Abstract
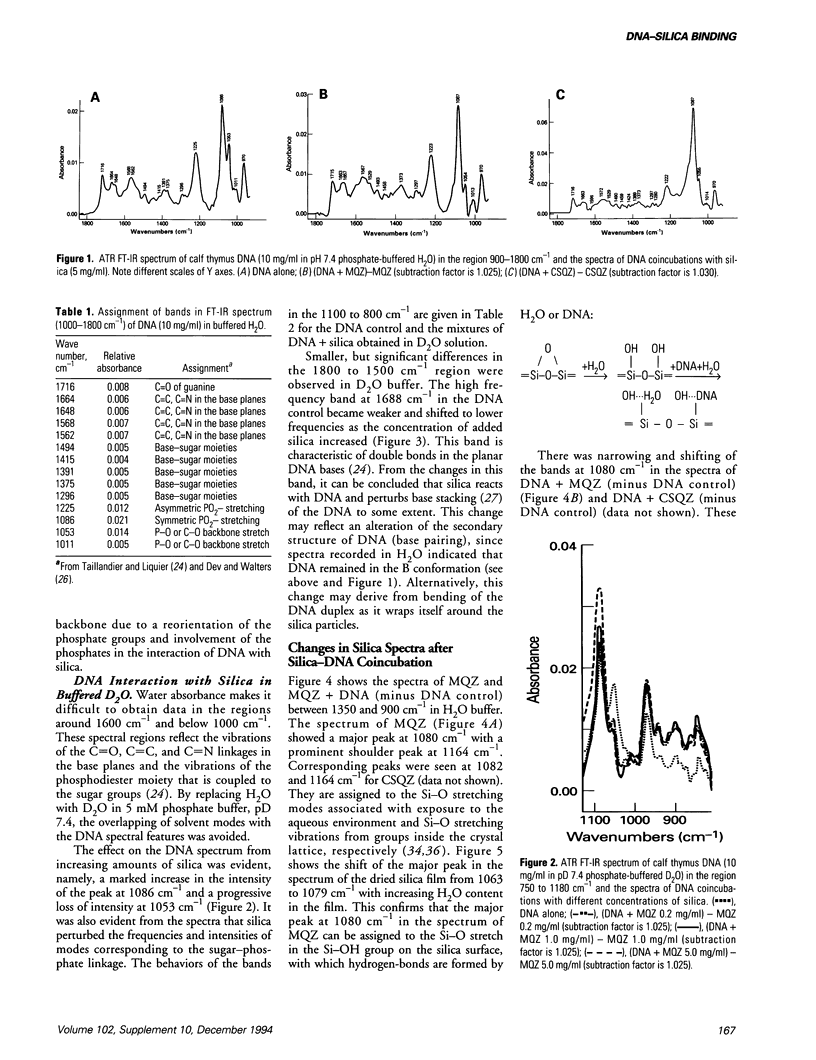
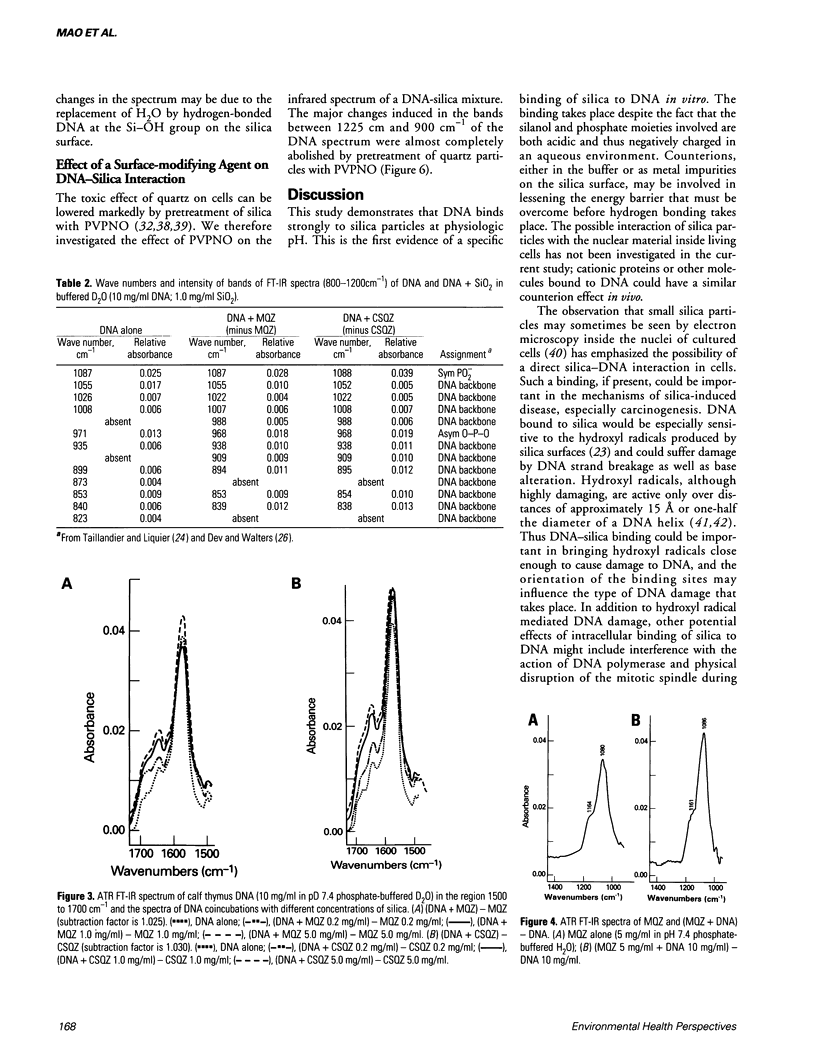
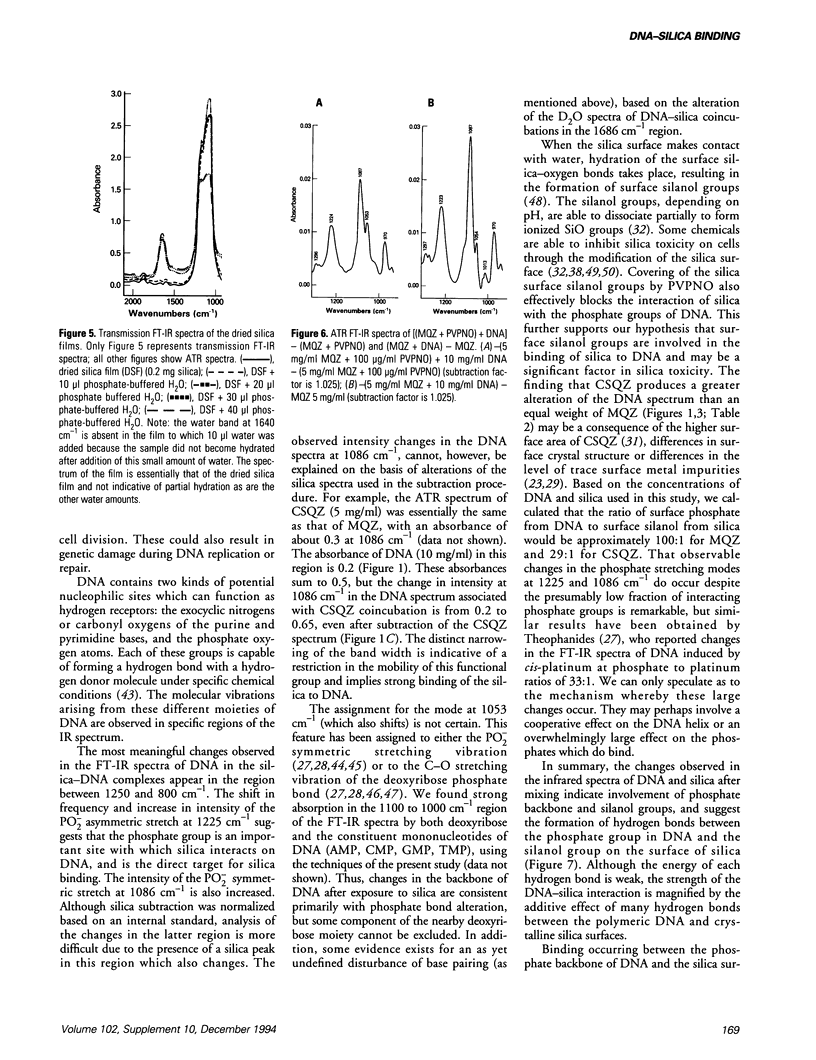
The interaction of DNA with crystalline silica in buffered aqueous solutions at physiologic pH has been investigated by Fourier-transform infrared spectroscopy (FT-IR). In aqueous buffer, significant changes occur in the spectra of DNA and silica upon coincubation, suggesting that a DNA-silica complex forms as silica interacts with DNA. As compared to the spectrum of silica alone, the changes in the FT-IR spectrum of silica in the DNA-silica complex are consistent with an Si-O bond perturbation on the surface of the silica crystal. DNA remains in a B-form conformation in the DNA-silica complex. The most prominent changes in the DNA spectrum occur in the 1225 to 1000 cm-1 region. Upon binding, the PO2- asymmetric stretch at 1225 cm-1 is increased in intensity and slightly shifted to lower frequencies; the PO2- symmetric stretch at 1086 cm-1 is markedly increased in intensity and the band at 1053 cm-1, representing either the phosphodiester or the C-O stretch of DNA backbone, is significantly reduced in intensity. In D2O buffer, the DNA spectrum reveals a marked increase in intensity of the peak at 1086 cm-1 and a progressive decrease in intensity of the peak at 1053 cm-1 when DNA is exposed to increasing concentrations of silica. The carbonyl band at 1688 cm-1 diminishes and shifts to slightly lower frequencies with increasing concentrations of silica. The present study demonstrates that crystalline silica binds to the phosphate-sugar backbone of DNA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. P., Monick M., Hunninghake G. W. Fibroblast proliferation induced by silica-exposed human alveolar macrophages. Am Rev Respir Dis. 1988 Jul;138(1):85–89. doi: 10.1164/ajrccm/138.1.85. [DOI] [PubMed] [Google Scholar]
- Daniel L. N., Mao Y., Saffiotti U. Oxidative DNA damage by crystalline silica. Free Radic Biol Med. 1993 May;14(5):463–472. doi: 10.1016/0891-5849(93)90103-2. [DOI] [PubMed] [Google Scholar]
- Daniel L. N., Mao Y., Vallyathan V., Saffiotti U. Binding of the cationic dye, Janus green B, as a measure of the specific surface area of crystalline silica in aqueous suspension. Toxicol Appl Pharmacol. 1993 Nov;123(1):62–67. doi: 10.1006/taap.1993.1221. [DOI] [PubMed] [Google Scholar]
