Abstract
An inducible fluorescent system based on GFP is presented that allows for the uncoupling of dendritic mRNA transport from subsequent protein synthesis at the single cell level. The iron-responsive element (IRE) derived from ferritin mRNA in the 5′-UTR of the GFP reporter mRNA renders translation of its mRNA dependent on iron. The addition of the full-length 3′-UTR of the Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα) after the stop codon of the GFP reading frame targets the reporter mRNA to dendrites of transfected fully polarized hippocampal neurons. As we show by time-lapse videomicroscopy, iron specifically turns on GFP reporter protein synthesis in a single transfected hippocampal neuron. We investigate whether GFP expression is affected—in addition to iron—by synaptic activity. Interestingly, synaptic activity has a clear stimulatory effect. Most importantly, however, this activity-dependent protein synthesis is critically dependent on the presence of the full-length 3′-UTR of CaMKIIα confirming that this sequence contains translational activation signals. The IRE-based system represents a new convenient tool to study local protein synthesis in mammalian cells where mRNA localization to a specific intracellular compartment occurs.
INTRODUCTION
Activity-dependent synaptic plasticity is an attractive mechanism that might explain such complex biological phenomena as formation and long-lasting storage of memory. It is thought that the strength of synapses is changed in response to activation of neurotransmitter receptors (Frey and Morris, 1997; Martin et al., 2000). Elegant experiments have shown that this modulation of synaptic strength is dependent on de novo protein synthesis (Stanton and Sarvey, 1984; Frey et al., 1988; Nguyen et al., 1994; Casadio et al., 1999). Interestingly, two different mechanisms contribute to this effect. First, translation occurs in the cell body of the postsynaptic neuron (Bailey et al., 1996). The resulting proteins are then actively transported to distal regions of the dendrites on demand. Second, the fact that both localized transcripts and components of the translational machinery have been detected in proximity of dendritic spines suggests that local dendritic protein synthesis could play a role in synaptic plasticity (Steward and Schuman, 2001). Feig and Lipton were the first to demonstrate that dendritic protein synthesis—visualized by incorporation of tritiated leucine—was increased by simultaneous stimulation of afferents and activation of acetylcholine receptors (Feig and Lipton, 1993). In hippocampal slices, BDNF-induced synaptic plasticity can be blocked by protein synthesis inhibitors (Kang and Schuman, 1996). It has been difficult to directly and unambiguously visualize local protein synthesis in intact CNS neurons experimentally, given the possibility that proteins translated in the cell body could be subsequently transported to the synapse. The first experimental approach to overcome this limitation has been to physically or optically separate dendrites from their cell bodies and then study potential local protein synthesis in those severed dendrites (Crino et al., 1998; Aakalu et al., 2001). In the first study, Crino et al. transfected a reporter mRNA into transected dendrites and showed that this compartment was still competent for translation. In the second study, Aakalu et al. transfected hippocampal neurons with a reporter construct coding for GFP flanked by the 5′- and 3′-UTR from CaMKIIα. Translation in dendrites was then analyzed by physical transection from cell bodies or by photo bleaching followed by fluorescence recovery. Interestingly, BDNF stimulated the translation of GFP in mechanically or optically isolated dendrites, suggesting that this reflects local protein synthesis independent of their cell bodies.
Here, we report a new fluorescent system based on GFP whose transcription is under the control of the ferritin promoter and which contains an iron-responsive element (IRE). Because translation is now under the control of iron, mRNA transport can therefore be uncoupled from subsequent protein synthesis. With this new fluorescent system, translation can be studied in a single living nerve cell, and it is even feasible to turn on protein synthesis within one compartment without affecting it in another. Furthermore, we show that GFP expression can be regulated by synaptic activity, specifically when the reporter transcript has been targeted to dendrites by the addition of the 3′-UTR of CaMKIIα containing a dendritic targeting element.
MATERIALS AND METHODS
Materials and Reagents
BDNF (25 ng/ml), l-glutamate (50 μM), and Lipofectamine 2000 were from Invitrogen/Life Technologies (Karlsruhe, Germany) and holo-transferrin (300 nM) was from Calbiochem-Novabiochem GmbH (Bad Soden, Germany). All other chemicals were purchased from Sigma (Munich, Germany): Actinomycin D (1 μM); APV (50 μM), iron chelator desferrioxamine mesylate (100 μM), CNQX (10 μM), ferric ammonium citrate (100 μM), and glycine (0.1 mM).
Constructs
A NLS-containing sequence (MKRPAATKKAGQAKKKKP) was cloned in frame with the first methionine of the cDNA encoding for GFP5 (accession no. AJ3069; construct 1, generous gift from Stephan Geley, ICRF, UK) or for GFP5 containing the full-length 3′-UTR of Ca2+/calmodulin-dependent protein kinase II alpha (CaMKIIα; Mayford et al., 1996) introduced after the stop codon (construct 2). The last 337 nucleotides of the rat ferritin promoter region and its 5′-UTR containing the IRE were amplified by PCR using the following primers: forward (containing an AseI restriction site) 5′-ccattaatctcagagacccaagagccg, reverse (containing an AgeI restriction site) 5′-caaccggtgatggcggctggggg. The PCR product was sequenced and cloned into pd2EGFP-N1 expression vector (BD Biosciences/Clontech, Heidelberg, Germany) that was cut with the same restriction enzymes to replace the CMV promoter by the ferritin promoter fragment. The destabilized GFP was then removed from the pd2EGFP-N1 vector using AgeI and NotI restriction enzymes and the vector was blunt-ended using T4 polymerase. The NLS-GFP cassettes (plus or minus the 3′-UTR) were then cloned inside the resulting expression vector and the orientation of the two inserts was confirmed by sequencing. The structure of the resulting mRNA was analyzed using the Zucker RNA folding program (Zucker and Stiegler, 1981).
In Situ Hybridization of Cultured Neurons
The NLS-GFP cDNA was subcloned into Bluescript KS (Stratagene, Heidelberg, Germany). Antisense and sense RNA probes labeled with digoxigenin were synthesized according to the manufacturer's protocol (Roche Diagnostics, Mannheim, Germany) by run-off transcription from restriction-digested plasmids with the T7 or T3 RNA polymerase, respectively. Fluorescent in situ hybridization (ISH) was performed as described by Blichenberg et al. (1999) with the following modifications. After fixation with 4% PFA, cells on coverslips were washed three times with PBS for 5 min at RT and then permeabilized for 3 min in PBS containing 0.1% (vol/vol) Triton X-100. Coverslips were then washed three times with PBS for 5 min at RT and subsequently prehybridized for 2 h at 50°C in 50% (vol/vol) deionized formamide, 5× SSC, 5× Denhardt's solution, 250 μg/ml Escherichia coli tRNA (Roche Diagnostics), 500 μg/ml denatured herring salmon sperm DNA. The solution was removed and the coverslips were then incubated overnight at 50°C with fresh hybridization solution containing 500 ng/ml in vitro–transcribed digoxigenin-labeled probes. The cells were subsequently washed at room temperature twice in 1× SSC and 0.1% (wt/vol) SDS for 10 min and then twice in 0.2× SSC, 0.1% (wt/vol) SDS at 50°C for 15 min each. The probes were detected using the HNPP fluorescent detection set (Roche Diagnostics) according to the manufacturer's description. Coverslips were mounted and processed as described below. The number of particles detected in dendrites was analyzed by choosing selected areas in the following way: first, only distal parts of dendrites with positive signals were selected and the threshold was set in a manner to detect individual particles. Then, the “analyze particles” feature of the NIH 1.6.2. imaging program (free shareware: http://rsb.info.nih.gov/nih-image/Default.html) was used to count particles (Σ: 180) from four dendrites each. The mean and the SD were calculated using the Microsoft Excel software.
