Abstract
Inherited mutations in PARK7, the gene encoding DJ-1, are associated with loss of protein function and early-onset parkinsonism. Like human DJ-1 (hDJ-1), Drosophila DJ-1b protects against oxidative insult and is modified with oxidation. We demonstrate that hDJ-1 rescues flies mutant for DJ-1b, and that a conserved cysteine residue in the fly protein (C104, analogous to C106 in hDJ-1) is critical for biological antioxidant function in vivo. Targeted mutagenesis suggests that modification of DJ-1b at this residue inactivates the protective activity of the protein against oxidative stress. Further studies show that DJ-1 modification increases dramatically with age in flies, mice, and humans, with aged flies showing strikingly increased susceptibility to oxidative stress and markedly enhanced DJ-1b modification upon oxidative challenge. Overoxidation of DJ-1 with age and exposure to oxidative toxins may lead to inactivation of DJ-1 function, suggesting a role in susceptibility to sporadic Parkinson’s disease.
Keywords: neurodegeneration, oxidative stress
Parkinson’s disease is the second most common neurodegenerative disorder (1) and is classified into two major subtypes: rare familial forms resulting from the inheritance of single gene mutations and the common sporadic disease with important environmental contributions (2–4). Age is the most significant risk factor for sporadic disease, although exposure to agricultural and environmental toxins, such as paraquat and rotenone, also increases risk (5, 6). A number of genes have been found associated with familial disease, although how these inherited disease genes may influence development of sporadic disease is not well understood.
Mutations in PARK7, which encodes DJ-1, generally occur in a recessive pattern resulting in loss of gene function and cause early-onset parkinsonism with abnormalities in the dopaminergic system resembling those of sporadic Parkinson’s disease (7, 8). The role of DJ-1 in oxidative stress (7) makes it a candidate to integrate genetic and environmental components critical for sporadic disease. Dopaminergic neurons are thought to have high basal levels of oxidative stress due to the highly reactive nature of dopamine (9–11). Exposure to environmental agents that induce further oxidative stress leads to selective dopaminergic neuron degeneration in animal models (6, 12–16), reflective of increased risk by such agents for Parkinson’s disease in humans (5, 12, 17–22).
Studies in cell culture and animal models suggest that DJ-1 protein responds to oxidative stress (23–32), shifting to more acidic species (33, 34). This includes oxidation of highly conserved cysteine residues, leading to the formation of cysteine sulfinic or sulfonic acids (35, 36). Given the potential importance of these residues in DJ-1 structure and/or function, their modification upon oxidative stress may have functional consequences relevant to the activity of the protein. Cell culture studies have suggested that DJ-1 function may be modulated by oxidation of cysteine residues (32), potentially leading to activation of chaperone activity (37, 38). Although studies in mice and Drosophila indicate that loss of DJ-1 leads to greater sensitivity to oxidative stress (28–30), functional effects of oxidative modification of DJ-1 in the animal in vivo are unknown. Here, we address functional consequences of oxidation, demonstrating that modification of DJ-1b at critical cysteine residues may inactivate protein function, and that age, a major risk factor for Parkinson’s disease, has a dramatic effect on the relative abundance of modified DJ-1 in flies, mice, and humans. These studies reveal how two risk factors (age and oxidative stress) may regulate DJ-1 protein activity, potentially contributing to sporadic Parkinson’s disease.
Results
Human DJ-1 (hDJ-1) Function Is Conserved in Drosophila.
In Drosophila, deletion of the gene encoding DJ-1b results in selective sensitivity to oxidative stress, a phenotype fully rescued by ubiquitous expression of DJ-1b in the DJ-1b deletion mutant background (29). Previous studies indicated that hDJ-1 protein may also play a protective role in the setting of oxidative stress (7, 28, 32). Therefore, we determined whether the DJ-1 pathway was conserved by testing the properties of the human protein in the context of the fly.
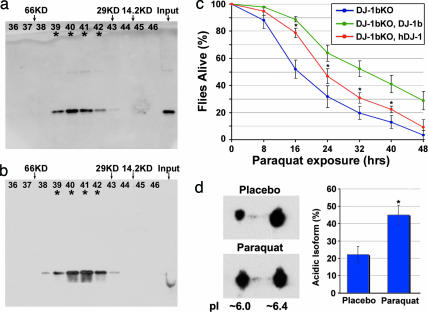
hDJ-1 forms a dimer critical for function (36, 39–41); therefore, we first determined whether hDJ-1 was dimeric in the fly in vivo. Gel filtration analysis showed that both fly DJ-1 and hDJ-1 displayed similar elution profiles, with DJ-1 immunoreactivity present in fractions corresponding to the size of the predicted dimer (38–40 kDa; Fig. 1a and b). This indicated that both hDJ-1 and fly DJ-1b are dimers upon isolation from intact flies.
Fig. 1.
