Abstract
Pharmacological studies have led to a model in which the phytohormone abscisic acid (ABA) may be positively transduced via protein phosphatases of the type 1 (PP1) or type 2A (PP2A) families. However, pharmacological evidence also exists that PP1s or PP2As may function as negative regulators of ABA signaling. Furthermore, recessive disruption mutants in protein phosphatases that function in ABA signal transduction have not yet been identified. A guard cell–expressed PP2A gene, RCN1, which had been characterized previously as a molecular component affecting auxin transport and gravity response, was isolated. A T-DNA disruption mutation in RCN1 confers recessive ABA insensitivity to Arabidopsis. The rcn1 mutation impairs ABA-induced stomatal closing and ABA activation of slow anion channels. Calcium imaging analyses show a reduced sensitivity of ABA-induced cytosolic calcium increases in rcn1, whereas mechanisms downstream of cytosolic calcium increases show wild-type responses, suggesting that RCN1 functions in ABA signal transduction upstream of cytosolic Ca2+ increases. Furthermore, rcn1 shows ABA insensitivity in ABA inhibition of seed germination and ABA-induced gene expression. The PP1 and PP2A inhibitor okadaic acid phenocopies the rcn1 phenotype in wild-type plants both in ABA-induced cytosolic calcium increases and in seed germination, and the wild-type RCN1 genomic DNA complements rcn1 phenotypes. These data show that RCN1 functions as a general positive transducer of early ABA signaling.
INTRODUCTION
The phytohormone abscisic acid (ABA) plays important roles in plant growth, development, and cellular signaling (Finkelstein and Zeevaart, 1994; Grill and Himmelbach, 1998; Koornneef et al., 1998; Finkelstein et al., 2002). ABA maintains seed dormancy, controls seed maturation, regulates vegetative growth, and mediates plant responses to various environmental stimuli such as stomatal closure during drought (Grill and Himmelbach, 1998; Koornneef et al., 1998; MacRobbie, 1998; Schroeder et al., 2001; Finkelstein et al., 2002). Previous molecular genetic approaches have led to the identification of several genes that function in ABA signal transduction (Koornneef et al., 1984; Giraudat et al., 1992; Finkelstein, 1994; Leung et al., 1994, 1997; Meyer et al., 1994; Rodriguez et al., 1998; Sheen, 1998; Finkelstein and Lynch, 2000; Li et al., 2000; Hugouvieux et al., 2001; Xiong et al., 2001b).
Guard cells in the leaf epidermis form stomates and regulate CO2 uptake into leaves for photosynthesis and control transpirational water loss. Guard cells integrate water status, hormonal stimuli, light, and other environmental conditions to regulate stomatal apertures for optimization of plant growth and have become a well-developed system in which to characterize events in early plant signaling cascades (for reviews, see MacRobbie, 1998; Schroeder et al., 2001).
Pharmacological studies have suggested that type 1 or 2A protein phosphatases (PP1 or PP2A) act as both negative and positive regulators of ABA signal transduction (Schmidt et al., 1995; Esser et al., 1997; Grabov et al., 1997; Hey et al., 1997; Pei et al., 1997; Wu et al., 1997). Studies showed that okadaic acid (OA), which inhibits PP1s or PP2As, promotes anion channel activation and ABA-induced stomatal closing in Vicia and Commelina (Schmidt et al., 1995). Replacement of cytosolic ATP with nonhydrolyzable analogs or depletion of cytosolic ATP disrupted ABA responses in guard cells, but only in the absence of OA. These data further implicate PP1- or PP2A-induced dephosphorylation events as negative regulators in ABA signaling (Schmidt et al., 1995). Consistent with this model, the PP1/PP2A inhibitor OA stimulated expression of the ABA-induced RD29A and KIN2 genes in tomato hypocotyl cells in the absence of ABA (Wu et al., 1997).
Conversely, studies in other systems showed that OA partially inhibited ABA induction of the PHAV1 gene in barley aleurone cells (Kuo et al., 1996), and in Arabidopsis guard cells, OA partially inhibited ABA activation of anion channels and stomatal closing (Pei et al., 1997). Together, these studies indicate that both positively transducing PP1s or PP2As (Kuo et al., 1996; Hey et al., 1997; Pei et al., 1997) and negatively regulating PP1s or PP2As (Schmidt et al., 1995; Esser et al., 1997; Grabov et al., 1997; Wu et al., 1997) might function at different locations in ABA signal transduction.
Further pharmacological evidence for conditionally active positively and negatively regulating protein phosphatases was obtained in pea guard cells: OA either inhibits or enhances ABA signaling depending on the physiological status or the history of stomates (Hey et al., 1997). When pea stomates are preopened, OA enhances ABA-induced stomatal closing (Hey et al., 1997), as in Vicia and Commelina (Schmidt et al., 1995; Esser et al., 1997). But the same study in pea showed that when stomates are closed initially, OA counteracts guard cell ABA responses and abolishes the ABA induction of dehydrin mRNA (Hey et al., 1997). In spite of several independent studies that implicate PP1s or PP2As pharmacologically in ABA signal transduction, genes that encode the proposed PP1 or PP2A protein phosphatases, as well as their locations within ABA signal cascades and their downstream targets, remain unknown.
In addition to PP1/PP2A-mediated ABA signal transduction, other phosphorylation/dephosphorylation enzymes have been shown to mediate ABA signaling in guard cells (for reviews, see Himmelbach et al., 1998; Leung and Giraudat, 1998; Assmann and Shimazaki, 1999). Previous studies have shown roles for type 2C protein phosphatases (PP2Cs), ABA-activated/ABA-responsive protein kinases (AAPK/ABRK), and Ca2+-dependent protein kinases in ABA signaling (Leung et al., 1994, 1997; Meyer et al., 1994; Li and Assmann, 1996; Sheen, 1996, 1998; Mori and Muto, 1997; Rodriguez et al., 1998; Li et al., 2000). Dominant mutants in the AAPK kinase disrupt the ABA activation of anion channels and ABA-induced stomatal closure (Li et al., 2000). The dominant mutations abi1-1 and abi2-1 impaired ABA-induced anion channel activation and ABA-induced stomatal closing (Pei et al., 1997) and have been shown to disrupt ABA signaling upstream of Ca2+ channel activation (Allen et al., 1999a; Murata et al., 2001). However, to date, no recessive gene deletion mutants in kinase and PP2C genes have been characterized that affect ABA signal transduction; therefore, neomorphic effects of the dominant mutants cannot be excluded unequivocally, although several lines of evidence support roles for the wild-type enzymes in ABA signaling (Li and Assmann, 1996; Leung et al., 1997; Mori and Muto, 1997; Sheen, 1998; Li et al., 2000; Merlot et al., 2001; for reviews, see Leung and Giraudat, 1998; Finkelstein et al., 2002).
Here, we identify a guard cell–expressed PP2A regulatory A subunit for which recessive gene disruption shows ABA insensitivity in Arabidopsis and that affects early events in guard cell ABA signal transduction. Further analyses show that this PP2A is required for ABA signal transduction during seed germination and ABA-induced gene expression, suggesting that the RCN1 is a general positive regulator of ABA signal transduction in Arabidopsis.
RESULTS
ABA-Insensitive Stomatal Response by Disruption of a Guard Cell–Expressed PP2A Gene
To identify guard cell–expressed PP2A genes, catalytic and regulatory subunit sequences of PP2As were aligned, and then degenerate oligomers designed from conserved regions were used to amplify guard cell–expressed PP2A genes using enriched Arabidopsis guard cell cDNA libraries (see Methods).
