Abstract
Gαo, the most abundant G protein in mammalian brain, occurs at least in two subforms, i.e., Gαo1 and Gαo2, derived by alternative splicing of the mRNA. A third Gαo1-related isoform, Gαo3, has been purified, representing about 30% of total Go in brain. Initial studies revealed distinct biochemical properties of Gαo3 as compared with other Gαo isoforms. In matrix-assisted laser desorption/ionization peptide mass mapping of gel-isolated Gαo1 and Gαo3, C-terminal peptides showed a difference of +1 Da for Gαo3. Nanoelectrospray tandem mass spectrometry sequencing revealed an Asp instead of an Asn at position 346 of Gαo3. Gel electrophoretic analysis of recombinant Gαo3 showed the same mobility as native Gαo3 but distinct to Gαo1. The conversion of 346Asn→Asp changed the signaling properties, including the velocity of the basal guanine nucleotide-exchange reaction, which points to the involvement of the C terminus in basal guanosine 5′-[γ-thio]triphosphate binding. No cDNA coding for Gαo3 was detected, suggesting an enzymatic deamidation of Gαo1 by a yet-unidentified activity. Therefore, Gα heterogeneity is generated not only at the DNA or RNA levels, but also at the protein level. The relative amount of Gαo1 and Gαo3 differed from cell type to cell type, indicating an additional principle of G protein regulation.
Heterotrimeric G proteins are known as membrane-tethered cellular switches linking a diverse set of heptahelical receptors to dozens of cellular effectors (1–3). Specificity within this signaling network depends on a structural diversity originating from multiple genes (4). Alternative splicing of transcripts increases heterogeneity, and at the protein level distinct αβγ compositions of G proteins allow further specificity and selectivity in signaling. Regulation of G protein activity is accomplished through interaction with heptahelical receptors, G protein-regulated effectors with GTPase-activating activity, or the recently identified regulators of G protein signaling. In addition, the function of an individual G protein is further regulated by posttranslational modifications induced by bacterial toxins, cellular kinases, or lipidation (5–9).
Many functions of G proteins have been elucidated despite poor expression of some isoforms. However Go proteins, which have been known for a long time as abundantly expressed proteins, still lack definition of a clear-cut function. In brain they are a major constituent in cells, making up 0.5–1% of the plasma membrane proteins and 10% of the membrane proteins in growth cones (10–12). Expression of Go is restricted to neuronal and endocrine systems and the heart (13). Transcription of the gene results in alternatively spliced products (14, 15) coding for Gαo1 and Gαo2, of which Gαo1 predominates (16). In view of the limited knowledge about their biological roles Go proteins were predicted to be a storage form of Gβγ complexes (17). Disruption of the Gαo gene in mice has pointed to a significant participation in the muscarinic regulation of Ca2+ channels in heart and a discrete involvement in neurotransmission in the nervous system (18, 19). Furthermore, a large body of evidence has implicated a role for Go proteins in modulation of voltage-gated calcium channels. It has been demonstrated that the inhibitory actions of Go on calcium channels are mediated by Gβγ (20–24). Go proteins also have been detected on endomembranes, suggesting specific roles in vesicular function (25). Recently, we have identified Gαo2 as a modulator of the monoamine uptake system in granules of chromaffin cells (26).
Interestingly, further heterogeneity of Gαo proteins is evident at the protein level (27–31). In addition to Gαo1 and Gαo2, a third Gαo isoform, Gαo3 or Gαoc, has been found in mammals (16, 31). This unknown protein represents the second most abundant Gαo isoform, comprising about 30% of total Gαo expressed in mammalian brain (16, 32).
This isoform diversity correlates with functional differences between the three Gαo proteins. Thus, activation of Gαo3 and Gαo1, but not of Gαo2, was observed on depolarization of rat brain synaptoneurosomes (56), whereas activated Gαo2, but neither Gαo1 nor Gαo3, inhibited the vesicular monoamine transporter system in PC-12 cells (26). In contrast to Gαo1 and Gαo2, infusion of purified Gαo3 failed to rescue receptor-induced calcium channel inhibition in pertussis toxin (PT)-treated GH3 cells (33).
Hitherto several fruitless attempts have been undertaken to identify the structural basis of Gαo3 (32, 34, 35). In the present study, the primary structures of Gαo isoforms were characterized by using matrix-assisted laser desorption/ionization (MALDI) and nanoelectrospray ionization tandem mass spectrometry (MS) (36). Gαo3 contains a single amino acid exchange (Asn→Asp) in the extreme C terminus as compared with Gαo1. This amino acid conversion is likely to explain the observed differences in the signal transduction properties of Gαo1 and Gαo3. Because we did not detect corresponding genetic information, we speculate that deamidation is an additional mechanism for regulation of G protein activity.
MATERIALS AND METHODS
Purification of G Proteins.
Isolation of G proteins and their Gα and Gβγ subunits from bovine brain membranes was reported previously (32). Final resolution of Gαo isoforms was achieved by ion exchange chromatography in fast protein liquid chromatography (FPLC) using a Mono Q HR 5/5 column (Amersham Pharmacia). Subsequent to an additional heptylamine-Sepharose chromatography step Gβγ complexes finally were purified by FPLC as for Gαo subunits (37).
Digestion of Purified Proteins and HPLC Separation of Peptides.
