Abstract
Very few examples of metabolic regulation are known in the gastric pathogen Helicobacter pylori. An unanticipated case was suggested, however, upon finding two types of metronidazole (Mtz)-susceptible strains: type I, in which frxA (which encodes a nitroreductase that contributes to Mtz susceptibility) is quiescent, and type II, in which frxA is well expressed. Here we report that inactivation of the fdxA ferredoxin gene (hp277) in type I strains resulted in high-level frxA expression (in effect, making them type II). However, fdxA null derivatives were obtained from only 6 of 32 type I strains tested that were readily transformed with an frxA::aphA marker. This suggested that fdxA is often essential. This essentiality was overcome in 4 of 20 strains by inactivating frxA, which suggested both that frxA overexpression is potentially deleterious and also that fdxA has additional, often vital roles. With type II strains, in contrast, fdxA null derivatives were obtained in 20 of 23 cases tested. Thus, fdxA is dispensable in most strains that normally exhibit (and tolerate) strong frxA expression. We propose that restraint of frxA expression helps maintain balanced metabolic networks in most type I strains, that other homeostatic mechanisms predominate in type II strains, and that these complex results constitute a phenotypic manifestation of H. pylori's great genetic diversity.
Helicobacter pylori, the gram-negative pathogen implicated in peptic ulcer disease and gastric cancer, chronically infects more than half of all people worldwide and is one of the most genetically diverse bacterial species (for reviews see references 14, 19,and 38). Independent clinical isolates typically differ by 3% or more in DNA sequences of representative housekeeping genes (2, 20) and by 5% or more in gene content (4, 5, 46). Any two independent clinical isolates are usually distinguishable from one another by DNA fingerprinting (3) or by sequencing a few housekeeping genes (2, 20).
Inspection of the two fully sequenced H. pylori genomes had revealed homologs of only a few of the regulatory genes known from other bacterial species. This had suggested that H. pylori might actually be relatively inflexible in a conventional regulatory sense; that is, it is hard wired for its special gastric niche (5, 16, 52). It seemed, however, that H. pylori would achieve phenotypic flexibility and diversity through mutation (56), interstrain and interspecies gene exchange and recombination (30, 51, 52), and frameshift mutations in repetitive sequences, the hallmark of highly mutable contingency genes (47, 52). More-recent studies have demonstrated considerable regulation of gene expression in response to growth phase and environmental parameters, such as acidity and concentrations or availability of iron, nickel, and other metals, and have identified more than a dozen genes with regulatory activity (15). Another case of metabolic regulation was suggested by our studies of susceptibility to the clinically important anti-H. pylori drug metronidazole (Mtz) (27, 28, 50). Susceptibility results from the action of one or two related nitroreductases that each mediate conversion of Mtz from harmless prodrug to hydroxylamine, a bactericidal and mutagenic agent; RdxA, which is abundant in essentially all Mtzs clinical isolates; and FrxA, which is present at only very low levels in most isolates (designated type I strains) but at higher levels in others (type II strains) (27, 28). RdxA and FrxA differ in substrate specificity (49), but their normal substrates, products, and roles (e.g., whether purely metabolic or protective against reactive nitrogen and oxygen metabolites that are produced in the host response to infection; see reference 40) are not known.
The two types of Mtzs strains can be distinguished provisionally in a forward mutation assay. Typically, Mtzr colonies are found at frequencies of about 10−4 in cultures of type I strains and are found at frequencies of ≤10−8 in cultures of type II strains. This reflects the need to inactivate just one gene (rdxA) rather than two genes (both rdxA and frxA) to achieve resistance (27, 28). Although frxA inactivation does not affect Mtz susceptibility when rdxA is functional, its inactivation in type I strains that are already mutated in rdxA usually increases resistance by about twofold (from 16 to 32 μg/ml). This illustrates that frxA is expressed, but only weakly, in type I strains. In accordance with this fact, frxA transcripts were detected by reverse transcriptase PCR (RT-PCR) in both type I and type II strains, but Northern blot analysis showed that they were abundant only in type II strains (27). In principle, the observed patterns of frxA expression might reflect differences in a regulatory site or in a trans-acting regulatory factor.
Here we identify a ferredoxin gene (fdxA; hp277 in the genome sequence) as a negative regulator of frxA gene expression and show that it is essential for many type I strains and that part of this essentiality can involve restraint of nitroreductase gene expression.
MATERIALS AND METHODS
H. pylori culture conditions.
H. pylori strains were grown on brain-heart infusion agar (BHI; Difco) supplemented with 7% horse blood, 0.4% Isovitalex, and the antibiotics amphotericin B (8 μg/ml) and trimethoprim (5 μg/ml) (this solution is referred to hereafter as BHI agar) (27). Mtz was added to this medium when needed at a concentration appropriate for the experiment, as detailed below. The plates were incubated at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). Transformation (electroporation) was carried out as follows: exponentially growing H. pylori cells were harvested from BHI agar (approximately 108 to 109 cells), washed twice in 10% glycerol, and suspended in a final volume of 80 μl of 10% glycerol at 4°C. Five micrograms of purified plasmid DNA or PCR fragment was added to the cells for 1 min on ice. The suspension of cells and DNA was transferred to a prechilled 0.2mm-gap electroporation cuvette and was subjected to single-pulse electroporation with an initial voltage of 2.5 kV in a Bio-Rad Gene Pulser, spread on BHI agar, and incubated for 24 h at 37°C. The cells were then transferred to BHI agar containing chloramphenicol (Cam) (15 μg/ml), kanamycin (Kan) (20 μg/ml), or Mtz (8 μg/ml), as appropriate, and were incubated for 3 to 10 days, as needed, to select for transformants.
Determination of Mtz susceptibility and resistance.
H. pylori cells growing exponentially on Mtz-free BHI agar were suspended in phosphate-buffered saline (PBS) buffer, a series of 10-fold dilutions of these cell suspensions was prepared, and 10 μl of each dilution was spotted on freshly prepared BHI agar containing various concentrations of Mtz (0, 0.2, 0.5, 1.5, 3, 8, 16, 32, 64, and 128 μg/ml) (essentially as described in references 27 and 28). The susceptibilities of strains to Mtz are described here in terms of MIC, defined operationally as the lowest of the Mtz concentrations listed above that reduces the efficiency of colony formation by at least 10-fold. When Mtz-resistant mutants were rare (<10−6) and accurate estimates of these frequencies were needed, culture aliquots were spread directly on the surface of an entire plate of Mtz-containing BHI agar. We used this culture dilution protocol here because it is more sensitive and reliable than traditional standard agar dilution or Etest methods for studying Mtz susceptibility (MIC) in H. pylori, as detailed in reference 27.
