Abstract
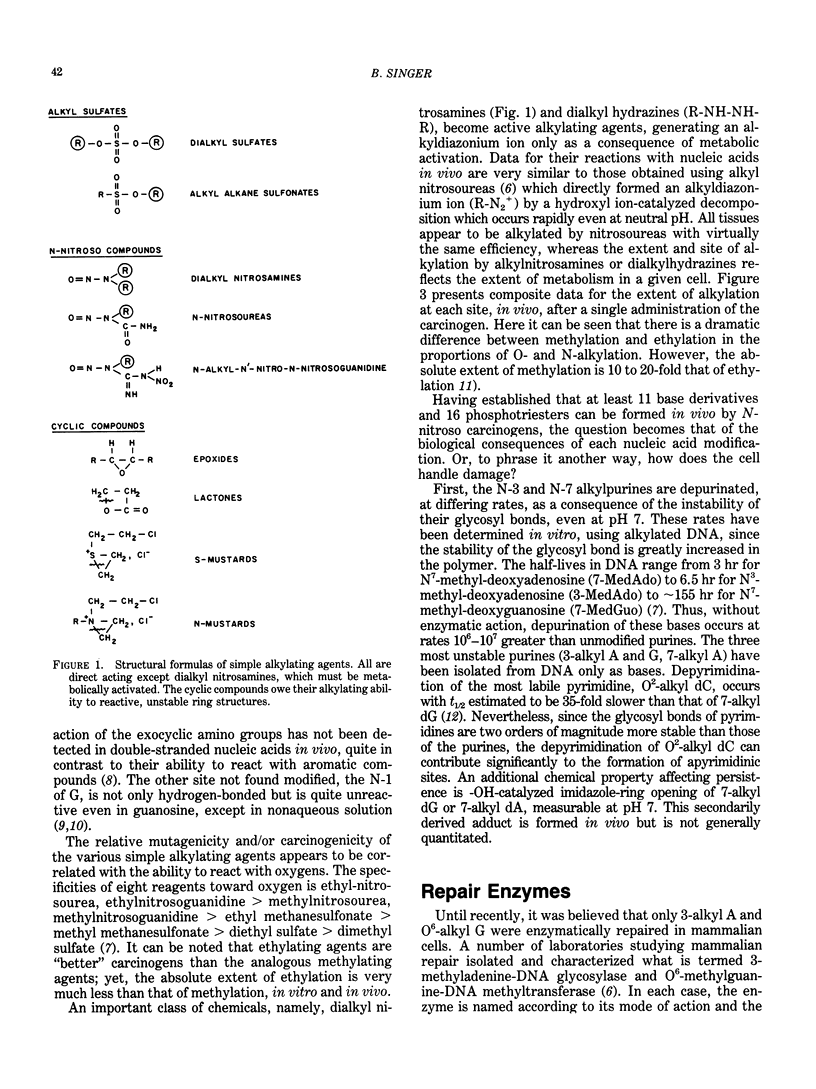
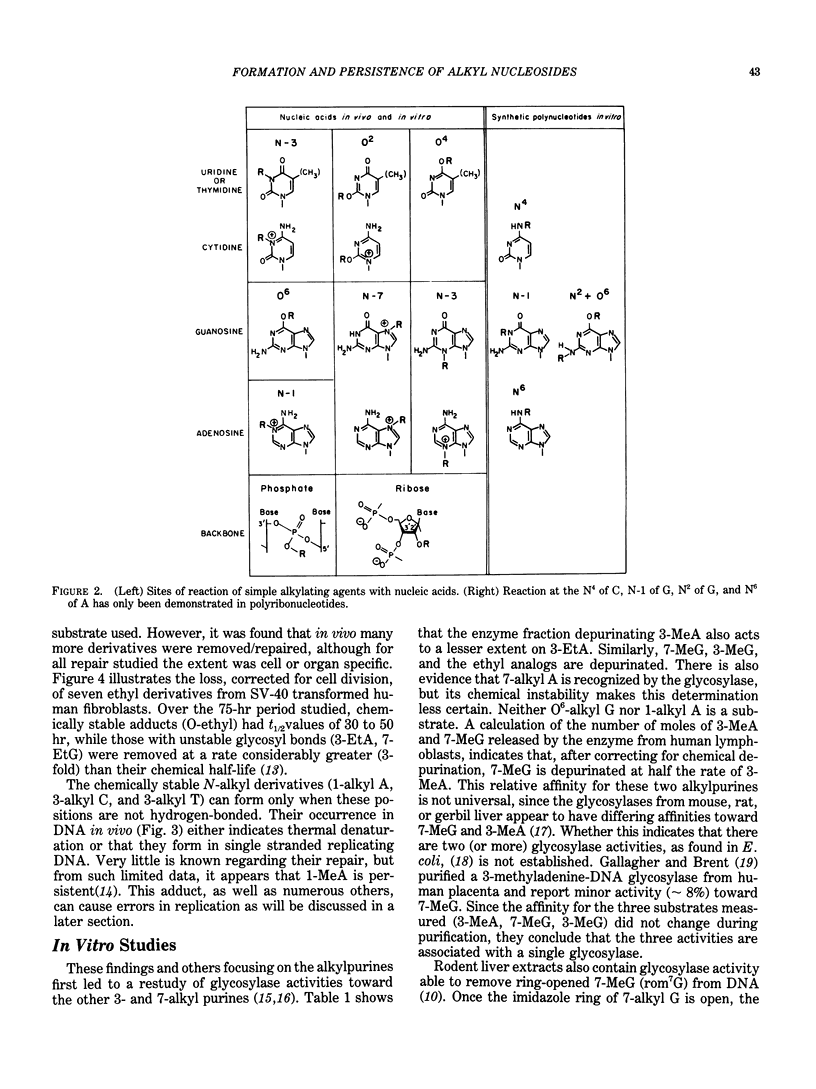
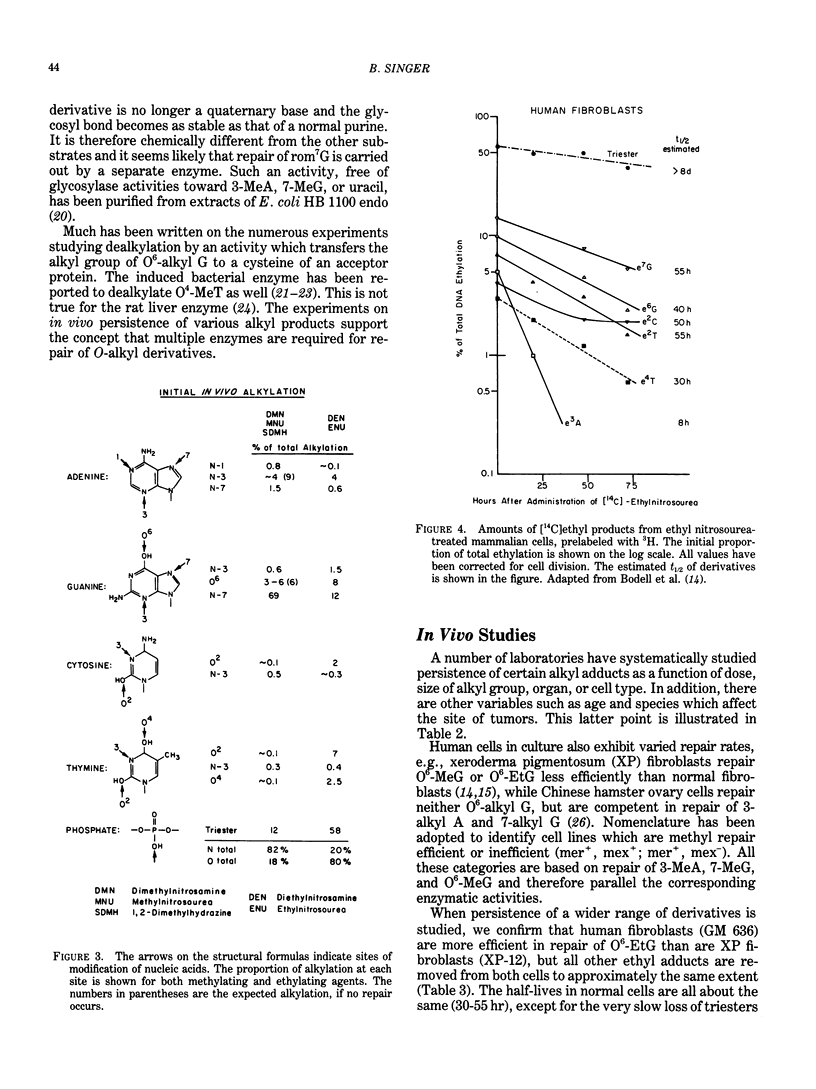
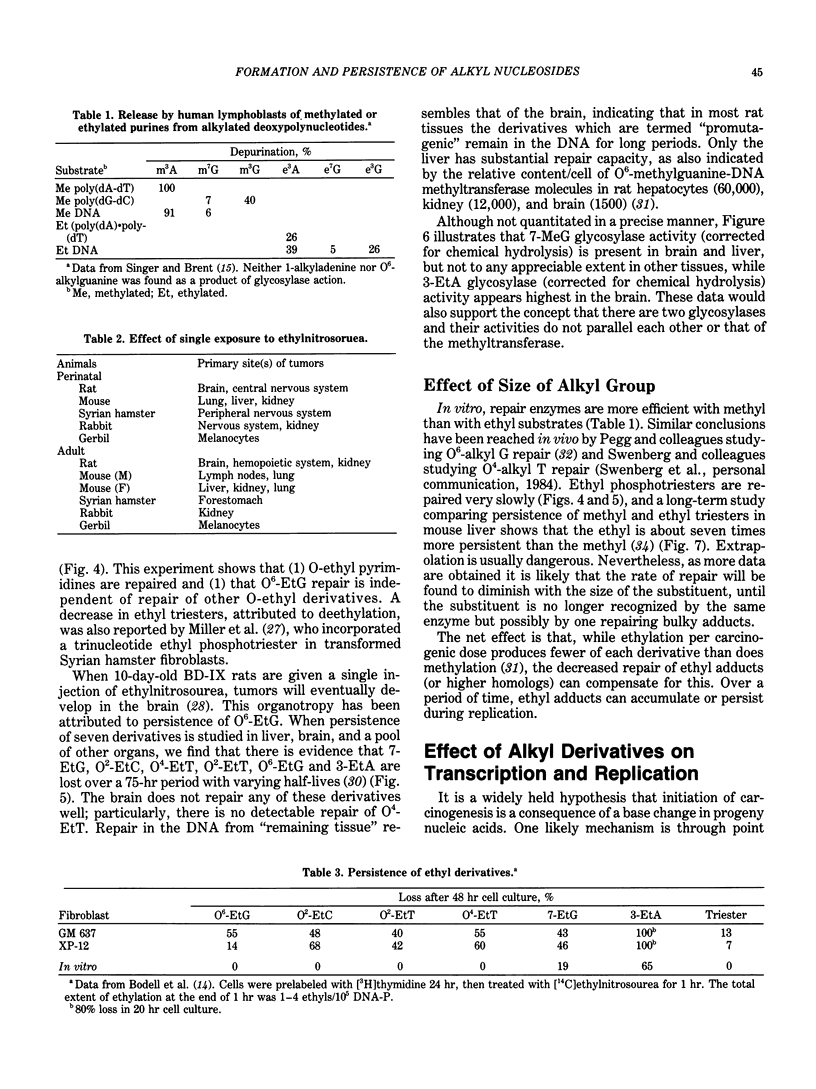
Alkylating agents are ubiquitous in the human environment and are continuously synthesized in vivo. Although many classes exist, interest has been focused on the N-nitroso compounds, since many are mutagens for bacteria, phage, and cells, and carcinogens for mammals. In contrast to aromatic amines and polyaromatic hydrocarbons which can react at carbons, simple alkylating agents react with nitrogens and oxygens: 13 sites are possible, including the internucleotide phosphodiester. However, only the N-nitroso compounds react extensively with oxygens. In vivo, most possible derivatives have been found after administration of methyl and ethyl nitroso compounds. The ethylating agents are more reactive toward oxygens than are the methylating agents and are more carcinogenic in terms of total alkylation. This is true regardless of whether or not the compounds require metabolic activation. It has been hypothesized that the level and persistence of specific derivatives in a "target" cell correlates with oncogenesis. However, no single derivative can be solely responsible for this complex process, since correlations cannot be made for even a single carcinogen acting on various species or cell types. Some derivatives are