Abstract
The Epstein-Barr virus-encoded latent infection membrane protein 1 (LMP1) is a pleiotropic protein, the activities of which include effects on cell transformation and phenotype, growth, and survival. The ability of LMP1 to mediate at least some of these phenomena could be attributed to the activation of the transcription factor NF-κB. LMP1 promotes NF-κB activation through the recruitment of the adapter protein TRAF2 and the formation of a dynamic multiprotein complex that includes the NF-κB kinase, the IκB kinases, and their downstream targets, IκBs and p105. In this study, we have identified the oncogenic kinase Tpl-2/Cot as a novel component of LMP1-induced NF-κB signaling. We show that Tpl-2 is expressed in primary biopsies from patients with nasopharyngeal carcinoma and Hodgkin's disease, where LMP1 is also found. Inducible expression of LMP1 promotes the activation of Tpl-2, and a catalytically inactive Tpl-2 mutant suppresses LMP1-induced NF-κB signaling. In colocalization and coimmunoprecipitation experiments, Tpl-2 and TRAF2 were found to interact with Tpl-2 functioning downstream of TRAF2. Consistent with this observation, catalytically inactive Tpl-2 also blocked CD40-mediated NF-κB activation, which largely depends on TRAF2. The ability of Tpl-2 to influence LMP1-induced NF-κB occurs through modulation of both IκBα and p105 functions. Furthermore, Tpl-2 was found to influence the expression of angiogenic mediators, such as COX-2 in LMP1-transfected cells. These data identify Tpl-2 as a component of LMP1 signaling downstream of TRAF2 and as a modulator of LMP1-mediated effects.
Epstein-Barr virus (EBV) is a human herpesvirus associated with several types of malignancy, including Burkitt's lymphoma, Hodgkin's disease (HD), lymphoproliferative disorders in immunocompromised individuals, undifferentiated nasopharyngeal carcinoma (NPC), and gastric cancer (23, 28, 50).
Among the nuclear and membrane viral proteins expressed as a consequence of EBV infection, the latent membrane protein 1 (LMP1) is essential for EBV-mediated primary B-cell transformation in vitro (27). In addition, LMP1 expression induces the oncogenic transformation of established fibroblast cell lines, such as Rat-1 and 3T3 (57), and suppresses senescence in primary mouse embryonic fibroblasts (60). In epithelial cells LMP1 blocks differentiation, a property which may be important in the pathogenesis of NPC (6). The ability of this viral protein to promote survival through the up-regulation of antiapoptotic proteins such as A20 and members of the Bcl-2 family may also contribute to its oncogenic properties (16, 21). Furthermore, expression of LMP1 in carcinoma cell lines induces the production of the angiogenic factors interleukin-8 (IL-8), prostaglandin E2, and the up-regulation of matrix metalloproteinases and vascular endothelial growth factor (12, 37, 61), suggesting that in addition to its transforming potential LMP1 may influence metastasis of EBV-associated tumors. Consistent with this notion, LMP1 expression in MDCK cells results in increased cell motility and invasive growth (29).
The signaling pathways that are activated by LMP1 and control its pleiotropic activities include the small GTPase Cdc42, the p38 mitogen-activated protein kinase (MAPK), and the JNK/AP-1 and JAK/STAT pathways, as well as the transcription factor NF-κB (for a review, see reference 14). The constitutive engagement of NF-κB appears to be responsible for many of the oncogenic properties of LMP1, including its ability to growth transform Rat-1 fibroblasts (19), to promote IL-8 production (12), and to up-regulate expression of cyclooxygenase-2 (COX-2), a key enzyme in the biosynthesis of prostaglandin E2 (37). In addition, suppression of NF-κB compromises the viability of EBV-transformed B-cell lines (2).
NF-κB activation requires the degradation of the inhibitory proteins IκBs and p105, which otherwise sequester NF-κB subunits in an inactive form in the cytoplasm. Degradation of IκBs and p105 relies on their phosphorylation by the IκB kinases IKKα and IKKβ and leads to the translocation of active p65 and p50 NF-κB subunits to the nucleus and transactivation of target genes (for a review, see reference 25). Two MAPK kinase kinases (MAPKKK), MEKK1 and NIK (NF-κB-inducing kinase), have so far been implicated in relaying inflammatory cytokine signals, such as those induced by IL-1, tumor necrosis factor (TNF), and CD40 ligand to the IKK-IκB-p105 complex. NIK also plays a role in LMP1 signaling upstream of IKKs but downstream of TRAFs, as a catalytically inactive NIK mutant has been shown to suppress LMP1- and TRAF2-induced IκBα phosphorylation and NF-κB transactivation (8, 9, 11, 24, 26, 48). TRAFs are believed to facilitate the assembly of a large IκB complex through their interaction with NIK. The mechanism of signal initiation in this multiprotein complex is yet unclear but it may involve oligomerization and autophosphorylation of NIK and/or other kinases that may directly affect or function in parallel with NIK.
The serine/threonine kinase Tpl-2/Cot, for example, appears to be a component of this complex, as it interacts with NIK and induces its phosphorylation and activation (33). Expression of Tpl-2 in human embryonic kidney (HEK) 293 or Jurkat T cells results in NF-κB activation (53), and a catalytically inactive form of this kinase suppresses CD3/CD28-mediated IκBα phosphorylation (33) and TNF-induced proteolysis of p105 in Jurkat cells (1). Tpl-2 is also critical for extracellular signal-regulated kinase (ERK) activation, which depends on the cooperative action of Tpl-2 and c-Raf1 in a multiprotein complex with Ras to promote phosphorylation of MEK1, the upstream kinase of ERK (10, 18, 40). Overexpression of Tpl-2 has also been shown to phosphorylate SEK1, thereby activating the JNK pathway (45). Activation of Tpl-2 has been implicated in generation of T-cell lymphomas in rats (39) and induction of morphological transformation in NIH 3T3 mouse fibroblasts (36). Tpl-2/Cot is also overexpressed at the RNA level in a number of human tumors, including gastric, colon, and breast cancers (15, 38, 47), and it regulates COX-2 expression (7; A. G. Eliopoulos et al., submitted for publication), further supporting its role in oncogenesis.
In this study we provide evidence suggesting that Tpl-2 is a component of the LMP1-induced NF-κB activation pathway. We show that Tpl-2 is commonly expressed in EBV-associated malignancies, such as NPC and HD, where LMP1 is also found. Inducible expression of LMP1 promotes the activation of Tpl-2, and expression of a catalytically inactive Tpl-2 mutant suppresses LMP1- and TRAF2-induced NF-κB activation without affecting LMP1-mediated Cdc42 signaling, which occurs in a TRAF2-independent fashion. The ability of a kinase-inactive Tpl-2 mutant to inhibit NF-κB activation and expression of COX-2 in LMP1-transfected cells identifies Tpl-2 as a modulator of LMP1-mediated activities.
MATERIALS AND METHODS
DNA constructs.
pSG5-based LMP1 (43) and LMP1 mutants Δ(332-386) and pSG5LMP1AxAxA have been previously described (11). A myc-tagged LMP1 expression vector was generated by PCR amplification of LMP1 coding sequences and insertion in frame into pRK5-myc (31) containing the peptide sequence MEQKLISEEDL, which is recognized by the anti-myc monoclonal antibody (MAb) 9E10 (Oncogene Research Products, Nottingham, United Kingdom). The myc-tagged p105 (59) was kindly provided by Takashi Fujita (Department of Tumor Cell Biology, The Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). To generate myc-tagged p105ΔN, amino acids (aa) 459 to 969 of p105 were PCR amplified and inserted in pRK5-myc. IκBα aa 1 to 62 were PCR amplified from a cytomegalovirus (CMV)-driven wild-type IκBα (gift from John Girdlestone, MRC Center for Immune Regulation, Birmingham, United Kingdom) and cloned in frame into a glutathione S-transferase (GST) vector (Pharmacia). The sequences of the primers used for these amplification reactions are available upon request. GFP-IκBαΔN was kindly provided by Martin Rowe (Cardiff, United Kingdom), and the wild-type and N-terminus-deleted TRAF2 expression vectors (26) were kindly provided by George Mosialos, Ken Kaye, and Eliott Kieff (Harvard Medical School, Boston, Mass.). FLAG-tagged TRAF2 (54) was a kind gift of Jun-Ichiro Inoue (The Institute of Medical Sciences, The University of Tokyo, Tokyo, Japan). Tpl-2 expression constructs have been previously described (5).
Cell culture, transfections, reporter assays, and EMSAs.
HEK 293 cells were cultured in Dulbecco's modified Eagle's (DME) medium supplemented with 10% fetal calf serum (FCS) and 2 mM glutamine. For transient transfections, 8 × 105 to 1 × 106 HEK 293 cells were plated out on a 25-cm2 flask and the following day they were transfected using a standard calcium phosphate technique. NIH 3T3 cells, cultured in DME with 5% FCS supplemented with 2 mM glutamine, were transfected using Lipofectamine (Gibco). Luciferase reporter and β-galactosidase assays were performed as previously described (13). Fifty nanograms each of a Rous sarcoma virus promoter-driven β-galactosidase-expressing plasmid and of 3Enh.κB-ConALuc reporter, which contains three tandem repeats of the NF-κB sites from the Igκ promoter, were routinely used to transfect 293 cells. Equal amounts of DNA were transfected in each experiment by the addition of empty vectors where necessary. For COX-2 reporter assays, HEK 293 cells were transfected with 0.6 μg of COX-2 promoter (−891 to +2)-luciferase construct (55) and 0.2 μg of Renilla plasmid (Promega) in the presence of effector plasmids and luciferase, and Renilla activities were determined 36 h posttransfection. Each experiment was performed at least three times, unless otherwise indicated, and in each individual assay duplicate determinations were performed. Electrophoretic mobility shift assays (EMSAs) for NF-κB and Sp1 were performed as previously described (13, 58).
