Abstract
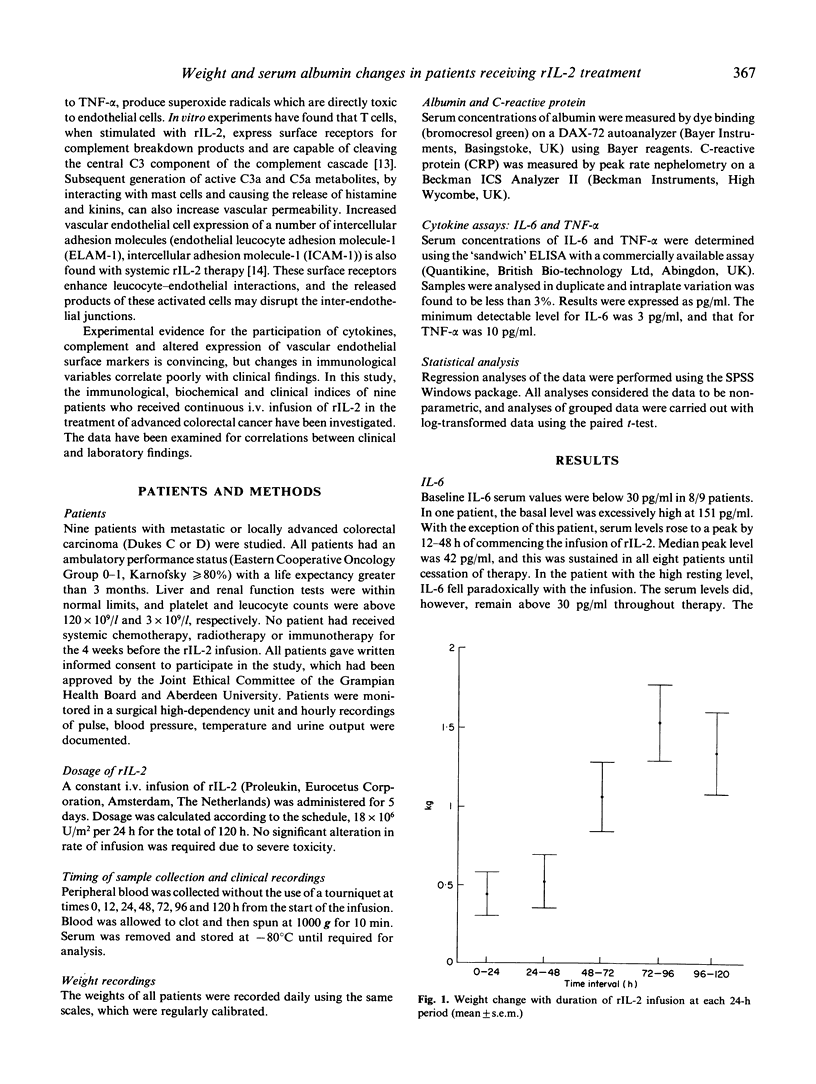
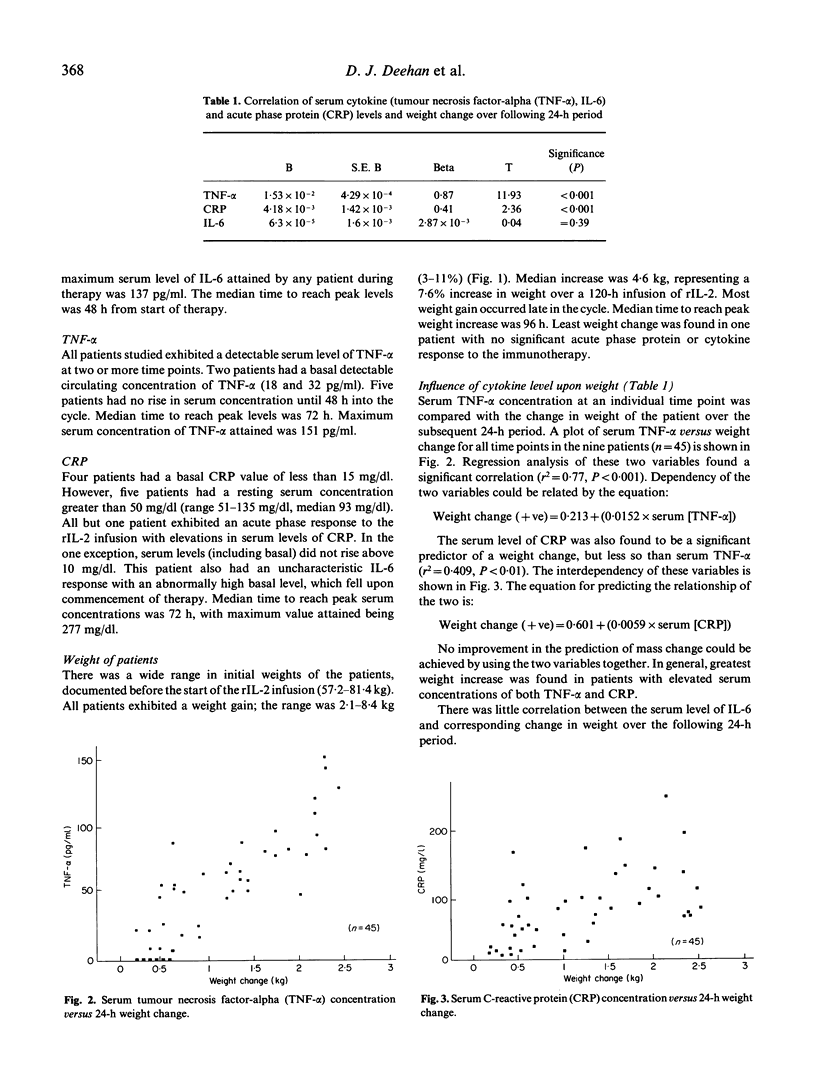
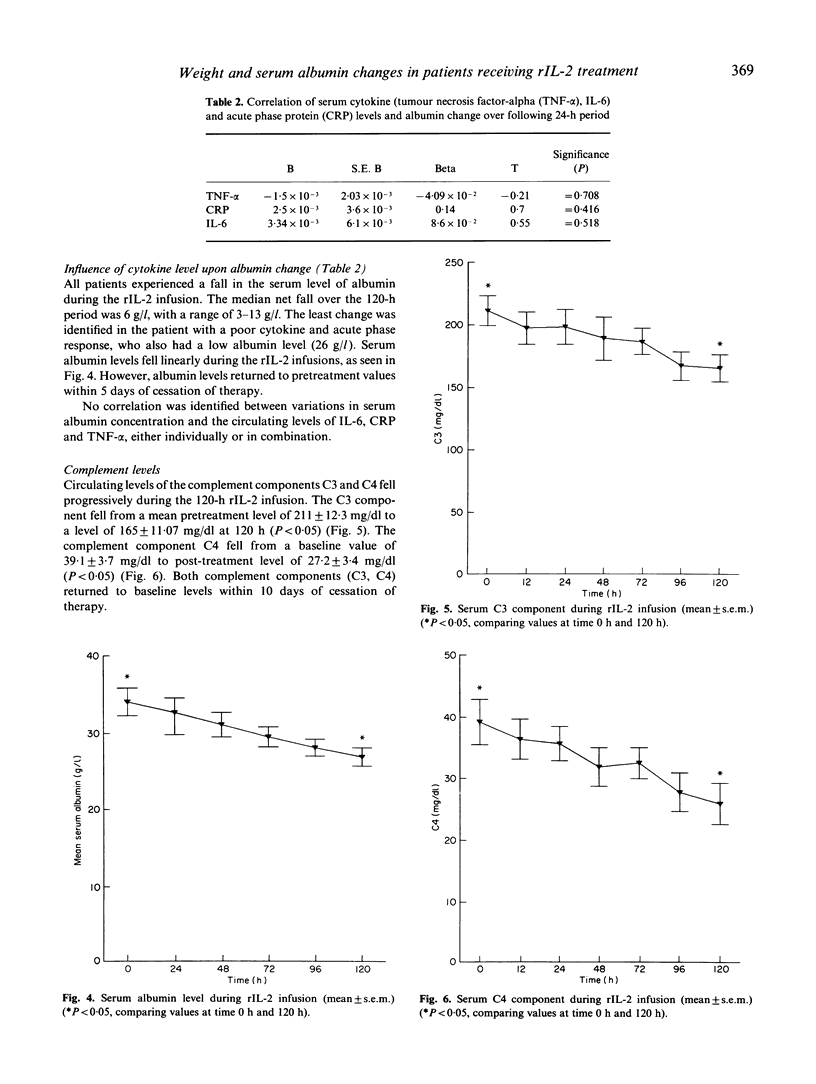
Recombinant IL-2 (rIL-2) has been used alone or in combination with other chemotherapeutic agents to enhance host defences against cancer. Prolonged administration of high doses, required for clinical efficacy, may precipitate serious dose-limiting toxicity. rIL-2-induced 'vascular leak syndrome' leads to hypotension, renal insufficiency, respiratory disturbances and other organ dysfunctions. Serial measurements of serum cytokines and the acute phase protein C-reactive protein (CRP) were performed on nine patients who received high-dose i.v. continuous therapy with rIL-2. The influence of these immunological parameters upon alterations in patients' weight and serum albumin, as indicators of toxicity, was assessed. All patients experienced weight increases during the cycle (3-11% of total body weight). The serum levels of tumour necrosis factor (TNF-alpha) and CRP were highly predictive of alterations in patients' weight (both P < 0.001), while no correlation was found with IL-6 and weight change. Serum albumin fell linearly throughout the infusion cycle, but this showed no correlation with variations in serum levels of IL-6, TNF-alpha, or CRP. The complement components C3 and C4 were significantly reduced at the end of the infusion, suggesting a possible role for this cascade system in mediating these clinical changes. The strong association between serum TNF-alpha and weight change, not previously documented, further supports the hypothesis that TNF-alpha is a key mediator in the pathogenesis of the 'vascular leak syndrome'.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Blay J. Y., Favrot M. C., Negrier S., Combaret V., Chouaib S., Mercatello A., Kaemmerlen P., Franks C. R., Philip T. Correlation between clinical response to interleukin 2 therapy and sustained production of tumor necrosis factor. Cancer Res. 1990 Apr 15;50(8):2371–2374. [PubMed] [Google Scholar]
