Abstract
Recently, we developed a series of cytotoxic peptide conjugates containing 14-O-glutaryl esters of doxorubicin (DOX) or 2-pyrrolino-DOX (AN-201). Serum carboxylesterase enzymes (CE) can partially hydrolyze these conjugates in the circulation, releasing the cytotoxic radical, before the targeting is complete. CE activity in serum of nude mice is about 10 times higher than in human serum. Thus, we found that the t1/2 of AN-152, an analog of luteinizing hormone-releasing hormone (LH-RH) containing DOX, at 0.3 mg/ml is 19.49 ± 0.74 min in mouse serum and 126.06 ± 3.03 min in human serum in vitro. The addition of a CE inhibitor, diisopropyl fluorophosphate (DFP), to mouse serum in vitro significantly (P < 0.01) prolongs the t1/2 of AN-152 to 69.63 ± 4.44 min. When DFP is used in vivo, 400 nmol/kg cytotoxic somatostatin analog AN-238 containing AN-201 is well tolerated by mice, whereas all animals die after the same dose without DFP. In contrast, DFP has no effect on the tolerance of AN-201. A better tolerance to AN-238 after DFP treatment is due to the selective uptake of AN-238 by somatostatin receptor-positive tissues. Our results demonstrate that the suppression of the CE activity in nude mice greatly decreases the toxicity of cytotoxic hybrids containing 2-pyrrolino-DOX 14-O-hemiglutarate and brings this animal model closer to the conditions that exist in humans. The use of DFP together with these peptide conjugates in nude mice permits a better understanding of their mechanism of action and improves the clinical predictability of the oncological and toxicological results.
Keywords: targeted chemotherapeutic agents, tolerance, esterase inhibitors, diisopropyl fluorophosphate
Doxorubicin (DOX) is the most widely used cytotoxic agent with a broad spectrum of antitumor activity (1). The strong antiproliferative effect of DOX is mainly due to its ability to intercalate into the DNA and break the strands of double helix by inhibiting topoisomerase II. However, its clinical efficacy is limited by toxic effects such as cardiomyopathy and myelosuppression, and the intrinsic or acquired resistance of cancer cells to DOX also reduces the response to DOX therapy. To exploit the full tumoricidal potential of DOX, diverse approaches have been tried, such as a more specific delivery of DOX to tumor cells (2–5) or the production of prodrugs that are activated mainly in tumor cells (6–8). The outcome of targeted chemotherapy greatly depends on two factors: the ability of the carrier molecule to selectively recognize malignant cells and the nature of the chemical linkage used for coupling the cytotoxic agent to the carrier. Ideally, the conjugate should be stable and inactive in the circulation, with the cytotoxic radical released in an active form in the target tumor tissue. Various chemical linkages have been tried to meet this goal, and various attempts yielded conjugates of DOX with increased antitumor activity and decreased toxicity in preclinical models (2–4). Because ester bonds are relatively stable and 14-O-esters of DOX have been reported to be effective as antitumor agents (9), we developed a series of targeted cytotoxic peptide conjugates in which DOX 14-O-hemiglutarate is linked to analogs of luteinizing hormone-releasing hormone (LH-RH) (10), bombesin (11), or somatostatin (SST) (12). We also developed a highly active derivative of DOX, 2-pyrrolino-DOX (AN-201) (13), which can be coupled to these peptide carriers by the same linkage (2, 10–12).
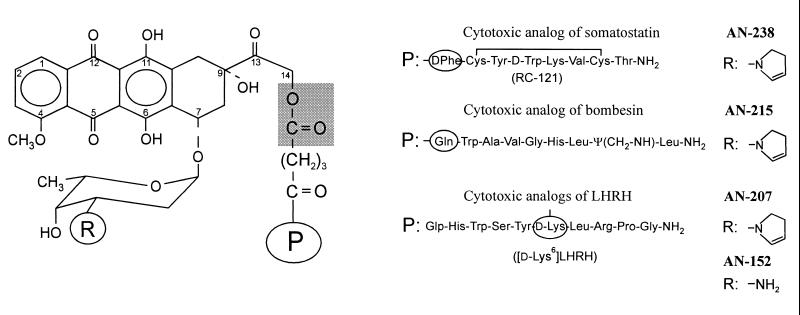
We have demonstrated that our targeted cytotoxic peptide conjugates are more active and less toxic in nude mice bearing xenografts of various human cancers than the cytotoxic moieties they contain (2). However, in male nude mice, the maximum tolerated doses (MTD) of AN-201 and its hybrids with LH-RH (AN-207), bombesin (AN-215), and SST (AN-238) (Fig. 1) were found to be similar, between 175 and 200 nmol/kg (2). In contrast, Copenhagen rats bearing R 3327-AT-1 rat Dunning prostate carcinoma tolerated a single i.v. injection of 300 nmol/kg SST analog AN-238, whereas 115–125 nmol/kg unconjugated AN-201 was lethal (14). Because serum carboxylesterase enzymes (CE) (EC 3.1.1.1) (15) can hydrolyze a certain percentage of the conjugates in the circulation and release DOX or AN-201 before they reach the target tissues, we assumed that a high activity of these enzymes in mice may explain our toxicological results (16). In fact, in preliminary experiments in vitro, we found that in the serum of nude mice, Copenhagen rats, and humans the t1/2 of cytotoxic LH-RH analog AN-152, which contains DOX 14-O-hemiglutarate (Fig. 1), was about 10, 30, and 120 min, respectively (16, 17).