Conservation of the DJ-1 pathway in Drosophila. (a and b) Gel-filtration analysis with samples from flies expressing hDJ-1 (a) or fly DJ-1b (b). Both hDJ-1 and fly DJ-1b eluted at a size consistent with dimer formation: hDJ-1 monomer ≈20 kDa, dimer predicted to be ≈40 kDa; fly DJ-1b monomer ≈19 kDa, dimer ≈38 kDa. (c) Rescue of sensitivity to paraquat of fly DJ-1b deletion mutant (DJ-1bKO, DJ-1b knock-out) with expression of hDJ-1. hDJ-1 was ubiquitously expressed with the driver line da-GAL4 in the background of the DJ-1b deletion (red). Compared to the control (driver alone in DJ-1b deletion background, blue) and to rescue with DJ-1b (DJ-1b expressed with da-GAL4 in the background of DJ-1b deletion, green), hDJ-1 partially rescues paraquat sensitivity. Mean ± SD; ∗, values significantly different from control in blue (P < 0.05, Student’s t test). (d) 2D analysis of hDJ-1 protein in flies exposed to placebo or paraquat-treated food for 10 days. Paraquat exposure increases the level of the more acidic isoform. Bar graph, mean ± SD; ∗, P = 0.015 (Student’s t test).
We then determined whether hDJ-1 had activity to rescue the fly DJ-1b deletion mutant. Exposure of DJ-1b mutants to paraquat normally results in dramatically increased sensitivity to the toxin; however, animals expressing hDJ-1 were now rescued, showing a reduction in sensitivity compared to deletion mutants (Fig. 1c). The hDJ-1 protein also responded biochemically in vivo to oxidative stress, with exposure of rescued animals to paraquat resulting in an increase in the more acidic isoform of hDJ-1 (from 22 ± 5% to 45 ± 6% with paraquat; Fig. 1d). These results demonstrate that the fly environment is compatible with hDJ-1 activity, and that both hDJ-1 and fly DJ-1 proteins retain important biochemical properties in vivo, highlighting conservation of the DJ-1 pathway.
Cysteine Residues Are Critical for Acidic Shift of DJ-1b in Response to Oxidative Stress.
A striking and conserved property of DJ-1b is the modification of the protein after exposure to oxidative stress: for hDJ-1, the cysteine residues (C46, C53, and C106) have been shown to be modified, resulting in a shift toward more acidic species (35, 36). Recent findings of increased acidic isoforms of DJ-1 in the brains of Parkinson’s patients support a role for DJ-1 modification in sporadic disease (42, 43). Therefore, we addressed DJ-1 modification and potential effects on function in vivo using Drosophila.
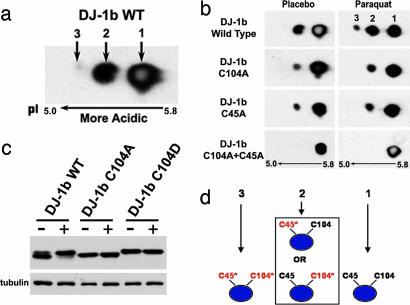
Modification of DJ-1b with paraquat exposure is detected both as a shift toward more acidic species by 2D gel electrophoresis and by decreased mobility by 1D SDS/PAGE. By 2D analysis, wild-type DJ-1b displayed three isoforms ranging in pI from ≈5.8 to 5.2 (Fig. 2a). Normally, the most basic isoform 1 was present in greatest quantity (68 ± 4%), followed by isoform 2 (32 ± 5%), with minor levels of the most acidic isoform 3 (≈1%). Upon exposure of flies to paraquat, isoforms 2 and 3 increased in relative abundance to 40.8 ± 4.2% and 7.1 ± 2.1%, respectively (Fig. 2b).
Fig. 2.
The cysteine residues in DJ-1b are critical for modification by paraquat. (a) Immunoblot (PA636 Ab, 1:1,000) of 2D samples from flies overexpressing wild-type DJ-1b. Three isoforms of DJ-1b are seen; only isoforms 1 and 2 are detectable with endogenous DJ-1b. Isoforms numbered 1, 2, and 3 from basic to acidic. (b) DJ-1b deletion flies expressing wild-type or mutant DJ-1b were exposed to placebo (−) or 2 mM (+) paraquat for 7 days. Samples separated by 2D and analyzed by immunoblot. Isoforms 1 (pI ≈5.8), 2 (pI ≈5.4), and 3 (pI ≈5.2) are indicated. (c) Flies expressing wild-type or mutant DJ-1b in the DJ-1b deletion background were exposed to placebo (−) or 2 mM (+) paraquat for 10 days and samples separated by 1D SDS/PAGE. The 1D shift of DJ-1b with paraquat is not seen with C104A and is constitutive with C104D. (d) Model for the 2D shift of DJ-1b upon oxidative stress. The blue ellipse represents DJ-1b protein, and the two unmodified cysteine residues are denoted in black. Modification of the cysteines is illustrated by a change to red and an asterisk. The data are consistent with isoform 1 representing unmodified DJ-1b; isoform 2, DJ-1b with a single modified cysteine residue of either C45 or C104; and isoform 3, DJ-1b with both cysteine residues modified.
To determine amino acid targets of modification, we performed mass spectrometry on DJ-1b from paraquat-treated Drosophila cells. Isoforms 1 and 2 were digested with trypsin and mass spectrometry analysis performed. This allowed 80% coverage of the protein, including a fragment containing C104. This approach revealed that DJ-1b became modified at C104 to cysteine sulfinic and sulfonic acid in isoform 2; modification of this peptide in isoform 1 was not detected (Fig. 7, which is published as supporting information on the PNAS web site). These results suggest that, as in hDJ-1, cysteine residues (minimally C104) are sites of modification upon oxidative stress.
