Abstract
Because DNA damage-inducible cell cycle checkpoints are thought to protect cells from the lethal effects of ionizing radiation, a better understanding of the mechanistic functions of cell cycle regulatory proteins may reveal new molecular targets for cancer therapy. The two major regulatory proteins of G2 arrest are Chk1 and p53. Yet, it is unclear how these two proteins interact and coordinate their functional roles during radiationinduced G2 arrest. To determine Chk1's role in p53-dependent G2 arrest, we used p53 proficient cells and examined expression of G2 arrest proteins under conditions in which G2 arrest was inhibited by the staurosporine analog, UCN-01. We found that UCN-01 inhibited both G1 and G2 arrest in irradiated p53 proficient cells. The arrest inhibition was associated with suppression of radiation-induced expression of both p21 and 14-3-3σ — two known p53-dependent G2 arrest proteins. The suppression occurred despite normal induction of p53 and normal phosphorylation of p53 at S20 and Cdc25C at S216 — the two known substrates of Chk1 kinase activity. In contrast, we showed that radiation-induced phosphorylation of Chk1 at S345 was associated with binding of Chk1 to p53, p21, and 14-3-3σ, and that UCN-01 inhibited S345 phosphorylation. We suggest that DNA damage-induced phosphorylation of Chk1 at S345, and subsequent p53 binding, links Chk1 with p53 downstream responses and may provide a coordinated interaction between DNA damage responses and cell cycle arrest functions.
Keywords: Chk1, p53, radiation, cell cycle arrest, UCN-01
Introduction
DNA damage-inducible cell cycle checkpoints are thought to play an important role in protecting cells from the lethal effects of ionizing radiation. This suggests that cell cycle regulatory proteins may be important therapeutic targets for sensitizing tumors to radiotherapy. The G1 checkpoint is the best-characterized checkpoint, but the G2 checkpoint is probably more important to cellular radioresistance. This is probably because premature entry into mitosis, without allowing time for repair of DNA damage, results in chromosome abnormalities that are lethal to cells [1]. A better understanding of the regulatory mechanisms of radiation-induced G2 arrest is needed to explore the full potential of targeting G2 arrest to enhance radiotherapy.
The human protein kinase, Chk1, is thought to play a central role in G2 arrest [2–6]. It has been proposed that its major mechanism is through phosphorylation of Cdc25C on S216; thereby marking Cdc25C for nuclear export and binding to 14-3-3 proteins. Sequestered outside the nucleus, Cdc25C cannot dephosphorylate and activate intranuclear Cdc2, the major initiation protein of mitosis [2,7–12]. Thus, Chk1's phosphorylation of Cdc25C promotes cell cycle arrest in G2 phase. Radiation-induced DNA damage usually initiates a strong cell cycle arrest in G2; however, it has been difficult to show that Chk1 kinase activity is increased by radiation [13,14].
Another protein thought to play a major role in cell cycle arrest is the transcriptional activator, p53. Unlike Chk1, however, p53 is well known to be activated by DNA damage. DNA damage increases the half-life of p53, and increases the expression of its downstream effector proteins, including p21 and 14-3-3σ. p21 is a well-characterized inhibitor of cyclin-dependent kinases and it is not only a potent inhibitor of G1 arrest, but also plays an important role in G2 arrest [15,16]. 14-3-3σ is the σ isoform of the 14-3-3 protein family — a family of binding proteins that has been implicated in a variety of intracellular regulatory functions, including cell cycle arrest [17]. p53's function in G2 arrest appears to be mediated primarily through the cooperative effects of p21 and 14-3-3σ. Cells deficient in expression of both p21 and 14-3-3σ, although proficient in p53, have defective G2 arrest and are highly sensitive to DNA-damaging agents [16].
An unanswered question is whether the Chk1 and p53 G2 arrest pathways work independently or communicate with each other in some way. Direct interaction with p53 is a possible means by which Chk1 might communicate with DNA damage response pathways and, thus, link Chk1's cell cycle effects to DNA damage-induced signal transduction. In fact, S20 of p53 is known to be one of the substrates for Chk1 kinase activity; however, it is not clear whether Chk1 regulates p53-dependent checkpoints through phosphorylating p53 at S20 in vivo. Furthermore, Chk1 has been shown to be phosphorylated in vivo in response to ionizing radiation; however, it is unclear what the functional consequences of Chk1 phosphorylation might be. One possibility is that phosphorylation alters Chk1's association with other proteins, similar to how Cdc25C's phosphorylation causes it to bind to 14-3-3 proteins. Therefore, we speculated that Chk1 phosphorylation, rather than just its kinase activity, might play an important role in communicating DNA damage response to downstream checkpoint proteins, particularly those in the p53 radiation response pathway.
To determine Chk1's role in p53-dependent cell cycle arrest, we used p53 proficient HCT116 human colon cancer cell lines, because they had previously been characterized for their normal G1 and G2 arrest phenotypes [18,19]. We examined expression of cell cycle checkpoint proteins in these cells under conditions in which radiation-induced cell cycle arrest was inhibited by the staurosporine analog, UCN-01. We found that UCN-01 inhibited both G1 and G2 arrest in irradiated cells. The arrest inhibition was associated with suppression of radiation-induced expression of both p21 and 14-3-3σ. The suppression occurred despite normal induction of p53, and normal phosphorylation of p53 at S20 and Cdc25C at S216. In contrast, we showed that radiation-induced phosphorylation of Chk1 at S345 promoted binding of Chk1 to p53, p21, and 14-3-3σ, and that UCN-01 inhibited S345 phosphorylation. We suggest that DNA damage-induced phosphorylation of Chk1 at S345, and subsequent p53 binding, links Chk1 with p53 downstream responses and may provide a coordinated interaction between DNA damage responses and cell cycle arrest functions.
Materials and Methods
Cell Lines and Cell Culture
Wild-type HCT116 human colon carcinoma cells (HCT116/p53+/+), which express normal p53, p21, and 14-3-3σ [19,20] and two derivatives, in which either p53 [15] or 14-3-3σ [19] genes had been deleted at both of their alleles through homologous recombination, were kindly provided by Dr. Bert Vogelstein (Howard Hughes Medical Institute, Johns Hopkins Oncology Center, Baltimore, MD). The cell lines were maintained in monolayer culture in McCoy's 5A modified medium supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 µg/ml).
