Abstract
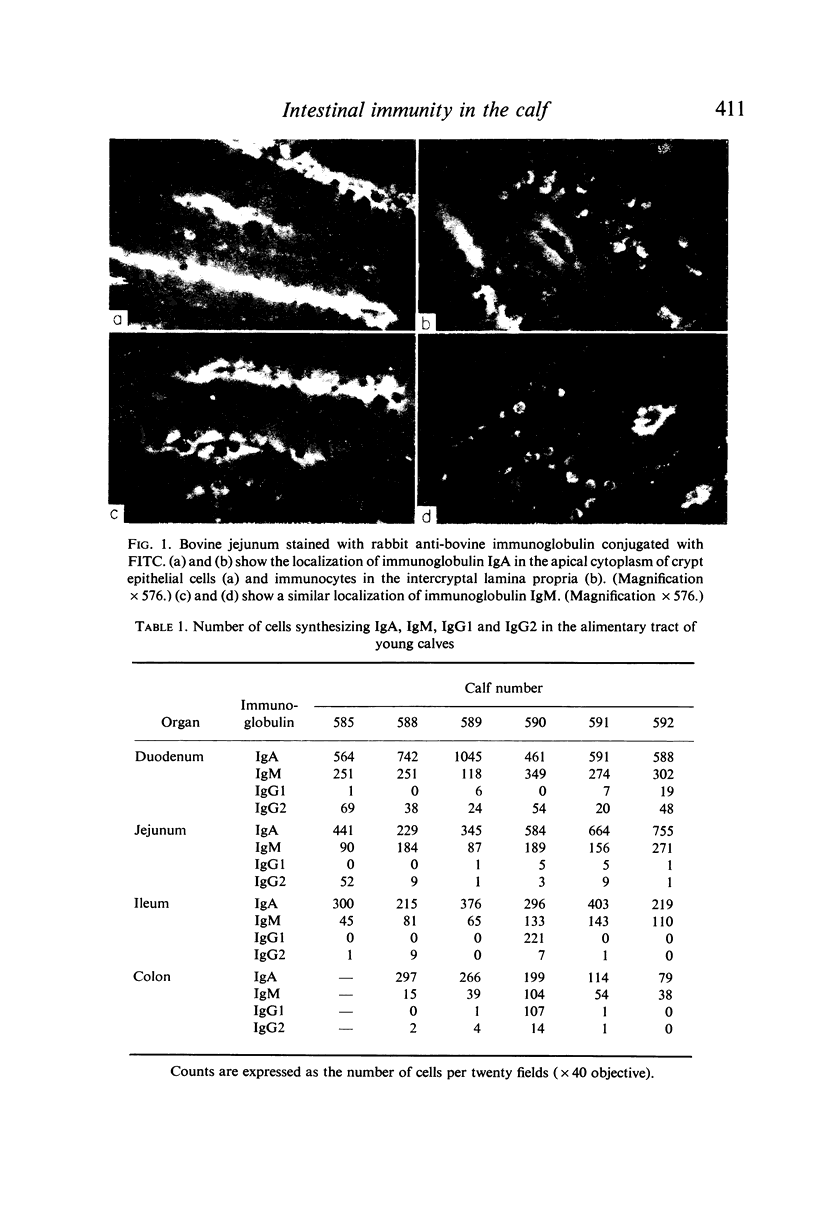
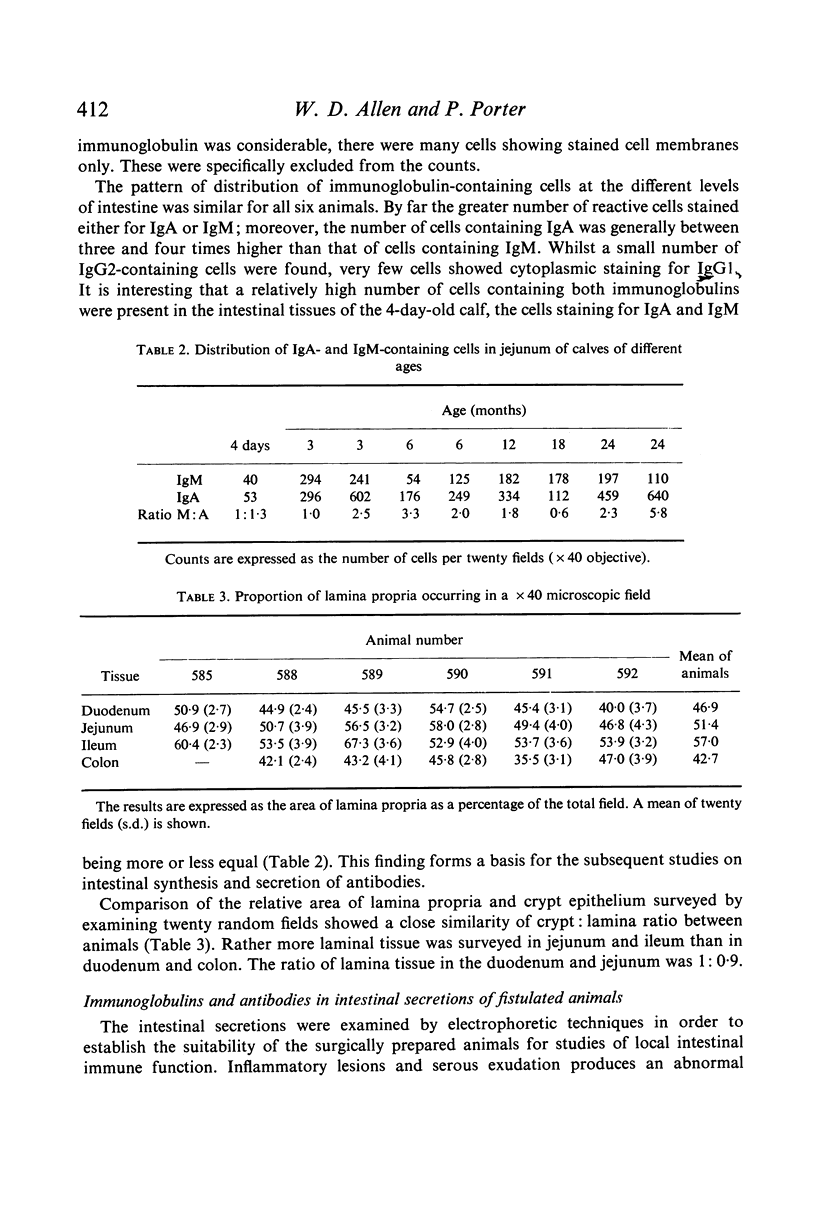
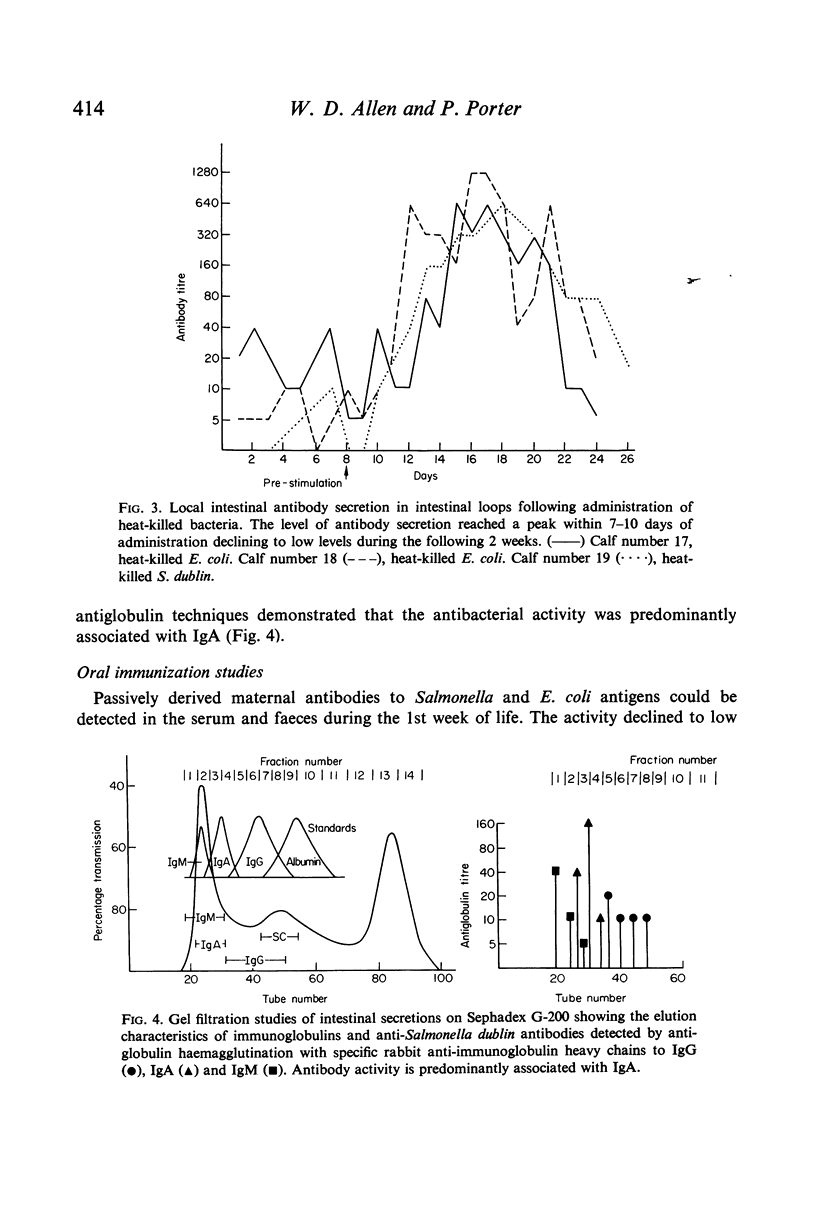
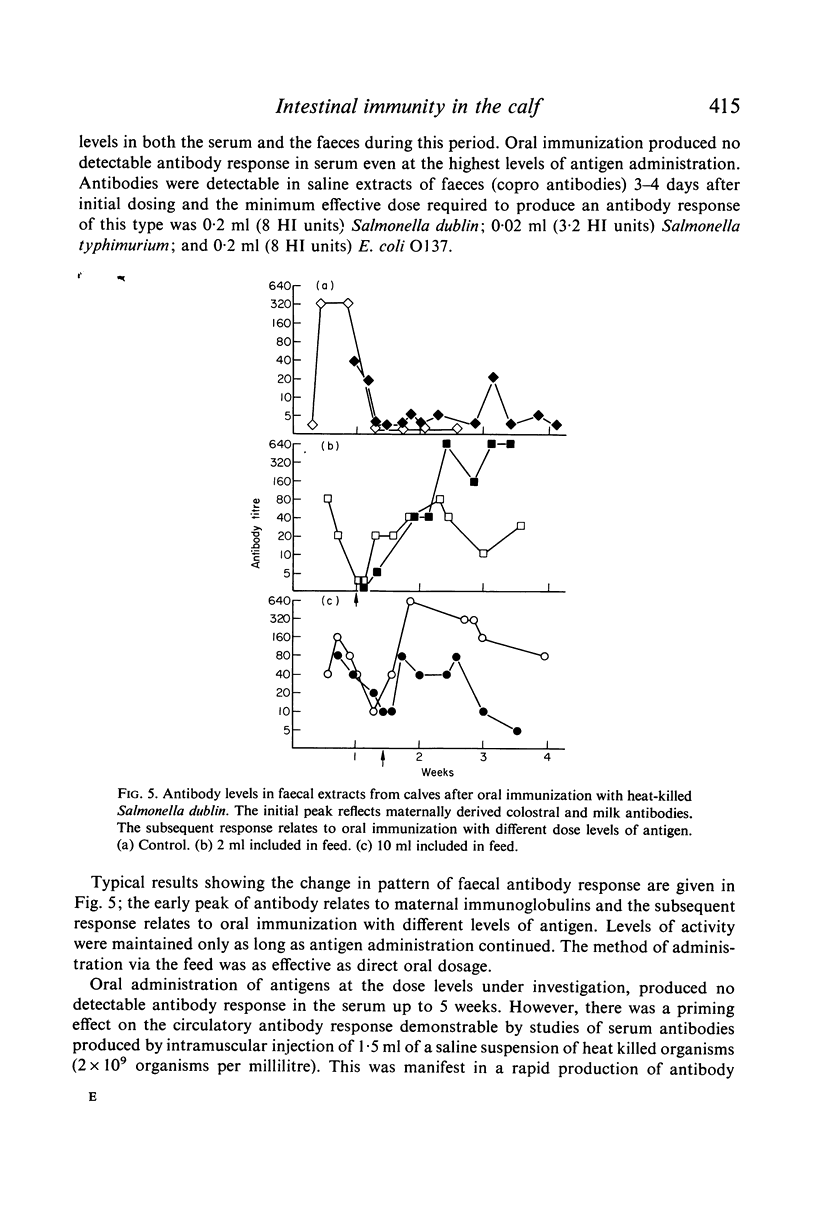
Immunofluorescent studies of intestinal tissues from young preruminant calves demonstrate the presence of two main populations of immunocytes synthesizing IgA and IgM. These cells had infiltrated the lamina propria of the intestine as early as 4 days of age. There was little evidence of any significant involvement of IgG1 in intestinal immune synthesis of calves at this age although activity was demonstrable in the ileum and colon of one calf. In general there were more IgG2-synthesizing cells than IgG1, but these were few compared with the main populations of IgA and IgM cells. Local antigenic stimulus to the intestinal mucosa of young fistulated calves using extracts of heat-killed Gram-negative bacteria produced antibody in the secretions over a period of approximately 3 weeks. A second administration of a similar antigenic dose produced a similar response indicating the requirement for continuous stimuli to maintain a measurable level of antibody secretion. Gel filtration and antiglobulin assays indicated that the antibacterial activity was predominantly associated with IgA and that IgM also played a significant role. Oral administration of bacterial antigens to colostrum-fed calves from 5 to 