chemically unstable, and the glycosyl bond is broken (3- and 7-alkylpurines), leaving apurinic sites which may be mutagenic. These, as well as most adducts, are recognized by different enzymatic activities which remove/repair at various rates and efficiencies depending on the number of alkyl derivatives, as well as enzyme content in the cell and recognition of the enzyme. Evaluation of human exposure requires early and sensitive methods to detect the initial damage and the extent of repair of each of the many promutagenic adducts.
Full text
PDF
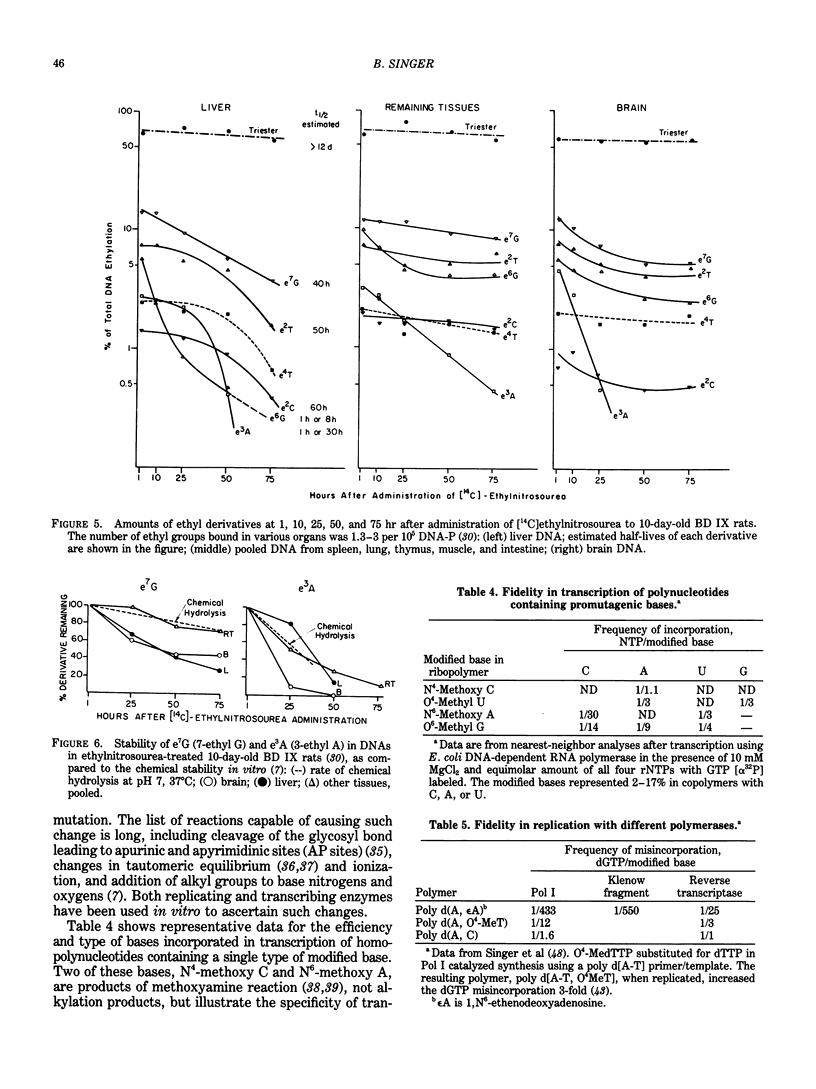
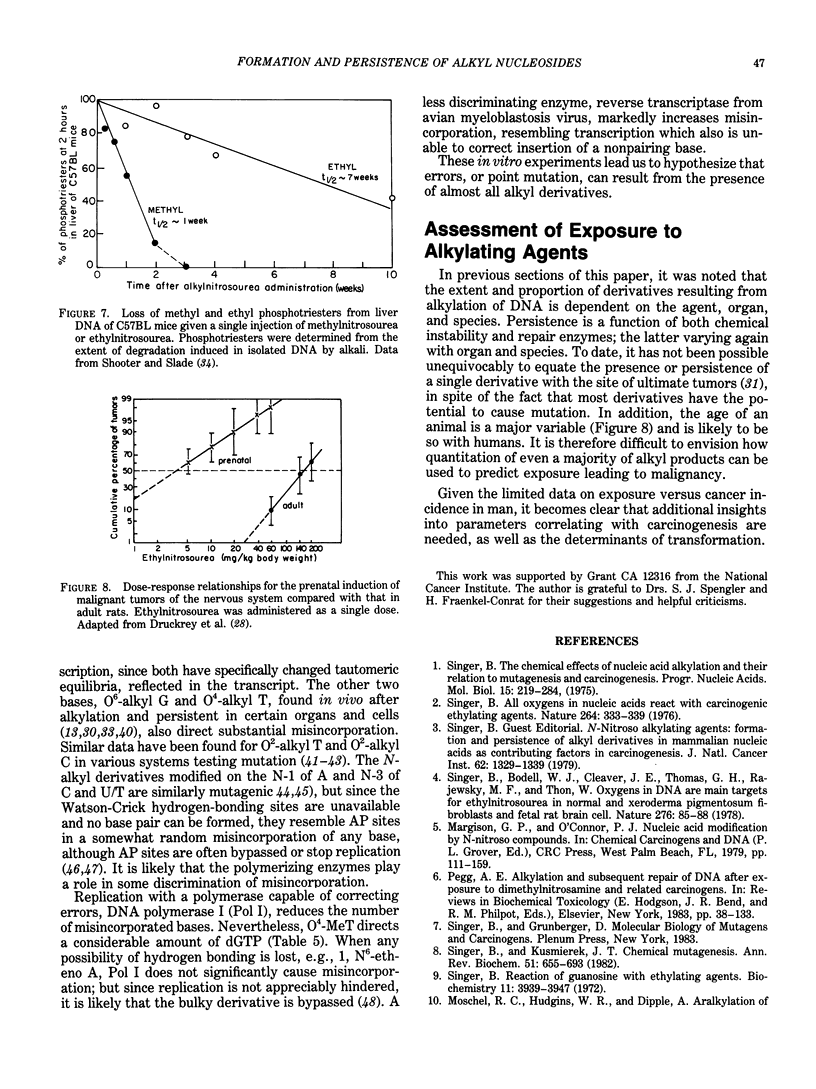

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamborschke S., O'Connor P. J., Margison G. P., Kleihues P., Maru G. B. DNA methylation by dimethylnitrosamine in the Mongolian gerbil (Meriones unguiculatus): indications of a deficient, noninducible hepatic repair system for O6-methylguanine. Cancer Res. 1983 Mar;43(3):1306–1311. [PubMed] [Google Scholar]
- Bodell W. J., Singer B., Thomas G. H., Cleaver J. E. Evidence for removal at different rates of O-ethyl pyrimidines and ethylphosphotriesters in two human fibroblast cell lines. Nucleic Acids Res. 1979 Jun 25;6(8):2819–2829. doi: 10.1093/nar/6.8.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M., Hewlins M. J. The reaction between hydroxylamine and cytosine derivatives. J Chem Soc Perkin 1. 1968;15:1922–1924. doi: 10.1039/j39680001922. [DOI] [PubMed] [Google Scholar]