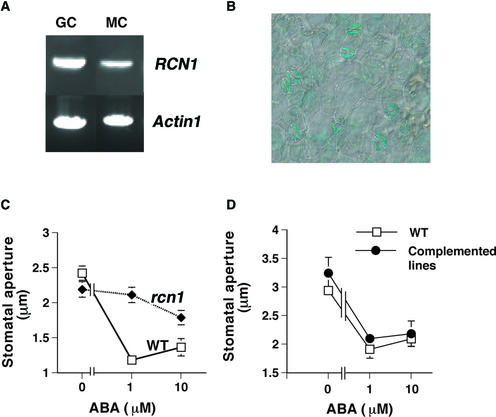
One of the genes encoding a PP2A that we identified from guard cell cDNA libraries was RCN1. RCN1, a PP2A regulatory A subunit, was characterized previously as a molecular component affecting auxin transport and gravitropism (Garbers et al., 1996; Rashotte et al., 2001). Loss-of-function rcn1 mutant seedlings, in which no full-length RCN1 transcript and no protein product were observed (Garbers et al., 1996; Deruère et al., 1999), showed defects in root and hypocotyl elongation and reduced PP2A activity, biochemically demonstrating that RCN1 is an activator of PP2A activity (Garbers et al., 1996; Deruère et al., 1999). To further test the expression of RCN1 in guard cells, we performed reverse transcription (RT) PCR with total RNA that was prepared independently from highly purified guard cells (>98% pure) and mesophyll cells (>96% pure). As shown in Figure 1A, RCN1 was expressed in both mesophyll cells and guard cells. The identities of RCN1 PCR products were confirmed by diagnostic restriction enzyme digestions (data not shown). Densitometry analysis of RCN1 expression levels suggested a higher expression in guard cells compared with mesophyll cells. Wild-type transgenic plants expressing the β-glucuronidase (GUS) reporter gene under the control of the RCN1 promoter (3 kb of the RCN1 upstream sequence; Deruère et al., 1999) also showed that RCN1 is expressed in guard cells (Figure 1B).
Figure 1.
RT-PCR and GUS Activity Analyses of RCN1 in Guard Cells and ABA-Insensitive Stomatal Responses in the rcn1 Mutant.
(A) RT-PCR with RNA extracted from highly purified guard cell (GC) and mesophyll cell (MC) protoplasts. The Actin1 gene was amplified as a control.
(B) The RCN1 promoter drives GUS activity in guard cells of wild-type plants expressing the RCN1-GUS fusion construct.
(C) Stomatal aperture measurements show that ABA-induced stomatal closing is reduced in the rcn1 T-DNA disruption mutant. Stomatal apertures were measured 3 h after the addition of 1 or 10 μM ABA. Error bars represent standard errors relative to three independent experiments with 36 stomata per data point.
(D) ABA-induced stomatal closing was observed in both wild-type and two independent rcn1-complemented lines (rcn1g17-1 and rcn1g8-1). Error bars represent standard errors of two independent experiments with 40 stomata per data point.
Error bars are smaller than symbols when not visible. Note that the light fluence rate was 83 μmol·m−2·s−1 in (C) and 125 μmol·m−2·s−1 in (D). WT, wild type.
Pharmacological studies have suggested that both positively transducing and negatively regulating PP1s or PP2As may function in ABA signal transduction (see Introduction). To determine whether an rcn1 disruption mutant carrying a T-DNA insertion (Garbers et al., 1996) has enhancing or inhibitory effects on the stomatal response to ABA, we first performed ABA-induced stomatal closing assays. Figure 1C shows that stomatal closing in rcn1 mutant plants was less sensitive to ABA than that in wild-type plants (Wassilewskija ecotype) at 1 and 10 μM ABA (P < 0.001). For complementation analysis, we used rcn1 plants transformed with a 7-kb genomic fragment containing the RCN1 gene under the control of its own promoter (1.6 kb of the RCN1 upstream sequence) (Garbers et al., 1996) in the hygromycin-resistant vector pCIT20 (Ma et al., 1992). The root-curling assay and PCR analyses in T2 generation seedlings showed cosegregation of the rescued phenotype with the RCN1 transgene (Garbers et al., 1996). Genomic DNA gel blot analyses with a hygromycin resistance marker DNA probe revealed a single T-DNA insertion carrying the complementing construct (J.M. Kwak, unpublished data). In addition, RT-PCR analyses showed that RCN1 expression was restored in the complemented lines (J.M. Kwak, unpublished data). Two independent T4 homozygous plant lines showed no difference in ABA sensitivity in stomatal aperture measurements compared with wild-type plants, showing complementation of the rcn1 phenotype (P > 0.58 at 1 μM ABA and P > 0.41 at 10 μM ABA; Figure 1D). The PP2A inhibitor OA inhibits ABA-induced stomatal closing in Arabidopsis (Pei et al., 1997), and the rcn1 mutation was shown to decrease PP2A activity in vivo (Deruère et al., 1999). Therefore, these results indicate that the RCN1 PP2A acts as a positive regulator in ABA signaling in Arabidopsis guard cells.
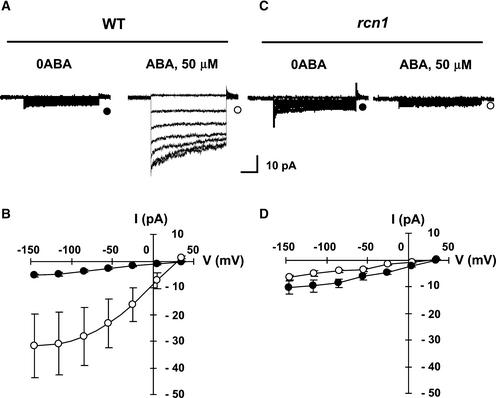
ABA Activation of Anion Channels Is Impaired in rcn1 Guard Cells
The activation of slow (S-type) anion channels is important for ABA-induced stomatal closing (Schroeder and Hagiwara, 1989; Schroeder et al., 1993), and ABA has been shown to activate S-type anion channels in guard cells (Grabov et al., 1997; Pei et al., 1997, 1998; Leonhardt et al., 1999; Li et al., 2000). We tested the ABA activation of S-type anion channels in rcn1 by preincubating guard cells in ABA and subsequently patch clamping the cells to assay for anion channel activities (Pei et al., 1997). The membrane potential was held at the anion equilibrium potential and stepped to membrane voltages ranging from −145 to +35 mV (Schroeder and Keller, 1992). As shown in Figures 2A and 2B, anion channel currents were activated by ABA in wild-type guard cells. By contrast, ABA failed to activate anion channels in rcn1 guard cells (Figures 2C and 2D). These data suggest that RCN1 is a subunit of the OA-sensitive protein phosphatase that is responsible for the OA inhibition of ABA-activated anion channels. Impairment in the ABA activation of anion channels in rcn1 is consistent with the ABA insensitivity of stomatal closing in rcn1 guard cells (Figure 1C). The finding that short-term exposure of wild-type guard cells to OA (Pei et al., 1997) mimics the rcn1 knockout phenotypes (partial inhibition of ABA-induced stomatal closing [Figure 1C] and anion channel activation [Figure 2]) suggests that ABA insensitivity in rcn1 is not attributable to a long-term effect of the rcn1 loss of function during guard cell development. Rather, these data suggest that RCN1 functions directly in ABA signaling.
Figure 2.
The rcn1 Mutation Impairs the ABA Activation of S-Type Anion Channels in Guard Cells.
(A) Whole cell recordings of anion channel currents in wild-type (WT) guard cells in the absence (left trace) or presence (right trace) of 50 μM ABA.
(B) Average peak current-voltage relationships for ABA activation of anion currents in wild-type cells as recorded in (A) (closed circles, 0 ABA, n = 8; open circles, 50 μM ABA, n = 10). Error bars represent standard errors.
(C) Whole-cell recordings of anion channel currents in rcn1 guard cells in the absence (left trace) or presence (right trace) of 50 μM ABA. ABA failed to activate anion channel currents in rcn1.
(D) Average peak current-voltage relationships for ABA activation of anion currents in rcn1 cells as recorded in (B) (closed circles, no ABA, n = 6; open circles, 50 μM ABA, n = 7). Error bars represent standard errors.