8 days of age produced a faecal antibody response, indicating that intestinal secretion could be successfully interrelated with the declining passive antibody to maintain an almost continuous level of intestinal antibody in early life.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. D., Porter P. The demonstration of immunoglobulins in porcine intestinal tissue by immunofluorescence with observations on the effect of fixation. Immunology. 1970 Jun;18(6):799–806. [PMC free article] [PubMed] [Google Scholar]
- Allen W. D., Porter P. The relative distribution of IgM and IgA cells in intestinal mucosa and lymphoid tissues of the young unweaned pig and their significance in ontogenesis of secretory immunity. Immunology. 1973 Mar;24(3):493–501. [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Maxwell C. F., Pierce C. S., Hylton M. B., Asofsky R., Kiddy C. A. Studies on the relative synthesis and distribution of IgA and IgG1 in various tissues and body fluids of the cow. J Immunol. 1972 Jul;109(1):38–46. [PubMed] [Google Scholar]
- Curtain C. C., Clark B. L., Dufty J. H. The origins of the immunoglobulins in the mucous secretions of cattle. Clin Exp Immunol. 1971 Feb;8(2):335–344. [PMC free article] [PubMed] [Google Scholar]
- FRETER R., GANGAROSA E. J. ORAL IMMUNIZATION AND PRODUCTION OF COPROANTIBODY IN HUMAN VOLUNTEERS. J Immunol. 1963 Dec;91:724–729. [PubMed] [Google Scholar]