For in-gel protein digestion of Gαo, bands of resolved proteins were cut from the gel, incubated in the presence of trypsin, chymotrypsin (Sigma), or Asp-N endoproteinase (Calbiochem) and prepared for MS as described (38). Peptides derived from Gαo1 and Gαo3 isoforms were separated by using an Applied Biosystems model 140B HPLC pump equipped with a 2.1 mm-by-150 mm reversed-phase column (Vydac C18, The Separations Group). Elution buffers consisted of (A) 0.1% trifluoroacetic acid and (B) 0.07% trifluoroacetic acid in 90% acetonitrile. A 120 μl/min linear gradient from 5% to 60% B was used. Peptide elution was monitored by UV absorption at 214 nm, and peptide-containing fractions were collected and subsequently subjected to MALDI MS analysis.
MS.
Mass analysis of crude peptide mixtures generated by in-gel digestion and of HPLC-purified peptides was accomplished by delayed extraction MALDI on a Bruker REFLEX time-of-flight mass spectrometer (Bruker-Franzen Analytik, Bremen) (38). Similarly, aliquots from extracted peptide mixtures or HPLC fractions were analyzed by MALDI. HPLC fractions were screened by using an automated MALDI data acquisition system developed at the European Molecular Biology Laboratory (38). Peptide sequencing was performed by nanoelectrospray tandem MS (36).
Analysis of Gαo mRNA.
Total RNA was isolated from rat brains by using the InViSorb RNA kit II (InViTek, Berlin). cDNAs were synthesized by SuperScript II RNAseH− Reverse Transcriptase (Gibco/BRL) using an oligo(dT) primer. Sequences encoding the C-terminal fragment of Gαo1 and Gαo3 were amplified by PCR. Primers (ARK Scientific Biosystems, Dieburg, Germany) used were 5′-G TCA CCC TTG ACC ATC TGC TTT CC-3′ and 5′-TCA GTA CAA GCC ACA GCC CCG GAG-3′, corresponding to nucleotides 1549–1572 and 1771–1748, respectively. PCR was performed in a final volume of 50 μl containing 20 mM Tris⋅Cl (pH 8.4), 50 mM KCl, 3 mM MgCl2, 200 μM of each dNTP, 100 pmol of each primer, and 250 milliunits of Taq DNA polymerase. The amplified DNA fragments were ligated into pGEM-T Easy (Promega). A total of 101 clones were isolated and analyzed on a model 377 sequencer (Perkin–Elmer).
Specific Cleavage of cDNA.
In three parallel experiments total mRNA from rat brains was amplified by reverse transcription–PCR. The oligonucleotide 5′-TC CTC AAC AAG AAA GAC CTC-3′, corresponding to nucleotides 1509–1528, was used as a forward primer in all cases. The reverse primers were designed to generate additional restriction cleavage sites when extended by Gαo1 (AclI used) and Gαo3 (AatII and EcoRV used) sequences. Accordingly, for amplification of Gαo1-encoding sequences the reverse primer was 5′-A GTA CAA GCC ACA GCC CCG GAG AAC GT-3′ (primer 1, nucleotides underlined: changed nucleotides to generate a new cleavage site), corresponding to nucleotides 1769–1743 in Gαo1-coding DNA. Because two different oligonucleotides were used to amplify possible cDNA sequences encoding Gαo3, primers 2 and 3 were 5′-A GTA CAA GCC ACA GCC CCG GAG GAC GT-3′ and 5′-A GTA CAA GCC ACA GCC CCG GAG GAT-3′, respectively, corresponding to nucleotides 1769–1745. Reactions were performed in 20 mM Tris⋅Cl, pH 8.8/10 mM KCl/10 mM (NH4)2SO4/2 mM MgSO4/0.1% Triton X-100/0.1 mg/ml BSA/200 μM of each dNTP/100 pmol of each primer/125 milliunits of Pfu DNA polymerase (Stratagene). The generation of cleavage sites by primer extension in PCR cycles was verified by a restriction digest (1 h, 37°C) with the appropriate enzymes. DNA amplified by primer 1 was digested with AclI, while for primer 2- and primer 3-derived PCR products AatII and EcoRV, respectively, were used. Internal controls added were pcDNA3 for PCR products obtained with primers 1 and 3 and pQE60 in the experiment using primer 2.
Expression and Purification of Recombinant Gαo1 and Gαo3.
For expression of recombinant proteins we used the cDNA of murine Gαo1, which is highly homologous to those of other mammalian species (39). No differences in splicing between murine and bovine Gαo1 transcripts were observed. Additionally, on the protein level murine and bovine Gαo1 and Gαo3 show similar apparent molecular masses and pI values (16, 32) although the deduced amino acid sequence of murine Gαo1 differs from bovine Gαo1 in six positions. Wild-type murine Gαo1 in the pCIS vector was a gift from M.I. Simon, CalTech, Pasadena, CA. PCR was performed as described above with primer 5′-GCG CAG CCC GCG AAT TCA GAT-3′, corresponding to nucleotides 830–850 of the pCIS vector, i.e., 41–21 nt upstream the Gαo1 insert, and reverse primer 5′-GCG TAA GCT TCA GTA CAA GCC GCA-3′, corresponding to nucleotides 1771–1757 of Gαo1, i.e., its C terminus. Asn at position 346 in Gαo1 was mutated to Asp by site-directed mutagenesis (italic letters) using the primer 5′-GCG TAA GCT TCA GTA CAA GCC ACA GCC CCG GAG ATT GTC GGC AAT GAT G-3′, corresponding to nucleotides 1771–1732. All clones constructed were confirmed by DNA sequencing. The amplified DNA fragments were used for construction of recombinant baculoviruses as described (37). Gαo isoforms were expressed together with Gβ1- and Gγ2-His6 and purified from Sf9 cell membranes as detailed elsewhere (40).
SDS/PAGE and Immunoblotting.
