Abstract
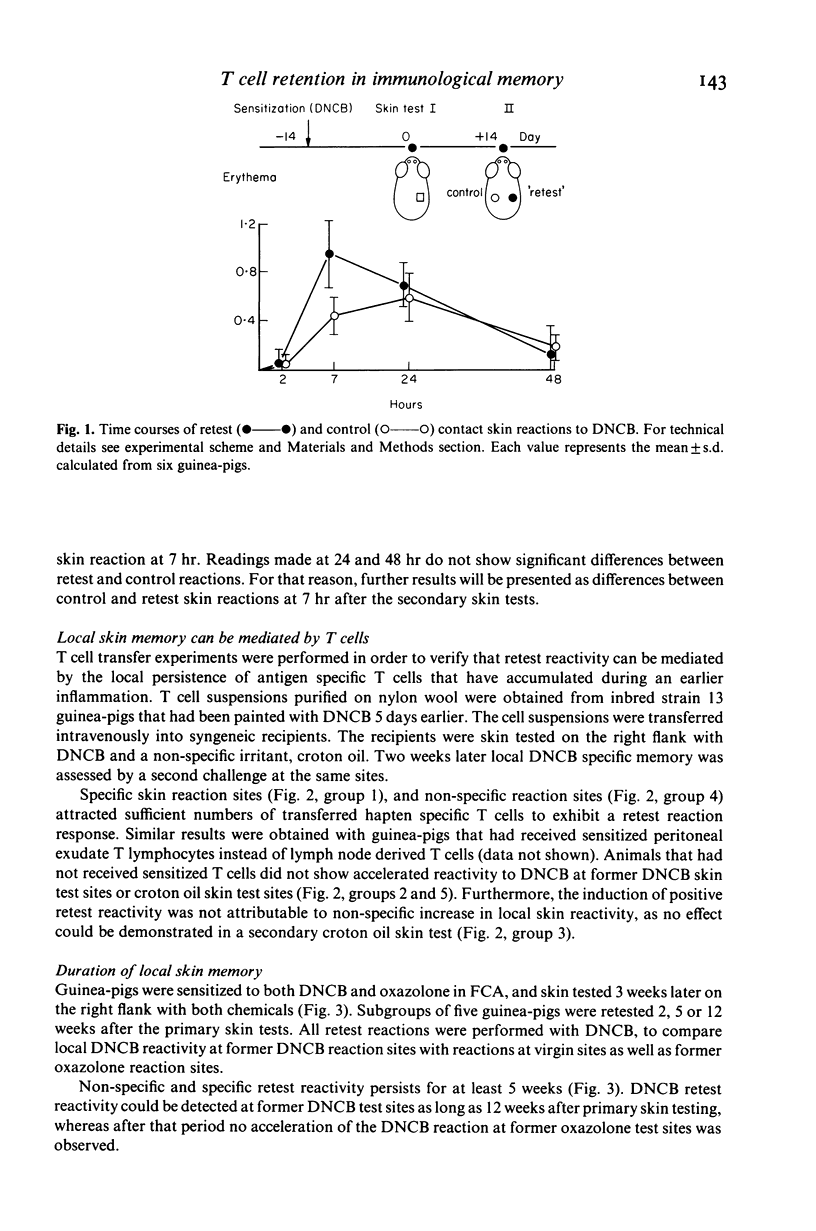
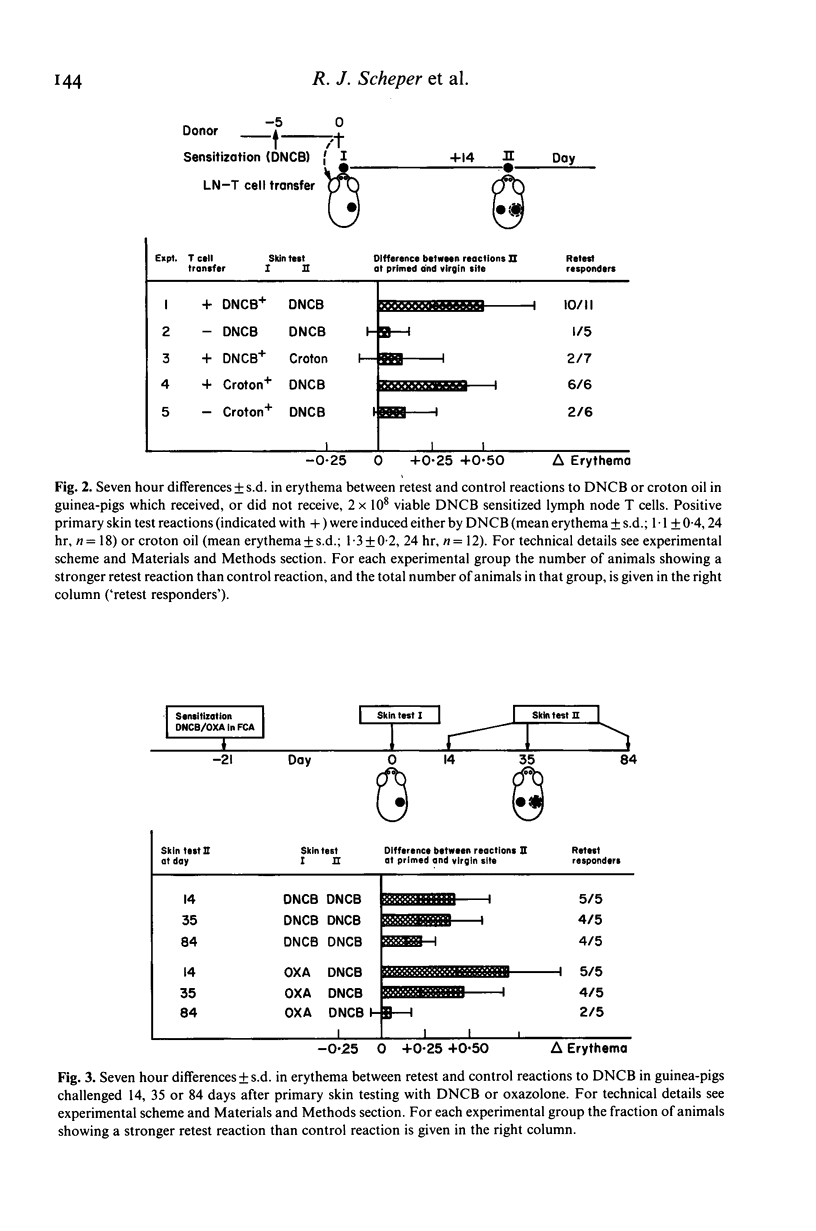
Using an experimental contact sensitivity model in guinea-pigs, evidence is presented that hapten (DNCB or oxazolone) specific T lymphocytes may persist for several months in previous sites of inflammation. Immunological memory, revealed by accelerated contact skin reactions upon retesting with the hapten, was limited to the original contact skin reaction sites. This 'local skin memory' to DNCB or oxazolone could be induced in both specific and non-specific skin inflammatory reactions, provided the animals had been sensitized to the hapten not longer than 2 weeks before. In animals which had been sensitized more than 1 month earlier, local skin memory could be induced if the animals received a booster application of hapten shortly (0-2 days) before primary skin testing. From these results we conclude that recently activated T cells may enter inflammatory sites non-specifically, producing specific local immunological memory. This memory may last several months. Accumulation of hapten specific T cells at inflammatory sites may be important in retest reactivity, in flare-up reactivity and in chronic inflammation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Baine Y., Thorbecke G. J. Induction and persistence of local B cell memory in mice. J Immunol. 1982 Feb;128(2):639–643. [PubMed] [Google Scholar]
- Bleumink E., Jansen L. H. Studies on flare and rash phenomena in guinea-pigs. Arch Dermatol Forsch. 1972;242(3):285–292. doi: 10.1007/BF00595414. [DOI] [PubMed] [Google Scholar]
- Dahlbäck K., Möller H. Flare-up of contract dermatitis to picryl chloride in the mouse. Acta Derm Venereol. 1981;61(4):342–344. [PubMed] [Google Scholar]
- Emeson E. E. Migratory behavior of lymphocytes with specific reactivity to alloantigens. II. Selective recruitment to lymphoid cell allografts and their draining lymph nodes. J Exp Med. 1978 Jan 1;147(1):13–24. doi: 10.1084/jem.147.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn L. E. The chronicity of inflammation and its significance in rheumatoid arthritis. Ann Rheum Dis. 1968 Mar;27(2):105–121. doi: 10.1136/ard.27.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Hopkins J., Reynolds J. Studies of efferent lymph cells from nodes stimulated with oxazolone. Immunology. 1980 Feb;39(2):141–149. [PMC free article] [PubMed] [Google Scholar]
