Abstract
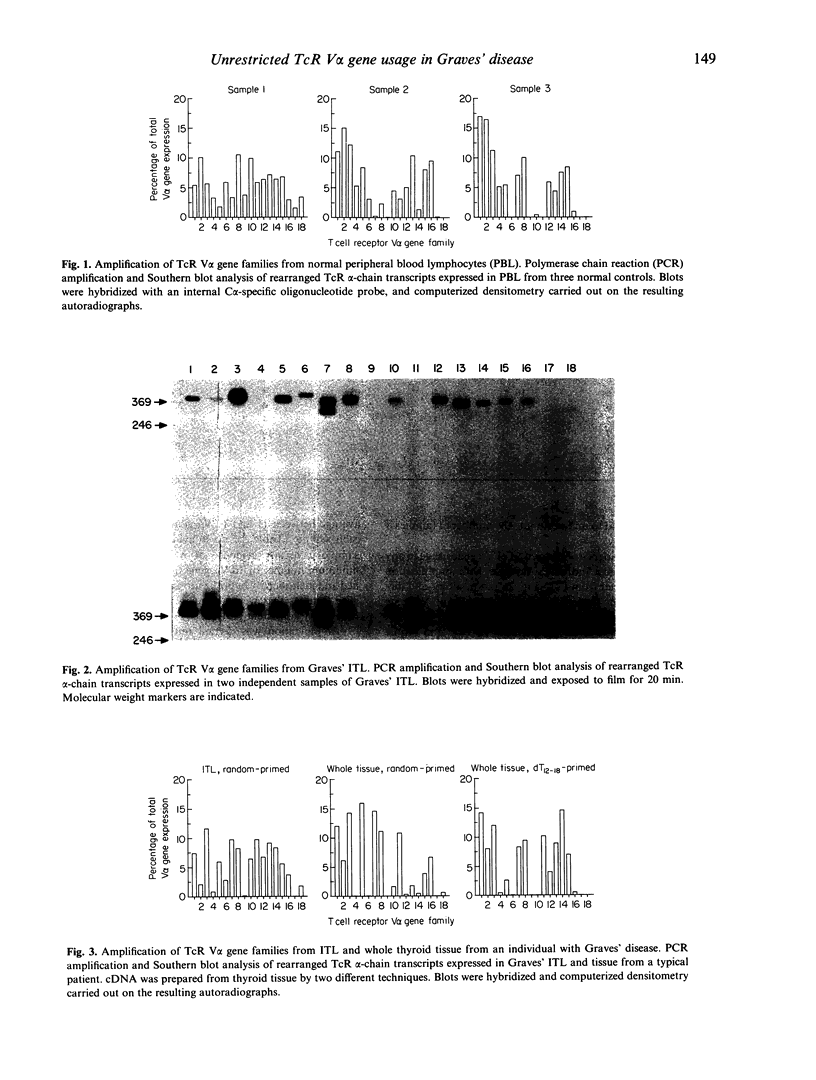
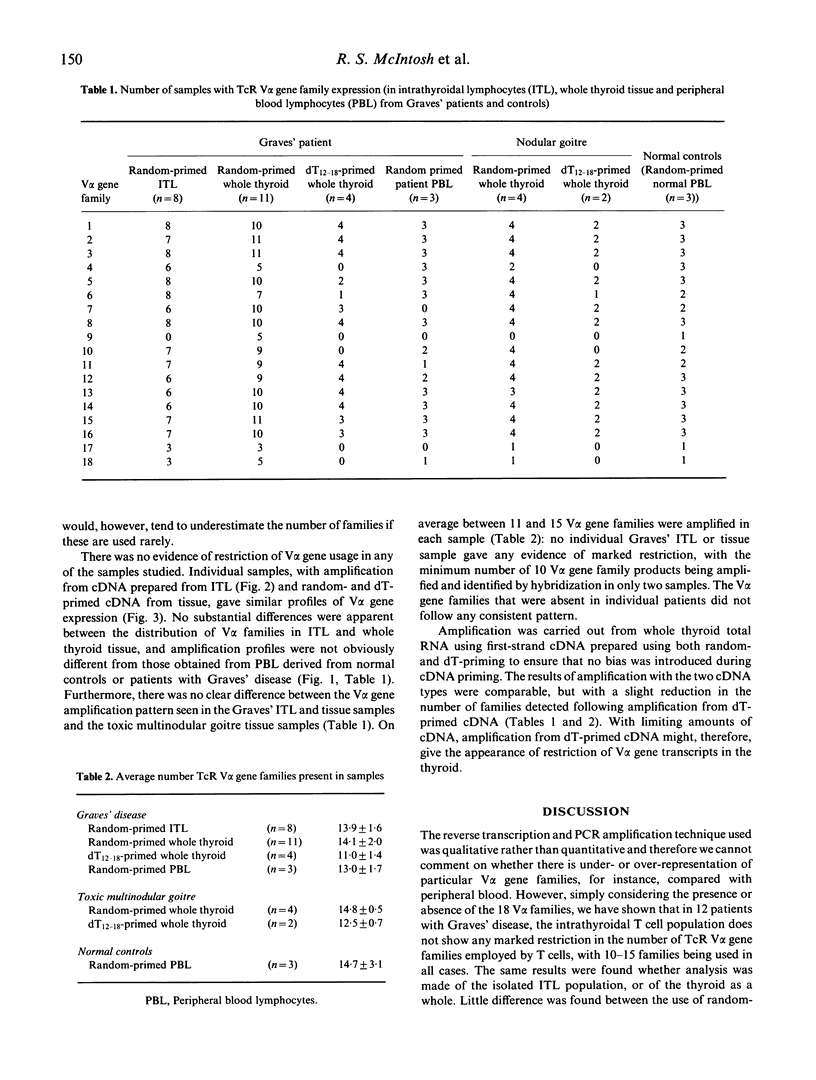
Recently it has been reported that the intrathyroidal T cells in Graves' disease display restriction in V alpha T cell receptor (TcR) gene family usage, although this is not found with TcR V beta gene families in the same individuals. We have performed a qualitative analysis of TcR V alpha family usage in 12 patients with Graves' disease by reverse transcription and polymerase chain reaction (PCR) amplification of RNA extracted from isolated, unstimulated intrathyroidal lymphocytes and from snap-frozen whole thyroid specimens. No restriction was observed, with 10-15 V alpha gene families being amplified in all cases. The pattern of usage was similar to that in peripheral blood lymphocytes derived from normal subjects (n = 3) and from patients with Graves' disease (n = 3), as well as that present in the thyroids of patients with non-autoimmune toxic multinodular goitre (n = 4). These results indicated that there is no marked restriction of the unselected intrathyroidal T cell population in patients with Graves' disease who have been treated with antithyroid drugs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Anderson G. D., Luthra H. S., David C. S. Influence of complement C5 and V beta T cell receptor mutations on susceptibility to collagen-induced arthritis in mice. J Immunol. 1989 Apr 1;142(7):2237–2243. [PubMed] [Google Scholar]
- Blackman M., Kappler J., Marrack P. The role of the T cell receptor in positive and negative selection of developing T cells. Science. 1990 Jun 15;248(4961):1335–1341. doi: 10.1126/science.1972592. [DOI] [PubMed] [Google Scholar]
- Brostoff S. W., Howell M. D. T cell receptors, immunoregulation, and autoimmunity. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 1):1–7. doi: 10.1016/0090-1229(92)90016-h. [DOI] [PubMed] [Google Scholar]
- Burns F. R., Li X. B., Shen N., Offner H., Chou Y. K., Vandenbark A. A., Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar V alpha and V beta chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989 Jan 1;169(1):27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica G. W., Cosulich M. E., Croci R., Ferrini S., Bagnasco M., Dirienzo W., Ferrini O., Bargellesi A., Giordano G. Thyroglobulin-induced T-cell in vitro proliferation in Hashimoto's thyroiditis: identification of the responsive subset and effect of monoclonal antibodies directed to Ia antigens. Clin Immunol Immunopathol. 1984 Aug;32(2):132–141. doi: 10.1016/0090-1229(84)90115-6. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Cohen L., Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991 Jul 25;325(4):238–244. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Martin A., Concepcion E. S., Graves P., Lahat N., Cohen W. L., Ben-Nun A. Evidence for selective accumulation of intrathyroidal T lymphocytes in human autoimmune thyroid disease based on T cell receptor V gene usage. J Clin Invest. 1992 Jan;89(1):157–162. doi: 10.1172/JCI115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan C. M., Feldmann M., Rapoport B., Londei M. Autoimmune thyroiditis and targeted anti-T cell immunotherapy in man. Autoimmunity. 1992;11(3):189–198. doi: 10.3109/08916939209035154. [DOI] [PubMed] [Google Scholar]
