Abstract
The latency-associated nuclear antigen 1 (LANA-1) of Kaposi's sarcoma-associated herpesvirus (KSHV) is required for the maintenance and replication of viral episomal DNA. The binding sites for nuclear heterochromatin and transcriptional repressor complexes are located in an amino-terminal region of LANA-1, whereas those for viral episomal DNA, p53, pRB, and members of the BRD/fsh family of nuclear proteins are located in its carboxy-terminal domain. LANA-1 activates or represses several cellular and viral promoters. In this report we show that a domain of 15 amino acids (amino acids 1129 to 1143), located close to the carboxy-terminal end of LANA-1, is required for the interaction of LANA-1 with nuclear heterochromatin or nuclear matrix, and for the ability of LANA-1 to activate the Epstein-Barr virus Cp promoter. LANA-1 proteins that are tightly associated with nuclear heterochromatin or matrix differ in molecular weight from LANA-1 proteins that can be dissociated from the nuclear matrix by high-salt buffers, suggesting that posttranslational modifications may determine the association of LANA-1 with nuclear heterochromatin or matrix.
Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (7) is a human type 2 gammaherpesvirus associated with all forms of Kaposi's sarcoma (KS) (5, 16, 31, 38) and linked with primary effusion lymphoma (6) and the plasma cell variant of multicentric Castleman's disease (41). KSHV persists in KS lesions (4, 12, 22, 35, 42), in a latent form with limited viral gene expression. Among the latent viral genes expressed in KS spindle cells, primary effusion lymphoma cells, and KSHV-infected B cells in multicentric Castleman's disease is latency-associated nuclear antigen 1 (LANA-1) (13, 23, 35), encoded by open reading frame 73 (orf73) (24, 25, 35).
LANA-1 is a nuclear protein which associates with nuclear heterochromatin during interphase and with chromosomes during mitosis (2, 9, 43, 44) and has been shown to bind to histone H1 (8).
LANA-1 appears to have multiple functions, of which the best delineated is its role in the replication and tethering to mitotic chromosomes of viral episomal DNA (2, 3, 18, 19, 44). For this purpose, LANA-1 binds to two 17-bp nucleotide motifs, LBS1 and LBS2, located in the terminal repeat of the KSHV genome, via a region in its C-terminal domain (3, 18, 19).
In addition, LANA-1 activates and/or represses several heterologous cellular and viral promoters (9, 17, 18, 26, 27, 28, 39). The mechanisms responsible for these effects are only partially understood but may involve the ability of LANA-1 to modulate the activity of, or interact with, cellular transcription factors such as CREB, CBP, and Sp1 (1, 26, 27, 34) (see Fig. 1). LANA-1 has also been reported to bind to p53 and repress the expression of p53-dependent promoters (17), as well as to interact with pRB and modulate the expression of E2F-dependent transcription (34). The binding to p53 and pRB appears to involve regions in the C-terminal half of LANA-1 (34). Similarly, a C-terminal domain (amino acids [aa] 1007 to 1055) binds to RING3/BRD2 and other members of the fsh/BRD family of nuclear factors (30, 33; unpublished observations). Given the reported role of BDF-1, a yeast member of this family, as a global modulator of transcription, it is thought that some of the four human homologues may also affect the transcription of cellular genes (29), and one member, HUNK1/MCAP, has been shown to be involved in the regulation of the cell cycle (11). The interaction of LANA-1 with RING3 leads to the phosphorylation of serine and threonine residues located between aa 951 and 1107 of LANA-1 (33), but the functional consequences of this are not yet understood. RING3 has been reported to interact with E2F and modulate E2F-dependent transcription (10); whether its interaction with LANA-1 affects this property is also unresolved.
FIG. 1.