Immunoprecipitation and kinase assays.
For IκBα kinase assays cells were lysed in 300 to 500 μl of kinase lysis buffer (20 mM Tris [pH 7.6], 0.5% Triton X-100, 250 mM NaCl, 3 mM EGTA, 3 mM EDTA, 2 mM sodium vanadate, 2 mM NaF, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 1 mM dithiothreitol [DTT]) for 20 min on ice. Cell debris was removed by centrifugation, and protein concentration was determined using a commercially available Bio-Rad protein assay. Endogenous IKKα was immunoprecipitated from 300 μg of total protein extracts using 5 μl of anti-IKKα antibody (H744; Santa Cruz) for 2 to 3 h and 25 μl of protein G-Sepharose (Pharmacia) for an additional 1 h. Following immunoprecipitation, beads were washed twice with kinase lysis buffer and twice with assay buffer (10 mM HEPES [pH 7.4], 12.5 mM β-glycerophosphate, 5 mM MgCl2, 1 mM MnCl2, 50 μM sodium vanadate, 2 mM NaF, 5 μM DTT, and 1 μg of leupeptin/ml). After the last wash, the beads were drained using a fine-gauge Hamilton syringe and resuspended in 50 μl of assay buffer containing 2 μg of GST-IκBα(1-62) substrate and 10 μCi of [γ-32P]ATP. Kinase reactions were carried out at 30°C for 30 min and stopped by addition of 25 μl of 3× Laemmli buffer. Samples were then analyzed on a sodium dodecyl sulfate (SDS)-12.5% polyacrylamide gel electrophoresis (PAGE) gel, and IκBα phosphorylation was measured on a phosphorimager. Coomassie blue staining verified the use of equal amounts of GST-IκBα(1-62) in these reaction mixtures. For Tpl-2 kinase assays, 106 HEK 293 cells carrying an ecdysone-regulatable LMP1 (12) were transfected with 0.3 μg of myc-tagged Tpl-2 by using a calcium phosphate method. Activation of Tpl-2 was determined 24 h later in 500 μg of cell lysates by autophosphorylation assays as previously described (5). Then, 200 μg of the same lysates were immunoprecipitated with 9E10 anti-myc MAb, analyzed on an SDS-10% PAGE gel, and probed with the M20 anti-Tpl-2 antibody. Coimmunoprecipitations were performed in 500 to 600 μg of 293 cell extracts. HEK 293 cells were transfected with 5 μg of TRAF2 in the presence of 5 μg of myc-tagged N17Cdc42, Tpl-2, or LMP1. At 24 h posttransfection, cells were lysed in a lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 3% glycerol, 1.5 mM EDTA, 0.5% NP-40, and protease inhibitors, and immunoprecipitations were performed overnight using 2 μg of anti-myc 9E10 MAb. Anti-myc immunoprecipitates were then analyzed with SDS-10% PAGE for TRAF2 by using the C20 anti-TRAF2 polyclonal antibody or for myc expression by using the 9E10 anti-myc epitope MAb.
Immunoblotting.
For LMP1 and TRAF2 immunoblotting, 25 to 40 μg of total cell lysates, isolated as described above, were analyzed by SDS-10% PAGE, and LMP1 or TRAF2 expression was detected with the anti-LMP1 MAb CS.1-4 (44) or the C20 anti-TRAF2 polyclonal antibody (Santa Cruz Biotechnology) and enhanced chemiluminescence (Amersham). myc-p105 and myc-p105ΔN expression was examined by immunoblotting by using the 9E10 anti-myc MAb at a 1:500 dilution in 80 μg of total cell lysates. Exogenous Tpl-2 was detected by using the 9E10 anti-myc MAb. Endogenous Tpl-2 expression was determined by analyzing 100 μg of total cell lysates by SDS-10% PAGE and probing with the anti-Tpl-2 polyclonal antibody M20 (Santa Cruz Biotechnology). According to the manufacturer and our unpublished observations, this antibody is unable to immunoprecipitate endogenous Tpl-2. COX-2 expression was detected in 50 μg of total lysates by SDS-7.5% PAGE, using the C-20 anti-COX-2 polyclonal antibody (Santa Cruz Biotechnology). The mouse MAb detecting β-actin was purchased from Sigma.
Immunohistochemical detection of Tpl-2.
Paraffin wax sections were deparaffinized and subjected to low-temperature antigen retrieval in 1 mM EDTA (pH 8.0), 0.1% Tween 20 on a hot plate stirrer at 65°C for 16 h. Following antigen retrieval, sections were washed in Tris-buffered saline (TBS; pH 7.6), and primary antibody to Tpl-2 (M20; Santa Cruz Biotechnology) was applied at a dilution of 1/200 for 1 h at room temperature (RT). Slides were washed in TBS (pH 7.6) containing 0.001% Tween 20. A universal streptavidin-biotin horseradish peroxidase kit (Binding Site Ltd., Birmingham, United Kingdom) was then applied to detect bound primary antibody. Briefly, this involved incubation in secondary antibody (diluted 1:100 in TBS) for 20 min followed by a wash in TBS-0.001% Tween 20. This was followed by incubation in tertiary reagent (also diluted 1:100) for 20 min. Following a final wash in TBS-0.001% Tween 20, peroxidase activity was visualized in diaminobenzidine for 5 min. Sections were then washed in tap water, counterstained in Mayer's hematoxylin, dehydrated, cleared, and mounted. Negative controls consisted of consecutive test sections in which primary antibody was replaced with nonimmune serum.
Microinjections and immunofluorescence microscopy.
Serum-deprived, subconfluent 3T3 fibroblasts were microinjected with plasmid DNA at a concentration of 0.2 mg/ml, as previously described (41). Cytoskeletal changes were visualized at 4 h after microinjection using tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin (Sigma), and LMP1 expression was determined by costaining with CS1-4. Vector-transfected cells were visualized by fluorescein isothiocyanate-dextran. Staining of microinjected cells for p65 NF-κB was performed as previously described (51) by using an anti-p65 polyclonal antibody from Santa Cruz Biotechnology. For colocalization immunofluorescence microscopy, BJAB cells were electroporated (280 V, 950 μF) with 8 μg of a FLAG-tagged TRAF2 expression vector, and 24 h later cell smears were air dried and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at RT. Following extensive washing in PBS (10 min, RT), cells were permeabilized in 0.1% Triton X-100 in PBS for exactly 3 min and then washed again in PBS for 5 min at RT. Cells were then incubated with the primary antibodies M2 anti-FLAG (Kodak/IBI) at 1:400 and M20 anti-Tpl-2 at 1:20 dilution in PBS supplemented with 10% heat-inactivated goat serum for 1 h at 37°C in a humidified chamber. Following extensive washing in PBS, the cells were incubated for 45 min with Oregon green-labeled anti-mouse immunoglobulin G (IgG; Molecular Probes, Leiden, The Netherlands) at 1:500 and TRITC-labeled anti-rabbit IgG (Sigma) at 1:80 dilution in PBS supplemented with 10% heat-inactivated goat serum. Following a 10-min wash in PBS, slides were mounted with DABCO anti-fade reagent and green and red fluorescence was visualized on a Nikon E600 digital microscope.
RESULTS
Tpl-2 is expressed in EBV-associated malignancies and is activated by LMP1 in epithelial cells.
To establish a role for Tpl-2 in LMP1 signaling, we first examined whether this kinase is expressed in EBV-associated malignancies. So far, there is no evidence at the protein level for Tpl-2 being expressed in human malignancies. To address this issue, paraffin wax sections from a total of 31 HD tumors and 23 undifferentiated NPC biopsies were immunostained for Tpl-2. All NPC specimens examined were positive for EBERs (EBV-encoded small nonpolyadenylated RNAs) as determined by in situ hybridization (data not shown), whereas only 12 of the HD tumors were EBER positive. Three EBER-positive NPCs and all 12 EBER-positive HD samples also expressed LMP1, as determined by immunostaining using the CS1-4 anti-LMP1 MAb.
