Abstract
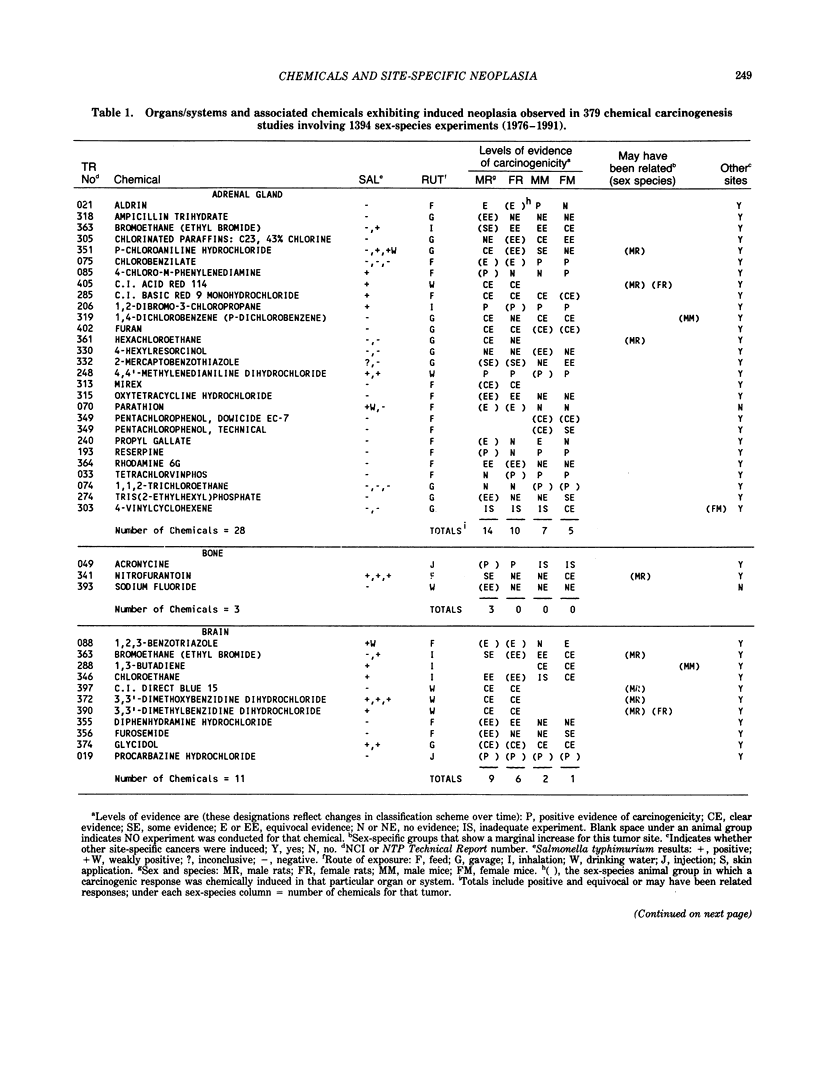
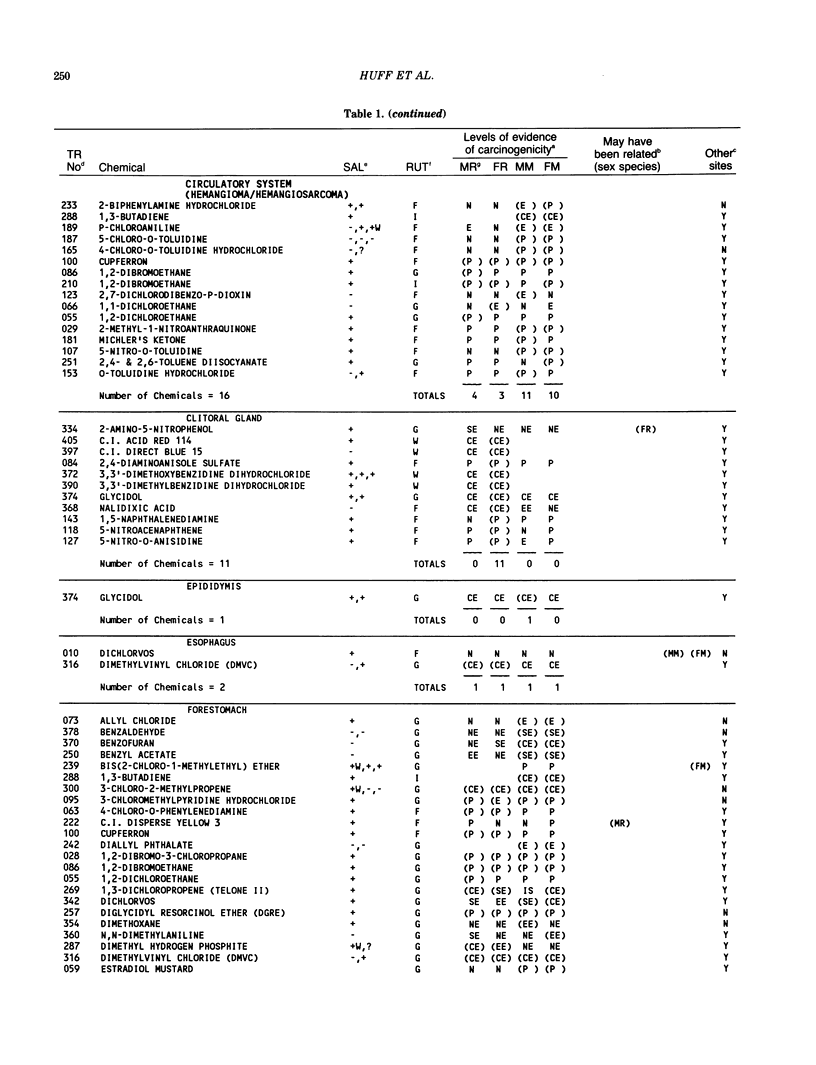
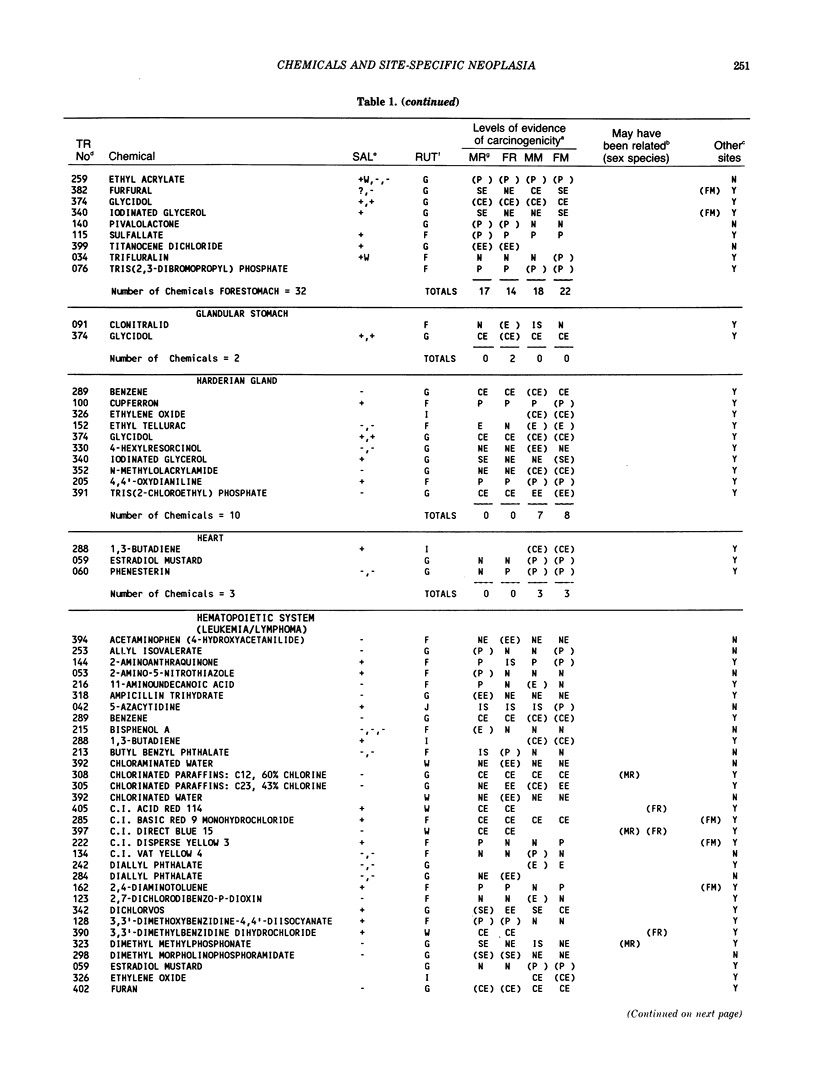
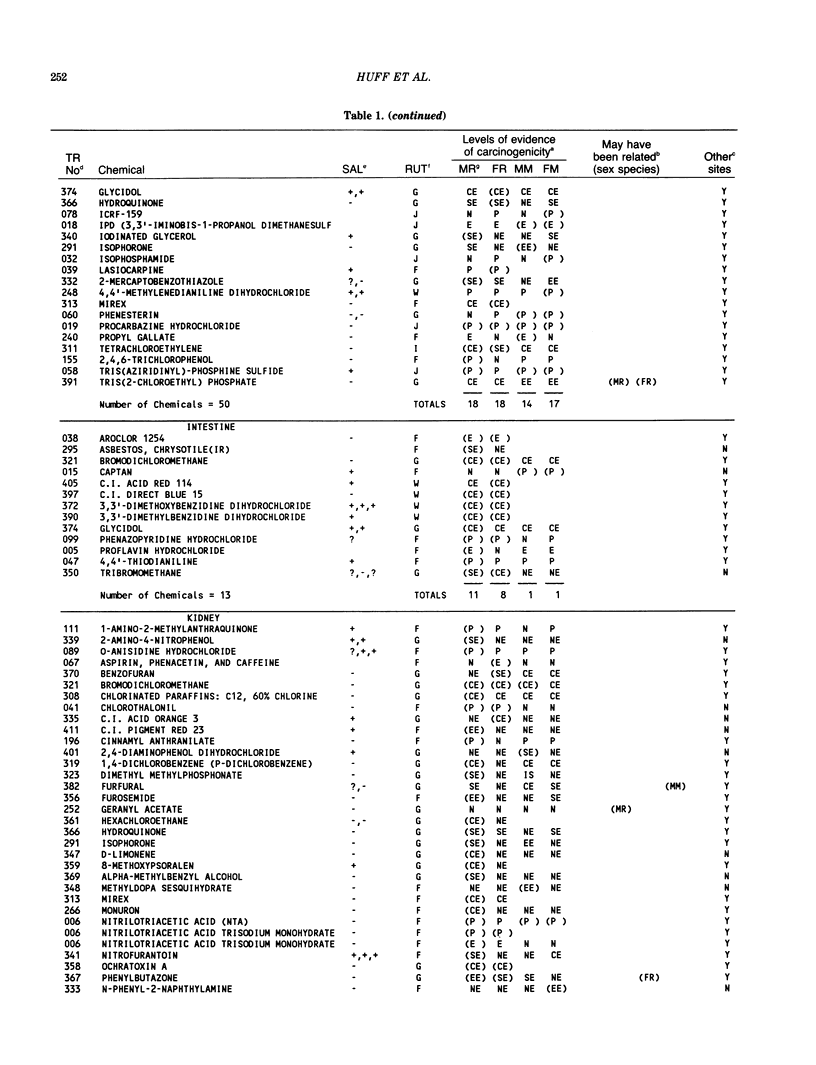
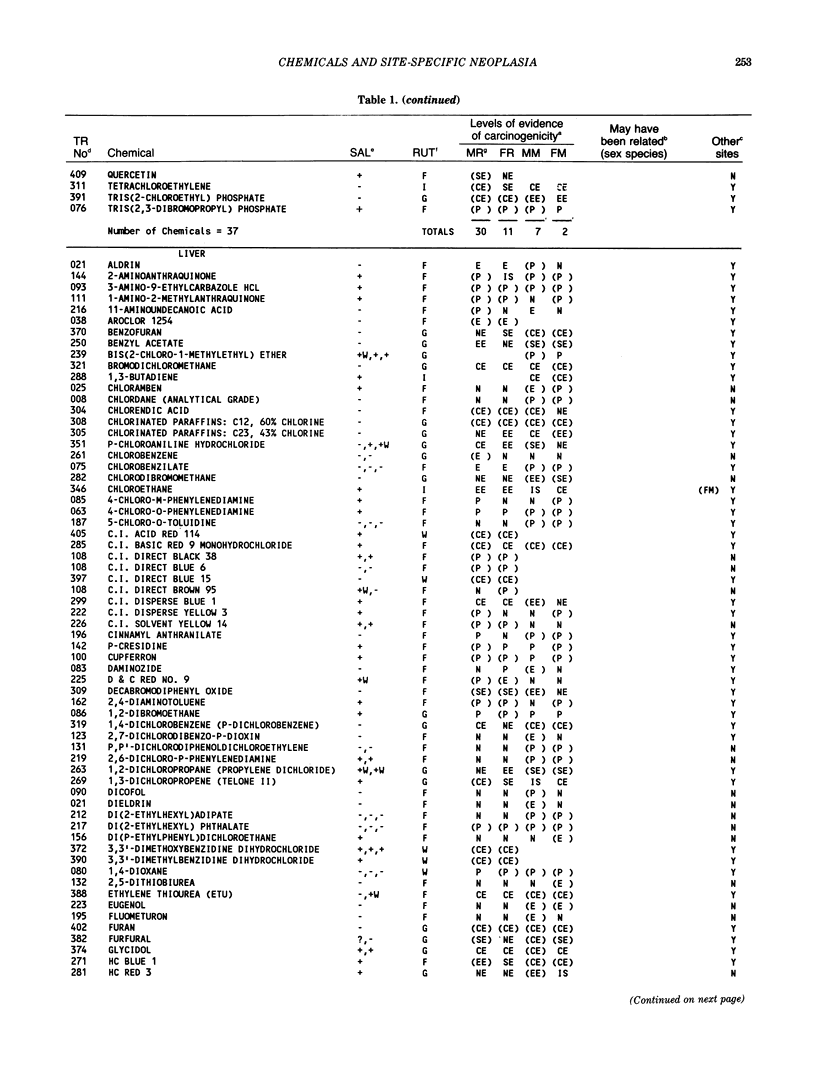
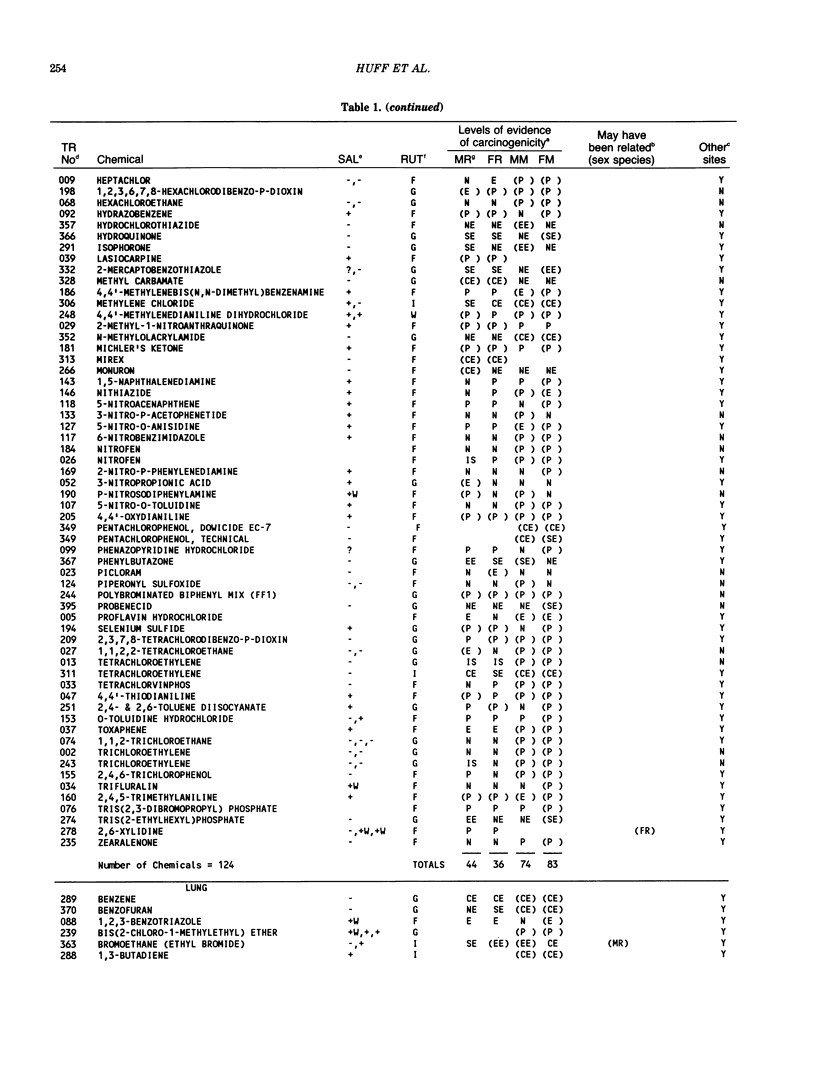
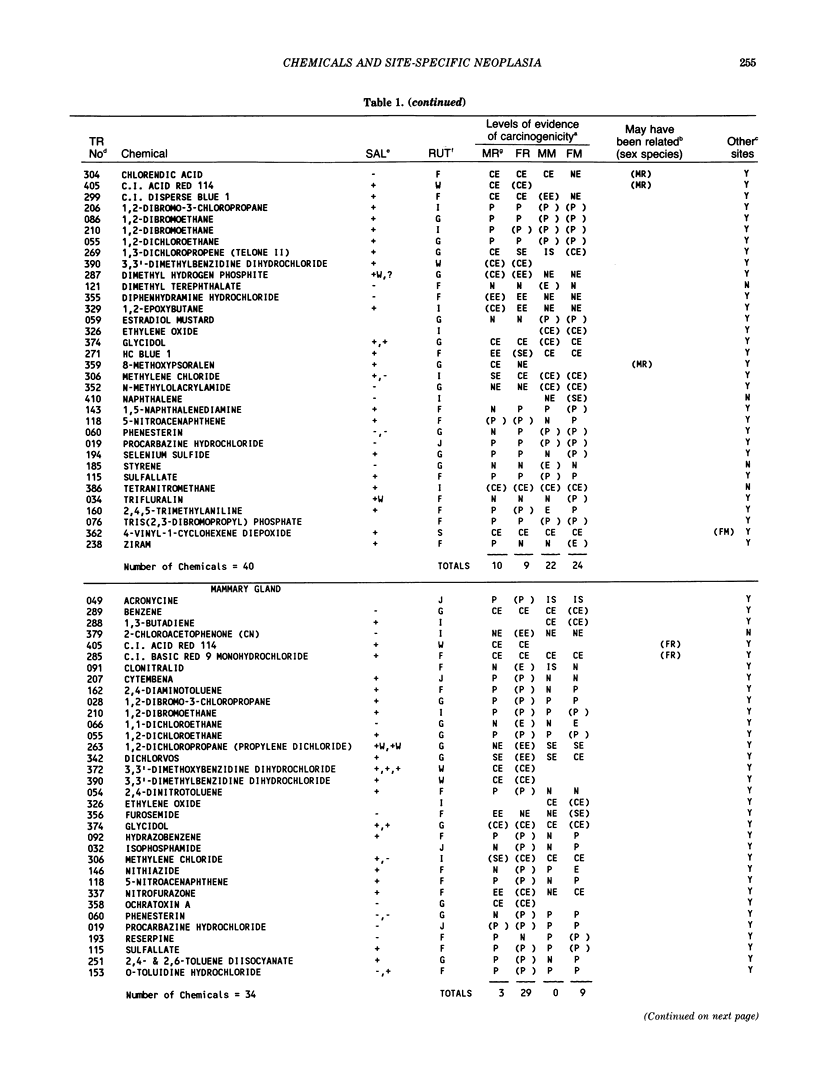
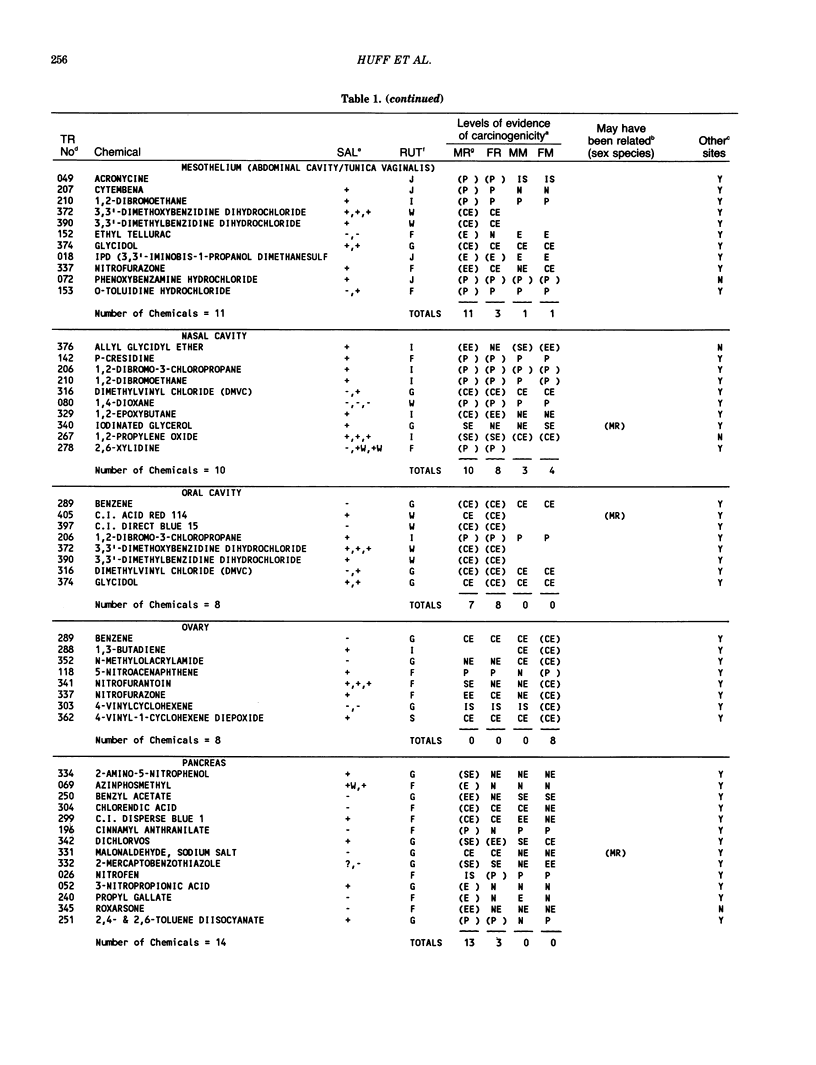
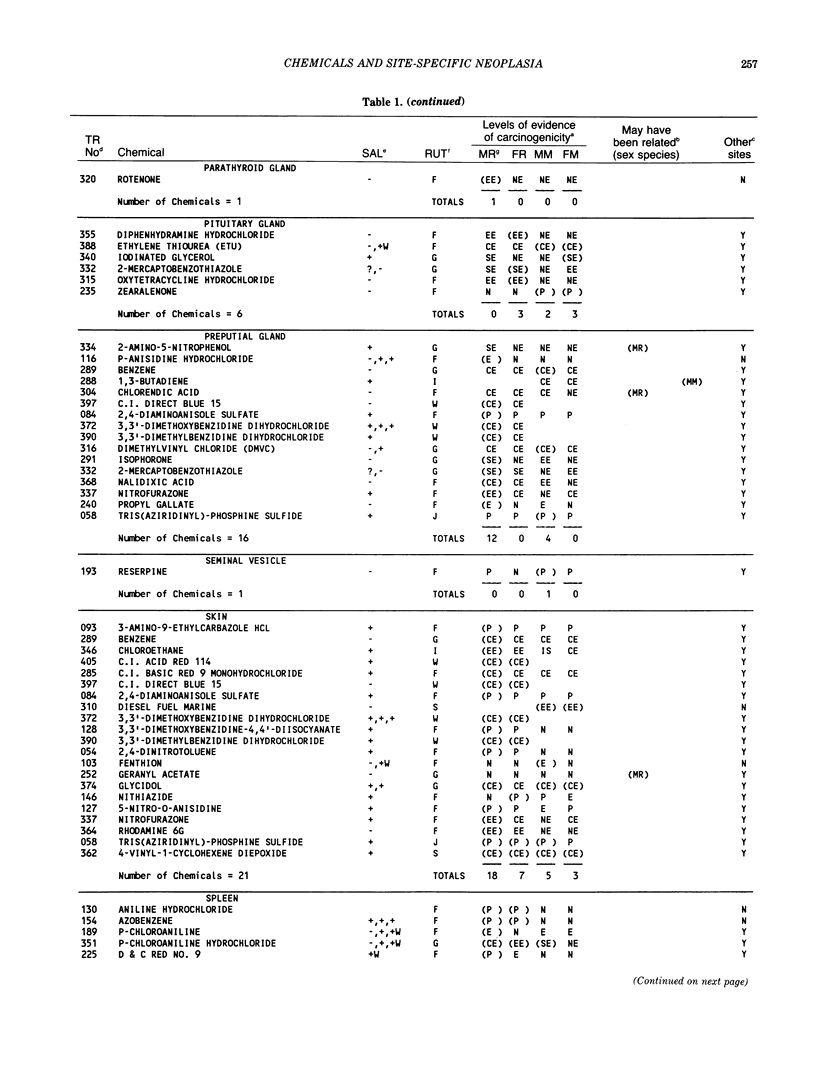
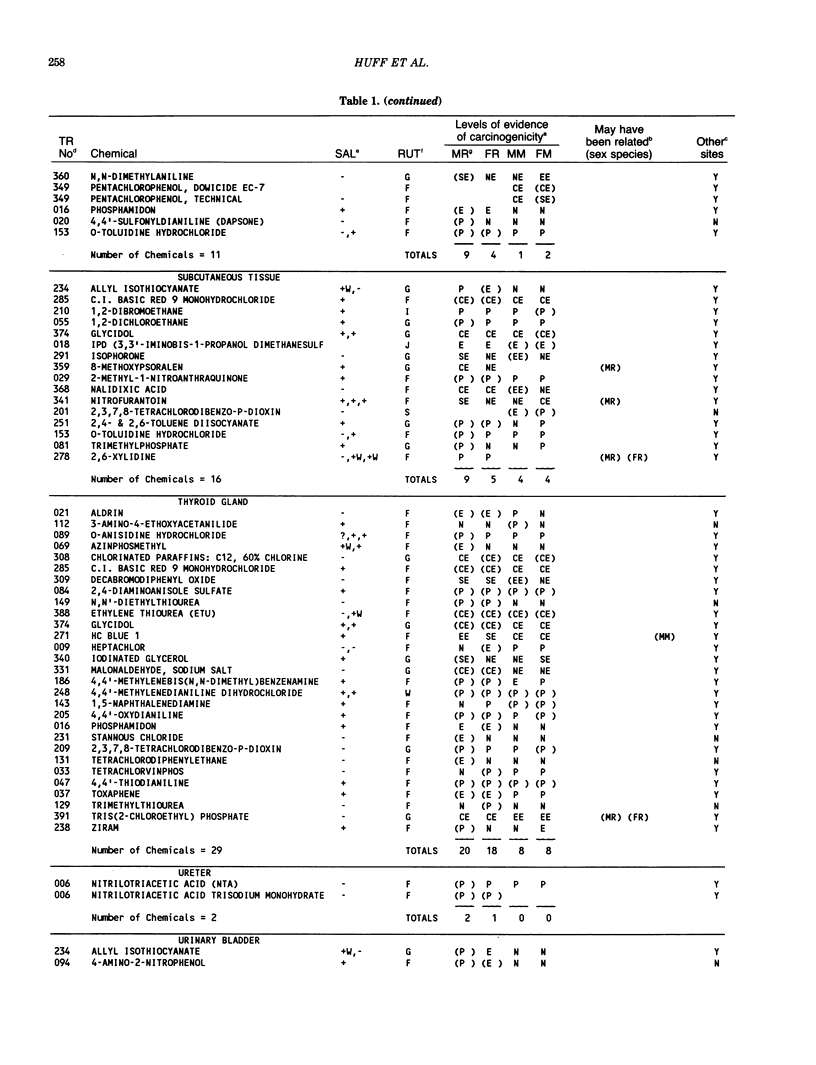
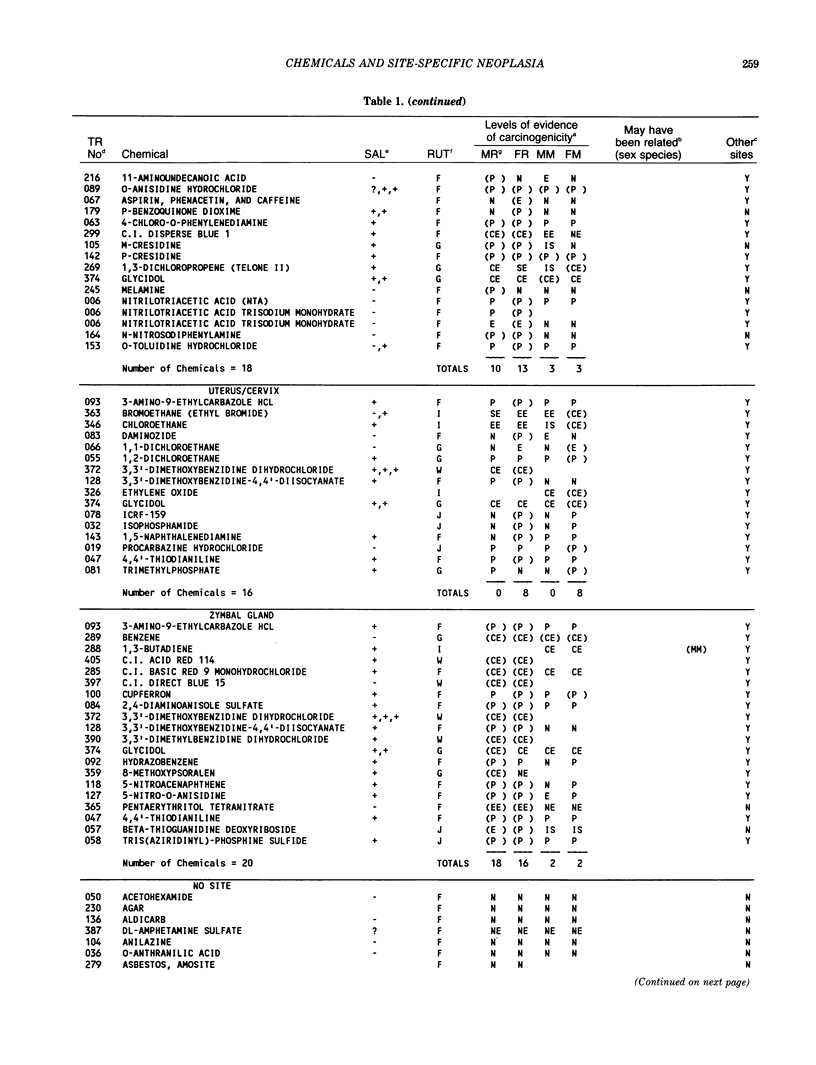
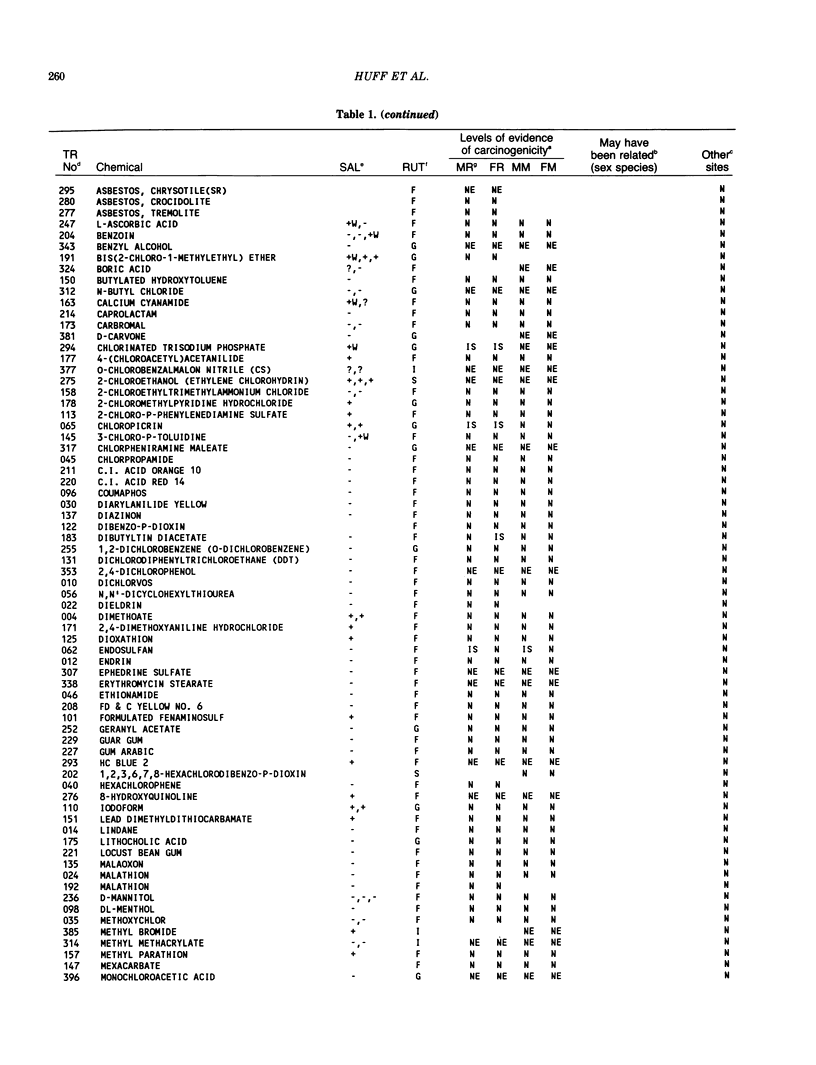
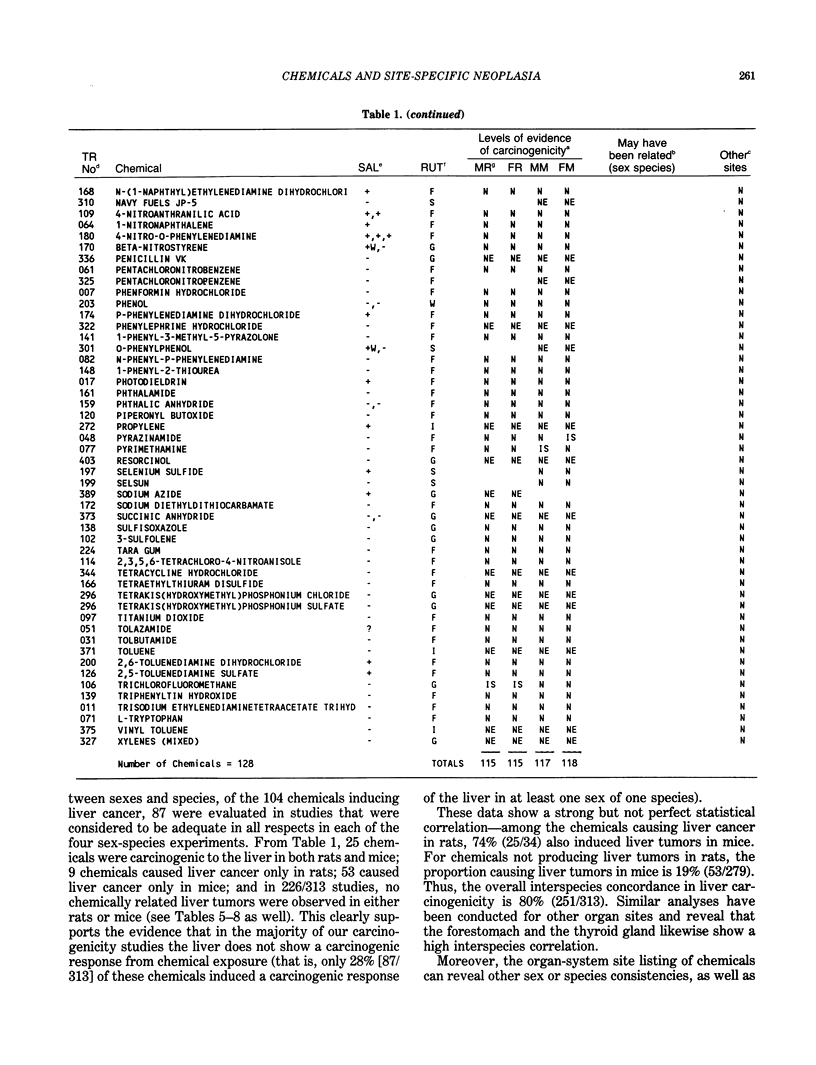
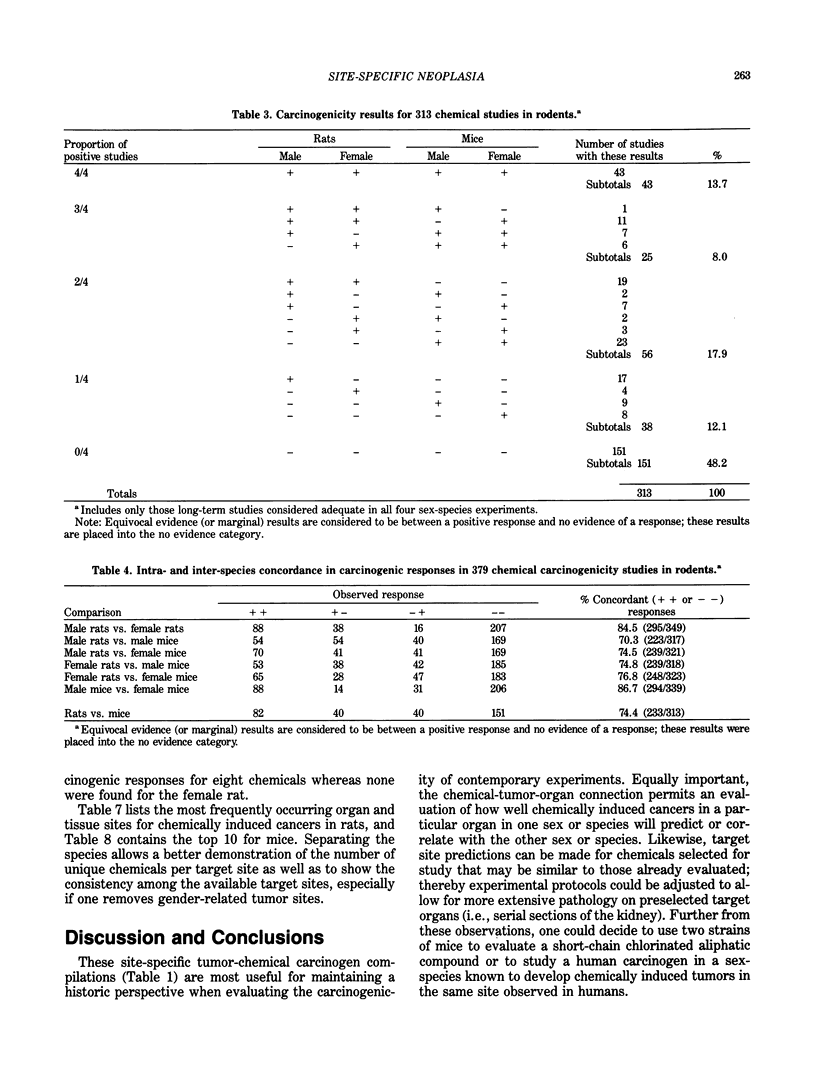
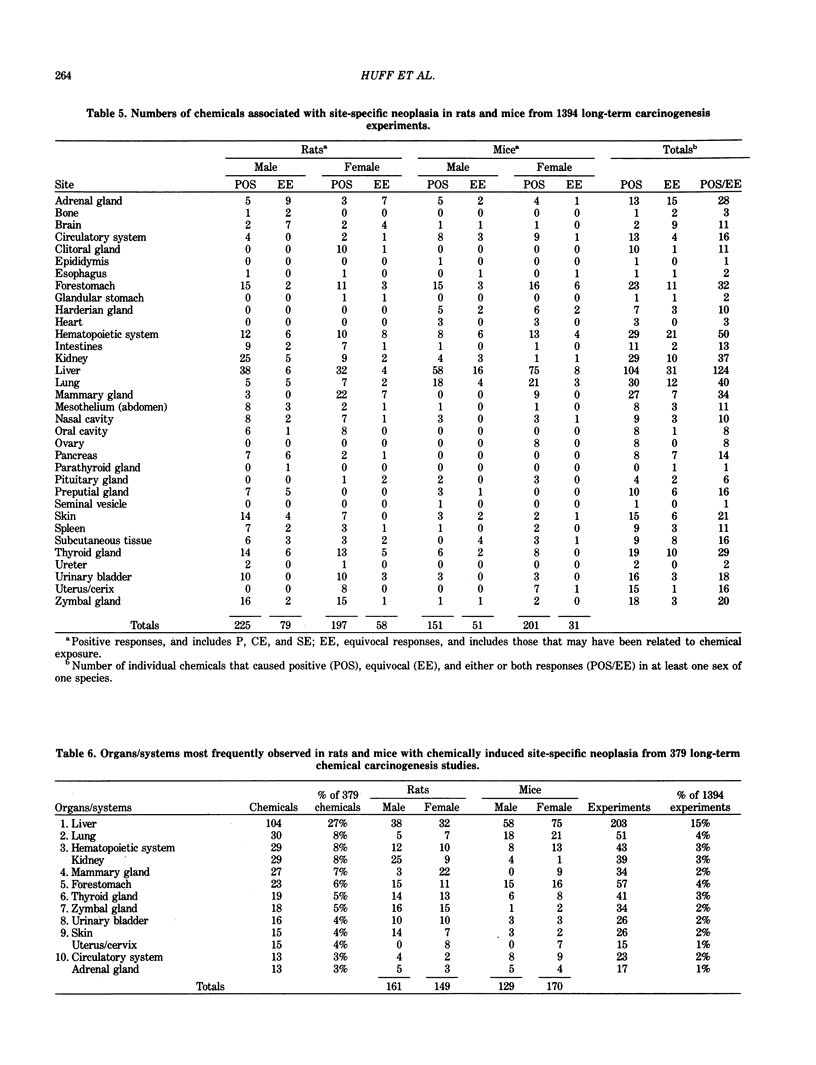
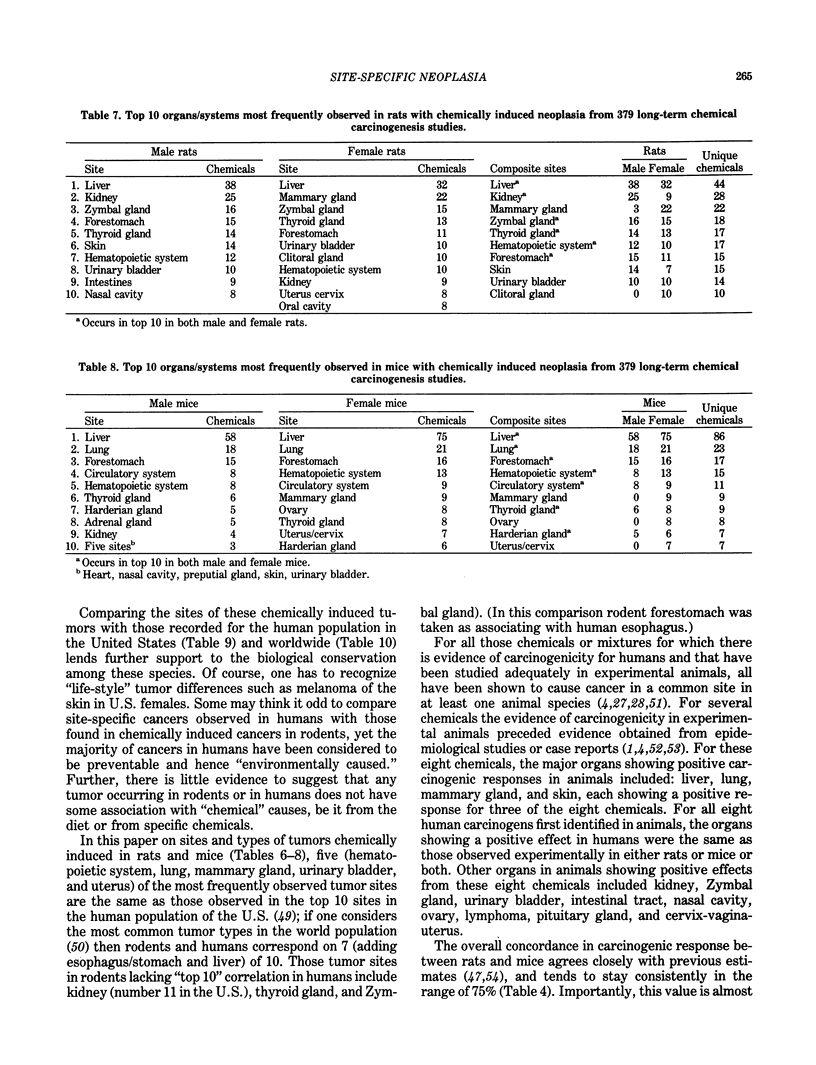
The carcinogenicity data base used for this paper originated in the late 1960s by the National Cancer Institute and since 1978 has been continued and made more comprehensive by the National Toxicology Program. The extensive files contain among other sets of information detailed pathology data on more