- Dolan M. E., Scicchitano D., Singer B., Pegg A. E. Comparison of repair of methylated pyrimidines in poly(dT) by extracts from rat liver and Escherichia coli. Biochem Biophys Res Commun. 1984 Aug 30;123(1):324–330. doi: 10.1016/0006-291x(84)90416-9. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Singer B. The chemical basis for the mutagenicity of hydroxylamine and methoxyamine. Biochim Biophys Acta. 1972 Mar 24;262(3):264–268. doi: 10.1016/0005-2787(72)90262-6. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P. E., Brent T. P. Further purification and characterization of human 3-methyladenine-DNA glycosylase. Evidence for broad specificity. Biochim Biophys Acta. 1984 Sep 10;782(4):394–401. doi: 10.1016/0167-4781(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Goth-Goldstein R. Repair of DNA damaged by alkylating carcinogens is defective in xeroderma pigmentosum-derived fibroblasts. Nature. 1977 May 5;267(5606):81–82. doi: 10.1038/267081a0. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Molecular and cellular mechanisms associated with pulse-carcinogenesis in the rat nerbous system by ethyinitrosourea: ethylation of nucleic acids and elimination rates of ethylated bases from the DNA of different tissues. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;82(1):37–64. doi: 10.1007/BF00304382. [DOI] [PubMed] [Google Scholar]