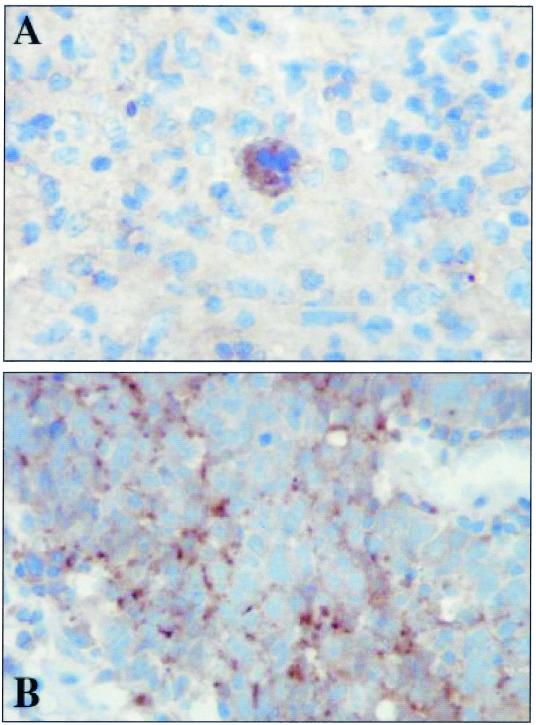
Strong expression of Tpl-2 was identified in malignant Hodgkin/Reed-Sternberg (HRS) cells from the majority of the HD cases (27 of 31), and both EBV-positive and EBV-negative samples expressed Tpl-2. In most sections, expression of Tpl-2 in HRS cells was considerably higher than in the surrounding nonmalignant cells. The staining pattern observed in HRS cells was cytoplasmic and granular in appearance (Fig. 1A). All NPC specimens expressed Tpl-2 in the tumor cells irrespective of EBV status. In the majority of cases, staining was cytoplasmic and granular (Fig. 1B), but in some sections diffuse cytoplasmic staining was observed. Taken together, these data demonstrate that Tpl-2 is expressed in EBV-associated tumors and could therefore serve as a potential effector of LMP1 signaling.
FIG. 1.
Tpl-2/Cot is detected in EBV-associated malignancies where LMP1 is commonly expressed. Shown is representative immunohistochemical staining of sections from patients with HD (A) and undifferentiated NPC (B), illustrating cytoplasmic localization of Tpl-2/Cot. Both sections shown were positive for LMP1.
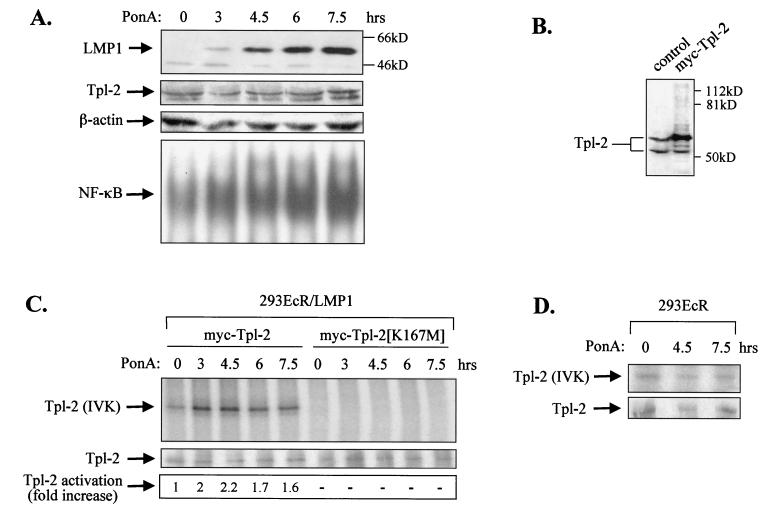
To determine whether LMP1 utilizes Tpl-2 as a signaling intermediate, we first examined the ability of LMP1 expression to promote Tpl-2 activation. For this purpose, HEK 293 cells carrying an ecdysone-regulatable LMP1 (293EcR/LMP1 [12]) were used. Addition of the ecdysone analogue ponasterone A in these cultures resulted in a time-dependent up-regulation of LMP1 expression, as determined by immunoblotting analysis using the CS1-4 MAb (Fig. 2A). LMP1 induction during this time course was followed by increased NF-κB DNA binding activity but did not affect endogenous expression of Tpl-2 or β-actin (Fig. 2A).
FIG. 2.
LMP1 promotes the activation of Tpl-2. (A) Induction of LMP1 in 293EcR/LMP1 cells carrying an ecdysone-regulatable LMP1, following addition of a 10 μM concentration of the ecdysone analogue ponasterone A (PonA; upper panel). Lysates from 293EcR/LMP1 cells treated with PonA or from untreated cultures were analyzed for endogenous Tpl-2 levels by using the anti-Tpl-2 polyclonal antibody M20 or for β-actin levels, as indicated. Nuclear extracts from parallel cultures were examined for NF-κB DNA binding activity by EMSA (lower panel). (B) HEK 293EcR/LMP1 cells were transfected with 0.3 μg of myc-tagged Tpl-2 per 106 cells and 24 h later were analyzed for Tpl-2 expression by immunoblotting, using the M20 anti-Tpl-2 polyclonal antibody (lane 2). Lysates from untransfected cultures (lane 1) were used to verify that, under these conditions, myc-Tpl-2 is expressed at near-physiological levels. (C) LMP1 promotes the activation of Tpl-2, as measured by enhanced autophosphorylation. HEK 293EcR/LMP1 cells were transfected with 0.3 μg of myc-tagged Tpl-2 (lanes 1 to 5) or its catalytically inactive mutant, Tpl-2[K167M] (lanes 6 to 10), and then stimulated with ponasterone A for 0, 3, 4.5, 6, or 7.5 h. Duplicate cultures from each time point were pulled together to minimize differences in transfection efficiencies and processed for in vitro kinase assays as described in Materials and Methods. Parallel anti-myc immunoprecipitates were probed for Tpl-2 using the M20 anti-Tpl-2 polyclonal antibody (middle panel). Relative levels of autophosphorylation were measured on a phosphorimager and are shown in the lower panel. (D) Parental HEK 293EcR cells were transiently transfected with 0.3 μg of myc-tagged Tpl-2 and then stimulated with 10 μM ponasterone A for 0, 4.5, or 7.5 h. Tpl-2 autophosphorylation was examined as described for panel C (upper panel). Anti-myc immunoprecipitates were also probed with the anti-Tpl-2 polyclonal antibody M20 (lower panel).
To determine whether inducible expression of LMP1 promotes the activation of Tpl-2, in the absence of an antibody which can immunoprecipitate the endogenous kinase, 293EcR/LMP1 cells were transfected with low amounts of a myc-tagged Tpl-2 expression vector. Tpl-2 protein levels were monitored by Western blot analysis of lysates from transfected cells or untransfected cultures by using an anti-Tpl-2 polyclonal antibody. This analysis verified that, under these conditions, myc-Tpl-2 is expressed at near-physiological levels (Fig. 2B). Tpl-2 activation was examined in lysates from myc-Tpl-2-transfected cells treated with ponasterone A for 0, 3, 4.5, 6, or 7.5 h by using in vitro kinase assays as described in Materials and Methods, and the relative increase in Tpl-2 autophosphorylation was evaluated. These experiments showed that Tpl-2 kinase activity was rapidly induced following addition of ponasterone A, reached a peak at 4.5 h of treatment, and remained at elevated levels for the rest of the time course (Fig. 2C, lanes 1 to 5). A kinase-inactive Tpl-2[K167M] mutant remained unresponsive to LMP1 induction (Fig. 2C, lanes 6 to 10). The increase in Tpl-2 autophosphorylation is caused by LMP1 and not by ponasterone A, as addition of this ecdysone analogue in myc-Tpl-2-transfected parental 293 EcR cultures did not result in the induction of Tpl-2 kinase activity (Fig. 2D).
Tpl-2 modulates LMP1-induced NF-κB activation but not Cdc42 activation.
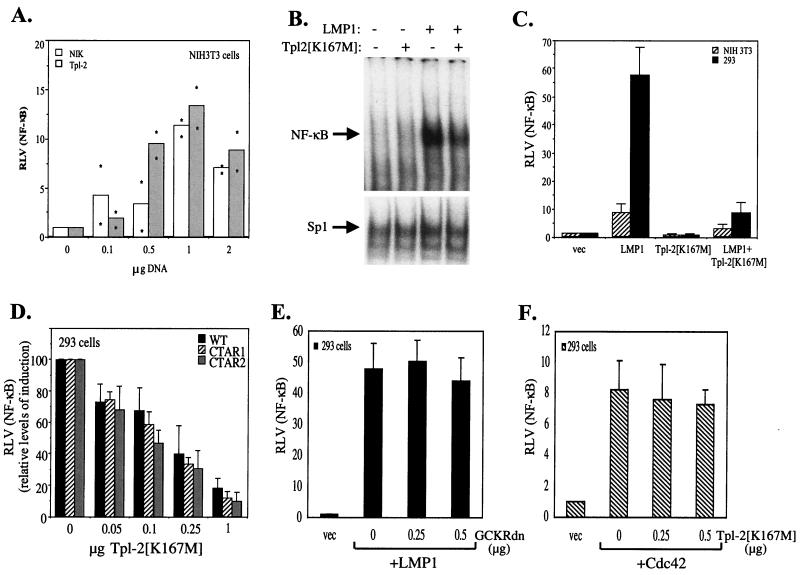
Previous studies have shown that expression of the MAPKKK Tpl-2 in T-cell lines and 293 cells results in NF-κB activation (33, 53). Consistent with these findings, transfection of wild-type Tpl-2 in NIH 3T3 fibroblasts was also found to induce NF-κB luciferase reporter activity (Fig. 3A). Interestingly, the levels of Tpl-2-mediated NF-κB transactivation were similar to those obtained with expression of NIK (Fig. 3A), a MAPKKK which functions downstream of TRAF2 to regulate NF-κB signaling.
FIG. 3.