- Girard J. P., de Kalbermatten A. Antibody activity in human duodenal fluid. Eur J Clin Invest. 1970 Nov;1(3):188–195. doi: 10.1111/j.1365-2362.1970.tb00616.x. [DOI] [PubMed] [Google Scholar]
- McClelland D. B., Samson R. R., Parkin D. M., Shearman D. J. Bacterial agglutination studies with secretory IgA prepared from human gastrointestinal secretions and colostrum. Gut. 1972 Jun;13(6):450–458. doi: 10.1136/gut.13.6.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochmann H., Ocklitz H. W., Hering L., Schmidt E. F., Richter H. Der Einfluss der Dosierung und des Booster-Reizes auf die Wirksamkeit der oralen Immunisierung gegen Dyspepsiecoli-Infektionen im aktiven Mäuseschutzversuch. Zentralbl Bakteriol Orig. 1971;216(1):24–31. [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Porter P., Allen W. D. Classes of immunoglobulins related to immunity in the pig. J Am Vet Med Assoc. 1972 Feb 15;160(4):511–518. [PubMed] [Google Scholar]
- Porter P. Immunoglobulins in bovine mammary secretions. Quantitative changes in early lactation and absorption by the neonatal calf. Immunology. 1972 Aug;23(2):225–238. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Allen W. D. Effect of oral immunisation with E coli antigens on post weaning enteric infection in the young pig. Vet Rec. 1974 Aug 3;95(5):99–104. doi: 10.1136/vr.95.5.99. [DOI] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Holme