- Blessing K., Park K. G., Heys S. D., King G., Eremin O. Immunopathological changes in the skin following recombinant interleukin-2 treatment. J Pathol. 1992 Jul;167(3):313–319. doi: 10.1002/path.1711670309. [DOI] [PubMed] [Google Scholar]
- Broom J., Fraser M. H., McKenzie K., Miller J. D., Fleck A. The protein metabolic response to short-term starvation in man. Clin Nutr. 1986 Feb;5(1):63–65. doi: 10.1016/0261-5614(86)90044-0. [DOI] [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Doi S., Saiki O., Hara T., Sugita T., Ha-Kawa K., Tanaka T., Hara H., Negoro S., Yabuuchi H., Kishimoto S. Administration of recombinant IL-2 augments the level of serum IgM in an IL-2 deficient patient. Eur J Pediatr. 1989 Jun;148(7):630–633. doi: 10.1007/BF00441517. [DOI] [PubMed] [Google Scholar]
- Ettinghausen S. E., Puri R. K., Rosenberg S. A. Increased vascular permeability in organs mediated by the systemic administration of lymphokine-activated killer cells and recombinant interleukin-2 in mice. J Natl Cancer Inst. 1988 Apr 6;80(3):177–188. doi: 10.1093/jnci/80.3.177. [DOI] [PubMed] [Google Scholar]
- Ferro T. J., Johnson A., Everitt J., Malik A. B. IL-2 induces pulmonary edema and vasoconstriction independent of circulating lymphocytes. J Immunol. 1989 Mar 15;142(6):1916–1921. [PubMed] [Google Scholar]
- Gaspari A. A., Lotze M. T., Rosenberg S. A., Stern J. B., Katz S. I. Dermatologic changes associated with interleukin 2 administration. JAMA. 1987 Sep 25;258(12):1624–1629. [PubMed] [Google Scholar]
- Gaynor E. R., Vitek L., Sticklin L., Creekmore S. P., Ferraro M. E., Thomas J. X., Jr, Fisher S. G., Fisher R. I. The hemodynamic effects of treatment with interleukin-2 and lymphokine-activated killer cells. Ann Intern Med. 1988 Dec 15;109(12):953–958. doi: 10.7326/0003-4819-109-12-953. [DOI] [PubMed] [Google Scholar]