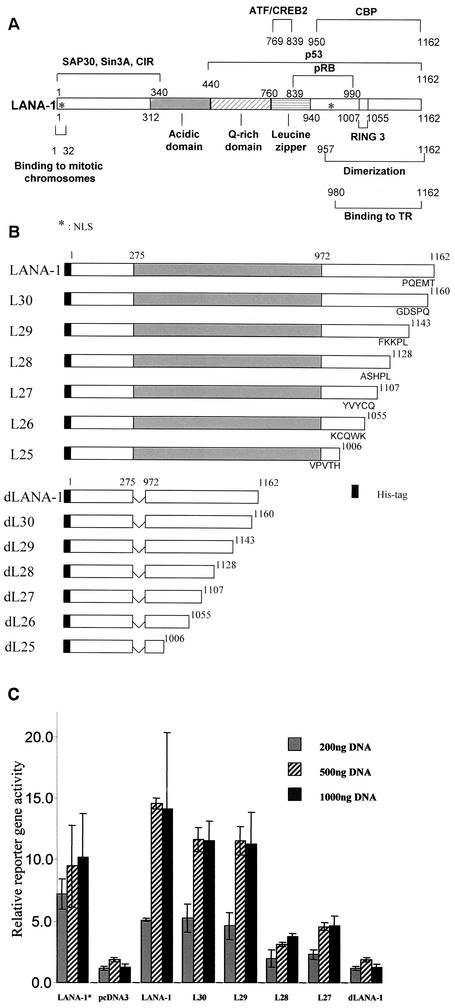
The region from aa 1129 to 1143 of LANA-1 is required for transcriptional activation of the EBV Cp promoter. (A) Binding sites for different nuclear proteins on LANA-1 and other functionally important regions, as far as they have been mapped at present. TR, terminal repeat in the KSHV genome; pRB, retinoblastoma protein; ✽, NLS, nuclear localization signal. (B) Deletion constructs of LANA-1 used in the present study showing the C-terminal 5 aa of each construct. (C) Activation of the EBV Cp promoter by LANA-1 deletion mutants. A total of 50 ng of a reporter construct containing 2.0 kb of EBV sequence upstream of the transcriptional start site of the EBV Cp promoter and a luciferase reporter gene were transfected into 293 cells, together with increasing concentrations of LANA-1, and the luciferase activity was measured. The relative activation of the LANA-1 constructs is shown. The experiment was repeated more than four times. A representative experiment is shown. Expression of the different LANA-1 constructs was checked with KS-positive human sera (not shown). LANA-1* represents a wild-type LANA-1 construct lacking a His tag.
The C-terminal region of LANA-1 also contains one of the two nuclear localization signals and mediates dimerization of LANA-1 in solution (39). The other nuclear localization signal in LANA-1 is located in its N-terminal region (aa 24 to 30) next to a domain (aa 5 to 22) that mediates attachment to nuclear chromatin during interphase and to mitotic chromosomes (32). Given that LANA-1 binds to viral episomal DNA via its C-terminal region (see above), it is therefore assumed that LANA-1 acts as a “bridge” between nuclear heterochromatin and viral episomal DNA. In addition, the N-terminal region has been shown to interact with transcriptional repressors such as SIN-3 (26) and to act as a transcriptional repressor when fused to a GAL4-binding domain (26). The central domain of LANA-1 contains several highly acidic repeat motifs, as well as a leucine zipper (37).
In the present study we examined the effect of a series of truncations of the C-terminal domain of LANA-1 on its ability to activate a heterologous promoter in an attempt to correlate the presence of previously reported interaction domains within LANA-1 with its ability to act as a transcriptional activator.
To produce whole LANA-1 and LANA-1-deletion constructs, the amino-terminal fragment of LANA-1 was first cloned from BCP-1 DNA by PCR with the primers orf73H6C (TAT GAA TTC AGA TCT CAC CAC CAT CAT CAC CAT GCG CCC CCG GGA ATG CGCCTG AGG TCG), containing a His tag motif, and orf73.1 (GTC CCC ATT ATC CTC GCC AGC). The amplified DNA fragment was inserted into Bluescript (Stratagene) by using EcoRI and BamHI. The resulting plasmid was named pBlueN. The whole LANA-1 C terminus was then cloned from BCP-1 DNA into pBlueN with primers 2B73 (TAT ACT AGT GAC GAT GAC CCA CAA CCT GGC CC) and 2A73 (CTC GAT GCG GCC GCT TAT GTC ATT TCC TGT GAG AG) containing SpeI and NotI sites, respectively (pBlueN-C). The C-terminal deletion constructs (L25 to L30) were amplified from pBlueN-C by PCR with primers containing SpeI and NotI sites. Coordinates for the C-terminal deletions are shown in Fig. 1B, and primer sequences are available from the authors on request. The numbering of amino acids in LANA-1 in the present study corresponds to the sequence published by Russo et al. (37). The acidic internal repeat was cloned into pBlueN-C or the N-C deletion constructs by using BamHI and NruI (aa 273 and 980, respectively). The internal repeat was obtained from a cosmid containing KSHV orf71, orf72, and orf73 (20). Finally, the whole LANA-1 construct or the LANA-1 deletion constructs were cloned into pcDNA3 (Invitrogen) by using HindIII and NotI. All constructs used in this work were sequenced to ensure that no sequence errors occurred during the cloning procedure.