For the in situ hybridization combined with immunocytochemistry, hippocampal neurons, pretreated with iron chelator for 12 h, were transfected and kept either in iron chelator or incubated in medium containing 100 μM of ferric ammonium citrate. For visualization of the in situ hybridization signal and immunostaining with GFP antibodies, the coverslips were incubated 1 h at room temperature with rhodamine-conjugated monoclonal anti-DIG (1:100; Roche) together with rabbit polyclonal anti-GFP antibodies (1:1000; Torrey Pines Biolab Inc., San Diego, CA). The coverslips were then incubated for 1 h at room temperature with anti-rabbit Alexa 488–conjugated secondary antibodies (Dianova, Hamburg, Germany). The coverslips were mounted using the ProLong Antifade kit (Molecular Probes, Leiden, The Netherlands). The same procedure was followed to perform ISH and immunostaining in BHK cells.
COS-7, BHK Cell Cultures, and Transient Transfection
COS-7 and BHK-21 cells were grown in minimal essential medium (MEM) and GMEM, respectively, supplemented with 10% fetal bovine serum and penicillin/streptomycin (Wickham et al., 1999). For immunofluorescence, cells were grown on poly-l-lysine coated (0.1 mg/ml) glass coverslips; for Western blotting, cells were grown in six-well plates (Costar, Cambridge, MA). Cells at 50–70% confluency were transfected with 1 μg of DNA per coverslip or 2 μg DNA per well using FuGENE-6 (Roche Diagnostics) according to the manufacturer's protocol, and expression of GFP was routinely analyzed 12 h upon transfection.
Actinomycin D Treatment
BHK cells were pretreated with iron chelator 6 h before transfection and incubated in the same medium for 14–16 h after transfection with 2 μg of DNA of construct 2, using FuGENE 6. Actinomycin D (Act D; 1 μM) was added 30 min before iron addition and the cells were incubated for 4 h before fixation. The cells were then counted as described later.
Hippocampal Cell Culture and Transient Transfection
Primary hippocampal neurons derived from E17 rat embryos were cultured as described in Kiebler et al. (1999) with the notable exception that cells were grown in N-MEM supplemented with B-27 (Invitrogen/Life Technologies, Karlsruhe, Germany) instead of N2 supplements. Adult primary hippocampal neurons (stage 5) were transfected using a modified Ca2+-phosphate precipitation protocol that has been described in detail in Köhrmann et al. (1999b). In brief, the pH of the neuronal N-MEM/B27 medium was adjusted to 7.00 before sterile filtration. AMPA/kainate receptor antagonist CNQX (10 μM; Sigma) was added to the medium to reduce excitotoxic death during the transfection procedure. The medium was allowed to equilibrate in 3-cm culture dishes at 5% CO2 for at least 1 h before transfection. The phosphate-containing buffer for transfection (2× BBS) was adjusted to a pH of 7.00. Air was bubbled into the final transfection mixture with an Eppendorf pipette for 1 min. The neurons on coverslips were then transferred into the preequilibrated neuronal medium (see above) with the cells facing up and the transfection mixture was added immediately. The dishes were subsequently incubated at 37°C and 2.5% CO2. The transfection was stopped after inspecting the size of the precipitate as described in Köhrmann et al. (1999b), typically 25–40 min after addition of the transfection mixture. The precipitate was washed off using Hanks' balanced saline (HBSS), and the coverslips were then transferred to the appropriate medium for the remainder of the experiment. Transfected hippocampal neurons were routinely analyzed as early as 5 h upon transfection (see also Wells et al., 2001).
Time-lapse Videomicroscopy of Living Hippocampal Neurons
Primary hippocampal neurons were cultured in video dishes (Willco Wells, Amsterdam, The Netherlands) as described above. Cells were transfected using Lipofectamine 2000 according to the manufacturer's protocol with the exception that 1 μg Lipofectamine 2000 was used per 1 μg DNA in 100 μl of medium. After 50 min of incubation, cells were washed twice with HBSS, and fresh medium was added; 4.5–6 h upon transfection, cells were transferred into the live chamber (for details see Köhrmann et al., 1999a), and 100 μM ferric ammonium citrate was added. Then, time-lapse videomicroscopy was performed 20–25 min upon iron addition as described in detail in Köhrmann et al. (1999a) with the following modifications. For fluorescence detection, a Roper scientific MicroMax Digital CCD-400B camera controlled by the Metamorph 4.6–5.0 imaging software package (Universal Imaging/Visitron Systems, Puchheim, Germany) was used. This software was then also used for image processing.
Pharmacological Treatment of Neurons
To ensure that the cells respond to the exogenously added iron into the medium, the following two controls were routinely performed. One set of cells (baseline value) was kept under low iron conditions for the whole duration of the experiment; the other set received ferric ammonium citrate (100 μM) after transfection (positive control). If cells were not responding appropriately to iron, the whole experiment was discarded.
For the initial experiments with COS-7 cells (Figure 1) and neurons (Figure 2), cells were either kept under either low iron conditions (iron chelator: desferrioxamine mesylate, 100 μM) or high iron conditions (ferric ammonium citrate, 100 μM, or holo-transferrin, 300 nM) for the whole time after transfection. Finally, cells were either processed for immunofluorescence or extracted for SDS-PAGE and Western blotting.
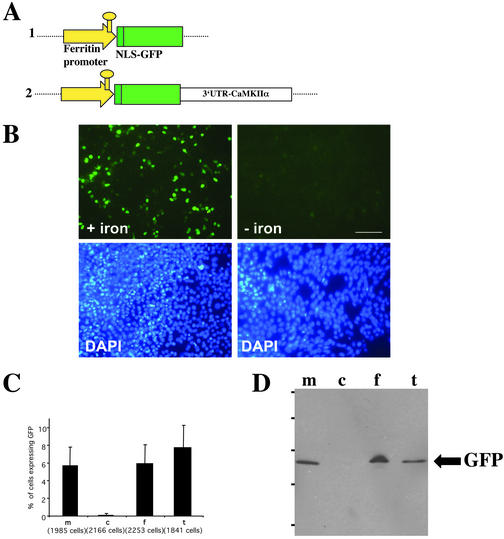
Figure 1.
Iron-dependent regulation of GFP expression in COS cells. (A) Schematic representation of the constructs used in this study. Construct 1: the ferritin promoter containing the IRE element drives the expression of the NLS-GFP. To target the resulting reporter mRNA to dendrites, the full-length 3′-UTR of the CaMKIIα (Mayford et al., 1996; Miller et al., 2002) was added downstream of the stop codon of the GFP cDNA (construct 2). (B) Representative example of a microscopic field containing COS cells that were transiently transfected with construct 2 and analyzed for GFP expression at the single cell level. In the presence of iron, COS cells express GFP, whereas in the presence of the iron chelator, translation is inhibited. The lower panels show the same fields of cells stained with the nuclear dye DAPI to quantify the total number of cells. (C) Quantitative analysis of COS cells expressing GFP in the presence of iron sources. The graph represents one of four independent experiments. (D) Western blot of COS cells that were transiently transfected with construct 2. m, normal medium; c, chelator; f, ferric ammonium citrate; t, holo-transferrin. GFP was detected by a polyclonal anti-GFP antibody. Bar, 100 μm.