H. pylori strains.
The H. pylori strains used here were Mtzs clinical isolates from diverse parts of the world. Most have been studied previously in other contexts (27, 28, 31, 39). The type I (frxA quiescent) and type II (frxA expressed) Mtzs H. pylori strains (as defined in references 27 and 28) that were used here and their origins are as follows. Type I strains included 26695 (from the United Kingdom) (52); TN2, GS3, HPK5, CPY6261, CPY6271, and CPY6311 (Japan); HUPB48, HUPB57, HUPB63, HUPB71, HUPB72, and HUPB77 (Spain); Lit11, Lit13, Lit50, Lit55, and Lit76 (Lithuania); Ind27, Ind66, Ind121, Ind92, Che5, and Che13 (India); HK192 (Hong Kong); and PeCan9a (Peru). Type II strains included SS1 (Australia); X47 (United States, mouse adapted, and ultimately from a domestic cat; also known as X47-2AL [18]); 88-3887 (United Kingdom, 26695-related) (29 and Fig. 1); 98QM3 (from domestic cat; D. Dailidiene, K. W. Simpson, and D. E. Berg, unpublished data); 2600, 2667, and 2714 (Texas) (32); Lit5-34, Lit28-1, Lit43, Lit66-1, Lit75-1, Lit102, Lit113, Lit119, Lit120, and Lit122 (Lithuania); Alas219, Alas381, and Alas10103 (Alaska); Ind31 (India); R10 (South Africa); and HK152 (Hong Kong).
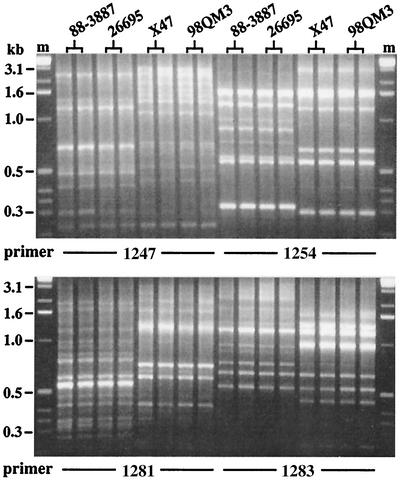
FIG. 1.
RAPD analysis of relatedness. RAPD tests were carried out on the related strain pairs 88-3887 and 26695 and also on X47 and 98QM3, as discussed in the text, by using primers 1247 (left eight lanes), 1254 (top right eight lanes), 1281 (bottom left), and 1283 (bottom right) (3). The first and second lanes in each set contain products of duplicate RAPD tests, carried out with 5 and 20 ng of template DNA, to ensure that any differences seen are reproducible (or to learn when they are not). Lanes labeled m contain 1-kb marker size standards from Gibco-BRL.
Five of these strains merit special comment. Strain 26695 (52) is nonmotile due to a frameshift mutation in the fliP flagellar assembly gene and had come from an initially mixed culture that also contained closely related motile cells, represented by strain 88-3887 (29). The relatedness of 26695 and 88-3887 is illustrated in randomly amplified polymorphic DNA (RAPD) fingerprint data (Fig. 1; no bands differed among some 25 scored in tests with four arbitrary primers). It was important for the present study that mutational tests suggested that 88-3887 expressed frxA (type II Mtzs phenotype), whereas 26695 did not (type I). Most critically, inactivation of strain 88-3887's rdxA gene (transformation with an rdxA::cat allele and selection for Camr, as described in references 21, 27, and 28) left it vulnerable to Mtz: it formed colonies 103-fold and 106-fold less efficiently on medium with 8 and 16 μg of Mtz per ml, respectively, than on medium with no Mtz or only 3 μg of Mtz per ml (MIC = 8 μg/ml). In contrast, rdxA inactivation in strain 26695 allowed this strain to form colonies with 100% efficiency on medium with up to 16 μg of Mtz per ml (MIC = 32 μg/ml). Inactivation of both rdxA and frxA resulted in a MIC of 64 μg/ml for both strains. These data showed that the residual Mtz susceptibility of rdxA-deficient 88-3887 involves its frxA gene, not some other function. We also note that frxA expression seemed weaker in 88-3887 than in several other type II strains in that rdxA inactivation in this strain did confer low-level Mtz resistance; it allowed colony formation with 100% efficiency on medium with 3 μg of Mtz per ml instead of just 1 or 1.5 μg per ml, as had been seen with SS1 and several other type II strains (27, 28). Also noteworthy are the type II strains X47 and 98QM3 because of differences in ease of fdxA inactivation (see below). Strain X47 derives from an H. pylori strain that had been isolated from a domestic cat and that was then adapted to mice by several sequential passages (18). 98QM3 was isolated from a member of the same cat colony some 5 years later by D. Dailidene, K. W. Simpson, and D. E. Berg and seemed closely related or identical to X47 in RAPD fingerprint (no bands differed among more than 25 scored in tests with four arbitrary primers; as in Fig. 1). Fifth, HUPB57 was considered type I because, even though rdxA inactivation resulted in an MIC of 64 μg/ml (higher than that seen for most strains after rdxA inactivation), inactivation of frxA as well as rdxA resulted in a higher level of resistance (MIC = 128 μg/ml).
DNA methods.
H. pylori genomic DNAs were isolated from confluent cultures grown on BHI agar by using a Qiamp Tissue kit (Qiagen Corporation, Chatsworth, Calif.) or a standard cetyltrimethylammonium bromide phenol method (4). RAPD fingerprint analysis was carried out essentially as described previously (3) in 25-μl reaction mixtures containing either 5 or 20 ng of genomic DNA (to assess reproducibility of patterns), 5 mM MgCl2, 20 pM concentrations of a given primer, 0.25 mM concentrations of each deoxynucleoside triphosphate, and 1 U of Biolase thermostable DNA polymerase (Midwest Scientific) in a solution containing 10 mM Tris-HCl (pH 8.3) and 50 mM KCl under the following cycling conditions: 45 cycles of 94°C, 1 min; 36°C, 1 min; and 72°C, 2 min. Gene-specific PCR was carried out in 20-μl volumes containing 1 to 10 ng of genomic DNA, 10 pmol of each primer, 1 U of Biolase, and 0.25 mmol of each deoxynucleoside triphosphate in standard PCR buffer. Gene-specific PCR entailed 2 min of preincubation at 94°C followed by 30 cycles of 94°C, 40 s; 58°C, 40 s; and 72°C for 1 min per kilobase pair, plus a final elongation step of 72°C for 10 min. The genetic structures of transformants were checked by PCR to verify that they had resulted from allelic replacement by using primers fdxA-F and fdxA-R, rdxA-F and rdxA-R, or frxA-F1 and frxA-R1, as appropriate (see Table 1 for primer sequences).
TABLE 1.