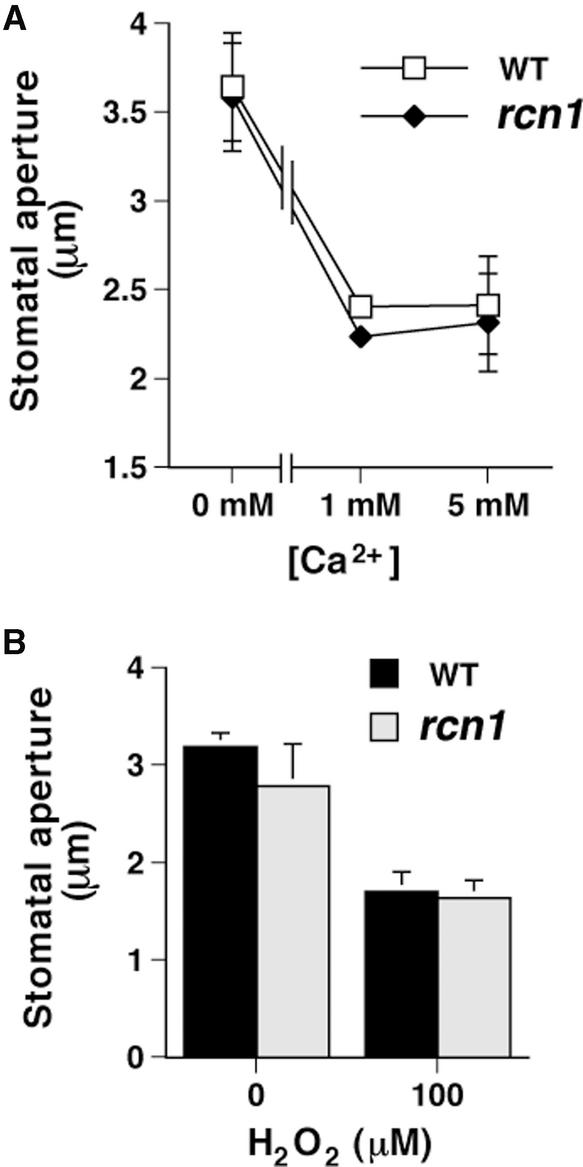
Mechanisms Downstream of Cytosolic Ca2+ Show Wild-Type Responses in the rcn1 Mutant
ABA-induced cytosolic Ca2+ concentration ([Ca2+]cyt) increases occur upstream of anion channel activation (Schroeder and Hagiwara, 1989; McAinsh et al., 1990; Allen et al., 1999a). Extracellular Ca2+ (McAinsh et al., 1995) and reactive oxygen species, a recently discovered second messenger of ABA signaling (Pei et al., 2000), induce increases in [Ca2+]cyt that mediate stomatal closure. To determine whether the rcn1 mutation can be bypassed by [Ca2+]cyt increases, we performed stomatal closing assays with extracellular Ca2+ and H2O2. As shown in Figure 3A, stomatal responses in the rcn1 mutant showed no significant difference compared with those of the wild type when they were treated with 1 and 5 mM external Ca2+ (P > 0.32 at 1 mM Ca2+ and P > 0.11 at 5 mM Ca2+). Furthermore, stomatal responses to 100 μM H2O2 were similar in rcn1 and the wild type (P > 0.54; Figure 3B). These results suggest that mechanisms downstream of [Ca2+]cyt increases, which lead to stomatal closure, may be largely functional in the rcn1 mutant.
Figure 3.
Calcium- and H2O2-Induced Stomatal Closing Movements Are Not Affected in rcn1.
(A) Stomatal apertures were measured 3 h after the addition of 0, 1, or 5 mM Ca2+ to the solution of wild-type (WT) and rcn1 stomata that had been preopened in white light. Error bars represent standard errors relative to three independent experiments with 60 stomata per data point.
(B) Stomatal apertures were measured 3 h after the addition of 100 μM H2O2 to the bath solution of wild-type and rcn1 stomata that had been preopened in light for 3 h. Error bars represent standard errors relative to three independent experiments with 80 (wild type) and 60 (rcn1) stomata per data point. The light fluence rate was 125 μmol·m−2·s−1.
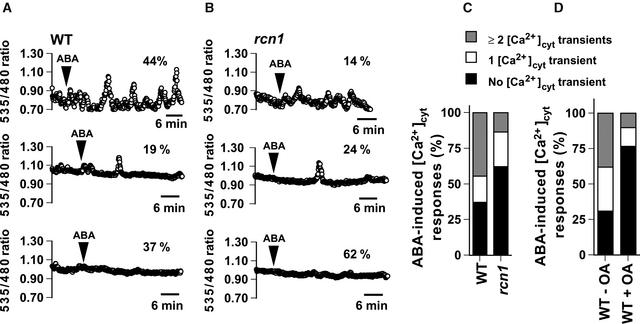
ABA-Induced Cytosolic Ca2+ Increases Are Reduced in rcn1 Guard Cells
One of the earliest measurable ABA signaling events in guard cells is an ABA-induced increase in [Ca2+]cyt (McAinsh et al., 1990, 1997; Schroeder and Hagiwara, 1990). Therefore, to determine whether the rcn1 mutation affects mechanisms upstream of [Ca2+]cyt increases in guard cells, we performed time-resolved [Ca2+]cyt imaging studies using wild-type and rcn1 plants expressing the Ca2+ reporter yellow cameleon 2.1 (Miyawaki et al., 1997; Allen et al., 1999b). Two independent wild-type lines and three independent rcn1 lines transformed with yellow cameleon 2.1 were used for [Ca2+]cyt imaging experiments. We treated guard cells with 5 μM ABA to investigate the responsiveness of ABA-induced [Ca2+]cyt increases in rcn1 because guard cells of rcn1 showed ABA insensitivity in stomatal movements (Figure 2B) and 5 μM ABA induced [Ca2+]cyt increases in wild-type guard cells (Wassilewskija ecotype).
Figures 4A and 4B show representative ABA-induced [Ca2+]cyt increases in wild-type and rcn1 guard cells. Five micromolar ABA induced two or more calcium transients in 44% of wild-type guard cells (n = 12 of 27 cells; Figures 4A, top trace, and 4C). By contrast, only 14% of rcn1 guard cells showed two or more calcium increases (n = 5 of 37 cells; Figures 4B, top trace, and 4C). Furthermore, in rcn1, 62% of guard cells showed no response to 5 μM ABA (n = 23 of 37 cells; Figures 4B, bottom trace, and 4C), whereas 37% of wild-type guard cells showed no response to 5 μM ABA during 40 to 45 min of recordings (n = 10 of 27 cells; Figures 4A, bottom trace, and 4C). Note that even at higher ABA concentrations, a background rate of ∼25 to 30% of wild-type guard cells showed no measurable ABA-induced [Ca2+]cyt increases under the imposed conditions (Hugouvieux et al., 2001). The number of cells that showed ABA-induced [Ca2+]cyt increases, including single Ca2+ transient responses, was reduced significantly in rcn1 guard cells (χ2 = 3.9, P < 0.03). Blind analyses of the same and unmarked data sets confirmed the reduced ABA induction of [Ca2+]cyt increases in rcn1 guard cells (see Methods). The average ABA-induced [Ca2+]cyt amplitude ratio changes were not significantly different between wild-type and rcn1 guard cells (211.6 ± 95.2 nM for the wild type and 222.0 ± 68.2 nM for rcn1; P > 0.71). However, the number of ABA-induced [Ca2+]cyt transients was reduced in rcn1 guard cells compared with the wild type, even when nonresponsive cells (no transients) were excluded from the analyses (2.6 ± 1.7 per recording for the wild type and 1.6 ± 0.9 per recording for rcn1; P < 0.05). Calcium imaging data indicate that the rcn1 mutation partially reduced the responsiveness of guard cells to ABA by reducing the probability of ABA-induced [Ca2+]cyt increases.
Figure 4.
ABA-Induced [Ca2+]cyt Increases Are Reduced in rcn1 Guard Cells.
(A) The fluorescence emission ratio (535/480 nm) shows examples of ABA-induced [Ca2+]cyt increases at 5 μM ABA in wild-type (WT) guard cells (n = 27 cells).
(B) The fluorescence emission ratio (535/480 nm) shows examples of ABA-induced [Ca2+]cyt increases at 5 μM ABA in rcn1 guard cells (n = 37 cells).
In (A) and (B), traces demonstrating two or more ABA-induced [Ca2+]cyt transients are shown at top, those demonstrating one [Ca2+]cyt transient are shown in the middle, and those demonstrating no clear [Ca2+]cyt transient are shown at bottom. Arrowheads indicate when cells were treated with 5 μM ABA. [Ca2+]cyt transients were counted when changes in [Ca2+]cyt ratios were ≥0.1 units.
(C) Stack column representation of the number of ABA-induced [Ca2+]cyt transients recorded in wild-type (n = 27) and rcn1 (n = 37) guard cells at 5 μM ABA.