Tpl-2 modulates LMP1-induced NF-κB activation. (A) Wild-type Tpl-2 induces levels of NF-κB transactivation similar to that of NIK. NIH 3T3 cells were transfected with a β-galactosidase-expressing plasmid and an NF-κB reporter in the presence of increasing amounts of NIK or Tpl-2 and 36 h later were analyzed for luciferase and β-galactosidase activities. Relative NF-κB activation (ratio of luciferase versus β-galactosidase activities; RLV) from two independent experiments is shown. Asterisks represent individual mean values of duplicate determinations from these experiments. (B) Kinase-inactive Tpl-2 inhibits LMP1-induced NF-κB binding activity in 293 cells, as determined by EMSAs (upper panel). The NF-κB complex shown contains p50-p65 heterodimers (data not shown). Nuclear extracts were also analyzed for Sp1 binding activity as a control (lower panel). (C) Kinase-inactive Tpl-2 inhibits LMP1-induced NF-κB transactivation. NIH 3T3 or HEK 293 cells were cotransfected with a galactosidase-expressing plasmid and an NF-κB reporter and 1 μg of pSG5-LMP1, in the presence of equivalent amounts of Tpl-2[K167M]. Relative NF-κB activation (ratio of luciferase versus β-galactosidase activities) (± standard deviation [SD]) from three independent experiments is shown. (D) Kinase-inactive Tpl-2 inhibits LMP1-, CTAR1 [LMP1Δ(332-386)]-, and CTAR2 (LMP1AxAxA)-induced NF-κB transactivation to similar extents. HEK 293 cells were transfected with 1 μg of pSG5-based vectors in the presence of reporter constructs and increasing amounts of Tpl-2[K167M]. To allow comparison of the inhibitory effect of kinase-inactive Tpl-2, RLVs have been normalized to 100 for each of the wild-type, CTAR1, and CTAR2 effects. In these experiments, the mean NF-κB activation values from the above LMP1 molecules were 60 (±5.4), 48.4 (±6.2), and 13 (±4). (E) Kinase-inactive GCKR does not affect LMP1-induced NF-κB transactivation. HEK 293 cells were transfected with reporter constructs and 1 μg of pSG5-LMP1, in the presence or absence of increasing amounts (0.25 or 0.5 μg) of dominant negative GCKR (GCKRdn). RLV was assessed as described for panel C. (F) Kinase-inactive Tpl-2 does not affect Cdc42-mediated NF-κB induction. HEK 293 cells were transfected with reporter constructs and 5 μg of pcDNA3-Cdc42, in the presence or absence of increasing amounts (0.25 or 0.5 μg) of dominant negative Tpl-2. RLV was assessed as described for panel C.
To examine the contribution of Tpl-2 to LMP1-mediated NF-κB activation, HEK 293 cells were cotransfected with 2.5 μg of pSG5-LMP1 in the presence or absence of an equivalent amount of Tpl-2[K167M], and the amount of NF-κB bound to a human immunodeficiency virus long terminal repeat NF-κB probe was evaluated using EMSAs. These experiments demonstrated that kinase-inactive Tpl-2 significantly suppressed LMP1-mediated NF-κB binding activity, while binding to a control Sp1 oligo probe remained essentially unaltered (Fig. 3B). A similar inhibitory effect of Tpl-2[K167M] was observed when using luciferase reporter assays in transiently transfected HEK 293 or NIH 3T3 cells (Fig. 3C).
LMP1 contains two domains in its cytoplasmic C terminus which are critical for NF-κB activation, namely the C-terminus activating region 1 (CTAR1; aa 187 to 231) and CTAR2 (aa 352 to 386) (22). The effect of kinase-inactive Tpl-2 on CTAR1- versus CTAR2-mediated NF-κB activation was evaluated by using luciferase reporter assays. HEK 293 cells were transfected with 1 μg of pSG5-based wild-type LMP1, LMP1Δ(332-386), which is deleted for CTAR2, or LMP1AxAxA, which contains a P204 xQ206 xT208 →AxAxA mutation in the TRAF-binding domain of CTAR1 and functions as a CTAR2 effector (11), in the presence or absence of increasing amounts of Tpl-2[K167M]. Analysis of reporter activity demonstrated that low amounts of this kinase-inactive mutant inhibited NF-κB signaling from both LMP1 domains (Fig. 3D). This phenomenon was specific for Tpl-2, as dominant negative mutants of other kinases, such as germinal center kinase-related protein (GCKR) (46) (Fig. 3E) or Cdc42 (41), do not influence LMP1-induced NF-κB transactivation. The specificity of the observed effects is further substantiated by the inability of catalytically inactive Tpl-2 to suppress NF-κB-dependent reporter activity induced by wild-type Cdc42 (Fig. 3E), which is mediated by an IKK-independent pathway (4).
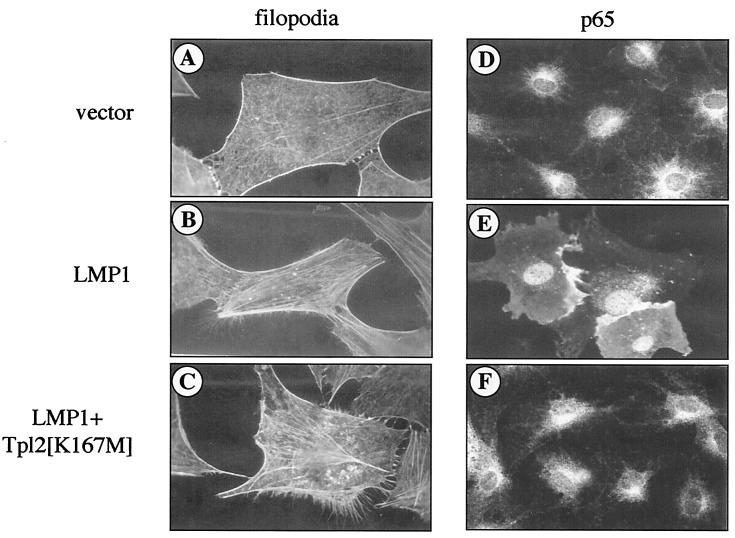
Recent work suggests that microinjection of LMP1 into serum-starved 3T3 cells leads to the reorganization of the actin cytoskeleton via a Cdc42-dependent but NF-κB-independent pathway (41). To determine whether Tpl-2 influences Cdc42-mediated filopodia formation, pSG5-LMP1 was microinjected into NIH 3T3 fibroblasts in the presence or absence of myc-tagged Tpl-2[K167M]. Consistent with previous findings, LMP1, but not vector control-injected cells, was found to contain filopodia extensions associated with lamellipodia as well as stress fibers (Fig. 4A and B). While these phenomena were inhibited by coexpression of a dominant negative Cdc42 (data not shown) (41), kinase-inactive Tpl-2 had no effect (Fig. 4C). However, this Tpl-2 mutant inhibited the nuclear translocation of the p65 subunit of NF-κB in 3T3 cells microinjected with LMP1 (Fig. 4D to F). We therefore conclude that Tpl-2 is a modulator of LMP1-induced NF-κB but not Cdc42 signaling.
FIG. 4.
Tpl-2 is not a component of LMP1-induced Cdc42 activation. Serum-starved 3T3 fibroblasts were microinjected with pSG5-LMP1 (B and E) or control vector (A and D), in the presence or absence of myc-tagged Tpl-2[K167M] (C and F), as described in Materials and Methods. Phalloidin staining revealed formation of filopodia extensions in LMP1-expressing cells (B), which was not affected by the presence of kinase-inactive Tpl-2 (C). Note also the extensive formation of stress fibers in these microinjected cells. Parallel experiments using dominant negative Cdc42 verified inhibition of these LMP1-mediated cytoskeletal changes (data not shown), in agreement with a previous report (41). Transfection of the pSG5 vector alone had no effect on actin reorganization (A). Immunostaining for the p65 subunit of NF-κB (D, E, and F) revealed p65 translocation to the nucleus of LMP1-expressing cells (E) but predominantly cytoplasmic staining in control cells or cells coinjected with LMP1 and Tpl-2[K167M] (panels D and F, respectively).
Tpl-2 coimmunoprecipitates with TRAF2 and regulates TRAF2-mediated signals.
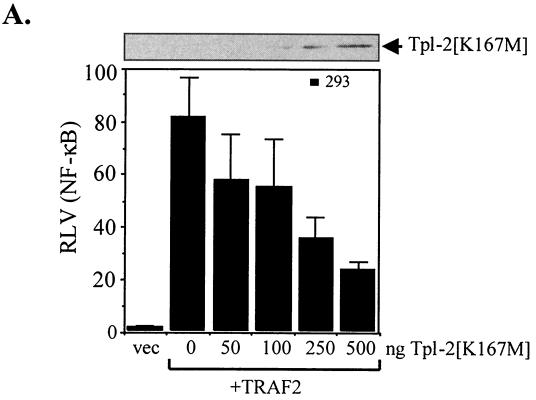
To position Tpl-2 to the LMP1-mediated signaling cascade, we examined the effects of kinase-inactive Tpl-2 on TRAF2-mediated NF-κB activation. Using luciferase reporter assays, we found that catalytically inactive Tpl-2 exerts a potent inhibitory effect on TRAF2-induced NF-κB activity (Fig. 5A). However, transfection of N-terminus-deleted TRAF2, which functions as a dominant negative inhibitor of LMP1-mediated NF-κB signals (26), had no effect on the ability of wild-type Tpl-2 to engage this pathway (data not shown). These data suggest that Tpl-2 functions downstream of TRAF2 to regulate LMP1-mediated NF-κB activation.
FIG. 5.