- Hemminki K., Falck K., Vainio H. Comparison of alkylation rates and mutagenicity of directly acting industrial and laboratory chemicals: epoxides, glycidyl ethers, methylating and ethylating agents, halogenated hydrocarbons, hydrazine derivatives, aldehydes, thiuram and dithiocarbamate derivatives. Arch Toxicol. 1980 Dec;46(3-4):277–285. doi: 10.1007/BF00310445. [DOI] [PubMed] [Google Scholar]
- Kröger M., Singer B. Ambiguity and transcriptional errors as a result of methylation of N-1 of purines and N-3 of pyrimidines. Biochemistry. 1979 Aug 7;18(16):3493–3500. doi: 10.1021/bi00583a009. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Shearman C. W., Loeb L. A. Mutagenesis in vitro by depurination of phiX174 dna. Nature. 1981 May 28;291(5813):349–351. doi: 10.1038/291349a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA repair enzymes. Annu Rev Biochem. 1982;51:61–87. doi: 10.1146/annurev.bi.51.070182.000425. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. G., Edington B. V., Schendel P. F. Inducible repair of phosphotriesters in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7380–7384. doi: 10.1073/pnas.80.24.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Karran P., Lindahl T. Inducible repair of O-alkylated DNA pyrimidines in Escherichia coli. EMBO J. 1984 Mar;3(3):545–550. doi: 10.1002/j.1460-2075.1984.tb01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Braiterman L. T., Ts'o P. O. Effects of a trinucleotide ethyl phosphotriester, Gmp(Et)Gmp(Et)U, on mammalian cells in culture. Biochemistry. 