Intercellular communication via diffusible chemical signals is well described for bacteria and functions to modulate a number of cellular processes. The perception and interpretation of these signals enables bacteria to sense their environment, leading to the coordinate expression of genes. The result of this communication is the proper and appropriate response of the bacterial community to its surroundings (74). Intercellular responses resulting from such signaling include the control of competence, sporulation, and virulence factor production (4, 29, 76). In some cases, the signaling processes not only occur between microbial populations but also occur between bacteria and members of higher eukaryotes. The symbiotic association between rhizobia and legumes is an example of such an interaction (68).
Bacteria of the genera Rhizobium, Mesorhizobium, Azorhizobium, Sinorhizobium, and Bradyrhizobium have the ability to infect the roots of leguminous plants, causing the formation of a new organ (i.e., nodule) and establishing a nitrogen-fixing symbiosis. Infection of the plant requires the products of the bacterial nodulation genes, which encode for the production of a lipochitin nodulation signal. This Nod signal, upon recognition by the plant, induces de novo organogenesis leading to nodule formation. The bacteria invade the root through root hairs and penetrate inside a plant-produced infection thread. Upon endocytosis into an infected cortical cell, the bacteria are enclosed in a membrane-bound symbiosome where they differentiate into bacteroids that are capable of fixing N2 into a form that the plant can utilize. In return, the bacteroids are supplied with an environment rich in carbon as an energy source.
The nature of the rhizobial life cycle presents a number of survival challenges. The bacteria must exist as a saprophyte in the soil, competing against other bacteria for sustenance. When the rhizobium encounters the rhizosphere, this presents another environment, richer in nutrients but with its own unique environmental challenges. In the presence of a compatible host root, the individual rhizobial cell must compete with members of its own species to gain successful entry into the host root to initiate the symbiosis. Its environment then changes again so that it must adapt to the rigors of the in planta and finally intracellular lifestyle. Due to these successive changes, study of rhizobial ecology has continued to be a major area of research interest. This work has practical implications, since a commercial industry exists to provide rhizobial inoculants for agricultural production. For example, the fact that commercial inoculants generally compete poorly against indigenous rhizobia for nodulation is considered a major limitation to improving the agricultural use of biological nitrogen fixation.
In this review, we focus on the symbiotic association of B. japonicum with its legume hosts (primarily soybean), focusing on both signaling and the integration of regulatory processes involved in this association. Specifically, we highlight the regulation of the bacterial nodulation genes, the surprising complexity of this regulation, and the integration of this system with the overall physiology and ecology of the organism.
nod GENE REGULATION AND THE NodD PARADIGM
Signaling between legume and its bacterial symbiont is essential for the coordinate expression of both plant and bacterial genes. The bacterial nodulation genes (nod, nol, noe) encode a key set of proteins involved in the establishment of this symbiotic relationship (12, 68). The nod genes are expressed specifically in response to plant-produced flavonoid compounds. Central to the regulation of the nod genes is NodD, a LysR-type regulator, which activates nod gene expression only in the presence of the flavonoid inducer (26, 40). The NodD protein binds to the nod box sequence upstream of the nod genes (16, 23, 32) and induces DNA bending (17, 18; R. Fisher, personal communication), leading to transcriptional activation upon recognition of the inducer. The chaperonin GroESL system is necessary for proper folding of NodD into its DNA binding-competent form. In addition, binding of NodD to the promoter is also increased in the presence of flavonoids (78). In general, the nod box contains highly conserved regions of 7, 5, and 25 bp (61). DNA bending of nod promoters was also reported to involve a histone-like protein, Px, in Rhizobium leguminosarum (41). In this case, the association of Px with the nod promoter leads to increased nod gene transcription by NodD. Multiple isoforms of NodD have been identified in rhizobia, and these vary among the rhizobial species, ranging from three copies of NodD in Sinorhizobium meliloti (33) to five copies in Rhizobium tropici (72). In S. meliloti these NodD proteins respond to different groups of flavonoids, suggesting that NodD redundancy allows the bacterium to infect multiple hosts secreting a wide range of flavonoids (38, 50, 54).
Two NodD proteins (NodD1 and NodD2) with distinctly different expression patterns and function were identified in B. japonicum. NodD1 functions as a positive transcriptional activator and responds to plant-produced isoflavones (genistein and daidzein) (3, 26, 40). In contrast, NodD2 represses nod gene expression (21, 22). NodD1 is autoregulated and inducible by genistein and daidzein, a feature that is uniquely differently from the constitutive expression of NodD found in most Rhizobium sp. (67). This induction is mediated by a divergent nod box sequence 5′ of the nodD1 gene (73). In addition to genistein and daidzein, nodD1 is also induced by glucosylated isoflavones (e.g., 6-O-malonyldaidzin and 6-O-malonyl genistin) (67). These glucosylated derivatives are unable to induce the expression of nodYABC transcription, thus providing the plant the potential to specifically regulate nodD1 expression.
While NodD remains the central regulator in nod gene regulation, additional regulators are now known to modulate the output of the nodulation genes. For instance, in S. meliloti a second LysR type regulator, SyrM, regulates nod gene expression in a flavonoid-independent fashion (38, 50). In B. japonicum, key regulatory roles are accorded to members of two other families (i.e., two-component regulators and a MerR-type regulator).
NodD AND BEYOND
Two-component regulation.