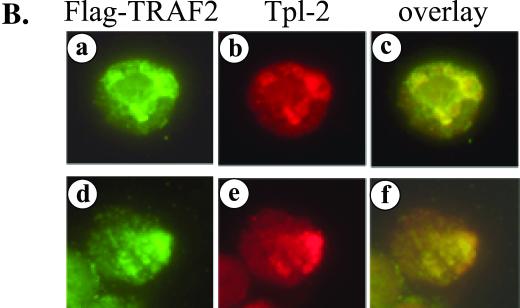
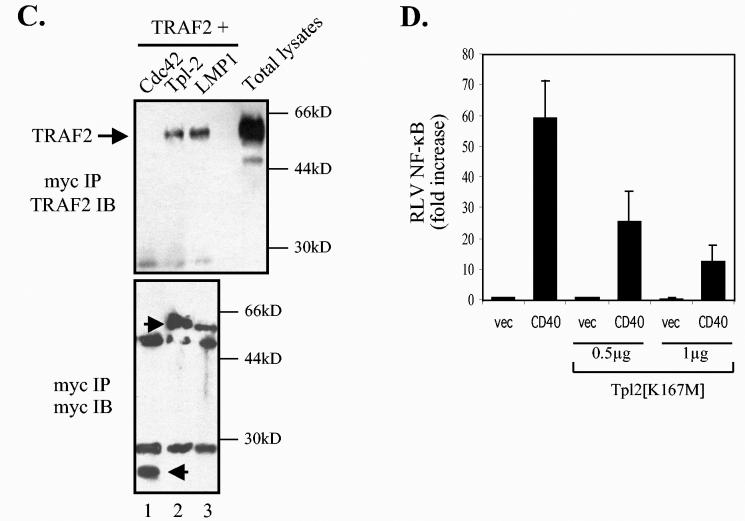
Tpl-2 functions downstream of TRAF2 in LMP1 signaling. (A) Kinase-inactive Tpl-2 inhibits TRAF2-mediated NF-κB activation in a concentration-dependent manner. HEK 293 cells were transfected with 1 μg of CMV-TRAF2 and increasing amounts of Tpl-2[K167M], and RLVs were determined as described in the legend for Fig. 3C. (B) TRAF2 colocalizes with endogenous Tpl-2. BJAB cells were transfected with FLAG-tagged TRAF2 and double-stained for TRAF2 using the anti-FLAG MAb M2 and for Tpl-2 using the anti-Tpl-2 polyclonal antibody M20. Green fluorescence (a and d) identifies FLAG-TRAF2 staining in two representative examples (a to c and d to f, respectively), red fluorescence (b and e) represents Tpl-2 staining, and yellow color (c and f) corresponds to the overlay of green and red fluorescence. (C) Tpl-2 coimmunoprecipitates with TRAF2. HEK 293 cells were transfected with 5 μg of CMV-TRAF2 in the presence of equivalent amounts of myc-tagged N17Cdc42, Tpl-2, or LMP1. Following immunoprecipitation (IP) with an anti-myc tag MAb (9E10), precipitates were analyzed for TRAF2 (upper panel) or myc expression (lower panel) by immunoblotting (IB). Total lysates (8%) were also analyzed to verify the migration of TRAF2 (upper panel). Arrowheads in the lower panel show the migration of immunoprecipitated N17Cdc42, Tpl-2, and LMP1 blotted with the anti-myc tag 9E10 MAb. (D) Kinase-inactive Tpl-2 inhibits CD40-mediated NF-κB activation. HEK 293 cells were transfected with 1 μg of pcDNA3-CD40 and increasing concentrations of Tpl-2[K167M], in the presence of a luciferase and β-galactosidase plasmid, and reporter activity was determined. Mean values (± SD) from three independent experiments are shown.
To explain the effects of Tpl-2[K167M] on TRAF2-induced signaling, we asked whether Tpl-2 is recruited to the TRAF2 signaling complex. To this end, EBV-negative BJAB lymphoma cells were transfected with a FLAG-tagged TRAF2 expression vector and subjected to dual immunofluorescence staining for TRAF2 by using an anti-FLAG MAb and for endogenous Tpl-2 by using an anti-Tpl-2 polyclonal antibody. These experiments demonstrated significant colocalization of the two proteins (Fig. 5B). The ability of TRAF2 to complex with Tpl-2 was confirmed in coimmunoprecipitation experiments. HEK 293 cells were transfected with TRAF2 or control vector in the presence of myc-tagged Tpl-2. Cell lysates were immunoprecipitated with an anti-myc tag antibody and analyzed for TRAF2 expression by immunoblotting. TRAF2 was found to coimmunoprecipitate with Tpl-2 in these assays (Fig. 5C). As a control, myc-tagged LMP1 but not Cdc42 also interacted with TRAF2 (Fig. 5C). We conclude that Tpl-2 modulates LMP1 signaling via association with TRAF2 and regulation of its functions. In agreement with these findings, kinase-inactive Tpl-2 also suppressed CD40-induced NF-κB transactivation (Fig. 5D), which is largely mediated by TRAF2 (43).
Tpl-2 regulates LMP1-mediated NF-κB activation by targeting p105 and IκBα signaling.
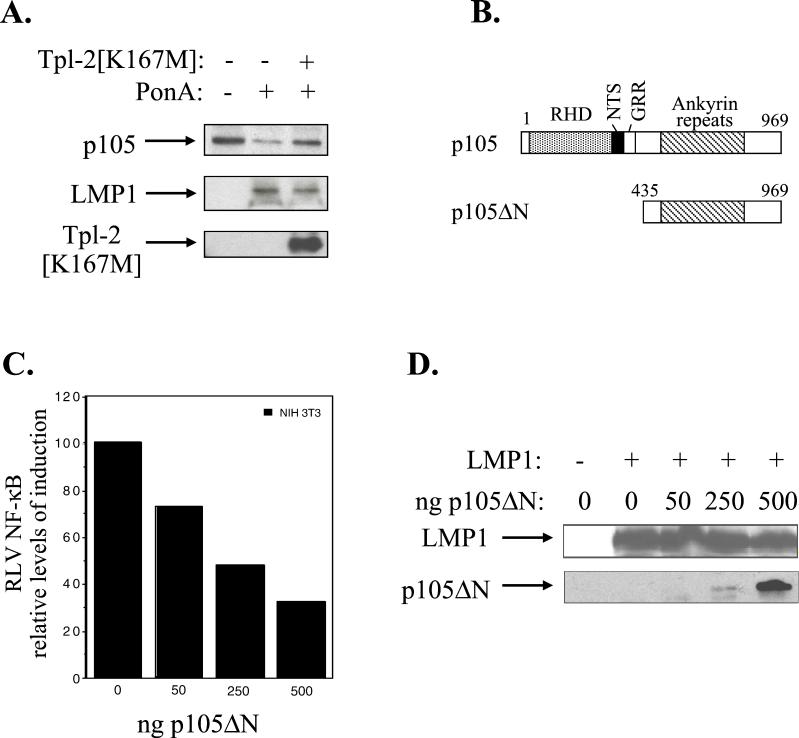
Tpl-2 has been implicated in the regulation of both p105 and IκBα NF-κB-inhibitory proteins, which retain the p50 and p65 NF-κB subunits to the cytoplasm (1, 33). The mechanisms by which p105 modulates p50 function are not fully understood but appear to be subject to both co- and posttranslational regulation (1, 20, 32, 35), with Tpl-2 promoting the degradation of p105 (1). To determine the contribution of Tpl-2 to p105 function in the context of LMP1, a CMV-driven myc-tagged p105 expression vector was transfected in 293EcR/LMP1 cells in the presence or absence of hemagglutinin (HA)-tagged Tpl-2[K167M]. Following a 9-h treatment with the ecdysone analogue ponasterone A, a significant up-regulation of LMP1 expression was observed which was not affected by coexpression of Tpl-2[K167M] (Fig. 6A, middle panel). The same lysates were also analyzed for expression of HA-Tpl-2 and myc-p105. It was found that induction of LMP1 leads to a reduction in myc-p105 levels, and this effect is significantly impaired in the presence of HA-tagged Tpl-2[K167M] (Fig. 6A, upper panel).
FIG. 6.
