Abstract
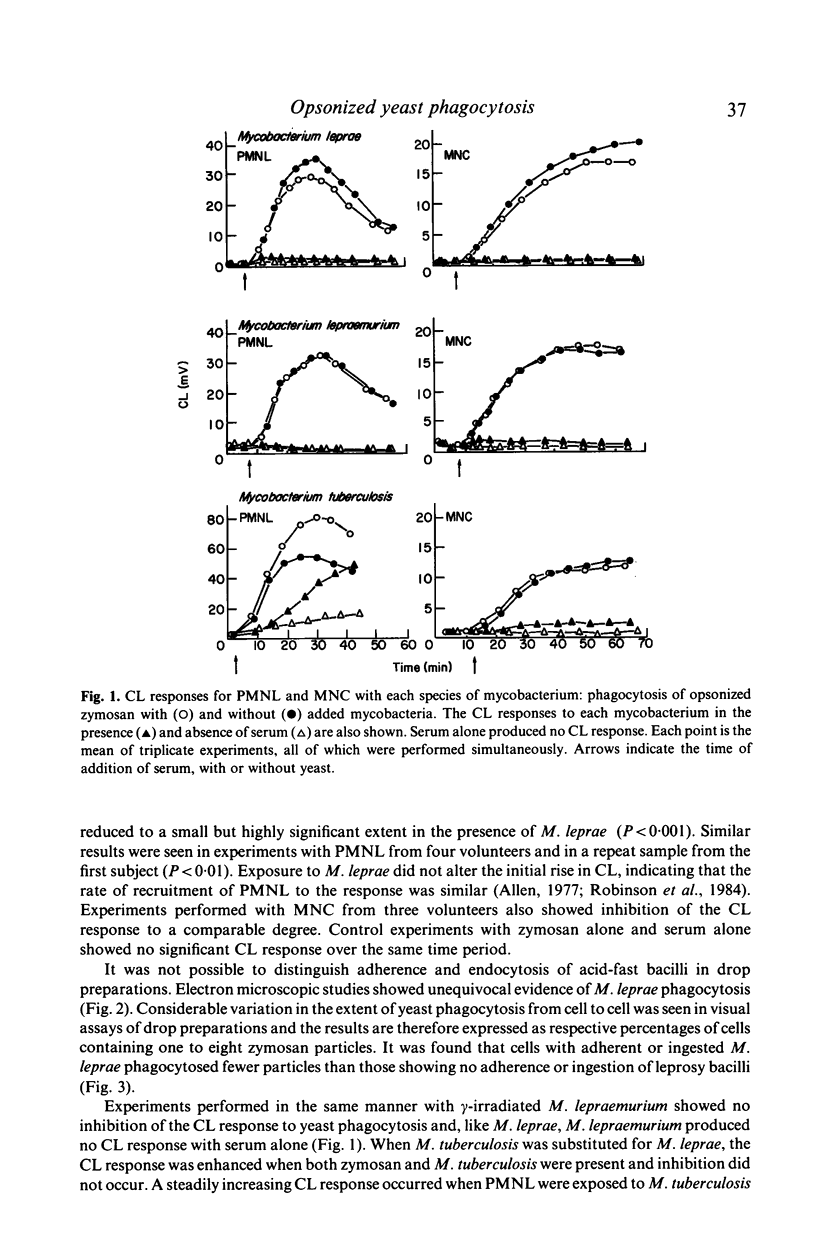
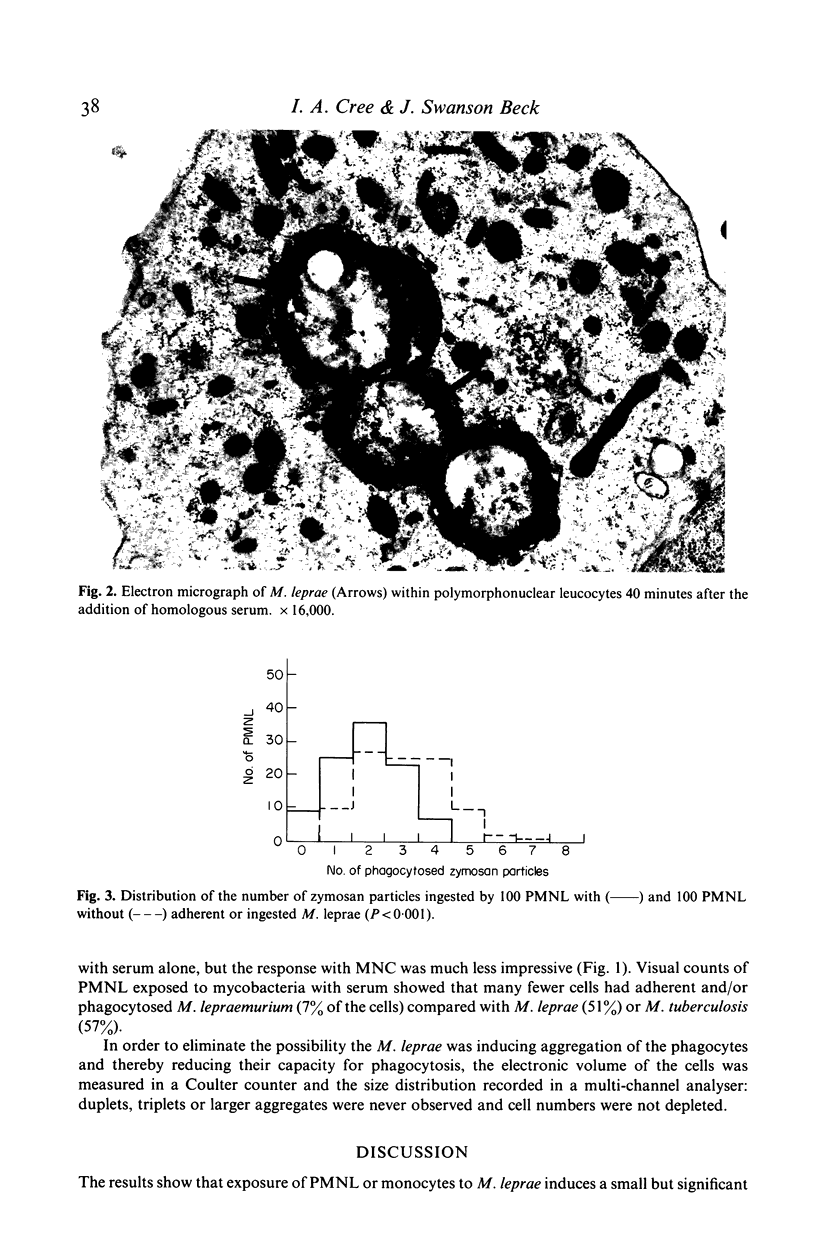
The influence of killed mycobacteria on the metabolic burst associated with phagocytosis of opsonized zymosan by normal human polymorphonuclear leucocytes (PMNL) or monocytes was studied by chemiluminescence (CL) measurements. M. leprae reproducibly reduced the peak and total CL of both types of phagocyte to a small, but highly significant extent. Electron microscopy showed that M. leprae were phagocytosed: cells with ingested or adherent M. leprae phagocytosed fewer zymosan particles. M. leprae did not cause aggregation of the phagocytes or quenching of CL. M. lepraemurium did not influence the CL response to zymosan. Addition of M. tuberculosis caused an increased response with PMNL but not with monocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdi T. J., Mistry N. F., Mahadevan P. R., Antia N. H. Alterations in the membrane of macrophages from leprosy patients. Infect Immun. 1983 Jul;41(1):121–127. doi: 10.1128/iai.41.1.121-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Hastings M. J., Petricevic I., Williams A. J., Cole P. J., Easmon C. S. The effect of culture media on the production and measurement of luminol-dependent chemiluminescence. Br J Exp Pathol. 1982 Apr;63(2):147–153. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Andrew P. W., Lowrie D. B. Release of superoxide and hydrogen peroxide from guinea-pig alveolar macrophages during phagocytosis of Mycobacterium bovis BCG. Adv Exp Med Biol. 1982;155:687–693. doi: 10.1007/978-1-4684-4394-3_75. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Shepard C. C. Toxic effect of the peroxidase-hydrogen peroxide-halide antimicrobial system on Mycobacterium leprae. Infect Immun. 