The first suggestion that nod gene regulation involved transcriptional activators other than NodD1 arose from the discovery that B. japonicum NodD1 mutants were still able to nodulate host plants (25). Resolution of this paradox was provided by the subsequent finding that NodVW provided an alternative pathway for nod gene activation (65). Originally identified as a host-specific gene by Göttfert et al. (25), NodVW is essential for the nodulation of cowpea, siratro, and mungbean, but not soybean. A possible explanation for the host-specific function of NodVW is that it specifically recognizes flavonoids produced by cowpea, siratro, and mungbean, but not soybean. In doing so, it provides B. japonicum the flexibility to nodulate a broader range of plants. Alternatively, the infection of cowpea, siratro, and mungbean may demand higher levels of Nod signal production than that needed for soybean infection. The combined efforts of NodD and NodVW would be necessary for increased Nod signal synthesis.
NodV and NodW represent members of the classical two-component regulatory family (25, 69) (Fig. 1). On detection of an environmental stimulus, autophosphorylation of the sensor kinase (i.e., NodV) occurs with subsequent transfer of the phosphoryl group to its cognate response regulator protein (i.e., NodW) (43). The phosphorylated response regulator is then primed to activate its target genes (nodYABC operon of B. japonicum). Phosphorylation of NodW, which likely occurs at the Asp70 residue in response to the isoflavonoid genistein, is critical for nod gene expression and nodulation. B. japonicum strains expressing NodWD70N (i.e., NodW that contains an aspartate-to-asparagine change at residue 70 and is therefore incapable of being phosphorylated) showed reduced levels of nod gene expression and were unable to nodulate mungbean (43). In addition to NodW, B. japonicum possesses a second response regulator (i.e., NwsB), which controls the expression of the nodulation genes. NwsB is very similar to NodW, sharing 65% amino acid identity and a conserved helix-turn-helix DNA binding motif (28). Given this similarity, it is not surprising that NwsB was first identified by its ability to suppress the nodulation defect of a NodW mutant. This suppression was possible only under conditions where NwsB was overexpressed from a strong, constitutive promoter. Under these circumstances, NwsB likely serves as an alternate response regulator in nod gene expression, thereby compensating for the loss of NodW. Indeed, we recently showed that an NwsB mutant demonstrated reduced levels of nod gene expression in response to the isoflavonoid genistein (44). Activation of NwsB would require phosphorylation by a sensor kinase. While this function is probably carried out by its cognate kinase NwsA, an additional sensor kinase(s) appears to activate NwsB. This view stems from work by Groß et al. (28), who discovered that a nodW nwsA double mutant overexpressing NwsB was still able to nodulate cowpea, siratro, and mungbean. Cross-talk between similar systems is possible, and given the similarity between NodW and NwsB, a likely candidate would be the isoflavonoid-perceiving sensor kinase NodV (25, 43, 65). The role of NwsB also appears to extend beyond the activation of the nodulation genes. More recently, we demonstrated that NwsB was involved in the quorum regulation of the nodulation genes (see below).
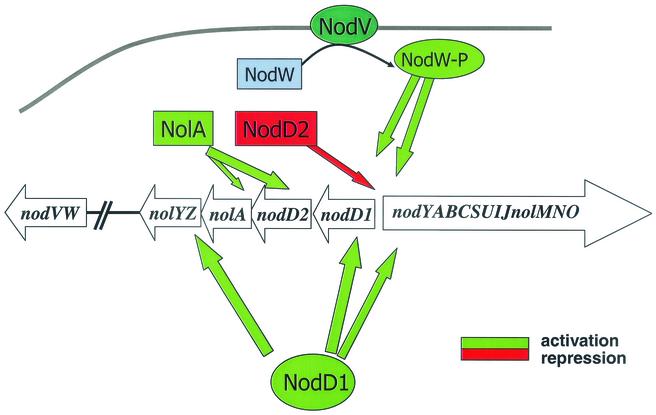
FIG. 1.
Model illustrating the key components involved in B. japonicum nod gene regulation. In response to genistein, the nod genes are activated by NodD1 and NodVW, resulting in the synthesis of the Nod signal. Negative regulation of the nod genes is mediated by NolA and NodD2. NolA regulates NodD2, which then represses the nod genes.
LysR and MerR regulators and their role in nod gene repression.
Although nod gene induction by flavonoids is required for nodulation, efficient nodulation occurs only when the nod genes are expressed in a fashion that is quantitatively, spatially, and temporally appropriate. Knight et al. (37) demonstrated that the strong, constitutive expression of the nodulation genes results in defective and reduced nodulation phenotypes on host plants. It is not surprising, therefore, that in addition to positive activation the nod genes are also subject to negative regulation (10, 15, 21, 39). In S. meliloti repression is mediated by NolR, while in Rhizobium sp. strain NGR234 and B. elkani NodD2 acts as a repressor. Mutations to these repressors result in aberrant and delayed nodulation phenotypes (15, 39). In B. japonicum, NolA and NodD2 form two key components in the negative regulation of the nodulation genes. Under appropriate conditions NolA induces NodD2 expression, which in turn represses the nodulation genes (Fig. 1).
NolA was first identified by Sadowsky et al. (62) and was classified as a genotype-specific gene since it was required for B. japonicum to nodulate restrictive soybean genotypes. NolA is a member of the MerR family of transcriptional regulators. Common among MerR members is the fact that the target genes contain promoters where the −35 and −10 sequences are separated by 19 bp rather than the usual 16 to 17 bp (30, 31, 55). As a result, no transcriptional activity would occur as both the −35 and −10 sequences could not simultaneously contact the RNA polymerase. Appropriate alignment of the −35 and −10 sequences occurs by DNA bending in response to interaction with the MerR transcriptional activator (2, 70).
