Abstract
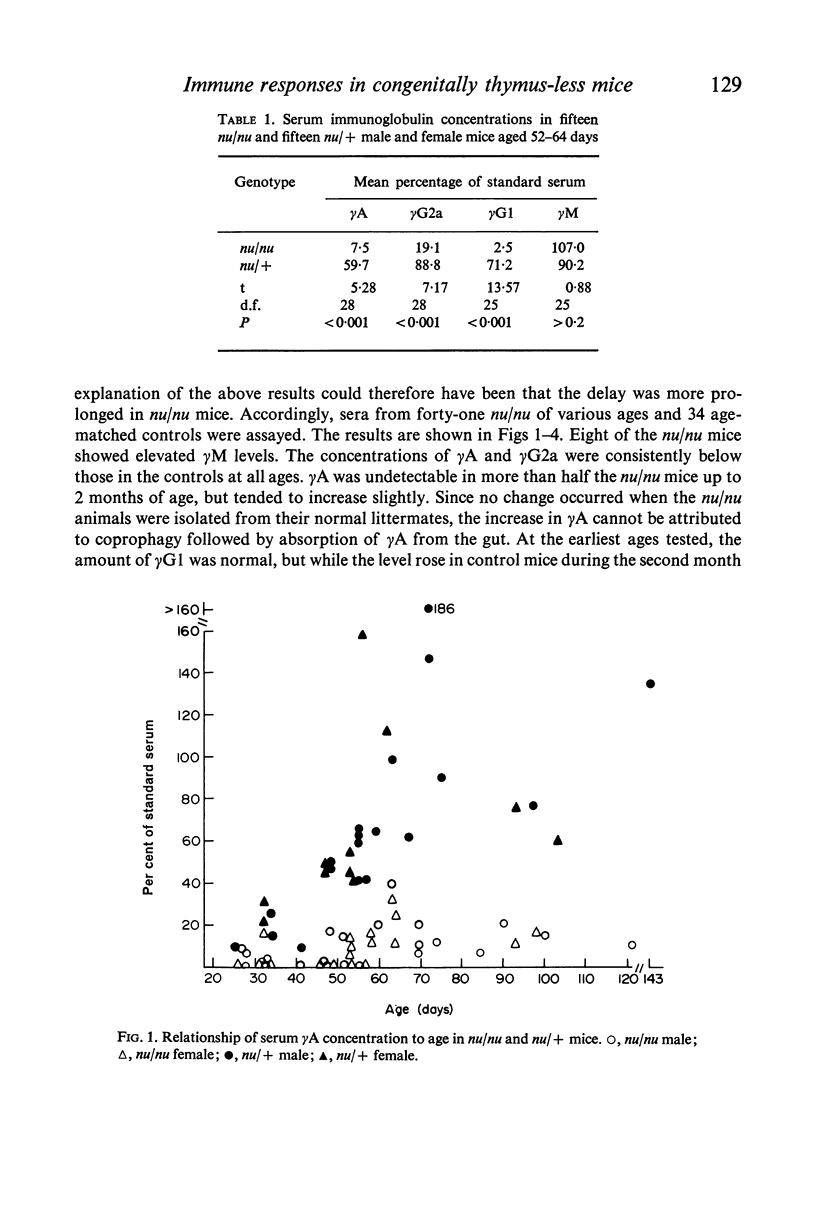
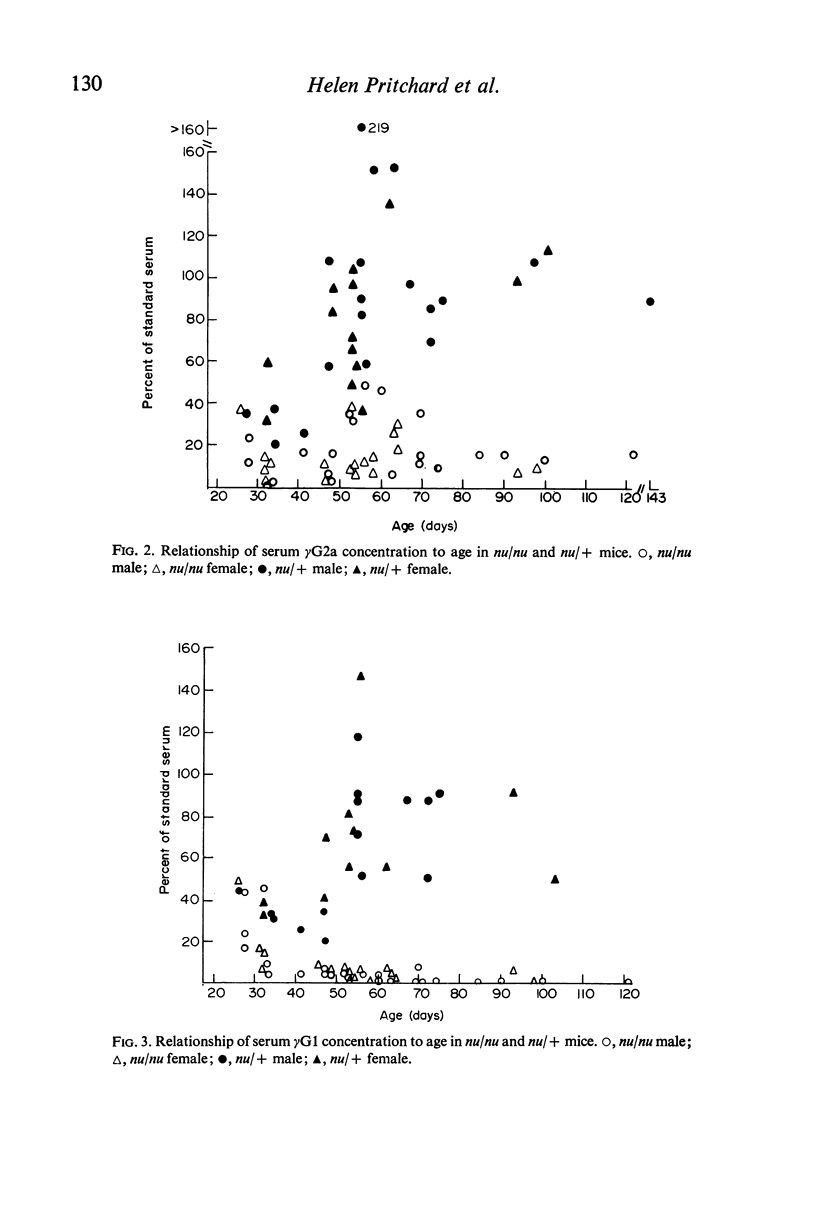
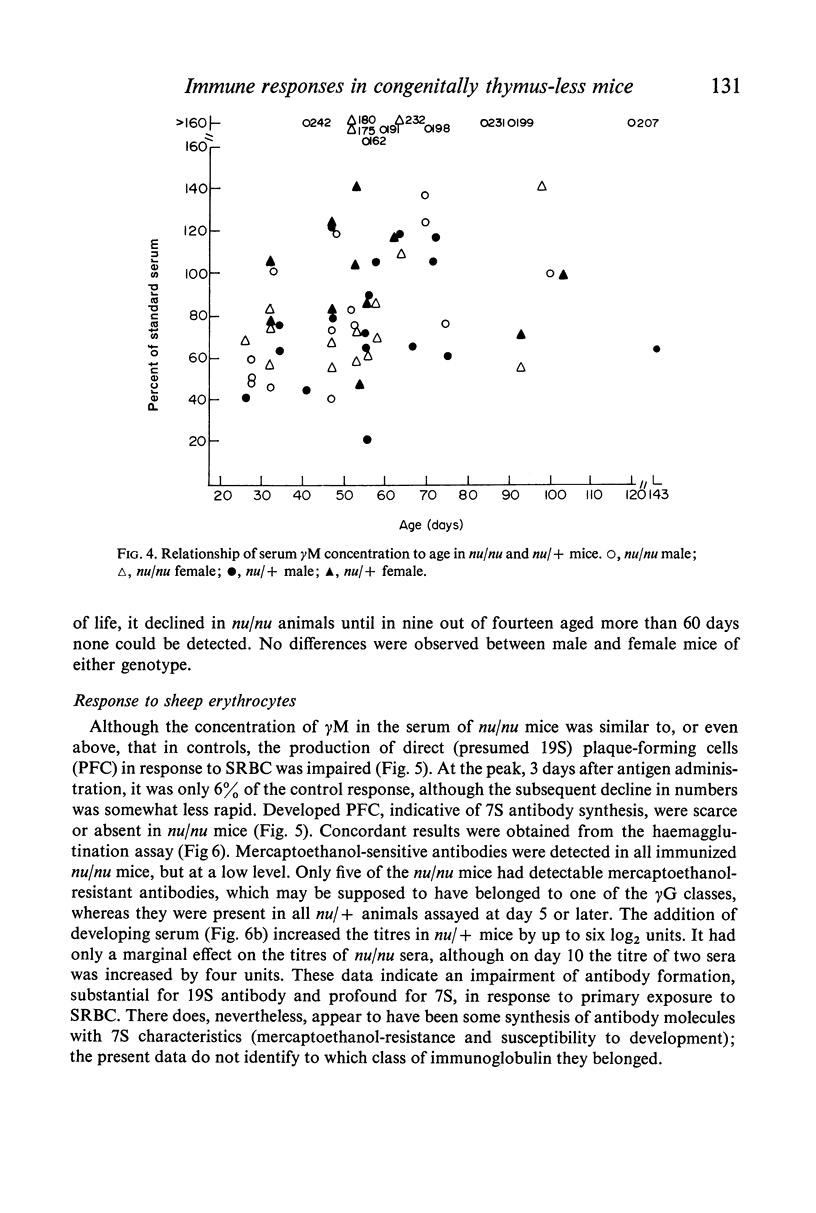
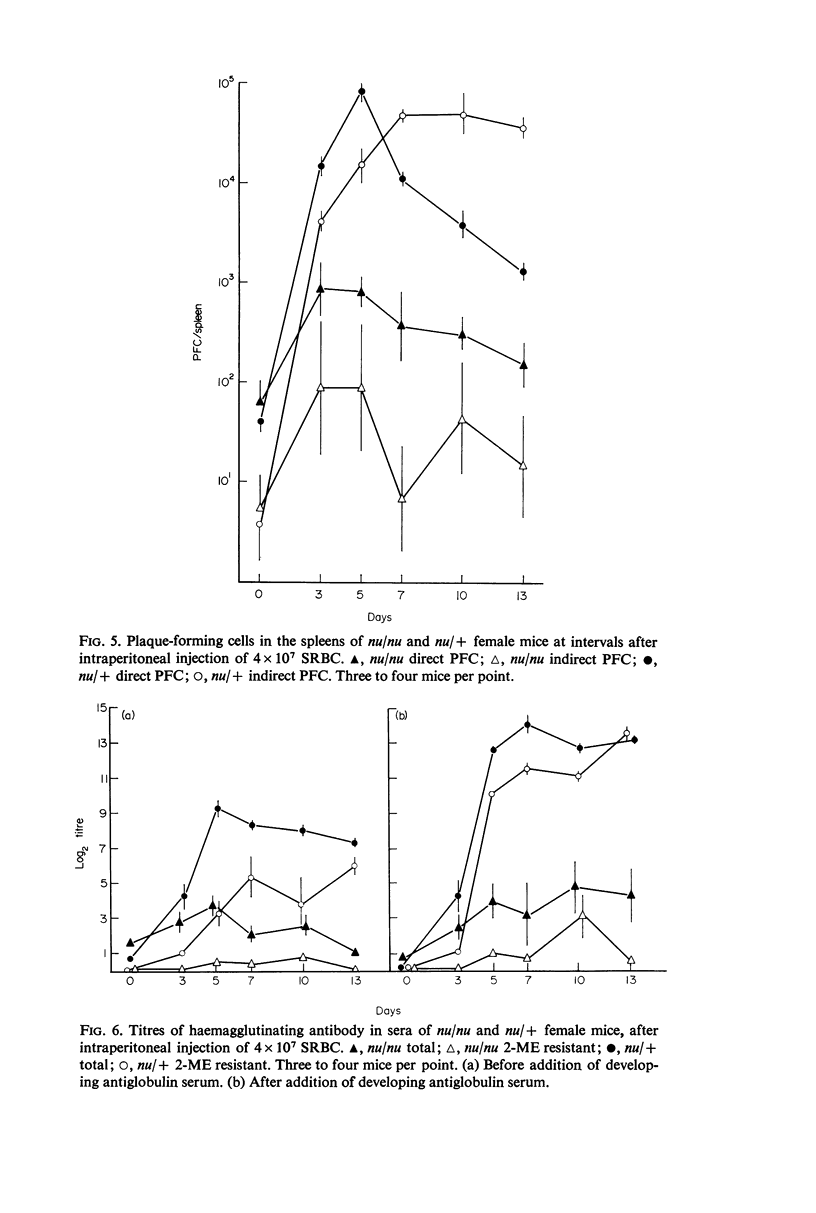
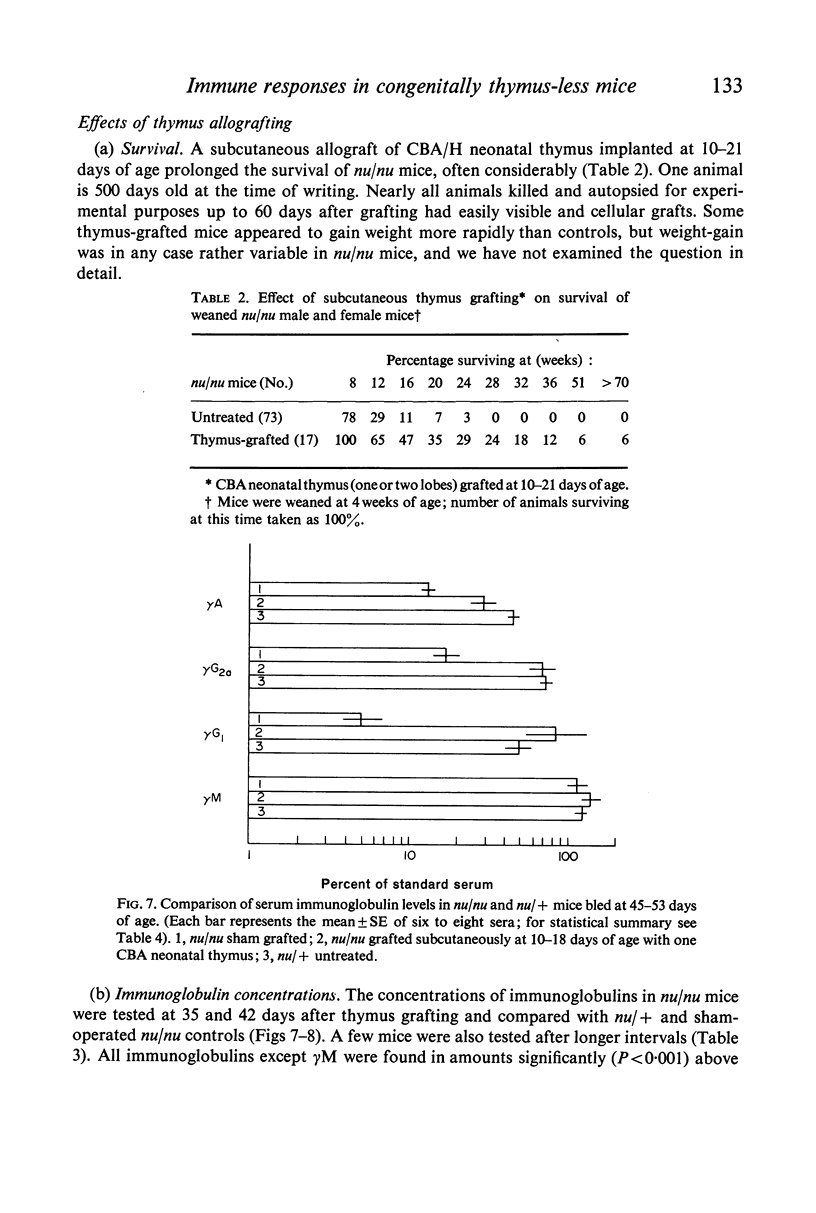
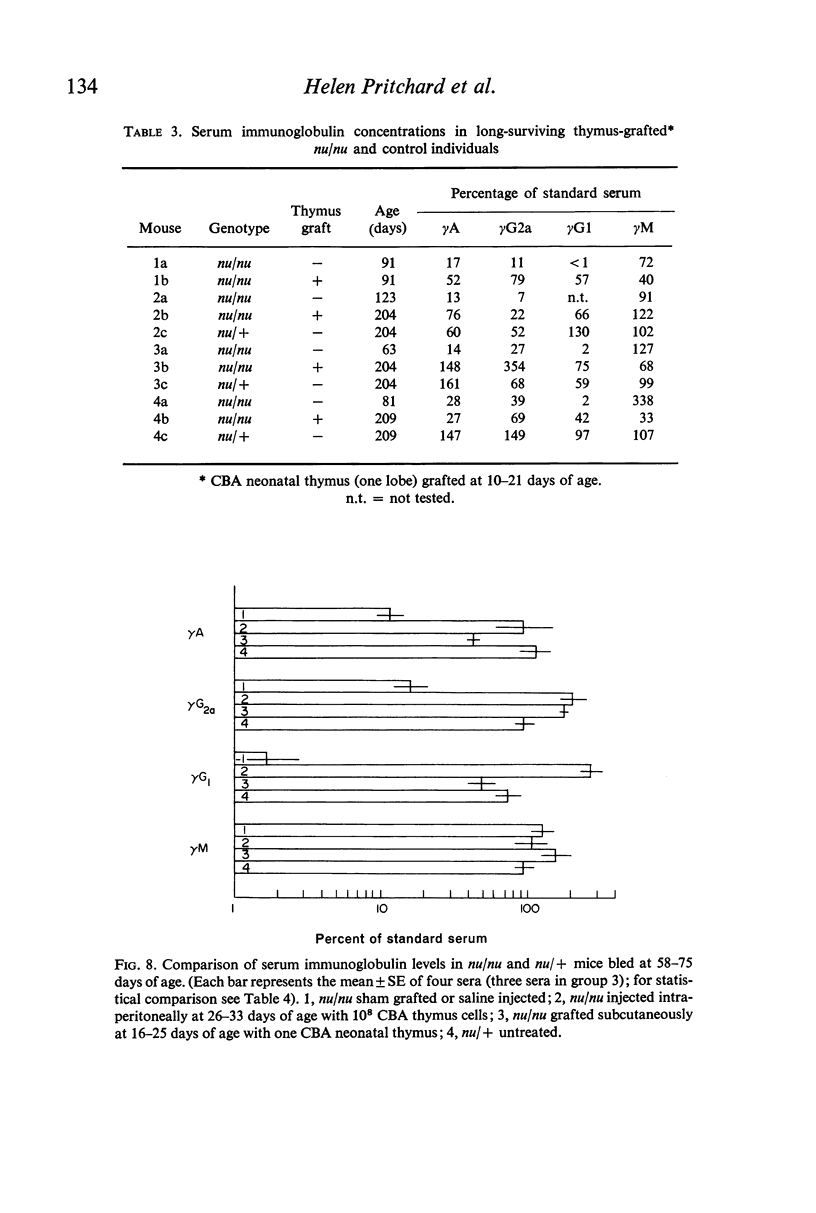
The concentrations of immunoglobulins in the sera of congenitally thymus-less `nude' (nu/nu) mice of various ages were studied by single radial diffusion and compared with those in phenotypically normal nu/+ controls. γM was present in normal concentrations. γA and γG2a concentrations were greatly reduced. γG1 was normal at the time of weaning (28 days of age), but fell progressively thereafter until it was indetectable in most animals more than 60 days old. The primary response to 4 × 107 sheep erythrocytes was studied by haemolytic plaque and serum haemagglutination techniques. Nu/nu mice made subnormal quantities of 19S and little or no 7S antibody. Subcutaneous grafting of neonatal