We then targeted both cysteine residues (C104 and C45, analogous to C106 and C46 in hDJ-1) for mutagenesis. These residues are fully conserved among all eukaryotic orthologs (44) and are known sites of oxidation in hDJ-1 (35, 36). Mutants were generated that substituted cysteine with alanine (C→A) or aspartic acid (C→D). Alanine substitution is predicted to be unable to become modified by oxidation, whereas aspartic acid substitution may simulate acidification by oxidation. Transgenic flies were generated, and lines selected that expressed the mutant proteins at levels comparable to that of the control DJ-1b transgene. We then expressed the transgenes in vivo in the DJ-1b deletion background. Flies were exposed to normal (placebo-supplemented) or paraquat-supplemented food and protein samples analyzed to determine modification profile.
Flies expressing either C45A or C104A displayed an altered isoform pattern: the mutant proteins showed a marked decrease in the amount of isoform 2 normally compared to the wild-type protein (11 ± 3% in C104A and 14 ± 2% in C45A, compared to 30 ± 4% with wild-type DJ-1b). The mutant proteins did display an increase in isoform 2 upon paraquat exposure (to 22 ± 3% in C104A and 20 ± 3% in C45A); however, isoform 3 was never detected with either mutant (Fig. 2b). These studies are consistent with a model by which the protein with a single cysteine can still oxidize to isoform 2, but by which both cysteines are required for isoform 3 (Fig. 2d). We confirmed this by mutating both cysteine residues to alanines (C45A.C104A); this double-mutant protein failed to shift despite exposure to paraquat (Fig. 2b). Therefore, these data suggested that both cysteines were necessary for acidic modification of DJ-1b.
We then examined the behavior of the C→D mutant proteins. As noted, aspartic acid is predicted to mimic constitutive acidic modification. Indeed, both C45D and C104D proteins displayed an increased level of the acidic isoforms, with or without exposure to paraquat (for both, >50% of total protein was isoform 2), and no additional isoforms were seen upon paraquat treatment (Fig. 8, which is published as supporting information on the PNAS web site). Given that the alanine mutants failed to shift, and that the aspartic acid mutants were constitutively shifted, these results indicate that C45 and C104 in DJ-1b are essential for acidic shift upon paraquat treatment. We also noted that C104 mutations affected the 1D migration pattern of the protein; C104A never shifted, whereas C104D migrated in a constitutively modified manner (Fig. 2c and Fig. 9, which is published as supporting information on the PNAS web site). These data indicate that the cysteine residues of DJ-1b are critical for protein modification upon oxidative stress and strongly suggest that the cysteines in fly DJ-1b protein, as in the human protein, are sites of modification.
C104 Is Critical for DJ-1b Function in Oxidative Stress.
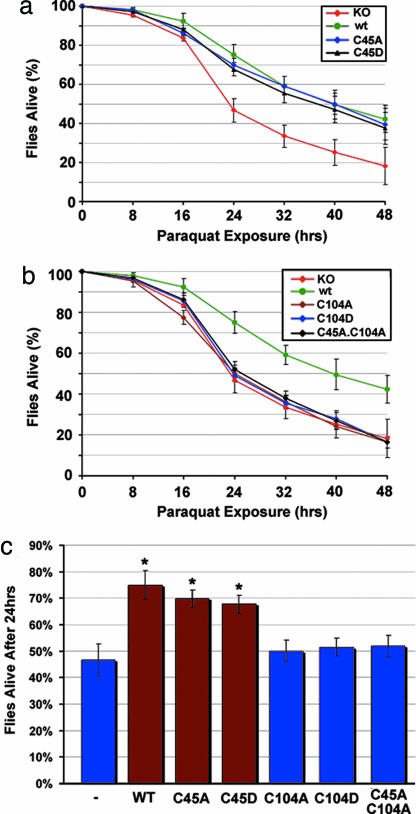
The significance of the cysteine residues and their modification was addressed by determining the rescue activity of normal and cysteine mutant forms of DJ-1b upon paraquat exposure of flies deleted for endogenous DJ-1b activity. These studies revealed that proteins mutant at C45 retained full activity to protect against paraquat exposure (Fig. 3a and c), but those mutant at C104 were strikingly defective, functioning as protein nulls (Fig. 3 b and c). Similar results were obtained with exposure to a second oxidative stress agent, hydrogen peroxide (data not shown). These data indicate that C104 appears crucial for the protective function of DJ-1b in vivo.
Fig. 3.
Cysteine 104 is critical for the antioxidant activity of DJ-1b in vivo. DJ-1b deletion mutant flies (KO) expressing DJ-1b mutant proteins with the da-GAL4 driver. The ability of DJ-1b mutant proteins to rescue paraquat sensitivity was assessed with survival curves of flies exposed to 20 mM paraquat. (a) Flies expressing C45A or C45D were fully rescued compared to flies expressing wild-type DJ-1b protein. (b) Flies expressing C104A, C104D, or C45A.C104A are not different from DJ-1b deletion mutant flies. (c) Bar graph showing the percent dead after 24 h, 20 mM paraquat. Mean ± SD; ∗, P < 0.05, compared to DJ-1b deletion (Student’s t test).