1984 May;44(2):534–536. doi: 10.1128/iai.44.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R. C., Gibbs J. H., Robertson A. J., Brown R. A., Beck J. S. A simple method for determining the extent of cellular contamination in peripheral blood lymphocyte preparations. J Immunol Methods. 1980;35(3-4):177–187. doi: 10.1016/0022-1759(80)90245-8. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Singh R., Nath I. Radiolabelled M. leprae resident in human macrophage cultures as an in vitro indicator of effective immunity in human leprosy. Clin Exp Immunol. 1982 Sep;49(3):517–522. [PMC free article] [PubMed] [Google Scholar]
- Robinson P., Wakefield D., Breit S. N., Easter J. F., Penny R. Chemiluminescent response to pathogenic organisms: normal human polymorphonuclear leukocytes. Infect Immun. 1984 Feb;43(2):744–752. doi: 10.1128/iai.43.2.744-752.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. K., Banerjee D. K. Hydrogen peroxide and superoxide production by peripheral blood monocytes in leprosy. Clin Exp Immunol. 1985 Apr;60(1):203–206. [PMC free article] [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Human bronchoalveolar lavage cells and luminol-dependent chemiluminescence. J Clin Pathol. 1981 Feb;34(2):167–171. doi: 10.1136/jcp.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Licht M. R., Craigmyle L. S., Silverstein S. C. Communication between receptors for different ligands on a single cell: ligation of fibronectin receptors induces a reversible alteration in the function of complement receptors on cultured human monocytes. J Cell Biol. 1984 Jul;99(1 Pt 1):336–339. doi: 10.1083/jcb.99.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]