Abstract
Reactive oxygen species (ROS) are constantly produced in biological tissues and play a role in various signalling pathways. Abnormally high ROS concentrations cause oxidative stress associated with tissue damage and dysregulation of physiological signals. There is growing evidence that oxidative stress increases with age. It has also been shown that the life span of worms, flies and mice can be significantly increased by mutations which impede the insulin receptor signalling cascade. Molecular studies revealed that the insulin-independent basal activity of the insulin receptor is increased by ROS and downregulated by certain antioxidants. Complementary clinical studies confirmed that supplementation of the glutathione precursor cysteine decreases insulin responsiveness in the fasted state. In several clinical trials, cysteine supplementation improved skeletal muscle functions, decreased the body fat/lean body mass ratio, decreased plasma levels of the inflammatory cytokine tumour necrosis factor α (TNF-α), improved immune functions, and increased plasma albumin levels. As all these parameters degenerate with age, these findings suggest: (i) that loss of youth, health and quality of life may be partly explained by a deficit in cysteine and (ii) that the dietary consumption of cysteine is generally suboptimal and everybody is likely to have a cysteine deficiency sooner or later.
Keywords: cysteine in vivo, redox status, muscle functions, immune functions, inflammatory cytokines, insulin signalling
1. Introduction
The popularity of antioxidative vitamins as dietary supplements may illustrate the growing public awareness that oxidative stress and oxygen radicals are important hazards to our health and play a major role in the ageing process. This review provides a critical evaluation of the evidence for a role of oxygen radicals and oxidative conditions in the ageing process and of the available options for therapeutic intervention.
(a) Oxygen radicals and redox signalling
Superoxide radicals () are constantly formed in most cells and tissues by enzymes such as NAD(P)H oxidases and by several other mechanisms (reviewed in Beckman & Ames 1998; Dröge 2002a). Superoxide can be converted into hydrogen peroxide and both together with several other related compounds are collectively called ‘reactive oxygen species’ (ROS). These chemically aggressive molecules react with different types of macromolecules and damage vital cell constituents such as DNA, proteins and lipids (reviewed in Harman 1981). The immune system, therefore, uses ROS as a first line of defence against environmental pathogens. The massive production of superoxide radicals and hydrogen peroxide by activated macrophages and neutrophils, commonly known as ‘oxidative burst’, was originally seen mainly as a mechanism to damage infective pathogens.
By altering the function of redox-sensitive signalling proteins, certain ROS also play an important role in biological signalling (reviewed in Dröge 2002a). In a study to determine potentially damaging effects of the ‘oxidative burst’ on immune cells, Roth & Dröge (1987) unexpectedly found that in activated T lymphocytes superoxide radicals or low micromolar concentrations of hydrogen peroxide upregulate the production of the immunologically important T cell protein interleukin-2 (figure 1). This was a surprising finding at that time, because superoxide radicals were previously considered only as mediators of structural damage. At least two transcription factors known to be involved in interleukin-2 production, namely nuclear factor κB (NF-κB) and activator protein-1 (AP-1) were subsequently shown to be activated under oxidative conditions (figure 2; reviewed in Dröge 2002a). NF-κB was the first eukaryotic transcription factor shown to be induced or enhanced by ROS in certain cell types (Schreck & Baeuerle 1991) and accordingly inhibited by antioxidants such as cysteine (Staal et al. 1990; Mihm et al. 1991). NF-κB is involved in various biological responses, including inflammatory reactions and the induced expression of lymphokines and cytokines. Tumour necrosis factor α (TNF-α) is one of the most prominent inflammatory cytokines (see also §4) which are induced by the transcription factor NF-κB and accordingly expressed under oxidative conditions (Verhasselt et al. 1998; reviewed in Dröge 2002a). The p65/p50 NF-κB heterodimer is activated by phosphorylation and degradation of its inhibitor IκB. The corresponding IκB kinase (IKK) is activated or enhanced under oxidative conditions through redox-sensitive components of the upstream signalling pathway. AP-1 is another widely used transcription factor and involved in various differentiation processes. In T lymphocytes AP-1 regulates the expression of the interleukin-2 gene and other immunologically relevant genes. AP-1 is typically composed of c-fos and c-jun proteins. Oxidative activation of AP-1 involves the activation of Jun-N-terminal kinase (JNK; Yoshizumi et al. 2000), a mitogen-activated protein kinase (MAPK) which phosphorylates and activates c-Jun (reviewed in Dröge 2002a). More recently, oxidative conditions have also been shown to activate the MAPK p38 and, to a lesser extent, the extracellular signal-regulated kinase 1 (ERK-1) and ERK-2 (reviewed in Dröge 2002a). The more detailed investigation revealed a remarkable redundance of redox-sensitive signalling molecules in these signalling pathways (reviewed in Dröge 2002a). Among other redox-sensitive targets, ROS directly stimulate certain protein tyrosine kinases, which are involved in these and other signalling cascades. In addition, ROS inactivate protein tyrosine phosphatases, which negatively regulate the effects of the tyrosine kinases (Dröge 2002a; see §3).
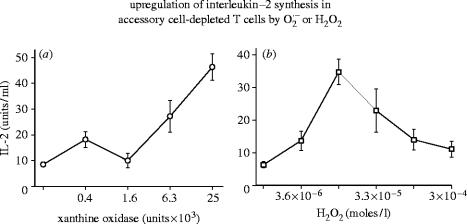
Figure 1.