Chemicals and Irradiation
UCN-01 (7-hydroxystaurosporine) was kindly provided by Dr. Robert J. Schultz of the Drug Synthesis and Chemistry Branch, National Cancer Institute, and was dissolved in dimethyl sulfoxide (DMSO) and stored at -20°C. All cell irradiation were performed using a J. L. Shepherd Mark I 137Cs irradiator (San Fernando, CA) at 1.5 to 3.0 Gy/min.
Cell Cycle Analysis
Cell cycle analysis, at 24 hours postirradiation, was performed as described previously [21,22]. Briefly, cells were treated with or without 100 nM of UCN-01 for 24 hours and irradiated at 10 Gy. Cells were then harvested 24 hours postirradiation and suspended as single cells in citrate/DMSO buffer [250 nM sucrose, 40 mM trisodium citrite, 5% DMSO (pH 7.6)]. Cells were stained with propidium iodide and analyzed by flow cytometry using FACSort (Becton Dickinson, Franklin Lakes, NJ). The cell cycle profiles were analyzed by Modfit software (Verity Software House).
Cell cycle analysis, at 6 hours postirradiation, was performed with a mitosis-specific flow cytometry assay, previously described by Juan and coworkers [23], which uses phosphorylation-specific antibody against H3 histones that are phosphorylated only during mitosis. Briefly, the cells were washed with phosphate-buffered saline (PBS) and fixed in suspension at a concentration of (4 to 6)x106 cells/ml in 2.0 ml of 1% formaldehyde in PBS for 15 minutes. The cells were then washed with PBS and resuspended in ice-cold 80% ethanol for up to 24 hours. After fixation, the cells were washed twice with PBS and then suspended in 1 ml of 0.25% Triton X-100 in PBS on ice for 5 minutes. After centrifugation, the cell pellet was suspended in 100 µl PBS containing 0.5 µg of anti-H3-p polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) and 1% bovine serum albumin (BSA) and incubated for 2 hours at room temperature. The cells were rinsed with PBS containing 1% BSA, then incubated with FITC-conjugated anti-rabbit antibody for 30 minutes in the dark. The cells were washed again and then resuspended in 5 µg/ml of propidium iodide (PI; Molecular Probes, Eugene, OR) and 0.1% RNase A (Sigma, St. Louis, MO) in PBS, and incubated at room temperature for 20 minutes before measurement.
Western Blot, Antibodies, Immunoprecipitation and Phosphorylation Assay
Cells were washed twice with ice-cold PBS and lysed with a Dounce homogenizer in lysis/wash buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P40, 0.5% sodium dexycholate, 1.0 tablet/25 ml of Complete® protease inhibitor cocktail (Boehringer Mannheim, Indianapolis, IN)]. Homogenized suspensions were centrifuged at 12,000g for 10 minutes to remove debris. The amounts of cell extracts used were adjusted for protein level by Western blot analysis as described previously [22], using the following antibodies: anti-p53 monoclonal antibody DO1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-p21 monoclonal antibody (WAF1) (Oncogene Research Products, Boston, MA), anti-14-3-3σ goat polyclonal antibody (N-14) (Santa Cruz Biotechnology), anti-Ku70 monoclonal antibody (N3H10) (NeoMarkers, Fremont, CA), anti-GAPDH polyclonal antibody (Trevigen, Gaithersburg, MD), anti-p53 monoclonal antibody DO7 (Oncogene Research Products), anti-Cdc25C monoclonal antibody TC113 (Oncogene Research Products), anti-Chk1 monoclonal antibody G4 (Santa Cruz Biotechnology). For immunoprecipitation, freshly prepared cell lysates were incubated for 2 hours at 4°C on a rotator with antibodies coupled to protein A Sepharose beads (20 µl) (Sigma). The beads were then washed three times with the lysis/wash buffer, and applied to SDS-PAGE.
Phosphorylation of p53 at S20 was assayed by immunoprecipitation as described previously [24,25], with S20p-p53, a polyclonal antibody (clone 430) obtained from New England Biolabs (Beverly, MA), which recognizes p53 phosphorylated on S20, followed by immunoblotting with DO7, which recognizes p53 irrespective of its phosphorylation state. Phosphorylation of p53 on S15 was assayed by immunoprecipitation with S15p-p53 antibody (Oncogene Research Products), a polyclonal antibody that recognizes p53 phosphorylated on S15, followed by immunoblotting with DO1. Immunoprecipitation of p53 was also performed using FL-393 polyclonal antibody (Santa Cruz Biotechnology), followed by detection with DO-1.
Phosphorylation of Cdc25C on S216 was assayed by immunoprecipitation with S216p-Cdc25C antibody (New England Biolabs), a polyclonal antibody that recognizes Cdc25C phosphorylated on S216, followed by immunoblotting with TC113 (Oncogene Research Products).
Phosphorylation of Chk1 on S345 was assayed by immunoprecipitation as described previously [5], with S345p-Chk1, a polyclonal antibody that recognizes Chk1 phosphorylated on S345, followed by immunoblotting with G4, a monoclonal antibody raised against full length of the Chk1 protein (Santa Cruz Biotechnology). Phosphorylation of Chk2 was assayed by measuring electrophoretic gel band shifts as described previously [26]. S345p-Chk1 and Chk2 antibodies were generously provided by Dr. Stephen J. Elledge (Howard Hughes Medical Institute, Baylor College of Medicine, Houston, TX).
Clonogenic Survival Assay
Cells were plated in T75 flasks and incubated approximately 16 hours. Cells were then treated with complete medium with or without UCN-01 at 100 nM for 24 hours. Cells were then irradiated with varying doses of gamma rays. After additional 24 hours incubation, cells were washed with PBS and collected following incubation with trypsin/EDTA. Various concentrations of cells were replated in drug-free medium in T-25 flasks, and then incubated for 12 days. Colonies were stained with crystal violet and counted. Colonies produced from the clones of surviving cells were counted. The fraction of surviving clonogenic cells was calculated by dividing the number of colonies by the number of cells seeded, and then normalizing the surviving fraction from irradiated cultures to the zero-dose surviving.
Results
Both G1 and G2 Checkpoints Were Abrogated in p53 Proficient Cells by UCN-01
The staurosporine analog, UCN-01, has been reported to be a potent inhibitor of the Chk1 pathway of G2 arrest [27–29]. We were interested in determining the effect of UCN-01 on p53-dependent cell cycle arrest functions. The HCT116 human colon cancer cell line was chosen for this study because wild-type cells have intact p53, p21, and 14-3-3σ genes and apparently normal p53-dependent checkpoint responses [18,19], whereas its p53-deficient isogenetic counterpart has been shown to fail to sustain both G1 and G2 arrest [15,30]. Hence, we used the wild-type HCT116 cells as a model to evaluate the effect of UCN-01 on radiation-induced p53-dependent cell cycle arrest.