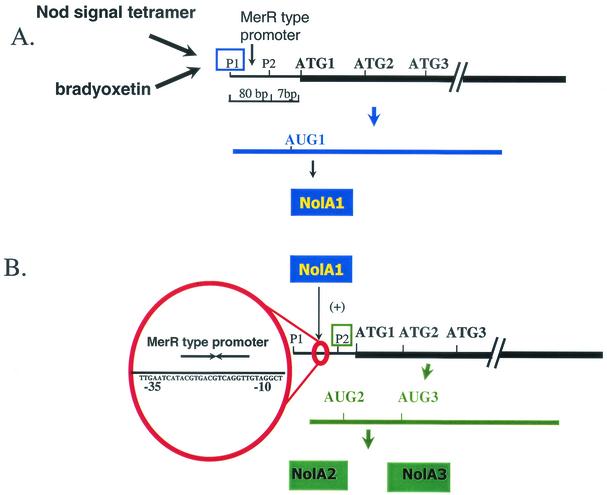
A unique feature of the nolA gene is that it encodes for three functionally distinct proteins (i.e., NolA1, NolA2, and NolA3) derived from three in-frame ATG initiation sites (47) (Fig. 2). Of the three proteins, only NolA1 possesses the N-terminal helix-turn-helix DNA binding motif. NolA1 regulates the expression of both NolA2 and NolA3. Expression of NolA1 is driven from a promoter, P1, located upstream of ATG1. NolA2 and NolA3 are obtained from translation of a second transcript derived from promoter P2. Consistent with the fact that NolA1 regulates NolA2 and NolA3, promoter P2 contains a putative NolA1 binding site similar to that found in MerR-type promoters. The exact functions of both NolA2 and NolA3 are less clear. Strong expression of NolA2 appeared to reduce nodulation efficacy on soybean, while NolA3 was essential for nodulation of the same host (47). It is possible that NolA2 may function to modulate gene expression with respect to NolA3 function. In this regard, it is interesting that a stem-loop structure surrounds the NolA2 translation initiation site. NolA2, by interacting with the stem-loop structure, could conceivably reduce NolA3 levels, thereby affecting nodulation. Clearly the presence of multiple forms of NolA suggests that nolA plays a key role in allowing B. japonicum to nodulate its plant hosts.
FIG. 2.
Regulation of NolA proteins. In the presence of the appropriate signal, nolA is transcribed from P1. This transcript is translated from AUG1, resulting in the synthesis of NolA1. NolA1 likely binds to the MerR promoter upstream of P1, resulting in the transcription of transcript 2. NolA2 and NolA3 are then obtained by translation from AUG2 and AUG3.
An interesting feature of the MerR regulators is that members of this family regulate genes in response to toxic compounds (1, 27, 31, 53). This raises the possibility that the genotype-specific properties (62) accorded to NolA may involve the detoxification of toxic compounds produced by certain soybean genotypes. Hypothetically, NolA expression could be induced in response to the toxin, leading to the expression of NolA3, which could sequester the compound to reduce toxicity. A similar model has been proposed for the MerR family protein TipA (31). In this case, TipA1 is the regulator, while TipA2 has been postulated to sequester the antibiotic thiostrepton, reducing the toxicity of this molecule.
In addition to regulating NolA2 and NolA3, NolA1 is also involved in controlling the expression of NodD2. Akin to the regulation of NolA2 and NolA3, a putative NolA1 binding site is present upstream of nodD2. As previously mentioned, NodD2 is a known repressor of the nodulation genes (15, 21, 22). Thus, NolA and NodD2 form key components in regulating the negative expression of the nodulation genes. The importance of both proteins is apparent in feedback regulation as well as quorum regulation of the nodulation genes.
FINE-TUNING EXPRESSION OF THE NODULATION GENES
Feedback regulation of the nodulation genes.
Feedback regulation is a common mechanism by which bacteria regulate the output of biosynthetic operons (for examples see references 5, 13, 24, and 77). This feedback regulation, which usually occurs in response to the biosynthetic end products, functions primarily to reduce or prevent unwanted production of these products. Recently we showed that the nodulation genes of B. japonicum are also feedback regulated by the Nod signal or components of the Nod signal (46). In B. japonicum the Nod signal produced contains either a tetrameric or pentameric chitin backbone (9). Curiously, only Nod signals containing a tetrameric backbone are able to feedback regulate the nodulation genes. This dependence on tetrameric chitin was demonstrated in several ways. First, the addition of exogenous chitin tetramer reduced the levels of nod gene expression. Little effect was noted with either chitin pentamer or other chitin oligomers tested (n = 1, 2, 3, 5, and 7). Second, only B. japonicum transconjugants expressing the nodC gene of S. meliloti showed reduced levels of nod gene expression, with no effect noted with transconjugants expressing the Sinorhizobium sp. strain NGR234 nodC gene. Work by several groups previously showed that the length of the Nod signal chitin backbone was determined primarily by the specific NodC chitin synthase (35, 60). Thus, while the NodC protein of S. meliloti produced tetrameric chitooligosaccharides, the Sinorhizobium sp. strain NGR234 NodC synthesized pentameric equivalents (6, 9, 58, 64). Since B. japonicum produces both tetrameric and pentameric Nod signals (9), our results suggest that B. japonicum senses only the levels of the chitotetraose molecules, which would only exceed the critical threshold when Nod signal synthesis was maximal. A high level of tetrameric Nod signal results in increased expression of NolA and NodD2, which then represses the nodYABC operon. The importance of this finding is reflected in results of Knight et al. (37), where overexpression of the Nod signals was found to result in delayed and aberrant nodule phenotypes. Indeed, aberrant nodule phenotypes were also found in legume plants infected with strains mutated in nolA or nodD2 (21, 22). Feedback regulation, through fine regulation of Nod signal synthesis, forms a key regulatory mechanism required for efficient and effective nodulation.
Quorum regulation of the nodulation genes.