SDS gels contained 6 M urea. Resolved proteins were transferred to poly(vinylidene difluoride) membranes (Immobilon P, Millipore) and detected with subtype-specific rabbit antisera (32).
35S-Guanosine 5′-[γ-Thio]Triphosphate (GTPγS) Binding and ADP Ribosylation of G Proteins by PT.
Purified G proteins were depleted from aluminium fluoride by dialyzing the samples against a buffer consisting of 20 mM Tris⋅Cl (pH 8.0), 1 mM EDTA, 20 mM 2-mercaptoethanol, 100 mM NaCl, 12 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 10 μM GDP. For analysis of differences in the time course of 35S-GTPγS (NEN) binding to Gαo isoforms we followed a published protocol (41). The time course of binding reactions was monitored at 25°C. Gαo isoforms were ADP-ribosylated in the presence of various concentrations of purified Gβγ complexes by using PT. Gα and Gβγ were incubated at indicated concentrations in 25 mM Tris⋅Cl, pH 8.0/1 mM EDTA/0.2% (wt/vol) Lubrol (Sigma)/2.5 mM MgCl2/2.5 mM ATP/30 μM 32P-NAD (5 × 106 cpm; NEN)/7.4 μg/ml activated PT (Calbiochem) for 30 sec at 32°C. Under these conditions ADP ribosylation was linear as a function of time and toxin concentration. For maximal ADP ribosylation, reactions were allowed to proceed for 3 h at 32°C or 2 days at 5°C (42). Samples were subjected to urea-SDS/PAGE. For quantification of 32P-incorporation, dilutions of the reaction mixture were spotted on nitrocellulose membranes and dried. Gel slabs and membranes were autoradiographed by using a BAS 1500 Fuji-imager (Raytest, Straubenhard, Germany).
Statistics.
A paired Student’s t test was used to compare results within individual experiments. Values quoted are means ± SD.
RESULTS AND DISCUSSION
Analysis of Purified Monomeric Gαo and Heterotrimeric Go Proteins.
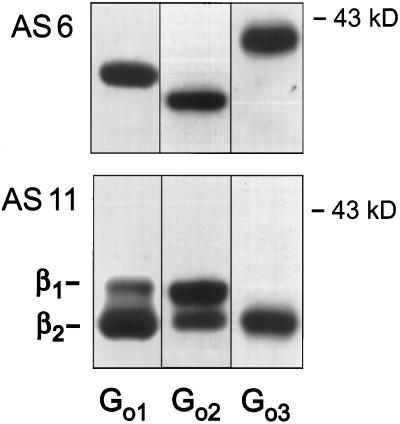
Previous work has identified Gαo3 as a major G protein present in mammalian brain of different species. It exhibits an apparent molecular weight similar to Gαo1 in standard SDS/PAGE, whereas addition of urea (4–6 M) results in distinct gel electrophoretic mobilities of these two Gα isoforms. Regardless of the species tested Gαo3 proteins exhibit a more acidic pI value and a slower mobility in urea-SDS/PAGE than Gαo1 and Gαo2 (16), allowing purification of this Gα isoform by conventional chromatographic approaches (32). Analysis of the three Gαo isoforms with specific antibodies excluded that Gαo3 represents a variant of the spliced isoform Gαo2. When purified as heterotrimers, Gαo isoforms have been reported to possess individual Gγ subunit profiles (31). To identify the Gβ isoforms copurifying with Gαo proteins, we separated heterotrimeric Gi/o proteins by fast protein liquid chromatography using Mono Q columns (Fig. 1). In accordance with previous reports, Go2 eluted in front of Go1 followed by Go3 (43–45). This pattern matches the previously communicated Gγ profile of the individual Gαo isoforms (31). However, the Gβ composition also reproducibly differed between the three purified Go proteins. Although Gαo1 and Gαo2 associated with Gβ1 and Gβ2 isoforms, Gαo3 almost exclusively copurified with Gβ2 (Fig. 1).
Figure 1.
Immunostain of Go proteins purified from bovine brain membranes. Proteins were resolved on urea-SDS/PAGE and immunoblotted with an anti-Gαo common antiserum (AS 6) (Upper) or a Gβ1- and Gβ2-detecting antiserum (AS 11) (Lower).
MS Analysis of Gαo Primary Structure.
Our previous work has indicated that native as well as denatured Gαo3 represents a more acidic and a slightly more hydrophilic protein compared with Gαo1 (refs. 16 and 32; T.E. and B.N., unpublished work). Although a difference in pI values of 0.15 units may suggest that Gαo3 could represent an ADP-ribosylated variant of Gαo1, the PT-sensitive cysteine at the C terminus was not modified. Subsequent biochemical studies also confirmed the absence of other posttranslational modifications such as phosphorylation, glycosylation, or lipidation. These initial results with Gαo proteins were observed among all species tested. Bovine brain membranes were chosen as the source for large-scale purification of Gαo proteins used in this study.