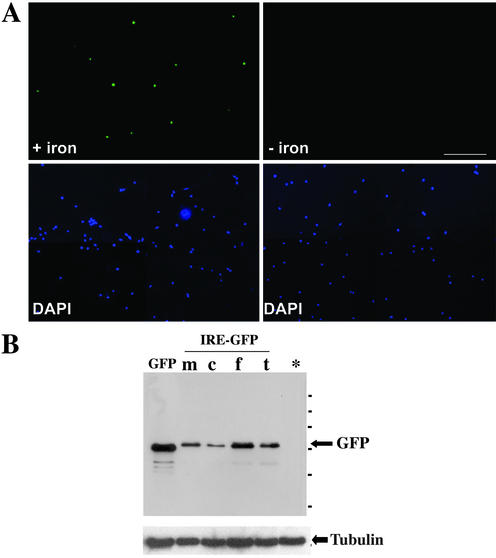
Figure 2.
Iron-dependent regulation of GFP expression in mature, primary hippocampal neurons. (A) Fluorescent picture of a representative microscopic field containing mature hippocampal neurons (10 DIV) that were transiently transfected with construct 2 and analyzed for GFP expression at the single cell level. In the presence of iron, neurons express GFP, whereas in the presence of the iron chelator, translation is inhibited. The bottom panels show the same fields of cells stained with the nuclear dye DAPI to quantify the total number of cells. (B) Detection of GFP in mature, transfected hippocampal neurons (10 DIV) by Western blot. Neurons were transiently transfected with either a construct expressing the commercially available GFP (lane 1) or alternatively, construct 2. m, normal medium; c, chelator; f, ferric ammonium citrate; t, holo-transferrin. *Untransfected neurons. Equal amounts of protein were loaded as demonstrated by the staining with anti-tubulin antibody (bottom panel). Bar, 100 μm.
For subsequent experiments with neurons (see Figures 6 and 7), cells were pretreated with chelator for 12 h before transfection to reduce background GFP expression. Different expression times for the two constructs (6 h for construct 2 or 9 h for construct 1, respectively) were chosen to warrant comparable levels of GFP expression because construct 1 is missing translational activator elements known to be present within the 3′-UTR of CaMKIIα (Wells et al., 2001; Miller et al., 2002). After transfection, neurons were transferred to low-iron conditions (N-MEM/B27 medium) or high-iron conditions (ferric ammonium citrate) for the remainder of the experiment. Neurons were then either mock-treated or treated with APV (50 μM) and CNQX (10 μM) for the whole time upon transfection. The following different chemical synaptic stimulation protocols were used: a, high KCl 30 mM (Rosa et al., 1985); b, glycine (0.1 mM) without Mg2+ for 3 min (Goldin et al., 2001); c, l-glutamate (50 μM) for 30 s (Malgaroli and Tsien, 1992); d, BDNF (25 ng/ml) for 60 min (Kang and Schuman, 1996); or e, a combination of l-glutamate (50 μM for 30 s) followed by 25 ng/ml BDNF for 1 h. Neurons were then transferred back into normal medium at 5% CO2 for 1 h. Finally, neurons were fixed, mounted, and analyzed for GFP expression at the single cell level.
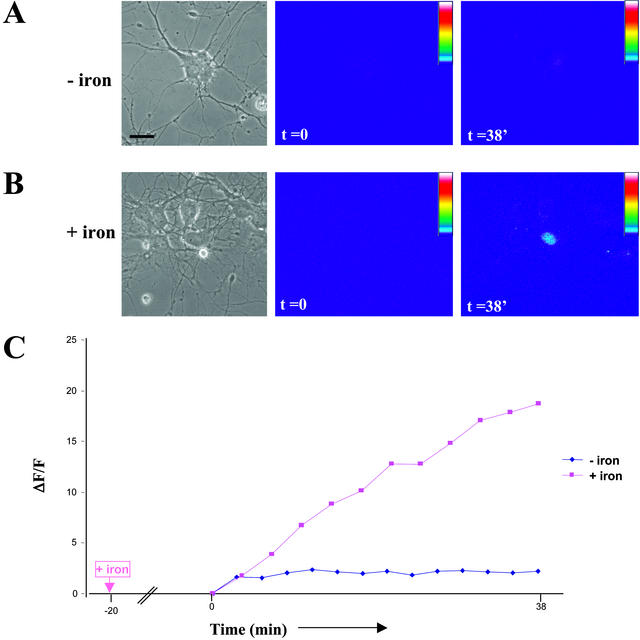
Figure 6.
Iron-dependent expression of GFP in living hippocampal neurons. Neurons were transfected in low iron-containing medium (see MATERIALS AND METHODS), ferric ammonium citrate was added upon transfection (4.5–6 h) or cells were mock-treated and time-lapse videomicroscopy was performed 20 min after the addition of iron. (A) Corresponding figure to Video 1: Little GFP expression in the absence of iron. The first panel shows a phase contrast picture of the observed field, the middle panel shows the first fluorescence picture (ΔF/F) taken at time point zero, and the right panel the same neuron at the end of the time-lapse video. Scale bar, 10 μm. (B). Corresponding figure to Video 2: Significant GFP expression in the presence of iron. The first panel shows a phase contrast picture of the observed field, the middle panel shows the first fluorescence picture (ΔF/F) taken at time point zero, and the right panel the same neuron at the end of the time-lapse video. Once iron has been added to the medium, a significant increase in fluorescence intensity over time was detected within minutes demonstrating that neurons started to express GFP. (C) Comparison of changes in fluorescence intensity over time for both conditions. The two curves within the presented graph represent the changes in fluorescence intensity over time of the two above neurons.
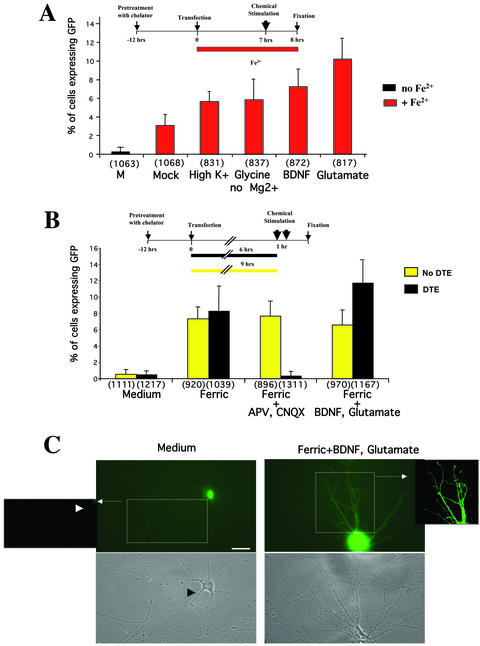
Figure 7.