Figure 1.
Molecular structure of cytotoxic analogs of SST (AN-238), bombesin (AN-215), and LH-RH (AN-152 and AN-207). Cytotoxic radicals doxorubicin (R = NH2) and 2-pyrrolinodoxorubicin (R = 2-pyrrolino) are linked through a glutaric acid spacer to the free amino groups of amino acids (circled) in the peptide carriers (P). The ester bond between the 14-OH group of the cytotoxic moiety and glutaric acid is indicated by a shaded area.
Based on these findings, our aim was to establish a preclinical model that shows more similarity to the clinical conditions. In this study we determined that the t1/2 of AN-152 in vitro in the serum of nude mice and men was 19 and 126 min, respectively. We also showed that, by adding diisopropyl fluorophosphate (DFP), an organophosphate inhibitor of CE, to mouse serum in vitro at a concentration corresponding to its i.v. MTD, the t1/2 of AN-152 can be prolonged to 70 min. In studies in vivo we established that tumor-free mice pretreated with DFP at its i.p. MTD can tolerate two consecutive injections of 400 nmol/kg AN-238 without an apparent toxicity, whereas animals receiving a single injection of 400 nmol/kg AN-238 without DFP die within 8 days. In contrast, the toxicity of AN-201 was not changed by DFP. The effect of DFP on the tolerance of mice to AN-207 and AN-215 as well as the effect of other esterase inhibitors on the toxicity of our hybrid analogs likewise were studied. Preliminary results on the toxicity and efficacy of 400 nmol/kg AN-238 in DFP-pretreated nude mice bearing SW-839 human renal cell carcinomas also are discussed.
Materials and Methods
Chemicals.
Cytotoxic radical AN-201 and cytotoxic peptide conjugates AN-152, AN-207, AN-215, and AN-238 as well as SST carrier analog RC-121 (Fig. 1) were synthesized in our laboratories (10–13). For in vivo experiments, the cytotoxic analogs were administered i.v. as described (16). DFP was purchased from Acros Organics (Fisher Scientific). Paraoxon, bis(4-nitrophenyl) phosphate (BNPP), and atropine sulfate were bought from Sigma.
Sample Preparation.
Human blood samples were obtained from healthy male volunteers, 23–43 years of age. Mice were anesthetized with methoxyflurane and blood was collected from abdominal vessels. Freshly taken whole blood was allowed to coagulate for 1 hr in an incubator at 37°C in 95% air/5% CO2 atmosphere with 100% relative humidity, and the serum was separated by centrifugation. Aliquots of 100 μl of human and mouse serum were kept in the incubator before being added to solutions of AN-152. The hydrolysis of AN-152 by CE in mouse serum was determined at different substrate concentrations by dissolving 10, 30, or 100 μg of AN-152 in 10 μl of 0.9% saline and adding 100 μl of mouse serum to each of these solutions. A comparative study on the hydrolysis of AN-152 in PBS (pH 7.4), human serum, and mouse serum with or without the addition of DFP was carried out at a substrate concentration of 30 μg in 100 μl. CE activity in mouse serum was inhibited by the addition of 6 μg of DFP in 6 μl of distilled water to 100 μl of serum. The samples were incubated for 10, 30, 60, and 120 min except for the study with mouse serum without DFP, where the incubations were done only for 10, 30, and 60 min. At the end of the incubation, 10 μl of glacial acetic acid was added to each sample to stop the hydrolysis.
HPLC Analysis.
The samples were applied on a Beckman analytical HPLC system equipped with model 168 diode array detector (Beckman Coulter). Separation of the intact peptide conjugate and the hydrolyzed cytotoxic radical, DOX, was carried out on a Vydac C8 column (250 × 4.6 mm; pore size, 300 Å; particle size, 10 μm). The UV absorption was detected at 480 nm, and the percentage of intact AN-152 was determined by analysis of the chromatograms by system gold chromatography software (Beckman Coulter).
