apo-DtxR(E175K) to a fully activated repressor at metal ion concentrations insufficient to activate wild-type DtxR. Moreover, comparative ANS binding assays suggest that, relative to wild-type apo-DtxR, apo-DtxR(E175K) is likely to adopt a somewhat different tertiary structure. Further, upon addition of transition metal ions, DtxR(E175K) undergoes structural rearrangements paralleling those of wild-type DtxR. Taken as a whole, these observations support the argument that the E175K substitution in DtxR results in a decrease of the threshold level of transition metal ion required for repressor activation.
When the constitutively active DtxR(E175K) was originally described (21), several hypotheses for its unusual phenotype were suggested. Experiments reported here confirm earlier in vivo observations; however, it is now clear that chelation with DP at concentrations low enough not to prohibit bacterial growth (e.g., 300 μM) fail to remove trace levels of iron from intact bacterial cells. While these trace iron levels are insufficient to activate wild-type apo-DtxR, the lower activation threshold of apo-DtxR(E175K) allows its activation under these conditions.
In vivo experiments using site-directed mutations in both the primary and ancillary metal ion-binding sites of DtxR and DtxR(E175K) clearly point to an important role for the ancillary cation-binding site in the phenotype of DtxR(E175K). Previous studies of wild-type DtxR have indicated that the ancillary site was not essential for DtxR activation (3). Only with the development of an improved in vivo assay system has the contribution of the ancillary site to DtxR activation become discernible (7). We have demonstrated here that the alanine substitution mutation of any of the residues comprising the ancillary cation-binding site (H79, E83, H98, E170, and Q173) abolishes the phenotypic distinction between DtxR and DtxR(E175K). Inherent in this observation is the suggestion that the ancillary metal ion-binding site is indeed able to affect the activation of DtxR. In fact, recent studies with DtxR(H79A) suggest that in the absence of a functional ancillary cation-binding site, higher concentrations of transition metal ion are necessary for repressor activation (A. D'Aquino, J. F. Love, J. Tetenbaum, J. R. Murphy, and D. Ringe, unpublished data). These observations are consistent with the hypothesis that the transition from apo-DtxR to fully activated repressor occurs through a multiple-step pathway. Because DtxR activation occurs as a result of cation coordination by the primary site, the ability of the E175K mutation to affect repressor cation sensitivity through the ancillary site strongly argues for a cooperative interaction between the two cation-binding sites.
Since the first reports of DtxR's crystal structure in 1995, a growing body of structural work on this repressor has been published. X-ray crystal studies of DtxR have included crystals complexed with a number of different cations (Ni2+, Co2+, Mn2+, Cd2+, and Zn2+) (14-16), cocrystals with operator DNA (2, 27), and structures of mutants of DtxR (10). Although real insight has been gleaned from a number of these structures (notably the DtxR-Ni2+-toxO holostructure (2, 27) and the structures of the C-terminal SH3-like domain (14, 15), many of the suppositions based on structural data alone await biological confirmation. For example, the data reported here are the first to demonstrate a functional significance for the two ancillary-binding-site ligands (E170 and Q173). The proposed role of sulfate or phosphate as a corepressor awaits direct functional analysis.
Within the population of structural data, there exists variability in the structure of amino acid side chains in the vicinity of the ancillary cation-binding site. While all structures agree that H79, E83, and H98 comprise ligands of this site, the two C-terminal ligands (E170 and Q173) from the SH3-like domain of the repressor have not been consistently observed coordinating at this site. The proposed anion-binding site (4, 14) is located adjacent to the ancillary cation-binding site, but in the structures that demonstrate a bound sulfate or phosphate, variability in its coordination is seen. Despite the structural promiscuity around the ancillary cation-binding site, a network of hydrogen bonds and salt bridges consistently connect it to residues of the primary cation-binding site. Thus, regardless of the precise structure in this region, communication between the two sites appears to be possible, permitting a cooperative relationship.