Upregulation of interleukin-2 (IL-2)synthesis in accessory cell-depleted T cells by or H2O2. IL-2 production in Concanavalin-A-stimulated accessory cell-depleted T cell populations in the presence of an -generating system (left panel) or hydrogen peroxide (right panel). For experimental details see Roth & Dröge (1987).
Figure 2.
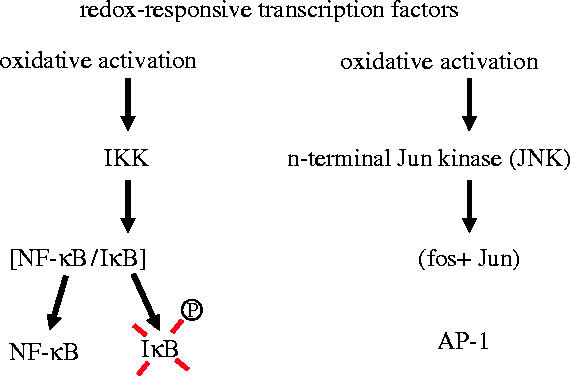
Redox-responsive transcription factors (schemtatic illustration). Nuclear factor-κB (NF-κB) is a p65/p50 heterodimer. It was the first transcription factor shown to be activated by ROS (see text). The activator protein-1 (AP-1) typically consists of Fos and Jun proteins and is activated under oxidative conditions through several redox-responsive signalling proteins upstream of N-termal Jun kinase (JNK).
(b) Response of signalling cascades to changes in the cellular thiol/disulfide redox status
The activation of NF-κB and AP-1 and several redox-sensitive signalling pathways known to be triggered by hydrogen peroxide are alternatively triggered by an oxidative change in the intracellular redox status (Galter et al. 1994; Kuge & Jones 1994; Hehner et al. 2000). The cytoplasm of most cells contains millimolar concentrations of glutathione, a tripeptide consisting of the amino acids glutamate, cysteine and glycine (figure 3; Meister & Anderson 1983; Sies 1999). As the cysteine moiety contains a thiol group (SH), glutathione can be converted by ROS into glutathione disulfide. Cysteine can similarly be oxidized to a disulfide called cystine. The thiol/disulfide ratio in the cytoplasm determines its redox status.
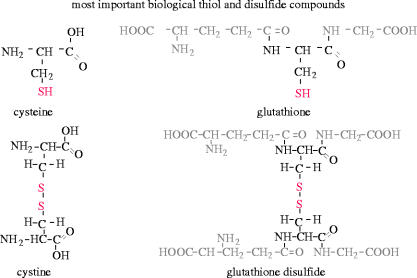
Figure 3.
Most important biological thiol and disulfide compounds of low molecular weight. Cysteine is a thiol-containing amino acid, which can be oxidized to the corresponding disulfide compound cystine. Glutathione is a tripeptide, consisting of glutamate, cysteine and glycine. Glutathione disulfide is formed by oxidation of glutathione.
Using the glutathione reductase inhibitor 1,3-bis-(2-chloroethyl)-1-nitrosourea (BCNU), Galter et al. (1994) studied the effect of the redox status on the activity of AP-1 and NF-κB. BCNU (10–100 μM) causes a substantial increase in intracellular glutathione disulfide concentration and a corresponding decrease in reduced glutathione (figure 4a). Under these conditions the transcription factor AP-1 is stimulated more than tenfold, as detected by the expression of a chloramphenicol-acetyltransferase (CAT) reporter construct under the control of 6 AP-1 binding sites (figure 4b). Analogous experiments have shown a similar stimulation of NF-κB activity (Galter et al. 1994). Hehner et al. (2000) reported that stimulation of lymphocytes by anti-CD3 and anti-CD28 antibodies, which are directed against the T cell receptor and the costimulatory CD28 receptor, respectively, causes by itself a substantial oxidative shift in the glutathione redox status which is further enhanced by the addition of either BCNU or hydrogen peroxide. The analysis of JNK and p38 MAPK activation revealed that the combination of anti-CD3 and anti-CD28 antibodies causes by itself a moderate phosphorylation of c-Jun and p38 MAPK, which is synergistically enhanced either by BCNU or by hydrogen peroxide (figure 5). Without the agonistic antibodies, BCNU or hydrogen peroxide cause only a moderate activation of these signalling proteins (Hehner et al. 2000). A similar synergistic enhancement by these antibodies in combination with BCNU has also been demonstrated in the phosphorylation of IKK and its substrate IκB, the inhibitor of NF-κB (figure 5, lower panels).
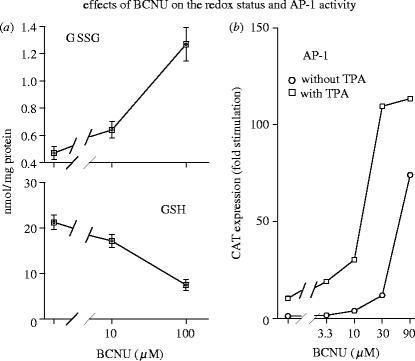
Figure 4.
Effects of BCNU on redox status and AP-1 activity. (a) Human T lineage cells (Molt-4) were treated with the glutathione reductase inhibitor BCNU at the indicated concentrations. After 3 h the cells were harvested, intracellular concentrations of total glutathione and glutathione disulfide were determined, and reduced glutathione (GSH) was calculated from the difference. (b) Molt-4 cells were transiently transfected with a CAT reporter construct driven by the thymidine kinase promoter under the control of 6 AP-1 binding sites. One day later the cells were treated with BCNU at the indicated concentrations and with or without TPA (10 ng ml−1). Transactivation was determined after 36 h by CAT assay. (For other details see Galter et al. 1994.)