Primers useda
| Primer | Sequence | Position of 5′ end |
|---|---|---|
| RAPD | ||
| 1254 | 5′-CCGCAGCCAA | |
| 1281 | 5′-AACGCGCAAC | |
| 1283 | 5′-GCGATCCCCA | |
| 1290 | 5′-GTGGATGCGA | |
| Gene-specific | ||
| fdxA-F1 | 5′-CGCTTGTTCAAGGCTCTGATG | 250 bp upstream of hp277 (fdxA) |
| fdxA-R1 | 5′-CGCTACAAACTCCAGCCGATT | 300 bp downstream of hp277 |
| fdxA-F2 | 5′-GCCTCGTTGCGTGAGCGTAT | 144th nt of hp277 (fdxA) |
| fdxA-R2 | 5′-CGCACGCAATGCATTCATCA | 18th nt of hp277 |
| frxRT-F | 5′-GGACAGAGAACAAGTGGTTGCTT | 3rd nt of hp642 (frxA) |
| frxRT-R | 5′-GCGAACCTAGAATTAGTGTCAT | 319th nt of hp642 |
| rdxART-F | 5′-GCATGCTGTGGTTGAATCTCAC | 367th nt of hp954 (rdxA) |
| rdxART-R | 5′-CGAGCGCCATTCTTGCAAGATGT | 42nd nt of hp954 |
| ureB-F | 5′-CGTCCGGCAATAGCTGCCATAGT | 781st nt of hp072 (ureB) |
| ureB-R | 5′-GTAGGTCCTGCTACTGAAGCCTTA | 340th nt of hp072 |
The genes are listed according to their numerical designations in the genome sequence database of strain 26695 (52). nt, nucleotide.
Mutant alleles used in strain construction.
The rdxA::cat, rdxAΔ111, and frxA::aphA alleles used to generate rdxA and frxA null mutant strains by DNA transformation and selection for transformants by resistance to chloramphenicol, metronidazole, and kanamycin, respectively, have been described previously (27, 28). An fdxA null allele was generated as follows: (i) PCR was used to amplify an 828-bp fdxA-containing DNA fragment from strain 26695 with primers fdxA-F1 and fdxA-R1 (Table 1); (ii) this fragment was cloned into a pBluescript plasmid vector (Stratagene); (iii) the resultant clone was linearized by PCR with primers fdxA-F2 and fdxA-R2 to delete 126 bp of fdxA; (iv) ligation of the linearized clone DNA was performed with a minimal cat cassette (44); and (v) plasmids containing cat cassette inserts were selected and PCR was used to identify one in which cat and fdxA are in the same orientation. This fdxA::cat DNA was used to generate fdxA null H. pylori strains by DNA transformation. It is important to note that the stem-loop structure that is just downstream of the open reading frame in many cat cassettes has been removed here. This cassette is considered nonpolar on distal gene expression, because its insertion between DNA segments encoding the β and β′ domains of the large β-β′ RNA polymerase subunit (normally fused in H. pylori) does not impair growth (44). An fdxA::aphA insertion allele that is probably polar on distal gene expression, because fdxA and aphA are in opposite orientations, was generated similarly by using the aphA cassette from the frxA::aphA allele.
Measurement of survival in stationary phase.
Concentrated suspensions of H. pylori cells that had been growing exponentially as overnight cultures on BHI agar medium were prepared in PBS buffer (about 2 × 109 cells per ml), and 20 μl (∼4 × 107 cells) was spread uniformly on the surface of fresh BHI agar (150-mm-diameter petri plate). The viability of this initial inoculum on each day, beginning at day three, was determined by suspending aliquots of confluent bacterial growth from these plates in PBS, measuring the optical density, and determining viable counts by quantitative culture (CFU per optical density unit).
RT-PCR analysis of mRNA levels.
Exponentially growing H. pylori strains were spread on BHI medium alone or with Mtz (0.2 μg/ml for SS1; 1.5 μg/ml for 26695). Following 2 days of incubation, bacterial cells were collected and total RNA was prepared by using a Qiagen RNeasy kit, as recommended by the manufacturer (Qiagen Corp). After elution from the RNeasy column, the RNA was treated with RNase-free DNaseI, extracted twice with phenol:chloroform, and extracted once with chloroform-isoamyl alcohol. It was then precipitated with ammonium acetate (final concentration of 2.5 M) and 2.5 volumes of ice-cold ethanol, washed in 75% ethanol, and resuspended in RNase-free water. The integrity of the 16S and 23S rRNA was checked on a 1% agarose gel. Genomic DNA contamination was checked by PCR with Taq DNA polymerase without RT. RT-PCR was carried out by using the One-Step RT-PCR kit (Gibco-BRL) and primers frxRT-F and frxRT-R (for frxA mRNA), rdxRT-F and rdxRT-R (for rdxA mRNA), and ureB-F and ureB-R (for ureB mRNA). RT-PCR was carried out in a volume of 50 μl in a Perkin-Elmer GeneAmp PCR system 2400 thermal cycler with the following conditions: 50°C for 20 min; 94°C for 2 min; and then 35 cycles of 94°C for 15 s, 58°C for 30 s, 72°C for 40 s, with a final incubation at 72°C for 10 min.
RESULTS
The fdxA gene (hp277 in the strain 26695 genome sequence [52]) encodes a [Fe4 S4]-type ferredoxin (5, 52), a carrier of reducing equivalents, that may variously accept, donate, shift, and/or store electrons in key steps in central metabolism (8). The possibility that this protein's redox potential might be low enough to reduce Mtz and thereby convert it from prodrug to bactericidal agent motivated our interest in testing whether fdxA inactivation affected Mtz susceptibility. Others (32) had also sought to study fdxA but were not able to obtain fdxA null mutant transformants in their laboratory strain (2600). They therefore concluded that fdxA is essential for viability (32). The cat resistance gene cassette they had inserted into fdxA was probably polar on distal gene expression (44); however, we suspected that one or more of the genes downstream of and probably cotranscribed with fdxA, which were annotated as guanosine pentaphosphate pyrophosphatase (hp278), lipopolysaccharide heptosyltransferase (hp279), and lipid A acyltransferase (hp280) (5, 52), might be essential. Accordingly, we decided to reexamine fdxA's importance for H. pylori.
A nonpolar fdxA null allele was generated in cloned DNA by replacing much of the fdxA sequence with a chloramphenicol resistance determinant (cat) that lacks transcription pause sites (44), and the resulting fdxA::cat DNA was used to transform the type I reference strain 26695. Hundreds of Camr transformant colonies were obtained, as is typical with other DNAs and this strain, although the colonies were slow growing (4 days, instead of 3, were needed to detect them by eye). PCR with fdxA-specific primers confirmed that these Camr transformants had resulted from replacement of a wild-type allele with the fdxA::cat allele (data not shown). Equivalent slow-growing fdxA null transformants were also obtained with the type II mouse-adapted strain SS1. In contrast, attempts to generate fdxA-deficient derivatives of these strains with a different null allele, fdxA::aphA, which is probably polar on distal gene expression because fdxA and aphA are in opposite orientations, were unsuccessful (no transformant colonies were scored as ≤0.01% of normal yield), presumably because of polarity on distal gene expression. We conclude that fdxA is not essential for viability in strains 26695 or SS1, although it probably contributes to the vigor of their growth.