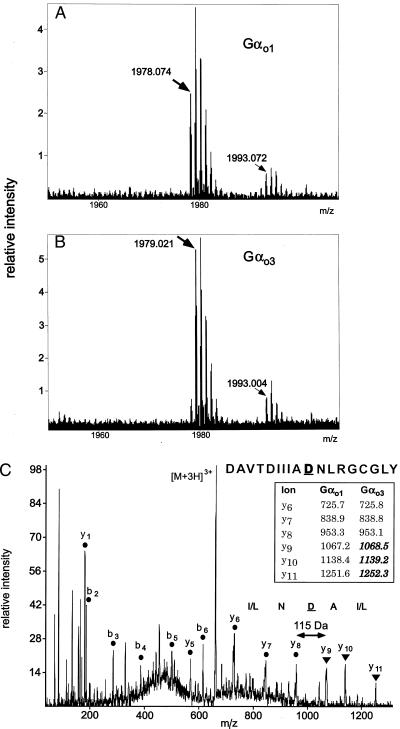
To identify the structural difference between the two proteins we enzymatically digested Gαo1 and Gαo3 and used MS. Tryptic and chymotryptic peptide mass maps of purified Gαo1 and Gαo3 were obtained by MALDI. As expected from previous work (32, 35) the tryptic peptide mass maps predominantly displayed peptides from the N-terminal domain of Gαo1 and Gαo3 and did not reveal any differences between the two isoforms in this region (underlined in Fig. 2). For both isoforms, a mass consistent with the myristoylated but unpalmitoylated N-terminal tryptic peptide was detected. In contrast, the chymotryptic peptide mass maps displayed peptides from the central and C-terminal domain of Gαo1 and Gαo3 (italicized in Fig. 2). A close inspection of the MALDI peptide mass maps revealed that the only significant difference was a mass increase of +0.95 Da of a peptide signal at m/z 1978.07 in Gαo1 to m/z 1979.02 in Gαo3 (Fig. 3 A and B). This peptide mass was assigned as the chymotryptic peptide DAVTDIIIANNLRGCGLY (predicted m/z 1978.01) generated from the extreme C terminus of Gαo1 by cleavage after 336Phe.
Figure 2.
Amino acid sequence of Gαo1 and Gαo3 covered by tryptic peptides (underlined) and chymotryptic peptides (italicized) assigned by MALDI peptide mass mapping or by MALDI analysis of HPLC fractions.
Figure 3.
Identification of the modified amino acid residue by MS. Part of MALDI MS peptide mass map obtained after chymotryptic digestion of Gαo1 (A) and Gαo3 (B). Peptides at m/z 1978.074 (Gαo1) and 1979.021 (Gαo3) were assigned as the normal and modified octadeca C-terminal peptide, respectively. Signals at m/z 1993.0 are of unknown origin but appear identical in both MALDI MS peptide mass maps. (C) Nanoelectrospray tandem mass spectrum obtained from the HPLC-isolated chymotryptic peptide from the Gαo3 isoform. Tandem MS of the triply charged peptide ions generated C-terminal (y type) and N-terminal (b type) peptide fragment ions. All y-type fragment ions containing amino acid residue 346 of Gαo3 displayed a mass shift of +1 Da as compared with Gαo1 (Inset). This finding identifies the modification as an Asn to Asp conversion in position 346, consistent with the amino acid sequence DAVTDIIIADNLRGCGLY where C is S-carbamidomethylcysteine.
To analyze the structural differences of the respective Gαo1 and Gαo3 chymotryptic peptides, we used nanoelectrospray tandem MS for peptide sequencing. It was not possible to detect the C-terminal Gαo3 peptide by direct nanoelectrospray mass analysis of the peptide mixture. Instead, the chymotryptic peptides were separated by narrow-bore reverse-phase HPLC followed by off-line analysis by automated MALDI MS to identify the fractions containing the native and modified C-terminal peptide. The m/z 1978 peptide was detected in a minor fraction eluting at 87.1 min (Fig. 3A) whereas the m/z 1979 peptide from Gαo3 was detected in a fraction eluting at 87.7 min (Fig. 3B). Isolated peptides from Gαo1 and Gαo3 were sequenced by nanoelectrospray tandem MS. In the Gαo1-derived peptide, a series of y-ion signals corresponding to cleavage of amide bonds confirmed that this chymotryptic peptide originated from the C terminus of Gαo with the predicted sequence (Fig. 3C, Inset). In the Gαo3-derived peptide, a mass shift of +1 Da was observed for all peptide fragment ions containing the amino acid in position 346, thereby localizing and identifying the modification as 346Asn→Asp (Fig. 3C). The finding that Gαo3 had an additional anionic residue was consistent with a difference in HPLC elution time between the two peptides (see above). A mass difference of +1 Da was observed exclusively in two distinct C-terminal peptide pairs of which one was obtained after cleavage with trypsin and one after digestion with chymotrypsin. Correspondingly, results from peptide mass mapping and determination of the total masses of Gαo1 and Gαo3 made additional variations between the two Gα isoforms unlikely.
Generation of Gαo3.