(D) Stack column representation of the number of ABA-induced [Ca2+]cyt transients recorded in wild-type guard cells at 5 μM ABA in the absence (WT − OA; n = 29 cells) or presence (WT + OA; n = 30 cells) of 1 μM OA.
We further tested whether the PP1/PP2A inhibitor OA applied to wild-type guard cells can mimic the rcn1 phenotype for ABA-induced [Ca2+]cyt increases. In the absence of OA, ABA triggered one or more calcium transients in 69% of guard cells (Wassilewskija ecotype) (n = 20 of 29 cells; 9 cells with one transient, 11 cells with two or more transients) (Figure 4D, WT − OA). However, in the presence of 1 μM OA, ABA triggered one or more calcium increases in only 23% of guard cells (n = 7 of 30 cells; four cells with one transient, three cells with two or more transients) (Figure 4D, WT + OA). Moreover, in the presence of 1 μM OA, 77% of guard cells showed no response to 5 μM ABA (n = 23 of 30 cells; Figure 4D); in the absence of OA, 31% of guard cells showed no response to 5 μM ABA (n = 9 of 29 cells; Figure 4D). These results show that 1 μM OA significantly reduced the number of guard cells displaying ABA-induced [Ca2+]cyt increases (χ2 = 12.27, P < 0.0005), demonstrating that OA applied to the wild type phenocopies the rcn1 phenotype.
The rcn1 Disruption Mutation Reduces ABA Sensitivity in Seeds
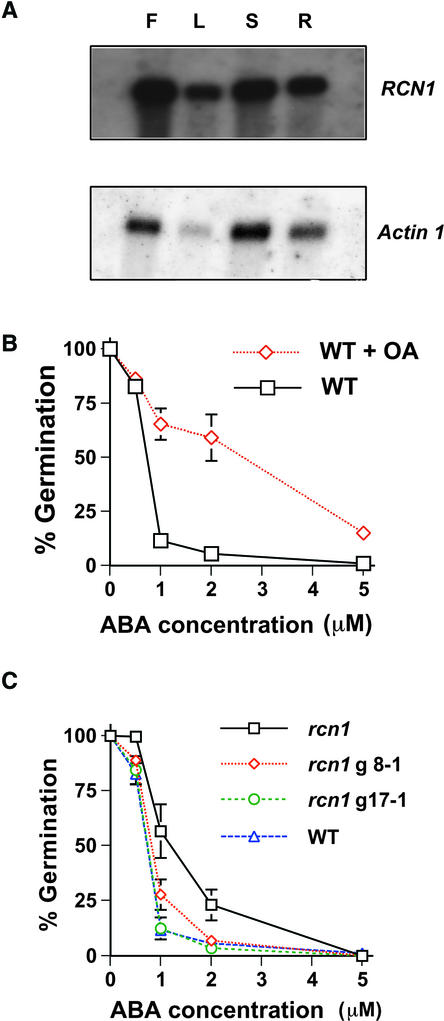
To examine the expression of RCN1 in other plant tissues, we performed RNA gel blot analyses with poly(A+) mRNA extracted from Arabidopsis flowers, leaves, stems, and roots. As shown in Figure 5A, RCN1 was expressed in all organs, suggesting additional roles for RCN1 (Corum et al., 1996; Garbers et al., 1996).
Figure 5.
OA and the rcn1 Mutation Both Impair ABA Inhibition of Arabidopsis Seed Germination.
(A) Tissue expression of the RCN1 gene. RCN1 transcript was detected in all organs examined (F, flower; L, leaf; S, stem; R, root). Two micrograms of poly(A+) RNA was transferred onto a nylon membrane. The blot was hybridized with 32P-labeled RCN1 and Actin1 cDNA.
(B) ABA inhibition of wild-type (WT) seed germination is reduced in the presence of 1 μM of OA. Error bars represent standard errors of three independent experiments with >360 seeds at each data point.
(C) The rcn1 mutation causes partial reduction in ABA sensitivity of seed germination. Wild-type seed germination rates were observed in two independent rcn1-complemented lines (rcn1g17-1 and rcn1g8-1). Symbols of the complemented rcn1g17-1 and rcn1g8-1 lines are overlapped with wild-type symbols when not visible. Error bars represent standard errors of three independent experiments with >360 seeds at each data point.
ABA mediates dormancy by inhibiting seed germination. We examined whether PP1 or PP2A may function in ABA-mediated inhibition of seed germination. Wild-type seed germination rates were measured on plates containing 0, 0.5, 1, 2, or 5 μM ABA in the absence and presence of OA. Seed germination was analyzed 5 days after transfer to a growth chamber preceded by exposure to 4°C for 4 days. As shown in Figure 5B, ABA inhibited the germination of wild-type Arabidopsis seeds. Interestingly, ABA inhibition of wild-type seed germination was reduced dramatically in the presence of 1 μM OA (P < 0.0005 at 1 μM ABA, P < 0.001 at 2 μM ABA, and P < 0.006 at 5 μM ABA). OA applied to seeds in the absence of added ABA did not affect seed germination, resulting in 99.7% germination (Figure 5B, 0 ABA). These results provide evidence that PP1s or PP2As also act as positive transducers of ABA signaling in Arabidopsis seeds.
To determine whether the loss of function of RCN1 affects the ABA regulation of seed germination in Arabidopsis, we investigated the effects of the rcn1 mutation on seed germination. ABA inhibition of seed germination showed a partial ABA insensitivity in the rcn1 mutant (P < 0.008 at 0.5 μM ABA, P < 0.01 at 1 μM ABA, and P < 0.02 at 2 μM ABA; Figure 5C). Two independent complemented lines of rcn1 showed seed germination rates similar to those of wild-type lines (for rcn1g17-1, P > 0.78 at 0.5 μM ABA, P > 0.88 at 1 μM ABA, and P > 0.20 at 2 μM ABA; for rcn1g8-1, P > 0.26 at 0.5 μM ABA, P > 0.05 at 1 μM ABA, and P > 0.58 at 2 μM ABA; Figure 5C). These results show that RCN1 contributes to the ABA inhibition of seed germination in Arabidopsis as well as to ABA signaling in guard cells. The stronger effect of OA on the ABA regulation of seed germination (Figure 5B) compared with the rcn1 phenotype (Figure 5C) indicates that additional (partially redundant) OA-sensitive protein phosphatases may function in ABA signaling in seeds.
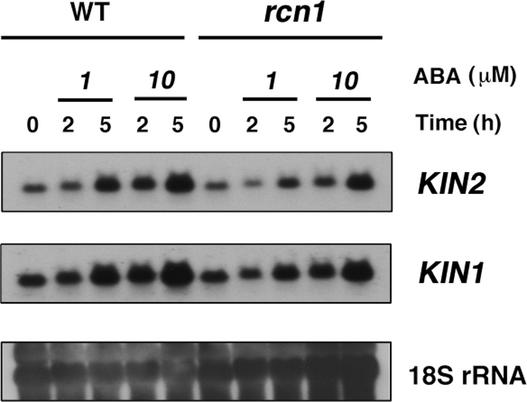
Reduced Expression of ABA-Responsive Genes in rcn1
To determine whether the rcn1 mutation also alters the expression of ABA-responsive genes, RNA gel blot analyses were performed. Total RNA was extracted from rosette leaves of wild-type and mutant plants sprayed with water or ABA. We hybridized RNA gel blots with probes that have been used to examine ABA-inducible gene transcription in ABA signaling mutants (Xiong et al., 2001a, 2001b). The rcn1 mutation reduced the ABA-induced transcript levels of KIN2, KIN1 (Figure 6), and RD29A (data not shown). Consistent with these results, ABA-induced transcript levels of these genes were enhanced in the ABA-hypersensitive mutants ade1 and fry1-1 (Foster and Chua, 1999; Xiong et al., 2001b).
Figure 6.
Transcript Levels of ABA-Responsive Genes Are Reduced in the rcn1 Mutant.
Wild-type (WT) and rcn1 plants were sprayed with 1 or 10 μM ABA 2 or 5 h before RNA isolation. Twenty micrograms of total RNA extracted from rosette leaves was transferred onto a nylon membrane. The blot was hybridized with 32P-labeled KIN2 or KIN1 cDNA. The same blot was hybridized with 32P-labeled 18S rDNA to show relative amounts of RNA samples.
