Abstract
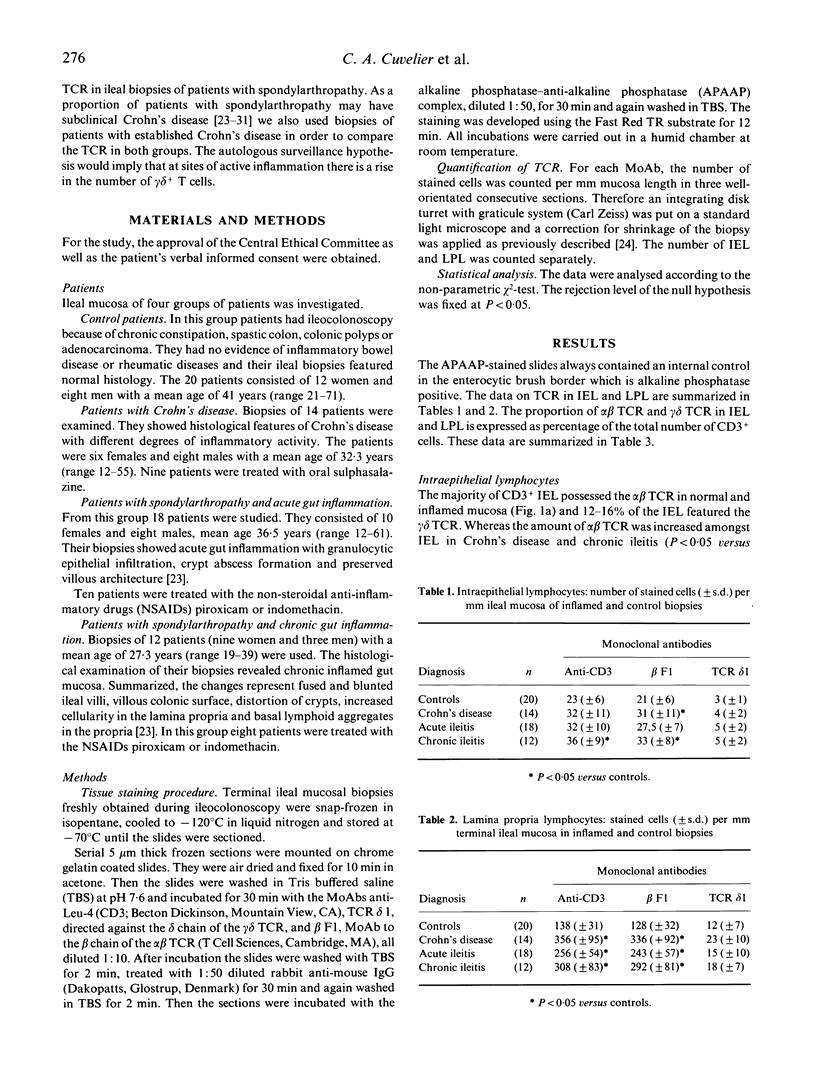
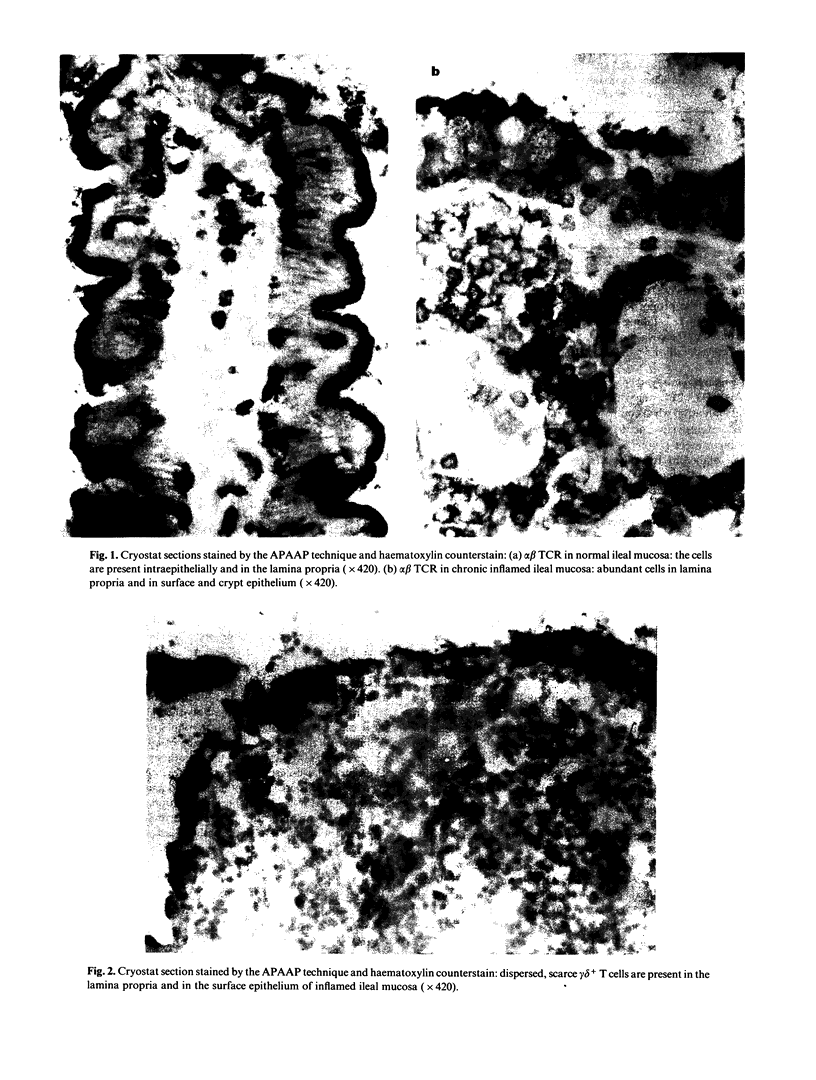
The expression of the alpha beta and gamma delta heterodimer of the T cell receptor (TCR) was studied in normal human ileal mucosa or in ileal biopsies featuring Crohn's disease or acute and chronic spondylarthropathy-related gut inflammation. With an immunohistochemical technique we demonstrated that the increase of mucosal lymphocytes per mm mucosa in Crohn's disease and spondylarthropathy-related ileitis is exclusively due to expansion of the alpha beta + T cell compartment. In Crohn's disease and chronic ileitis observed in some spondylarthropathy patients the alpha beta + T cells were increased amongst intraepithelial lymphocytes (IEL). The lamina propria lymphocytes (LPL) were augmented in all studied inflammatory conditions. The gamma delta + T cells showed no changes in IEL or LPL and their proportions were not altered. They were evenly dispersed throughout the ileal mucosa and did not seem to participate in the inflammatory process. This study confirms that gamma delta T cells are a distinct subset in the intestinal mucosa. The increase in alpha beta + T cells suggests augmented mucosal antigen handling and involvement of the major histocompatibility complex in the pathogenesis of spondylarthropathy-related gut inflammation and Crohn's disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bland P. W., Whiting C. V. Antigen processing by isolated rat intestinal villus enterocytes. Immunology. 1989 Dec;68(4):497–502. [PMC free article] [PubMed] [Google Scholar]
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Born W., Hall L., Dallas A., Boymel J., Shinnick T., Young D., Brennan P., O'Brien R. Recognition of a peptide antigen by heat shock--reactive gamma delta T lymphocytes. Science. 1990 Jul 6;249(4964):67–69. doi: 10.1126/science.1695022. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Riberdy J., Ang S. L., Seidman J. G., Devlin P., Krangel M. S. Two forms of the T-cell receptor gamma protein found on peripheral blood cytotoxic T lymphocytes. Nature. 1987 Feb 19;325(6106):689–694. doi: 10.1038/325689a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Jarry A., Brousse N., Lisowska-Grospierre B., Guy-Grand D., Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987 Sep;17(9):1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Cuvelier C., Barbatis C., Mielants H., De Vos M., Roels H., Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987 Apr;28(4):394–401. doi: 10.1136/gut.28.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier C., Dierick A. M., Mielants H., De Vos M., Veys E., Roels H. Ileal mucosal mononuclear cells in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1990 Mar-Apr;8(2):137–143. [PubMed] [Google Scholar]