(A) Activation of GFP expression upon chemical synaptic stimulation of mature neurons. Mature hippocampal neurons were pretreated with chelator before transfection to ensure low GFP background expression. After transient transfection with construct 2, neurons were transferred to iron-containing medium and treated as depicted in the scheme on top of A. Transfected neurons were either mock-treated in normal medium or chemically stimulated in high potassium (30 mM for 5 min), glycine (0.1 mM) without Mg2+ for 3 min, BDNF (25 ng/ml) for 60 min, or l-glutamate (50 μM) for 30 s. As control, neurons were not exposed to iron after transfection (column 1). After stimulation, coverslips containing transfected neurons were transferred back to iron-containing medium. After 1 h, transfected neurons were then fixed, mounted, and analyzed for GFP expression at the single cell level. Although iron induces the expression of GFP, chemical stimulation of neurons using the mentioned drugs boosts GFP expression to various extents as shown in the graph. (B) Synaptic, activity-dependent translation of GFP requires the presence of a DTE. Neurons were transiently transfected either with either construct 1 (yellow columns) or construct 2 (black columns) and then returned into either iron-containing medium or iron-containing medium in the presence of APV (50 μM) and CNQX (10 μM) to reduce endogenous synaptic activity. Control neurons did not receive any iron source (labeled: “medium”). To synaptically stimulate transfected neurons, cells were then treated in the following manner. On 6 h (for construct 2) or 9 h (construct 1) after transfection, neurons were pulsed with L-glutamate (50 μM) for 30 s and then transferred back to iron-containing medium for 1 h in the presence of BDNF (25 ng/ml, 60 min). Finally, neurons were fixed, mounted and analyzed for GFP expression at the single-cell level. (C) Representative pictures of GFP expression of hippocampal neurons transfected with construct 2. In low iron-containing medium, low expression of GFP can be observed (left panel). Autofluorescence can be recorded in the proximal dendrites (arrowhead). Strong GFP signal in the distal dendritic compartment can be detected upon chemical stimulation signal (right panel, magnification). Background was subtracted to better illustrate the difference in signal intensities for “low iron” and “stimulated” conditions (boxes). Scale bar = 10 μm.
Immunocytochemistry, Fluorescence Microscopy, and Image Analysis
Immunocytochemistry was essentially carried out as described in Kiebler et al. (1999). For fluorescence microscopy, the following setup was used: a Zeiss Axiophot microscope using either a 63×, 40×, or 10× Plan-Apochromat objective, standard FITC, rhodamine, and GFP-bandpass filters, a 100-W mercury arc lamp, and a Cohu CCD Camera (Chromaphor, Duisburg, Germany) controlled by the NIH Image 1.6.2. software package. Figures were assembled using Adobe Photoshop 5.5 or higher. For Figure 7C image acquisition and processing were performed using the setup described in the paragraph “Time-lapse videomicroscopy.” High-power images (see insets) were obtained by subtracting the background using Metamorph 5.0 software.
Quantification of GFP-expressing Cells
The protocol used to transfect hippocampal neurons has been shown to selectively transfect neurons but not glia cells (Köhrmann et al., 1999a, 1999b, and M. Köhrmann, B.G., unpublished results). DAPI staining allowed us to identify glia cells by the size and morphology of the nucleus; these cells were not counted. In each independent experimental set, a person quantified twenty randomly chosen representative microscopic fields without being aware of the experimental treatments. In brief, GFP-expressing cells were counted using either the 40× objective (for COS-7 and BHK cells) or the 10× objective (for neurons). DAPI staining of the nuclei was used to count the total number of cells per field. The graphs represent the ratio of GFP-expressing neurons compared with the total number of cells. The mean and the SD were calculated using the Microsoft Excel software.
RT-PCR
BHK grown in six-well plates were preincubated either with iron chelator or mock-treated and then transfected either with construct 1 or cotransfected with both construct 1 and a plasmid expressing a hemagglutinin (HA)-tagged Staufen1 protein (Duchaîne et al., 2000). Cells coming from the same set of transfection were processed both for Western blotting as described before and at the same time for RT-PCR. In detail, total RNA was extracted using RNeasy Mini extraction KIT (Qiagen, Hilden, Germany). Total RNA, 1.5 μg, was retrotranscribed using the RevertAid First Strand cDNA synthesis Kit (MBI Fermentas, St. Leon-Rot, Germany) according to the manufacturer's instructions. PCR was then performed using the following primers: GFP forward: 5′-atgggtaaaggagaagaactt; GFP reverse: 5′-ggaagcttttgtatagttcatcca; β-actin forward: 5′-ttcgcgggcgacgatgctcc; β-actin reverse: 5′-caggtccagacgcaggatgg; HA forward: 5′-cggccgcatcttttacc; HA reverse: 5′-attacgtaatctggaacgtcatatg. To test the linearity of the PCR reaction, aliquots of the amplified products were collected at different numbers of cycles (unpublished data).
Western Blotting
The following antibodies were used: rabbit anti-ferritin antibodies (dilution 1:100, Roche Diagnostics, Mannheim, Germany), rabbit anti-GFP antibodies (dilution 1:400, BD Biosciences/Clontech, Heidelberg, Germany, or alternatively, 1:2000, Molecular Probes, Leiden, The Netherlands), mouse monoclonal anti-tubulin-α antibodies (dilution 1:2000 up to 1:10,000, Sigma) and monoclonal anti-HA (1:2000, Roche) antibodies. As secondary antibodies, HRP-coupled donkey anti-rabbit Ig antibodies (1:1000 in blocking buffer) and HRP-coupled donkey anti-mouse Ig antibodies (1:1000 in blocking buffer; both antibodies are from Amersham-Pharmacia, Freiburg, Germany) were used.
COS-7, BHK and neurons grown in six-well plates were transfected as described above. After the relevant expression time (routinely 12 h upon transfection), cells were briefly washed with prewarmed PBS (for COS cells) or HBSS (for neurons) and lysed in 0.1% SDS, and proteins were TCA-precipitated. Equal amounts of proteins as determined by a protein quantification assay (Bio-Rad, Munich, Germany) were separated by 12% SDS-PAGE and blotted onto nitrocellulose. Nonspecific binding sites were blocked by incubation for 30 min in blocking buffer (TBS/5% low fat milk powder), and then filters were incubated overnight at 4°C with the relevant primary antibodies. Intermediate washing steps were carried out with TBS, TBS/0.1% Triton X-100, and TBS for 5 min each. Detection of bound antibodies was performed with HRP-coupled secondary antibodies (1:1000 in blocking buffer for 60 min) followed by ECL detection (Amersham-Pharmacia).
RESULTS
To uncouple dendritic transport of a reporter mRNA from immediate protein synthesis, we adapted the IRE system from M. Hentze and colleagues (Hentze et al., 1987) to GFP. Two constructs were assembled (Figure 1A): construct 1 included an NLS-containing GFP under control of the rat ferritin promoter; in the construct 2, the 3′-UTR of the vector was replaced by the full-length 3′-UTR of CaMKIIα (Mayford et al., 1996). The rat ferritin promoter element was derived from the ferritin gene whose mRNA has been shown to be dendritically localized (Ishimoto et al., 2000). This sequence contains a 32-nucleotide IRE that allows for the binding of iron responsive element binding proteins (IRP1–2; Rouault et al., 1988; Henderson and Kühn, 1995; LaVaute et al., 2001). Both IRP1 and IRP2 are expressed in the cortex, hippocampus and striatum (Siddappa et al., 2002). The NLS was added to the GFP sequence in order to avoid diffusion of the protein into the periphery of the cell.
