Abstract
We studied the effect of superoxide dismutase (SOD) on murine collagen-induced arthritis (CIA), an animal model of human rheumatoid arthritis (RA). Among SOD derivatives studied, only gelatin-SOD conjugate which has prolonged half life in vivo was effective to suppress the development of CIA, while native SOD or gelatin carrier alone was ineffective. Interestingly, pyran polymer-conjugated SOD which also has a long half life showed no suppressive effect on the disease. No significant effect on immune response against type II collagen (CII) was found in any of the experimental groups. In addition, induction of suppressor cells was not detected in spleen or lymph node cells of the gelatin-SOD-treated group. Therefore, these results suggest that oxygen radicals may have an important role in the effector phase of the immune response to manifest this chronic autoimmune polyarthritis. Thus, the use of appropriate antioxidants for the treatment of human RA may be rationalized.
Full text
PDF

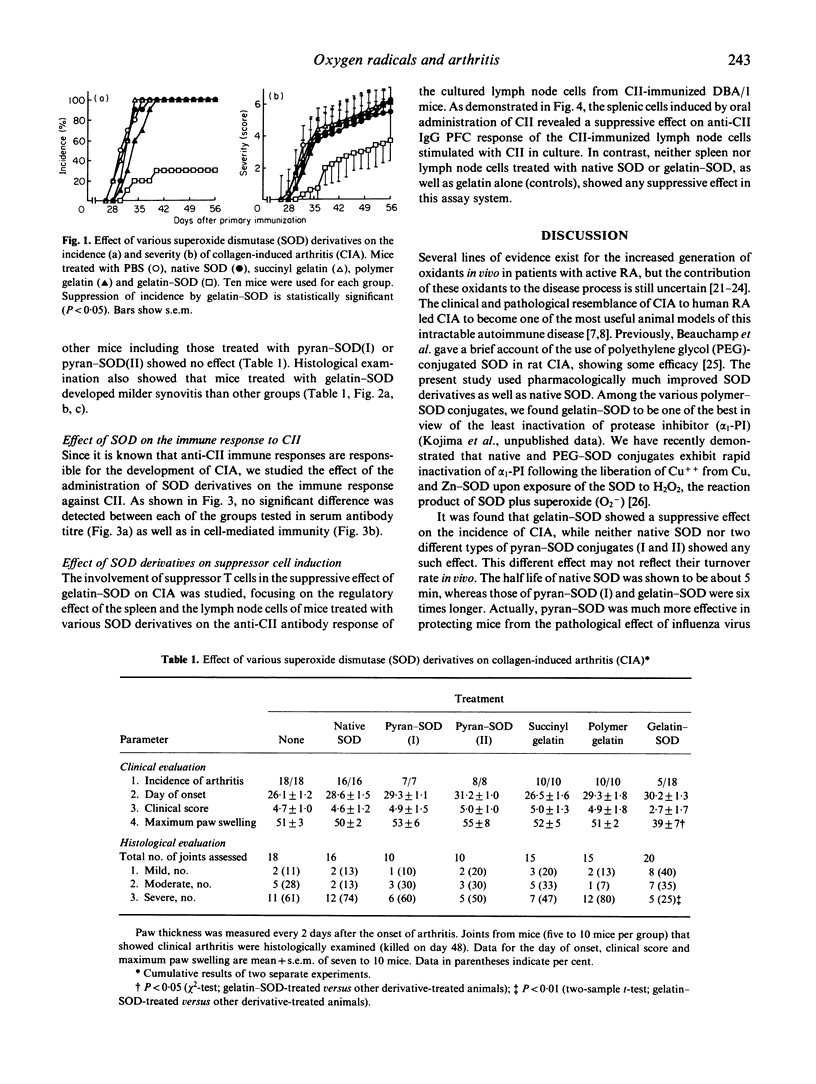
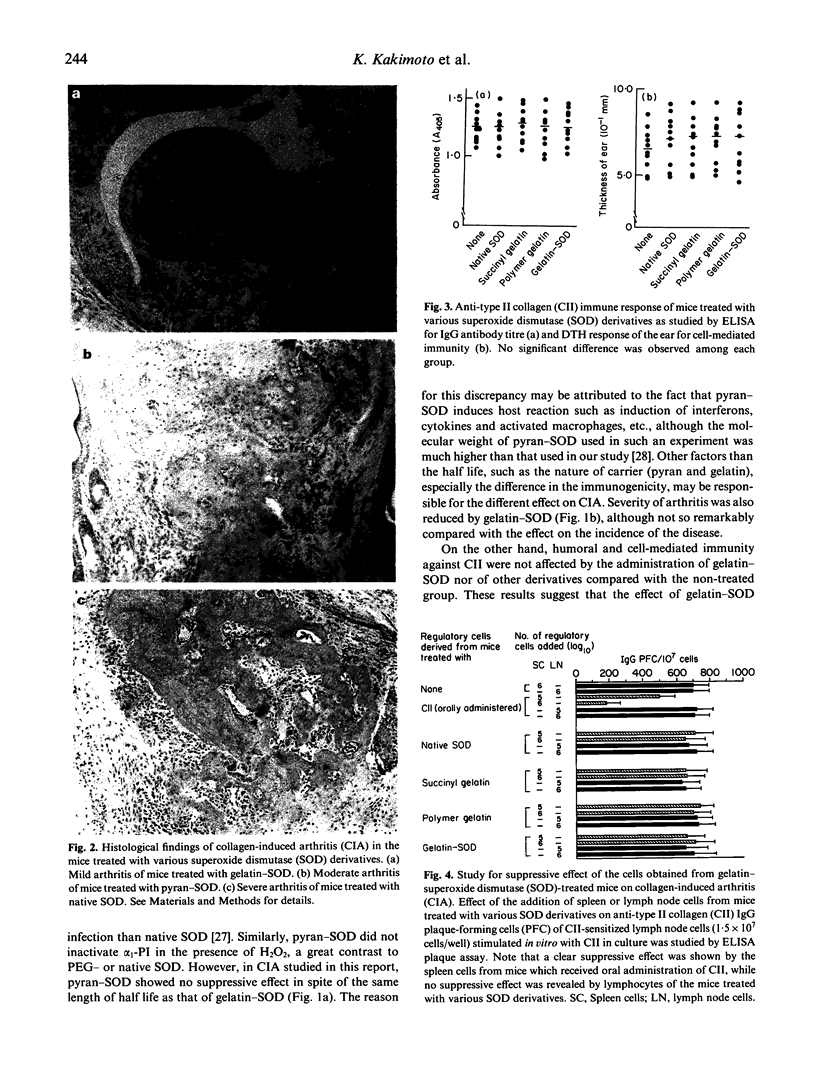


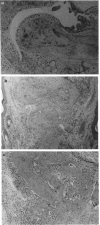
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemond P., Swaak A. J., Penders J. M., Beindorff C. M., Koster J. F. Superoxide production by polymorphonuclear leucocytes in rheumatoid arthritis and osteoarthritis: in vivo inhibition by the antirheumatic drug piroxicam due to interference with the activation of the NADPH-oxidase. Ann Rheum Dis. 1986 Mar;45(3):249–255. doi: 10.1136/ard.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Farmer L. M., Watt G., Glatt M., Blaettler A., Loutis N., Feige U. Delayed type hypersensitivity (DTH) to type II collagen (CII) in DBA-1 mice. Clin Exp Immunol. 1986 Aug;65(2):329–335. [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Harth M., Keown P. A., Orange J. F. Monocyte dependent excited oxygen radical generation in rheumatoid arthritis: inhibition by gold sodium thiomalate. J Rheumatol. 1983 Oct;10(5):701–707. [PubMed] [Google Scholar]
- Hurst N. P., Bessac B., Nuki G. Monocyte superoxide anion production in rheumatoid arthritis: preliminary evidence for enhanced rates of superoxide anion production by monocytes from patients receiving penicillamine, sodium aurothiomalate and corticosteroids. Ann Rheum Dis. 1984 Feb;43(1):28–33. doi: 10.1136/ard.43.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto K., Katsuki M., Hirofuji T., Iwata H., Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988 Jan 1;140(1):78–83. [PubMed] [Google Scholar]
- Kay N. E., Douglas S. D. Monocyte metabolic activation in patients with rheumatoid arthritis. Proc Soc Exp Biol Med. 1979 Jul;161(3):303–306. doi: 10.3181/00379727-161-40541. [DOI] [PubMed] [Google Scholar]