DISCUSSION
The recessive T-DNA disruption mutant rcn1 reduces the ABA responsiveness of Arabidopsis seed germination, ABA-induced stomatal closing, ABA activation of S-type anion channels, and ABA-induced gene expression. These findings suggest that RCN1 is a general component of ABA signaling. The rcn1 mutation partially reduces sensitivities to ABA by decreasing the probability of ABA-induced [Ca2+]cyt increases in guard cells (Figure 4), whereas experimental [Ca2+]cyt increases analyzed here bypass rcn1, resulting in stomatal closure in rcn1 (Figure 3). The subtlety of the ABA response phenotype (30 to 40% higher germination frequencies at 1 μM ABA) could explain why mutations in the RCN1 gene have not been isolated previously in forward genetic ABA response screens that set a higher threshold for facilitated mutant isolation.
RCN1 Functions in ABA-Induced [Ca2+]cyt Increases
In Arabidopsis suspension cells, recent studies have shown that ABA-induced [Ca2+]cyt increases and anion channel activation are important for the ABA induction of RAB18 gene expression (Ghelis et al., 2000a, 2000b). Furthermore, antisense suppression of the phospholipase C, AtPLC1, and sense expression of the inositol 1,4,5-trisphosphate 5-phosphatase, AtIP5PII, as well as the fry1 mutation in an inositol polyphosphate 1-phosphatase implicate Ca2+ signaling in the ABA inhibition of seedling emergence (Sanchez and Chua, 2001) and ABA-induced RD29A::luciferase expression (Xiong et al., 2001b). These findings suggest that cytosolic Ca2+ may be a general second messenger for several ABA responses.
Calcium imaging experiments showed that the rcn1 mutation impairs ABA-induced [Ca2+]cyt increases in guard cells (Figure 4). Recent findings with experimentally imposed Ca2+ oscillations in guard cells showed that the degree of stomatal closure depends on the number of [Ca2+]cyt transients (Allen et al., 2001). The probability of ABA-induced [Ca2+]cyt increases and the number of ABA-induced [Ca2+]cyt transients were reduced significantly in rcn1 guard cells. Stomatal movement experiments showed that experimental increases in [Ca2+]cyt in rcn1 can restore wild-type responses (Figure 3A).
ABA is known to induce stomatal closing via multiple pathways (Allan et al., 1994; Grabov et al., 1997; Allen et al., 1999a; Pei et al., 2000). A previous study with the abi1-1 and abi2-1 PP2C mutants demonstrated that the addition of external calcium results in stomatal closing in both the wild type and PP2C mutants by activating processes downstream of [Ca2+]cyt increases (Allen et al., 1999a). These data showed that abi1-1 and abi2-1 can be bypassed in the stomatal closing pathway. Furthermore, because abi1-1 and abi2-1 disrupt the ABA activation of plasma membrane Ca2+ channels, the ability to impose [Ca2+]cyt increases by adding external Ca2+ suggests that the external Ca2+-induced Ca2+ oscillation pathway differs from the ABA-induced Ca2+ oscillation pathway (Allen et al., 1999a; Murata et al., 2001). This hypothesis is strengthened by the finding that the det3 mutant affects the external Ca2+ pathway but not the ABA signaling pathway (Allen et al., 2000). Based on these previous findings, stomatal movement assays (Figure 3A) suggest that the impairment in ABA-induced stomatal closing in the rcn1 mutant is bypassed by external calcium.
One micromolar OA phenocopies the rcn1 phenotype in wild-type guard cells with ABA-induced [Ca2+]cyt increases (Figure 4D), which correlates with findings that rcn1 has reduced PP2A activity (Deruère et al., 1999). Together with reactive oxygen species–induced stomatal closing assays (Figure 3B), these data indicate that RCN1 may act upstream of reactive oxygen species and/or in a parallel branch of the ABA signaling network in guard cells (Blatt, 2000; Schroeder et al., 2001). The location of RCN1 relative to ABA-induced Ca2+ release mechanisms (Leckie et al., 1998; Staxen et al., 1999) and ABA-activated plasma membrane Ca2+ channels (Hamilton et al., 2000; Pei et al., 2000; Murata et al., 2001) remains to be determined.
Cross-Talk in RCN1-Mediated Signaling
Recent studies have demonstrated that a single signal transduction protein can mediate cross-talk between two or more different hormone response pathways (Alonso et al., 1999; Ephritikhine et al., 1999; Beaudoin et al., 2000; Ghassemian et al., 2000; Lu and Fedoroff, 2000). For example, the Arabidopsis sax1 mutant showed altered sensitivities to ABA, auxin, and gibberellins, but normal responses to these hormones were restored by exogenous application of brassinosteroid (Ephritikhine et al., 1999). The rcn1 mutant was shown previously to be sensitive to the auxin transport inhibitor naphthylphthalamic acid in Arabidopsis (Garbers et al., 1996). The rcn1 mutant exhibits defects in auxin transport, which may cause abnormal auxin distribution (Rashotte et al., 2001). Our analyses of early signaling events show that RCN1 functions in the early ABA signal transduction cascade. Our data provide additional evidence that a single molecular component can function in both auxin transport and ABA hormone response pathways.
PP2As are heterotrimeric holoenzymes that constitute a family of Ser/Thr phosphatases. A catalytic C subunit and a regulatory A subunit make up the heterodimeric AC core enzyme, which is associated with another regulatory B-type subunit (Janssens and Goris, 2001). In Arabidopsis, genes encoding three A subunits, five C subunits, two B subunits, eight B′ subunits, and six B′′ subunits have been identified. Given the numbers of PP2A subunit genes, permutations in the specific PP2A holoenzyme composition could provide diversity in PP2A activity (Janssens and Goris, 2001). In Arabidopsis, the yeast two-hybrid system and in vitro binding assays revealed that one A subunit can interact with two B′ subunits and two C subunits (Haynes et al., 1999), suggesting many possibilities in PP2A holoenzyme composition. Therefore, it is possible that the RCN1 PP2A subunit forms complexes with more than one PP2A catalytic subunit in vivo, which could contribute to cross-talk among RCN1 functions.
All five C subunit genes are expressed in seedlings and all organs of Arabidopsis (Ariño et al., 1993; Pérez-Callejón et al., 1993; Casamayor et al., 1994). RNA gel blot analyses show that three A subunit, two B subunit, one B′′ subunit, and five B′ subunit genes also are expressed ubiquitously (Rundle et al., 1995; Corum et al., 1996; Latorre et al., 1997; Haynes et al., 1999; Camilleri et al., 2002; Terol et al., 2002). However, GUS reporter gene fusion experiments showed that the promoters of one C and two B subunit genes have some tissue specificity in seedlings and flowers (Thakore et al., 1999). In addition, whole-mount in situ hybridization and promoter-GUS reporter fusions showed some tissue specificity of RCN1 in roots, shoots, and etiolated hypocotyls (Deruère et al., 1999).
RCN1 as a Positive Transducer of ABA Signaling
Reversible phosphorylation events play important roles in ABA signal transduction. Genetic screens have yielded two genetically dominant ABA-insensitive Arabidopsis mutants, abi1-1 and abi2-1 (Koornneef et al., 1984), in which two genes encoding PP2C have a point mutation (Leung et al., 1994, 1997; Meyer et al., 1994; Rodriguez et al., 1998). The mutant alleles of these genes are dominant, which supports the proposed redundancy of PP2Cs (Leung et al., 1997; Rodriguez et al., 1998). It is possible that the wild-type ABI1 and ABI2 proteins have additional functions. Because intragenic suppressors of the abi1-1 and abi2-1 mutants showed reduced or no protein phosphatase activity in vitro (Gosti et al., 1999; Merlot et al., 2001) and recessive ABA hypersensitivity was reported in a double mutant of both suppressors (Merlot et al., 2001), these PP2Cs have been proposed to act as negative regulators of ABA signaling (Gosti et al., 1999; Merlot et al., 2001). Furthermore, overexpression of the constitutively active ABI1 and AtPP2C mutant isoforms in maize mesophyll protoplasts inhibited ABA-induced gene expression, also suggesting that PP2Cs are negative regulators of ABA signaling (Sheen, 1998). All suppressor mutants in abi1-1 and abi2-1 have point mutations downstream of the dominant G180D (abi1-1) and G168D (abi2-1) sites, and no stop codon or frameshift alleles have been isolated. Research with gene disruption/ silencing mutants should allow further analysis of the model that the wild-type ABAI1 and ABI2 PP2Cs function as negative regulators of ABA signaling (Sheen, 1998; Merlot et al., 2001).