To analyze the effect of the LANA-1 truncations in transcriptional activation, we designed a construct containing 2.0 kb of the Epstein-Barr virus (EBV) Cp promoter upstream of the transcriptional start site which contained the EBNA-2 response elements but lacked the EBV origin of replication. This was done by cloning into pGL2Basic (Promega) a PCR amplification product of 2 kb of the EBV Cp promoter from a plasmid containing the EBV 5.0 Cp promoter (14). All transfections were carried out by using FuGENE (Roche). To normalize the transfection efficiency, the cells were also transfected with pCMV-β-Gal and β-galactosidase activity was detected by using a plate reader (Anthos htIII). Luciferase activity was measured as recommended by the manufacturer's manual with a luminometer (Lumat LB9501; Berthold). The experiments were performed in triplicates.
Cotransfection of the LANA-1 constructs and the EBV Cp promoter into 293 cells showed that removal of the last 34 aa, but not removal of the last 19 aa, abolished LANA-1-mediated activation of this reporter plasmid (Fig. 1C). These results identified a region of 15 aa (aa 1129 to 1143) required for LANA-1-mediated transcriptional activation in this system. This region is located outside the domain of aa 1007 to 1055 previously identified as containing a binding site for RING3 (33).
We also deleted the central repeat region of LANA-1 from a full-length LANA-1 expression construct (dLANA-1), as well as from the series of C-terminal truncation mutants (delL25 to delL30) (Fig. 1B) and tested these constructs for their ability to activate the Cp promoter. Deletion of the internal repeat region from full-length LANA-1 and the C-terminal truncation mutants eliminated the activation of the Cp promoter (Fig. 1C and not shown).
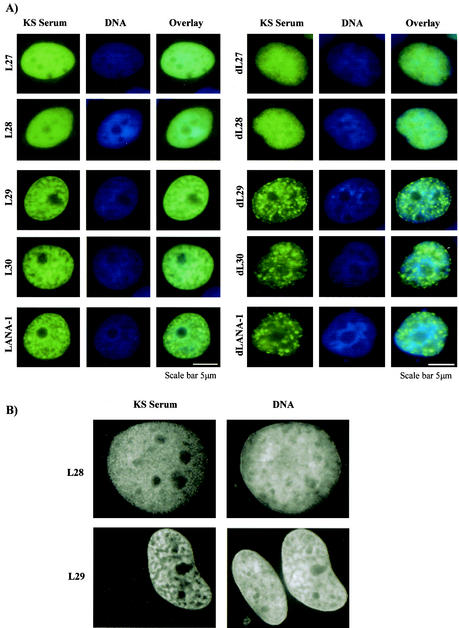
We next examined the nuclear localization and intranuclear distribution of the various LANA-1 mutants. Immunofluorescence analysis was performed as previously described (40) on cells grown on coverslips 40 h after transfection. LANA-1 wild-type and mutants were detected with KSHV-positive patient serum (1:300) and secondary rabbit anti-human fluorescein isothiocyanate-conjugated antibody (1:40) in phosphate-buffered saline containing 2% fetal bovine serum. The cellular DNA was stained with Hoechst 33258 (Sigma). All mutant proteins localized to the nucleus, a finding in line with the fact that both previously identified nuclear localization signals of LANA-1 (see Fig. 1A) were retained in these constructs. However, we noticed marked differences in their intranuclear distribution. As previously reported (30, 32, 44), full-length LANA-1 associates with nuclear heterochromatin and displays a heterochromatin-like staining pattern in transfected cells (Fig. 2). In a series of C-terminal truncations, this heterochromatin-like staining pattern is lost in the mutant (L28) that terminates at aa 1128 and which shows a diffuse nuclear localization (Fig. 2). The region from aa 1129 to 1143 appears to be crucial for the heterochromatin association, since mutant L29, terminating at aa 1143, shows the normal heterochromatin association of full-length LANA-1 (Fig. 2A). The transition between the heterochromatin-associated staining pattern and a diffuse nuclear distribution is seen even more clearly in the case of the series of deletion mutants that lack the internal repeat (Fig. 2A). The dLANA-1 protein, containing the N-terminal and C-terminal regions of LANA-1, is localized in heterochromatin-associated nuclear speckles (Fig. 2A). After successive deletion of the C-terminal end of this protein to aa 1128, this speckled nuclear localization changes to a diffuse intranuclear distribution of the truncated protein. This change in nuclear distribution was observed in MCF-7 cells (Fig. 2), as well as in L cells, 293 cells, and HeLa cells (not shown) and therefore does not appear to be cell type dependent.