- Gemlo B. T., Palladino M. A., Jr, Jaffe H. S., Espevik T. P., Rayner A. A. Circulating cytokines in patients with metastatic cancer treated with recombinant interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1988 Oct 15;48(20):5864–5867. [PubMed] [Google Scholar]
- Greaves M. F., Brown J., Molgaard H. V., Spurr N. K., Robertson D., Delia D., Sutherland D. R. Molecular features of CD34: a hemopoietic progenitor cell-associated molecule. Leukemia. 1992;6 (Suppl 1):31–36. [PubMed] [Google Scholar]
- Grimm E. A., Ramsey K. M., Mazumder A., Wilson D. J., Djeu J. Y., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. II. Precursor phenotype is serologically distinct from peripheral T lymphocytes, memory cytotoxic thymus-derived lymphocytes, and natural killer cells. J Exp Med. 1983 Mar 1;157(3):884–897. doi: 10.1084/jem.157.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Director E. P., Rosenberg S. A. Induction of endogenous cytokine-mRNA in circulating peripheral blood mononuclear cells by IL-2 administration to cancer patients. J Immunol. 1989 Jul 15;143(2):736–739. [PubMed] [Google Scholar]
- Kragel A. H., Travis W. D., Steis R. G., Rosenberg S. A., Roberts W. C. Myocarditis or acute myocardial infarction associated with interleukin-2 therapy for cancer. Cancer. 1990 Oct 1;66(7):1513–1516. doi: 10.1002/1097-0142(19901001)66:7<1513::aid-cncr2820660713>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lee R. E., Lotze M. T., Skibber J. M., Tucker E., Bonow R. O., Ognibene F. P., Carrasquillo J. A., Shelhamer J. H., Parrillo J. E., Rosenberg S. A. Cardiorespiratory effects of immunotherapy with interleukin-2. J Clin Oncol. 1989 Jan;7(1):7–20. doi: 10.1200/JCO.1989.7.1.7. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Rayner A. A., Ettinghausen S. E., Vetto J. T., Seipp C. A., Rosenberg S. A. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986 Dec 15;58(12):2764–2772. doi: 10.1002/1097-0142(19861215)58:12<2764::aid-cncr2820581235>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Macdonald D., Gordon A. A., Kajitani H., Enokihara H., Barrett A. J. Interleukin-2 treatment-associated eosinophilia is mediated by interleukin-5 production. Br J Haematol. 1990 Oct;76(2):168–173. doi: 10.1111/j.1365-2141.1990.tb07867.x. [DOI] [PubMed] [Google Scholar]