The present study demonstrates that in addition to PP2Cs, PP2As function in early ABA signal transduction. rcn1 used in our study is a recessive ABA-insensitive gene disruption mutant that is complemented by the wild-type RCN1 gene. A previous biochemical and pharmacological study showed that wild-type RCN1 protein increases PP2A activity in vivo and that rcn1 shows reduced PP2A activity (Deruère et al., 1999). ABA responses in the wild type were inhibited by short-term OA exposure (Figures 4D and 5B) (Pei et al., 1997), which correlates with rcn1 mutant phenotypes in ABA inhibition of seed germination, ABA-induced [Ca2+]cyt increases, ABA-induced stomatal closing, and ABA activation of anion channels in Arabidopsis guard cells (Figures 1B and 2). These data provide evidence that RCN1 is a positive transducer of ABA signal transduction.
Pharmacological research with guard cells from other species and with other cell types has suggested that other PP1s or PP2As may function as negative regulators of ABA signal transduction (Schmidt et al., 1995; Esser et al., 1997; Grabov et al., 1997; Wu et al., 1997). Other yet-to-be-identified PP1 or PP2A genes or protein complexes may act as negative regulators of ABA signaling. Our genetic and cell biological analyses have allowed a distinction between pharmacologically proposed PP1s and PP2As by showing that the RCN1 PP2A positively mediates ABA signal transduction.
Conclusions
In conclusion, we have identified a guard cell–expressed PP2A regulatory A subunit gene, RCN1, which functions in ABA signal transduction. The rcn1 loss-of-function mutation confers general ABA insensitivity to Arabidopsis and impairs ABA-induced [Ca2+]cyt increases in guard cells, ABA activation of anion channels, and ABA-induced stomatal closing. Short-term exposure to the PP2A inhibitor OA in wild-type Arabidopsis phenocopies rcn1, further suggesting that RCN1 functions in ABA signal transduction. Furthermore, ABA inhibition of seed germination and ABA induction of gene expression are reduced in rcn1, suggesting that RCN1 is a general transducer of ABA signaling.
METHODS
Identification of the RCN1 Gene in Guard Cells
To identify guard cell–expressed type 2A protein phosphatase (PP2A) genes, catalytic and regulatory subunit sequences of PP2A genes were aligned and then two conserved regions of PP2A regulatory A subunits were selected: AH/YVLLPPLE (for the sense primer) and DVRY/FFANQA (for the antisense primer). The degenerate oligomers designed from these sequences were 5′-GCIYAYGTIYTIYTICCICCIYTIGA-3′ (sense primer) and 5′-GCYTGRTTIGCRAARTAI-CKIACRTC-3′ (antisense primer). Total RNA was extracted from guard cell–enriched epidermal strips as described (Hugouvieux et al., 2001). Guard cell cDNA libraries were synthesized from guard cell total RNA (1 to ∼2 μg) using the First-Strand cDNA Synthesis Kit according to the manufacturer's instructions (Amersham Pharmacia Biotech). PCR was performed in a 50-μL mixture (sense and antisense primers at 200 nM, 1 × ExTaq polymerase buffer [Takara, Otsu, Japan], each deoxynucleotide triphosphate at 200 μM, 5 ng of cDNA, and 2.5 units of ExTaq polymerase). The PCR mixture was denatured at 94°C for 4 min followed by 35 cycles of amplification (94°C for 30 s, 45°C for 30 s, and 72°C for 3 min). PCR products were purified and cloned into the pGEM-T Easy vector (Promega). Sequencing of inserts was performed as described (Kwak et al., 1997).
Stomatal Aperture Measurements
Leaves of 5- to 6-week-old wild-type, rcn1, and rcn1-complemented Arabidopsis thaliana plants (T4 progeny of rcn1g17-1 and rcn1g8-1) (Garbers et al., 1996) were incubated in white light (fluence rate of 125 or 83 μmol·m−2·s−1) for 2.5 h in stomatal opening solution containing 5 mM KCl, 50 μM CaCl2, and 10 mM Mes/Tris, pH 6.15, except for the experiment illustrated in Figure 1C, in which 30 mM KCl, 50 μM CaCl2, and 10 mM Mes/Tris, pH 6.15, was used as described by Pei et al. (1997). In the present study, stomatal apertures were measured in the focal plane of the outer edges of guard cells in epidermal strips. For calcium-induced stomatal closing experiments, the same opening solution without any calcium was used. Stomatal apertures were measured 3 h after abscisic acid (ABA), calcium, or H2O2 was added. Standard errors were calculated relative to the square root of the number of stomatal aperture experiments. Statistical significance was determined using Student's t test (two-tailed distribution, two samples assuming equal variance) and Excel software (version 98; Microsoft, Redmond, WA).
Reverse Transcription PCR and β-Glucuronidase Activity Analyses
Leaves of 5- to 6-week-old wild-type Arabidopsis plants were used to prepare guard cell and mesophyll cell protoplasts as described (Leonhardt et al., 1997). Protoplast preparations with high purity (>97% for guard cells and >95% for mesophyll cells) were used to extract total RNA using Trizol reagent (Life Technologies, Rockville, MD). First-strand cDNA was synthesized using the First-Strand cDNA Synthesis Kit (Amersham Pharmacia Biotech). The PCR mixture was prepared as described above and denatured at 94°C for 4 min followed by 27 cycles of amplification (94°C for 30 s, 57°C for 30 s, and 72°C for 2 min). Primers used for PCR were as follows: Actin1-5, 5′-GGCCGATGGTGAGGATATTCAGCCACTTG-3′; Actin1-3, 5′-TCG-ATGGACCTGACTCATCGTACTCACTC-3′; RCN1-51, 5′-CCGACG-CCTGGATCGTGATTTGATTCGA-3′; and RCN1-31, 5′-CAATTCAGG-ATTGTGCTGCTGTGGAACCA-3′. β-Glucuronidase activity was assayed on 12-day-old wild-type transgenic seedlings grown on Murashige and Skoog (1962) plates as described (Hugouvieux et al., 2001).
Patch-Clamp Analyses
Arabidopsis guard cell protoplasts were isolated enzymatically from leaves of 5- to 6-week old wild-type and rcn1 plants as described previously (Kwak et al., 2001). Whole-cell recordings of guard cells were conducted as described (Pei et al., 1997). The pipette solution contained 150 mM CsCl, 5.87 mM CaCl2, 2 mM MgCl2, 6.7 mM EGTA, 5 mM Mg-ATP, and 10 mM Hepes/Tris, pH 7.1. The bath solution contained 30 mM CsCl, 1 mM CaCl2, 2 mM MgCl2, and 10 mM Mes/Tris, pH 5.6. Osmolalities were adjusted to 500 mmol/kg for the pipette solution and to 485 mmol/kg for the bath solution. Seal resistance was >10 GΩ. The holding potential was +30 mV. Subsequent voltage steps were decreased by 30 mV per pulse. To measure the effects of the upstream ABA signaling pathway on slow anion channel activities, protoplasts were preincubated at 22°C with 50 μM ABA for 2 h before patch-clamp recordings (Pei et al., 1997). Leak currents were not subtracted.