We used two different flow cytometry methods to measure cell cycle arrest. To simultaneously assess G1 and G2 arrest we used a standard method of quantitating DNA histograms of asynchronous cell populations at 24 hours postirradiation, and measured changes in cell cycle distribution between G1, S, and G2/M [21]. In this assay, postirradiation S phase depletion provided a reliable index of G1 arrest, but the combined G2 and M phases obscured accurate measurement of G2 arrest. Therefore, we also used a highly sensitive M-phase-specific three-dimensional assay, which allowed us to measure G2 arrest as early as 6 hours postirradiation. This technique, originally described by Juan and coworkers [23], also uses cell number and DNA content as the first two dimensions; however, a third dimension uses antibody against histones phosphorylated during mitosis to distinguish M from G2 cells. We look for a drop in the number of cells in mitosis, caused by an arrest at the G2/M border, as an indication of G2 arrest. By 6 hours postirradiation, wild-type cells that were in mitosis at the time of irradiation have already transited into G1, and M phase has not yet been repopulated due to an arrest in G2. Thus, G2 arrest can be detected as a depletion of M phase cells at 6 hours. When G2 arrest is inhibited, cells continue to cycle through mitosis, and M phase is not depleted.
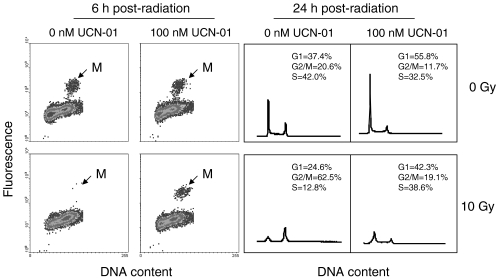
When wild-type HCT116 cells were irradiated with 10 Gy in the absence of UCN-01, it resulted in the expected G1 arrest at 24 hours, as shown by a depletion of S phase cells (S phase from 42.0% to 12.8%) (Figure 1). Additionally, a substantial G2 arrest was suggested, as shown by accumulation of cells in G2/M (G2/M phase from 20.6% to 62.5%). Treatment of the cells with UCN-01, however, inhibited both G1 arrest and G2 arrest, and the cell cycle distributions of the radiation/UCN-01-treated cells appeared very similar to untreated controls, indicating that UCN-01 inhibits both G1 and G2 arrest in p53 proficient cells.
Figure 1.
UCN-01 abrogates both G1 and G2 arrest in p53 proficient cells. Flow cytometric analysis of human HCT116 cells in the absence or presence of 100 nM of UCN-01 and in the absence or presence of 10 Gy, at 6 and 24 hours. G2 arrest at 6 hours was measured as a depletion in M phase cells by a three-dimensional flow cytometry technique, which distinguishes M phase cells based on both DNA content and staining with antibody to mitosis-specific phosphorylated histone H3 (see Materials and Methods section). G2 arrest at 24 hours was measured as an accumulation of cells in G2/M phase through conventional DNA histogram flow cytometry. Cell cycle distributions, shown in upper right of panel, were calculated from the histograms for each irradiation/drug condition.
The inhibition of G2 arrest by UCN-01 was confirmed with mitosis-specific flow cytometry (Figure 1). At 6 hours postirradiation without UCN-01 there was a complete loss of M phase cells due to G2 arrest. In the presence of UCN-01, however, mitotic cells were still apparent in irradiated cultures, indicating an inhibition of G2 arrest by the drug.
Downregulation of Radiation-Induced Increases in p21 and 14-3-3σ by UCN-01 is Dependent on p53 Status
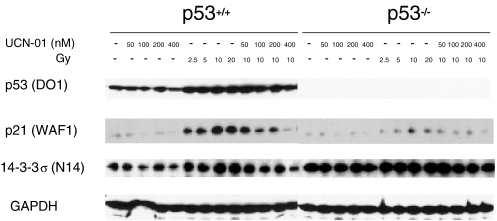
Because p21 and 14-3-3σ proteins are known to participate in G2 arrest [19,20,31], we next looked at the effect of UCN-01 on expression of those proteins. We found that UCN-01 significantly downregulated DNA damage-mediated p21 and 14-3-3σ induction in cells with wild-type p53 (Figure 2). The finding that UCN-01 inhibits p21 induction is consistent with its ability to inhibit G1 as well as G2 arrest. p21 is a strong inhibitor of cdk2, a major initiator of progression from G1 into S. Failure to induce p21 would be expected to abrogate G1 arrest, and our flow cytometry data supports this.
Figure 2.
UCN-01 downregulation of p21 and 14-3-3σ. UCN-01 downregulates radiation-mediated p21 and 14-3-3σ induction only in p53 proficient cells without affecting p53 protein levels, as shown by Western blot. Radiation induction of p21 and 14-3-3σ in the absence or presence of varying concentration of UCN-01 is indicated. Cells were treated with UCN-01 for 24 hours and then irradiated. Cells were then harvested 24 hours postirradiation. GAPDH was used as a control of loading.
The reduction in p21 and 14-3-3σ levels could not, however, be attributed to a mere reduction in p53 expression, because p53 remained significantly elevated in irradiated cells, even at UCN-01 concentrations that strongly inhibit p21 and 14-3-3σ (Figure 2). p53 induction was confirmed by Western blotting of whole-cell extracts with two different p53 antibodies (DO7 and DO1) (Figure 3, A and B), and by p53 immunoprecipitation (DO1) followed by p53 immunoblotting (FL-393) (data not shown). In contrast, when p53-/- cells were irradiated, induction of p21 and 14-3-3σ was largely absent, yet UCN-01 did not diminish their baseline levels (Figure 2), consistent with the idea that p21 and 14-3-3σ induction are p53 dependent, and that inhibition of their expression by UCN-01 is also dependent on p53 status. However, unlike for p21 that has a baseline similar to p53+/+ cells, 14-3-3σ baseline levels were much higher relative to that in p53+/+ cells (Figure 2). This overexpression of 14-3-3σ was itself not able to induce arrest, as these cells continued to cycle at rates similar to p53+/+ cells, consistent with the notion that 14-3-3σ helps sustain, rather than initiate, arrest [19].