- Lee W. Y., Sehon A. H., Akerblom E. Suppression of reaginic antibodies with modified allergens. IV. Induction of suppressor T cells by conjugates of polyethylene glycol (PEG) and monomethoxy PEG with ovalbumin. Int Arch Allergy Appl Immunol. 1981;64(1):100–114. [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. Chemical and biological characterization of succinyl neocarzinostatin. J Antibiot (Tokyo) 1974 May;27(5):303–311. doi: 10.7164/antibiotics.27.303. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McNeil J. D., Wiebkin O. W., Betts W. H., Cleland L. G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985 Nov;44(11):780–789. doi: 10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry P., Grootveld M., Lunec J., Blake D. R. Oxidative damage to lipids within the inflamed human joint provides evidence of radical-mediated hypoxic-reperfusion injury. Am J Clin Nutr. 1991 Jan;53(1 Suppl):362S–369S. doi: 10.1093/ajcn/53.1.362S. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972 Dec 19;11(26):4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- Nurcombe H. L., Bucknall R. C., Edwards S. W. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann Rheum Dis. 1991 Mar;50(3):147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Akaike T., Hamamoto T., Suzuki F., Hirano T., Maeda H. Oxygen radicals in influenza-induced pathogenesis and treatment with pyran polymer-conjugated SOD. Science. 1989 May 26;244(4907):974–976. doi: 10.1126/science.2543070. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regelson W. The biological activity of the synthetic polyanion, pyran copolymer (diveema, MVE, 46015) and the heterocyclic bis DEAE fluorenone derivative, tilorone and congeners: clinical and laboratory effects of these agents as modulators of host resistance. Pharmacol Ther. 1981;15(1):1–44. doi: 10.1016/0163-7258(81)90014-0. [DOI] [PubMed] [Google Scholar]
- Sato K., Akaike T., Kohno M., Ando M., Maeda H. Hydroxyl radical production by H2O2 plus Cu,Zn-superoxide dismutase reflects the activity of free copper released from the oxidatively damaged enzyme. J Biol Chem. 1992 Dec 15;267(35):25371–25377. [PubMed] [Google Scholar]
- Schrier D., Gilbertsen R. B., Lesch M., Fantone J. The role of neutrophils in type II collagen-induced arthritis in rats. Am J Pathol. 1984 Oct;117(1):26–29. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J Immunol Methods. 1983 Feb 25;57(1-3):301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- Seki N., Sudo Y., Yoshioka T., Sugihara S., Fujitsu T., Sakuma S., Ogawa T., Hamaoka T., Senoh H., Fujiwara H. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988 Mar 1;140(5):1477–1484. [PubMed] [Google Scholar]
- Situnayake R. D., Thurnham D. I., Kootathep S., Chirico S., Lunec J., Davis M., McConkey B. Chain breaking antioxidant status in rheumatoid arthritis: clinical and laboratory correlates. Ann Rheum Dis. 1991 Feb;50(2):81–86. doi: 10.1136/ard.50.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]