CBA/H thymus at 10–25 days of age was followed by increased survival of nu/nu mice and by the appearance of normal concentrations of γG2a and γG1 globulins and near-normal concentrations of γA. Intraperitoneal injection of 108 CBA/H thymus cells resulted in still higher concentrations of these three proteins. It is concluded that the establishment of normal serum concentrations of γA, γG2a and, especially, γG1 depends upon the presence of funtional T-lymphocytes. γM shows no such T-dependence.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNASON B. G., STCYRCDE V., SHAFFNER J. B. A COMPARISON OF IMMUNOGLOBULINS AND ANTIBODY PRODUCTION IN THE NORMAL AND THYMECTOMIZED MOUSE. J Immunol. 1964 Dec;93:915–925. [PubMed] [Google Scholar]
- Bazin H., Duplan J. F. Modifications du taux des immunoglobulines chez des souris thymectomisées à l'age adulte et irradiées. Rev Fr Etud Clin Biol. 1966 Dec;11(10):987–1000. [PubMed] [Google Scholar]
- Bazin H., Malet F. The metabolism of different immunoglobulin classes in irradiated mice. Immunology. 1969 Sep;17(3):345–365. [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Savin M. C., Micklem H. S. Separation of mouse immunoglobulins on the basis of water-solubility on Sephadex G-25. J Chromatogr. 1968 Apr 9;34(2):180–185. doi: 10.1016/0021-9673(68)80034-2. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Lespinats G., Adam C., Salomon J. C. Immunoglobulins in intact, immunized, and contaminated axenic mice: study of serum IgA. J Immunol. 1971 Dec;107(6):1647–1655. [PubMed] [Google Scholar]
- Benveniste J., Lespinats G., Salomon J. C. Study of immunoglobulins in axenic mice thymectomized at birth. Proc Soc Exp Biol Med. 1969 Mar;130(3):936–940. doi: 10.3181/00379727-130-33692. [DOI] [PubMed] [Google Scholar]
- Claflin A. J., Smithies O., Meyer R. K. Antibody responses in bursa-deficient chickens. J Immunol. 1966 Nov;97(5):693–699. [PubMed] [Google Scholar]