- Marks J. D., Marks C. B., Luce J. M., Montgomery A. B., Turner J., Metz C. A., Murray J. F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am Rev Respir Dis. 1990 Jan;141(1):94–97. doi: 10.1164/ajrccm/141.1.94. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Vachino G., van der Meer J. W., Numerof R. P., Adams S., Cannon J. G., Bernheim H. A., Atkins M. B., Parkinson D. R., Dinarello C. A. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988 Nov;8(6):426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985 Jul;135(1):646–652. [PubMed] [Google Scholar]
- Nijsten M. W., de Groot E. R., ten Duis H. J., Klasen H. J., Hack C. E., Aarden L. A. Serum levels of interleukin-6 and acute phase responses. Lancet. 1987 Oct 17;2(8564):921–921. doi: 10.1016/s0140-6736(87)91413-9. [DOI] [PubMed] [Google Scholar]
- Norton J., Sloane J. P., Delia D., Greaves M. F. Reciprocal expression of CD34 and cell adhesion molecule ELAM-1 on vascular endothelium in acute cutaneous graft-versus-host disease. J Pathol. 1993 Jun;170(2):173–177. doi: 10.1002/path.1711700213. [DOI] [PubMed] [Google Scholar]
- Ognibene F. P., Rosenberg S. A., Lotze M., Skibber J., Parker M. M., Shelhamer J. H., Parrillo J. E. Interleukin-2 administration causes reversible hemodynamic changes and left ventricular dysfunction similar to those seen in septic shock. Chest. 1988 Oct;94(4):750–754. doi: 10.1378/chest.94.4.750. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Ramos O. F., Algarra I., Sármay G., Yefenof E., Gergely J., Klein E. Lymphocytes stimulated by allogeneic B cell lines cleave the third component of complement and fix C3 fragments. Their nonspecific lytic capacity is elevated against complement receptor type 2-carrying targets. J Immunol. 1989 Jan 1;142(1):217–223. [PubMed] [Google Scholar]