1977 May 3;16(9):1988–1996. doi: 10.1021/bi00628a036. [DOI] [PubMed] [Google Scholar]
- Scherer E., Timmer A. P., Emmelot P. Formation by diethylnitrosamine and persistence of O4-ethylthymidine in rat liver DNA in vivo. Cancer Lett. 1980 Jul;10(1):1–6. doi: 10.1016/0304-3835(80)90058-0. [DOI] [PubMed] [Google Scholar]
- Shooter K. V., Slade T. A. The stability of methyl and ethyl phosphotriesters in DNA in vivo. Chem Biol Interact. 1977 Dec;19(3):353–361. doi: 10.1016/0009-2797(77)90057-6. [DOI] [PubMed] [Google Scholar]
- Singer B., Abbott L. G., Spengler S. J. Assessment of mutagenic efficiency of two carcinogen-modified nucleosides, 1,N6-ethenodeoxyadenosine and O4-methyldeoxythymidine, using polymerases of varying fidelity. Carcinogenesis. 1984 Sep;5(9):1165–1171. doi: 10.1093/carcin/5.9.1165. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Bodell W. J., Cleaver J. E., Thomas G. H., Rajewsky M. F., Thon W. Oxygens in DNA are main targets for ethylnitrosourea in normal and xeroderma pigmentosum fibroblasts and fetal rat brain cells. Nature. 1978 Nov 2;276(5683):85–88. doi: 10.1038/276085a0. [DOI] [PubMed] [Google Scholar]