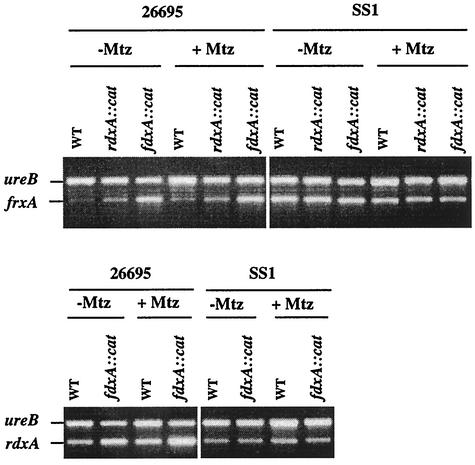
Three sets of results indicated that fdxA inactivation increased nitroreductase gene expression in strain 26695. First, Mtzr mutants were found in cultures of fdxA null derivatives of strain 26695 at frequencies of ≤10−8 (Table 2). This contrasts with a frequency of about 10−4, which is characteristic of 26695 wild type, in which just one gene (rdxA) needs to be inactivated to achieve Mtz resistance (27, 28, 50). Second, 26695 derivatives with null alleles of rdxA and fdxA (frxA functional) or of frxA and fdxA (rdxA functional) each remained Mtzs but gave rise to Mtzr mutants at frequencies of about 10−4 rather than ≤10−8. A triple mutant, containing null alleles of both rdxA and frxA as well as of fdxA (rdxAΔ111, frxA::aphA, fdxA::cat), was Mtzr (Table 2). Thus, inactivation of frxA as well as rdxA was needed to render the fdxA null derivative of 26695 resistant to Mtz. Third, RT-PCR indicated that fdxA inactivation increased the frxA mRNA level about fivefold relative to that of a ureB internal standard (Fig. 2). The rdxA transcript level also seemed to be increased about twofold in the fdxA-null derivative, which suggested that fdxA might help regulate both nitroreductase genes. In contrast, fdxA inactivation in SS1, which normally expresses frxA at a high level, did not affect frxA or rdxA mRNA levels (Fig. 2). Further tests revealed similar mRNA levels in cultures grown with sublethal levels of Mtz (see the legend of Fig. 2). Collectively, these outcomes support the view that the nitroreductase gene expression level inferred from Mtz susceptibility patterns reflects bacterial genotype (fdxA status) per se but not induction of gene expression by Mtz or the cellular damage that it causes.
TABLE 2.
Efficiency of colony formation on Mtz-containing mediuma
| Strain | Mtzr frequency |
|---|---|
| 26695 wild type (type I) | ∼10−4 |
| 26695 rdxA | ∼1.0 |
| 26695 frxA | ∼10−4 |
| 26695 fdxA | <10−8 |
| 26695 fdxA rdxA | ∼10−4 |
| 26695 fdxA frxA | ∼10−4 |
| 26695 fdxA rdxA frxA | ∼1.0 |
| SS1 wild type (type II) | <10−8 |
Efficiency of colony formation on BHI agar containing 8 μg of Mtz per ml. When this efficiency is less than 1.0 (which implies that the strain is fully resistant to Mtz), this frequency generally corresponds to frequencies of new Mtzr mutants, generated and selected by the mutagenic and bactericidal properties of Mtz once activated.
FIG. 2.
RT-PCR analysis of mRNA levels. H. pylori cells were grown, RNA was extracted, and RT-PCR was carried out as detailed in Materials and Methods. Threshold deleterious levels of Mtz were included in BHI agar where indicated (0.2 μg/ml for strain SS1; 1.5 μg/ml for strain 26695). WT, wild type.
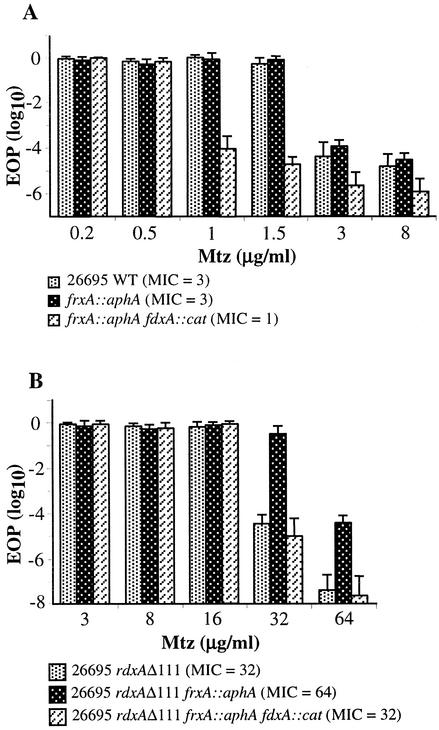
It is noteworthy that the rdxA frxA double mutant derivative of 26695 was fully resistant to 32 μg of Mtz per ml (100% efficiency of colony formation), whereas the isogenic rdxA frxA fdxA triple mutant exhibited full resistance only up to 16 μg of Mtz per ml in each of three independent trials (Fig. 3). The quantitative differences in efficiency of colony formation by double and triple mutants on plates with the critical 32 μg of Mtz per ml were seen when cells of each strain were spotted on the same plate. The greater susceptibility of the triple mutant might reflect either increases in other enzymes that also activate Mtz (28) or a nonspecific effect of the fdxA mutant's less vigorous growth.
FIG. 3.
Profiles of susceptibility to Mtz of strain 26695 wild type (WT) and isogenic mutant derivatives of it. Each test was carried out at least three times.
fdxA is essential in most but not all type I Mtzs strains.
The generality of findings with strains 26695 was tested by using 31 additional transformable strains that had been classified as type I by forward mutation tests (see Materials and Methods). Expected yields of transformants (generally at least hundreds of Camr colonies) were obtained with only two of them (Ind121 and HUPB57) (Table 3). Mutational tests showed that they each resembled 26695 in that fdxA inactivation in them caused a decrease in the Mtzr mutant frequency from approximately 10−4 to <10−8 and a need to inactivate both frxA and rdxA, rather than only rdxA, to achieve Mtz resistance. Thus, fdxA inactivation seemed to have turned on frxA expression in these two type I strains.
TABLE 3.
Efficiency of recovery of fdxA::cat (null) transformants depends on bacterial genotype
| Frequency of recovery of fdxA null transformants | Fraction of strains | Examples |
|---|---|---|
| Type I strains | ||
| Readily obtaineda | 3 of 32 | 26695, Ind121, HUPB57 |
| Obtained with difficultyb | 3 of 32 | Lit055, CPY6271, HUPB48 |
| Obtained only after frxA inactivation | 4 of 20 | Ind27, Ind66, Chen13, Lit050 |
| Not obtained after frxA inactivationc | 16 of 20 | Variousd |
| Obtained only after frxA and rdxA inactivation | 1 of 2 | HK192e |
| Type II strains | ||
| Readily obtained | 20 of 23 | SS1, 98QM3, 88-3887 and othersf |
| Not obtained | 3 of 23 | X47, HK152, R10 |
Readily obtained indicates that the yield of Camr fdxA null transformants was similar to that of Kanr frxA null transformants generated with frxA::aphA DNAs in the same transformation mix.