than 400 long-term (most often 24 month) chemical carcinogenesis studies, comprised of nearly 1600 individual experiments having at least 10 million tissue sections that have been evaluated for toxicity and carcinogenicity. Using the current data set of 379 studies made up of 1394 experiments, we have compiled listings of chemicals having like carcinogenic target sites for each of the 34 organs or systems for which histopathology diagnoses have been recorded routinely. The most common tumor site is the liver (15% of all experiments), followed in rank order by: lung, hematopoietic system and kidneys, mammary glands, forestomach, thyroid glands, Zymbal glands, urinary bladder, skin and uterus/cervix, and circulatory system and adrenal glands. These compilations are most useful for maintaining a historic perspective when evaluating the carcinogenicity of contemporary experiments. Equally important, the chemical-tumor-organ connection permits an evaluation of how well chemically induced cancers in a particular organ in one sex or species will predict or correlate with the other sex or species. Using liver cancers as an example, the overall interspecies concordance is 80%. Likewise target site predictions can be made for chemicals selected for study that may be similar to those already evaluated; thereby experimental protocols could be adjusted to allow, for example, more extensive pathology on preselected target organs (i.e., serial sections of the kidney). Further from these observations, one could decide to use two strains of mice to evaluate a short-chain chlorinated aliphatic compound or to study a human carcinogen in a sex-species known to develop chemically induced tumors in the same site observed in humans. Structural classes of chemicals having a propensity for certain organs can be easily identified from these data. Sex-species responders to particular induced cancers become clearly evident, such as in the ovary of female mice or in the kidney of male rats.