- Singer B., Brent T. P. Human lymphoblasts contain DNA glycosylase activity excising N-3 and N-7 methyl and ethyl purines but not O6-alkylguanines or 1-alkyladenines. Proc Natl Acad Sci U S A. 1981 Feb;78(2):856–860. doi: 10.1073/pnas.78.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Fraenkel-Conrat H., Kuśmierek J. T. Preparation and template activities of polynucleotides containing O2- and O4-alkyluridine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1722–1726. doi: 10.1073/pnas.75.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Kröger M., Carrano M. O2- and O4-alkyl pyrimidine nucleosides: stability of the glycosyl bond and of the alkyl group as a function of pH. Biochemistry. 1978 Apr 4;17(7):1246–1250. doi: 10.1021/bi00600a018. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T., Fraenkel-Conrat H. In vitro discrimination of replicases acting on carcinogen-modified polynucleotide templates. Proc Natl Acad Sci U S A. 1983 Feb;80(4):969–972. doi: 10.1073/pnas.80.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Singer B., Pergolizzi R. G., Grunberger D. Synthesis and coding properties of dinucleoside diphosphates containing alky pyrimidines which are formed by the action of carcinogens on nucleic acids. Nucleic Acids Res. 1979 Apr;6(4):1709–1719. doi: 10.1093/nar/6.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. Reaction of guanosine with ethylating agents. Biochemistry. 1972 Oct 10;11(21):3939–3947. doi: 10.1021/bi00771a017. [DOI] [PubMed] [Google Scholar]