Evaluation of Data.
The percentage of intact AN-152 at 10, 30, and 60 min was used to calculate the t1/2 of the conjugate in mouse serum at 0.1, 0.3, and 1.0 mg/ml concentrations. The t1/2 values for AN-152 in PBS (pH 7.4), human serum, and mouse serum in the presence of DFP were calculated between 0 and 120 min. The calculations were done by linear regression analysis of the log concentrations vs. time. The area under the curve (AUC) for the concentration of intact AN-152 in serum was calculated by using the trapezoidal rule between 0 and 120 min.
Statistical analyses were performed by using Student's two-tailed t test.
In Vivo Studies.
Animals.
Male athymic (Ncr nu/nu) nude mice were purchased from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD) and housed in laminar airflow cabinets under pathogen-free conditions with a 12-hr light/12-hr dark schedule and fed autoclaved standard chow and water ad libitum. All experiments were performed in accordance with institutional guidelines for animal care.
Inhibition of CE by DFP.
For the inhibition of CE, 10 mg (9.5 μl) of DFP was dissolved in 10 ml of distilled water and 200 μl of this solution was injected i.p. to mice weighing 30 g [MTD, 6 mg/kg body weight (BW)]. To avoid possible side effects related to inhibition of cholinesterases, atropine sulfate was given i.m. (200 μg in 100 μl of distilled water) immediately after the injection of DFP. Cytotoxic compound AN-238, AN-215, AN-207, or AN-201 was administered i.v. 30 min after the injection of DFP.
Tolerance studies.
To investigate the effect of DFP on the tolerance of mice to AN-238 and AN-201, single injections of AN-238 or AN-201 at 400 nmol/kg were given to mice (three to four per group) with and without DFP pretreatment. The tolerance to LH-RH analog AN-207 at 300 and 400 nmol/kg and bombesin analog AN-215 at 400 nmol/kg was determined only after DFP treatment. Other mice pretreated with DFP received 200 μg of carrier peptide RC-121 i.v. 10 min before injection of AN-238 at 400 nmol/kg. For the assessment of tolerance to repetitive administration of high doses of AN-238 after inhibition of CE, injections of 300 or 400 nmol/kg of AN-238 were given to two groups of mice pretreated with DFP, and the treatment regimen was repeated twice as their BW returned to the initial values.
Studies with other esterase inhibitors.
Paraoxon was injected s.c. into mice at various doses between 0.4 and 2.0 mg/kg, followed by the i.m. injection of 200 μg of atropine in 100 μl of distilled water. Thirty minutes later, single injections of AN-238 at 225, 300, 350, or 400 nmol/kg were administered. BNPP was dissolved in 30 μl of 2-propanol and diluted with 5.5% (wt/vol) aqueous d-mannitol. Animals received 100–450 mg/kg BNPP i.p. 60 min before the i.v. administration of cytotoxic analog AN-201, AN-238, or AN-215 at 400-nmol/kg doses.
Results
In Vitro Hydrolysis of AN-152.
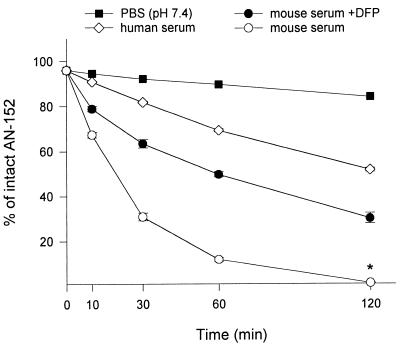
The effect of CE on the rate of deconjugation of AN-152 in the serum of nude mice was studied at 0.1, 0.3, and 1.0 mg/ml concentrations of AN-152, and the t1/2 of the conjugate was calculated to be 19.99 ± 0.97, 19.48 ± 0.72, and 44.89 ± 11.59 min, respectively. Further kinetic studies on the hydrolysis of cytotoxic peptide conjugate AN-152 at a concentration of 0.3 mg/ml were carried out in PBS (pH 7.4), human serum, and mouse serum pretreated with DFP (Fig. 2). Only a slight deconjugation occurred in PBS (pH 7.4) as characterized by a t1/2 of 477.06 min. The rate of hydrolysis of AN-152 in mouse serum (t1/2 = 19.48 ± 0.72 min) was significantly higher than in human serum (t1/2 = 126.06 ± 3.03 min; P < 0.001). The addition of DFP to mouse serum could significantly prolong the t1/2 of AN-152 to 69.63 ± 4.44 min (P < 0.01), which is still only ≈50% of that found in human serum. The concentration of DFP used corresponded to its i.v. MTD (3 mg/kg) (18), and the calculation was based on a value for mice of 50 ml of serum/kg of BW. The inhibition of CE in mouse serum also increased the amount of intact cytotoxic conjugate available for targeting within 120 min, as shown by a marked increase of AUC from 8.51 ± 0.33 to 19.22 ± 0.43 mg × min × ml−1 (P < 0.001). This value represents 74.8% of the AUC for AN-152 in human serum (25.70 ± 0.19). However, t1/2 as well as AUC values in DFP-pretreated mouse serum were still significantly lower than in human serum (Table 1).