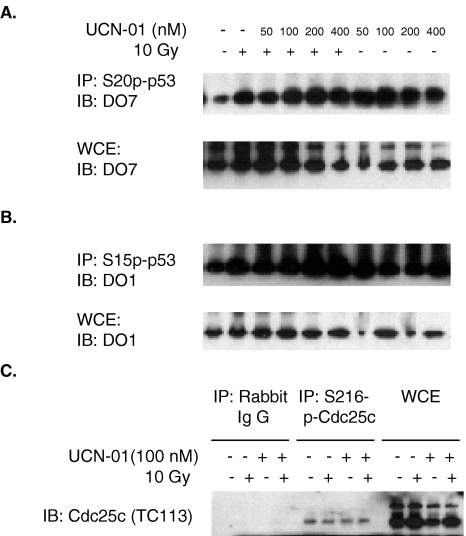
Figure 3.
Phosphorylation of p53 on S20 and S15 and phosphorylation of Cdc25C on S216 in HCT116/p53+/+ cells. After treatment with varying concentration of UCN-01 for 24 hours, cells were irradiated at 10 Gy and harvested 24 hours post-10 Gy for the whole-cell extract (WCE). (A) Phosphorylation of p53 on S20 in response to DNA damage and UCN-01 was performed by immunoprecipitation (IP) with antibody against S20p-p53 followed by an immunoblotting (IB) with p53 antibody, DO7. p53 protein levels were monitored by direct IB with DO7 without IP. (B) Phosphorylation of p53 on S15 in response to DNA damage and UCN-01 was performed by IP with antibody against S15p-p53 followed by an IB with p53 antibody, DO1. p53 protein levels were monitored by direct IB with DO1 without IP. (C) Phosphorylation of Cdc25C on S216 in response to DNA damage and UCN-01 was performed by IP with antibody against S216p-Cdc25C followed by an IB with Cdc25C antibody, TC113. Cdc25C protein levels were monitored by direct IB with TC113 without IP. Nonimmune rabbit serum was used as a control for the IP.
Phosphorylations of p53 on S20 and S15 are not Inhibited by UCN-01
The above results suggest that UCN-01 suppressed p53-dependent transactivation of p21 and 14-3-3σ by a mechanism that did not alter the intracellular levels of p53. Because Chk1 has been shown to phosphorylate p53 at S20 in vitro, and because UCN-01 has also been shown to inhibit Chk1 kinase activity on a synthetic substrate in vitro [28,29], inhibition of Chk1 phosphorylation of S20 was considered a possible in vivo mechanism by which UCN-01 might reduce the transactivation of p21 and 14-3-3σ.
In vivo phosphorylation of p53 on S20 was assayed by immunoprecipitation with an S20-p53 phosphorylation-specific antibody followed by immunoblotting with DO7, a monoclonal p53 antibody, as described previously [24,25]. In response to DNA damage, p53 was phosphorylated on S20, consistent with previous reports [24,25]. Phosphorylation of p53 on S20, however, was not inhibited in the presence of UCN-01 (Figure 3A). Actually, when adjusted for p53 protein level, an increase in phosphorylation was seen in the presence of high concentrations of UCN-01. At these concentrations, the checkpoints were completely abrogated, suggesting that phosphorylation of p53 on S20 was insufficient to induce G2 arrest.
ATM directly phosphorylates p53 on S15 in response to DNA damage [32,33]. Ataxia-telangiectasia cells, which are mutated in ATM, show defective G1 and G2 arrest accompanied by an attenuated p53 and p21 response [34,35], similar to the effects of UCN-01 on cell cycle arrest, leaving the possibility that ATM kinase activity was inhibited by UCN-01. Despite the previous report that UCN-01 does not inhibit ATM's PI-3 kinase activity on a peptide substrate in vitro [36], we investigated the possibility that UCN-01-mediated checkpoint abrogation and downregulation of p21 and 14-3-3σ in vivo might result from inhibiting phosphorylation of p53 on S15.
In response to DNA damage, p53 was phosphorylated on S15, consistent with previous reports [32,33]. However, as for S20, phosphorylation of p53 on S15 was not inhibited in the presence of UCN-01 (Figure 3B). In fact, a slight increase of phosphorylation was seen (Figure 3B). These results suggest that DNA damage-mediated phosphorylation of p53 at both S15 and S20 are normal in the presence of UCN-01, and that the pronounced reduction of p21 and 14-3-3σ, as well as the inhibition of cell cycle arrest could not be attributed to hypophosphorylation at either of these two sites.
Phosphorylation of Cdc25c on S216 is not Changed in Response to Either Radiation or UCN-01
Besides S20 on p53, the other known substrate for Chk1 is S216 on Cdc25C [2,8–10]. This pathway is the best characterized cell cycle checkpoint mechanism governing G2 arrest and has been the pathway most strongly implicated for UCN-01's effects on the cell cycle [28]. As mentioned above, phosphorylation of Chk1 is thought to activate Chk1 kinase activity, resulting in phosphorylation of S216 on the phosphatase Cdc25C, which causes its nuclear export and initiation of G2 arrest [2,7–12].
Although Chk1's role in this cell cycle checkpoint is well established, it has not been shown that this checkpoint is responsive to radiation, and Chk1 kinase activity does not appear to be activated by ionizing radiation [13,14]. Thus, the phosphorylation of Cdc25C seems to be uncoupled from the radiation response. Nevertheless, we sought to determine whether UCN-01 inhibited phosphorylation of Cdc25C on S216. To determine the status of S216, we used a phosphorylation-specific antibody against the S216 of Cdc25C. Our result shows that phosphorylation of Cdc25C on S216 was not changed in response to either radiation or UCN-01 (Figure 3C). Therefore, it is highly unlikely that phosphorylation of Cdc25C on S216 by Chk1 regulates radiation-induced G2 arrest.