While the precise mechanism of how the E175K mutation impacts the activity of the ancillary cation-binding site of DtxR remains unclear, the proximity of the mutation to two ancillary-site ligands suggests an intimate relationship with this cation-binding site. The simplest explanation is that the side chain of K175 is able to increase the affinity of metal ion binding at the ancillary site. Metal ion dissociation from the ancillary site might be slower, resulting in DtxR(E175K) existing for longer periods in a conformation more favorable for activation. We initially raised the possibility that the positively charged lysine side chain could act as an iron mimetic at the ancillary site (21). However, if such an interaction were responsible for the unusual phenotype of DtxR(E175K), it is likely that individual residues in the ancillary site would demonstrate a hierarchy of importance when metal ions are chelated. In addition, the site-directed mutation of the negatively charged glutamates would be expected to be more deleterious than mutation of the neutral histidines or glutamine. Instead, all mutants behave identically when grown in the presence of DP. Furthermore, ancillary-site mutations in DtxR and DtxR(E175K) grown without DP demonstrate remarkable similarity, suggesting that this site has the same function in both the wild-type and mutant repressors.
The data presented here also suggest a more intimate role of the C-terminal SH3-like domain in the metal ion-mediated activation of DtxR. We report biological evidence that the C-terminal SH3-like domain of DtxR does in fact contribute two ligands to the ancillary cation-binding site. In addition, it appears that certain residues in this domain (e.g., position 175) are able to modulate repressor activation by affecting behavior of at least the ancillary cation-binding site. Others have reported the SH3-like fold found in the C-terminal domain (14, 26), as well as the ability of this isolated domain to bind in vitro a proline-rich peptide mimicking residues 125 to 139 of DtxR (26). The elucidation of the overall biological role of the C-terminal SH3-like domain in the activation of DtxR remains a focus of active research in many laboratories.
Homologues of DtxR have been found in many pathogenic gram-positive microorganisms, including S. aureus (5), S. mutans (20), and T. pallidum (13). The closest relative to DtxR is IdeR from M. tuberculosis (19), and both metal ion-dependent repressors are believed to recognize similar operator sequences and to regulate similar regulons (8, 11, 19). In fact, when merodiploid M. tuberculosis cells were engineered to express DtxR(E175K), the resulting mycobacteria were significantly less virulent in mouse models of tuberculosis (8). This work highlights the potential for therapeutic modulation of virulence determinant expression. By studying the molecular mechanisms of transition metal ion activation of DtxR, we ultimately hope to identify new approaches for the modulation of gene expression and the development of novel methods for therapeutic intervention.
Acknowledgments
We thank Timothy Logan and Donald G. L. Caspar, Florida State University, for helpful discussions.
This work was supported by Public Health Service grant AI-21628 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Boyd, J., M. N. Oza, and J. R. Murphy. 1990. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. USA 87:5968-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, C. S., A. White, J. Love, J. R. Murphy, and D. Ringe. 2000. Methyl groups of thymine bases are important for nucleic acid recognition by DtxR. Biochemistry 39:10397-10407. [DOI] [PubMed] [Google Scholar]
- 3.Ding, X., H. Zeng, N. Schiering, D. Ringe, and J. R. Murphy. 1996. Identification of the primary ion-activation sites of the diphtheria tox repressor by X-ray crystallography and site-directed mutational analysis. Nat. Struct. Biol. 3:382-387. [DOI] [PubMed] [Google Scholar]
- 4.Goranson-Siekierke, J., E. Pohl, W. G. J. Hol, and R. K. Holmes. 1999. Anion-coordinating residues at binding site 1 are essential for the biological activity of the diphtheria toxin repressor. Infect. Immun. 67:1806-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horsburgh, M. J., S. J. Wharton, A. G. Cox, E. Ingham, S. Peacock, and S. J. Foster. 2002. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 44:1269-1386. [DOI] [PubMed] [Google Scholar]
- 6.Lee, J. H., T. Wang, K. Ault, J. Liu, M. P. Schmitt, and R. K. Holmes. 1997. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 65:4273-4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Love, J. F., and J. R. Murphy. 2002. Design and development of a novel genetic probe for the analysis of repressor-operator interactions. J. Microbiol. Methods 51:63-72. [DOI] [PubMed] [Google Scholar]
