Abstract
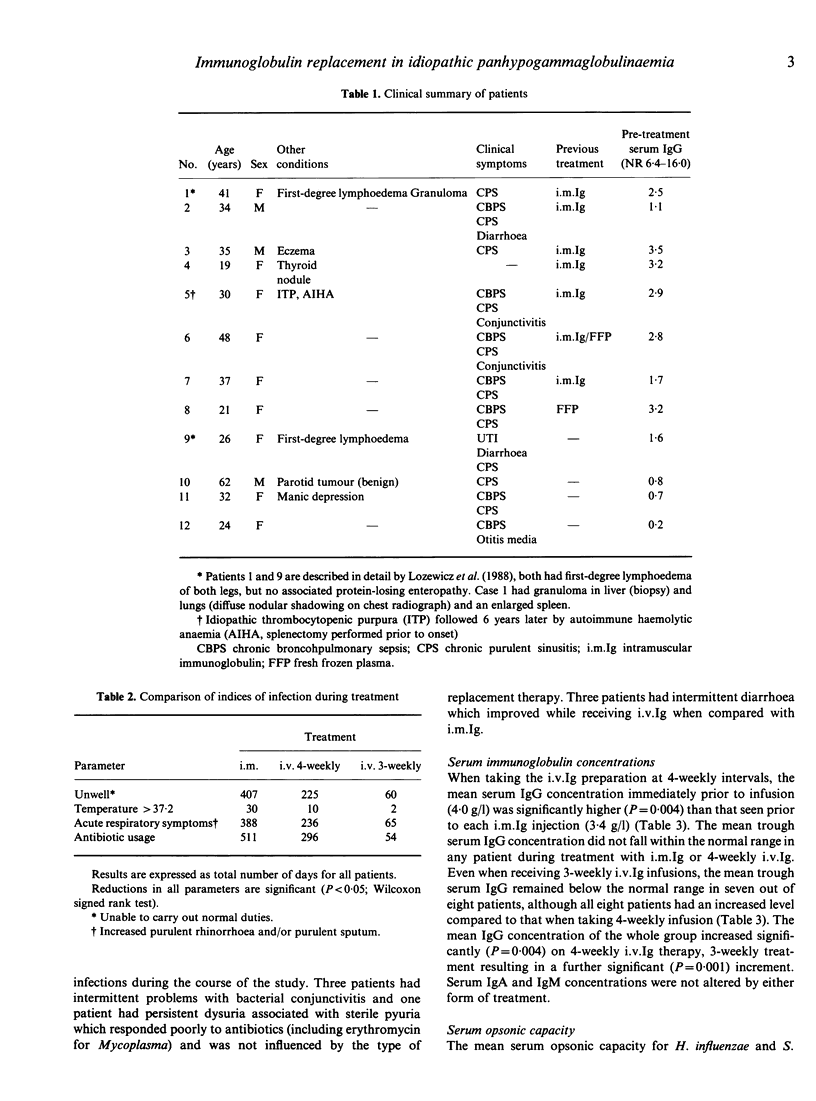
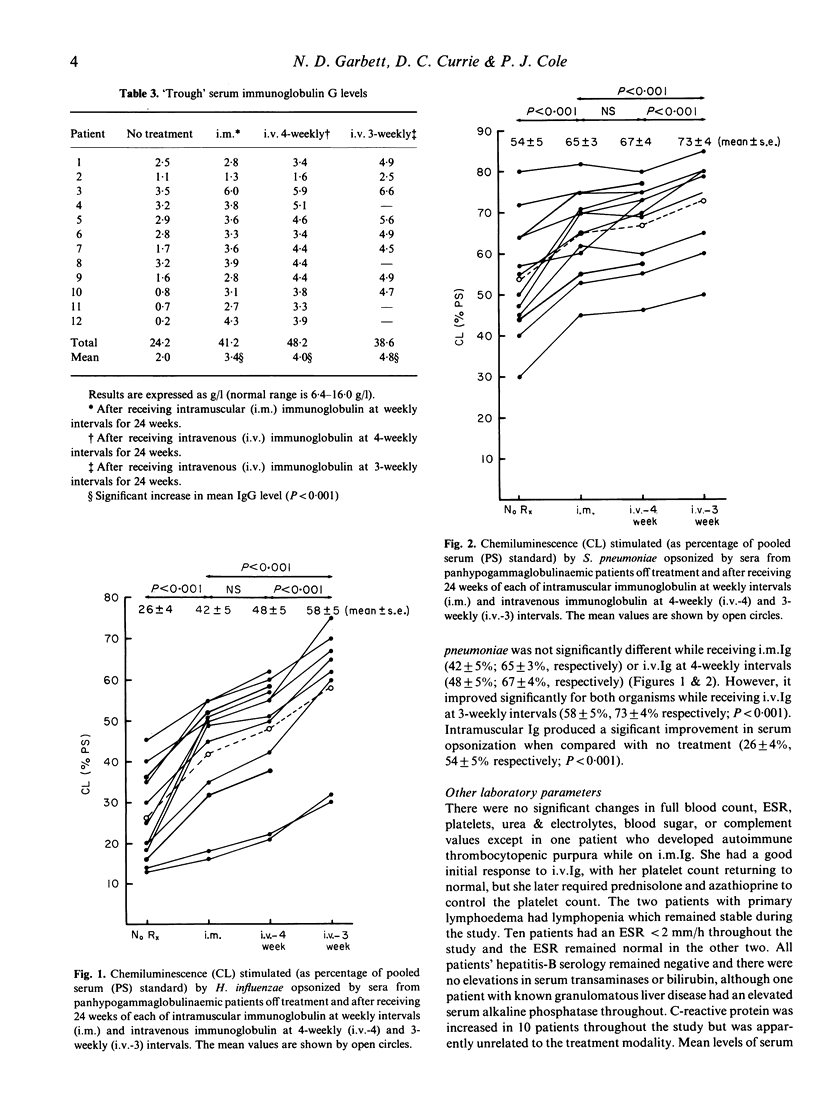
A prospective, randomized, cross-over study was performed to evaluate the safety and clinical efficacy of an immunoglobulin preparation for intravenous use (Intraglobin F) in comparison with the standard intra-muscular preparation. Twelve patients with idiopathic adult-onset panhypogammaglobulinaemia received intravenous Intraglobin F infusions of 300 mg/kg body weight every 4 weeks, or standard intramuscular injections of 25 mg/kg body weight every week (following a 4-week loading phase of 50 mg/kg/week) in random order for 24 weeks each with a 'washout' period between. At the end of this comparative trial eight of the patients received a further 24 weeks treatment with Intraglobin F at 300 mg/kg body weight every 3 weeks. Patients were assessed by diary cards, interview, laboratory screening (including assessment of serum opsonic capacity), chest and sinus radiographs, and full lung function tests. There were statistically significant, favourable changes in clinical parameters and trough serum IgG levels using the intravenous preparation but no other significant differences with respect to the two preparations. The eight patients who subsequently received the 3-weekly intravenous regimen improved further in these parameters, including serum IgG levels and serum opsonic capacity. Thirty per cent of infusions were associated with mild adverse reactions related to the rate of administration but one patient was withdrawn due to anaphylactoid reaction to her fifth infusion. Ten of the eleven remaining patients elected to continue intravenous therapy at the end of the trial. We conclude that Intraglobin F is preferable to the standard intramuscular preparations for control of idiopathic adult-onset panhypogammaglobulinaemia but that infusion intervals should be tailored to the individual's clinical response and results of appropriate functional in vitro assays, eg. opsonization.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARANDUN S., KISTLER P., JEUNET F., ISLIKER H. Intravenous administration of human gamma-globulin. Vox Sang. 1962;7:157–174. doi: 10.1111/j.1423-0410.1962.tb03240.x. [DOI] [PubMed] [Google Scholar]
- Bernatowska E., Madaliński K., Janowicz W., Weremowicz R., Gutkowski P., Wolf H. M., Eibl M. M. Results of a prospective controlled two-dose crossover study with intravenous immunoglobulin and comparison (retrospective) with plasma treatment. Clin Immunol Immunopathol. 1987 May;43(2):153–162. doi: 10.1016/0090-1229(87)90123-1. [DOI] [PubMed] [Google Scholar]