Obtained with difficulty indicates that the yield of Camr fdxA null transformants was <1% that of Kanr frxA null transformants generated in the same transformation mix. In these cases, some Camr transformants did not contain allelic replacements of the wild-type fdxA allele by the null mutant allele and thus may have resulted from illegitimate recombination or some other mutation event.
Not obtained also indicates that the yield of Camr fdxA null transformants was <1% that of Kanr frxA null transformants generated in the same transformation mix. We do not know if the not obtained class differs from the obtained with difficulty class.
These strains were TN2, GS3, HPK5, CPY6261, and CPY6311 (Japan); HUPB63, HUPB71, HUPB72, and HUPB77 (Spain); A-11, A-13, and O76 (Lithuania); Ind92 and Chen5 (India); HK192 (HongKong); and PeCan9a (Peru).
Incorporation of frxA::aphA and rdxAΔ111 null mutations into HK192 allowed recovery of slow-growing fdxA null transformants in high yield, whereas incorporation of these mutations into PeCan9a did not allow fdxA null transformants.
These additional strains were Lit5, Lit28-1, Lit43, Lit66-1, Lit75-1, Lit102, Lit113, Lit119, Lit120, Lit122, Alas219, Alas381, Alas10103, 2600, 2667, 2714, and Ind31.
Just a few Camr transformants were also obtained from 3 of the other 29 type I isolates (Lit055, CPY6271, and HUPB48), yields that were, in each case, less than 1% of those obtained with frxA::aphA DNA used as an internal control in the same transformation mixes. PCR tests indicated that some of these rare Camr transformants still retained the wild-type fdxA allele, suggesting that the fdxA::cat DNA had been inserted at another locus, perhaps by an illegitimate (mutation-like) recombination event (data not shown). However, at least one exceptional Camr transformant of each lineage was found by PCR to contain fdxA::cat in place of the resident wild-type fdxA gene, and these transformants were studied further. Inactivation of fdxA in these three strains had also, in each case, caused reduction in frequencies of Mtzr mutants in young cultures from ∼10−4 to ≤10−8. The Mtzr mutant frequencies were increased again (to ∼10−4) by further derivatives in which frxA had been inactivated. This indicated that fdxA also helped down-regulate frxA expression in these three exceptional type I strains.
No Camr transformants of any of the other 26 type I strains were obtained in repeated transformations with fdxA::cat DNA. In each case, the use of frxA::aphA DNA as a parallel control or as an internal control in the same transformation mix resulted in 100 or more Kanr transformants (Table 3), and PCR tests showed that each strain did contain an fdxA gene. Thus, fdxA seemed to be essential in most type I strains. It also seemed that this essentiality could be relieved by suppressor mutations in at least some strains (Lit055, CPY6271, and HUPB48). The ease of generating fdxA null derivatives of strains 26695, Ind121, and HUPB57 suggested that possibly equivalent suppressors already preexist at low frequency in natural populations.
fdxA is dispensable in most type II Mtzs strains.
Replacement of resident fdxA alleles with the fdxA::cat null allele was achieved readily in 20 of 23 type II strains tested (Table 3), although in each case fdxA null transformant colonies grew less rapidly than did colonies of their fdxA-proficient parents. Among the type II strains in which fdxA was readily inactivated were (i) strain 2600, in which fdxA-null derivatives had first been sought but without success (32), probably because of transcription polarity (noted above); (ii) 88-3887, a 26695-related strain that, remarkably, is type II in its Mtz-susceptible phenotype (26695 is type I); and 98QM3, but not X47, which is closely related to 98QM3 (see Fig. 1 and Materials and Methods).
No Camr transformant colonies were obtained from 3 of 23 type II isolates in repeated trials with fdxA::cat DNA (nonpolar null allele) (X47, a North American isolate, HK152 from Hong Kong, and R10 from South Africa), despite obtaining many Kanr transformants with frxA::aphA control DNA. Each of these three strains was retested and was confirmed as type II; their Mtzs phenotypes were changed from stable (≤10−8 Mtzr) to metastable (∼10−4 Mtzr) by inactivation of either rdxA or frxA, and they became Mtzr if both rdxA and frxA were inactivated. Among these fdxA-requiring strains was X47, which is remarkable because fdxA null derivatives of the closely related strain 98QM3 were readily obtained. This indicates that small differences in background genotype may determine whether fdxA is essential or not.
Premature death in stationary phase.
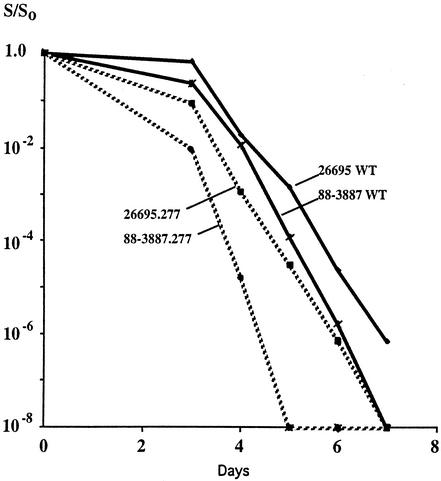
H. pylori cells began dying soon after reaching stationary phase. Although death in stationary phase is not understood, we suspected that it might be accelerated by disturbance of metabolic networks. Accordingly, the survivals of the wild-type strains 26695 and 88-3887 were compared with those of their isogenic fdxA derivatives (Fig. 4). In each case, fdxA null derivatives tended to die more rapidly than their wild-type parents in stationary phase, but the kinetics of death seemed strain specific. By the time that the viabilities of wild-type 88-3887 and 26695 had declined to about 1% of the initial level, the viabilities of their fdxA null derivatives had declined another ∼400-fold and ∼20-fold, respectively (Fig. 4). This was seen in each of two independent trials, each with an independently constructed fdxA null transformant. The fdxA null derivative of the unrelated SS1 strain also died prematurely in stationary phase, with a severity similar to that of strain 26695 but not 88-3887 (data not shown). Given that 88-3887 and 26695 are closely related, it would seem that effects of fdxA on fitness can also be strongly affected by small differences in background genotype.
FIG. 4.
Kinetics of death in stationary phase. Young exponentially growing cells were spread on BHI agar and were incubated. Aliquots were withdrawn daily, and efficiencies of colony formation, relative to culture optical density, were determined. WT, wild type.
Lethality of fdxA inactivation can involve frxA.