Figure 2.
Hydrolysis of cytotoxic LHRH analog AN-152 after incubation at 37°C in a 95% air/5% CO2 atmosphere with 100% humidity in PBS (pH 7.4), human serum, and in mouse serum with or without CE inhibitor DFP. The percentage of intact AN-152 was determined by HPLC as described in Materials and Methods. *, Value obtained by extrapolation.
Table 1.
Kinetics of the hydrolysis of cytotoxic LH-RH conjugate AN-152 at the concentration of 0.3 mg/ml in human and mouse serum with or without CE-inhibitor DFP
| Serum | t1/2, min | AUC, mg × min × ml−1 |
|---|---|---|
| Mouse | 19.49 ± 0.74* | 8.51 ± 0.33* |
| Mouse + DFP | 69.63 ± 4.44† | 19.22 ± 0.43‡ |
| Human | 126.06 ± 3.03‡§ | 25.70 ± 0.19‡¶ |
t1/2 and AUC values were calculated between 0 and 120 min.
Concentration of drug at 120 min in mouse serum was obtained by extrapolation.
P < 0.01 vs. mouse serum.
P < 0.001 vs. mouse serum.
P < 0.001 vs. mouse + DFP.
P < 0.01 vs. mouse + DFP.
The Effect of DFP on the Tolerance of Nude Mice to AN-201 and Its Peptide Conjugates.
Studies with AN-201 and AN-238.
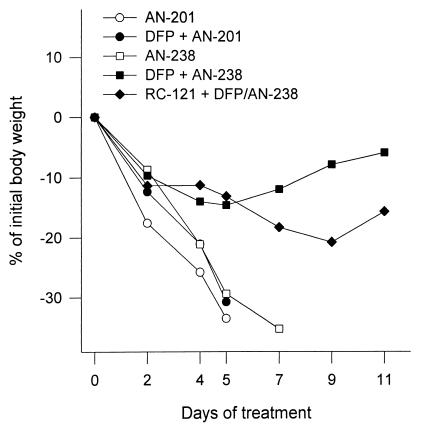
A single administration of 400 nmol/kg AN-238 or AN-201 to nude mice without DFP pretreatment caused a severe loss in BW and a 100% mortality within 8 days (Fig. 3). Pretreatment with the CE inhibitor, DFP, given i.p. at its MTD (6 mg/kg) (18) caused a remarkable improvement in tolerance to AN-238 at 400 nmol/kg, as shown by a 0% mortality and a <20% loss in BW. These animals regained their initial BW within 17 days. In contrast, pretreatment with DFP did not reduce the toxicity of AN-201 at 400 nmol/kg (Fig. 3).
Figure 3.
The effect of CE inhibitor DFP on the tolerance of AN-201 and cytotoxic SST analog AN-238 by nude mice as assessed by the loss in BW. AN-201 and AN-238 were injected i.v. at 400 nmol/kg to untreated nude mice or to animals that received an i.p. injection of DFP at 6 mg/kg (MTD). AN-238 also was given to DFP-pretreated mice after blockade of SST receptors with an excessive dose of carrier SST analog RC-121 (200 μg i.v., 10 min before AN-238 injection).
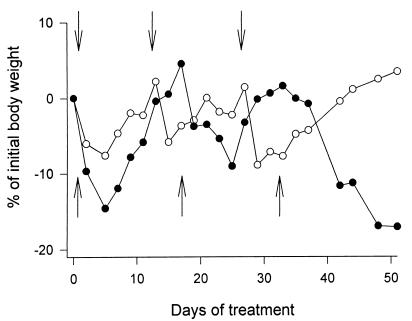
To investigate whether the improved tolerance to AN-238 after DFP treatment is the result of a better targeting and a more selective uptake of the conjugate by SST receptor-positive tissues, an experiment with blockade of these receptors was designed. After treatment with DFP, a group of animals received a large dose (200 μg per animal i.v.) of SST carrier peptide RC-121 before the administration of 400 nmol/kg AN-238. Despite the inhibition of CE, the treatment with AN-238 caused a substantial loss in BW and a 50% mortality within 11 days (Fig. 3). Fig. 4 illustrates the tolerance to three consecutive injections of 300 or 400 nmol/kg AN-238 in DFP-pretreated mice. The second and the third injections of AN-238 were given when the BW returned to the initial value. The average recovery time in mice treated with 300 nmol/kg AN-238 was ≈14 days, and the loss in BW was <10%. After the first and the second injections of 400 nmol/kg AN-238, the loss in BW was <20% and recovery occurred within 17 days. However, the third injection of 400 nmol/kg caused progressive loss in BW with 100% mortality within the following 4 weeks.