FIG. 2.
Intranuclear distribution of LANA-1 deletion mutants. MCF7 cells grown on glass coverslips were transfected with LANA-1 and individual LANA-1 deletion mutants (see Fig. 1 for a description of mutants). At 2 days after transfection, cells were stained with serum from a patient with KS and fluorescence-conjugated secondary antibody. (A) Staining for LANA-1 (green), and DNA (Hoechst 33258), and overlay. (B) Black-and-white photograph showing the difference in nuclear staining pattern between L29 (heterochromatin-associated pattern) and L28 (diffuse nuclear staining).
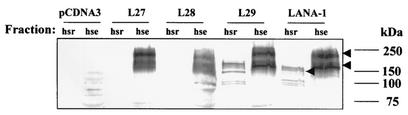
To confirm these results biochemically, we investigated whether the association of LANA-1, or LANA-1 truncation mutants, with nuclear heterochromatin would be resistant to washes in high-salt buffers. Transfected 293 T cells were lysed for 30 min in a low-ionic-strength buffer in the presence of small amounts of NP-40 medium (5% glycerol, 1% NP-40, 0.2 mM EDTA, 10 mM Tris-HCl [pH 7.9], leupeptin [50 μM], benzamidin [200 μM], aprotinin [100 U/ml], pepstatin A [1 μM], phenylmethylsulfonyl fluoride [1 mM]), and after separation of the nuclear material by centrifugation, LANA-1 was mostly recovered in the pellet (not shown). When the lysate was further incubated with 300 μl of hypertonic buffer (5% glycerol, 1% NP-40, 10 mM Tris-HCl, 500 mM KCl) for 15 min on ice, centrifuged at 15,700 × g for 10 min at 4°C, two fractions were obtained. The supernatant (soluble fraction) containing both cytoplasmic and nuclear soluble proteins was transferred to a clean tube. The pellet (matrix-associated fraction) was washed thoroughly with 500 μl of hypertonic buffer, pelleted as described above, and incubated with 100 μg of DNase I/ml for 1 h on ice. The protein was then loaded into a polyacrylamide gel, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were performed as previously described (40). LANA-1 was detected by using KSHV-positive patient serum (1:500) and secondary rabbit anti-human horseradish peroxidase-conjugated antibody (1:1,000), followed by enhanced chemiluminescence (Perkin-Elmer Life Sciences). Using this fractionation protocol, we can distinguish between a high-salt-extractable (hse) fraction and a high-salt-resistant (hsr) fraction of LANA-1 (Fig. 3). The hse form of LANA-1 has a higher molecular weight than the tightly heterochromatin or nuclear matrix-associated hsr form. This most likely suggests that the hse and hsr forms of LANA-1 have different posttranslational modifications.
FIG. 3.
Association of LANA-1 and LANA-1 deletion mutants with fraction of the nuclear matrix that is resistant to extraction in high-salt buffers. 293T cells were transfected with LANA-1-expressing constructs, lysed in low-ionic-strength buffer containing 1% NP-40, followed by the addition of a buffer containing 500 mM KCl and the hsr fraction of the nuclear material pelleted by centrifugation as described in the text. After the pellet was washed in high-salt buffer, the hsr pellet and hse supernatant were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting, with serum from a Kaposi's sarcoma patient to detect LANA-1. Arrowheads indicate the position of the two different LANA-1 isoforms in the hse and hsr fractions.