Calcium Imaging Analyses
Four- to 6-week old wild-type (two independent lines) and rcn1 (three independent lines) plants stably transformed with the yellow cameleon construct p35SYC2.1 were used for calcium imaging analyses as described (Allen et al., 1999b; Hugouvieux et al., 2001). Epidermal strips were incubated in white light (fluence rate of 125 μmol· m−2·s−1) for 2.5 h in stomatal opening solution containing 5 mM KCl, 50 μM CaCl2, and 10 mM Mes/Tris, pH 6.15, before recordings. To test okadaic acid (OA) effects on ABA-induced cytosolic Ca2+ concentration ([Ca2+]cyt) increases in wild-type guard cells, epidermal strips were incubated in the dark for 2.5 h in the same solution in the absence or presence of 1 μM OA before recordings. Background fluorescence was measured in guard cell-less epidermal domains (∼20% of cameleon fluorescence intensity) and was subtracted from the epidermal field before ABA application. Chloroplast fluorescence in guard cells was not observed because of changes in the optical setup and the use of higher-cameleon-expressing plants (Allen et al., 1999b). Statistical analyses demonstrated that the average fluorescence baseline ratio before ABA application was not significantly different in wild-type (0.87 ± 0.15) and rcn1 (0.83 ± 0.14; P > 0.12) plants. [Ca2+]cyt transients were counted when the change in [Ca2+]cyt ratios was ≥0.1 units above the baseline. More than 50% of cells showed spontaneous Ca2+ transients and were excluded from analyses (Allen et al., 1999b). Cells showing stable [Ca2+]cyt ratios during the first 7 min were exposed to ABA. Unmarked and mixed complete data sets were analyzed as blind tests in which the identities of the plant lines (wild-type or rcn1) under investigation were not known to two independent researchers, confirming the reduced ABA responsiveness in rcn1 (V. Hugouvieux, χ2 = 3.9, P < 0.03; J. Young, χ2 = 4.9, P < 0.03).
Seed Germination Analyses
Seeds of wild-type, rcn1, and two independent rcn1-complemented lines were plated on one-fourth-strength Murashige and Skoog (1962) medium containing 0, 0.5, 1, 2, or 5 μM ABA. Seeds were stratified at 4°C for 4 days and then transferred to a growth chamber (24°C under a 16-h-light/8-h-dark regime). Seed germination rates were scored after 5 days in the growth chamber. To test the effect of OA, 1 μM OA was added to medium containing the indicated ABA concentrations.
RNA Gel Blot Analyses
Total RNA was extracted from flowers, leaves, stems, and roots of 5- to 6-week-old wild-type plants as described above. Poly(A+) RNA was further purified using the μMACS mRNA Isolation Kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. To determine ABA-inducible gene expression, total RNA was extracted from rosette leaves of wild-type and rcn1 plants sprayed with 1 or 10 μM ABA for 2 and 5 h. Total and poly(A+) RNA were separated on a 1.2% (w/v) denaturing agarose gel and then transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech). The blots were hybridized with 32P-labeled RCN1, KIN1, or KIN2 cDNA. Blots were washed as described (Kwak et al., 1997). 32P-labeled Actin1 cDNA or 18S rDNA was used to show relative amounts of RNA samples.
Upon request, all novel materials described in this article will be made available in a timely manner for noncommercial research purposes. No restrictions or conditions will be placed on the use of any materials described in this article that would limit their use for noncommercial research purposes.
Acknowledgments
We thank Michel Wang for help with rcn1 stomatal aperture measurements, Gethyn Allen for advice with calcium imaging experiments, and Véronique Hugouvieux and Jared Young for blind analyses of calcium imaging data. This research was supported by National Science Foundation (MCB 0077791) and National Institutes of Health (R01GM60396-01) grants to J.I.S. and fellowships to J.M.K. and N.L. from the Human Frontier Science Program Organization. We dedicate this work to the memory of our friend and colleague, Gethyn Allen.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.003335.
References
- Allan, A.C., Fricker, M.D., Ward, J.L., Beale, M.H., and Trewavas, A.J. (1994). Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell 6, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffmann, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999. a). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11, 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999. b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19, 735–747. [DOI] [PubMed] [Google Scholar]
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Ariño, J., Pérez-Callejón, E., Cunillera, N., Camps, M., Posas, F., and Ferrer, A. (1993). Protein phosphatases in higher plants: Multiplicity of type 2A phosphatases in Arabidopsis thaliana. Plant Mol. Biol. 21, 475–485. [DOI] [PubMed] [Google Scholar]
- Assmann, S.M., and Shimazaki, K. (1999). The multisensory guard cell: Stomatal responses to blue light and abscisic acid. Plant Physiol. 119, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin, N., Serizet, C., Gosti, F., and Giraudat, J. (2000). Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt, M.R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol. 16, 221–241. [DOI] [PubMed] [Google Scholar]
- Camilleri, C., Azimzadeh, J., Pastuglia, M., Bellini, C., Grandjean, O., and Bouchez, D. (2002). The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell 14, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamayor, A., Pérez-Callejón, E., Pujol, G., Ariño, J., and Ferrer, A. (1994). Molecular characterization of a fourth isoform of the catalytic subunit of protein phosphatase 2A from Arabidopsis thaliana. Plant Mol. Biol. 26, 523–528. [DOI] [PubMed] [Google Scholar]
- Corum, J.W.I., Hartung, A.J., Stamey, R.T., and Rundle, S.J. (1996). Characterization of DNA sequences encoding a novel isoform of the 55 kDa B regulatory subunit of the type 2A protein serine/threonine phosphatase of Arabidopsis thaliana. Plant Mol. Biol. 31, 419–427. [DOI] [PubMed] [Google Scholar]
- Deruère, J., Jackson, K., Garbers, C., Söll, D., and DeLong, A. (1999). The RCN1-encoded A subunit of protein phosphatase 2A increases phosphatase activity in vivo. Plant J. 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Ephritikhine, G., Fellner, M., Vannini, C., Lapous, D., and Barbier-Brygoo, H. (1999). The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J. 18, 303–314. [DOI] [PubMed] [Google Scholar]
- Esser, J.E., Liao, Y.-J., and Schroeder, J.I. (1997). Characterization of ion channel modulator effects on ABA- and malate-induced stomatal movements: Strong regulation by kinase and phosphatase inhibitors, and relative insensitivity to mastoparans. J. Exp. Bot. 48, 539–550. [DOI] [PubMed] [Google Scholar]
- Finkelstein, R.R. (1994). Mutations at two new Arabidopsis ABA response loci are similar to abi3 mutations. Plant J. 5, 765–771. [Google Scholar]
- Finkelstein, R.R., Gampala, S.S.L., and Rock, C.D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14 (suppl.), S15.–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., and Lynch, T.J. (2000). The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., and Zeevaart, J.A.D. (1994). Gibberellin and abscisic acid biosynthesis and response. In Arabidopsis, E.M. Meyerowitz and C.R. Somerville, eds (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), pp. 523–553.