To study whether transcription and subsequent translation of the reporter mRNA is now iron dependent, COS-7 cells were transiently transfected with construct 2 and analyzed for GFP expression at the single-cell level (Figure 1, B and C). In the presence of iron, COS cells expressed GFP, whereas in the presence of iron chelator, translation was inhibited. Figure 1B compares similar microscopic fields of transfected COS-7 cells. The bottom panels show the same fields stained with the nuclear dye DAPI to quantify the total number of cells. Figure 1C shows the quantitative analysis of one representative experiment (n = 4). In low iron conditions (c), 0.06 ± 0.2% of COS-7 cells expressed GFP, whereas in normal, iron-containing medium (m), 93 times more cells (5.63 ± 2.09%) expressed GFP. In cells treated with iron sources such as ferric ammonium citrate (f) or holo-transferrin (t), this value further increased to 5.91 ± 2.13% or 7.72 ± 2.52%, respectively. A Western blot of COS-7 cells transiently transfected with construct 2 corroborated the iron dependence of GFP expression at the biochemical level (Figure 1D).
Conditions were then adapted to mature polarized hippocampal neurons (8–10 DIV), that were transiently transfected with a Ca2+-phosphate protocol (Köhrmann et al., 1999a, 1999b) and either incubated in iron containing medium including ferric ammonium citrate or mock-treated. In these experiments, neurons were analyzed for early GFP expression at the single cell level (Figure 2A). Because hippocampal neurons are relatively small cells with a representative cell body diameter of ∼10 to 15 μm, the nuclei of these neurons only appear as small green dots in this figure. This experiment clearly demonstrates that GFP expression in neurons is now exclusively detected in the presence of iron in the medium. The bottom panels identify the total number of nuclei in these fields to ensure equal cell numbers for both experimental conditions. A Western blot of transiently transfected hippocampal neurons demonstrated this significant increase in GFP expression when cells were exposed to increased iron levels (Figure 2B). Given the low expression time required for achieving early expression in neurons (as little as 5 h) compared with that for COS-7 cells (12 h, see Figure 1), addition of chelator after transfection still led to a residual expression in neurons. To circumvent this problem, neurons were pretreated for 12 h with chelator before transfection.
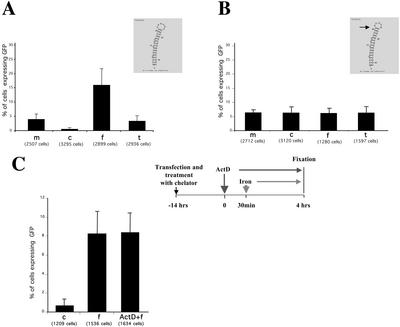
We then went on to investigate the most efficient conditions to switch on GFP expression. For this reason, neurons were transfected with construct 1 and either kept in normal medium (m), transferred into iron chelator (c, desferrioxamine mesylate), or into two different, iron-containing media (f, ferric ammonium citrate; t, holo-transferrin). The quantitative analysis of such an experiment is documented in Figure 3A. In conditions where iron is basically absent (c), 0.35 ± 0.30% of neurons expressed GFP, whereas in normal, low iron-containing medium (m), 11 times more cells (3.77 ± 1.69%) expressed GFP. However, this value can vary significantly, because it is critically dependent on endogenous synaptic activity (see below) of one given neuronal culture. In hippocampal neurons treated with exogenous iron sources this expression value further increased to 15.78 ± 5.71% (ferric ammonium citrate) or to 3.16 ± 1.63% (holo-transferrin), respectively. As an additional control for the iron-dependent translation of GFP, the functional IRE in construct 1 was inactivated by deleting an essential nucleotide (ΔC165, Rouault et al., 1988; Goossen et al., 1990). As shown in Figure 3B, the translation of the reporter construct is now independent of the level of iron in the medium. Taken together, these experiments in both fibroblasts and neurons clearly demonstrate that the IRE functions as a switch. This places the expression of GFP under the control of iron allowing us to assess protein synthesis in a single mammalian cell.
Figure 3.
Quantitative analysis of iron-dependent regulation of GFP expression in mature, primary hippocampal neurons. (A) GFP expression under the control of a functional IRE. Transfected hippocampal neurons with construct 1 were either kept in normal medium (M), transferred into chelator (c, desferrioxamine mesylate), or into two different, iron-containing media (f, ferric ammonium citrate; t, holo-transferrin). The graph represents one of three independent experiments. The small inset shows the predicted structure of the IRE element used. (B) GFP expression under the control of a nonfunctional IRE. The graph represents one of three independent experiments. Hippocampal neurons were transfected with a variant of construct 1 containing a single nucleotide deletion affecting the secondary structure of the IRE (see small inset). The mutant IRE is unable to bind the iron-responsive element binding protein (IRP), therefore making the system insensitive to iron levels. Otherwise, treatments were as described in A. (C) Actinomycin D treatment of BHK cells does not interfere with the upregulation of GFP in an iron dependent manner. The graph represents one of three independent experiments. Experiments using hippocampal neurons yielded similar results (unpublished data).
To demonstrate that the increase of translation of the reporter transcript was due to the already transcribed mRNA and not due to a rise of transcription rate, we induced translation upon iron stimulation after addition of actinomycin D (Act D), which inhibits RNA transcription (Figure 3C). BHK cells were preincubated with iron chelator for 6 h and then transfected with construct 2 and incubated for 14 h in iron chelator containing medium at 37°C/5% CO2. Two dishes were then either incubated in the same medium in the presence of Act D (see MATERIALS AND METHODS) or mock-treated; ferric ammonium citrate was then added 30 min later to both sets. The cells were fixed 3.5 h after the iron pulse. One dish was incubated in the iron chelator–containing medium during all the experiment as negative control. As shown in Figure 3C (left panel), the amount of cells responding to iron was comparable in either presence or absence of Act D. Similar results were obtained in neurons (unpublished data). This finding is in agreement with a published study (Schalinske et al., 1998) and suggests that the up-regulation of IRE-dependent translation of reporter mRNA does not involve new synthesis of mRNA.
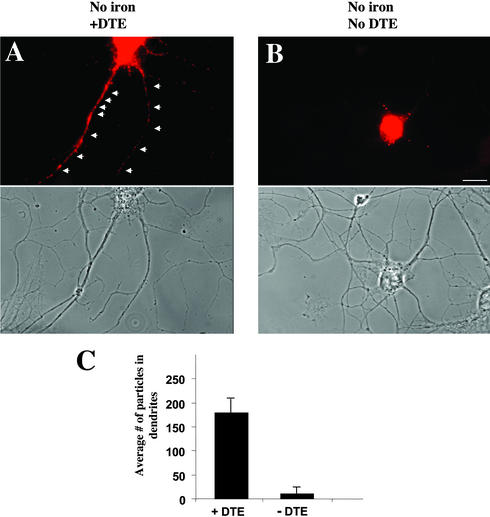
We next asked the question whether this system could be useful to restrict protein synthesis to a specific compartment of a mammalian cell by targeting the reporter mRNA inside the cell to its destination. As an example, we replaced the intrinsic 3′-UTR of the vector with the 3′-UTR of the CaMKIIα that has been shown to be sufficient and necessary for direct localization of the reporter mRNA to distal dendrites (Mayford et al., 1996; Rook et al., 2000; Blichenberg et al., 2001). To ensure that the chimerical mRNA of construct 2 was indeed localized to distal dendrites even in the absence of iron, ISH was performed against the GFP reporter mRNA in polarized hippocampal neurons that had been transiently transfected with constructs containing or lacking the CaMKIIα 3′-UTR and were kept in low iron-containing medium (Figure 4). Whereas the reporter mRNA containing the 3′-UTR of CaMKIIα (construct 2) clearly localized to the dendritic compartment (Figure 4A), the control mRNA missing a dendritic targeting element (DTE) was restricted to the cell body (Figure 4B). No signal was detected when the control ISH using the sense probe was performed (unpublished data). The graph in Figure 4C shows the quantitative analysis of the average number of fluorescent particles detected in the dendritic compartment of hippocampal neurons (see MATERIALS AND METHODS).