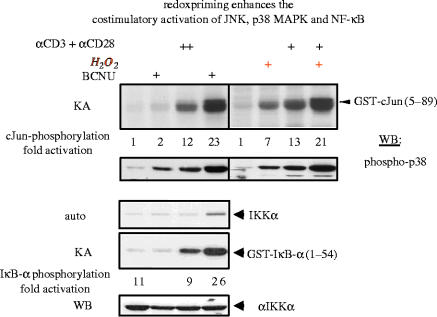
Figure 5.
Redox priming enhances the costimulatory activation of JNK, p38 MAPK and NF-κB. Jurkat cells were incubated with or without BCNU (10 μM) or hydrogen peroxide (30 μM) and activated with or without agonistic anti-CD3/anti-CD28 antibodies. Upper panels: Cells were lysed and tested for cJun phosphorylation by immune complex kinase assays and for p38 MAPK phosphorylation by immunoblotting. Lower panels: Endogenous IκB kinase α (IKKα) was immunoprecipitated and analysed either for its kinase activity (KA), using recombinant GST-IκB-α as a substrate or by Western blotting (WB) for the occurrence of IKKα. A longer exposure of the gel displaying the phosphorylation of IKK is also shown (Auto). (For other details see Hehner et al. 2000.)
The list of signalling processes known to respond to changes in the thiol/disulfide redox status has been growing in recent years and includes among others the bacterial OxyR system, the insulin receptor kinase activity (see §3b), Src family kinases, protein tyrosine phosphatases, and signalling events involved in replicative senescence (reviewed in Dröge 2002a). The reversible oxidative inhibition of protein tyrosine phosphatases and certain lipid phosphatases has been shown to involve a redox-sensitive cysteine moiety in the catalytic site (Bartford et al. 1995; Berrett et al. 1999a,b). The oxidative conversion of cysteine into sulfenic acid by hydrogen peroxide renders the enzyme inactive (figure 6). The chemically highly reactive sulfenic acid moiety, in turn, interacts spontaneously with intracellular glutathione or another thiol to yield a mixed disulfide, which again is enzymatically inactive. Importantly, the active protein tyrosine phosphatase can be inactivated not only by ROS but also by an oxidative shift in the cellular glutathione redox status (reviewed in Dröge 2002a). The active phosphatase is eventually regenerated through reduction by thioredoxin (figure 6).
Figure 6.
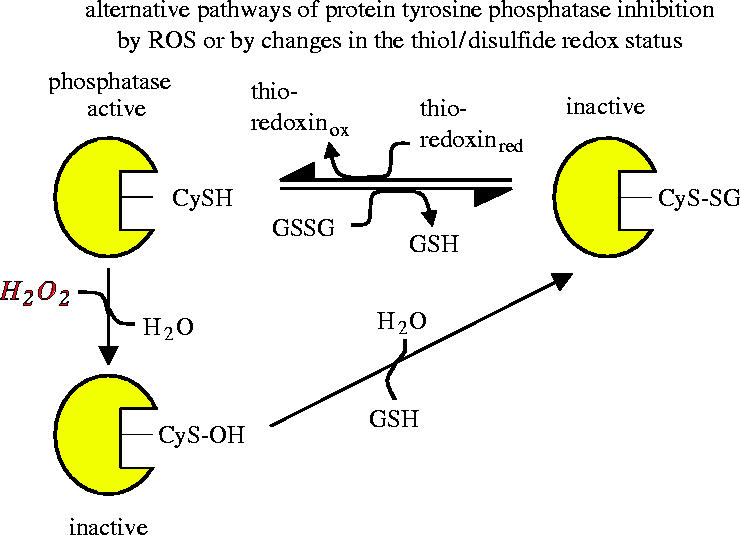
Alternative pathways of protein tyrosine phosphatase inhibition by ROS or by changes in the thiol/disulfide redox status. A cysteine residue in the catalytic site of the phosphatase is critical for its catalytic activity. Inactivation can occur either by reaction with hydrogen peroxide to form a sulfenic acid derivative or by reaction with glutathione disulfide, resulting in glutathionylation of the critical cysteine residue. Reactivation of the phosphatase may occur by reaction with reduced glutathione or other thiol compounds (schematic illustration based on Barford et al. 1995; Barrett et al. 1999a,b).
(c) Key message of section 1
ROS play an important role as mediators in biological signalling processes but are also potentially harmful for vital cell constituents of the host. As several of the redox-sensitive signalling proteins respond not only to the chemical modification by ROS but also by oxidation through an oxidative shift in the thiol/disulfide redox status of the cell, important physiological processes may become dysregulated by local or systemic changes in the thiol redox status. To ensure that ROS can function as signalling molecules without excessive tissue damage, ROS are rapidly scavenged in healthy subjects by certain antioxidants, including glutathione.
2. Impeding insulin receptor signalling to increase life span
(a) Mechanisms involved in life span extension in mutant strains of worms, fruit flies and mice
One of the most seminal findings in ageing research has been the discovery by Cynthia Kenyon and colleagues that the life span of a little worm (Caenorhabditis elegans) was increased 2.5 fold and more by mutating a single gene (Kenyon et al. 1993). In the human species, such an increase would correspond to a life span of more than 200 years. Subsequent biochemical studies revealed that mutant strains of worms, flies and mice with increased life span often showed an increased resistance to oxidative stress (Orr & Sohal 1994; Lin et al. 1998; Parkes et al. 1998; Honda & Honda 1999; Lee et al. 1999; Migliaccio et al. 1999; Taub et al. 1999), thus suggesting a mechanistic link between oxidative stress and ageing. More importantly, mutant animals with an extended life span often turned out to have a mutation in the signalling pathway of the insulin receptor or related receptors (reviewed in Guarente & Kenyon 2000; Longo & Finch 2003). Mutations of the insulin receptor in Drosophila have also been shown to ameliorate the age-related decline in cardiac performance (Wessells et al. 2004), implying that this signalling cascade determines the ageing-related deterioration of organ function at least in the fruit fly.