- De Sousa M. A., Parrott D. M., Pantelouris E. M. The lymphoid tissues in mice with congenital aplasia of the thymus. Clin Exp Immunol. 1969 Jun;4(6):637–644. [PMC free article] [PubMed] [Google Scholar]
- Fahey J. L., Barth W. F., Law L. W. Normal immunoglobulins and antibody response in neonatally thymectomized mice. J Natl Cancer Inst. 1965 Oct;35(4):663–678. [PubMed] [Google Scholar]
- Flanagan S. P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., PARROTT D. M., EAST J. STUDIES ON GLOBULIN AND ANTIBODY PRODUCTION IN MICE THYMECTOMIZED AT BIRTH. Immunology. 1964 Jul;7:419–439. [PMC free article] [PubMed] [Google Scholar]
- Kindred B. Antibody response in genetically thymus-less nude mice injected with normal thymus cells. J Immunol. 1971 Nov;107(5):1291–1295. [PubMed] [Google Scholar]
- Kindred B. Immunological unresponsiveness of genetically thymusless (nude) mice. Eur J Immunol. 1971 Jan;1(1):59–61. doi: 10.1002/eji.1830010114. [DOI] [PubMed] [Google Scholar]
- Koltay M., Kinsky R. G., Arnason B. G., Schaffner J. B. Immunoglobulins and antibody formation in mice during the graft versus host reaction. Immunology. 1965 Dec;9(6):581–590. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M. Absence of thymus in a mouse mutant. Nature. 1968 Jan 27;217(5126):370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M., Flisch P. A. Estimation of PFC and serum haemolysin response to SRBC in 'nude' mice. Immunology. 1972 Jan;22(1):159–164. [PMC free article] [PubMed] [Google Scholar]
- Pantelouris E. M., Hair J. Thymus dysgenesis in nude (nu nu) mice. J Embryol Exp Morphol. 1970 Nov;24(3):615–623. [PubMed] [Google Scholar]
- Pantelouris E. M. Observations on the immunobiology of 'nude' mice. Immunology. 1971 Feb;20(2):247–252. [PMC free article] [PubMed] [Google Scholar]