- 8.Manabe, Y. C., B. J. Saviola, L. Sun, J. R. Murphy, and W. R. Bishai. 1999. Attenuation of virulence in Mycobacterium tuberculosis expressing a constitutively active iron repressor. Proc. Natl. Acad. Sci. USA 96:12844-12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 10.Pohl, E., J. Goranson-Siekierke, M. K. Choi, T. Rooslid, R. K. Holmes, and W. G. J. Hol. 2001. Structures of three diphtheria toxin repressor (DtxR) variants with decreased repressor activity. Acta Crystallogr. Sect. D 57:619-627. [DOI] [PubMed] [Google Scholar]
- 11.Pohl, E., R. K. Holmes, and W. G. J. Hol. 1999. Crystal structure of the iron-dependent regulator (IdeR) from Mycobacterium tuberculosis shows both metal binding sites fully occupied. J. Mol. Biol. 285:1145-1156. [DOI] [PubMed] [Google Scholar]
- 12.Pohl, E., R. K. Holmes, and W. G. J. Hol. 1999. Crystal structure of a cobalt-activated diphtheria toxin repressor-DNA complex reveals a metal-binding SH3-like domain. J. Mol. Biol. 292:653-667. [DOI] [PubMed] [Google Scholar]
- 13.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu, X., E. Pohl, R. K. Holmes, and W. G. J. Hol. 1996. High-resolution structure of the diphtheria toxin repressor complexed with cobalt and manganese reveals an SH3-like third domain and suggests a possible role of phosphate as co-repressor. Biochemistry 35:12292-12302. [DOI] [PubMed] [Google Scholar]
- 15.Qiu, X., C. L. Verline, S. Zhang, M. P. Schmitt, R. K. Holmes, and W. G. J. Hol. 1995. Three dimensional structure of the diphtheria toxin repressor in complex with divalent cation co-repressors. Structure 3:87-100. [DOI] [PubMed] [Google Scholar]
- 16.Schiering, N., X. Tao, H. Zeng, J. R. Murphy, G. A. Petsko, and D. Ringe. 1995. Structures of the apo- and the metal ion-activated forms of the diphtheria tox repressor from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. USA 92:9843-9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt, M. P. 1997. Transcription of the Corynebacterium diphtheriae hmuO gene is regulated by iron and heme. Infect. Immun. 65:4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt, M. P., and R. K. Holmes. 1994. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J. Bacteriol. 176:1141-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt, M. P., M. Predich, L. Doukhan, I. Smith, and R. K. Holmes. 1995. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect. Immun. 63:4284-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spatafora, G., M. Moore, S. Landgren, E. Stonehouse, and S. Michalek. 2001. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology 147:1599-1610. [DOI] [PubMed] [Google Scholar]
- 21.Sun, L., J. C. vanderSpek, and J. R. Murphy. 1998. Isolation and characterization of iron-independent positive dominant mutants of the diphtheria toxin repressor DtxR. Proc. Natl. Acad. Sci. USA 95:14985-14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tao, X., J. Boyd, and J. R. Murphy. 1992. Specific binding of the diphtheria tox regulatory element DtxR to the tox operator requires divalent heavy metal ions and a 9-base-pair interrupted palindromic sequence. Proc. Natl. Acad. Sci. USA 89:5897-5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao, X., and J. R. Murphy. 1992. Binding of the metalloregulatory protein DtxR to the diphtheria tox operator requires heavy metal ion and protects the palindromic sequence from DNase I digestion. J. Biol. Chem. 267:21761-21764. [PubMed] [Google Scholar]
- 24.Tao, X., H. Zeng, and J. R. Murphy. 1995. Transition metal ion activation of DNA binding by the diphtheria tox repressor requires the formation of stable homodimers. Proc. Natl. Acad. Sci. USA 92:6803-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Twigg, P. D., G. Parthasarthy, L. Guerrero, T. M. Logan, and D. L. D. Caspar. 2001. Disordered to ordered folding in the regulation of diphtheria toxin repressor activity. Proc. Natl. Acad. Sci. USA 98:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, G., G. P. Wylie, P. D. Twigg, D. L. D. Caspar, J. R. Murphy, and T. M. Logan. 1999. Solution structure and peptide binding studies of the C-terminal Scr homology 3-like domain of the diphtheria toxin repressor protein. Proc. Natl. Acad. Sci. USA 96:6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White, A., X. Ding, J. C. vanderSpek, J. R. Murphy, and D. Ringe. 1998. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature 394:502-506. [DOI] [PubMed] [Google Scholar]