- Dayan C. M., Londei M., Corcoran A. E., Grubeck-Loebenstein B., James R. F., Rapoport B., Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby A. D., Sinclair A. K., Osborne-Lawrence S. L., Zeldes W., Kan L., Fox D. A. Clonal heterogeneity of synovial fluid T lymphocytes from patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6206–6210. doi: 10.1073/pnas.86.16.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisfalen M. E., DeGroot L. J., Quintans J., Franklin W. A., Soltani K. Microsomal antigen-reactive lymphocyte lines and clones derived from thyroid tissue of patients with Graves' disease. J Clin Endocrinol Metab. 1988 Apr;66(4):776–784. doi: 10.1210/jcem-66-4-776. [DOI] [PubMed] [Google Scholar]
- Giegerich G., Pette M., Meinl E., Epplen J. T., Wekerle H., Hinkkanen A. Diversity of T cell receptor alpha and beta chain genes expressed by human T cells specific for similar myelin basic protein peptide/major histocompatibility complexes. Eur J Immunol. 1992 Mar;22(3):753–758. doi: 10.1002/eji.1830220319. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E., Acha-Orbea H. The V-region disease hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989 May;10(5):164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Winters S. T., Olee T., Powell H. C., Carlo D. J., Brostoff S. W. Vaccination against experimental allergic encephalomyelitis with T cell receptor peptides. Science. 1989 Nov 3;246(4930):668–670. doi: 10.1126/science.2814489. [DOI] [PubMed] [Google Scholar]
- Katzin W. E., Fishleder A. J., Tubbs R. R. Investigation of the clonality of lymphocytes in Hashimoto's thyroiditis using immunoglobulin and T-cell receptor gene probes. Clin Immunol Immunopathol. 1989 May;51(2):264–274. doi: 10.1016/0090-1229(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Kaulfersch W., Baker J. R., Jr, Burman K. D., Ahmann A. J., D'Avis J. C., Waldmann T. A. Immunoglobulin and T cell antigen receptor gene arrangements indicate that the immune response in autoimmune thyroid disease is polyclonal. J Clin Endocrinol Metab. 1988 May;66(5):958–963. doi: 10.1210/jcem-66-5-958. [DOI] [PubMed] [Google Scholar]
- Keystone E. C., Minden M., Klock R., Poplonski L., Zalcberg J., Takadera T., Mak T. W. Structure of T cell antigen receptor beta chain in synovial fluid cells from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Dec;31(12):1555–1557. doi: 10.1002/art.1780311213. [DOI] [PubMed] [Google Scholar]