Mutation at C104 or in vivo modification of this residue by oxidation could lead to functional inactivation by affecting the activity of the protein or by altering critical biochemical properties of the protein such as stability or dimerization. Control experiments indicated similar stability and dimerization of modified DJ-1b and the mutant proteins, although C45D was difficult to express at comparable levels in vivo (Figs. 10 and 11, which are published as supporting information on the PNAS web site). These biochemical and functional studies revealed that DJ-1b is modified in vivo at C104, and that mutation of C104, including aspartic acid mutation that by 2D and 1D analyses mimics constitutively modified DJ-1b, renders the protein nonfunctional in protection from oxidative stress. These studies therefore suggested the possibility that oxidation of DJ-1b by oxidative stress impairs DJ-1b protein function, that is, that overoxidation of DJ-1b may inactivate the protein.
DJ-1 Modification Increases with Age in Flies and Humans.
These studies indicated that the status of DJ-1b modification at C104 can be assessed by 1D SDS/PAGE (see Fig. 2c), and that overoxidation may indicate functional inactivation. We then determined whether any disease-associated phenotypes showed altered levels of modified DJ-1b, thus potentially indicating situations where DJ-1 may be functionally inactive. The level of DJ-1b modification was assessed in various fly models. Most revealed little effect, except for superoxide dismutase mutant flies with neurodegeneration; this finding parallels a recent report of a DJ-1 mutation in a family showing parkinsonism accompanied by amytrophic lateral sclerosis (ref. 45; Fig. 12, which is published as supporting information on the PNAS web site). However, in the course of these studies, we noted a striking effect of age on the status of DJ-1b modification. We pursued this relationship given that age is a critical risk factor for Parkinson’s disease.
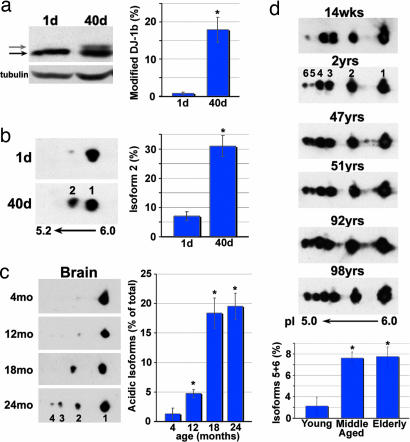
First, we compared the modification status of endogenous DJ-1b between young (1-day-old) and older (40-day-old) flies in several isogenic lines. This analysis revealed a striking and consistent increase in the amount of modified DJ-1b with age: for example, whereas 0.8 ± 0.4% of DJ-1b was modified in 1-day-old flies of line 5905, 17.9 ± 3.4% was modified in 40-day-old flies (Fig. 4a). 2D gel analysis confirmed an increase in isoform 2 in older flies, with a level of 7.1 ± 1.4% of DJ-1b in isoform 2 in young flies, increasing to 31.0 ± 3.5% in 40-day-old flies (Fig. 4b; in these and other studies, we did not see a change in the overall level of protein).
Fig. 4.
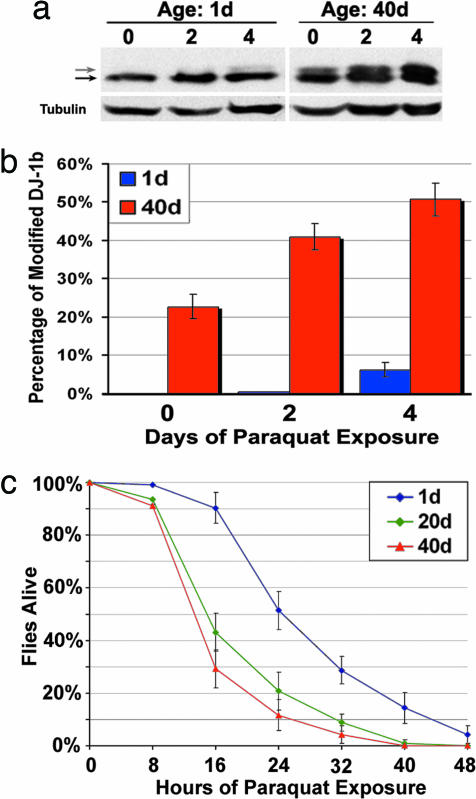
DJ-1 modification increases with age in flies, mice, and humans. (a) Samples from young (1-day-old) and old (40-day-old) flies, run on SDS/PAGE and immunoblotted (PA925 Ab, 1:250). Tubulin, loading control. Black arrow highlights unmodified DJ-1b, and gray arrow indicates modified DJ-1b. Bar graph, percentage of modified DJ-1b, mean ± SD; ∗, P < 0.05 (Student’s t test). (b) Immunoblot of samples from young (1-day-old) and old (40-day-old) flies separated by 2D gel analysis. Bar graph, percentage of DJ-1b present as isoform 2, mean ± SD; ∗, P < 0.05 (Student’s t test). Results were similar in two different isogenic fly lines (BL-5905 and BL-6326). (c) Immunoblot analysis of DJ-1, separated by 2D gel, from forebrain samples of mice of increasing age (4, 12, 18, and 24 months). Results were similar in two different mouse strains (B6D2F1 and C57BL/6). Bar graph, percentage of DJ-1 in acidic isoforms (2, 3, and 4), mean ± SD; ∗, P < 0.05 (Student’s t test). (d) Immunoblot analysis of samples from postmortem human cortex, from individuals of increasing age. Bar graph indicates percentage of hDJ-1 present as isoforms 5 and 6 from each age group (young = 14 weeks to 2 years, middle-aged = 47–51 years, elderly = 92–98 years), mean ± SD, total of nine human brain samples; ∗, P < 0.05 (Student’s t test).