Insulin receptor signalling (schematically illustrated in figure 7) involves phosphatidylinositol 3-kinase (PI3K), (3,4,5)P3-dependent kinase 1 (PDK1), the kinase Akt/PKB, and the target of rapamycin (TOR or mTOR in mammals). It is negatively regulated by phosphatases including PTP1B, PTEN and SHIP2. An increase in the life span of the nematode worm C. elegans was achieved by mutations in daf-2, age-1 and pdk-1 which encode an insulin receptor autologue, PI3K, and a PDK1 homologue, respectively (Kenyon et al. 1993; Morris et al. 1996; Kimura et al. 1997; Paradis et al. 1999). Mutations in daf-18, which encodes a homologue of PTEN phosphatase, or an increase in TOR activity were found to suppress life span extension (Dorman et al. 1995; Larsen et al. 1995; Vellai et al. 2003). TOR/mTOR is needed to stimulate many functions including protein synthesis (Oldham & Hafen 2003), but downregulates the autophagic/lysosomal protein catabolism, and the expression of sirtuin proteins (sir2.1 and mammalian SIRT1; reviewed in Tallóczy et al. 2002; Dröge 2003; Hekimi & Guarente 2003). Sirtuin proteins regulate a diverse set of pathways which all have an impact on ageing. Autophagy is also required for life span extension (Melendez et al. 2003) and plays an important role in the maintenance of cellular integrity by removing damaged mitochondria and other organelles (reviewed in Dröge 2003). Life span extension was also shown to require the transcription factor DAF-16, a member of the FOXO (forkhead box) transcription factors, which are also negatively regulated by the insulin receptor signalling pathway (reviewed in Brunet et al. 1999; Tran et al. 2003; Murphy et al. 2004). DAF-16 and its mammalian counterparts control various genes and metabolic processes (Carlsson & Mahlapuu 2002; Kops et al. 2002; Bois & Grosveld 2003; Hribal et al. 2003; Nemoto & Finkel 2004). Specifically, FOXO1 and FOXO3 were shown to induce the expression of proteins involved in proteasomal proteolysis (Sacheck et al. 2004; Sandri et al. 2004; Stitt et al. 2004). This function is complementary to autophagy as it accounts for the proteolytic turnover of long-lived myofibrillar proteins which are not degraded through the autophagic/lysosomal pathway (Solomon & Goldberg 1996).
Figure 7.
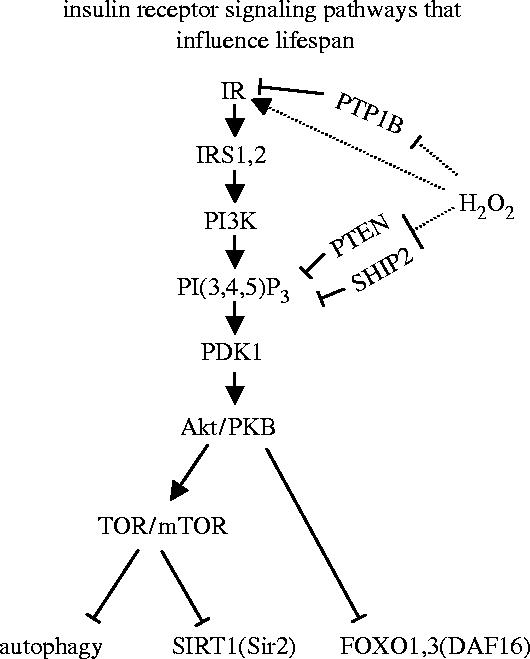
Insulin receptor signalling pathways that influence life span. Binding of insulin leads to autophosphorylation and activation of the insulin receptor kinase (IR) and recruitment of insulin-receptor substrates (IRS-1, IRS-2). Phosphatidylinositol 3-kinase (PI3K) converts phosphatidylinositol(4,5)diphosphate (PI(4,5)P2) into phosphatidylinositol(3,4,5)triphosphate (PI(3,4,5)P3). This binds and activates phosphoinositide-dependent protein kinase 1 (PDK1), which phosphorylates the serine/threonine kinase Akt1. Akt1 is activated by binding to PI(3,4,5)P3 at the cell membrane and by phosphorylation. Akt1 stimulates the target of rapamycin (TOR or the mammalian mTOR) and protein synthesis but is an inhibitor of several other functions, including autophagy, SIRT1 activity and FOXO transcription factor activity. Insulin receptor signalling activity is downregulated by protein tyrosine phosphatase 1B (PTB1B), which dephosphorylates the IRK domain, and by the phosphatase and tensine homologue on chromosome 10 (PTEN) and SH2-domain-containing inositol phosphatase (SHIP2) both of which dephosphorylate PI(3,4,5)P3. Hydrogen peroxide enhances the autophosphorylation of IRK and inhibits the activity of the three phosphatases.