Radiation-Induced Phosphorylation of Chk1 on S345 is p53-Independent and Inhibited by UCN-01
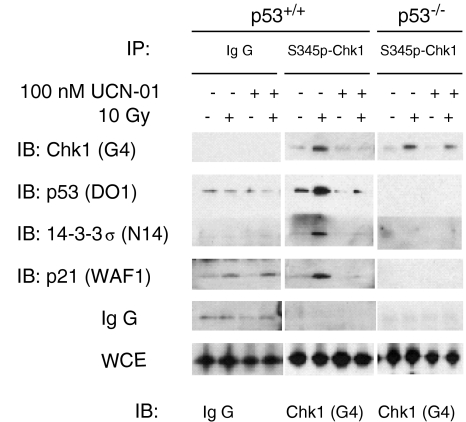
Chk1 is known to be phosphorylated in response to ionizing radiation at S345 in an ATR-regulated (and possibly ATM-regulated) manner [5]. Despite the lack of effect of UCN-01 on Chk1's kinase activity on known substrates, we wondered whether UCN-01 might inhibit phosphorylation of Chk1 itself at S345. To test this, phosphorylation of Chk1 on S345 was assayed by immunoprecipitation, with a phosphorylation-specific antibody raised against S345 of Chk1 in the absence and presence of UCN-01 in both p53+/+ and p53-/- cells. In response to radiation, Chk1 was phosphorylated on S345 regardless of p53 status (Figure 4), which was consistent with a previous report [5]. We also found that UCN-01 inhibited the radiation-induced S345 phosphorylation at drug doses comparable to those that inhibited cell cycle arrest.
Figure 4.
DNA damage-mediated phosphorylation of Chk1 at S345 is associated with p53-dependent checkpoint proteins. Phosphorylation of Chk1 on S345 in response to DNA damage and UCN-01 was measured by IP with antibody against S345p-Chk1 followed by an IB with Chk1 antibody (G4). The complexes of phosphorylated form of Chk1 with p53, 14-3-3σ, p21, and IgG were assayed by immunoblotting the membrane with anti-p53 monoclonal antibody (DO-1), anti-14-3-3σ goat polyclonal antibody (N-14), anti-p21 monoclonal antibody (WAF1), and anti-mouse IgG. The nonimmune serum was used as a control for the IP. Nonimmune serum did not precipitate Chk1, p53, 14-3-3σ or p21. (The amounts of extracts used for IPs were adjusted to have equal Chk1 levels in all lanes. This was confirmed by direct immunoblotting of whole-cell extracts with G4 anti-Chk1 and anti-mouse IgG antibodies, as shown in the lower bracketed section.)
We further probed the immunoprecipitation blots and found that there was a significant coimmunoprecipitation of p53 in irradiated wild-type cells, and the precipitates contained 14-3-3σ and p21 as well. This coprecipitation was inhibited by UCN-01, because UCN-01 inhibited Chk1 phosphorylation at S345 (Figure 4). Chk1 was also phosphorylated in p53-/- cells, yet coprecipitation of p21 and 14-3-3σ was not seen, even though baseline levels of these proteins (Figure 2) were sufficiently high to detect associations. These results suggest that S345 phosphorylated Chk1 physically associates with p53, and with downstream cell cycle arrest proteins, in a p53-dependent manner. Thus, protein-protein interactions may provide a link between Chk1 and p53 that is independent of Chk1's kinase activity.
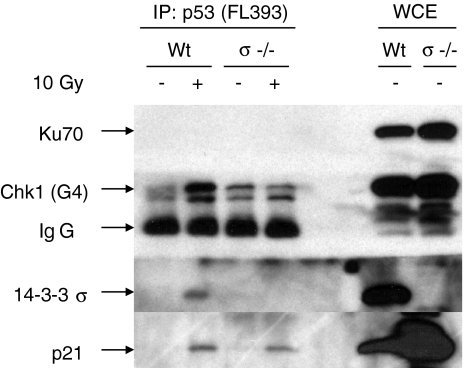
To confirm that there was a radiation-induced association of Chk1 with p53, a reciprocal immunoprecipitation was performed in which p53 antibody (FL393), rather than Chk1, was immunoprecipitated and Chk1, p21, and 14-3-3σ were probed for by Western blot (Figure 5). Once again, Chk1 showed a radiation-induced coprecipitation with p53, as well as 14-3-3σ and p21, supporting the previous observation that Chk1 and p53 physically associate with p53 following irradiation. In contrast, Ku70 — an abundant DNA repair protein for radiation-induced DNA strand breaks that has been reported to be upregulated in a p53/ATM-dependent fashion [37] — did not coprecipitate with p53.
Figure 5.
Chk1, 14-3-3σ, and p21 coprecipitates with p53 in wild-type cells following irradiation. Wild-type and 14-3-3σ knockout HCT 116 cells were irradiated to 10 Gy and extracts were immunoprecipitated with p53 antibody, FL393 (rabbit), followed with immunoblotting with mouse antibodies for Chk1 (G4), 14-3-3σ (N-14), p21 (WAF1), and Ku70 (N3H10). [Because p53 and Chk1 are of similar size and cannot be probed on the same blot, equivalent p53 precipitation was confirmed by Western analysis on a separate blot using p53 antibody, DO1 (mouse). The cross-reacting rabbit IgG band (from FL393) on this blot serves as an internal loading control. Analysis of whole-cell extracts showed Ku70 to be highly expressed in HCT116 cells.]
The recent availability of a 14-3-3σ knockout HCT116 cell line allowed us to test the importance of 14-3-3σ to Chk1 binding to p53. When wild-type HCT116 cells were compared to the isogenic 14-3-3σ knockout line, the radiation-induced Chk1 binding to p53 appeared to be 14-3-3σ dependent, whereas the p21 binding was not (Figure 5). Thus, p53 and 14-3-3σ binding to Chk1 might be interdependent on one another, whereas p21 binding to Chk1 is only dependent on p53.
p53 is Required for UCN-01-Mediated Radiosensitization
UCN-01 has been reported to preferentially radiosensitize a HPV-E6-expressing tumor cell line relative to the p53 wild-type parent cells [38], and this finding was attributed to UCN-01's abrogation of the G2 checkpoint. However, cells lacking p53 have since been shown to fail to sustain G2 arrest and yet were not radiosensitive [30]. Nevertheless, 14-3-3σ and p21 double-mutant cells, expressing wild-type p53, have been reported to be highly radiosensitive [16], suggesting that p53 may be required for both cell cycle arrest and radiosensitivity. Because UCN-01's suppression of 14-3-3σ and p21, but not p53, mimics the double-mutant situation in terms of gene expression, we tested UCN-01's ability to radiosensitize p53 wild-type and mutated HCT116 cells.