Figure 4.
Intracellular localization of the mRNA encoding for the reporter gene GFP. Fluorescent in situ hybridization (ISH) using an antisense probe specific for GFP mRNA. Mature hippocampal neurons were transfected with construct 2 containing the full-length 3′-UTR of the Ca2+/calmodulin-dependent protein kinase IIα and ISH was performed 5 h posttransfection (A) or with construct 1 missing the dendritic targeting element (DTE) (B). The mRNA resulting from construct 2 localizes to the dendritic compartment (arrowheads), whereas the GFP mRNA of neurons transfected with construct 1 is restricted to the cell body. (C) Quantitative analysis of particles detected in dendrites of hippocampal neurons in cell culture after ISH. Three cells were analyzed for each experiment. Bar, 10 μm.
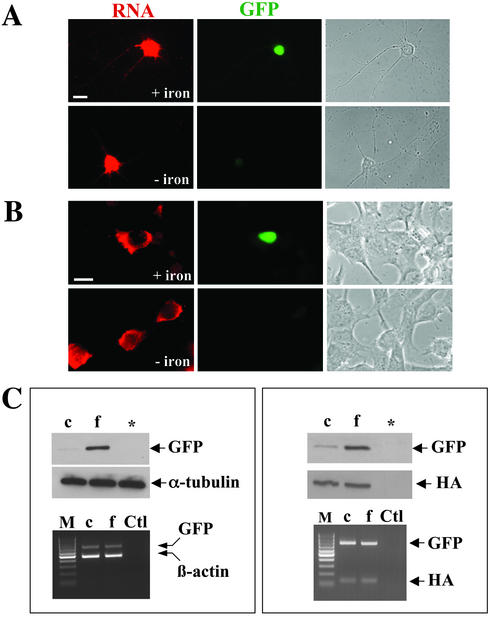
To show that mRNA is transcribed independently of the iron level, we performed ISH followed by immunocytochemistry using anti-GFP antibodies. Hippocampal neurons were transfected with construct 2 and incubated in medium containing either iron chelator or iron (Figure 5A). The same experiment was done in parallel in BHK cells (Figure 5B). In both neurons and BHK, mRNA expression can be detected despite the different levels of iron in the medium. GFP expression, in contrast, could only be observed upon addition of iron. To further exclude a possible effect of iron on transcription, we transfected BHK cells with construct 1 and performed RT-PCR and Western blotting in parallel. Figure 5C (left panel) shows that GFP expression is upregulated by addition of iron, the level of the reporter transcript, however, remained unaffected. As another control, cells were cotransfected with Staufen1-HA to rule out any effect of the iron chelator treatment on transfection rates (Figure 5C, right panel). Although GFP expression varied due to the experimental conditions, the expression level of both the transcript as well as the control protein was unaffected. Taken together, these experiments clearly demonstrate that the dendritic transport of a given reporter transcript can be uncoupled from its translation (and subsequent transport of the newly synthesized reporter protein into dendrites) through the presence of the IRE and the 3′-UTR of CaMKIIα. The IRE keeps the mRNA in a translationally inactive state, so that transport and translation of the reporter are uncoupled. Apparently, both the presence of the IRE in the reporter transcript and the low iron concentration in the medium do not interfere with the dendritic localization of the transcript.
Figure 5.
Iron-dependent posttranscriptional regulation of GFP expression in mammalian cells. ISH was combined with immunocytochemistry for both hippocampal neurons (A) and BHK cells (B). In C, RT-PCR on transfected BHK cells was combined with Western blotting. The level of RNA detected by ISH or RT-PCR was not affected by iron. In contrast, both on the fluorescence (A+B) as well as on the biochemical level (C), there was a significant increase in GFP expression upon iron addition. To exclude any variability of transfection efficiencies, cells were cotransfected with construct 1 and a control plasmid encoding for Staufen1-HA (C, right panel). Although Staufen1-HA was equally expressed under the chosen experimental conditions, GFP expression was significantly upregulated in the presence of iron. M, marker (100-base pair ladder, MBI Fermentas); c, chelator; f, ferric ammonium citrate; *untransfected cells; Ctl, negative control, no reverse transcriptase was added during the RT-PCR step.
We then went on to investigate whether this new fluorescent reporter could be used in living hippocampal neurons in culture to assay for iron-dependent protein synthesis. Neurons were transfected in low iron-containing medium, and time-lapse videomicroscopy was performed after addition of ferric ammonium citrate, or mock-treated, 4.5–6 h posttransfection (Figure 6). As the control video shows (Figure 6, A and C), there is little background expression of GFP when iron was basically absent and there was no increase in fluorescence intensity over time. In contrast, when iron was added, neurons started to express GFP within minutes upon iron addition (Figure 6, B and C). The first expression of GFP was always detected within the nucleus because of the presence of the NLS. Taken together, these experiments indicate that the new fluorescent reporter can be successfully used to monitor protein synthesis in individual living hippocampal neurons.
The significant variations in the GFP expression level in neurons, kept in iron-containing media (see Figures 2 and 3) prompted us to further evaluate whether synaptic activity occurring in fully polarized, mature hippocampal neurons might affect protein synthesis. Because many different chemical stimulation protocols exist for neurons, we sought to determine which protocol gave the most robust and most reproducible stimulation of GFP expression in transfected hippocampal neurons. To ensure low GFP background expression in the subsequent experiments, neurons were pretreated at least 12 h with iron chelator before transfection. Additionally, we chose a short time of expression (5–9 h; see Wells et al., 2001) to avoid a potential saturation of the endogenous iron-regulatory system. Then, cells were transferred back into iron-containing medium and the following various chemical stimulation protocols were tested (Figure 7A). Transfected neurons were either depolarized by high potassium (30 mM KCl for 5 min), chemically stimulated by a short pulse of either l-glutamate (50 μM for 30 s) or glycine (0.1 mM without Mg2+ for 3 min), or they were exposed to BDNF (25 ng/ml for 60 min). As controls, neurons were not exposed to iron after transfection or mock-treated in iron-containing medium. Upon stimulation, neurons were kept in iron-containing medium for 1 h, fixed, and analyzed for GFP expression. Interestingly, all stimulation protocols yielded a boost in GFP expression in transfected neurons to various extents as shown in the graph (Figure 7A). In detail, 0.24 ± 0.4% of cells expressed GFP in the absence of iron; when iron was present, 3.07 ± 1.19% of cells were GFP positive. Because the efficiency of stimulation was somewhat variable for the number of cells expressing GFP (values range from 5.65 ± 1.84% to 10.19 ± 2.17%, see Figure 3A), we decided to combine two protocols in the remainder of the experiments (see below). Taken together, this line of experiments clearly demonstrated that protein synthesis in transfected hippocampal neurons is not only dependent on the presence of iron, but also on synaptic activity of a given neuronal culture. This could easily explain why we often detected a significant variation in GFP expression in neurons in the presence of iron, because these transfected cells are part of a dense, electrically active neuronal network (Goslin et al., 1998).