It is reasonable to assume that autophagy and FOXO-dependent ubiquitin ligases also play an important role in humans by removing damaged long-lived cellular constituents. This is obviously important for the integrity of postmitotic tissues. But there are two caveats. Firstly, upregulation of autophagy and FOXO transcription factors would have to be well balanced as uncontrolled autophagy can lead to cell death (Klionsky & Emr 2000; Gozuacik & Kimchi 2004; Shao et al. 2004), and high levels of FOXO1 and FOXO3 activity were shown to be involved in cancer cachexia (McKinnell & Rudnicki 2004; Sacheck et al. 2004; Sandri et al. 2004; Stitt et al. 2004). Secondly, in view of the well-known positive functions of the insulin receptor-dependent signal it is unlikely that the permanent downregulation of this signalling cascade by genetic or other methods may be the best strategy to increase life span. This conclusion applies to C. elegans and Drosophila and even more to humans. In higher organisms, a substantial response to insulin is required after food intake to ensure glucose homeostatis and to stimulate protein synthesis in the postprandrial (fed) state. In humans, downregulation of the insulin receptor signalling cascade is, therefore, only found in the postabsorptive (fasted) state (reviewed in Dröge 2003), implying that substantial induction of autophagy, SIRT1, FOXO1 and FOXO3 activities is largely restricted to this state. Accordingly, any attempt to further downregulate insulin receptor signalling in humans should, therefore, be restricted to the fasted state. How can this be done? Recent reports have shown that the insulin-independent basal activity of the insulin receptor is weak but clearly detectable and determined to a large extent by the redox status of the individual cell.
(b) The basal insulin receptor kinase activity is increased under oxidative conditions
In analogy to the redox-sensitive signalling pathways described in §1, the basal insulin receptor tyrosine kinase activity is strongly increased by small concentrations of hydrogen peroxide (i.e. 60 μM), or by an oxidative shift in the intracellular glutathione redox status (figure 8; Schmid et al. 1998; Schmitt et al. 2005). However, the enhancing effect of hydrogen peroxide operates only in the presence of physiologically relevant concentrations of ADP (i.e. 70 μM), which is both a product and an inhibitor of the kinase reaction (figure 9). Hydrogen peroxide practically attenuates the inhibitory effect of ADP.
Figure 8.
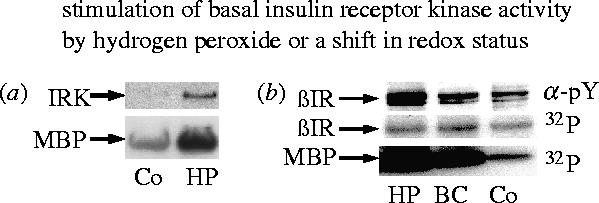
Stimulation of basal insulin receptor kinase activity by hydrogen peroxide or a shift in glutathione redox status. (a) Phosphorylation of recombinant IRK protein and myelin basic protein (MBP) substrate. The recombinant IRK protein was incubated at 30 °C for 20 min with or without 60 μM hydrogen peroxide (HP), then for 30 min with MBP and 1 mM ATP, and finally for 20 min with 32P-ATP and MBP. (b) Intact CHO-HIR cells were cultured with 50 μM hydrogen peroxide (HP), 80 μM 1-(2-chloroethyl)-3-(2-hydroxyethyl)-1-nitrosourea, a specific inhibitor of glutathione reductase (BC), or no additives (Co). The insulin receptor was purified by immunoprecipitation and assayed for autophosphorylation (βIR) or substrate phosphorylation (MBP) by 32P-incorporation or phosphotyrosine antibody (α-PY; data taken from Schmitt et al. 2005).
Figure 9.
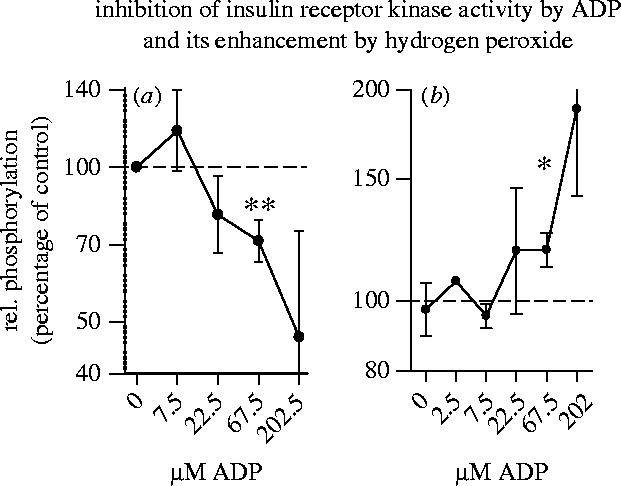
Inhibition of insulin receptor kinase activity by ADP and its enhancement by hydrogen peroxide. Recombinant IRK protein was incubated with or without 60 μM hydrogen peroxide and with or without the indicated concentrations of ADP. (a) shows the relative phosphorylation of myelin basic protein (MBP) substrate in cultures without hydrogen peroxide. (b) shows the relative enhancement of MBP phosphorylation by hydrogen peroxide as compared to controls without hydrogen peroxide. (For other details see Schmitt et al. 2005.)
In skeletal muscle tissue, cytoplasmic ADP is rapidly converted into ATP by cytoplasmic creatine kinase in combination with relative high concentrations of phosphocreatine. In muscle tissue basal insulin receptor kinase activity is, therefore, expected to be less inhibited by ADP and accordingly stronger than in fat cells under reducing conditions. This difference disappears if the inhibitory effect of ADP is attenuated by oxidative conditions. Accordingly, oxidative enhancement of insulin receptor kinase activity is likely to operate preferentially in cells with little creatine and creatine kinase activity such as adipose tissue. That the insulin reactivity of this tissue is particularly relevant for longevity is suggested by the finding that mice with fat-specific insulin receptor knockout also showed an increased life span (Blüher et al. 2003).