Figure 4.
The tolerance of DFP-pretreated nude mice to chronic treatment with cytotoxic SST analog AN-238 as assessed by the loss in BW. AN-238 was administered at 300 nmol/kg (○) on days 0, 14, and 28 (arrows pointing down) or at 400 nmol/kg (●) on days 0, 17, and 34 (arrows pointing up). Mice that received 3 × 400 nmol/kg showed progressive loss in BW after the last injection and died within 4 weeks.
Studies with AN-215 and AN-207.
After a pretreatment with DFP, all three mice survived the administration of a single dose of 400 nmol/kg cytotoxic bombesin analog AN-215. Two mice suffered a severe loss in BW, but recovered within 3 weeks. In contrast, AN-207 at 400 nmol/kg killed all the animals within 7 days. However, the treatment with 300 nmol/kg AN-207 was well tolerated.
In Vivo Studies with Paraoxon.
Table 2 summarizes the effect of increasing doses of paraoxon on the tolerance of mice to various doses of AN-238. Paraoxon was lethal after a single s.c. administration of 1.5- or 2.0-mg/kg doses and ineffective in improving the tolerance of mice to AN-238 at doses ≤1.0 mg/kg. However, when the animals received paraoxon at a dose of 1.0 mg/kg, followed by another injection of 0.5 mg/kg 15 min later, four of five animals survived the treatment with 225 and 300 nmol/kg AN-238. Nevertheless, a mouse that received 400 nmol/kg AN-238 died 11 days after treatment.
Table 2.
EFfect of pretreatment with paraoxon on the tolerance of nude mice to AN-238
| Dose of AN-238,* nmol/kg, i.v. | Paraoxon, mg/kg, s.c.
|
|||||
|---|---|---|---|---|---|---|
| 0.4 | 0.8 | 1.0 | 1.0 + 0.5† | 1.5 | 2.0 | |
| Survival rate after injection of paraoxon‡ | ||||||
| 2/2 | 5/5 | 4/4 | 11/11 | 1/5 | 1/3 | |
| Survival rate after injection of AN-238‡ | ||||||
| 225 | — | — | — | 4/5 | — | — |
| 300 | — | — | — | 4/5 | — | — |
| 350 | — | 0/3 | 0/2 | — | 0/1 | 0/1 |
| 400 | 0/2 | 0/2 | 0/2 | 0/1 | — | — |
Various doses of AN-238 were administered 30 min after pretreatment with paraoxon.
These animals received paraoxon in two consecutive injections: 1.0 and 5.0 mg, 15 min part.
Number of animals surviving/number of animals treated.
In Vivo Studies with BNPP.
To investigate the tolerance of mice to BNPP, groups of two mice were given i.p. injections at 300- or 450-mg/kg doses. No apparent toxicity was observed, and all animals survived the treatment. LD50 for BNPP in mice was reported to be 410 mg/kg i.p. (15). To study the effect of BNPP on the tolerance of mice to AN-238, 300 mg/kg BNPP was given i.p. to three mice 1 hr before the injection of AN-238 at 400 nmol/kg. Two mice died within 24 hr and the third died 2 days after treatment. A lower dose of 250 mg/kg of BNPP then was given to seven mice. One hour later, four mice received cytotoxic bombesin analog AN-215 at 400 nmol/kg and three were given AN-201 at 400 nmol/kg. In the group treated with AN-215, one animal died within 24 hr and three died within 2 days after treatment. In the group that received AN-201, all three animals were dead within 2 days. No signs of cholinergic hyperactivity or loss in BW were observed in these groups. In addition, 100, 150, or 200 mg/kg BNPP was given to groups of three mice followed by 400 nmol/kg AN-238. All these animals suffered a severe loss in BW, and eight of nine mice died within 10 days, but one animal in the group given 200 mg/kg BNPP survived.