- Hashim G. A., Yee N., Ramey W. G. The kinetics of T lymphocyte subpopulations in guinea-pigs sensitized with allogeneic transplantation antigens. Clin Exp Immunol. 1980 Sep;41(3):533–540. [PMC free article] [PubMed] [Google Scholar]
- Hopkins J., McConnell I., Lachmann P. J. Specific selection of antigen-reactive lymphocytes into antigenically stimulated lymph nodes in sheep. J Exp Med. 1981 Mar 1;153(3):706–719. doi: 10.1084/jem.153.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W. Nonrecirculating memory T lymphocytes in cellular resistance to infection. Cell Immunol. 1980 Oct;55(2):499–505. doi: 10.1016/0008-8749(80)90181-1. [DOI] [PubMed] [Google Scholar]
- Leber P. D., Milgrom M., Cohen S. Eosinophils in delayed hypersensitivity skin reaction sites. Immunol Commun. 1973;2(6):615–620. doi: 10.3109/08820137309022832. [DOI] [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., MCCLUSKEY J. W. STUDIES ON THE SPECIFICITY OF THE CELLULAR INFILTRATE IN DELAYED HYPERSENSITIVITY REACTIONS. J Immunol. 1963 Mar;90:466–477. [PubMed] [Google Scholar]
- Mitchell J. C. The angry back syndrome: eczema creates eczema. Contact Dermatitis. 1975 Aug;1(4):193–194. doi: 10.1111/j.1600-0536.1975.tb05380.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Fukushiro S., Gotoh M., Kohda M., Namba M., Tanioku K. Studies on the retest reaction in contact sensitivity to DNCB. Dermatologica. 1978;157(1):13–20. doi: 10.1159/000250803. [DOI] [PubMed] [Google Scholar]
- Polak L., Frey J. R., Turk J. L. Studies on the effect of systemic administration of sensitizers to guinea-pigs with contact sensitivity to inorganic metal compounds. V. Studies on the mechanism of the 'flare up' reaction. Clin Exp Immunol. 1970 Nov;7(5):739–744. [PMC free article] [PubMed] [Google Scholar]