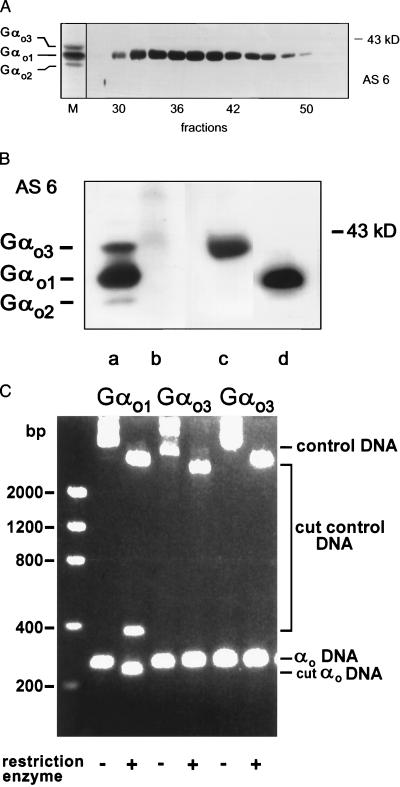
Thus far, we have shown that Gαo3 differs from Gαo1 by conversion of 346Asn→Asp. This amino acid exchange might have resulted from an artificial deamidation of Gαo1 during purification and analysis of the proteins. To address such an instability of the γ-amide bond, we rechromatographed isolated Gαo1 devoid of Gαo3 on a Mono Q column by using the identical conditions as for purification of Gαo3. As seen in Fig. 4A only a single band was visible on immunoblots of the eluted fractions, excluding a purification artifact. Although a mass difference of +1 Da was seen exclusively in two distinct C-terminal peptide pairs after cleavage with different enzymes, this observation does not exclude the presence of an additional variation between Gαo1 and Gαo3. However, peptide mass mapping covered the entire sequence with the exception of two amino acids for both isoforms (Fig. 2). In addition we could exclude major differences in molecular weight by nanoelectrospray MS analysis of intact proteins of Gαo1 and Gαo3, which showed similar total masses for the two isoforms. To test the effect of the observed amino acid exchange we replaced the coding triplet 346 (AAC) of the murine cDNA of Gαo1 by GAC using site-directed mutagenesis. This replacement would result in translation of Asp instead of Asn at position 346. Gαo1- and Gαo3-encoding cDNAs were used to generate recombinant baculoviruses. After infection with these viruses, Sf9 cells overexpressed Gαo-immunoreactive proteins that showed electrophoretic properties identical to their respective native murine counterparts (Fig. 4B). To confirm the presence of 346Asp instead of Asn in recombinant Gαo3 we subjected the purified protein to digestion by chymotrypsin and endoprotease Asp-N, and monitored these reactions by MS. Inspection of the MALDI peptide mass maps after chymotrypsin treatment of recombinant Gαo3 revealed a peptide signal at m/z 1979.0 instead of m/z 1978. As for native Gαo3 this peptide mass was assigned as the chymotryptic peptide DAVTDIIIADNLRGCGLY generated from the C terminus of recombinant Gαo3. We also confirmed that endoprotease Asp-N cleaved between 345Ala and 346Asp of recombinant Gαo3 to generate a peptide signal at m/z 1067.6 corresponding to the C-terminal peptide 346DNLRGCGLY (data not shown). Hence, an exchange of a single amino acid, i.e., 346Asn→Asp, by site-directed mutagenesis resulted in expression of a recombinant protein with gel electrophoretic properties similar to native Gαo3 but distinct from both native and recombinant Gαo1 (Fig. 4B).
Figure 4.
(A) Immunoblot of fractions containing Gαo1 but not Gαo3 collected after rechromatography of purified Gαo1 using antiserum AS 6. The migration pattern of a purified mixture of three Gαo isoforms is shown on the left (M). (B) AS 6-stained immunoblot showing the mobilities of native murine brain Gαo isoforms (lane a) in comparison to recombinant murine Gαo1 (lane d) and Gαo3 (lane c). Lane b shows the absence of proteins after wild-type baculovirus infection of Sf9 cells. Apparent molecular mass of a marker protein is indicated. (C) Cleavage of Gαo-specific PCR products and control DNA by restriction enzymes. Total mRNA from rat brains was amplified by reverse transcription–PCR. While the forward primer was identical in all three experiments, reverse primers differed to generate additional restriction cleavage sites for AclI in the case of Gαo1, or AatII and EcoRV for the two possible Gαo3-specific sequences. The presence of cleavage sites generated by primer extension in PCR cycles was verified by a restriction digest with the appropriate enzymes. The amplified cDNAs and resulting cleavage products were separated on agarose gels and visualized by ethidium bromide staining. In the case of Gαo1 the intact PCR product (−; αo DNA) was 260 bp in length, whereas the cleaved product (+; cut αo DNA) had a length of 233 bp. The internal controls (control DNA) added were pcDNA3 for PCR products obtained with primers 1 and 3 and pQE60 in the experiment using primer 2. Note that cleavage of pcDNA3 by AclI resulted in an additional product of 373 bp (third lane from left). Positions of DNA standards are shown on the left.
Having confirmed the sequence of a major G protein in brain we sought to identify the basis for its generation. Previous analysis of the genomic sequences has revealed the existence of transcripts encoding only Gαo1 and Gαo2 (39, 46). Disruption of this gene in mice resulted in complete loss of Gαo-specific immunoreactivity in homozygous knockout animals (18, 19). Therefore, the genetic information for Gαo3 must be located on this Gαo gene. Accordingly, we performed reverse transcription–PCR on total mRNA obtained from rat brain. Guided by the sequence of Gαo1 we generated cDNAs encoding C-terminal regions of Gαo1 and Gαo3 to identify a Gαo3-specific transcript. However, cloning and sequencing of 101 of these constructs yielded only nucleotide sequences coding for the C terminus of Gαo1 but failed to detect any Gαo3-coding cDNA. In a second independent and complementary approach Gαo1- and Gαo3-specific primers were used to generate additional restriction cleavage sites when extended by Gαo1 and Gαo3 sequences. Because of a degeneration for the Asp code two alternative triplets had to be considered for Gαo3. Analysis of the cleavage sites formed in the PCR products again showed no Gαo3-specific cDNA (Fig. 4C). Only the Gαo1-specific product was cleaved whereas both Gαo3-specific products remained unaffected. In contrast, plasmid DNA supplied in the same tube as the PCR product was cut by the respective enzymes. Therefore, processes such as allelic variations, alternative splicing, or mRNA editing are unlikely to generate Gαo3 at the transcriptional level.
Functional Consequences of Gαo1 Deamidation.