One explanation for fdxA's essentiality in many type I strains and dispensability in most type II strains supposes that high FrxA nitroreductase levels are deleterious in strains of particular genotypes. This idea is based on indications that inactivation of fdxA caused increased frxA expression in at least some type I strains, that fdxA seemed to be essential in most type I strains, and that fdxA was dispensable in most type II strains. To test this idea, we made frxA null (frxA::aphA) transformants of 20 representative fdxA-requiring type I strains and transformed frxA null derivatives of each lineage with fdxA::cat DNA. Camr transformant colonies were obtained at normal frequency in four lineages (Ind27, Ind66, Chen13, and Lit050) (Table 3). In each case, the colonies obtained were much smaller and slower growing than those made by the parental (frxA-deficient but fdxA-functional) strain. PCR tests of two single-colony isolates and of pools of 20 to 50 transformants from each lineage demonstrated the expected allelic replacement (original fdxA allele by fdxA::cat allele) in each case. This suggests that fdxA's essentiality in some type I strains reflects the ability of its gene product to down-regulate frxA gene expression. However, the inability to obtain fdxA null transformants of frxA null derivatives of 16 of these 20 selected type I strains and the slow growth of fdxA null transformants, when obtained, indicated that fdxA must have additional role(s).
DISCUSSION
fdxA, an often essential regulatory gene.
The possibility of metabolic regulation of nitroreductase gene expression in H. pylori emerged first with the finding of two types of Mtzs clinical isolates: type I, in which frxA is relatively quiescent; and type II, in which frxA is highly expressed (27, 28). Here we report that (i) fdxA (hp277, ferredoxin gene) helped down-regulate frxA expression in some type I H. pylori strains; (ii) the fdxA gene was essential for viability in many of them; (iii) this essentiality reflected a need to restrain frxA expression, at least in some cases; and (iv) fdxA was dispensable in most type II strains (which naturally express frxA). The complexity of these results—the inability to predict with certainty how any one strain will behave based on findings with other strains—provides a new phenotype-level illustration of H. pylori's great genetic diversity and may give insight into how this gastric pathogen evolves and interacts with its human hosts during long-term chronic infection.
The involvement of fdxA in down-regulating frxA expression was most evident in six type I strains that tolerated fdxA inactivation; in each case, this caused a need to mutate frxA (along with rdxA) to achieve Mtz resistance. The repeated failure to obtain fdxA null transformants of most other type I strains, however, indicated that FdxA was often essential. Although a failure to obtain fdxA null transformants of strain 2600 had also been interpreted as indicating fdxA essentiality (32), that particular result can now be ascribed to polarity on distal gene expression, because fdxA null derivatives of strain 2600 were readily generated here by using a nonpolar fdxA::cat allele.
The requirement for fdxA was overcome in 4 of 20 strains by inactivating their frxA genes. This suggested that FdxA protein also regulated frxA expression in these strains and that keeping frxA quiescent was an adaptive trait for them. Indications of additional roles for fdxA included our inability to obtain fdxA null transformants in most type I strains, even after making them frxA deficient; the slow growth of fdxA null transformants (although some of this might also be ascribed to residual polarity of the cat cassette used to inactivate fdxA); and their premature death in stationary phase. This additional role(s) may include carriage of reducing equivalents for multiple metabolic reactions and possibly also regulating expression of additional genes (8, 9).
In light of fdxA's essentiality in most type I strains, it was striking that fdxA null derivatives were obtained in most type II strains, the class that normally exhibits (and tolerates) strong frxA expression. This might reflect either (i) the presence in them of genes with equivalent or compensatory functions (e.g., additional ferredoxins) and their absence from most type I strains or (ii) the presence in them of naturally occurring suppressor mutations, perhaps equivalent to the suppressors invoked above to explain the rare fdxA null transformants of three type I strains (Lit055, CPY6271, and HUPB48). An illustration that rather small differences in background genotype might determine whether fdxA is essential or not was provided by studies of two closely related strains, X47 (fdxA requiring) and 98QM3 (fdxA independent) (Fig. 1).
Consequences of increasing frxA expression and fdxA inactivation.
Although the normal role of FrxA nitroreductase is not known (e.g., whether it is strictly metabolic or protective against reactive metabolites produced in the host response to infection), we suggest that the detrimental effect of excess frxA expression in H. pylori strains of certain genotypes stems from changes in metabolite pools. In one model, excess FrxA might cause a potentially injurious metabolite to accumulate to toxic levels, analogous to that seen with 2-ketobutyrate, sugar phosphates, and 3′-phosphoadenoside 5′-phosphosulfate (PAPS) in certain mutant strains of enteric bacteria (33, 34, 41). In an alternative model, excess FrxA might cause depletion of a critical intermediate or end product, analogous to starvation variously for succinyl-coenzyme A, caused by excess glutamate dehydrogenase and a resultant siphoning of most alpha ketoglutarate into glutamate synthesis (26); or for several serine-derived metabolites, caused by excess serine deaminase and a resultant siphoning of most serine into pyruvate synthesis (6). In our experiments, the tolerance of high nitroreductase levels in most type II strains and a few type I strains might stem from differences in levels of other metabolic enzymes that, in a toxicity model, consume the metabolite, interfere with its synthesis, or produce an antidote or that, in an intermediate depletion model, increase flux through undersupplied pathways or activate alternative modes of synthesis of the essential end product. Such flexibility, the compensation of deleterious effects of one metabolic alteration by changes in other metabolic functions, is a familiar theme in traditional biochemical genetics (see, for example, references 6, 10, 11, and 26).
How FdxA might act.
Two models for FdxA-mediated down-regulation of frxA expression seem attractive. One invokes a ferredoxin-mediated effect on a metabolite that itself is regulatory; e.g., ferredoxin-dependent synthesis of a corepressor or consumption of an inducer. A second model envisions direct action of FdxA itself and is suggested by studies of the FdI ferredoxin of Azotobacter vinelandii. This ferredoxin interacts with a pyruvate dehydrogenase subunit and enables it to bind the fpr promoter and block fpr gene transcription (45). Other useful precedents include various larger iron-sulfur proteins, such as SoxR, aconitase-iron regulatory protein, and IscR, which bind specific RNA or DNA sequences or participate in protein-protein interactions in reactions that also depend critically on oxidation states and/or iron binding to their iron-sulfur centers and that thus can respond sensitively to environmental and intracellular cues (9, 48).
Evolutionary inferences.
The complexity among H. pylori strains of patterns of fdxA essentiality and frxA regulation illustrates, at the phenotypic level, H. pylori's extraordinary genetic diversity. Much of this diversity may reflect accumulation of numerous genetic differences, many of which may have quantitative effects on metabolite flux in one or more biochemical pathways. The following possible sources of this diversity have been much discussed: general mutation (56), frameshifts in repetitive sequences in contingency genes (47), and recombination within and between strains (24, 29, 51, 52). We suggest that the present level of diversity also reflects several additional features: (i) H. pylori's mode of transmission, which is preferentially intrafamilial and occurs efficiently in early childhood (7, 13, 23, 36); (ii) the tendency of infections to persist for decades; and (iii) the rarity of new infections in adulthood (1, 37, 55). These three features create a highly fragmented bacterial population and diminish competition among strains from unrelated persons and selection for any one or few potentially most-fit genotypes (selective sweeps [22]). These features would promote genetic drift even if all people were identical physiologically. Given human diversity in traits that may be important to individual H. pylori strains (17, 24, 35, 43), we also imagine that at least subtly different phenotypes may be selected in different infected people. These features of H. pylori and of human populations create, in effect, rugged evolutionary landscapes (12, 53, 57). The chance of ingestion, especially in infancy, as much as any near-ideal match between bacterial genotype and particular host physiology may dictate which H. pylori strain becomes established in any new human host. This feature should often result in selection for adaptive changes that make each infecting strain better suited for its present host. The selection for adaptive changes may continue for years, in part because gastric physiology changes with age and in response to chronic infection. Adaptation will often involve many small steps and operate along different trajectories in different strains and infected people (12, 43, 54, 57)—Jacob's concept of evolution by tinkering (25). The resultant constellations of quantitative trait determinants should, in turn, affect the chance that a given strain will productively infect a particular human host and the chance that persistent infection will lead to overt disease.