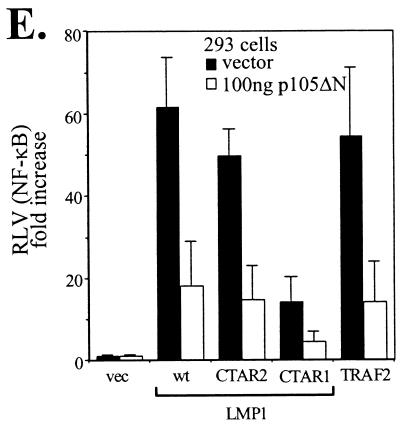
LMP1-induced Tpl-2-mediated p105 degradation contributes to NF-κB transactivation. (A) Kinase-inactive Tpl-2 inhibits LMP1-mediated p105 degradation. HA-tagged Tpl-2[K167M] (1 μg) and 3 μg of myc-tagged p105 were transfected in 293EcR/LMP1 cells. Following treatment with the ecdysone analogue ponasterone A for 9 h, cell lysates were isolated and analyzed for LMP1, Tpl-2, or myc-p105 expression by immunoblotting. (B) Structure of p105 and the N-terminus-deleted, nondegradable mutant used in this study. RHD, rel-homology domain; NTS, nuclear translocation signal; GRR, glycine-rich region. Both constructs carry a myc tag in their N termini. (C) Transfection of p105ΔN in NIH 3T3 cells inhibits LMP1-induced NF-κB activation. The relative decrease in NF-κB luciferase values (RLVs) is shown in histogram form, in which LMP1 has been given the arbitrary value of 100. The actual increase in NF-κB transactivation induced by LMP1 in this representative assay was 8.5-fold. (D) Identical lysates from the experiment shown in panel C were analyzed for LMP1 expression in order to verify that the inhibitory effects of p105ΔN on LMP1-induced NF-κB activation were not due to altered LMP1 levels (upper panel). Deleted p105 expression was assessed by immunoblot analysis using anti-myc tag 9E10 MAb (lower panel). (E) Transfection of a low plasmid concentration (100 ng) of p105ΔN significantly inhibits NF-κB transactivation induced by 1 μg of LMP1, LMP1 CTAR1 [LMP1Δ(332-386)], or LMP1 CTAR2 (LMP1AxAxA) in HEK 293 cells. The effects of this deleted p105 mutant on TRAF2-mediated NF-κB reporter activity are also shown. Data (RLVs) are the mean values (± SD) from three independent experiments.
To determine whether p105 degradation is important for LMP1-mediated NF-κB transactivation, presumably through the release of active p50 NF-κB to the nucleus, we generated a myc-tagged N-terminus-deleted p105 molecule (myc-p105ΔN; Fig. 6B), which cannot be degraded by the proteasome (1). Reporter assays demonstrated that low plasmid concentrations of p105ΔN suppressed LMP1-mediated NF-κB transactivation in NIH 3T3 cells in the absence of an effect on LMP1 expression, as determined by immunoblot analysis of the same lysates (Fig. 6C and D). Furthermore, expression of p105ΔN significantly inhibited wild-type LMP1, CTAR1, or CTAR2, as well as TRAF2-mediated NF-κB transactivation in HEK 293 cells (Fig. 6E). Parallel experiments using equal amounts of an N-terminus-deleted IκBα, which cannot be phosphorylated and degraded by LMP1, demonstrated that this mutated IκBα conferred a more potent inhibitory effect than p105ΔN and almost completely suppressed LMP1- and TRAF2-mediated NF-κB activation (data not shown). Overall, these data demonstrate the involvement of Tpl-2 in LMP1- and TRAF2-induced NF-κB signaling through modulation of p105 function.
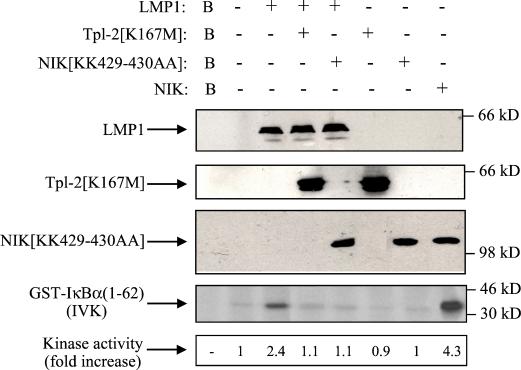
To determine whether Tpl-2 also influences LMP1-mediated IκBα phosphorylation, an essential step for its degradation, HEK 293 cells were transiently transfected with LMP1 in the presence or absence of equal amounts of kinase-inactive Tpl-2. Endogenous IKKα, which mediates phosphorylation of Ser32 and Ser36 of IκBα, was immunoprecipitated from lysates from these cultures and kinase activity was determined using GST-IκBα (aa 1 to 62) as the substrate in an in vitro kinase assay.
While LMP1 was found to induce a 2.1-fold increase in IκBα phosphorylation, coexpression of Tpl-2[K167M] consistently inhibited this effect (Fig. 7, panel 4, lanes 3 and 4). Kinase-inactive NIK (NIK[KK429-430AA]) also blocked LMP1-mediated IκBα phosphorylation, in agreement with a previous report (48), while transfection of wild-type NIK induced significant endogenous IKK kinase activity (Fig. 7, panel 4). We therefore conclude that Tpl-2 influences LMP1-induced NF-κB by targeting signaling pathways which regulate both the inhibitory p105 and IκBα proteins.
FIG. 7.
Involvement of Tpl-2 in LMP1-mediated IκBα activation. HEK 293 cells were transiently transfected with 2.5 μg of pSG5-LMP1 or control vector in the presence or absence of equal amounts of kinase-inactive Tpl-2 (lanes 4 and 6) or NIK (lanes 5 and 7). Lysates were subjected to immunoblot analysis for LMP1, Tpl-2, or NIK expression (upper three panels). Endogenous IKKα was immunoprecipitated using the H744 polyclonal anti-IKKα antibody (Santa Cruz Biotechnology) and analyzed for kinase activity in an in vitro kinase assay (IVK) using GST-IκBα(1-62) as the substrate. As a positive control, lysates from wild-type NIK-transfected cells were used (lane 8). IκBα phosphorylation was measured on a phosphorimager, and relative levels of kinase activity are shown. In three independent experiments, LMP1 induced a mean of 2.1 (±0.4)-fold increase in IκBα activation, while kinase-inactive Tpl-2 consistently suppressed this effect by more than 85%. Background phosphorylation (B, lane 1) from mock immunoprecipitation (in the absence of IKKα antibody) is also shown. Expression of wild-type Tpl-2 has been previously shown to induce IκBα phosphorylation in 293 cells (33).
Tpl-2 modulates the expression of the angiogenic factor COX-2.
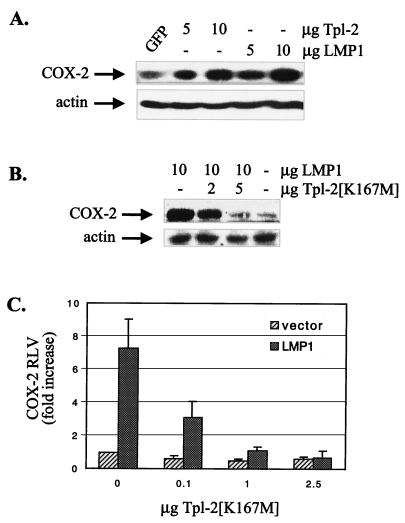
Recent work suggests an important role for LMP1 in the regulation of the angiogenic factor COX-2 in epithelial cells. LMP1-induced COX-2 up-regulation and COX-2 promoter activity critically depend on NF-κB, as a constitutively active IκBα mutant which cannot be phosphorylated and degraded suppressed these LMP1-mediated effects (37). We have found that expression of Tpl-2 in HEK 293 cells promoted the up-regulation of COX-2 to levels similar to those observed following LMP1 expression (Fig. 8A). Transfection of a green fluorescent protein (GFP)-encoding plasmid was used as a negative control for COX-2 expression, and β-actin was used as a loading control in these immunoblots. Transfection of HEK 293 cells with LMP1 in the presence of catalytically inactive Tpl-2[K167M] resulted in a significant inhibition of LMP1-mediated COX-2 induction (Fig. 8B). Consistent with the effects of Tpl-2[K167M] on LMP1-induced NF-κB activation and COX-2 protein levels, we have found that this dominant negative Tpl-2 also modulates COX-2 promoter activity. Transfection of HEK 293 cells with LMP1 leads to a sevenfold increase in COX-2 promoter activity. Coexpression of LMP1 and myc-tagged Tpl-2[K167M] at a ratio of 1:0.04 significantly suppressed reporter activity, which was completely abolished at a 1:1 ratio (Fig. 8C). Taken together, these data suggest that Tpl-2 modulates the ability of LMP1 to promote the expression of the angiogenic factor COX-2.
FIG. 8.
Tpl-2 modulates the expression of the angiogenic factor COX-2. (A) LMP1 and Tpl-2 expression in HEK 293 cells transduces signals leading to COX-2 up-regulation. Cells were transfected with increasing amounts of LMP1, Tpl-2, or GFP-expressing vectors as indicated and 36 h later total lysates were analyzed for COX-2 or β-actin expression by immunoblotting. (B) Catalytically inactive Tpl-2 inhibits the ability of LMP1 to induce COX-2 expression. HEK 293 cells were transfected with 10 μg of LMP1 in the absence or presence of increasing amounts of Tpl-2[K167M] as indicated, and 36 h later total lysates were analyzed for COX-2 or β-actin expression by immunoblotting. (C) Tpl-2[K167M] suppresses LMP1-induced COX-2 promoter activity. HEK293 cells were cotransfected with 0.6 μg of COX-2 promoter-luciferase reporter construct, 0.2 μg of Renilla plasmid, 2.5 μg of pSG5-LMP1, and increasing amounts of Tpl-2[K167M], as indicated. The fold increase in RLVs (ratio of luciferase versus Renilla values) is shown.
DISCUSSION
The EBV-encoded LMP1 is a pleiotropic protein, the activities of which include the oncogenic transformation of rodent fibroblast cell lines, up-regulation of antiapoptotic proteins and cell surface markers, cytokine production, and differentiation blockade in epithelial cells. LMP1 is also essential for EBV-induced B-cell immortalization in vitro and is expressed in a number of EBV-associated malignancies. Genetic and biochemical evidence correlates many of these phenotypic changes and growth-transforming properties with activation of the transcription factor NF-κB (for a review, see reference 3). NF-κB activation by LMP1 requires recruitment of TRAF2 to the cytoplasmic C terminus of the protein (26). TRAF2 lacks intrinsic kinase activity and promotes NF-κB signaling by acting as a platform for the formation of a high-molecular-weight catalytic complex containing NIK, IKKs, and the inhibitory proteins IκBs and p105 among other molecules.