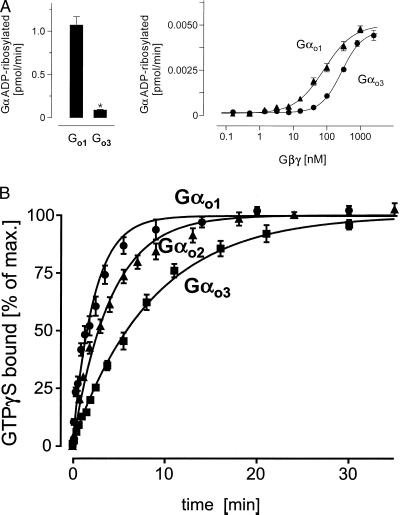
The 346Asn→Asp exchange observed between Gαo1 and Gαo3 alters the charge of the C terminus. C-terminal regions of Gα were found important for receptor and effector coupling (3). Therefore deamidation of Gαo1 may affect biochemical and cellular signaling properties of this isoform. It has been observed that infusion of Gαo1 and Gαo2, but not Gαo3, restored receptor-mediated calcium channel inhibition in cultured cells after stimulation with various agonists (32, 33). Alterations in the C terminus also could affect the sensitivity of Gα toward PT, as PT ADP-ribosylates 351Cys close to position 346. We previously have noticed that under standard conditions Go3 appeared to be less well 32P-ADP-ribosylated by PT than Go1 (32). Therefore, we studied initial rates of PT-mediated ADP ribosylation of Gαo1 and Gαo3 (Fig. 5A). Short-term incubation revealed significant differences in the velocity of ADP ribosylation between Gαo1 and Gαo3 (Fig. 5A, Left) whereas no differences in maximal incorporation of 32P-ADP into Gαo1 and Gαo3 were observed after prolonged incubation of Gα in the presence of equimolar amounts of Gβγ complexes (data not shown). Because we observed that the three purified Go proteins differed in their Gβ composition (Fig. 1), we could not exclude that the difference in velocity of ADP ribosylation was caused by a different interaction of Gαo1 and Gαo3 with Gβγ dimers. Thus, differences in PT-mediated 32P-ADP ribosylation could be secondary to αβγ heterotrimer formation rather than representing an immediate consequence of the different C termini. Hence, we carried out a series of experiments in which Gαo1 and Gαo3 were incubated with increasing concentrations of Gβγ complexes. In the presence of excess Gβγ, we observed similar maximum rates of 32P-ADP ribosylation between Gαo1 and Gαo3 (Fig. 5A, Right). Thus, increasing the Gβγ concentration abolished differences in reaction velocity, indicating that Gαo1 and Gαo3 have different affinities to Gβγ. Accordingly, the EC50 values were 60 nM and 280 nM Gβγ for Gαo1 and Gαo3, respectively. Therefore, we speculate that the different rates of PT-mediated ADP ribosylation observed between Gαo1 and Gαo3 are the result of differences in affinity to Gβγ. Evidence from cross-linking studies suggest an interaction of the C terminus of Gα with Gγ (47).
Figure 5.
(A) PT-mediated ADP ribosylation. Gαo isoforms (350 nM, Left; 4 nM, Right) were ADP-ribosylated in the presence of stoichiometric (Left) or increasing concentrations (Right) of Gβγ complexes using PT. The reactions were stopped by addition of an equal volume of 2× concentrated electrophoresis sample buffer and subjected to urea-SDS/PAGE. For quantification of 32P-incorporation dilutions of the reaction mixture were spotted on nitrocellulose membranes and dried. Gel slabs and membranes were autoradiographed and analyzed by using a PhosphorImager. Data shown are mean values ± SD (n = 3) from one typical experiment of three (∗, P < 0.01). (B) Time course of 35S-GTPγS binding to three purified Gαo isoforms. The appropriate Gα was added to the reaction mixture in a final concentration of 3–5 nM in the presence of 1 mM EDTA at 25°C. The binding reaction was carried out at 50 nM 35S-GTPγS, yielding a total binding of 110,000 to 180,000 cpm. The reaction was stopped at the indicated time points by diluting samples with ice-cold buffer followed by filtration through nitrocellulose. Filters were washed and counted in a liquid scintillator counter. Nonspecific binding was less than 5% of the total (3,600 to 7,800 cpm). Shown are mean values ± SD (n = 3) from one typical experiment of three.
It is well established that changes in the C-terminal sequence affect the specificity of G protein-receptor coupling and activation kinetics (48). In the activated GTPγS-bound state, the extreme C terminus has been found to contact the switch II region of Gα (49). We therefore compared the activation reactions of the Gαo isoforms. Previously, it was reported that heterotrimeric Go1 and Go2 differed in their GTPγS binding rates (41). We confirmed these results (not shown) and extended them by testing Gα monomers of all three Gαo isoforms (Fig. 5B). Gαo1 and Gαo2 still exhibited a faster GTPγS binding than Gαo2, whereas Gαo3 bound GTPγS with the slowest rate. This observation is in agreement with suggestions that the C terminus of Gα is functionally involved in conformational changes of Gα during its activation on GTP binding. The C terminus contains the α5 helix, which is partially unwound during activation (50, 51) and has been proposed to function as an internal GDP dissociation inhibitor (52). Interestingly, 346Asn, as the last residue of the α5 helix in the inactive GDP-bound state, contributes to the stability of the helix. Because Asn, but not Asp, is known as a favored amino acid for this position, the substitution by a carboxylate for the amide is likely to alter the stability of this α5 helix, affecting the GDP/GTP-exchange reaction (53). In this context it was interesting to notice significant differences in the time course of GTPγS binding between Gαo isoforms (Fig. 5B). Our findings support the idea that closely related G protein isoforms display significant differences in the signal transduction cascade caused by differences in activation kinetics.