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Chemical carcinogenesis: too many rodent carcinogens. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7772–7776. doi: 10.1073/pnas.87.19.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman G. A., Eustis S. L. Proliferative lesions of the exocrine pancreas in male F344/N rats. Environ Health Perspect. 1984 Jun;56:213–217. doi: 10.1289/ehp.8456213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra R. S., Huff J. E., Schwetz B. S., Selkirk J. An overview of prechronic and chronic toxicity/carcinogenicity experimental study designs and criteria used by the National Toxicology Program. Environ Health Perspect. 1990 Jun;86:313–321. doi: 10.1289/ehp.9086313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustis S. L. The sequential development of cancer: a morphological perspective. Toxicol Lett. 1989 Dec;49(2-3):267–281. doi: 10.1016/0378-4274(89)90037-4. [DOI] [PubMed] [Google Scholar]
- Gart J. J., Krewski D., Lee P. N., Tarone R. E., Wahrendorf J. Statistical methods in cancer research. Volume III--The design and analysis of long-term animal experiments. IARC Sci Publ. 1986;(79):1–219. [PubMed] [Google Scholar]
- Gold L. S., Bernstein L., Magaw R., Slone T. H. Interspecies extrapolation in carcinogenesis: prediction between rats and mice. Environ Health Perspect. 1989 May;81:211–219. doi: 10.1289/ehp.8981211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Rao G. N., Arnold J. E., Boorman G. A., McConnell E. E. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1985 Nov;75(5):975–984. doi: 10.1093/jnci/75.5.975. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E. Species correlation in long-term carcinogenicity studies. Cancer Lett. 1987 Oct 30;37(2):125–132. doi: 10.1016/0304-3835(87)90154-6. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J. E., Zeiger E., McConnell E. E. Comparative results of 327 chemical carcinogenicity studies. Environ Health Perspect. 