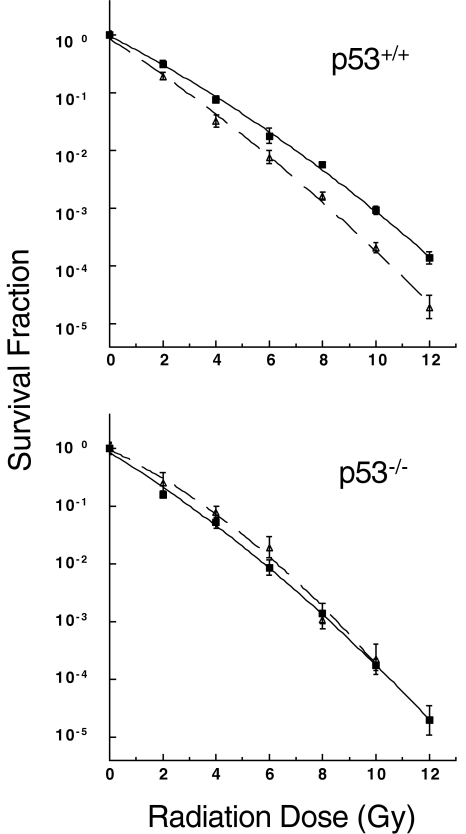
Cells were treated with 100 nM of UCN-01 for 24 hours, irradiated with increasing doses of radiation, and assayed for clonogenic survival. Our results demonstrated that UCN-01 specifically radiosensitized the p53+/+ cells (Figure 6).
Figure 6.
p53 is required for UCN-01 mediated radiosensitization. Colony-formation assay was performed on HCT116 p53+/+ (top panel) and p53-/- (bottom panel) as described in the Materials and Methods section. After treatment with 0 nM of UCN-01 (closed square) or 100 nM of UCN-01 (open triangle) for 24 hours, cells were irradiated at varying dose and replated 24 hours postirradiation in drug-free medium. Colonies were stained and counted 12 days later. There were three replicate plates at each dose point within an experiment, and the experiments were done in triplicate. Error bars represent ± one standard error of the mean.
These findings are consistent with the notion that p21 and 14-3-3σ cooperate in G2 arrest and that loss of expression of both proteins, in the presence of wild-type p53, results in clonogenic radiosensitization [16]. Also, the observation that UCN-01-mediated radiosensitization is p53-dependent is consistent with our findings that p21 and 14-3-3σ suppression is p53-dependent.
Chk2 Pathway is not Inhibited by UCN-01
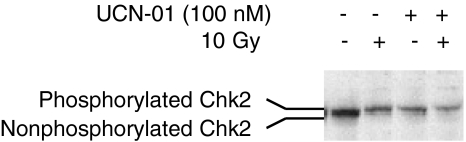
Chk2 is a functional homolog of Chk1 that has also been shown to phosphorylate p53 at S20 [25,39], and is thought to be a major activator of p53 [25,39–42]. Like Chk1, Chk2 is known to be phosphorylated on irradiation treatment, and cells lacking Chk2 failed to sustain G2 arrest [42]. We wanted to determine whether UCN-01 inhibited radiation-induced phosphorylation of Chk2 as well as Chk1. Chk2 phosphorylation in response to radiation and UCN-01 was measured by electrophoretic band shift (Figure 7). Results showed that radiation-induced phosphorylation of Chk2 was not inhibited by UCN-01. In fact, UCN-01 alone appeared to induce phosphorylation, although it is not clear whether UCN-01 directly stimulated phosphorylation of Chk2, or that phosphorylation was a consequence of a persistent DNA damage signal due to unresolved DNA strand breakage in cells with disrupted checkpoints. These findings are consistent with our earlier observation that UCN-01 enhanced p53 phosphorylation at S20, and suggest that the Chk2/p53 pathway remains functional in the presence of UCN-01, yet is unable to induce cell cycle arrest. This further supports the notion that Chk1, and not Chk2, is affected by UCN-01.
Figure 7.
Phosphorylation of Chk2 in response to DNA damage and UCN-01. Cells were treated with UCN-01 and/or radiation. Phosphorylation of Chk2 was shown as a shift relative to the untreated sample analyzed by Western blot with an anti-Chk2 antibody.
Discussion
Interactions between Chk1 and p53 Cell Cycle Arrest Pathways
A substantial body of evidence implicates Chk1 as a central player in the G2 checkpoint in human cells, yet the nature of its involvement in radiation-induced G2 arrest is unclear. Nevertheless, Chk1 has been shown to be phosphorylated in a radiation dose-dependent manner at S345 [5,43] — a consensus sequence for radiation signal transduction kinases ATM and ATR — and ATR has been shown to regulate phosphorylation at S345 in both an ionizing and UV radiation-dependent manner [5]. This strongly implicates S345 of Chk1 as a mediator of radiation-induced G2 arrest function, yet it has not been shown that this phosphorylation alters the kinase activity of Chk1, at least on its best known substrate, Cdc25C. This raises the question as to what the functional consequences of Chk1 phosphorylation might be.
Unlike Chk1, however, the transcriptional activity of p53 is well known to be stimulated by DNA damage. DNA damage increases the half-life of p53, and increases the expression of its downstream cell cycle effector proteins, including p21 and 14-3-3σ [20,44]. p21 is a well-characterized inhibitor of cyclin-dependent kinases. It is not only a potent inducer of G1 arrest [18,44–46], but also plays an important role in G2 arrest [15]. 14-3-3σ has been shown to cooperate with p21 in sustaining G2 arrest [16]. Thus, p53's promotion of G2 arrest is thought to be mediated primarily through the cooperative effects of these two downstream cell cycle effector proteins [16,19,20]. HCT116 cells deficient in expression of both p21 and 14-3-3σ, yet proficient in p53, have shortened G2 arrest [16]. It should be noted, however, that there are some reports that exogenous expression of wild-type p53 in p53-mutated tumors can actually promote the release of cells from radiation-induced G2 arrest [47,48], suggesting that p53 induction may exert negative as well as positive regulation on G2 arrest. At this time, the molecular mechanisms underlying p53's putative negative regulation of G2 are not known.
An important question is whether the Chk1 pathway for G2 arrest interacts with the p53-mediated G2 arrest pathway, and what the mechanism of that interaction might be. Direct interaction with p53 is a possible means by which Chk1 might communicate with DNA damage response pathways and, thus, link Chk1's cell cycle effects to DNA damage-induced signal transduction. In fact, S20 of p53 is known to be one of the substrates for Chk1 kinase activity [39]; however, it is not clear whether Chk1 regulates p53-dependent cell cycle checkpoints through phosphorylating p53 at S20 in vivo. We show here that p53 at S20 remains normally phosphorylated even when G2 arrest has been completely inhibited by UCN-01.