This basal activity of the insulin receptor signalling cascade is further enhanced by hydrogen peroxide through the inhibition of the protein tyrosine phosphatase PTP1B and the lipid phosphatases PTEN and SHIP (see figure 6). If active, these phosphatases downregulate insulin receptor signalling. They are readily inactivated, however, by oxidation of a redox-sensitive cysteine residue in their active site (see §1b; Barford et al. 1995; Denu & Tanner 1998; Barrett et al. 1999a,b; Haj et al. 2002; Lee et al. 2002; Salmeen et al. 2003).
The physiological relevance of the interrelated effects of redox status and ADP has been confirmed by the results of a clinical study on non-diabetic obese subjects (Hildebrandt et al. 2004), indicating that the basal insulin receptor signalling activity is decreased in subjects supplemented with N-acetylcysteine (see §4b).
(c) Key message of section 2
A series of recent reports strongly suggests that impeding insulin receptor signalling facilitates the optimal activation of several independent mechanisms which all have an impact on the speed of ageing. Two of these mechanisms appear to be involved in the degradation of damaged cell constituents such as mitochondria and myofibrillar proteins and may thus be needed to maintain the integrity of postmitotic tissues.
In healthy human subjects downregulation of insulin recpetor signalling is only found in the postabsorptive (fasted) state, implying that under healthy conditions maximum autophagy, SIRT1 and FOXO transcription factor activities are also largely restricted to the fasted state. Any attempt to further downregulate the insulin receptor signalling cascade in humans must also be restricted to the fasted state. The insulin independent basal insulin receptor tyrosine kinase activity is strongly increased by small concentrations of hydrogen peroxide or by an oxidative shift in the intracellular thiol/disulfide redox status. Treatment with antioxidants is, therefore, viewed as a viable option to ‘adjust’ the basal insulin receptor signalling activity in humans to an appropriate level in the upper normal range without compromising glucose clearance in the postprandial state.
3. Oxidative stress
(a) Oxidative stress as a change in redox balance
To ensure that ROS can function as signalling molecules and yet to minimize tissue damage, ROS are rapidly scavenged in healthy young subjects by antioxidants such as glutathione and the vitamins A, C and E. If either the production of ROS is increased or the level of antioxidants is decreased, ROS may reach abnormally high concentrations. This condition is called ‘oxidative stress’ and is implicated in numerous diseases. Its pathological consequences may involve direct oxidative tissue damage or the dysregulation of signalling processes (reviewed in Dröge 2002a). A popular theory states that the damaging effects of ROS may play a key role in the mechanism of ageing (Harman 1956). This theory may now be extended to include the dysregulation of signalling processes by abnormally high ROS concentrations and, last but not least, also by an oxidative shift in redox status.
(b) Evidence for an age-related increase in oxidative stress and a decrease in glutathione and cysteine concentrations
To directly measure the concentration of oxygen radicals or hydrogen peroxide in living tissue is difficult. But there is indirect evidence that oxidative stress increases with age. Lipid peroxidation and the oxidative damage of proteins and DNA increase with age (Fraga et al. 1990; Lucas & Szweda 1998; Inal et al. 2001; Kasapoglu & Ozben 2001; Levine & Stadtman 2001; Balkan et al. 2002; Cakatay et al. 2003; Traverso et al. 2003; van der Loo et al. 2003). The concentrations of antioxidants including serum and tissue levels of vitamin E and plasma concentrations of vitamin C were shown to decrease with age (Scrofano et al. 1998; Balkan et al. 2002; Kumaran et al. 2003; De Cabo et al. 2004), and an age-related decrease in the intracellular glutathione content was found in the brain tissue from rats and mice (Oubidar et al. 1996; Pallardó et al. 1999; Sasaki et al. 2001), in retinal glial cells from guinea pigs (Paasche et al. 1998), spleen cells from mice (Furukawa et al. 1987) and in the liver and kidney from rats (Arivazhagan et al. 2001).
In humans, an age-related oxidative shift in the ratio of reduced to oxidized glutathione, i.e. the glutathione redox status, has been demonstrated in whole blood (i.e. mainly in erythrocytes; Samiec et al. 1998; Erden-Inal et al. 2002), and peripheral blood mononuclear cells (mainly lymphocytes; Hernanz et al. 2000; Lenton et al. 2000). The mean plasma cysteine/cystine redox status of human subjects shows a significant oxidative shift between the third and the ninth decade of life (see figure 10; Hack et al. 1998; Hildebrandt et al. 2002b). This age-related oxidative shift is accompanied by a decrease in the plasma glutathione level (Nuttall et al. 1998; Samiec et al. 1998) and a decrease in the ratio of reduced versus oxidized forms of plasma albumin and other thiol/disulfide redox couples (Rothschild et al. 1988; Halliwell & Gutteridge 1990; Jones et al. 2000, Dröge 2002b,c). Responses of signalling cascades to changes in redox status (see §1b) are, therefore, not merely experimental artefacts. As the thiol/disulfide redox status shifts to more oxidative conditions in old age, there is inevitably a shift in the set points of physiological signals.
Figure 10.