We also examined whether the C-terminal LANA-1 truncation mutants also occur in an hse and an hsr form. As shown in Fig. 3, C-terminal truncation to aa 1128 leads to the disappearance of the hsr form and L28, as well as all shorter truncated proteins, are found exclusively in the “extractable” fraction. In contrast L29, truncated to aa 1143, behaves like the full-length protein, being found in both the “extractable” and the “residual” fractions. This result supports the observations on heterochromatin association made by immunofluorescence analyses and suggests that the region encompassing aa 1129 and 1143 influences nuclear heterochromatin association.
A previous study (32) had identified a heterochromatin-binding region of LANA-1 within its N-terminal region (aa 5 to 22), whereas the C-terminal 230 aa of LANA-1 have been reported to bind to a defined sequence motif in the terminal repeat of the viral genome (3, 18, 19). To investigate whether the region between aa 1129 and 1143 contained an additional interaction site for heterochromatin and to explore which of these regions is responsible for the presence of LANA-1 in the “residual” fraction after high-salt extraction, we fused both regions to enhanced green fluorescent protein (EGFP) and determined their intranuclear localization by fluorescence microscopy and fractionation of nuclei from transfected cells by high-salt washes.
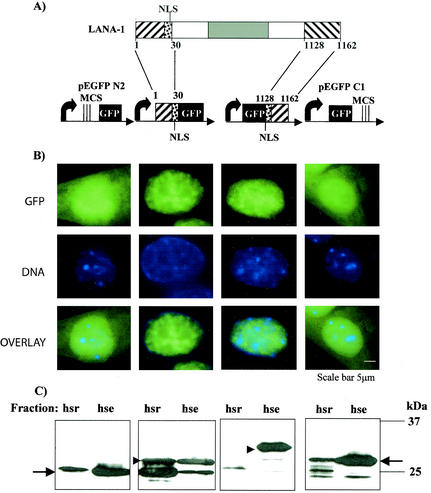
To produce LANA-1 fragments fused to EGFP, pEGFP-N2 and pEGFP-C1 (Clontech) plasmids were used. The primers 73NBglF (TTA AAG ATC TCG AGG ATG GCG CCC CCG GGA ATG) and 73HindIIIRev (TTA AAA GCT TTC CGG AGA CCT GTT TCG TTT C), containing BglII and HindIII sites, respectively, were used to amplify the first 30 aa of LANA-1. This fragment was cloned into pEGFP-N2 and expressed as a fusion protein with LANA-1 aa 1 to 30 positioned upstream of EGFP (Fig. 4A). The C-terminal 40 aa (aa 1128 to 1162) were amplified with primers 73C2BglF (TTA AAG ATC TAG GAA ACG AAA CAG GGT CTC CGG AAG CTA GTC ACC CCC CTG) and 73C2HindR (TTA AAA GCT TTT ATG TCA TTT CCT GTG GAG), containing BglII and HindIII sites, respectively, cloned into pEGFP-C1 and expressed as a fusion protein downstream of EGFP (Fig. 4A). The oligonucleotide 73C2BglF also contains the sequence coding for the LANA-1 N-terminal nuclear localization signal (aa 24 to 30). Therefore, both EGFP-LANA-1 fusion proteins contain the same LANA-1 nuclear localization signal.
FIG. 4.
Association of aa 1 to 30 and aa 1128 to 1162 of LANA-1 with nuclear heterochromatin. (A) Diagram of EGFP fusion constructs used in this experiment. The region from aa 1 to 30 of LANA-1, containing a previously described (32) binding site (aa 1 to 24) for nuclear heterochromatin and a nuclear localization signal (aa 24 to 30), was fused upstream of EGFP, by using the vector EGFPN2, and aa 1128 to 1162 of LANA-1, representing its C-terminal end (see Fig. 1) were fused downstream of EGFP by using the vector EGFPC1. MCS, multiple cloning site; NLS, nuclear localization signal. (B) Intranuclear localization of EGFP fusion proteins in L cells. Cells were transfected with the constructs indicated, and the intracellular localization of the corresponding proteins was analyzed by fluorescence. (C) Association of aa 1 to 30 and aa 1128 to 1162 of LANA-1 with the hsr and hse nuclear fractions. The experiment was carried out as described in the legend to Fig. 3, except that an antibody to EGFP was used to stain the Western blots. Arrows indicate the position of EGFP expressed from the pEGFP-N2 (left panel) and pEGFP-C1 (right panel) vectors. Arrowheads indicate the positions of aa1-30/EGFP and EGFP/aa1128-1162 fusion proteins.