- Dev S. B., Keller J. T., Rha C. K. Secondary structure of 11 S globulin in aqueous solution investigated by FT-IR derivative spectroscopy. Biochim Biophys Acta. 1988 Nov 23;957(2):272–280. doi: 10.1016/0167-4838(88)90283-x. [DOI] [PubMed] [Google Scholar]
- Dev S. B., Walters L. Fourier transform infrared spectroscopy for the characterization of a model peptide-DNA interaction. Biopolymers. 1990 Jan;29(1):289–299. doi: 10.1002/bip.360290131. [DOI] [PubMed] [Google Scholar]
- Doelman C. J., Leurs R., Oosterom W. C., Bast A. Mineral dust exposure and free radical-mediated lung damage. Exp Lung Res. 1990 Jan;16(1):41–55. doi: 10.3109/01902149009064698. [DOI] [PubMed] [Google Scholar]
- Ghio A. J., Hatch G. E. Lavage phospholipid concentration after silica instillation in the rat is associated with complexed [Fe3+] on the dust surface. Am J Respir Cell Mol Biol. 1993 Apr;8(4):403–407. doi: 10.1165/ajrcmb/8.4.403. [DOI] [PubMed] [Google Scholar]
- Ghio A. J., Kennedy T. P., Whorton A. R., Crumbliss A. L., Hatch G. E., Hoidal J. R. Role of surface complexed iron in oxidant generation and lung inflammation induced by silicates. Am J Physiol. 1992 Nov;263(5 Pt 1):L511–L518. doi: 10.1152/ajplung.1992.263.5.L511. [DOI] [PubMed] [Google Scholar]
- Haris P. I., Chapman D. Does Fourier-transform infrared spectroscopy provide useful information on protein structures? Trends Biochem Sci. 1992 Sep;17(9):328–333. doi: 10.1016/0968-0004(92)90305-s. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Kennedy T. P., Dodson R., Rao N. V., Ky H., Hopkins C., Baser M., Tolley E., Hoidal J. R. Dusts causing pneumoconiosis generate .OH and produce hemolysis by acting as Fenton catalysts. Arch Biochem Biophys. 1989 Feb 15;269(1):359–364. doi: 10.1016/0003-9861(89)90118-5. [DOI] [PubMed] [Google Scholar]
- Klockars M., Hedenborg M., Vanhala E. Effect of two particle surface-modifying agents, polyvinylpyridine-N-oxide and carboxymethylcellulose, on the quartz and asbestos mineral fiber-induced production of reactive oxygen metabolites by human polymorphonuclear leukocytes. Arch Environ Health. 1990 Jan-Feb;45(1):8–14. doi: 10.1080/00039896.1990.9935917. [DOI] [PubMed] [Google Scholar]