D. W., Horsfield S. Escherichia coli antigens as dietary additives for oral immunisation of pigs: trials with pig creep feeds. Vet Rec. 1973 Jun 16;92(24):630–636. doi: 10.1136/vr.92.24.630. [DOI] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Noakes D. E., Allen W. D. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974 Nov;27(5):841–853. [PMC free article] [PubMed] [Google Scholar]
- Porter P., Noakes D. E. Immunoglobulin IgA in bovine serum and external secretions. Biochim Biophys Acta. 1970 Jul 27;214(1):107–116. doi: 10.1016/0005-2795(70)90074-7. [DOI] [PubMed] [Google Scholar]
- ROY J. H., PALMER J., SHILLAM K. W., INGRAM P. L., WOOD P. C. The nutritive value of colostrum for the calf. 10. The relationship between the period of time that a calfhouse has been occupied and the incidence of scouring and mortality in young calves. Br J Nutr. 1955;9(1):11–20. doi: 10.1079/bjn19550005. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Jones E. W., Bush L. J. Maternal transport of immunoglobulins to the calf. J Dairy Sci. 1971 Sep;54(9):1323–1323. [PubMed] [Google Scholar]
- Wernet P., Breu H., Knop J., Rowley D. Antibacterial action of specific IgA and transport of IgM, IgA, and IgG from serum into the small intestine. J Infect Dis. 1971 Aug;124(2):223–226. doi: 10.1093/infdis/124.2.223. [DOI] [PubMed] [Google Scholar]