- Buckley R. H. Humoral immunodeficiency. Clin Immunol Immunopathol. 1986 Jul;40(1):13–24. doi: 10.1016/0090-1229(86)90065-6. [DOI] [PubMed] [Google Scholar]
- Burks A. W., Sampson H. A., Buckley R. H. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N Engl J Med. 1986 Feb 27;314(9):560–564. doi: 10.1056/NEJM198602273140907. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Siegal F. P., Smithwick E. M., Lion-Boulé A., Cunningham-Rundles S., O'Malley J., Barandun S., Good R. A. Efficacy of intravenous immunoglobulin in primary humoral immunodeficiency disease. Ann Intern Med. 1984 Oct;101(4):435–439. doi: 10.7326/0003-4819-101-4-435. [DOI] [PubMed] [Google Scholar]
- Eibl M. M., Cairns L., Rosen F. S. Safety and efficacy of a monomeric, functionally intact intravenous IgG preparation in patients with primary immunodeficiency syndromes. Clin Immunol Immunopathol. 1984 Apr;31(1):151–160. doi: 10.1016/0090-1229(84)90200-9. [DOI] [PubMed] [Google Scholar]
- Hosking C. S., Roberton D. M. Epidemiology and treatment of hypogammaglobulinemia. Birth Defects Orig Artic Ser. 1983;19(3):223–227. [PubMed] [Google Scholar]
- Janeway C. A., Rosen F. S. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med. 1966 Oct 13;275(15):826–831. doi: 10.1056/NEJM196610132751508. [DOI] [PubMed] [Google Scholar]
- Lozewicz S., Morris G., Garbett N., Browse N., Slavin B., Cole P. Acquired common variable hypogammaglobulinaemia and lymphoedema. Postgrad Med J. 1988 Jan;64(747):63–65. doi: 10.1136/pgmj.64.747.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A., Schnoz M., Barandun S. Build-up maintenance of IgG serum concentrations with intravenous immunoglobulin in patients with primary humoral immunodeficiency. Vox Sang. 1982;43(4):212–219. doi: 10.1111/j.1423-0410.1982.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Munro C. S., Stanley P. J., Cole P. J. Assessment of biological activity of immunoglobulin preparations by using opsonized micro-organisms to stimulate neutrophil chemiluminescence. Clin Exp Immunol. 1985 Jul;61(1):183–188. [PMC free article] [PubMed] [Google Scholar]
- Nolte M. T., Pirofsky B., Gerritz G. A., Golding B. Intravenous immunoglobulin therapy for antibody deficiency. Clin Exp Immunol. 1979 May;36(2):237–243. [PMC free article] [PubMed] [Google Scholar]