Discussion
Recently, we developed a series of targeted cytotoxic peptide conjugates consisting of DOX 14-O-hemiglutarate or 2-pyrrolino-DOX 14-O-hemiglutarate linked to peptide hormone carriers such as analogs of LH-RH, SST, and bombesin (Fig. 1) (2, 10–12). In these conjugates, the glutaric acid spacer is linked to the cytotoxic radicals through an ester bond (Fig. 1, shaded area) that is sensitive to hydrolysis by CE in blood. Although we could demonstrate repeatedly in oncological studies in nude mice that our targeted cytotoxic peptide conjugates are significantly more active and less toxic than their respective cytotoxic radicals (2, 14, 16, 17), the MTDs of the conjugates and their radicals were found to be similar, being between 175 and 200 nmol/kg. In contrast, Copenhagen rats showed a much higher tolerance to cytotoxic SST analog AN-238 than to cytotoxic radical AN-201 (14). This was explained by a longer t1/2 of the conjugate in Copenhagen rats than in nude mice and, consequently, a better targeting that is a more specific delivery of the cytotoxic agent to SST receptor-positive tissues (14). In a preliminary study in vitro we showed that the t1/2 of AN-152, an LH-RH analog containing DOX (Fig. 1), in the serum of nude mice, Copenhagen rats, and men was about 10, 30, and 120 min, respectively. Because human cancer lines can be grown as xenografts in nude mice, this is the most widely used model for the preclinical evaluation of anticancer drugs. However, because of differences between the enzymatic activity in laboratory animals and humans, the results of preclinical studies have to be interpreted with caution (19–21). Fortunately, the availability of organophosphate CE inhibitors makes it possible to adjust the nude mouse model to the human conditions at least with regard to CE activity. However, it has to be emphasized strongly that no such manipulations would be performed in human subjects who eventually might be treated with cytotoxic hybrid analogs. The use of CE inhibitors is necessary only to bring the nude mouse model closer to human conditions and render it adequate for preclinical evaluation of the cytotoxic peptide conjugates.
In the first experiment, we determined in vitro the rate of hydrolysis of AN-152 in mouse serum at 0.1, 0.3, and 1.0 mg/ml concentrations. Our results showed that t1/2 of AN-152 at 0.1 and 0.3 mg/ml concentrations was basically the same, ≈20 min (Table 1). The difference between these data and our earlier finding of ≈10 min probably is due to differences in the sample preparation. Thus, in our previous study the serum samples were not stored in the incubator before the addition of AN-152, and the lower concentration of CO2 in the atmosphere could have resulted in a higher pH of the serum and a higher rate of hydrolysis during the first few minutes of the incubation.
The t1/2 of AN-152 in human serum at 0.3 mg/ml drug concentration was found to be 126 min, which is similar to our previous result of ≈120 min. By the addition of DFP to mouse serum at a concentration corresponding to its i.v. MTD, the t1/2 of AN-152 could be extended to ≈70 min. Comparison of the corresponding AUC values (Table 1), which reflect the total amount of available AN-152 within 120 min, shows that the addition of DFP could raise the bioavailability of AN-152 in the mouse from 32% of the human value to 75%.
To investigate whether inhibition of CE in vivo would lead to a higher tolerance to AN-238, as observed in Copenhagen rats, we injected DFP-pretreated mice with 400 nmol/kg AN-238, which is lethal without pretreatment. We found that a single administration or two consecutive injections of AN-238 at this high dose were well tolerated by mice, but the animals could not recover from the third injection (Figs. 3 and 4). However, treatment with three consecutive injections of AN-238 at 300 nmol/kg was well tolerated (Fig. 4). As expected, the pretreatment with DFP did not affect the tolerance to AN-201 (Fig. 3). These results demonstrate that a suppression of the CE activity in mice is accompanied by an increased tolerance to AN-238. We assumed that, in analogy to the situation in Copenhagen rats, the higher tolerance of DFP-pretreated mice to AN-238 is a result of a better targeting and accumulation of the cytotoxic agent in SST receptor-positive tissues (14). To substantiate this theory, we designed an experiment in which the receptors for SST were blocked by i.v. injection of a large dose of carrier SST analog RC-121 (200 μg per animal) before the injection of the high dose of 400 nmol/kg AN-238 to DFP-pretreated mice. It was expected that the competitive blocking of the receptors by the carrier would prevent the uptake of the conjugate by receptor-positive tissues and, eventually, the cytotoxic radical would be hydrolyzed in the circulation, leading to a higher toxicity. Accordingly, two of four animals in this experiment died because of toxicity and two survived but suffered a severe loss of BW before the recovery. These results strongly support our theory that the lower toxicity of AN-238 in mice with suppressed CE activity is due to the selective uptake of the conjugate by SST receptor-positive tissues and also provide evidence that AN-238 is targeted to SST receptors. A possible damage to such tissues is apparently less detrimental than myelosuppression caused by the unconjugated radical AN-201. It is of interest that RC-121 injected s.c. at 500 μg per animal did not affect the toxicity of AN-238 in DFP-pretreated mice and higher doses than 200 μg per animal RC-121 given i.v. proved to be lethal.