- Singer B., Spengler S., Bodell W. J. Tissue-dependent enzyme-mediated repair or removal of O-ethyl pyrimidines and ethyl purines in carcinogen-treated rats. Carcinogenesis. 1981;2(10):1069–1073. doi: 10.1093/carcin/2.10.1069. [DOI] [PubMed] [Google Scholar]
- Singer B., Sági J., Kuśmierek J. T. Escherichia coli polymerase I can use O2-methyldeoxythymidine or O4-methyldeoxythymidine in place of deoxythymidine in primed poly(dA-dT).poly(dA-dT) synthesis. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4884–4888. doi: 10.1073/pnas.80.16.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. The chemical effects of nucleic acid alkylation and their relation to mutagenesis and carcinogenesis. Prog Nucleic Acid Res Mol Biol. 1975;15(0):219–284. [PubMed] [Google Scholar]
- Spengler S., Singer B. Effect of tautomeric shift on mutation: N4-methoxycytidine forms hydrogen bonds with adenosine in polymers. Biochemistry. 1981 Dec 8;20(25):7290–7294. doi: 10.1021/bi00528a037. [DOI] [PubMed] [Google Scholar]
- Swenberg J. A., Dyroff M. C., Bedell M. A., Popp J. A., Huh N., Kirstein U., Rajewsky M. F. O4-ethyldeoxythymidine, but not O6-ethyldeoxyguanosine, accumulates in hepatocyte DNA of rats exposed continuously to diethylnitrosamine. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1692–1695. doi: 10.1073/pnas.81.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]