Given this striking effect of age in flies, we then determined whether an increase in DJ-1 modification occurred with age in mice and humans. 2D gel analysis was performed on brain samples from two distinct mouse strains (B6D2F1 and C57BL/6) of increasing age (4–24 months). Murine DJ-1 showed up to four isoforms of the protein. Analysis revealed an increase in acidic isoforms 2–4 with age, from 1 ± 1% in mice of 4 months to 19.6 ± 2.2% in 2-yr-old mice (Fig. 4c). A similar increase in acidic species also occurred in skin samples, although isoforms 3 and 4 were not increased as dramatically as in brain (Fig. 13, which is published as supporting information on the PNAS web site). Results were similar in both strains.
We then analyzed postmortem samples of human cortex from individuals of increasing age. HDJ-1 displayed up to six distinct isoforms with 2D analysis. Comparison of young to older individuals revealed a significant increase in the relative amounts of the most acidic isoforms 5 and 6 with age: 2.2 ± 1.7% in young individuals (14 wks to 2 yrs) to 11.2 ± 1.1% in middle-aged and 11.5 ± 1.8% in elderly individuals (36–92 yrs; Fig. 4d). These results suggest that age is associated with increased levels of acidic DJ-1 isoforms, thus increased modification of DJ-1, in flies, mice, and humans. Taken together with our findings to indicate that modification of DJ-1 may inactivate protein function, these results suggest that DJ-1 activity may become compromised with age.
Modification of DJ-1b in Aged Flies Increases Dramatically upon Oxidative Insult, and Aged Flies Are More Vulnerable to Oxidative Stress.
The increase in acidic forms of DJ-1 in flies, mice, and humans with age was ≈10–30% of the protein, with a majority of the protein remaining unmodified. This suggested that, despite the increase in acidic DJ-1 isoforms, DJ-1 function may remain largely unaltered. We therefore addressed the effect of age combined with oxidative insult on DJ-1 modification and function. These studies revealed a striking effect on the extent of DJ-1 modification by oxidative stress with age and a dramatically enhanced sensitivity to paraquat in older flies.
Upon exposure to paraquat, much more relative DJ-1b protein was modified in older flies compared to younger flies: whereas 1-day-old flies showed an increase in modified DJ-1b from 0% to 6.3 ± 1.8% with paraquat, 40- to 50-day-old flies showed an increase in modified DJ-1b from a basal level of 22.7 ± 3.2% to 50.7 ± 4.3% upon paraquat exposure (Fig. 5a and b). Thus, not only was more DJ-1b modified with age in the absence of exposure to paraquat, but significantly more DJ-1b became modified upon exposure of the animals to the oxidative toxin.
Fig. 5.
Flies of advanced age display increased DJ-1b modification and increased sensitivity to paraquat. (a) Immunoblot of samples prepared from young (1-day-old) and old (40-day-old) flies exposed to 2 mM paraquat for 0, 2, and 4 days (PA925 Ab, 1:250). Black arrow indicates unmodified DJ-1b, and gray arrow is modified DJ-1b. Tubulin, loading control. (b) Bar graph illustrating the percentage of modified DJ-1b in young (blue) and old (red) flies challenged with paraquat for the indicated number of days. (c) Survival curve of flies of increasing age (1 day old, blue; 20 days old, green; 40 days old, red) exposed to 20 mM paraquat. Results were similar in two isogenic backgrounds [BL-5905 (shown) and BL-6326].
We then tested the sensitivity of flies of increasing age to paraquat. These experiments revealed that the animals showed a striking and progressive increase in sensitivity to paraquat with age. Whereas 1-day-old flies showed normal sensitivity, flies 20 and 40 days of age were dramatically more sensitive to paraquat; this result was similar in two different isogenic fly strains (Fig. 5c). Taken together with the biochemical findings, these results suggest that increased modification of DJ-1, which is potentially indicative of functional compromise, may contribute to increased susceptibility to oxidative stress, and thus to disease, with age.
Discussion
These studies reveal a critical role for C104 in DJ-1b function and suggest that overoxidation of this residue may inactivate protein function. Moreover, modification of DJ-1 normally occurs with age in flies, mice, and humans. The increased oxidation status of the protein may lead to functional consequences: aged flies mimic DJ-1 mutant flies in response to paraquat, showing a strikingly increased sensitivity. These studies raise the possibility that DJ-1 function becomes compromised with age due to increased oxidation of the protein. This suggests a potential mechanism by which altered activity of normal DJ-1 protein, with age and exposure to oxidative toxins, may contribute to development of sporadic Parkinson’s disease.