Fluorescence analysis of L cells transfected with these constructs indicated that both fusion proteins localized to the nucleus, as intended. The heterochromatin-associated staining pattern typical of LANA-1 (Fig. 2) was seen for the aa1-30/EGFP fusion protein (Fig. 4B), whereas the aa1128-1162/EGFP fusion protein showed a more diffuse nuclear localization (Fig. 4B). When nuclei of cells transfected with these constructs were fractionated by using the high-salt wash protocol and the two fractions were analyzed on Western blots with a monoclonal antibody to EGFP, we found that, as for unfused LANA-1 (Fig. 3), the aa1-30/EGFP fusion protein was present in both the extractable and the residual fractions (Fig. 4C). In contrast, the aa1122-1162/EGFP fusion protein could be completely extracted from the nuclear matrix-heterochromatin by high-salt washes. This result suggests that the previously identified (32) amino-terminal heterochromatin-binding site is responsible for the tight association of LANA-1 with nuclear heterochromatin that is resistant to washes with high-salt buffers. In contrast, these results do not support the existence of an additional heterochromatin-binding site within aa 1129 to 1143, although a weaker binding site cannot be excluded. Thus, although the deletion of aa 1129 to 1143 from the complete LANA-1 protein removes the tight association with nuclear heterochromatin, these amino acids are not, on their own, sufficient to mediate this tight interaction. This region seems to modulate the tight association with nuclear heterochromatin through an as-yet-unknown mechanism. The observation that the same region affects the ability of LANA-1 to activate a heterologous viral promoter could suggest that LANA-1-mediated transcriptional activation is linked to its interaction with nuclear heterochromatin.
The current view is that LANA-1 interacts with nuclear heterochromatin via aa 5 to 22 (32). We could confirm that this amino-terminal region of LANA-1 can target EGFP to nuclear heterochromatin (Fig. 4B) and extend this observation by demonstrating that it also determines the association of LANA-1 with a component of the nuclear heterochromatin or matrix that is resistant to extraction by 500 mM KCl (Fig. 4C). The tightly heterochromatin- or nuclear matrix-bound form of LANA-1 has a slightly reduced molecular weight compared to the form of LANA-1 that is easily extracted under these conditions (Fig. 3). This observation suggests that posttranslational modifications could modulate the tight association of LANA-1 with nuclear heterochromatin or nuclear matrix. The nature of this posttranslational modification is currently unknown but could involve phosphorylation or conjugation with ubiquitin or one of the three isoforms of SUMO.
Given the finding that aa 1 to 30 are sufficient to mediate attachment to a high-salt-concentration-resistant component of the nuclear structure, it appears at first surprising that deletion of aa 1129 to 1143 should abolish this tight association with nuclear heterochromatin or nuclear matrix. Our results also do not suggest that aa 1122 to 1162 contain an additional binding site for nuclear heterochromatin, although a low-affinity interaction could probably not be ruled out. A possible explanation therefore is that deletion of aa 1129 to 1143 induces conformational changes in LANA-1 that lead to a concealment of the amino-terminal chromatin-binding region or that this region interacts with another nuclear protein capable of modulating the interaction between LANA-1 and the nuclear matrix. Based on our earlier report (33) that aa 1007 to 1055 contain a binding site for RING3, it would seem unlikely that RING3 or one of its homologues (33) represents this factor. However, in our earlier report (33), we noted binding of RING3 to glutathione S-transferase-LANA-1 fusion protein containing aa 1046 to 1162, suggesting that RING3 may interact with more than one domain in LANA-1.