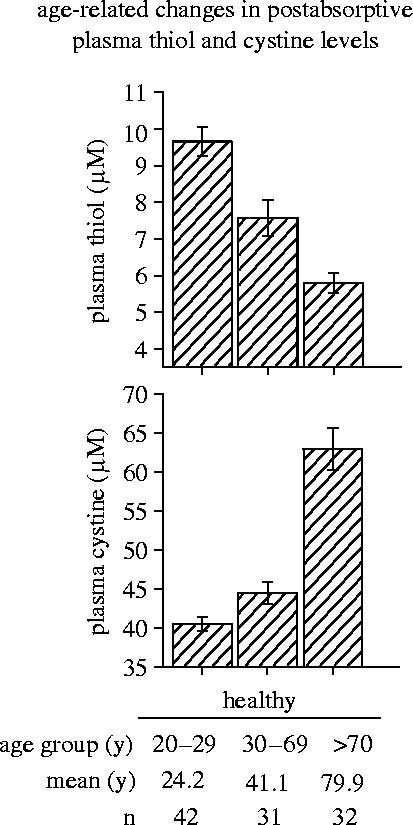
Age-related changes in postabsorptive plasma thiol and cystine levels. Postabsorptive plasma amino acid and acid-soluble thiol levels have been determined in the plasma from the cubital vein of randomly selected healthy human subjects of both sexes (unpublished data from Wulf Hildebrandt, see also Hack et al. 1998).
It is important to note, however, that reports on ‘total plasma cysteine concentrations’ or ‘total plasma homocysteine concentrations’ (Brattström et al. 1994; Jacob et al. 1999; Bates et al. 2002) are not directly related to the plasma thiol concentration because the terms ‘total cysteine’ and ‘total homocysteine’ include in these cases oxidized and protein-bound forms of these compounds.
(c) Key message of section 3
An abnormal increase in ROS production or a decrease in antioxidant concentrations leads to ‘oxidative stress’. Several lines of evidence indicate that oxidative stress increases with age and that the intracellular glutathione concentration of certain cells and tissues decreases accordingly.
4. Effects of cysteine supplementation
As the age-related increase in oxidative stress does not formally prove that oxidative stress actually causes ageing, it needs to be shown that age-related degenerative processes can be ameliorated by raising antioxidant levels. This can be done by supplementation of antioxidants like vitamins C or E or by raising the endogenous glutathione concentration. Several clinical investigations on the effects of cysteine as a glutathione precursor have been published. Some of these are summarized below.
(a) Most important sources of cysteine
The amino acid cysteine has been termed a ‘semi-essential’ amino acid as humans can synthesize cysteine from the sulphur-containing amino acid methionine only to a limited and generally not sufficient extent. If cysteine and methionine are taken together, an adult person in Western countries consumes, on the average, proteins equivalent to 2–4 g cysteine per day (Breitkreutz et al. 2000a). A series of recent clinical studies has shown that cysteine supplementation on top of the normal protein diet has several positive effects, implying that the ‘normal’ dietary intake of cysteine may be suboptimal although the average intake of calories in Western countries is generally considered superoptimal.
The amino acid cysteine is easily oxidized and, therefore, relatively unstable on the shelf. Its disulfide cystine is poorly soluble in water and not well absorbed. It is also important to note that most cells and tissues exhibit high transport activity only for the reduced form of cysteine but relatively low transport activity for the relatively large disulfide cystine (Sato et al. 1999). Most clinical studies on cysteine supplementation have, therefore, been performed either with the relatively stable synthetic cysteine derivative N-acetylcysteine, or with a naturally derived cysteine-rich undenatured whey protein isolate. At least under certain conditions, the whey protein isolate has been shown to increase the intracellular glutathione concentration of lymphocytes in humans (figure 11, left panel; Lands et al. 1999; Middleton et al. 2004) and of heart muscle tissue in mice (Bounous & Gold 1991; Bartfay et al. 2003). In a placebo-controlled clinical trial, treatment of human immunodeficiency virus (HIV) infected subjects with 3–8 g N-acetylcysteine per day for eight weeks was shown to cause a significant increase in whole blood glutathione levels (Herzenberg et al. 1997). However, as a low glutathione concentration is not generally considered as a disease, the increase in glutathione level is also not a generally accepted target for therapeutic intervention in clinical medicine. The following sections are, therefore, dealing with the effects of N-acetylcysteine or undenatured whey protein on the insulin receptor signalling activity and several other clinical parameters relevant to ageing (see table 1).
Figure 11.
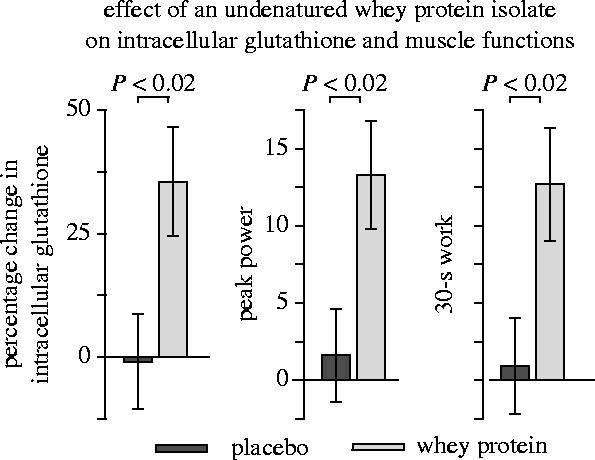
Effect of undenatured whey protein on intracellular glutathione and muscle functions. The data indicate the relative change (%) in the intracellular total glutathione concentration of lymphocytes, peak power, and 30 s work capacity of healthy young subjects during a three month treatment and observation period. (For other details see Lands et al. 1999.)
Table 1.