- Pennycuik P. R. Unresponsiveness of nude mice to skin allografts. Transplantation. 1971 Apr;11(4):417–418. doi: 10.1097/00007890-197104000-00012. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Pritchard H., Micklem H. S. Immune responses in congenitally thymus-less mice. I. Absence of response to oxazolone. Clin Exp Immunol. 1972 Jan;10(1):151–161. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Wortis H. H. Thymus dependence of theta-bearing cells in the peripheral lymphoid tissues of mice. Immunology. 1970 Jun;18(6):931–942. [PMC free article] [PubMed] [Google Scholar]
- Reed N. D., Jutila J. W. Immune response of congenitally thymusless mice to heterologous erythrocytes. Proc Soc Exp Biol Med. 1972 Apr;139(4):1234–1237. doi: 10.3181/00379727-139-36337. [DOI] [PubMed] [Google Scholar]
- Rygaard J. Immunobiology of the mouse mutant "Nude". Preliminary investigations. Acta Pathol Microbiol Scand. 1969;77(4):761–762. [PubMed] [Google Scholar]
- Taylor R. B., Wortis H. H. Thymus dependence of antibody response: variation with dose of antigen and class of antibody. Nature. 1968 Nov 30;220(5170):927–928. doi: 10.1038/220927a0. [DOI] [PubMed] [Google Scholar]
- Torrigiani G. Quantitative estimation of antibody in the immunoglobulin classes of the mouse. II. Thymic dependence of the different classes. J Immunol. 1972 Jan;108(1):161–164. [PubMed] [Google Scholar]
- Wortis H. H. Immunological responses of 'nude' mice. Clin Exp Immunol. 1971 Feb;8(2):305–317. [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Nehlsen S., Owen J. J. Abnormal development of the thymus in "nude" mice. J Exp Med. 1971 Sep 1;134(3 Pt 1):681–692. doi: 10.1084/jem.134.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortis H. H., Taylor R. B., Dresser D. W. Antibody production studied by means of the LHG assay. I. The splenic response of CBA mice to sheep erythrocytes. Immunology. 1966 Dec;11(6):603–616. [PMC free article] [PubMed] [Google Scholar]