The altered toxicity pattern of AN-238 in mice with suppressed CE activity also was demonstrated by another study, in which mice bearing relatively large (≈400 mm3 in size) xenografts of SST receptor-positive SW-839 human renal cancer were given 400 nmol/kg AN-238 after treatment with DFP. Two of the five animals died within 48 hr. Most tumors shrank by 30–50% during the first few days after treatment (unpublished data). Because no mortality was observed in tumor-free animals receiving the same treatment, it is most likely that the cause of death was related to metabolic abnormalities associated with abrupt tumor breakdown. This view is supported by reports that tumor lysis syndrome can be a potentially lethal condition in patients with hematopoietic malignancies after effective treatment with high doses of anticancer agents (22).
We also tested whether our cytotoxic bombesin analog, AN-215, and cytotoxic LH-RH analog, AN-207, could be tolerated at high doses after treatment with DFP. We found that although AN-215 was tolerated at 400 nmol/kg, all animals (three of three) treated with 400 nmol/kg AN-207 died within 7 days. However, 300 nmol/kg AN-207 was well tolerated by mice after DFP treatment whereas the MTD for AN-207 or AN-215 in male nude mice without DFP was determined to be 200 nmol/kg (2).The difference between the tolerance of AN-215 and AN-207 was anticipated because the receptors for bombesin are found in various organs, in contrast to LH-RH receptors that are present in high concentration mainly in the pituitary gland. As a consequence of the limited number of LH-RH receptors in normal tissues, cytotoxic LH-RH analogs would bind mainly to cancerous cells and may cause fewer side effects than other targeted analogs. Although specific toxicity to the pituitary was shown in rats after treatment with AN-207, the pituitary function recovered within 3 weeks (23).
The effects of other inhibitors of CE on the toxicity of cytotoxic peptide conjugates also were studied. Table 2 shows that paraoxon improves only marginally the tolerance of mice to AN-238 at a concentration that caused cholinergic hyperactivity. This finding is in accordance with results demonstrating that paraoxon is more specific than DFP to acetylcholinesterases (EC 3.1.1.7) (24). We also tested a selective inhibitor of CE, BNPP, that has very low affinity to acetylcholinesterases (15). Mice injected i.p. with 300 or 450 mg/kg BNPP showed no signs of increased parasympathetic activity and survived without apparent toxicity. However, animals that received 300 mg/kg BNPP, followed by 400 nmol/kg SST analog AN-238, died within 2 days. A similar mortality pattern was observed when animals were treated with 250 mg/kg BNPP followed by 400 nmol/kg bombesin analog AN-215 or cytotoxic radical AN-201. These animals did not suffer a loss in BW characteristic of the toxicity caused by AN-201, and we can only assume that the deaths are related to a pharmacological interaction between the cytotoxic radical and BNPP at a high dose. However, animals receiving BNPP at 100, 150, or 200 mg/kg followed by 400 nmol/kg AN-238 suffered a severe loss in BW, and eight of nine animals died within 10 days. The only surviving mouse was from the group that received 200 mg/kg BNPP, indicating some CE inhibitory effect of BNPP at this concentration. The eight deaths probably were caused by an insufficient inhibition of CE by the low dose of BNPP.
In conclusion, the findings reported in this paper demonstrate that the very high CE activity in serum of nude mice, which is about 10 times higher than in human beings (19), can be inhibited by DFP, which results in a significantly longer t1/2 and a better targeting for the cytotoxic peptide conjugates. The inhibition of CE in nude mice by DFP is very reproducible. The use of this model may permit a better understanding of the mechanism of action of the targeted hybrid analogs and improve the clinical predictability of the oncological and toxicological results.
Acknowledgments
We thank Prof. J. Engel and Dr. T. Nolte (Degussa-Hüls and Asta Medica, Frankfurt am Main, Germany) for their help in the preparation of this manuscript. Some work described in this paper was supported by the Medical Research Service of the Veterans Affairs. Tulane University has applied for a patent on the cytotoxic analogs cited in this paper, and A.N. and A.V.S. are coinventors on that patent.