The rich environment of the rhizosphere can support large bacterial populations. Therefore, it is not surprising that the nod genes were recently discovered to be regulated in a population density-dependent fashion (48). This population dependence is similar to quorum sensing regulation found for gram-negative and gram-positive bacteria. Quorum regulation evokes population on the basis of behavioral changes in response to levels of diffusible, bacterium-produced molecules. Typically, homoserine lactones (AHLs) play this role in gram-negative bacteria (20, 52, 75, 76) while modified peptides are the quorum signal in gram-positive bacteria (36). These molecules accumulate with increasing population density and trigger gene expression when threshold levels are obtained (i.e., when the bacterial population is quorate). AHL-dependent LuxI/LuxR quorum regulatory systems have been identified in many bacterium-plant interactions (45). The importance of LuxI/LuxR was originally elucidated in Vibrio fischeri (51). LuxI synthesizes the AHL signal, while LuxR recognizes this signal and regulates transcription of quorum-controlled genes. To date, some evidence points to quorum control as a mode of regulation in rhizobium-plant symbiosis (45). For example, AHLs have been identified in a number of rhizobial strains, and multiple AHLs are produced by rhizobial species (7). In addition, several LuxRI-type loci have been identified (e.g., two Lux-type loci in S. meliloti [51]) and, in some cases, have been shown to affect symbiotic phenotypes, such as nodule number and symbiotic nitrogen fixation (11).
Examination of nod gene expression revealed that the nodulation genes are maximally induced by genistein at low population densities, and induction is significantly reduced at high cell population densities (48). However, this apparent quorum regulation is not mediated by LuxI/LuxR but by NolA and NodD2 (48) (Fig. 3). Consistent with this, both NolA and NodD2 accumulate with increasing population density. In addition, nolA mutations result in derepression of the nodYABC operon, even at high culture densities. This population density dependence is mediated by an extracellular signal molecule (termed CDF, for cell density factor; see below) that accumulates in high-population cultures. More recently a two-component regulator, NwsB, was shown to mediate this population dependence (44) (Fig. 3). Both nolA and nodD2-lacZ expression remained low in NwsB mutant cultures grown to a high population density. In line with this, the levels of nodY-lacZ induction by genistein were unaffected by changes in population density. It is interesting that NwsB is important for both nod gene induction and the population density repression of the nodulation genes. In this regard, NwsB may function as a switch that results in either activation or repression of nod gene expression. Loh et al. (44) demonstrated that the population density defect of the NwsB mutant could be suppressed by constitutive expression of NodW. Moreover, suppression occurred with both NodW and the nonphosphorylated NodWD70N mutant protein (see above). Thus, while phosphorylation of NodW is requisite for nod gene induction, phosphorylation was not essential for quorum expression of nolA and nodD2. We speculate that a similar phosphorylation requirement occurs with NwsB, with the phosphorylation state of NwsB determining its role in either activation or quorum repression of the nod genes.
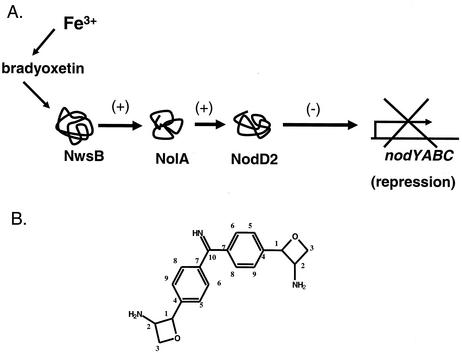
FIG. 3.
(A) Components involved in the population density-dependent regulation of the nod genes. In response to bradyoxetin, NwsB regulates the expression of NolA and NodD2. The expression of NodD2 leads to the repression of the nodYABC operon in B. japonicum. In addition, expression of bradyoxetin is known to be regulated in response to iron. The addition of iron leads to reduced NolA expression and a concomitant increase in nod gene expression. (B) Structure of bradyoxetin.
It is well established that the nodulation genes are not expressed in nodules (66). However, the mechanism for this repression was unknown. Loh et al. (48) rationalized that as bacteria are packed in symbiosomes in the nodule, this high cell-to-volume ratio would mimic an environment of high population density. This, in turn, would lead to quorum-based repression of the nod genes. Indeed, by using a nodY-GUS fusion the authors demonstrated that nod gene expression remained high in nodules infected with a NolA mutant. In contrast, the nod genes were repressed in wild-type nodules. Therefore, in planta control of the nodulation genes likely occurs via NolA-mediated quorum control. Conclusive evidence for the role of quorum control in bacteroids would, however, involve similar studies using a quorum mutant defective in CDF production (see below). To date, the genetics surrounding CDF production are not known and remain to be elucidated.
Nodulation efficiency: does more inoculant equal increased nodulation?
One limitation to the use of commercial rhizobium inoculant is the competition that inoculants face from indigenous soil bacteria. Though more efficient in nodulation, these inoculants seldom occupy more than a few percentages of nodules. One approach to overcome this competition would be to apply the commercial inoculants at high population densities. However, Lohrke et al. (49) showed that very high levels of B. japonicum inoculant actually resulted in significantly lower nodulation. The discovery that nod gene expression is modulated in a population density fashion has clear implications for inoculant use. A survey of commercial inoculants, which are prepared with bacteria grown in large fermentors to high population densities, revealed that all of those tested contained significant levels of a nolA inducer (CDF) (48). Therefore, it is likely that these inoculants show reduced nodulation efficacy due to the action of NolA and NodD2, which would reduce the level of nod gene expression. If true, then a B. japonicum strain insensitive to the nolA (quorum) signal should nodulate better than the wild type at high population density. Indeed, competition assays with the population-insensitive B. japonicum NwsB mutant revealed that, at high population densities, this mutant was better able to compete with the wild type for nodule occupancy (44).
Bradyoxetin: a new symbiotic signal.