- Foster, R., and Chua, N.-H. (1999). An Arabidopsis mutant with deregulated ABA gene expression: Implications for negative regulator function. Plant J. 17, 363–372. [DOI] [PubMed] [Google Scholar]
- Garbers, C., DeLong, A., Deruère, J., Bernasconi, P., and Söll, D. (1996). A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO J. 15, 2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Ghassemian, M., Nambara, E., Cutler, S., Kawaide, H., Kamiya, Y., and McCourt, P. (2000). Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Cornel, D., Miginiac, E., Rona, J.-P., and Sotta, B. (2000. a). Abscisic acid specific expression of RAB18 involves activation of anion channels in Arabidopsis thaliana suspension cells. FEBS Lett. 474, 43–47. [DOI] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Miginiac, E., and Sotta, B. (2000. b). Abscisic acid plasmalemma perception triggers a calcium influx essential for RAB18 gene expression in Arabidopsis thaliana suspension cells. FEBS Lett. 483, 67–70. [DOI] [PubMed] [Google Scholar]
- Giraudat, J., Hauge, B.M., Valon, C., Smalle, J., Parcy, F., and Goodman, H.M. (1992). Isolation of the Arabidopsis ABI3 gene by positional cloning. Plant Cell 4, 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosti, F., Beaudoin, N., Serizet, C., Webb, A.A.R., Vartanian, N., and Giraudat, J. (1999). ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11, 1897–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov, A., Leung, J., Giraudat, J., and Blatt, M.R. (1997). Alteration of anion channel kinetics in wild-type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J. 12, 203–213. [DOI] [PubMed] [Google Scholar]
- Grill, E., and Himmelbach, A. (1998). ABA signal transduction. Curr. Opin. Plant Biol. 5, 412–418. [DOI] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Kohler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, J.G., Hartung, A.J., Hendershot, J.D., Passingham, R.S., and Rundle, S.J. (1999). Molecular characterization of the B′ regulatory subunit gene family of Arabidopsis protein phosphatase 2A. Eur. J. Biochem. 260, 127–136. [DOI] [PubMed] [Google Scholar]
- Hey, S.J., Bacon, A., Burnett, E., and Neill, S.J. (1997). Abscisic acid signal transduction in epidermal cells of Pisum sativum L. Argenteum: Both dehydrin mRNA accumulation and stomatal responses require protein phosphorylation and dephosphorylation. Planta 202, 85–92. [Google Scholar]
- Himmelbach, A., Iten, M., and Grill, E. (1998). Signalling of abscisic acid to regulate plant growth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux, V., Kwak, J.M., and Schroeder, J.I. (2001). An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell 106, 477–487. [DOI] [PubMed] [Google Scholar]
- Janssens, V., and Goris, J. (2001). Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., Leon-Kloosterziel, K.M., Schwartz, S.H., and Zeevaart, J.A.D. (1998). The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem. 36, 83–89. [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61, 377–383. [Google Scholar]
- Kuo, A., Cappelluti, S., Cervantes-Cervantes, M., Rodriguez, M., and Bush, D.S. (1996). Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 8, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak, J.M., Kim, S.A., Hong, S.W., and Nam, H.G. (1997). Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris. Planta 202, 9–17. [DOI] [PubMed] [Google Scholar]
- Kwak, J.M., Murata, Y., Baizabal-Aguirre, V.M., Merrill, J., Wang, M., Kemper, A., Hawke, S.D., Tallman, G., and Schroeder, J.I. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127, 473–485. [PMC free article] [PubMed] [Google Scholar]
- Latorre, K.A., Harris, D.M., and Rundle, S.J. (1997). Differential expression of three Arabidopsis genes encoding the B′ regulatory subunit of protein phosphatase 2A. Eur. J. Biochem. 245, 156–163. [DOI] [PubMed] [Google Scholar]
- Leckie, C.P., McAinsh, M.R., Montgomery, L., Priestley, A.J., Staxen, I., Webb, A.A.R., and Hetherington, A.M. (1998). Second messengers in guard cells. J. Exp. Bot. 49, 339–349. [Google Scholar]
- Leonhardt, N., Marin, E., Vavasseur, A., and Forestier, C. (1997). Evidence for the existence of a sulfonylurea-receptor-like protein in plants: Modulation of stomatal movements and guard cell potassium channels by sulfonylureas and potassium channel openers. Proc. Natl. Acad. Sci. USA 94, 14156–14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt, N., Vavasseur, A., and Forestier, C. (1999). ATP binding cassette modulators control abscisic acid–regulated slow anion channels in guard cells. Plant Cell 11, 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., Bouvier-Durand, M., Morris, P.-C., Guerrier, D., Chefdor, F., and Giraudat, J. (1994). Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264, 1448–1452. [DOI] [PubMed] [Google Scholar]
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 199–222. [DOI] [PubMed] [Google Scholar]
- Leung, J., Merlot, S., and Giraudat, J. (1997). The Arabidopsis ABSCISIC ACID-INSENSITIVE 2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9, 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., and Assmann, S.M. (1996). An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8, 2359–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wang, X.-Q., Watson, M.B., and Assmann, S.M. (2000). Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287, 300–303. [DOI] [PubMed] [Google Scholar]
- Lu, C., and Fedoroff, N. (2000). A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12, 2351–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H., Yanofsky, M.F., Klee, H.J., Bowman, J.L., and Meyerowitz, E.M. (1992). Vectors for plant transformation and cosmid libraries. Gene 117, 161–167. [DOI] [PubMed] [Google Scholar]
- MacRobbie, E.A. (1998). Signal transduction and ion channels in guard cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 1475–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343, 186–188. [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1997). Calcium ions as second messengers in guard cell signal transduction. Physiol. Plant. 100, 16–29. [Google Scholar]
- McAinsh, M.R., Webb, A.R., Taylor, J.E., and Hetherington, A.M. (1995). Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot, S., Gosti, F., Guerrier, D., Vavasseur, A., and Giraudat, J. (2001). The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 25, 295–303. [DOI] [PubMed] [Google Scholar]
- Meyer, K., Leube, M.P., and Grill, E. (1994). A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264, 1452–1455. [DOI] [PubMed] [Google Scholar]
- Miyawaki, A., Llopis, J., Heim, R., McCaffery, J.M., Adams, J.A., Ikura, J.A., and Tsien, R.Y. (1997). Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887. [DOI] [PubMed] [Google Scholar]
- Mori, I., and Muto, S. (1997). Abscisic acid activates a 48-kilodalton protein kinase in guard cell protoplasts. Plant Physiol. 113, 833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Murata, Y., Pei, Z.-M., Mori, I.C., and Schroeder, J.I. (2001). Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 13, 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Ghassemian, M., Kwak, C.M., McCourt, P., and Schroeder, J.I. (1998). Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290. [DOI] [PubMed] [Google Scholar]
- Pei, Z.-M., Kuchitsu, K., Ward, J.M., Schwarz, M., and Schroeder, J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9, 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.-M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Pérez-Callejón, E., Casamayor, A., Pujol, G., Clua, E., Ferrer, A., and Ariño, J. (1993). Identification and molecular cloning of two homologues of protein phosphatase X from Arabidopsis thaliana. Plant Mol. Biol. 23, 1177–1185. [DOI] [PubMed] [Google Scholar]
- Rashotte, A.M., DeLong, A., and Muday, G.K. (2001). Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell 13, 1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, P.L., Benning, G., and Grill, E. (1998). ABI2, a second protein phosphatase 2C involved in abscisic acid signal transduction in Arabidopsis. FEBS Lett. 421, 185–190. [DOI] [PubMed] [Google Scholar]
- Rundle, S.J., Hartung, A.J., Corumi, J.W.I., and O'Neill, M. (1995). Characterization of a cDNA encoding the 55 kDa B regulatory subunit of Arabidopsis protein phosphatase 2A. Plant Mol. Biol. 28, 257–266. [DOI] [PubMed] [Google Scholar]
- Sanchez, J.-P., and Chua, N.-H. (2001). Arabidopsis PLC1 is required for secondary responses to abscisic acid signals. Plant Cell 13, 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, C., Schelle, I., Liao, Y.J., and Schroeder, J.I. (1995). Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc. Natl. Acad. Sci. USA 92, 9535–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Allen, G.J., Hugouvieux, V., Kwak, J.M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338, 427–430. [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1990). Repetitive increases in cytosolic calcium of guard cells by abscisic acid activation of nonselective calcium permeable channels. Proc. Natl. Acad. Sci. USA 87, 9305–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., and Keller, B.U. (1992). Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl. Acad. Sci. USA 89, 5025–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Schmidt, R.C., and Sheaffer, J. (1993). Identification of high-affinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard cells. Plant Cell 5, 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen, J. (1996). Ca2+-dependent protein kinases and stress signal transduction in plants. Science 274, 1900–1902. [DOI] [PubMed] [Google Scholar]
- Sheen, J. (1998). Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc. Natl. Acad. Sci. USA 95, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staxen, I., Pical, C., Montgomery, L.T., and Gray, J.E. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96, 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terol, J., Bargues, M., Carrasco, P., Pérez-Alonso, M., and Paricio, N. (2002). Molecular characterization and evolution of the protein phosphatase 2A B′ regulatory subunit family in plants. Plant Physiol. 129, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakore, C.U., Livengood, A.J., Hendershot, J.D.I., Corum, J.W., LaTorre, K.A., and Rundle, S.J. (1999). Characterization of the promoter region and expression pattern of three Arabidopsis protein phosphatase type 2A subunit genes. Plant Sci. 147, 165–176. [Google Scholar]
- Wu, Y., Kuzma, J., Marechal, E., Graeff, R., Lee, H.C., Foster, R., and Chua, N.-H. (1997). Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278, 2126–2130. [DOI] [PubMed] [Google Scholar]
- Xiong, L., Ishitani, M., Lee, H., and Zhu, J.-K. (2001. a). The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress– and osmotic stress–responsive gene expression. Plant Cell 13, 2063–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L., Lee, B.-h., Ishitani, M., Lee, H., Zhang, C., and Zhu, J.-K. (2001. b). FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 15, 1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]