Abbreviations
- AUC
area under the curve
- BNPP
bis(4-nitrophenyl) phosphate
- BW
body weight
- CE
carboxylesterase enzymes
- DFP
diisopropyl fluorophosphate
- DOX
doxorubicin
- LH-RH
luteinizing hormone-releasing hormone
- MTD
maximum tolerated dose
- SST
somatostatin
References
- 1.Weiss R B. Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- 2.Schally A V, Nagy A. E J Endocrinol. 1999;141:1–14. doi: 10.1530/eje.0.1410001. [DOI] [PubMed] [Google Scholar]
- 3.Arap W, Pasqualini R, Ruoslahti E. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 4.Trail P A, Willner D, Lasch S J, Henderson A J, Hofstead S, Casazza A M, Firestone R A, Hellström I, Hellström K E. Science. 1993;261:212–215. doi: 10.1126/science.8327892. [DOI] [PubMed] [Google Scholar]
- 5.Vasey P A, Kaye S B, Morrison R, Twelves C, Wilson P, Duncan R, Thomson A H, Murray L S, Hilditch T E, Murray T, et al. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 6.Denmeade S R, Nagy A, Gao J, Lilja H, Schally A V, Isaacs J T. Cancer Res. 1998;58:2537–2540. [PubMed] [Google Scholar]
- 7.Bakina E, Wu Z, Rosenblum M, Farquhar D. J Med Chem. 1997;40:4013–4018. doi: 10.1021/jm970066d. [DOI] [PubMed] [Google Scholar]
- 8.Niculescu-Duvaz I, Niculescu-Duvaz D, Friedlos F, Spooner R, Martin J, Springer C J. J Med Chem. 1999;42:2485–2489. doi: 10.1021/jm980696v. [DOI] [PubMed] [Google Scholar]
- 9.Israel M, Modest E J, Frei E. Cancer Res. 1975;35:1365–1368. [PubMed] [Google Scholar]
- 10.Nagy A, Schally A V, Armatis P, Szepesházi K, Halmos G, Kovacs M, Zarandi M, Groot K, Miyazaki M, Jungwirth A, et al. Proc Natl Acad Sci USA. 1996;93:7269–7273. doi: 10.1073/pnas.93.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy A, Armatis P, Cai R-Z, Szepesházi K, Halmos G, Schally A V. Proc Natl Acad Sci USA. 1997;94:652–656. doi: 10.1073/pnas.94.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagy A, Schally A V, Halmos G, Armatis P, Cai R-Z, Csernus V, Kovács M, Koppán M, Szepesházi K, Kahán Z. Proc Natl Acad Sci USA. 1998;95:1794–1799. doi: 10.1073/pnas.95.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy A, Armatis P, Schally A V. Proc Natl Acad Sci USA. 1996;93:2464–2469. doi: 10.1073/pnas.93.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppán M, Nagy A, Schally A V, Arencibia J M, Plonowski A, Halmos G. Cancer Res. 1998;58:4132–4137. [PubMed] [Google Scholar]
- 15.Krisch K. In: The Enzymes. Boyer P D, editor. Vol. 5. New York: Academic; 1971. pp. 43–69. [Google Scholar]
- 16.Plonowski A, Schally A V, Nagy A, Sun B, Szepesházi K. Cancer Res. 1999;59:1947–1953. [PubMed] [Google Scholar]
- 17.Miyazaki M, Nagy A, Schally A V, Lamharzi N, Halmos G, Szepesházi K, Groot K, Armatis P. J Natl Cancer Inst. 1997;89:1803–1809. doi: 10.1093/jnci/89.23.1803. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran B V. Biochem Pharm. 1966;15:169–175. doi: 10.1016/0006-2952(66)90057-8. [DOI] [PubMed] [Google Scholar]
- 19.Kaliste-Korhonen E, Tuovinen K, Hänninen O. Human Exp Toxicol. 1996;15:972–978. doi: 10.1177/096032719601501205. [DOI] [PubMed] [Google Scholar]
- 20.van Tellingen O, Nooijen W J, Schaaf L J, van der Valk M, van Asperen J, Henrar R E C, Beijnen J H. Cancer Res. 1998;58:2410–2416. [PubMed] [Google Scholar]
- 21.Inaba M, Kobayashi T, Tashiro T, Sakurai Y, Maruo K, Ohnishi Y, Ueyama Y, Nomura T. Cancer. 1989;64:1577–1582. doi: 10.1002/1097-0142(19891015)64:8<1577::aid-cncr2820640803>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 22.Hande K R, Garrow G C. Am J Med. 1993;94:133–139. doi: 10.1016/0002-9343(93)90174-n. [DOI] [PubMed] [Google Scholar]
- 23.Kovács M, Schally A V, Nagy A, Koppán M, Groot K. Proc Natl Acad Sci USA. 1997;94:1420–1425. doi: 10.1073/pnas.94.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaustad R, Sletten K, Løvhaug D, Fonnum F. Biochem J. 1991;274:693–697. doi: 10.1042/bj2740693. [DOI] [PMC free article] [PubMed] [Google Scholar]