A small molecule, CDF, that accumulates in the conditioned medium of B. japonicum cultures was shown to induce NolA expression. Preliminary structural and molecular analysis of the molecule suggests that it was unique and neither an AHL nor a modified peptide. The chemical structure of CDF was recently elucidated and was shown to be 2-(4-{[4-(3-aminooxetan-2-yl) phenyl](imino) methyl}phenyl)oxetan-3-ylamine, otherwise designated bradyoxetin (42) (Fig. 3B). B. japonicum joins a growing list of bacteria that utilize non-AHL-type signaling molecules. Examples of these molecules include 3-OH palmitic acid methyl ester of Ralstonia solanacearum (19), Pseudomonas aeruginosa PQS (2-heptyl-3-hydroxy-4-quinolone [57]), and Vibrio harveyi AI-2, a boron-containing furanone signal (8).
Bradyoxetin possesses an oxetane ring structure that is most similar to that of oxetin, an antibiotic produced by Streptomyces sp. strain OM2317, which can inhibit the growth of Bacillus subtilis and Piricularia oryzae (56). Oxetin also possesses herbicidal activity against both alfalfa and turnip. Given that NolA is a member of the MerR family (see above), it is interesting to speculate that bradyoxetin may, in addition to regulating nod gene activity, have antibiotic activity, inhibiting growth of competing bacteria in the rhizosphere. By analogy to other MerR regulators (e.g., TipA), NolA may regulate genes that allow B. japonicum to detoxify bradyoxetin, thus conferring a competitive advantage in the rhizosphere.
Besides oxetin, the structure of bradyoxetin is also similar to that of mugeneic acid, a known siderophore. Indeed, bradyoxetin synthesis was found to be iron regulated, with maximal production under iron-depleted conditions (42). Consistent with the fact that bradyoxetin regulates nod gene expression, a corresponding increase in nolA expression and a concomitant decrease in nod gene expression was noted under iron-starved conditions. This result is significant, as iron is essential for proper development of the root nodule (34). Iron is part of the active site of nitrogenase and is, therefore, essential for symbiotic nitrogen fixation. As discussed above, NolA is expressed in nodules and clearly represses in planta nod gene expression, likely due to the production of bradyoxetin by bacteroids inside the symbiosome. Therefore, the realization that iron controls bradyoxetin production argues that bacteroids live in an environment of low iron availability. Since nitrogenase clearly functions in bacteroids, the data argue that iron is quickly sequestered during bacteroid development resulting in low free-iron levels, which results in bradyoxetin synthesis. Does bradyoxetin function as a siderophore in symbiosomes? Unlike normal siderophores, experiments indicated that bradyoxetin has a very low affinity for Fe3+ (42). However, since the nodule is a low partial O2 environment, the iron present may be in a ferrous form and therefore a low-affinity siderophore (possibly bradyoxetin) may suffice.
Bradyoxetin: a common signal for α-Proteobacteria?
Assayed by the ability to induce nolA-lacZ expression, bradyoxetin activity appears to be widespread among Rhizobium species (42). Indeed, bradyoxetin activity was found in extracts of all α-Proteobacteria tested, suggesting that its synthesis may be a feature of this phylogenetic group. It is interesting that besides members that are plant symbionts, α-Proteobacteria also include known animal pathogens (e.g., Bartonella and Brucella spp.) that form similar symbiosome-like associations within host cells (63, 71). In this connection, a comparative analysis of fully sequenced genomes of α-Proteobacteria revealed the presence of sequences that are common among endosymbionts and plant and animal pathogens. Another common feature is the importance of iron for host invasion and growth. Indeed, animal pathogens possess a wide array of regulatory mechanisms to acquire and respond to this metal (59). Given these parallels, it is interesting to speculate that bradyoxetin-like molecules may also play key roles in host-pathogen interactions. Thus, bradyoxetin may not be just a rhizobial symbiotic signal but rather a chemical signal that is required for the association of other α-Proteobacteria with host cells.
CONCLUSION AND FUTURE DIRECTIONS
Proper expression of the nodulation genes is critical for the establishment of a nitrogen-fixing symbiosis. Therefore, it is not surprising to find that multiple mechanisms regulate and fine-tune B. japonicum nod gene expression. This regulation involves members of three global regulatory families (i.e., MerR, two-component, and LysR). Moreover, it also includes input from several signals derived from both symbiotic partners. For example, in addition to the plant-produced isoflavone nod gene inducers, feedback regulation by tetrameric Nod signals and response to iron and cell density affect nod gene regulation. Preliminary work in our laboratory indicates that a more productive association between B. japonicum and its legume host can be achieved by manipulating nod gene expression. For example, use of a population density-insensitive strain as inoculant resulted in more competitive nodulation. Analysis of B. japonicum nod gene control led to the discovery of bradyoxetin, a unique signaling molecule involved in symbiotic nitrogen fixation. Judged by the ability to induce a nolA-lacZ fusion, bradyoxetin-like molecules are common among α-Proteobacteria, some of which are intracellular pathogens. Therefore, it is interesting to speculate that bradyoxetin-like molecules may play a crucial role in the pathogenicity of these bacteria. Research on AHL-based quorum control has clearly identified these systems as targets for controlling bacterial pathogen infections (14). Therefore, bradyoxetin-mediated control systems could also make promising targets for disrupting infection by α-proteobacterial pathogens (e.g., Brucella spp.).
From the first discovery of the role of NodD in controlling B. japonicum nod gene transcription, models for nod gene control in this bacterium have steadily become more complex. This complexity likely arose from the need for this organism to adapt and survive in multiple environments and to finely control Nod signal production, which is critical to symbiotic invasion. Continuing research on the mechanism of gene control in B. japonicum holds promise not only for basic research discoveries but also for practical application to commercial inoculant production. With the growing realization of the commonality among pathogenic bacteria of plants and animals, discoveries, such as the discovery of bradyoxetin, may also affect our ability to fight disease.