Although the C-terminal 205 aa of LANA-1 have been shown to contain a region required for dimerization (39), we do not feel that dimerization is required for the tight (i.e., hsr) attachment to nuclear heterochromatin, since the EGFP fusion protein containing aa 1 to 30 showed this tight attachment, suggesting that tight heterochromatin attachment is possible in the absence of the LANA-1 dimerization domain. It is therefore in our view more likely that posttranslational modifications of the C-terminal domain, or its interaction with another nuclear component, would modulate the ability of LANA-1 to interact tightly with nuclear heterochromatin via its amino-terminal aa 5 to 22 and its role as a transcriptional activator.
The short stretch of sequence between aa 1129 and 1143, AGNLQSSIVKFKKPL, contains two serine and three lysine residues, putative targets, respectively, for phosphorylation, ubiquitinylation, and sumoylation. We have mutated residues 1132 to 1141 in pairs to alanine and examined their ability to activate the Cp promoter and their nuclear distribution: none of these mutants had lost the ability to interact with nuclear heterochromatin by immunofluorescence or in the high-salt fractionation assay. Mutant KK1140/1141AA was markedly (and the other mutants only moderately) impaired in the ability to activate the Cp promoter (not shown). This finding could suggest that structural defects or interaction with an unidentified ligand, rather than a single posttranslational modification, could be the basis for the effect of aa 1129 to 1143.
The link between the heterochromatin association of LANA-1 and its ability to activate a heterologous promoter in 293 cells suggests that LANA-1 could activate transcription by targeting chromatin structures. LANA-1 appears to be a promiscuous transactivator of several cellular and viral genes (21, 34, 36) and has been shown to interact functionally with the transcription factors CREB/ATF, CBP, and SP1 (1, 26, 27). In addition, we have recently reported the observation that LANA-1-transfected cells appear to have an altered heterochromatin pattern (30). Other viral transactivators, such as the herpes simplex virus type 1 ICP0 protein, have been shown to modulate transcriptional activation of other viral promoters by relieving repressive nuclear structures through involvement of the nuclear proteasome machinery (15).
Based on the observations reported here it is therefore conceivable that LANA-1 could help to convert regions of transcriptionally inactive heterochromatin to become transcriptionally active.
A recent report (21) showed that deletion mutants of LANA-1 containing either aa 1 to 950 or aa 301 to 942, i.e., lacking the region of aa 1129 to 1143 investigated here, could activate the EBV LMP-1 promoter in the B-cell line BJAB. We also found that aa 276 to 971 are crucial for the activation of the EBV Cp promoter in 293 cells and that their absence changes the intranuclear distribution pattern of LANA-1 but does not abolish its association with nuclear heterochromatin. It is thus possible that aa 276 to 971, although not required for the targeting to nuclear heterochromatin, contribute to heterochromatin modifications that are necessary for transcriptional activation. The region from aa 276 to 971 contains an acidic repeat region, a glutamine-rich repeat region and the leucine zipper. LANA-1 has been shown to interact with histone H1 (8), and it is thus possible that such interaction is responsible for chromatin modulation. Given the observation by Groves et al. (21) that aa 301 to 942 are sufficient for the activation of the LMP-1 promoter in BJAB cells, it is thus conceivable that, depending on the promoter or cell type studied, different regions of LANA-1 contribute to the activation of heterologous promoters.
Acknowledgments
We thank Paul Farrel for the EBV Cp 5.0 construct.
This work was sponsored by the Deutsche Forschungsgemeinschaft (Schu 1432-1/1).