It had been reported that Chk1 in fission-yeast was phosphorylated after DNA damage, and the phosphorylated form of Chk1 bound to yeast 14-3-3 proteins [43]. This suggested that a mechanism other than mere activation of Chk1 kinase activity might be involved in a DNA damage-induced G2 arrest function, and that the mechanism may involve protein-protein interactions. This is reminiscent of other checkpoint mechanisms that rely on protein binding, such as Cdc25C binding to 14-3-3 proteins. Because both Chk1 and p53 are required for normal DNA damage checkpoints, we wondered whether radiation-induced phosphorylation of Chk1 S345 might result in physical association between Chk1 and p53. Our findings support this notion, and suggest an association between p53, 14-3-3σ, and p21. In agreement with the study of Liu and coworkers [5], we showed that Chk1 was phosphorylated on S345 in response to radiation regardless of p53 status. However, the coprecipitation of p21 and 14-3-3σ with the phosphorylated Chk1 was found only in p53+/+ cells, even though the p53-/- cells expressed ample amounts of 14-3-3σ and p21, suggesting that p53 was centrally important to the association of these proteins. In reciprocal experiments, where p53 was precipitated, Chk1 coprecipitation appeared to be 14-3-3σ dependent, suggesting that both p53 and 14-3-3σ are required for binding. Interestingly, p21 binding was not dependent on 14-3-3σ. Thus, p53 and 14-3-3σ binding to Chk1 might be interdependent on one another, whereas p21 binding to Chk1 is only dependent on p53.
At this point it is not clear how important phosphorylation of Chk1 is to the radiation-induced G2 arrest pathway. Our results showing that phosphorylated Chk1 physically associates with p53 may provide a new mechanism for the Chk1 G2 arrest pathway to interact with the p53-mediated G2 arrest pathway, and may reveal another level of control of radiation-induced arrest functions. The issue of Chk1's radiation-inducible protein associations and the role of these associations in radiation-induced G2 arrest need to be further investigated.
Mechanistic Clues from UCN-01
Studies of the role of Chk1 in radiation-induced G2 arrest have been hampered because Chk1-deficient cells are not viable. Therefore, we were intrigued by reports that the staurosporine analog, UCN-01 — a potent inhibitor of G2 arrest and a protein kinase inhibitor — acted by targeting Chk1 [27–29]. Furthermore, it was claimed that UCN-01 specifically radiosensitized p53-deficient cells [38], suggesting a possible interactive role for Chk1 and p53 in radiation-induced G2 arrest.
Although Chk1 has been reported to be the relevant target for UCN-01, it is also known that the kinase inhibition by UCN-01 is more broad than simply Chk1 (including protein kinase C [49]) and that radiosensitization potential is variable among cell types. Nevertheless, UCN-01 remains a potent inhibitor of G2 arrest (IC50≤50 nM) with radiosensitization activity that is modified by p53 status. These results, coupled with our observation that UCN-01 was also a potent inhibitor of radiation-induced increases in p21 and 14-3-3σ, stimulated us to further investigate the of effects of this drug on cell cycle regulatory proteins, as a tool to find clues to critical protein targets for cell cycle arrest inhibition.
Despite the fact that UCN-01 is a kinase inhibitor with questionable target specificity, of the known phosphorylation sites on major proteins controlling G2 cell cycle arrest only S345 of Chk1 is known to be both radiation inducible and UCN-01 inhibitable. Thus, regardless of the kinase activity, which is targeted by UCN-01, its inhibition of S345 may be an important mediator of its radiation-induced cell cycle effects. These findings suggest that phosphorylation of Chk1 at S345 and the suppression of p21/14-3-3σ may be linked, and that this linkage may be mediated by binding of S345 phosphorylated Chk1 to p53, 14-3-3σ, and possibly other proteins. These are provocative findings need to be further explored in genetically based cell models.
Cell Cycle Proteins as Radiotherapeutic Targets
Because cell cycle arrest is a downstream event of signal transduction processes, in which some of the upstream effector proteins are known, it is intuitively logical that targeting the known upstream transducing proteins would inhibit cell cycle arrest functions and promote cell killing. However, many of these transducing proteins produce multiple signals, some of which even promote programmed cell death. It is increasingly becoming evident that cell cycle regulation and cell death processes are orchestrated in pathways with common protein players. Furthermore, many of these processes are modulated by downstream feedback proteins, which further complicate mechanisms. Thus, the fate of a DNA-damaged cell may very well depend on how faithfully the different players fulfill their functional roles and the overall balance of those events.
In terms of p53, it is possible to view the radioresponse of p53 as a competition between cell cycle arrest and cell death [50,51]. The normal elevation of p53 levels following irradiation, even while downstream cell cycle arrest proteins are inhibited, such as we saw for UCN-01, may allow p53 to remain active in cell killing even though its arrest functions are inhibited. This could explain why we only saw UCN-01 radiosensitization in p53 wild-type cells. This situation could have some general implications for cell cycle targeting strategies. Because cell cycle proteins may be players in both arrest and death function, there may be therapeutic benefits to be gained by specifically targeting downstream functions that have segregated to cell cycle arrest, rather than proteins upstream of both arrest and death functions. In this way, cell cycle arrest and cell killing might be uncoupled and manipulated for therapeutic advantage. This notion is supported by a report of a 14-3-3σ and p21 double-knockout cell line showing a loss of radiation-induced cell cycle arrest accompanied by increased clonogenic radiosensitivity, whereas p53 single knockouts had radiation survival comparable to wild-type cells [16]. In any event, downstream cell cycle arrest proteins may prove to be very important targets for enhancing radiotherapy and other DNA damage-based cancer treatments. This area needs further investigation.
Acknowledgements
We thank Bert Vogelstein of the Howard Hughes Medical Institute and the Johns Hopkins Oncology Center for generously providing the cell lines used for this study. We thank Stephen Elledge of the Howard Hughes Medical Institute and the Baylor College of Medicine for providing antibody to Chk1 and Karen Cresswell for analysis of flow cytometry data. We thank Anatoly Dritschilo, Mira Jung, Usha Kasid, Vicente Notario, Mark Smulson, and Sunghan Yoo for helpful discussions.
Footnotes
This work was supported by grant P01-CA74175 from the National Cancer Institute of the National Institutes of Health, U.S. Department of Health and Human Services. Flow cytometry work was supported by the Flow Cytometry/Cell Sorting Shared Resource of the Lombardi Cancer Center, U.S. Public Health Service grant 2P30-CA51008.