Acknowledgments
A.K.M. and J.-Y.J. contributed equally to the experiments described here.
We thank Bob Bender, Bob LaRossa, G. Balakrish Nair, Elaine Newman, Paul Robben, and Kenny Simpson for stimulating discussions, and we thank many colleagues and collaborators for strains used in the present studies.
This research was supported by grants from the U.S. Public Health Service (AI38166, AI49161, DK53727, and P30 DK52574) and from the Canadian Institutes for Health Research (grant number ROP37514).
REFERENCES
- 1.Abu-Mahfouz, M. Z., V. M. Prasad, P. Santogade, and A. F. Cutler. 1997. Helicobacter pylori recurrence after successful eradication: 5-year follow-up in the United States. Am. J. Gastroenterol. 92:2025-2028. [PubMed] [Google Scholar]
- 2.Achtman, M., T. Azuma, D. E. Berg, Y. Ito, G. Morelli, Z. J. Pan, S. Suerbaum, S. A. Thompson, A. van der Ende, and L. J. van Doorn. 1999. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol. Microbiol. 32:459-470. [DOI] [PubMed] [Google Scholar]
- 3.Akopyanz, N., N. O. Bukanov, T. U. Westblom, S. Kresovich, and D. E. Berg. 1992. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 20:5137-5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akopyants, N. S., A. Fradkov, L. Diatchenko, J. E. Hill, P. D. Siebert, S. A. Lukyanov, E. D. Sverdlov, and D. E. Berg. 1998. PCR-based subtractive hybridization and differences in gene content among strains of Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:13108-13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 6.Ambartsoumian, G., R. D'Ari, R. T. Lin, and E. B. Newman. 1994. Altered amino acid metabolism in lrp mutants of Escherichia coli K12 and their derivatives. Microbiology 140:1737-1744. [DOI] [PubMed] [Google Scholar]
- 7.Bamford, K. B., J. Bickley, J. S. Collins, B. T. Johnston, S. Potts, V. Boston, R. J. Owen, and J. M. Sloan. 1993. Helicobacter pylori: comparison of DNA fingerprints provides evidence for intrafamilial infection. Gut 34:1348-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beinert, H., R. H. Holm, and E. Münck. 1997. Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277:653-659. [DOI] [PubMed] [Google Scholar]
- 9.Beinert, H., and P. J. Kiley. 1999. Fe-S proteins in sensing and regulatory functions. Curr. Opin. Chem. Biol. 3:152-157. [DOI] [PubMed] [Google Scholar]
- 10.Berg, C. M., and J. J. Rossi. 1974. Proline excretion and indirect suppression in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 118:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg, C. M., M. D. Wang, N. B. Vartak, and L. Liu. 1988. Acquisition of new metabolic capabilities: multicopy suppression by cloned transaminase genes in Escherichia coli K-12. Gene 65:195-202. [DOI] [PubMed] [Google Scholar]
- 12.Burch, C. L., and L. Chao. 1999. Evolution by small steps and rugged landscapes in the RNA virus phi6. Genetics 151:921-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalkauskas, H., D. Kersulyte, I. Cepuliene, V. Urbonas, D. Ruzeviciene, A. Barakauskiene, A. Raudonikiene, and D. E. Berg. 1998. Genotypes of Helicobacter pylori in Lithuanian families. Helicobacter 3:296-302. [PubMed] [Google Scholar]
- 14.Cover, T. L., D. E. Berg, M. J. Blaser, and H. L. T. Mobley. 2001. H. pylori pathogenesis, p. 509-558. In E. A. Groisman (ed.), Principles of bacterial pathogensis. Academic Press, New York, N.Y.
- 15.de Vries, N., A. H. M. van Vliet, and J. G. Kusters. 2001. Gene regulation, p. 321-334. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. American Society for Microbiology, Washington, D.C.
- 16.Doig, P., B. L. de Jonge, R. A. Alm, E. D. Brown, M. Uria-Nickelsen, B. Noonan, S. D. Mills, P. Tummino, G. Carmel, B. C. Guild, D. T. Moir, G. F. Vovis, and T. J. Trust. 1999. Helicobacter pylori physiology predicted from genomic comparison of two strains. Microbiol. Mol. Biol. Rev. 63:675-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois, A., D. E. Berg, E. T. Incecik, N. Fiala, L. M. Heman-Ackah, J. Del Valle, M. Yang, H. P. Wirth, G. I. Perez-Perez, and M. J. Blaser. 1999. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology 116:90-96. [DOI] [PubMed] [Google Scholar]
- 18.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 20.Garner, J. A., and T. L. Cover. 1995. Analysis of genetic diversity in cytotoxin-producing and non-cytotoxin-producing Helicobacter pylori strains. J. Infect. Dis. 172:290-293. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin, A., D. Kersulyte, G. Sisson, S. J. O. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 22.Guttman, D. S., and D. E. Dykhuizen. 1994. Detecting selective sweeps in naturally occurring Escherichia coli. Genetics 138:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han, S. R., H. C. Zschausch, H. G. Meyer, T. Schneider, M. Loos, S. Bhakdi, and M. J. Maeurer. 2000. Helicobacter pylori: clonal population structure and restricted transmission within families revealed by molecular typing. J. Clin. Microbiol. 38:3646-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilver, D., A. Arnqvist, J. Ogren, I-M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. The Helicobacter pylori Lewis b blood group antigen binding adhesin revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 25.Jacob, F. 1977. Evolution and tinkering. Science 196:1161-1166. [DOI] [PubMed] [Google Scholar]
- 26.Janes, B. K., P. J. Pomposiello, A. Perez-Matos, D. J. Najarian, T. J. Goss, and R. A. Bender. 2001. Growth inhibition caused by overexpression of the structural gene for glutamate dehydrogenase (gdhA) from Klebsiella aerogenes. J. Bacteriol. 183:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong, J. Y., A. K. Mukhopadhyay, J. K. Akada, D. Dailidiene, P. S. Hoffman, and D. E. Berg. 2001. Roles of FrxA and RdxA nitroreductases of Helicobacter pylori in susceptibility and resistance to metronidazole. J. Bacteriol. 183:5155-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong, J. Y., A. K. Mukhopadhyay, D. Dailidiene, Y. Wang, B. Velapatiño, R. H. Gilman, A. J. Parkinson, G. B. Nair, B. C. Y. Wong, S. K. Lam, R. Mistry, I. Segal, Y. Yuan, H. Gao, T. Alarcon, M. L. Brea, Y. Ito, D. Kersulyte, H.-K. Lee, Y. Gong, A. Goodwin, P. S. Hoffman, and D. E. Berg. 2000. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes cause moderate and high-level metronidazole resistance in Helicobacter pylori. J. Bacteriol. 182:5082-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Josenhans, C., K. A. Eaton, T. Thevenot, and S. Suerbaum. 2000. Switching of flagellar motility in Helicobacter pylori by reversible length variation of a short homopolymeric sequence repeat in fliP, a gene encoding a basal body protein. Infect. Immun. 68:4598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersulyte, D., H. Chalkauskas, and D. E. Berg. 1999. Emergence of recombinant strains of Helicobacter pylori during human infection. Mol. Microbiol. 31:31-43. [DOI] [PubMed] [Google Scholar]