To test whether the observed increase in GFP expression in transfected hippocampal neurons was due to this endogenous synaptic activity, one set of cells was kept quiescent in iron-containing medium in the presence of the NMDA antagonist APV and the AMPA/kainate antagonist CNQX (Figure 7B). This treatment resulted in a significant decrease in the number of cells expressing GFP compared with electrically active cells (0.30 ± 0.54% compared with 8.25 ± 3.05%). This further supports the idea that protein synthesis in neurons, in addition to iron in the medium, is critically dependent on synaptic activity. When another set of transfected hippocampal neurons was chemically stimulated by a short pulse of l-glutamate followed by a 1-h exposure to BDNF, GFP expression was boosted to a maximal extent (11.74 ± 2.71%), yielding a 39-fold stimulation of translation compared with electrically silent neurons.
To evaluate the function of the CaMKIIα 3′-UTR in synaptic-activity–dependent translation, polarized hippocampal neurons were transiently transfected in parallel (Figure 7B) with either a reporter construct that codes for a mRNA containing the full-length 3′-UTR of CaMKIIα (construct 2, black bars) or for the same mRNA with a canonical nonlocalized 3′-UTR (construct 1, yellow bars). The addition of the CaMKIIα 3′-UTR serves as a dendritic targeting element (DTE) that localizes the reporter mRNA to distal dendrites as shown in Figure 4A. In the presence of this DTE (Figure 7B, black bars), translation was clearly regulated by chemical synaptic activation. In contrast, neurons that had been transfected with construct 1 (which does not contain the DTE), now express GFP independently of their synaptic activity (Figure 7B, yellow bars).
Figure 7C shows representative examples of GFP fluorescence detected in hippocampal neurons transfected with construct 2 upon incubation in different media. Although in low iron containing medium a background level of GFP could be detected in the nucleus (“Medium”), chemical stimulation boosted the expression of the reporter construct and signals in the dendritic compartment could be also detected (Figure 7C, right panel and high magnification inset).
DISCUSSION
The identification of IREs in a variety of mRNAs (reviewed in Hentze and Kühn, 1996) led to the establishment of both cell-free (Paraskeva et al., 1999) and cell-dependent translational assays (De Gregorio et al., 1999). This in turn led to a molecular understanding of the vertebrate iron metabolism and of the translational regulation in general. This system has been previously used in mammalian cells that were transiently transfected by an IRE-containing construct and the expression of the reporter conveniently measured by assaying for enzymatic activities, e.g., CAT, β-Gal, or luciferase (De Gregorio et al., 1999). To study translation in a living single cell, we chose the rat ferritin promoter that contains such an IRE element to drive the expression of GFP. With this new reporter construct it is possible to regulate GFP expression, because protein synthesis is now dependent on the presence of iron. When an iron source, e.g., ferric ammonium citrate or holo-transferrin, is added locally by microperfusion to cells, translation can be studied in individual cells.
Interestingly, there are alternative mechanisms inside a polarized cell to regulate translation. An elegant example is the localization of a given transcript to a specific compartment of a cell, thereby restricting new protein synthesis to where it is needed (for a review see St. Johnston, 1995). In the last 10 years, many different targeting elements derived from such localized transcripts have been identified (Kuhl and Skehel, 1998). We therefore decided to combine both mechanisms of translational regulation to target a reporter mRNA to a specific compartment of a mammalian cell and to be able to regulate the subsequent local protein synthesis through changes in the intracellular iron level. Because we were specifically interested in studying local protein synthesis in living neurons, we took advantage of the dendritic targeting element within the 3′-UTR of the CaMKIIα mRNA (Mayford et al., 1996; Mori et al., 2000; Blichenberg et al., 2001). This element localizes the mRNA of the IRE-GFP construct to dendrites of polarized neurons. Translation, however, is exclusively switched on in the presence of iron allowing us to uncouple mRNA transport from subsequent translation in a polarized, living neuron. This is of great importance because local protein synthesis may now be visualized in an intact, living neuron discriminating locally synthesized proteins from proteins made in the cell body and then subsequently transported into dendrites (reviewed in Martin et al., 2000; Smith et al., 2001). More importantly, the underlying biochemical pathways of local protein synthesis may now be studied in detail.
When we actually transfected living hippocampal neurons with this IRE-GFP construct containing the DTE of CaMKIIα, the translation of GFP was not only dependent on the presence of iron, but also on synaptic activity. This finding indicated that the presence of iron is necessary but not sufficient to induce GFP expression in neurons when the reporter mRNA has been targeted to dendrites before. In this case, endogenous electrical activity in mature hippocampal neurons or induced synaptic activity led to a significant boost of GFP expression. However, if the DTE is missing, translation of the GFP message is independent of synaptic activity. Our results can be interpreted in two ways. First, the DTE targets the mRNA to distal dendrites and therefore to synapses. Synaptic activity could then induce local protein synthesis. This is in accordance with previous studies showing that local protein synthesis might be regulated by synaptic activity (Feig and Lipton, 1993; Kang and Schuman, 1996; Martin et al., 1997; Casadio et al., 1999; Aakalu et al., 2001). Second, the CaMKIIα 3′-UTR has two independent functions in neurons. In addition to its established role in dendritic mRNA transport (Mayford et al., 1996; Rook et al., 2000; Blichenberg et al., 2001; Miller et al., 2002; this study, Figure 4) it contains so far unknown translational activation elements (Miller et al., 2002) that may render the translation of that particular transcript dependent on synaptic activity. In mice lacking this CaMKIIα 3′-UTR, the dendritic targeting of CaMKIIα mRNA is disrupted. Consequently, CaMKIIα protein is greatly reduced in both hippocampal homogenates as well as in purified PSD fractions, indicating that the lack of mRNA targeting causes this significant reduction of protein in dendrites (Miller et al., 2002). Most importantly, however, these mice show a reduction in late phase long-term potentiation and impairments in several forms of associative learning, suggesting that local protein synthesis contributes to synaptic plasticity. Another example of such a translational regulation is the well-established role of the cytoplasmic polyadenylation binding protein (CPEB) that is necessary for cytoplasmic polyadenylation-induced translation, thereby regulating local translation of CaMKIIα mRNA at activated synapses (Wu et al., 1998; Wells et al., 2001).
Taken together, we think that with this novel fluorescent assay, a number of important questions can be addressed in living mammalian cells. One important application will certainly be high-resolution, time-lapse videomicroscopy of living cells to study the molecular effects of local protein synthesis in real time. In particular we expect that this assay will then allow us to unravel the molecular mechanisms of how local protein synthesis may be regulated by synaptic activity in an activated dendritic spine thereby extending existing studies (Fischer et al., 1998; Engert and Bonhoeffer, 1999) to the molecular level.
Supplementary Material
ACKNOWLEDGMENTS
We thank Philippe Ascher, Carlos Dotti, Martina Mucken-Thaler, and Matthias Hentze for useful comments and suggestions. This work was supported by grants from the HFSPO (RG 325/2001) and the SFB446 (Teilprojekt A16) to M.K.