The 346Asn→Asp exchange changes the physicochemical and functional properties of Gαo1, increasing diversity in Go-dependent cellular signaling. Moreover, we obtained preliminary evidence that deamidation is not restricted to Gαo1. In this context, the observation that Escherichia coli cytotoxic necrotizing factor-1 deamidates 63Gln of Rho proteins, yielding a constitutively active Rho, may be of relevance (54, 55). Our data suggest that mammals also express deamidases, which convert Gαo1 to Gαo3. This hypothesis is supported by the observation that cell lines generate Gαo1 and Gαo3 in varying relative amounts. For instance, HIT cells express Gαo1 and Gαo3 in roughly equal amounts whereas RIN or GH3 cells express only Gαo1 (16, 33). Moreover, although the relative concentrations of all Gαo isoforms have been found to be roughly constant among total brain membrane preparations of various species (16), more detailed analysis has shown that relative expression levels of Gαo1/Gαo3 varied considerably among cells or in distinct regions of the brain (28–30). Furthermore, the Gαo1/Gαo3-concentration ratios changed on development of Alzheimer’s disease, suggesting different functional control of Gαo isoforms (K. Kolasa, personal communication). The reported differences in functional properties of Gαo3 encourage further studies to establish deamidation as an additional regulatory mechanism of G protein function.
Acknowledgments
We thank Antje Tomschegg for superb technical assistance. We are indebted to Drs. David Garbers (Dallas), Doris Koesling, and Güutes Schultz (Berlin) for helpful discussions, and Drs. Alfred G. Gilman (Dallas), Peter Gierschik (Ulm), and Alfred Wittinghofer (Dortmund) for critical reading of an earlier version of the manuscript. We are grateful to Dr. Matthias Wilm (European Molecular Biology Laboratory) for expert assistance with nanoelectrospray tandem MS. O.N.J. was supported by a postdoctoral fellowship from the European Union Biotechnology Program. This work was supported by Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- GTPγS
guanosine 5′-[γ-thio]triphosphate
- MALDI
matrix-assisted laser desorption/ionization
- MS
mass spectrometry
- PT
pertussis toxin
References
- 1.Gilman A G. Biosci Rep. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- 2.Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 3.Hamm H E. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 4.Simon M I, Strathmann M P, Gautam N. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 5.Ui M. Trends Pharmacol Sci. 1984;5:277–279. [Google Scholar]
- 6.Moss J, Vaughan M. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorf W P, Pitcher J A, Luttrell D K, Linder M E, Kurose H, Parsons S J, Caron M G, Lefkowitz R J. Proc Natl Acad Sci USA. 1992;89:5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wedegaertner P B, Wilson P T, Bourne H R. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F L, Casey P J. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 10.Neer E J, Lok J M, Wolf L G. J Biol Chem. 1984;259:14222–14229. [PubMed] [Google Scholar]
- 11.Sternweis P C, Robishaw J D. J Biol Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- 12.Strittmatter S M, Valenzuela D, Kennedy T E, Neer E J, Fishman M C. Nature (London) 1990;266:836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- 13.Strathmann M, Wilkie T M, Simon M I. Proc Natl Acad Sci USA. 1990;87:6477–6481. doi: 10.1073/pnas.87.17.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand P, Sanford J, Rudolph U, Codina J, Birnbaumer L. J Biol Chem. 1990;265:18576–18580. [PubMed] [Google Scholar]
- 15.Hsu W H, Rudolph U, Sanford J, Bertrand P, Olate J, Nelson C, Moss L G, Boyd A E, Codina J, Birnbaumer L. J Biol Chem. 1990;265:11220–11226. [PubMed] [Google Scholar]
- 16.Spicher K, Nürnberg B, Jäger B, Rosenthal W, Schultz G. FEBS Lett. 1992;307:215–218. doi: 10.1016/0014-5793(92)80770-h. [DOI] [PubMed] [Google Scholar]
- 17.Iñiguez-Lluhi J, Kleuss C, Gilman A G. Trends Cell Biol. 1993;3:230–236. doi: 10.1016/0962-8924(93)90122-h. [DOI] [PubMed] [Google Scholar]
- 18.Valenzuela D, Han X, Mende U, Fankhauser C, Mashimo H, Huang P, Pfeffer J, Neer E J, Fishman M C. Proc Natl Acad Sci USA. 1997;94:1727–1732. doi: 10.1073/pnas.94.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang M, Gold M S, Boulay G, Spicher K, Peyton M, Brabet P, Srinivasan Y, Rudolph U, Ellison G, Birnbaumer L. Proc Natl Acad Sci USA. 1998;95:3269–3274. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diversé-Pierluissi M, Goldsmith P K, Dunlap K. Neuron. 1995;14:191–200. doi: 10.1016/0896-6273(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 21.Herlitze S, Garcia D E, Mackie K, Hille B, Scheuer T, Catterall W A. Nature (London) 1996;380:258–262. doi: 10.1038/380258a0. , and correction (1996) 381, 172. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S R. Nature (London) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 23.DeWaard M, Liu H, Walker D, Scott V E S, Gurnett C A, Campbell K P. Nature (London) 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 24.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nürnberg B, Ahnert-Hilger G. FEBS Lett. 1996;389:61–65. doi: 10.1016/0014-5793(96)00584-4. [DOI] [PubMed] [Google Scholar]