1987 Oct;74:229–235. doi: 10.1289/ehp.8774229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K. Statistical issues in the design, analysis and interpretation of animal carcinogenicity studies. Environ Health Perspect. 1984 Dec;58:385–392. doi: 10.1289/ehp.8458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseman J. K., Tharrington E. C., Huff J. E., McConnell E. E. Comparison of site-specific and overall tumor incidence analyses for 81 recent National Toxicology Program carcinogenicity studies. Regul Toxicol Pharmacol. 1986 Jun;6(2):155–170. doi: 10.1016/0273-2300(86)90031-0. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Eustis S. L., Haseman J. K. Occurrence and relevance of chemically induced benign neoplasms in long-term carcinogenicity studies. Cancer Metastasis Rev. 1989 Jun;8(1):1–22. doi: 10.1007/BF00047055. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Moore J. A. Carcinogenesis studies design and experimental data interpretation/evaluation at the National Toxicology Program. Prog Clin Biol Res. 1984;141:43–64. [PubMed] [Google Scholar]
- Huff J., Haseman J., Rall D. Scientific concepts, value, and significance of chemical carcinogenesis studies. Annu Rev Pharmacol Toxicol. 1991;31:621–652. doi: 10.1146/annurev.pa.31.040191.003201. [DOI] [PubMed] [Google Scholar]
- Maronpot R. R., Haseman J. K., Boorman G. A., Eustis S. E., Rao G. N., Huff J. E. Liver lesions in B6C3F1 mice: the National Toxicology Program, experience and position. Arch Toxicol Suppl. 1987;10:10–26. doi: 10.1007/978-3-642-71617-1_2. [DOI] [PubMed] [Google Scholar]
- McConnell E. E., Solleveld H. A., Swenberg J. A., Boorman G. A. Guidelines for combining neoplasms for evaluation of rodent carcinogenesis studies. J Natl Cancer Inst. 1986 Feb;76(2):283–289. [PubMed] [Google Scholar]
- Muir C. S. Epidemiology, basic science, and the prevention of cancer: implications for the future. Cancer Res. 1990 Oct 15;50(20):6441–6448. [PubMed] [Google Scholar]
- Parkin D. M., Lärä E., Muir C. S. Estimates of the worldwide frequency of sixteen major cancers in 1980. Int J Cancer. 1988 Feb 15;41(2):184–197. doi: 10.1002/ijc.2910410205. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Huff J. Refinement of long-term toxicity and carcinogenesis studies. Fundam Appl Toxicol. 1990 Jul;15(1):33–43. doi: 10.1016/0272-0590(90)90160-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatis L., Aitio A., Wilbourn J., Shuker L. Human carcinogens so far identified. Jpn J Cancer Res. 1989 Sep;80(9):795–807. doi: 10.1111/j.1349-7006.1989.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomatis L. The predictive value of rodent carcinogenicity tests in the evaluation of human risks. Annu Rev Pharmacol Toxicol. 1979;19:511–530. doi: 10.1146/annurev.pa.19.040179.002455. [DOI] [PubMed] [Google Scholar]