In this study we have demonstrated that the oncogenic MAPKKK Tpl-2 is a component of LMP1-mediated NF-κB signaling. LMP1 promotes the activation of Tpl-2, and expression of catalytically inactive Tpl-2 dramatically inhibits LMP1-induced NF-κB activation as measured by reporter assays and EMSAs (Fig. 3). The extent of this inhibition is similar to the known effects of a kinase-inactive NIK mutant on LMP1-induced NF-κB induction (11, 48) and emphasizes the role of Tpl-2 in LMP1 signaling. This is further supported by the observation that Tpl-2 is recruited in the TRAF2 signaling complex and affects its NF-κB-inducing properties (Fig. 5). Our findings, coupled with the reported ability of Tpl-2 to interact with NIK (33), raise the possibility that TRAF2 forms a higher-order complex containing NIK, Tpl-2, and perhaps other MAPKKKs together with IKK molecules, thus creating a microenvironment which facilitates signal initiation and amplification. The inhibitory effect of kinase-inactive Tpl-2 on CD3/CD28-induced NF-κB activation (33), which is TRAF2 independent, suggests that the interaction between Tpl-2 and TRAF2 is probably indirect and is mediated by NIK. The aggregation of Tpl-2 molecules in this complex may result in their autophosphorylation and increased catalytic activity towards NIK (33).
By virtue of these interactions, Tpl-2 may control both IκBα and p105 functions. Indeed, we have found that kinase-dead Tpl-2 inhibits p105 degradation as well as IKKα activity towards IκBα in LMP1-expressing cells. Consistent with our results, IKKs have been recently shown to phosphorylate p105, in addition to their established substrate IκBα, and TNF treatment promotes the degradation of p105 (20). The contribution of p105 to LMP1- and TRAF2-induced NF-κB activation is substantiated by the effects of a nondegradable p105 mutant (p105ΔN), which was found to inhibit LMP1- and TRAF2-induced NF-κB signals (Fig. 6), providing further evidence for the involvement of p105 in LMP1-mediated NF-κB signaling.
A recent study suggests that NIK may not be essential for NF-κB activation by LMP1. Thus, LMP1-induced NF-κB is not affected in aly/aly mouse embryo fibroblasts carrying a NIK mutation which eliminates the interaction of NIK with IKKα but not IKKβ (34). While this finding does not exclude a role for NIK in LMP1-mediated NF-κB signaling, it suggests that other kinases may compensate for IKK activation in this cell type. Such a redundancy is possible and is exemplified by a recent study on the complementary role of TRAFs in TNF receptor signaling. Thus, although neither TRAF2 nor TRAF5 appears to be uniquely responsible for TNF-induced NF-κB activation, which occurs normally in TRAF2 or TRAF5 knockout mouse embryo fibroblasts, cells lacking both proteins are severely impaired in TNF-induced NF-κB activation (49). Furthermore, NIK has been shown to be essential for CD40-induced NF-κB activation in a cell-type-dependent manner, as CD40 ligation stimulates NF-κB in dendritic but not B cells isolated from aly/aly mice (17). The levels of expression of NIK, Tpl-2, and other components of the IκB kinase complex in different tissues may also affect their relative contributions to NF-κB signaling. Activated Ras has been reported to negatively influence Tpl-2/Cot kinase activity (18). Although Ras mutations are rare in EBV-associated malignancies (30, 52), we cannot exclude the possibility that the effects of Tpl-2 on NIK phosphorylation and activation and Tpl-2 involvement in LMP1-mediated NF-κB signaling are also under negative regulation by Ras or other kinases in a cell-type-dependent manner. This may also provide a possible explanation for the observation that Tpl-2 activation is transient while NF-κB activity is sustained in HEK 293EcR/LMP1 cells induced to express LMP1 (Fig. 2).
As Tpl-2 was found to regulate TRAF2-mediated signaling, we would anticipate that this oncogenic kinase should not affect any LMP1-activated, TRAF2-independent phenomena. LMP1 expression in fibroblasts and cell lines of epithelial and B-cell origins promotes filopodia formation (14, 56, 57) via a Cdc42-dependent pathway (41). This small GTPase, together with its downstream targets Rho and Rac, is involved in various cellular processes such as cytokinesis, adhesion, and cell polarity. Previous work demonstrated that LMP1-induced Cdc42 activation in 3T3 cells occurs independently of TRADD or TRAF2 signaling (41). In line with these findings, we have found that microinjection of kinase-inactive Tpl-2 does not influence the ability of LMP1 to induce filopodia formation (Fig. 4), providing further proof that the Cdc42 and NF-κB signaling pathways are primarily independent. This observation also raises the possibility that Tpl-2 does not affect the metastatic potential of LMP1 related to cell motility and adhesion, which are regulated by the small GTPases. However, we have found that Tpl-2 modulates the expression of two angiogenic factors, COX-2 (Fig. 8) and IL-8 (data not shown). COX-2 is overexpressed in a number of tumors, including NPCs, where Tpl-2 is also found. LMP1 expression correlates with COX-2 expression in vivo and up-regulates COX-2 in vitro via a pathway which critically depends on NF-κB activation (37). Consistent with this observation, we have found that Tpl-2 expression in HEK 293 cells results in COX-2 induction and that a kinase-inactive Tpl-2 mutant suppresses the ability of LMP1 to induce COX-2 protein and promoter activity. These data indicate that Tpl-2 may play a role in LMP1-induced angiogenesis and metastasis.
Overall, our data demonstrate that Tpl-2 is a regulator of LMP1-induced NF-κB activity downstream of TRAF2. As Tpl-2 is known to engage the ERK and JNK pathways, in addition to that of NF-κB, it is possible that it may also affect other LMP1-mediated signals. This possibility is currently under investigation. Taking into account the contribution of NF-κB to oncogenesis and the role of Tpl-2 in this process (5, 42), our findings that Tpl-2 influences LMP1-induced NF-κB activation may have important implications for our understanding of the transforming abilities of this viral protein.
Acknowledgments
We are grateful to Elliot Kieff, Ken Kaye, George Mosialos, Martin Rowe, Takashi Fujita, and John Girdlestone for providing us with plasmids, to Alan Hall and Axel Puls for helpful advice on the microinjection experiments, and to Christos Tsatsanis for helpful discussions.
This work was supported by a Medical Research Council Career Development Award to A.G.E., by Cancer Research UK grants 2091 and 2658 to A.G.E. and L.S.Y., and by NIH grant RO1 CA38047 to P.N.T.
REFERENCES
- 1.Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. Tpl-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 397:363-368. [DOI] [PubMed] [Google Scholar]
- 2.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cell lines. Proc. Natl. Acad. Sci. USA 97:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahir-McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene 18:6959-6964. [DOI] [PubMed] [Google Scholar]
- 4.Cammarano, M. S., and A. Minden. 2001. Dbl and the Rho GTPases activate NF-κB by IκB kinase (IKK)-dependent and IKK-independent pathways. J. Biol. Chem. 276:25876-25882. [DOI] [PubMed] [Google Scholar]
- 5.Ceci, J. D., C. P. Patriotis, C. Tsatsanis, A. M. Makris, R. Kovatch, D. A. Swing, N. A. Jenkins, P. N. Tsichlis, and N. G. Copeland. 1997. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 11:688-700. [DOI] [PubMed] [Google Scholar]
- 6.Dawson, C. W., A. B. Rickinson, and L. S. Young. 1990. Epstein-Barr virus latent membrane protein inhibits human epithelial cell differentiation. Nature (London) 344:777-780. [DOI] [PubMed] [Google Scholar]
- 7.de Gregorio, R., M. A. Iniguez, M. Fresno, and S. Alemany. 2001. Cot kinase induces cyclooxygenase-2 expression in T cells through activation of the nuclear factor of activated T cells. J. Biol. Chem. 276:27003-27009. [DOI] [PubMed] [Google Scholar]
- 8.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. Kleijner, E. Kieff, and G. Mosialos. 1996. TRAF1, TRAF2, and TRAF3 effect NF-κB activation by an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation. Mol. Cell. Biol. 16:7098-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devergne, O., E. C. McFarland, G. Mosialos, K. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumitru, C. D., J. D. Ceci, C. Tsatsanis, D. Kontoyiannis, K. Stamatakis, J. H. Lin, C. Patriotis, N. A. Jenkins, N. G. Copeland, G. Kollias, and P. N. Tsichlis. 2000. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103:1071-1083. [DOI] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., S. M. S. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eliopoulos, A. G., N. J. Gallagher, S. M. S. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 MAPK pathway by Epstein-Barr virus encoded latent membrane protein 1 (LMP1) co-regulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 13.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene 14:2899-2916. [DOI] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435-444. [DOI] [PubMed] [Google Scholar]
- 15.Erny, K. M., J. Peli, J. F. Lambert, V. Muller, and H. Diggelmann. 1996. Involvement of the Tpl-2/cot oncogene in MMTV tumorigenesis. Oncogene 13:2015-2020. [PubMed] [Google Scholar]
- 16.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garceau, N., Y. Kosaka, S. Masters, J. Hambor, R. Shinkura, T. Honjo, and R. J. Noelle. 2000. Lineage-restricted function of nuclear factor κB-inducing kinase (NIK) in transducing signals via CD40. J. Exp. Med. 191:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagemann, D., J. Troppmair, and U. R. Rapp. 1999. Cot proto-oncoprotein activates the dual specificity kinases MEK-1 and SEK-1 and induces differentiation of PC12 cells. Oncogene 18:1391-1400. [DOI] [PubMed] [Google Scholar]
- 19.He, Z. M., B. Z. Xin, X. H. Yang, C. P. Chan, and L. Cao. 2000. Nuclear factor-kappa B activation is involved in LMP1-mediated transformation and tumorigenesis of Rat-1 fibroblasts. Cancer Res. 60:1845-1848. [PubMed] [Google Scholar]
- 20.Heissmeyer, V., D. Krappmann, F. G. Wulczyn, and C. Scheidereit. 1999. NF-κB p105 is a target of IκB kinases and controls signal induction of Bcl-3-p50 complexes. EMBO J. 18:4766-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. B. Rickinson. 1991. Induction of bcl-2 expression by the Epstein-Barr virus latent membrane protein-1 protects infected B cells from programmed cell death. Cell 65:1107-1115. [DOI] [PubMed] [Google Scholar]
- 22.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein 1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 23.Imai, S., S. Koizumi, M. Sugiura, M. Tokunaga, Y. Uemura, N. Yamamoto, S. Tnaka, E. Sato, and T. Osato. 1994. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc. Natl. Acad. Sci. USA 91:9131-9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izumi, K. M., E. C. McFarland, A. T. Ting, E. Riley, B. Seed, and E. D. Kieff. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karin, M. 1999. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene 18:6867-6874. [DOI] [PubMed] [Google Scholar]
- 26.Kaye, K. M., O. Devergne, J. N. Harada, K. M. Izumi, R. Yalamanchili, E. Kieff, and G. Mosialos. 1996. Tumour necrosis factor receptor associated factor 2 is a mediator of NF-κB activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc. Natl. Acad. Sci. USA 93:11085-11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2396. In B. C. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven Press, New York, N.Y.