- Robbins R. A., Russ W. D., Rasmussen J. K., Clayton M. M. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987 Mar;135(3):651–658. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraya K. A., Balkwill F. R. Temporal sequence and cellular origin of interleukin-2 stimulated cytokine gene expression. Br J Cancer. 1993 Mar;67(3):514–521. doi: 10.1038/bjc.1993.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slungaard A., Vercellotti G. M., Walker G., Nelson R. D., Jacob H. S. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990 Jun 1;171(6):2025–2041. doi: 10.1084/jem.171.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Suter P. M., Suter S., Girardin E., Roux-Lombard P., Grau G. E., Dayer J. M. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992 May;145(5):1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- Thijs L. G., Hack C. E., Strack van Schijndel R. J., Nuijens J. H., Wolbink G. J., Eerenberg-Belmer A. J., Van der Vall H., Wagstaff J. Activation of the complement system during immunotherapy with recombinant IL-2. Relation to the development of side effects. J Immunol. 1990 Mar 15;144(6):2419–2424. [PubMed] [Google Scholar]
- Vachino G., Gelfand J. A., Atkins M. B., Tamerius J. D., Demchak P., Mier J. W. Complement activation in cancer patients undergoing immunotherapy with interleukin-2 (IL-2): binding of complement and C-reactive protein by IL-2-activated lymphocytes. Blood. 1991 Nov 15;78(10):2505–2513. [PubMed] [Google Scholar]
- Volanakis J. E. Complement activation by C-reactive protein complexes. Ann N Y Acad Sci. 1982;389:235–250. doi: 10.1111/j.1749-6632.1982.tb22140.x. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Shiozaki H., Miura Y., Hitoshi Y., Tominaga A., Takatsu K., Kasahara T. Role of IL-5 in IL-2-induced eosinophilia. In vivo and in vitro expression of IL-5 mRNA by IL-2. J Immunol. 1990 Aug 1;145(3):873–877. [PubMed] [Google Scholar]