References
- 1.Bedford JS, Mitchell JB, Griggs HG, Bender MA. Radiation-induced cellular reproductive death and chromosome aberrations. Radiat Res. 1978;76:573–586. [PubMed] [Google Scholar]
- 2.Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to CdK regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, Elledge SJ. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–1671. doi: 10.1126/science.286.5442.1166. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Gatei M, O'Connell MJ, Khanna KK, Bugg SJ, Hogg A, Scott SP, Hobson K, Lavin MF. Chk1 complements the G2/M checkpoint defect and radiosensitivity of ataxia-telangiectasia cells. Oncogene. 1999;18:249–256. doi: 10.1038/sj.onc.1202257. [DOI] [PubMed] [Google Scholar]
- 5.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 6.Takai H, Tominaga K, Motoyama N, Minamishima YA, Nagahama H, Tsukiyama T, Ikeda K, Nakayama K, Nakanishi M, Nakayama K. Aberrant cell cycle checkpoint function and early embryonic cell death in Chk1(-/-) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 7.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 8.Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 9.Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, Wu Z, Stephenson MT, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- 10.Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- 11.Dalal SN, Schweitzer CM, Gan J, DeCaprio JA. Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol Cell Biol. 1999;19:4465–4479. doi: 10.1128/mcb.19.6.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Girona A, Furnari B, Mondesert O, Russell P. Nuclear localization of Cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature. 1999;397:172–175. doi: 10.1038/16488. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko Y, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasaw M, Tachibana A, Ikeda K, Nakanishi Cell cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene. 1999;18:3673–3681. doi: 10.1038/sj.onc.1202706. [DOI] [PubMed] [Google Scholar]
- 14.Zhou B-BS, Elledge S. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- 15.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 16.Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G2/M checkpoint. Genes Dev. 2000;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 17.Atken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological functions and domain structure. Biochem Soc Trans. 1995;23:605–611. doi: 10.1042/bst0230605. [DOI] [PubMed] [Google Scholar]
- 18.Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 19.Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3σ is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 20.Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 21.Vindelov LL, Christensen IJ, Nissen NI. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983;3:323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- 22.Tian H, Wittmack EK, Jorgensen TJ. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679–684. [PubMed] [Google Scholar]
- 23.Juan G, Traganos F, James WM, Ray JM, Roberge M, Sauve DM, Anderso H, Darzynkiewicz Z. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry. 1998;32:71–77. doi: 10.1002/(sici)1097-0320(19980601)32:2<71::aid-cyto1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 24.Chehab NH, Malikzay A, Stavridi ES, Halazonetis TD. Phosphorylation of Ser-20 mediates stabilization of human p53 in response to DNA damage. Proc Nat Acad Sci USA. 1999;96:13777–13782. doi: 10.1073/pnas.96.24.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chehab NH, Malikzay A, Appel M, Halazonetis TD. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 2000;14:278–288. [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 27.Busby EC, Leistritz DF, Abraham RT, Karnitz LM, Sarkaria JN. The radiosensitizing agent 7-hydroxystaurosporine (UCN-01) inhibits the DNA damage checkpoint kinase hChk1. Cancer Res. 2000;60:2108–2112. [PubMed] [Google Scholar]
- 28.Graves PR, Yu L, Schwartz JK, Gales J, Sausville EA, O'Connor PM, Piwnica-Worms H. The Chk1 protein kinase and the Cdc25C regulatory pathways are targets of the anticancer agent UCN-01. J Biol Chem. 2000;275:5600–5605. doi: 10.1074/jbc.275.8.5600. [DOI] [PubMed] [Google Scholar]
- 29.Jackson JR, Gilmartin A, Imburgia C, Winkler JD, Marshall LA, Roshak A. An indolocarbazole inhibitor of human checkpoint kinase (Chk1) abrogates cell cycle arrest caused by DNA damage. Cancer Res. 2000;60:566–572. [PubMed] [Google Scholar]
- 30.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J. Cell-cycle arrest versus cell death in cancer therapy. Nat Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- 32.Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1676. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 33.Canman CE, Lim D-S, Cimprick KA, Taya Y, Tamai K, Sakaguchi K, Appell E, Kastan MB, Siliciano JD. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–1678. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 34.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 35.Morgan SE, Kastan M. p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res. 1997;71:1–25. doi: 10.1016/s0065-230x(08)60095-0. [DOI] [PubMed] [Google Scholar]
- 36.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 37.Brown KD, Lataxes TA, Shangary S, Mannino JL, Giardina JF, Chen J, Baskaran R. Ionizing radiation exposure results in up-regulation of Ku70 via a p53/ataxia-telangiectasia-mutated protein-dependent mechanism. J Biol Chem. 2000;275:6651–6656. doi: 10.1074/jbc.275.9.6651. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O'Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88:956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 39.Shieh SY, Ahn J, Tamai K, Taya Y, Prives C. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Development. 2000;14:289–300. [PMC free article] [PubMed] [Google Scholar]
- 40.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang XL, Annan RS, Lu Q, Faucette LF, Scott GF, Li XT, Carr SA, Johnson RK, Winkler JD, Zhou BBS. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 41.Tominaga K, Morisaki H, Kaneko Y, Fujimoto A, Tanaka T, Ohtsubo M, Hirai M, Okayama H, Ikeda K, Nakanishi M. Role of human Cds1 (Chk2) kinase in DNA damage checkpoint and its regulation by p53. J Biol Chem. 1999;274:31463–31467. doi: 10.1074/jbc.274.44.31463. [DOI] [PubMed] [Google Scholar]
- 42.Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Liu TH, Walworth NC. Association of Chk1 with 14-3-3 proteins is stimulated by DNA damage. Genes Dev. 1999;13:675–685. doi: 10.1101/gad.13.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 45.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 46.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 47.Guillouf C, Rosselli F, Krishnaraju K, Moustacchi E, Hoffman B, Liebermann DA. p53 involvement in control of G2 exit of the cell cycle: role in DNA damage-induced apoptosis. Oncogene. 1995;10:2263–2270. [PubMed] [Google Scholar]
- 48.Schwartz D, Almog N, Peled A, Goldfinger N, Rotter V. Role of wild type p53 in the G2 phase: regulation of the gamma-irradiation-induced delay and DNA repair. Oncogene. 1997;15:2597–2607. doi: 10.1038/sj.onc.1201436. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann J. Modulation of protein kinase C in antitumor treatment. Rev Physiol Biochem Pharmacol. 2001;142:1–96. doi: 10.1007/BFb0117491. [DOI] [PubMed] [Google Scholar]
- 50.Oren M. Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol. 1994;5:221–227. [PubMed] [Google Scholar]
- 51.Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]