Acknowledgments
This work was supported by National Science Foundation grant MCB-0108955 and by Liphatech Inc.
REFERENCES
- 1.Ahmed, M., C. M Borsch, S. S. Taylor, N. Vasquez-Laslop, and A. A. Neyfakh. 1994. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J. Biol. Chem. 269:28506-28513. [PubMed] [Google Scholar]
- 2.Ansari, A. Z., M. L Chael, and T. V. O'Halloran. 1992. Allosteric underwinding of DNA is a critical step in positive control of transcription by Hg-MerR. Nature 355:87-89. [DOI] [PubMed] [Google Scholar]
- 3.Banfalvi, Z., A. Nieuwkoop, M. Schell, L. Besl, and G. Stacey. 1988. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol. Gen. Genet. 214:420-424. [DOI] [PubMed] [Google Scholar]
- 4.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 5.Blasi, F., and C. B. Bruni. 1981. Regulation of the histidine operon: translation-controlled transcription termination (a mechanism common to several biosynthetic operons). Curr. Top. Cell Regul. 19:1-45. [DOI] [PubMed] [Google Scholar]
- 6.Carlson, R. W., J. Sanjuan, U. R. Bhat, J. Glushka, H. P. Spaink, H. W. Wijfjes, A. N. van Brussel, T. J. W. Stokkermans, N. K. Peters, and G. Stacey. 1993. The structures and biological activities of the lipo-oligosaccharide nodulation signals produced by type I and type II strains of Bradyrhizobium japonicum. J. Biol. Chem. 268:18372-18381. [PubMed] [Google Scholar]
- 7.Cha, C., P. Gao, Y.-C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 9.Cohn, J., T. Stokkermans, V. Kumar Kolli, R. B. Day, J. Dunlap, R. Carlson, D. Hughes, N. K. Peters, and G. Stacey. 1999. Aberrant nodulation response of Vigna umbellata to a Bradyrhizobium japonicum NodZ mutant and nodulation signals. Mol. Plant-Microbe Interact. 12:766-773. [DOI] [PubMed] [Google Scholar]
- 10.Cren, M., A. Kondorosi, and E. Kondorosi. 1995. NolR controls expression of the Rhizobium meliloti nodulation genes involved in the core Nod factor synthesis. Mol. Microbiol. 15:733-747. [DOI] [PubMed] [Google Scholar]
- 11.Daniels, R., D. E. De Vos, J. Desair, G. Raedschelders, E. Luyten, V. Rosemeyer, C. Verreth, E. Schoeters, J. Vanderleyden, and J. Michiels. 2002. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 277:462-468. [DOI] [PubMed] [Google Scholar]
- 12.Day, R. B., J. Loh, J. R. Cohn, and G. Stacey. 2000. Signal exchange involved in the establishment of the Bradyrhizobium-legume symbiosis, p. 385-414. In E. Triplett (ed.), Prokaryotic nitrogen fixation, a model system for the analysis of a biological process. Horizon Scientific Press, Norfolk, United Kingdom.
- 13.Delorme, C., S. D. Ehrlich, and P. Renault. 1999. Regulation of expression of the Lactococcus lactis histidine operon. J. Bacteriol. 181:2026-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 15.Fellay, R., M. Hanin, G. Montorzi, J. Frey, C. Freiberg, W. Golinowski, C. Staehelin, W. J. Broughton, and S. Jabbouri. 1998. nodD2 of Rhizobium sp. NGR234 is involved in the repression of the nodABC operon. Mol. Microbiol. 27:1039-1050. [DOI] [PubMed] [Google Scholar]
- 16.Fisher, R. F., T. T. Egelhoff, J. T. Mulligan, and S. R. Long. 1988. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 2:282-293. [DOI] [PubMed] [Google Scholar]
- 17.Fisher, R. F., and S. R. Long. 1993. Interactions of NodD at the nod box: NodD binds to two distinct sites on the same face of the helix and induces a bend in the DNA. J. Mol. Biol. 233:336-348. [DOI] [PubMed] [Google Scholar]
- 18.Fisher, R. F., and S. R. Long. 1989. DNA footprint analysis of the transcriptional activator proteins NodD1 and NodD3 on inducible nod gene promoters. J. Bacteriol. 171:5492-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flavier, A. B., S. J. Clough, M. A. Schell, and T. P. Denny. 1997. Identification of 3-hydroxypalmitic acid methyl ester as a novel autoregulator controlling virulence in Ralstonia solanacearum. Mol. Microbiol. 26:251-259. [DOI] [PubMed] [Google Scholar]
- 20.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 21.Garcia, M. L., J. Dunlap, J. Loh, and G. Stacey. 1996. Phenotypic characterization and regulation of the nolA gene of Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 9:625-635. [DOI] [PubMed] [Google Scholar]
- 22.Gillette, W. K., and G. H. Elkan. 1996. Bradyrhizobium (Arachis) sp. strain NC92 contains two nodD genes involved in the repression of nodA and a nolA gene required for the efficient nodulation of host plants. J. Bacteriol. 178:2757-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goethals, K., M. van Montagu, and M. Holsters. 1992. Conserved motifs in a divergent nod box of Azorhizobium caulinodans ORS571 reveal a common structure in promoters regulated by LysR-type proteins. Proc. Natl. Acad. Sci. USA 89:1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberger, R. F. 1974. Autogenous regulation of gene expression. Science 183:810-816. [DOI] [PubMed] [Google Scholar]
- 25.Göttfert, M., P. Groß, and H. Hennecke. 1990. Proposed regulatory pathway encoded by the nodV and nodW genes, determinants of host specificity in Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. USA 87:2680-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Göttfert, M., D. Holzhauser, and H. Hennecke. 1992. Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDA110. Mol. Plant-Microbe Interact. 5:257-265. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg, J. T., P. Monach, J. H. Chou, D. Josephy, and B. Demple. 1993. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc. Natl. Acad. Sci. USA 87:6181-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groß, P., P. Michel, H. Hennecke, and M. Göttfert. 1993. A novel response-regulator is able to suppress the nodulation defect of a Bradyrhizobium japonicum nodW mutant. Mol. Gen. Genet. 241:531-541. [DOI] [PubMed] [Google Scholar]
