Abstract
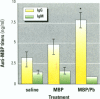
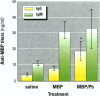
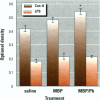
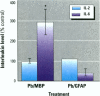
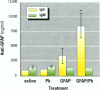
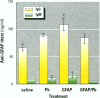
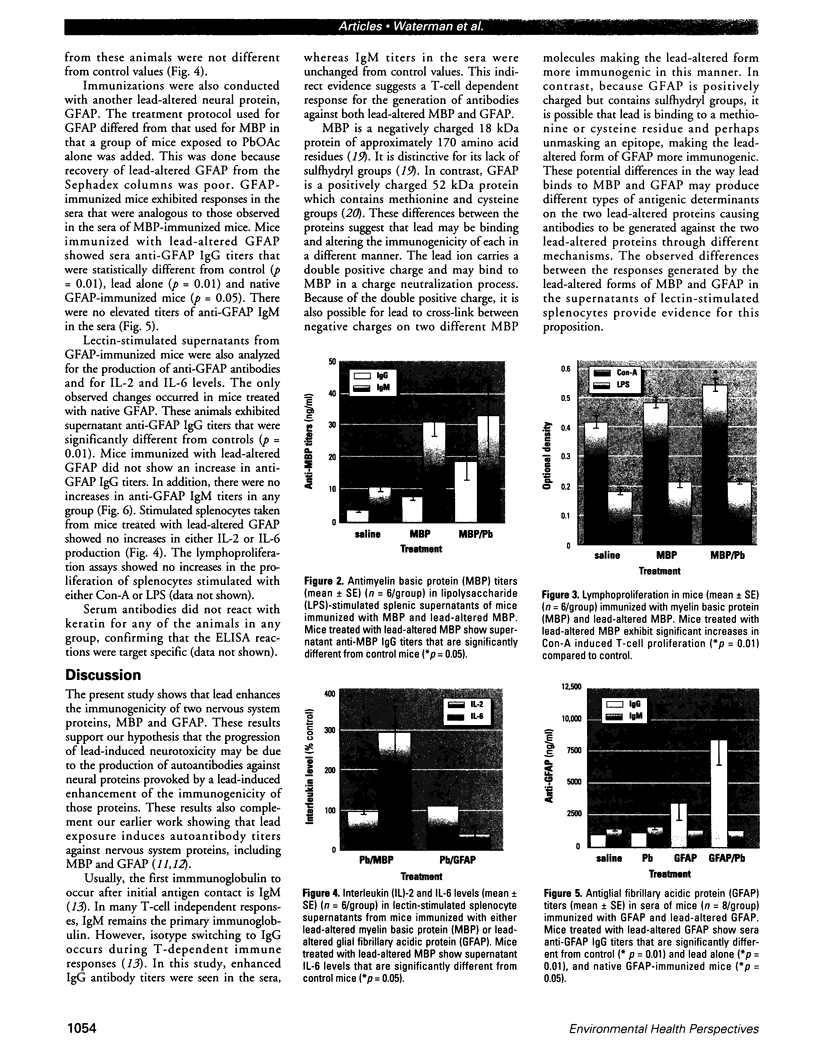
Some heavy metals have been suspected of playing a role in the pathogenesis of nervous system diseases such as multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer's disease. In these disorders, autoantibodies against neural proteins are evident at some stage of the disease. Lead is known to affect both the immune and nervous systems. Work in our laboratory has shown that lead exposure leads to the production of autoantibodies against neural proteins, including myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP). We hypothesize that lead aggravates neurological disease by enhancing the immunogenicity of nervous system proteins, including MBP and GFAP. To test this hypothesis, lead-altered protein was prepared by incubating MBP or GFAP with lead acetate for 24 hr. On days 0, 14, and 28, mice received inoculations with either saline, native protein, or lead-altered protein. Anti-MBP and anti-GFAP, isotypes IgM and IgG, were measured in sera by ELISA on day 38. Sera of mice treated with lead-altered MBP had statistically higher anti-MBP IgG titers than both control and native MBP-immunized mice. An analogous response was seen in mice immunized with lead-altered GFAP. Supernatants from lectin-stimulated splenocytes were also examined for antibody titers and for interleukin 2 (IL-2) and interleukin 6 (IL-6) levels. A significant increase in IL-6 production was seen in mice immunized with lead-altered MBP but not with lead-altered GFAP. No changes were observed in the IL-2 levels of mice immunized with either lead-altered protein.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baig S., Olsson T., Höjeberg B., Link H. Cells secreting antibodies to myelin basic protein in cerebrospinal fluid of patients with Lyme neuroborreliosis. Neurology. 1991 Apr;41(4):581–587. doi: 10.1212/wnl.41.4.581. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992 Jul;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Cash E., Weerth S., Voltz R., Kornhuber M. Cells of cerebrospinal fluid of multiple sclerosis patients secrete antibodies to myelin basic protein in vitro. Scand J Immunol. 1992 Jun;35(6):695–701. doi: 10.1111/j.1365-3083.1992.tb02977.x. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Lee S. C., Mattiace L. A., Yen S. H., Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993 Jan;7(1):75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Fontana A., Grieder A., Arrenbrecht S., Grob P. In vitro stimulation of glia cells by a lymphocyte-produced factor. J Neurol Sci. 1980 Apr;46(1):55–62. doi: 10.1016/0022-510x(80)90043-x. [DOI] [PubMed] [Google Scholar]
- Frohman E. M., Frohman T. C., Dustin M. L., Vayuvegula B., Choi B., Gupta A., van den Noort S., Gupta S. The induction of intercellular adhesion molecule 1 (ICAM-1) expression on human fetal astrocytes by interferon-gamma, tumor necrosis factor alpha, lymphotoxin, and interleukin-1: relevance to intracerebral antigen presentation. J Neuroimmunol. 1989 Jul;23(2):117–124. doi: 10.1016/0165-5728(89)90030-1. [DOI] [PubMed] [Google Scholar]
- Górny M., Losy J., Wender M. Przeciwciała przeciw kwaśnemu białku włkienkowemu gleju (GFAP) w płynie mózgowo-rdzeniowym chorych na stwardnienie rozsiane i inne choroby neurologiczne. Neurol Neurochir Pol. 1990 Jan-Apr;24(1-2):17–22. [PubMed] [Google Scholar]