- Lesur O., Cantin A. M., Tanswell A. K., Melloni B., Beaulieu J. F., Bégin R. Silica exposure induces cytotoxicity and proliferative activity of type II pneumocytes. Exp Lung Res. 1992 Mar-Apr;18(2):173–190. doi: 10.3109/01902149209031679. [DOI] [PubMed] [Google Scholar]
- Manfait M., Theophanides T. Fourier transform infrared spectra of cells treated with the drug adriamycin. Biochem Biophys Res Commun. 1983 Oct 14;116(1):321–326. doi: 10.1016/0006-291x(83)90417-5. [DOI] [PubMed] [Google Scholar]
- Menghini R. Genotoxicity of active oxygen species in mammalian cells. Mutat Res. 1988 May;195(3):215–230. [PubMed] [Google Scholar]
- Miller B. E., Dethloff L. A., Gladen B. C., Hook G. E. Progression of type II cell hypertrophy and hyperplasia during silica-induced pulmonary inflammation. Lab Invest. 1987 Nov;57(5):546–554. [PubMed] [Google Scholar]
- Mohr S. C., Sokolov N. V., He C. M., Setlow P. Binding of small acid-soluble spore proteins from Bacillus subtilis changes the conformation of DNA from B to A. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):77–81. doi: 10.1073/pnas.88.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash T., Allison A. C., Harington J. S. Physico-chemical properties of silica in relation to its toxicity. Nature. 1966 Apr 16;210(5033):259–261. doi: 10.1038/210259a0. [DOI] [PubMed] [Google Scholar]
- Nolan R. P., Langer A. M., Harington J. S., Oster G., Selikoff I. J. Quartz hemolysis as related to its surface functionalities. Environ Res. 1981 Dec;26(2):503–520. doi: 10.1016/0013-9351(81)90226-7. [DOI] [PubMed] [Google Scholar]
- Nyberg P. Polyvinylpyridine-N-oxide and carboxymethyl cellulose inhibit mineral dust-induced production of reactive oxygen species by human macrophages. Environ Res. 1991 Aug;55(2):157–164. doi: 10.1016/s0013-9351(05)80172-0. [DOI] [PubMed] [Google Scholar]
- Pairon J. C., Jaurand M. C., Kheuang L., Janson X., Brochard P., Bignon J. Sister chromatid exchanges in human lymphocytes treated with silica. Br J Ind Med. 1990 Feb;47(2):110–115. doi: 10.1136/oem.47.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandurangi R. S., Seehra M. S., Razzaboni B. L., Bolsaitis P. Surface and bulk infrared modes of crystalline and amorphous silica particles: a study of the relation of surface structure to cytotoxicity of respirable silica. Environ Health Perspect. 