REFERENCES
- 1.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 2.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff, C., and R. A. Weiss. 1998. Kaposi's sarcoma-associated herpesvirus. Adv. Cancer Res. 75:57-86. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 8.Cotter, M. A., and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 9.Cotter, M. A., C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 10.Denis, G. V., C. Vaziri, N. Guo, and D. V. Faller. 2000. RING3 kinase transactivates promoters of cell cycle regulatory genes through E2F. Cell Growth Differ. 11:417-424. [PMC free article] [PubMed] [Google Scholar]
- 11.Dey, A., J. Ellenberg, A. Farina, A. E. Coleman, T. Maruyama, S. Sciortino, J. Lippincott-Schwartz, and K. Ozato. 2000. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2-to-M transition. Mol. Cell. Biol. 20:6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, T. J., P. J. Farrell, and S. Swaminathan. 1996. Molecular genetic analysis of Epstein-Barr virus Cp promoter function. J. Virol. 70:1695-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R. D. 1999. A surprising role for the proteasome in the regulation of herpesvirus infection. Trends Biochem. Sci. 24:293-295. [DOI] [PubMed] [Google Scholar]
- 16.Fakhari, F. D., and D. P. Dittmer. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213-6223. [DOI] [PMC free article] [PubMed]
- 17.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 18.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 20.Glenn, M., L. Rainbow, F. Aurade, A. Davsion, and T. F. Schulz. 1999. Identification of a multiply spliced gene of KSHV/HHV8 with similarities to the latent membrane proteins of EBV. J. Virol. 73:6953-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groves, A. K., M. A. Cotter, C. Subramanian, and E. S. Robertson. 2001. The latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus activates two major essential Epstein-Barr virus latent promoters. J. Virol. 75:9446-9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iscovich, J., P. Boffetta, S. Franceschi, E. Azizi, and R. Sarid. 2000. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer 88:500-517. [PubMed] [Google Scholar]
- 23.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269:335-344. [DOI] [PubMed] [Google Scholar]
- 24.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kellam, P., C. Boshoff, D. Whitby, S. Matthews, R. A. Weiss, and S. J. Talbot. 1997. Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1:19-29. [PubMed] [Google Scholar]
- 26.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 28.Lim, C., Y. Gwack, S. Hwang, S. Kim, and J. Choe. 2001. The transcriptional activity of cAMP response element-binding protein-binding protein is modulated by the latency associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Biol. Chem. 276:31016-31022. [DOI] [PubMed] [Google Scholar]
- 29.Matangkasombut, O., R. M. Buratowski, N. W. Swilling, and S. Buratowski. 2000. Bromodomain factor 1 corresponds to a missing piece of yeast TFIID. Genes Dev. 14:951-962. [PMC free article] [PubMed] [Google Scholar]
- 30.Mattsson, K., C. Kiss, G. M. Platt, G. R. Simpson, E. Kashuba, G. Klein, T. F. Schulz, and L. Szekely. 2002. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J. Gen. Virol. 83:179-188. [DOI] [PubMed] [Google Scholar]
- 31.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with or without HIV infection. N. Engl. J. Med. 338:1181-1185. [DOI] [PubMed] [Google Scholar]
- 32.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platt, G. M., G. R. Simpson, S. Mittnacht, and T. F. Schulz. 1999. Latent nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts with RING3, a homolog of the Drosophila female sterile homeotic (fsh) gene. J. Virol. 73:9789-9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 35.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S.-J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulz, T. F. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J. Antimicrob. Chemother. 43(T3):15-27. [DOI] [PubMed] [Google Scholar]
- 39.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson, G. R., T. F. Schulz, D. Whitby, P. M. Cook, C. Boshoff, L. Rainbow, M. R. Howard, S. J. Gao, R. A. Bohenzky, P. Simmonds, C. Lee, A. de Ruiter, A. Hatzakis, R. S. Tedder, I. V. Weller, R. A. Weiss, and P. S. Moore. 1996. Prevalence of Kaposi's sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet 348:1133-1138. [DOI] [PubMed] [Google Scholar]
- 41.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, and L. Degos. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 42.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szekely, L., F. Chen, N. Teramoto, B. Ehlin-Henriksson, K. Pokrovskaja, A. Szeles, A. Manneborg-Sandlund, M. Lowbeer, E. T. Lennette, and G. Klein. 1998. Restricted expression of Epstein-Barr virus (EBV)-encoded, growth transformation-associated antigens in an EBV- and human herpesvirus type 8-carrying body cavity lymphoma line. J. Gen. Virol. 79:1445-1452. [DOI] [PubMed] [Google Scholar]
- 44.Szekely, L., C. Kiss, K. Mattsson, E. Kashuba, K. Pokrovskaja, A. Juhasz, P. Holmvall, and G. Klein. 1999. Human herpesvirus-8-encoded LNA-1 accumulates in heterochromatin-associated nuclear bodies. J. Gen. Virol. 80:2889-2900. [DOI] [PubMed] [Google Scholar]