- 29.Hardman, A. M., G. S. A. B. Stewart, and P. Williams. 1998. Quorum sensing and the cell-cell communication dependent regulation of gene expression in pathogenic and non-pathogenic bacteria. Antonie Van Leeuwenhoek 74:199-210. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo, E., and B. Demple. 1994. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 13:138-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes, D. J., J. L. Caso, and C. J. Thompson. 1993. Autogenous transcriptional activation of a thiostrepton-induced gene in Streptomyces lividans. EMBO J. 12:3183-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong, G. F., J. E. Burn, and A. W. B. Johnston. 1987. Evidence that DNA involved in the expression of nodulation (nod) genes in Rhizobium binds to the product of the regulatory gene nodD. Nucleic Acids Res. 15:9677-9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honma, M. A., M. Asomaning, and F. M. Ausubel. 1990. Rhizobium meliloti nodD genes mediate host-specific activation of nodABC. J. Bacteriol. 172:901-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston, A. W. B., J. K. H. Yeoman, and M. Wexler. 2001. Metals and the rhizobial-legume symbiosis-uptake, utilization and signaling. Adv. Microb. Physiol. 45:114-156. [DOI] [PubMed] [Google Scholar]
- 35.Kamst, E., J. Pilling, L. M. Raamsdonk, B. J. J. Lugtenberg, and H. Spaink. 1997. Rhizobium nodulation protein NodC is an important determinant of chitin oligosaccharide chain length in Nod factor biosynthesis. J. Bacteriol. 179:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleerebezem, M., L. E. N. Quadri, O. P. Kuipers, and W. M. de Vos. 1997. Quorum sensing by peptide pheromones and two-component signal-transduction systems in gram-positive bacteria. Mol. Microbiol. 24:895-904. [DOI] [PubMed] [Google Scholar]
- 37.Knight, C. D., L. Rossen, J. G. Robertson, B. Wells, and J. A. Downie. 1986. Nodulation inhibition of Rhizobium leguminosarum multicopy nodABC genes and analysis of early stages of plant infection. J. Bacteriol. 166:552-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondorosi, E., M. Buire, M. Cren, M. Iyer, B. Hoffman, and A. Kondorosi. 1991. Involvement of the syrM gene and nodD3 gene of Rhizobium meliloti in nod gene activation and in optimal nodulation of the plant host. Mol. Microbiol. 5:3035-3048. [DOI] [PubMed] [Google Scholar]
- 39.Kondorosi, E., J. Gyuris, J. Schmidt, M. John, E. Duda, B. Hoffmann, J. Schell, and A. Kondorosi. 1989. Positive and negative regulation of nod gene expression in Rhizobium meliloti is required for optimal nodulation. EMBO J. 8:1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosslak, R. M., R. Bookland, J. Barker, H. E. Paaren, and E. R. Applebaum. 1987. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc. Natl. Acad. Sci. USA 84:7428-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu, S. T., W. Z. Chang, H. M. Cao, H. L. Hu, Z. H. Chen, F. D. Ni, H. F. Lu, and G. F. Hong. 1998. A HU-like protein binds to specific sites within nod promoters of Rhizobium leguminosarum. J. Biol. Chem. 273:20568-20574. [DOI] [PubMed] [Google Scholar]
- 42.Loh, J., R. W. Carlson, W. S. York, and G. Stacey. 2002. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. Proc. Natl. Acad. Sci. USA 99:14446-14451. [DOI] [PMC free article] [PubMed]
- 43.Loh, J., M. Garcia, and G. Stacey. 1997. NodV and NodW, a second flavonoid recognition system regulating nod gene expression in Bradyrhizobium japonicum. J. Bacteriol. 179:3013-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh, J., D. Lohar, B. Andersen, and G. Stacey. 2002. A two-component regulator mediates population density dependent expression of the Bradyrhizobium japonicum nodulation genes. J. Bacteriol. 184:1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loh, J., E. Pierson, L. S. Pierson III, G. Stacey, and A. Chatterjee. 2002. Quorum sensing in plant associated bacteria. Curr. Opin. Plant Biol. 5:285-290. [DOI] [PubMed]
- 46.Loh, J., and G. Stacey. 2001. Feedback regulation of the Bradyrhizobium japonicum nodulation genes. Mol. Microbiol. 41:1357-1364. [DOI] [PubMed] [Google Scholar]
- 47.Loh, J., M. G. Stacey, M. J. Sadowsky, and G. Stacey. 1999. The Bradyrhizobium japonicum nolA gene encodes three functionally distinct proteins. J. Bacteriol. 181:1544-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh, J., J. P. Y. Yuen-Tsai, A. Welborn, and G. Stacey. 2001. Population density-dependent regulation of the Bradyrhizobium japonicum nodulation genes. Mol. Microbiol. 42:37-46. [DOI] [PubMed] [Google Scholar]
- 49.Lohrke, S. M., C. J. Madrzak, H.-G. Hur, A. K. Judd, J. H. Orf, and M. J. Sadowsky. 2000. Inoculum density-dependent restriction of nodulation in the soybean-Bradyrhizobium japonicum symbiosis. Symbiosis 29:59-70. [Google Scholar]
- 50.Maillet, F., F. Debelle, and J. Denarie. 1990. Role of the nodD and syrM genes in the activation of the regulatory gene nodD3 and of the common and host-specific nod genes of Rhizobium meliloti. Mol. Microbiol. 4:1975-1984. [DOI] [PubMed] [Google Scholar]