- 26.Ahnert-Hilger G, Nürnberg B, Exner T, Schäfer T, Jahn R. EMBO J. 1998;17:406–413. doi: 10.1093/emboj/17.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi I, Shibasaki H, Takahashi K, Kikkawa S, Ui M, Katada T. FEBS Lett. 1989;257:177–180. doi: 10.1016/0014-5793(89)81815-0. [DOI] [PubMed] [Google Scholar]
- 28.Granneman J G, Kapatos G. J Neurochem. 1990;54:1995–2001. doi: 10.1111/j.1471-4159.1990.tb04903.x. [DOI] [PubMed] [Google Scholar]
- 29.Mullaney I, Milligan G. J Neurochem. 1990;55:1890–1898. doi: 10.1111/j.1471-4159.1990.tb05773.x. [DOI] [PubMed] [Google Scholar]
- 30.Seaquist E, Neal A R, Shoger K D, Walseth T F, Robertson R P. Diabetes. 1992;41:1390–1399. doi: 10.2337/diab.41.11.1390. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox M D, Dingus J, Balcueva E A, McIntire W E, Mehta N D, Schey K L, Robishaw J D, Hildebrandt J D. J Biol Chem. 1995;270:4189–4192. doi: 10.1074/jbc.270.9.4189. [DOI] [PubMed] [Google Scholar]
- 32.Nürnberg B, Spicher K, Harhammer R, Bosserhoff A, Frank R, Hilz H, Schultz G. Biochem J. 1994;300:387–394. doi: 10.1042/bj3000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degtiar V, Harhammer R, Nürnberg B. J Physiol. 1997;502:321–333. doi: 10.1111/j.1469-7793.1997.321bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inanobe A, Shibasaki H, Takahashi K, Kobayashi I, Tomita U, Ui M, Katada T. FEBS Lett. 1990;263:369–372. doi: 10.1016/0014-5793(90)81416-l. [DOI] [PubMed] [Google Scholar]
- 35.Shibasaki H, Kozasa T, Takahashi K, Inanobe A, Kaziro Y, Ui M, Katada T. FEBS Lett. 1991;285:268–270. doi: 10.1016/0014-5793(91)80814-j. [DOI] [PubMed] [Google Scholar]
- 36.Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, Fotsis T, Mann M. Nature (London) 1996;379:466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 37.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. J Biol Chem. 1998;273:7024–7029. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 38.Jensen O N, Shevchenko A, Mann M. In: Protein Analysis by Mass Spectrometry. Creighton T E, editor. Oxford: IRL; 1997a. pp. 29–57. [Google Scholar]
- 39.Tsukamoto T, Toyama R, Itoh H, Kozasa T, Matsuoka M, Kaziro Y. Proc Natl Acad Sci USA. 1991;88:2974–2978. doi: 10.1073/pnas.88.8.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozasa T, Gilman A G. J Biol Chem. 1995;270:1734–1741. doi: 10.1074/jbc.270.4.1734. [DOI] [PubMed] [Google Scholar]
- 41.Padrell E, Carty D J, Moriarty T M, Hildebrandt J D, Landau E M, Iyengar R. J Biol Chem. 1991;259:13806–13813. [PubMed] [Google Scholar]
- 42.Graf R, Mattera R, Codina J, Evans T, Ho Y K, Estes M K, Birnbaumer L. Eur J Biochem. 1992;210:609–619. doi: 10.1111/j.1432-1033.1992.tb17461.x. [DOI] [PubMed] [Google Scholar]
- 43.Goldsmith P, Backlund P S, Rossiter K, Carter A, Milligan G, Unson C G, Spiegel A. Biochemistry. 1988;27:7085–7090. doi: 10.1021/bi00418a062. [DOI] [PubMed] [Google Scholar]
- 44.Lang J. Eur J Biochem. 1989;183:687–692. doi: 10.1111/j.1432-1033.1989.tb21099.x. [DOI] [PubMed] [Google Scholar]
- 45.Codina J, Carty D J, Birnbaumer L, Iyengar R. Methods Enzymol. 1991;195:177–188. doi: 10.1016/0076-6879(91)95164-f. [DOI] [PubMed] [Google Scholar]
- 46.Murtagh J J, Eddy R, Shows T B, Moss J, Vaughan M. Mol Cell Biol. 1991;11:1146–1155. doi: 10.1128/mcb.11.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaillancourt R R, Dhanasekaran N, Johnson G L, Ruoho A E. Proc Natl Acad Sci USA. 1990;87:3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iiri T, Farfel Z, Bourne H R. Nature (London) 1998;394:35–38. doi: 10.1038/27831. [DOI] [PubMed] [Google Scholar]
- 49.Sprang S R. Annu Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 50.Kisselev O G, Kao J, Ponder J W, Fann Y C, Gautam N, Marshall G R. Proc Natl Acad Sci USA. 1998;95:4270–4275. doi: 10.1073/pnas.95.8.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka T, Kohno T, Kinoshita S, Mukai H, Itoh H, Ohya M, Miyazawa T, Higashijima T, Wakamatsu K. J Biol Chem. 1998;273:3247–3252. doi: 10.1074/jbc.273.6.3247. [DOI] [PubMed] [Google Scholar]
- 52.Okamoto T, Murayama Y, Strittmatter S M, Katada T, Asano S, Ogata E, Nishimoto I. J Biol Chem. 1994;269:13756–13759. [PubMed] [Google Scholar]
- 53.Richardson J S, Richardson J D. Science. 1988;240:1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- 54.Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Nature (London) 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt G, Sehr P, Wilm M, Selzer J, Mann M, Aktories K. Nature (London) 1997;387:725–729. doi: 10.1038/42735. [DOI] [PubMed] [Google Scholar]
- 56.Anis, Y., Nürnberg, B., Reiss, N., Naor, Z., Visochek, L. & Cohen-Armon, M. (1999) J. Biol. Chem., in press. [DOI] [PubMed]