Cysteine oxidation alters the function of proteins ranging from enzymes to transcriptional regulators and is emerging as a posttranslational signaling mechanism in cells that may be as common as phosphorylation (46, 47). Reports on hDJ-1 strongly suggest that all three cysteine residues can become oxidized with exposure to oxidative stress agents (35, 36, 43). Two of these cysteine residues are conserved in all eukaryotic orthologs of DJ-1 protein, including fly DJ-1b. In DJ-1b, both the C45 and C104 are necessary for the acidic isoelectric shift in response to paraquat exposure. Rescue analysis in vivo revealed that C104, but not C45, was essential for protective function in vivo. This finding indicates that C104 is critical to the activity of DJ-1b, and mutation of this residue renders the protein inactive. The D substitution leads to a residue that is molecularly similar to overoxidized cysteine (cysteine sulfinic and/or sulfonic acid), with the main difference of a carbon atom in place of the sulfur. The C104D protein behaved biochemically identical to modified DJ-1b protein, by both 1D and 2D gel analysis, yet had no activity in vivo. We suggest that this mutation reflects the functional activity of the overoxidized protein. An alternative interpretation is that C104 is simply a critical amino acid; further studies of protein activity in vitro, using specific mutant forms and oxidized protein, will help address this issue. Moreover, once genes are defined that modulate the oxidation status of DJ-1b in vivo to arrest the protein in the animal in one oxidation state or another, this can be further tested. That overoxidation of the protein inactivates function is supported by recent studies in vitro: although mild oxidation of C106 in hDJ-1 activates chaperone activity toward α-synuclein, overoxidation inactivates function (38). Thus, situations of dramatically increased modification of DJ-1, such as aging and oxidative stress, may lead to the compromise of DJ-1 protein function through overoxidation.
The phenomenon involving inactivation of a protein by the toxic insult it protects against has precedent in the peroxiredoxins (Prxs). These proteins are involved in the reduction of hydrogen peroxide and organic hydroperoxides through the action of a catalytic cysteine residue (46) but are markedly susceptible to inactivation by these same hydroperoxides through overoxidation (to sulfinic acid) of the catalytic cysteine residue (48, 49). Both DJ-1 and the Prxs are small proteins with broad cytoplasmic expression involved in biological protection from oxidative stress, which contain critical functional cysteine residues susceptible to overoxidation. There is evidence that DJ-1 may quench hydrogen peroxide activity in vitro (24), likely through the oxidation of reactive cysteines. Furthermore, DJ-1 may harbor chaperone activity that is activated through modification of critical cysteines (38). Our studies suggest that, whereas oxidation may activate DJ-1-protective functions, overoxidation may lead to molecular inactivation of DJ-1 function. Potentially, a delicate balance of inactive and active forms of the protein may determine the overall protective activity in the animal.
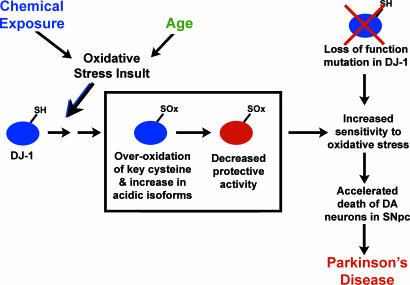
We observed a consistent increase in DJ-1 modification with age in multiple lines of flies and mice. Notably, we also observed an increase in acidic isoforms of DJ-1 with age in human brain samples, despite the heterogeneous genetic background. Older flies were also strikingly more susceptible to DJ-1b modification and death with paraquat. These findings indicate that aging leads to increased modification of the DJ-1 protein, which in turn may contribute to increased susceptibility to oxidative stress, a mechanism of DJ-1 inactivation with age that could contribute to the development of sporadic Parkinson’s disease (Fig. 6). The profiles of DJ-1 protein in control vs. late-onset Parkinson’s patients support this idea, where increased levels of modified DJ-1 are seen (42, 43). Increased modification of C104 (C106 in hDJ-1) may functionally impair DJ-1 activity, leading to decreased protein function. Modification of C45 (C46 in hDJ-1) may not compromise protein activity but may affect stability. Such loss of DJ-1 protein activity, like the loss of DJ-1 function through mutation in familial parkinsonism, would lead to increased susceptibility to oxidative stressors, potentially accelerated loss of dopaminergic neurons, and ultimately disease. These findings underscore findings with a second parkinsonism protein, parkin, whose cysteine modification results in decreased protein stability (50). These studies suggest a role for DJ-1 in susceptibility to sporadic Parkinson’s disease, with the increase in DJ-1 modification with age being a dramatic example of the potentially damaging effects of aging on protein function relevant to disease. Therapeutics to augment DJ-1 activity, inhibiting or minimizing DJ-1 overoxidation, may be a promising approach for neurodegenerative diseases in which oxidative stress plays a significant role.
Fig. 6.
Model of DJ-1 loss of function by modification and mutation in Parkinson’s disease. Oxidative stress resulting from environmental exposure or aging leads to oxidation of DJ-1 (blue, active DJ-1) at key cysteine residues (SOx) and the inactivation of DJ-1 biological activity to protect against oxidative stress (red, inactive DJ-1). This is proposed to lead to increased sensitivity to oxidative stress, accelerated loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and contribute to the development of sporadic Parkinson’s disease. In inherited parkinsonism due to loss of DJ-1 gene (top right), cells have increased sensitivity to oxidative stress from initial stages, leading to accelerated loss of dopaminergic neurons and Parkinson’s disease.