- Polak L., Turk J. L., Frey J. R. Studies on contact hypersensitivity to chromium compounds. Prog Allergy. 1973;17:145–226. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Vernes A., Delabre M., Biguet J. Experimental study of resistance to infection by Trichophyton mentagrophytes: demonstration of memory skin cells. J Invest Dermatol. 1980 Apr;74(4):205–209. doi: 10.1111/1523-1747.ep12541727. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Parrott D. M., Bruce R. G. The accumulation of immunoblasts in extravascular tissues including mammary gland, peritoneal cavity, gut and skin. Immunology. 1978 Aug;35(2):415–423. [PMC free article] [PubMed] [Google Scholar]
- Scheper R. J., Noble B., Parker D., Turk J. L. The value of an assessment of erythema and increase in thickness of the skin reaction for a full appreciation of the nature of delayed hypersensitivity in the guinea pig. Int Arch Allergy Appl Immunol. 1977;54(1):58–66. doi: 10.1159/000231808. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Oort J. Rosette-forming cells and the immunological response after DNCB, DNP-carrier and oxazolone sensitization. Immunology. 1974 Feb;26(2):269–280. [PMC free article] [PubMed] [Google Scholar]
- van Beusekom H. J., van de Putte L. B., van den Berg W. B., van den Broek W. J., Buijs W. C. Antigen handling in antigen-induced joint inflammation: kinetics of a second intra-articularly injected dose of antigen in an already established antigen-induced joint inflammation. Immunology. 1981 Sep;44(1):153–161. [PMC free article] [PubMed] [Google Scholar]
- van Maarsseveen A. C., Bomhof G., Scheper R. J. Demonstration of accelerated and increased migration inhibition factor release in vivo in a PPD retest reaction. Int Arch Allergy Appl Immunol. 1982;67(1):1–6. doi: 10.1159/000232979. [DOI] [PubMed] [Google Scholar]
- van den Berg W. B., van Maarsseveen A. C., Mullink H., Scheper R. J. Accumulation of T cells and local anti-PPD antibody production in lymphokine-mediated chronic peritoneal inflammation in the guinea pig. J Pathol. 1980 Sep;132(1):23–33. doi: 10.1002/path.1711320104. [DOI] [PubMed] [Google Scholar]