- 29.Kim, K.-R., T. Yoshizaki, H. Miyamori, K. Hasegawa, T. Horikawa, M. Furukawa, S. Harada, M. Seiki, and H. Sato. 2000. Transformation of Madin-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 19:1764-1771. [DOI] [PubMed] [Google Scholar]
- 30.Knowles, D. M., E. Cesarman, A. Chadburn, G. Frizzera, J. Chen, E. A. Rose, and R. E. Michler. 1995. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 85:552-565. [PubMed] [Google Scholar]
- 31.Lamarche, N., N. Tapon, L. Stowers, P. D. Burbelo, P. Aspenstrom, T. Bridges, J. Chant, and A. Hall. 1996. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87:519-529. [DOI] [PubMed] [Google Scholar]
- 32.Lin, L., G. N. DeMartino, and W. C. Greene. 1998. Cotranslational biogenesis of NF-κB p50 by the 26S proteasome. Cell 92:819-828. [DOI] [PubMed] [Google Scholar]
- 33.Lin, X., E. T. Cunningham, Y. Mu, R. Geleziunas, and W. C. Greene. 1999. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity 10:271-280. [DOI] [PubMed] [Google Scholar]
- 34.Luftig, M. A., E. Cahir-McFarland, G. Mosialos, and E. Kieff. 2001. Effects of the NIK aly mutation on NF-κB activation by the Epstein-Barr virus latent infection membrane protein, lymphotoxin beta receptor, and CD40. J. Biol. Chem. 276:14602-14606. [DOI] [PubMed] [Google Scholar]
- 35.Mercurio, F., J. A. DiDonato, C. Rosette, and M. Karin. 1993. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev. 7:705-718. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi, J., T. Higashi, H. Mukai, T. Ohuchi, and T. Kakunaga. 1991. Structure and transforming potential of the human Cot oncogene encoding a putative protein kinase. Mol. Cell. Biol. 11:4088-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohara, R., S. Hirota, H. Onoue, S. Nomura, Y. Kitamura, and K. Toyoshima. 1995. Identification of the cells expressing COT proto-oncogene messenger RNA. J. Cell Sci. 108:97-103. [DOI] [PubMed] [Google Scholar]
- 39.Patriotis, C., A. Makris, S. E. Bear, and P. N. Tsichlis. 1993. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc. Natl. Acad. Sci. USA 90:2251-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patriotis, C., A. Makris, J. Chernoff, and P. N. Tsichlis. 1994. Tpl-2 acts in concert with Ras and Raf-1 to activate mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 91:9755-9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puls, A., A. G. Eliopoulos, C. D. Nobes, T. Bridges, L. S. Young, and A. Hall. 1999. Activation of the small GTPase Cdc42 by the inflammatory cytokines TNFα and IL-1, and by the Epstein-Barr virus transforming protein LMP1. J. Cell Sci. 112:2983-2992. [DOI] [PubMed] [Google Scholar]
- 42.Rayet, B., and C. Gelinas. 1999. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 43.Rothe, M., V. Sarma, V. M. Dixit, and D. V. Goeddel. 1995. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269:1424-1427. [DOI] [PubMed] [Google Scholar]
- 44.Rowe, M., H. S. Evans, L. S. Young, K. Hennessy, E. Kieff, and A. B. Rickinson. 1987. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J. Gen. Virol. 68:1575-1586. [DOI] [PubMed] [Google Scholar]
- 45.Salmeron, A., T. B. Ahmad, G. W. Carlile, D. Pappin, R. P. Narsimhan, and S. C. Ley. 1996. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 15:817-826. [PMC free article] [PubMed] [Google Scholar]
- 46.Shi, C.-S., and J. H. Kehrl. 1997. Activation of stress-activated protein kinase/cJun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem. 272:32102-32107. [DOI] [PubMed] [Google Scholar]
- 47.Sourvinos, G., C. Tsatsanis, and D. A. Spandidos. 1999. Over-expression of the Tpl-2/Cot oncogene in human breast cancer. Oncogene 18:4968-4973. [DOI] [PubMed] [Google Scholar]
- 48.Sylla, B. S., S. C. Hung, D. M. Davidson, E. Hatzivassiliou, N. L. Malinin, D. Wallach, T. D. Gilmore, E. Kieff, and G. Mosialos. 1998. Epstein-Barr virus-transforming protein latent membrane protein 1 activates transcription factor NF-κB through a pathway that includes the NF-κB-inducing kinase and the IκB kinases IKKα and IKKβ. Proc. Natl. Acad. Sci. USA 95:10106-10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tada, K., T. Okazaki, S. Sakon, T. Kobarai, K. Kurosawa, S. Yamaoka, H. Hashimoto, T. W. Mak, H. Yagita, K. Okumura, W. C. Yeh, and H. Nakano. 2001. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-κB activation and protection from cell death. J. Biol. Chem. 276:36530-36534. [DOI] [PubMed] [Google Scholar]
- 50.Takada, K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapon, N., K. Nagata, N. Lamarche, and A. Hall. 1998. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-κB signalling pathways. EMBO J. 17:1395-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trumper, L., M. Pfreundschuh, G. Jacobs, F. von Bonin, U. Loftin, P. Moller, and H. Daus. 1996. N-ras genes are not mutated in Hodgkin and Reed-Sternberg cells: results from single cell polymerase chain-reaction examinations. Leukemia 10:727-730. [PubMed] [Google Scholar]
- 53.Tsatsanis, C., C. Patriotis, and P. N. Tsichlis. 1998. Tpl-2 induces IL-2 expression in T cell lines by triggering multiple signaling pathways that activate NFAT and NF-κB. Oncogene 17:2609-2618. [DOI] [PubMed] [Google Scholar]
- 54.Tsukamoto, N., N. Kobayashi, S. Azuma, T. Yamamoto, and J.-I. Inoue. 1999. Two differently regulated nuclear factor κB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA 96:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wadleigh, D. J., S. T. Reddy, E. Kopp, S. Ghosh, and H. R. Herschman. 2000. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 275:6259-6266. [DOI] [PubMed] [Google Scholar]
- 56.Wang, D., D. Leibowitz, F. Wang, C. Gregory, A. Rickinson, R. Larson, T. Springer, and E. Kieff. 1988. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 62:4173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 58.Wang, T., W. P. Lafuse, and B. S. Zwilling. 2001. NFκB and Sp1 elements are necessary for maximal transcription of toll-like receptor 2 induced by Mycobacterium avium. J. Immunol. 167:6924-6932. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe, N., T. Iwamura, T. Shinoda, and T. Fujita. 1997. Regulation of NFκB1 proteins by the candidate oncoprotein BCL-3: generation of NF-κB homodimers from the cytoplasmic pool of p50-p105 and nuclear translocation. EMBO J. 16:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang, X., Z. He, B. Xin, and L. Cao. 2000. LMP1 of Epstein-Barr virus suppresses cellular senescence associated with the inhibition of p16(INK4a) expression. Oncogene 19:2002-2013. [DOI] [PubMed] [Google Scholar]
- 61.Yoshizaki, T., H. Sato, M. Furukawa, and J. S. Pagano. 1998. The expression of matrix metelloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc. Natl. Acad. Sci. USA 95:3621-3626. [DOI] [PMC free article] [PubMed] [Google Scholar]