- Ochs H. D., Buckley R. H., Pirofsky B., Fischer S. H., Rousell R. H., Anderson C. J., Wedgwood R. J. Safety and patient acceptability of intravenous immune globulin in 10% maltose. Lancet. 1980 Nov 29;2(8205):1158–1159. doi: 10.1016/s0140-6736(80)92594-5. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Fischer S. H., Wedgwood R. J., Wara D. W., Cowan M. J., Ammann A. J., Saxon A., Budinger M. D., Allred R. U., Rousell R. H. Comparison of high-dose and low-dose intravenous immunoglobulin therapy in patients with primary immunodeficiency diseases. Am J Med. 1984 Mar 30;76(3A):78–82. doi: 10.1016/0002-9343(84)90324-3. [DOI] [PubMed] [Google Scholar]
- Pirofsky B., Campbell S. M., Montanaro A. Individual patient variations in the kinetics of intravenous immune globulin administration. J Clin Immunol. 1982 Apr;2(2 Suppl):7S–14S. doi: 10.1007/BF00918361. [DOI] [PubMed] [Google Scholar]
- Pirofsky B. Clinical use of a new pH 4.25 intravenous immunoglobulin preparation (gamimune-N). J Infect. 1987 Jul;15 (Suppl 1):29–37. doi: 10.1016/s0163-4453(87)92447-9. [DOI] [PubMed] [Google Scholar]
- Pirofsky B. Intravenous immune globulin therapy in hypogammaglobulinemia. A review. Am J Med. 1984 Mar 30;76(3A):53–60. doi: 10.1016/0002-9343(84)90320-6. [DOI] [PubMed] [Google Scholar]
- Primary immunodeficiency diseases. Report of a World Health Organization scientific group. Clin Immunol Immunopathol. 1986 Jul;40(1):166–196. [PubMed] [Google Scholar]
- Prince A. M., Stephan W., Brotman B. beta-propiolactone/ultraviolet irradiation: a review of its effectiveness for inactivation of viruses in blood derivatives. Rev Infect Dis. 1983 Jan-Feb;5(1):92–107. doi: 10.1093/clinids/5.1.92. [DOI] [PubMed] [Google Scholar]
- Prince A. M., Stephan W., Dichtelmüller H., Brotman B., Huima T. Inactivation of the Hutchinson strain of non-A, non-B hepatitis virus by combined use of beta-propiolactone and ultraviolet irradiation. J Med Virol. 1985 Jun;16(2):119–125. doi: 10.1002/jmv.1890160204. [DOI] [PubMed] [Google Scholar]
- Roberton D. M., Hosking C. S., Efthimiou H., Wright S., Upton H., Colgan T., Hartman L., Schiff P. Decreased incidence of adverse infusion reactions in hypogammaglobulinemic children receiving low pH intravenous immunoglobulin. Aust N Z J Med. 1987 Oct;17(5):495–500. doi: 10.1111/j.1445-5994.1987.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Roifman C. M., Lederman H. M., Lavi S., Stein L. D., Levison H., Gelfand E. W. Benefit of intravenous IgG replacement in hypogammaglobulinemic patients with chronic sinopulmonary disease. Am J Med. 1985 Aug;79(2):171–174. doi: 10.1016/0002-9343(85)90006-3. [DOI] [PubMed] [Google Scholar]