- 51.Marketon, M. M., and J. E. Gonzalez. 2002. Identification of two quorum-sensing systems in Sinorhizobium meliloti. J. Bacteriol. 184:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 53.Misra, K. T. 1992. Bacterial resistance to inorganic mercury salts and organomercurials. Plasmid 27:4-16. [DOI] [PubMed] [Google Scholar]
- 54.Mulligan, J. T., and S. R. Long. 1989. A family of activator genes regulates expression of the Rhizobium meliloti nodulation genes. Genetics 122:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Halloran, T. V., B. Frantz, M. K. Shin, D. M. Ralston, and J. G. Wright. 1989. The MerR heavy metal receptor mediates positive activation in a topologically novel transcription complex. Cell 56:119-129. [DOI] [PubMed] [Google Scholar]
- 56.Omura, S., M. Murata, N. Imamura, Y. Iwai, H. Tanaka, A. Furusaki, and T. Matsumoto. 1984. Oxetin, a new antimetabolite from an actinomycete fermentation, isolation, structure and biological activity. J. Antibiot. 37:1324-1332. [DOI] [PubMed] [Google Scholar]
- 57.Pesci, E. C., J. B. J. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price, N. P. J., B. Relic, F. Talmont, A. Lewin, D. Prome, S. G. Pueppke, F. Maillet, J. Denarie, J.-C. Prome, and W. J. Broughton. 1992. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol. Microbiol. 6:3575-3584. [DOI] [PubMed] [Google Scholar]
- 59.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 60.Roche, P., F. Maillet, C. Plazanet, F. Debelle, M. Ferro, G. Truchet, J.-C. Prome, and J. Denarie. 1996. The common nodABC genes of Rhizobium meliloti are host-range determinants. Proc. Natl. Acad. Sci. USA 93:15305-15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rostas, K., E. Kondorosi, B. Horvath, A. Simoncsits, and A. Kondorosi. 1986. Conservation of the extended promoter regions of nodulation genes in rhizobia. Proc. Natl. Acad. Sci. USA 83:1757-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadowsky, M. J., P. B. Cregan, M. Göttfert, A. Sharma, D. Gerhold, F. Rodriguez-Quinones, H. H. Keyser, H. Hennecke, and G. Stacey. 1991. The Bradyrhizobium japonicum nolA gene and its involvement in the genotype-specific nodulation of soybeans. Proc. Natl. Acad. Sci. USA 88:637-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez, D. O., R. O. Zandomeni, S. Carvero, R. E. Verdun, E. Pierrou, P. Faccio, G. Diaz, S. Lanzavecchia, F. Aguero, A. C. C. Frasch, S. G. E. Andersson, O. L. Rossetti, O. Grau, and R. Ugalde. 2001. Gene discovery through genomic sequencing of Brucella abortus. Infect. Immun. 69:865-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanjuan, J., R. W. Carlson, H. P. Spaink, U. R. Bhat, W. M. Barbour, J. Glushka, and G. Stacey. 1992. A 2-O-methylfucose moiety is present in the lipooligosaccharide nodulation signal of Bradyrhizobium japonicum. Proc. Natl. Acad. Sci. USA 89:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanjuan, J., P. Groß, M. Göttfert, H. Hennnecke, and G. Stacey. 1994. NodW is essential for the full expression of the common nodulation genes in Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 7:364-369. [Google Scholar]
- 66.Schlaman, H. R. M., B. Horvath, E. Vigenboom, R. J. H. Okker, and B. J. J. Lugtenberg. 1991. Suppression of nodulation gene expression in bacteroids of Rhizobium leguminosarum biovar vicae. J. Bacteriol. 173:4277-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smit, G., V. Puvanesarajah, R. W. Carlson, W. M. Barbour, and G. Stacey. 1992. Bradyrhizobium japonicum nodD1 can be specifically induced by soybean flavonoids that do not induce the nodYABCSUIJ operon. J. Biol. Chem. 267:310-318. [PubMed] [Google Scholar]
- 68.Spaink, H. P. 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54:257-288. [DOI] [PubMed] [Google Scholar]
- 69.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69: 183-215. [DOI] [PubMed] [Google Scholar]
- 70.Summers, A. O. 1992. Untwist and shout: a heavy metal-responsive transcriptional regulator. J. Bacteriol. 174:3097-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ugalde, R. A. 1999. Intracellular lifestyle of Brucella spp. common genes with other animal pathogens, plant pathogens, and endosymbionts. Microbes Infect. 1:1211-1219. [DOI] [PubMed] [Google Scholar]
- 72.Van Rhijn, P. J., B. Feys, C. Verreth, and J. Vanderleyden. 1993. Multiple copies of nodD in Rhizobium tropici CIAT899 and BR816. J. Bacteriol. 175:438-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang, S.-P., and G. Stacey. 1991. Studies of the Bradyrhizobium japonicum nodD1 promoter: a repeated structure for the nod box. J. Bacteriol. 173:3356-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Winans, S. C., and B. L. Bassler. 2002. Mob psychology. J. Bacteriol. 174:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whitehead, N. A., A. M. L. Barnard, H. Slater, N. J. L. Simpson, and G. P. C. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 76.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 77.Yanofsky, C., T. Platt, I. P. Crawford, B. P. Nichols, G. E. Christie, H. Horowitz, M. van Cleemput, and A. M. Wu. 1981. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 9:6647-6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeh, K. C., M. C. Peck, and S. R. Long. 2002. Luteolin and GroESL modulate in vitro activity of NodD. J. Bacteriol. 184:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]