Materials and Methods
Drosophila Stocks.
Transgenic flies for expression of wild-type hDJ-1 and various DJ-1 mutants were generated by cloning the coding sequence of the genes into the pUAST vector, sequencing the constructs for verification, and introduction into the Drosophila germline using standard methods. All crosses and experiments were performed at 25°C. Mutations were made with the QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). Exposure to toxic compounds was performed as described (29).
Protein Analysis.
Twenty fly heads or four whole flies were homogenized in 100 μl of Laemmli buffer, separated by 12.5% SDS/PAGE, and transferred to nitrocellulose. Antibodies used were as follows: PA636 (ref. 29; guinea pig anti-DJ-1b, 1:1,000), PA691 (rabbit anti-hDJ-1; ref. 51), goat anti-guinea pig HRP (1:5,000, Chemicon, Temecula, CA), and donkey anti-rabbit HRP (1:2,500, Chemicon). For 2D analysis, samples were homogenized in 8 M urea and 2% CHAPS. For fly samples, four flies in 100 μl of PBS buffer were used. For mouse and human brain samples, 0.2 g of tissue was homogenized in 600 μl of buffer. After centrifugation at 16,000 × g for 10 min at 4°C, the supernatant was transferred to a new tube. The protocol provided by the manufacturer (Invitrogen, Carlsbad, CA, ZOOM System) was then followed.
For further details, see Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank A. Cashmore for comments, B. Giasson for help with gel filtration studies, and K. Speicher and T. Beer of the Wistar Proteomics Facility. Funding for this study was provided by the National Institutes of Health (N.M.B. and H.I.), a neurodegeneration training grant from the University of Pennsylvania, and a National Research Service Award from the National Institutes of Health (to M.C.M.). We thank the University of Pennsylvania National Institutes of Health/National Institute of Aging (Grant P30 AG010124) for human brain tissue samples and the National Institute on Aging Aged Mouse Colony for aged mouse tissue samples. N.M.B. is an Investigator of the Howard Hughes Medical Institute.
Abbreviation
- hDJ-1
human DJ-1.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Lang A. E., Lozano A. M. N. Engl. J. Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Dawson T. M., Dawson V. L. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 3.Paisan-Ruiz C., Jain S., Evans E. W., Gilks W. P., Simon J., van der Brug M., Lopez de Munain A., Aparicio S., Gil A. M., Khan N., et al. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Valente E. M., Abou-Sleiman P. M., Caputo V., Muqit M. M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A. R., Healy D. G., et al. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 5.Liou H. H., Tsai M. C., Chen C. J., Jeng J. S., Chang Y. C., Chen S. Y., Chen R. C. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- 6.Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. Nat. Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 7.Bonifati V., Rizzu P., van Baren M. J., Schaap O., Breedveld G. J., Krieger E., Dekker M. C., Squitieri F., Ibanez P., Joosse M., et al. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 8.van Duijn C. M., Dekker M. C., Bonifati V., Galjaard R. J., Houwing-Duistermaat J. J., Snijders P. J., Testers L., Breedveld G. J., Horstink M., Sandkuijl L. A., et al. Am. J. Hum. Genet. 2001;69:629–634. doi: 10.1086/322996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stokes A. H., Hastings T. G., Vrana K. E. J. Neurosci. Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Floor E., Wetzel M. G. J. Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 11.Yoritaka A., Hattori N., Uchida K., Tanaka M., Stadtman E. R., Mizuno Y. Proc. Natl. Acad. Sci. USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenamyre J. T., Betarbet R., Sherer T. B. Parkinsonism Relat. Disord. 2003;9(Suppl. 2):S59–S64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 13.Sherer T. B., Kim J. H., Betarbet R., Greenamyre J. T. Exp. Neurol. 2003;179:9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- 14.Hoglinger G. U., Feger J., Prigent A., Michel P. P., Parain K., Champy P., Ruberg M., Oertel W. H., Hirsch E. C. J. Neurochem. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- 15.Alam M., Schmidt W. J. Behav. Brain Res. 2002;136:317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 16.Betarbet R., Canet-Aviles R. M., Sherer T. B., Mastroberardino P. G., McLendon C., Kim J. H., Lund S., Na H. M., Taylor G., Bence N. F., et al. Neurobiol. Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Uversky V. N. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 18.Kopin I. J., Markey S. P. Annu. Rev. Neurosci. 1988;11:81–96. doi: 10.1146/annurev.ne.11.030188.000501. [DOI] [PubMed] [Google Scholar]