- Cuvelier C., Mielants H., De Vos M., Veys E., Roels H. Immunoglobulin containing cells in terminal ileum and colorectum of patients with arthritis related gut inflammation. Gut. 1988 Jul;29(7):916–925. doi: 10.1136/gut.29.7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier C., Mielants H., De Vos M., Veys E., Roels H. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut. 1990 May;31(5):545–549. doi: 10.1136/gut.31.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M., Cuvelier C., Mielants H., Veys E., Barbier F., Elewaut A. Ileocolonoscopy in seronegative spondylarthropathy. Gastroenterology. 1989 Feb;96(2 Pt 1):339–344. doi: 10.1016/0016-5085(89)91557-6. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989 Dec;30(6):665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Happ M. P., Kubo R. T., Palmer E., Born W. K., O'Brien R. L. Limited receptor repertoire in a mycobacteria-reactive subset of gamma delta T lymphocytes. Nature. 1989 Dec 7;342(6250):696–698. doi: 10.1038/342696a0. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Zhu B. Y., Chess L., Knowles D. M. Flow cytometric and immunohistochemical characterization of the gamma/delta T-lymphocyte population in normal human lymphoid tissue and peripheral blood. Am J Pathol. 1990 Feb;136(2):357–367. [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S. H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990 Mar 1;171(3):667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Eisenhardt D., Salomon P., Bauer W., Plous R., Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., De Vos M. Subclinical involvement of the gut in undifferentiated spondylarthropathies. Clin Exp Rheumatol. 1989 Sep-Oct;7(5):499–504. [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Joos R., Cuvelier C., De Vos M. Repeat ileocolonoscopy in reactive arthritis. J Rheumatol. 1987 Jun;14(3):456–458. [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Joos R., Noens L., Cuvelier C., De Vos M. HLA antigens in seronegative spondylarthropathies. Reactive arthritis and arthritis in ankylosing spondylitis: relation to gut inflammation. J Rheumatol. 1987 Jun;14(3):466–471. [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Joos R., Suykens S., Cuvelier C., De Vos M. Familial aggregation in seronegative spondyloarthritis of enterogenic origin. A family study. J Rheumatol. 1986 Feb;13(1):126–128. [PubMed] [Google Scholar]