- 31.Kersulyte, D., A. K. Mukhopadhyay, B. Velapatiño, W. W. Su, Z. J. Pan, C. Garcia, V. Hernandez, Y. Valdez, R. S. Mistry, R. H. Gilman, Y. Yuan, H. Gao, T. Alarcon, M. Lopez Brea, G. B. Nair, A. Chowdhury, S. Datta, M. Shirai, T. Nakazawa, R. Ally, I. Segal, B. C. Y. Wong, S. K. Lam, F. Olfat, T. Boren, L. Engstrand, O. Torres, R. Schneider, J. E. Thomas, S. Czinn, and D. E. Berg. 2000. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 182:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon, D. H., F. A. El-Zaatari, M. Kato, M. S. Osato, R. Reddy, Y. Yamaoka, and D. Y. Graham. 2000. Analysis of rdxA and involvement of additional genes encoding NAD(P)H flavin oxidoreductase (FrxA) and ferredoxin-like protein (FdxB) in metronidazole resistance of Helicobacter pylori. Antimicrob. Agents Chemother. 44:2133-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaRossa, R. A. 1996. Mutant selections linking physiology, inhibitors and genotypes, p. 2527-2587. In F.C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 34.LaRossa, R. A., and T. K. Van Dyk. 1987. Metabolic mayhem caused by 2-ketoacid imbalances. Bioessays 7:125-130. [DOI] [PubMed] [Google Scholar]
- 35.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malaty, H. M., D. Y. Graham, P. D. Klein, D. G. Evans, E. Adam, and D. J. Evans. 1991. Transmission of Helicobacter pylori infection. Studies in families of healthy individuals. Scand. J. Gastroenterol. 26:927-932. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell, H. M., P. Hu., Y. Chi, M. H. Chen, Y. Y. Li, and S. L. Hazell. 1998. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China). Gastroenterology 114:256-261. [DOI] [PubMed] [Google Scholar]
- 38.Mobley, H. L. T., G. L. Mendz, and S. L. Hazell (ed.). 2001. Helicobacter pylori physiology and genetics. ASM Press, Washington D.C. [PubMed]
- 39.Mukhopadhyay, A. K., D. Kersulyte, J. Y. Jeong, S. Datta, Y. Ito, A. Chowdhury, S. Chowdhury, A. Santra, S. K. Bhattacharya, T. Azuma, G. B. Nair, and D. E. Berg. 2000. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J. Bacteriol. 182:3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuwald, A. F., B. R. Krishnan, I. Brikun, S. Kulakauskas, K. Suziedelis, T. Tomcsanyi, T. S. Leyh, and D. E. Berg. 1992. cysQ, a gene needed for cysteine synthesis in Escherichia coli K-12 only during aerobic growth. J. Bacteriol. 174:415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohta, T. 2002. Inaugural Article: near-neutrality in evolution of genes and gene regulation. Proc. Natl. Acad. Sci. USA 99:16134-16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penn, D. J., K. Damjanovich, and W. K. Potts. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl. Acad. Sci. USA 99:11260-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raudonikiene, A., N. Zakharova, W. W. Su, J. Y. Jeong, L. Bryden, P. S. Hoffman, D. E. Berg, and K. Severinov. 1999. Helicobacter pylori with separate beta- and beta′-subunits of RNA polymerase is viable and can colonize conventional mice. Mol. Microbiol. 32:131-138. [DOI] [PubMed] [Google Scholar]
- 45.Regnstrom, K., S. Sauge-Merle, K. Chen, and B. K. Burgess. 1999. In Azotobacter vinelandii, the E1 subunit of the pyruvate dehydrogenase complex binds fpr promoter region DNA and ferredoxin I. Proc. Natl. Acad. Sci. USA 96:12389-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders, N. J., J. F. Peden, D. W. Hood, and E. R. Moxon. 1998. Simple sequence repeats in the Helicobacter pylori genome. Mol. Microbiol. 27:1091-1098. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz, C. J., J. L. Giel, T. Patschkowski, C. Luther, F. J. Ruzicka, H. Beinert, and P. J. Kiley. 2001. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc. Natl. Acad. Sci. USA 98:14895-14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sisson, G., A. Goodwin, A. Raudonikiene, N. J. Hughes, A. K. Mukhopadhyay, D. E. Berg, and P. S. Hoffman. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 46:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sisson, G., J. Y. Jeong, A. Goodwin, L. Bryden, N. Rossler, S. Lim-Morrison, A. Raudonikiene, D. E. Berg, and P. S. Hoffman. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori rdxA (nitroreductase) gene. J. Bacteriol. 182:5091-5096. [DOI] [PMC free article] [PubMed]
- 51.Suerbaum, S., et al. 1998. Free recombination within Helicobacter pylori. Proc. Natl. Acad. Sci. USA 95:12619-12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomb, J. F., et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 53.Travisano, M., J. A. Mongold, A. F. Bennett, and R. E. Lenski. 1995. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267:87-90. [DOI] [PubMed] [Google Scholar]
- 54.Travisano, M., J. A. Mongold, and R. E. Lenski. 1995. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267:87-90. [DOI] [PubMed] [Google Scholar]
- 55.van der Hulst, R. W., et al. 1997. Helicobacter pylori reinfection is virtually absent after successful eradication. J. Infect. Dis. 176:196-200. [DOI] [PubMed] [Google Scholar]
- 56.Wang, G., M. Z. Humayun, and D. E. Taylor. 1999. Mutation as an origin of genetic variability in Helicobacter pylori. Trends Microbiol. 7:488-493. [DOI] [PubMed] [Google Scholar]
- 57.Wright, S. 1982. The shifting balance theory and macroevolution. Annu. Rev. Genet. 16:1-19. [DOI] [PubMed] [Google Scholar]