- 19.Fall P. A., Fredrikson M., Axelson O., Granerus A. K. Mov. Disord. 1999;14:28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Gorell J. M., Johnson C. C., Rybicki B. A., Peterson E. L., Richardson R. J. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- 21.Semchuk K. M., Love E. J., Lee R. G. Neurology. 1993;43:1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.Vanacore N., Nappo A., Gentile M., Brustolin A., Palange S., Liberati A., Di Rezze S., Caldora G., Gasparini M., Benedetti F., et al. Neurol. Sci. 2002;23(Suppl. 2):S119–S20. doi: 10.1007/s100720200098. [DOI] [PubMed] [Google Scholar]
- 23.Ooe H., Taira T., Iguchi-Ariga S. M., Ariga H. Toxicol. Sci. 2005;88:114–126. doi: 10.1093/toxsci/kfi278. [DOI] [PubMed] [Google Scholar]
- 24.Taira T., Saito Y., Niki T., Iguchi-Ariga S. M., Takahashi K., Ariga H. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi-Niki K., Niki T., Taira T., Iguchi-Ariga S. M., Ariga H. Biochem. Biophys. Res. Commun. 2004;320:389–397. doi: 10.1016/j.bbrc.2004.05.187. [DOI] [PubMed] [Google Scholar]
- 26.Yokota T., Sugawara K., Ito K., Takahashi R., Ariga H., Mizusawa H. Biochem. Biophys. Res. Commun. 2003;312:1342–1348. doi: 10.1016/j.bbrc.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 27.Martinat C., Shendelman S., Jonason A., Leete T., Beal M. F., Yang L., Floss T., Abeliovich A. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim R. H., Smith P. D., Aleyasin H., Hayley S., Mount M. P., Pownall S., Wakeham A., You-Ten A. J., Kalia S. K., Horne P., et al. Proc. Natl. Acad. Sci. USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meulener M., Whitworth A. J., Armstrong-Gold C. E., Rizzu P., Heutink P., Wes P. D., Pallanck L. J., Bonini N. M. Curr. Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 30.Park J., Kim S. Y., Cha G. H., Lee S. B., Kim S., Chung J. Gene. 2005;361C:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 31.Zhou W., Freed C. R. J. Biol. Chem. 2005;280:43150–43158. doi: 10.1074/jbc.M507124200. [DOI] [PubMed] [Google Scholar]
- 32.Canet-Aviles R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., Cookson M. R. Proc. Natl. Acad. Sci. USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitsumoto A., Nakagawa Y. Free Radic. Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 34.Mitsumoto A., Nakagawa Y., Takeuchi A., Okawa K., Iwamatsu A., Takanezawa Y. Free Radic. Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 35.Kinumi T., Kimata J., Taira T., Ariga H., Niki E. Biochem. Biophys. Res. Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 36.Wilson M. A., Collins J. L., Hod Y., Ringe D., Petsko G. A. Proc. Natl. Acad. Sci. USA. 2003;100:9256–9261. doi: 10.1073/pnas.1133288100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shendelman S., Jonason A., Martinat C., Leete T., Abeliovich A. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W., Zhu M., Wilson M. A., Petsko G. A., Fink A. L. J. Mol. Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Honbou K., Suzuki N. N., Horiuchi M., Niki T., Taira T., Ariga H., Inagaki F. J. Biol. Chem. 2003;278:31380–31384. doi: 10.1074/jbc.M305878200. [DOI] [PubMed] [Google Scholar]
- 40.Tao X., Tong L. J. Biol. Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- 41.Huai Q., Sun Y., Wang H., Chin L. S., Li L., Robinson H., Ke H. FEBS Lett. 2003;549:171–175. doi: 10.1016/s0014-5793(03)00764-6. [DOI] [PubMed] [Google Scholar]
- 42.Bandopadhyay R., Kingsbury A. E., Cookson M. R., Reid A. R., Evans I. M., Hope A. D., Pittman A. M., Lashley T., Canet-Aviles R., Miller D. W., et al. Brain. 2004;127:420–430. doi: 10.1093/brain/awh054. [DOI] [PubMed] [Google Scholar]
- 43.Choi J., Sullards M. C., Olzmann J. A., Rees H. D., Weintraub S. T., Bostwick D. E., Gearing M., Levey A. I., Chin L. S., Li L. J. Biol. Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandyopadhyay S., Cookson M. R. BMC Evol. Biol. 2004;4:6–9. doi: 10.1186/1471-2148-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annesi G., Savettieri G., Pugliese P., D’Amelio M., Tarantino P., Ragonese P., La Bella V., Piccoli T., Civitelli D., Annesi F., et al. Ann. Neurol. 2005;58:803–807. doi: 10.1002/ana.20666. [DOI] [PubMed] [Google Scholar]
- 46.Poole L. B., Karplus P. A., Claiborne A. Annu. Rev. Pharmacol. Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- 47.Wood Z. A., Poole L. B., Karplus P. A. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 48.Jeong W., Park S. J., Chang T. S., Lee D. Y., Rhee S. G. J. Biol. Chem. 2006;281:14400–14407. doi: 10.1074/jbc.M511082200. [DOI] [PubMed] [Google Scholar]
- 49.Schroder E., Littlechild J. A., Lebedev A. A., Errington N., Vagin A. A., Isupov M. N. Struct. Folding Des. 2000;8:605–615. doi: 10.1016/s0969-2126(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 50.LaVoie M. J., Ostaszewski B. L., Weihofen A., Schlossmacher M. G., Selkoe D. J. Nat. Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 51.Meulener M. C., Graves C. L., Sampathu D. M., Armstrong-Gold C. E., Bonini N. M., Giasson B. I. J. Neurochem. 2005;93:1524–1532. doi: 10.1111/j.1471-4159.2005.03145.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.