- Mielants H., Veys E. M. The gut in the spondyloarthropathies. J Rheumatol. 1990 Jan;17(1):7–10. [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H. Immunology. Antigens for gamma/delta T cells. Nature. 1989 Jun 1;339(6223):342–343. doi: 10.1038/339342a0. [DOI] [PubMed] [Google Scholar]
- Savilahti E., Reunala T., Mäki M. Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut. 1992 Feb;33(2):206–211. doi: 10.1136/gut.33.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., Diss T. C., MacDonald T. T. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor gamma/delta heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989 Jul;19(7):1335–1338. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G. Human T-cell receptor expression. Nature. 1989 Feb 2;337(6206):416–416. doi: 10.1038/337416a0. [DOI] [PubMed] [Google Scholar]
- Strominger J. L. Developmental biology of T cell receptors. Science. 1989 May 26;244(4907):943–950. doi: 10.1126/science.2658058. [DOI] [PubMed] [Google Scholar]
- Takagaki Y., DeCloux A., Bonneville M., Tonegawa S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989 Jun 29;339(6227):712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Calabrese A., Smart C. J., Oakes D. J., Howdle P. D., Crabtree J. E., Losowsky M. S., Lancaster F., Boylston A. W. Gamma delta T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Heliobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin Exp Immunol. 1991 Jun;84(3):440–444. [PMC free article] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Smart C. J., Oakes D. J., Howdle P. D., Malizia G., Campana D., Boylston A. W. Expression of T-cell receptors TcR1 (gamma/delta) and TcR2 (alpha/beta) in the human intestinal mucosa. Immunology. 1989 Sep;68(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Ullrich R., Schieferdecker H. L., Ziegler K., Riecken E. O., Zeitz M. gamma delta T cells in the human intestine express surface markers of activation and are preferentially located in the epithelium. Cell Immunol. 1990 Jul;128(2):619–627. doi: 10.1016/0008-8749(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Viney J., MacDonald T. T., Spencer J. Gamma/delta T cells in the gut epithelium. Gut. 1990 Aug;31(8):841–844. doi: 10.1136/gut.31.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]