1990 Jun;86:327–336. doi: 10.1289/ehp.9086327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Hu X. N., Vallyathan V. The chemical properties of silica particle surface in relation to silica-cell interactions. J Toxicol Environ Health. 1989;27(4):435–454. doi: 10.1080/15287398909531314. [DOI] [PubMed] [Google Scholar]
- Shi X. L., Dalal N. S., Vallyathan V. ESR evidence for the hydroxyl radical formation in aqueous suspension of quartz particles and its possible significance to lipid peroxidation in silicosis. J Toxicol Environ Health. 1988;25(2):237–245. doi: 10.1080/15287398809531205. [DOI] [PubMed] [Google Scholar]
- Spiethoff A., Wesch H., Wegener K., Klimisch H. J. The effects of Thorotrast and quartz on the induction of lung tumors in rats. Health Phys. 1992 Jul;63(1):101–110. doi: 10.1097/00004032-199207000-00011. [DOI] [PubMed] [Google Scholar]
- Summerton J., Hoenig S. The mechanism of hemolysis by silica and its bearing on silicosis. Exp Mol Pathol. 1977 Feb;26(1):113–128. doi: 10.1016/0014-4800(77)90071-5. [DOI] [PubMed] [Google Scholar]
- Taillandier E., Liquier J. Infrared spectroscopy of DNA. Methods Enzymol. 1992;211:307–335. doi: 10.1016/0076-6879(92)11018-e. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Kang J. H., Van Dyke K., Dalal N. S., Castranova V. Response of alveolar macrophages to in vitro exposure to freshly fractured versus aged silica dust: the ability of Prosil 28, an organosilane material, to coat silica and reduce its biological reactivity. J Toxicol Environ Health. 1991 Jul;33(3):303–315. doi: 10.1080/15287399109531529. [DOI] [PubMed] [Google Scholar]
- Vallyathan V., Shi X. L., Dalal N. S., Irr W., Castranova V. Generation of free radicals from freshly fractured silica dust. Potential role in acute silica-induced lung injury. Am Rev Respir Dis. 1988 Nov;138(5):1213–1219. doi: 10.1164/ajrccm/138.5.1213. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Wiessner J. H., Mandel N. S., Sohnle P. G., Hasegawa A., Mandel G. S. The effect of chemical modification of quartz surfaces on particulate-induced pulmonary inflammation and fibrosis in the mouse. Am Rev Respir Dis. 1990 Jan;141(1):111–116. doi: 10.1164/ajrccm/141.1.111. [DOI] [PubMed] [Google Scholar]