- Hashim G. A. Myelin basic protein: structure, function and antigenic determinants. Immunol Rev. 1978;39:60–107. doi: 10.1111/j.1600-065x.1978.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Hsu B. L., Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991 Feb;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Lawrence D. A. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect Immun. 1981 Jan;31(1):136–143. doi: 10.1128/iai.31.1.136-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Liu W., Dickson D. W., Brosnan C. F., Berman J. W. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993 Apr 1;150(7):2659–2667. [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. J., Jr, Lawrence D. A. Lead, a major environmental pollutant, is immunomodulatory by its differential effects on CD4+ T cells subsets. Toxicol Appl Pharmacol. 1991 Oct;111(1):13–23. doi: 10.1016/0041-008X(91)90129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. J., Jr, Lawrence D. A. The heavy metal lead exhibits B cell-stimulatory factor activity by enhancing B cell Ia expression and differentiation. J Immunol. 1990 Jul 15;145(2):671–677. [PubMed] [Google Scholar]
- Mecocci P., Parnetti L., Donato R., Santucci C., Santucci A., Cadini D., Foà E., Cecchetti R., Senin U. Serum autoantibodies against glial fibrillary acidic protein in brain aging and senile dementias. Brain Behav Immun. 1992 Sep;6(3):286–292. doi: 10.1016/0889-1591(92)90049-t. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Nakamura K., Takeda M., Tanaka T., Tanaka J., Kato Y., Nishinuma K., Nishimura T. Glial fibrillary acidic protein stimulates proliferation and immunoglobulin synthesis of lymphocytes from Alzheimer's disease patients. Methods Find Exp Clin Pharmacol. 1992 Mar;14(2):141–149. [PubMed] [Google Scholar]
- Nisticò G., De Sarro G. Is interleukin 2 a neuromodulator in the brain? Trends Neurosci. 1991 Apr;14(4):146–150. doi: 10.1016/0166-2236(91)90086-a. [DOI] [PubMed] [Google Scholar]
- Olsson T., Baig S., Höjeberg B., Link H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann Neurol. 1990 Feb;27(2):132–136. doi: 10.1002/ana.410270207. [DOI] [PubMed] [Google Scholar]
- Singh V. K., Yang Y. Y., Singh E. A. Immunoblot detection of antibodies to myelin basic protein in Alzheimer's disease patients. Neurosci Lett. 1992 Nov 23;147(1):25–28. doi: 10.1016/0304-3940(92)90766-z. [DOI] [PubMed] [Google Scholar]
- Snyder C. A. The neuroendocrine system, an opportunity for immunotoxicologists. Environ Health Perspect. 1989 May;81:165–166. doi: 10.1289/ehp.8981165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Nakamura K., Takeda M., Tada K., Suzuki H., Morita H., Okado T., Hariguchi S., Nishimura T. Enzyme-linked immunosorbent assay for human autoantibody to glial fibrillary acidic protein: higher titer of the antibody is detected in serum of patients with Alzheimer's disease. Acta Neurol Scand. 1989 Dec;80(6):554–560. doi: 10.1111/j.1600-0404.1989.tb03926.x. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Catz I. Diagnostic value of cerebrospinal fluid anti-myelin basic protein in patients with multiple sclerosis. Ann Neurol. 1986 Jul;20(1):20–25. doi: 10.1002/ana.410200105. [DOI] [PubMed] [Google Scholar]
- Weber W., Jingwu Z., Borst J., Buurman W. Antibody cross-reactivity between myelin basic protein and CD3 antigen of T cells. Implications for autoimmunity. Ann N Y Acad Sci. 1988;540:470–471. doi: 10.1111/j.1749-6632.1988.tb27138.x. [DOI] [PubMed] [Google Scholar]