- Lehmann P. V., Forsthuber T., Miller A., Sercarz E. E. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992 Jul 9;358(6382):155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- Lipoldova M., Londei M., Grubeck-Loebenstein B., Feldmann M., Owen M. J. Analysis of T-cell receptor usage in activated T-cell clones from Hashimoto's thyroiditis and Graves' disease. J Autoimmun. 1989 Feb;2(1):1–13. doi: 10.1016/0896-8411(89)90103-0. [DOI] [PubMed] [Google Scholar]
- MacKenzie W. A., Schwartz A. E., Friedman E. W., Davies T. F. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987 Apr;64(4):818–824. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., Daha M. R., de Vries R. R., van den Elsen P. J., Breedveld F. C. Dominant T-cell receptor beta-chain gene rearrangements indicate clonal expansion in the rheumatoid joint. Scand J Immunol. 1990 Jan;31(1):121–126. doi: 10.1111/j.1365-3083.1990.tb02750.x. [DOI] [PubMed] [Google Scholar]
- Nakano N., Kikutani H., Nishimoto H., Kishimoto T. T cell receptor V gene usage of islet beta cell-reactive T cells is not restricted in non-obese diabetic mice. J Exp Med. 1991 May 1;173(5):1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksenberg J. R., Stuart S., Begovich A. B., Bell R. B., Erlich H. A., Steinman L., Bernard C. C. Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature. 1990 May 24;345(6273):344–346. doi: 10.1038/345344a0. [DOI] [PubMed] [Google Scholar]
- Savill C. M., Delves P. J., Kioussis D., Walker P., Lydyard P. M., Colaco B., Shipley M., Roitt I. M. A minority of patients with rheumatoid arthritis show a dominant rearrangement of T-cell receptor beta chain genes in synovial lymphocytes. Scand J Immunol. 1987 Jun;25(6):629–635. doi: 10.1111/j.1365-3083.1987.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N., Freeman M. A., Weetman A. P. T cell responses to synthetic TSH receptor peptides in Graves' disease. Clin Exp Immunol. 1992 Sep;89(3):468–473. doi: 10.1111/j.1365-2249.1992.tb06982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N., Freeman M., Weetman A. P. T cell responses to synthetic thyroid peroxidase peptides in autoimmune thyroid disease. Clin Exp Immunol. 1991 Oct;86(1):56–60. doi: 10.1111/j.1365-2249.1991.tb05773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Van Laar J. M., Miltenburg A. M., Verdonk M. J., Daha M. R., De Vries R. R., Van den Elsen P. J., Breedveld F. C. Lack of T cell oligoclonality in enzyme-digested synovial tissue and in synovial fluid in most patients with rheumatoid arthritis. Clin Exp Immunol. 1991 Mar;83(3):352–358. doi: 10.1111/j.1365-2249.1991.tb05642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark A. A., Hashim G., Offner H. Immunization with a synthetic T-cell receptor V-region peptide protects against experimental autoimmune encephalomyelitis. Nature. 1989 Oct 12;341(6242):541–544. doi: 10.1038/341541a0. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., McGregor A. M. Autoimmune thyroid disease: developments in our understanding. Endocr Rev. 1984 Spring;5(2):309–355. doi: 10.1210/edrv-5-2-309. [DOI] [PubMed] [Google Scholar]