- Roifman C. M., Levison H., Gelfand E. W. High-dose versus low-dose intravenous immunoglobulin in hypogammaglobulinaemia and chronic lung disease. Lancet. 1987 May 9;1(8541):1075–1077. doi: 10.1016/s0140-6736(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Schiff R. I., Rudd C., Johnson R., Buckley R. H. Use of a new chemically modified intravenous IgG preparation in severe primary humoral immunodeficiency: clinical efficacy and attempts to individualize dosage. Clin Immunol Immunopathol. 1984 Apr;31(1):13–23. doi: 10.1016/0090-1229(84)90185-5. [DOI] [PubMed] [Google Scholar]
- So A., Brenner M. K., Hill I. D., Asherson G. L., Webster A. D. Intravenous gammaglobulin treatment in patients with hypogammaglobulinaemia. Br Med J (Clin Res Ed) 1984 Nov 3;289(6453):1177–1178. doi: 10.1136/bmj.289.6453.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedgwood R. J., Rosen F. S. Gamma-globulin replacement therapy in immunodeficiency. Clin Immunol Immunopathol. 1987 May;43(2):151–152. doi: 10.1016/0090-1229(87)90122-x. [DOI] [PubMed] [Google Scholar]
- Wells J. V., Buckley R. H., Schanfield M. S., Fudenberg H. H. Anaphylactic reactions to plasma infusions in patients with hypogammaglobulinemia and anti-IgA antibodies. Clin Immunol Immunopathol. 1977 Sep;8(2):265–271. doi: 10.1016/0090-1229(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Hastings M. J., Easmon C. S., Cole P. J. Factor affecting the in vitro assessment of opsonization: a study of the kinetics of opsonization using the technique of phagocytic chemiluminescence. Immunology. 1980 Dec;41(4):903–911. [PMC free article] [PubMed] [Google Scholar]
- Yap P. L., McClelland D. B. An evaluation of the safety of three intravenous immunoglobulin preparations in patients with primary hypogammaglobulinaemia. J Infect. 1986 Jan;12(1):5–10. doi: 10.1016/s0163-4453(86)94717-1. [DOI] [PubMed] [Google Scholar]
- van Furth R., Leijh P. C., Klein F. Correlation between opsonic activity for various microorganisms and composition of gammaglobulin preparations for intravenous use. J Infect Dis. 1984 Apr;149(4):511–517. doi: 10.1093/infdis/149.4.511. [DOI] [PubMed] [Google Scholar]