Abbreviations used:
- APV
dl-2-Amino-5-phosphonovaleric acid
- Act D
actinomycin D
- BDNF
brain derived neurotrophic factor
- CaMKIIα
Ca2+/calmodulin-dependent protein kinase II alpha
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DIV
days in vitro
- DTE
dendritic targeting element
- GFP
green fluorescent protein
- IRE
iron-responsive element
- IRP
iron responsive element binding protein
- ISH
in situ hybridization
- UTR
untranslated region
- NLS
nuclear localization signal
Footnotes
Online version of this article contains video materials. Online version of this article is available at www.molbiolcell.org.
DOI: 10.1091/mbc.E02–08–0505.
REFERENCES
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blichenberg A, Schwanke B, Rehbein M, Garner CC, Richter D, Kindler S. Identification of a cis-acting dendritic targeting element in MAP2 mRNAs. J Neurosci. 1999;19:8818–8829. doi: 10.1523/JNEUROSCI.19-20-08818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blichenberg A, Rehbein M, Muller R, Garner CC, Richter D, Kindler S. Identification of a cis-acting dendritic targeting element in the mRNA encoding the alpha subunit of Ca2+/calmodulin-dependent protein kinase II. Eur J Neurosci. 2001;13:1881–1888. doi: 10.1046/j.0953-816x.2001.01565.x. [DOI] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Crino P, Khodakhah K, Becker K, Ginsberg S, Hemby S, Eberwine J. Presence and phosphorylation of transcription factors in developing dendrites. Proc Natl Acad Sci USA. 1998;95:2313–2318. doi: 10.1073/pnas.95.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Preiss T, Hentze MW. Translation driven by an eIF4G core domain in vivo. EMBO J. 1999;18:4865–4874. doi: 10.1093/emboj/18.17.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaîne T, Wang HJ, Luo M, Steinberg SV, Nabi IR, DesGroseillers L. A novel murine Staufen isoform modulates the RNA content of Staufen complexes. Mol Cell Biol. 2000;20:5592–5601. doi: 10.1128/mcb.20.15.5592-5601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- Feig S, Lipton P. Pairing the cholinergic agonist carbachol with patterned Schaffer collateral stimulation initiates protein synthesis in hippocampal CA1 pyramidal cell dendrites via a muscarinic, NMDA-dependent mechanism. J Neurosci. 1993;13:1010–1021. doi: 10.1523/JNEUROSCI.13-03-01010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Knutti D, Matus A. Rapid actin-based plasticity in dendritic spines. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Goldin M, Segal M, Avignone E. Functional plasticity triggers formation and pruning of dendritic spines in cultured hippocampal networks. J Neurosci. 2001;21:186–193. doi: 10.1523/JNEUROSCI.21-01-00186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B, Caughman SW, Harford JB, Klausner RD, Hentze MW. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990;9:4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low-density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge, MA: MIT Press; 1998. pp. 339–370. [Google Scholar]
- Henderson BR, Kuhn LC. Differential modulation of the RNA-binding proteins IRP-1 and IRP-2 in response to iron. IRP-2 inactivation requires translation of another protein. J Biol Chem. 1995;270:20509–15. doi: 10.1074/jbc.270.35.20509. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Caughman SW, Rouault TA, Barriocanal JG, Dancis A, Harford JB, Klausner RD. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238:1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Kühn LC. Molecular control of vertebrate iron metabolism: mRNA-based regulatory circuits operated by iron, nitric oxide, and oxidative stress. Proc Natl Acad Sci USA. 1996;93:8175–8182. doi: 10.1073/pnas.93.16.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto T, Fujimori K, Kasai M, Taguchi T. Dendritic translocation of the rat ferritin H chain mRNA. Biochem Biophys Res Commun. 2000;272:789–793. doi: 10.1006/bbrc.2000.2857. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Hemraj I, Verkade P, Köhrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrmann M, Luo M, Kaether C, DesGroseillers L, Dotti CG, Kiebler MA. Microtubule-dependent recruitment of Staufen-green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol Biol Cell. 1999a;10:2945–2953. doi: 10.1091/mbc.10.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrmann M, Haubensak W, Hemraj I, Kaether C, Lessmann VJ, Kiebler MA. Fast, convenient, and effective method to transiently transfect primary hippocampal neurons. J Neurosci Res. 1999b;58:831–835. [PubMed] [Google Scholar]
- Kuhl D, Skehel P. Dendritic localization of mRNAs. Curr Opin Neurobiol. 1998;8:600–606. doi: 10.1016/s0959-4388(98)80087-1. [DOI] [PubMed] [Google Scholar]
- LaVaute T, et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- Malgaroli A, Tsien RW. Glutamate-induced long-term potentiation of the frequency of miniature synaptic currents in cultured hippocampal neurons. Nature. 1992;357:134–139. doi: 10.1038/357134a0. [DOI] [PubMed] [Google Scholar]
- Martin KC, Casadio A, Zhu HEY, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Martin KC, Barad M, Kandel ER. Local protein synthesis and its role in synapse-specific plasticity. Curr Opin Neurobiol. 2000;10:587–592. doi: 10.1016/s0959-4388(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci USA. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKII-alpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Mori Y, Imaizumi K, Katayama T, Yoneda T, Tohyama M. Two cis-acting elements in the 3′ untranslated region of alpha-CaMKII regulate its dendritic targeting. Nat Neurosci. 2000;3:1079–1084. doi: 10.1038/80591. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Paraskeva E, Gray NK, Schlager B, Wehr K, Hentze MW. Ribosomal pausing and scanning arrest as mechanisms of translational regulation from cap-distal iron-responsive elements. Mol Cell Biol. 1999;19:807–816. doi: 10.1128/mcb.19.1.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook MS, Lu M, Kosik KS. CaMKII alpha 3′ untranslated region-directed mRNA translocation in living neurons: visualization by GFP linkage. J Neurosci. 2000;20:6385–6393. doi: 10.1523/JNEUROSCI.20-17-06385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P, Hille A, Lee RW, Zanini A, De Camilli P, Huttner WB. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985;101:1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Hentze MW, Caughman SW, Harford JB, Klausner RD. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988;241:1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Schalinske KL, Chen OS, Eisenstein RS. Iron differentially stimulates translation of mitochondrial aconitase and ferritin mRNAs in mammalian cells. Implications for iron regulatory proteins as regulators of mitochondrial citrate utilization. J Biol Chem. 1998;273:3740–3746. doi: 10.1074/jbc.273.6.3740. [DOI] [PubMed] [Google Scholar]
- Siddappa AJ, Rao RB, Wobken JD, Leibold EA, Connor JR, Georgieff MK. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68:761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- Smith WB, Aakalu G, Schuman EM. Local protein synthesis in neurons. Curr Biol. 2001;11:R901–R903. doi: 10.1016/s0960-9822(01)00548-6. [DOI] [PubMed] [Google Scholar]
- St. Johnston D. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sarvey JM. Blockage of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Wells DG, Dong X, Quinlan EM, Huang YS, Bear MF, Richter JD, Fallon JR. A role for the cytoplasmic polyadenylation element in NMDA receptor-regulated mRNA translation in neurons. J Neurosci. 2001;21:9541–9548. doi: 10.1523/JNEUROSCI.21-24-09541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham L, Duchaine T, Luo M, Nabi IR, DesGroseillers L. Mammalian staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol Cell Biol. 1999;19:2220–2230. doi: 10.1128/mcb.19.3.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of alpha-CaMKII mRNA at synapses. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]
- Zucker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.