Key results from clinical studies.
| N-acetyl cysteine (NAC) | whey protein isolate (IMMUNOCAL) | |
|---|---|---|
| skel. muscle functions ↑ | frail elderly subjects (five force parameters, seven neuromotor fcts) | young healthy subjects (peak power, 30 s work, isokinetic cycling) |
| body cell mass ↑ (skel. muscle mass) | cancer patients | n.t. |
| body fat ↓ | healthy subjects | healthy subjects |
| immunological functions ↑ | HIV patients (NK, T cell prolif.) | hepatitis B patients (NK, serum IL-2) |
| tumour necrosis factor (TNF) ↓ | frail elderly subjects | n.t. |
| erythropoietin (EPO) ↑ | healthy subjects | n.t. |
| hypoxic ventilatory response (HVR) ↑ | healthy subjects | n.t. |
| plasma albumin↑ | HIV patients, cancer patients | n.t. |
(b) Modulation of the basal insulin receptor activity
In line with the redox regulation of the insulin receptor tyrosine kinase activity and the coregulatory effect of ADP (Schmid et al. 1998; Schmitt et al. 2005; see §2b), it was found that the basal insulin responsiveness can be altered by treatment with N-acetylcysteine together with or without creatine. A placebo-controlled double-blind study on the effects of N-acetylcysteine in combination with creatine in non-diabetic obese patients (Hildebrandt et al. 2004) revealed that the basal insulin reactivity in the postabsorptive (fasted) state was decreased by N-acetylcysteine in combination with placebo but increased by N-acetylcysteine in combination with creatine (see figure 12). The homeostasis model assessment/insulin resistance index which is essentially a measure of fasting plasma glucose levels multiplied by fasting plasma insulin concentrations was used as a widely accepted inverse indicator of basal insulin reactivity (Matthews et al. 1985). Incidentally, cysteine supplementation with or without creatine had also a significant effect on the glucose clearance capacity as determined by the oral glucose tolerance test (figure 13). According to anecdotal data it is possible to guide the doses of cysteine and creatine in such a way that the basal insulin receptor activity is decreased and the postabsorptive glucose level is adjusted to a value in the upper normal range (i.e. about 95 mg dl−1) without substantially compromising glucose clearance capacity. The study by Hildebrandt et al. also suggested that the absence or presence of creatine had no significant effect on fat tissue. This is tentatively explained by the fact that creatine is mainly utilized by brain and muscle tissues but not by fat cells (reviewed in Hildebrandt et al. 2004). As ADP is rapidly converted into ATP by cytoplasmic creatine kinase in brain and muscle tissues but not in fat cells, insulin receptor activity of muscle tissue is expected to be less inhibited and accordingly stronger than that of adipocytes at least under reducing conditions. But this difference is attenuated under oxidative conditions which attenuate the redox-sensitive inhibitory effect of ADP (Schmitt et al. 2005).
Figure 12.
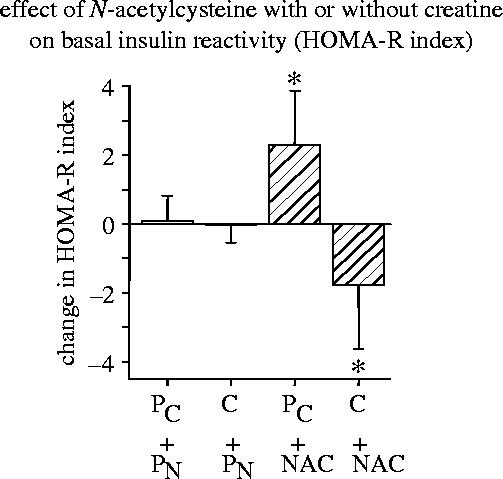
Effect of N-acetylcysteine (NAC) with or without creatine (C) on basal insulin reactivity. The homeostasis model assessment/insulin resistance index (HOMA-R) was used as a widely accepted measure of basal insulin reactivity (Matthews et al. 1985). It was calculated by the formula: HOMA-R=fasting plasma glucose (mg dl−1)×fasting plasma insulin concentration (mU ml−1)×405−1. The data show the absolute changes (mean±s.e.m.) in the HOMA-R index between baseline and terminal examination in the four treatment groups: . (For other details see Hildebrandt et al. 2004.)
Figure 13.
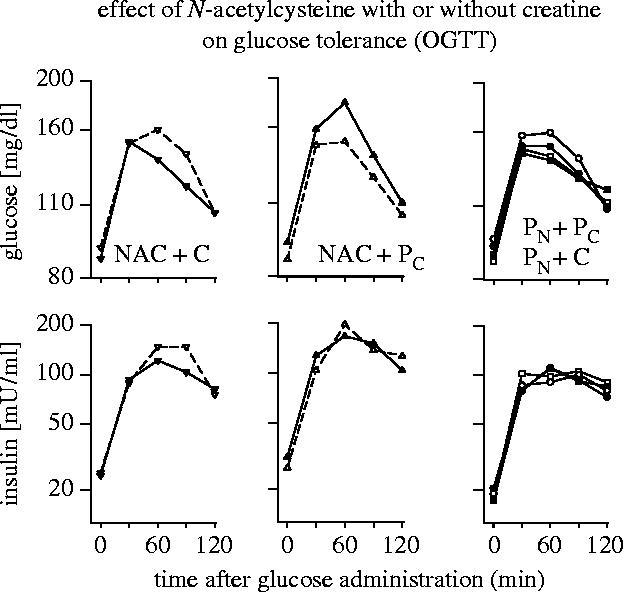
Effect of N-acetylcysteine with or without creatine on glucose tolerance (OGTT). Non-diabetic obese subjects have been treated with a combination of N-acetylcysteine+creatine (NAC+C; left panel), N-acetylcysteine+placebocreatine (middle panel), placeboNAC+creatine, or with placeboNAC+placebocreatine (right panel). The data show glucose and insulin concentrations at different times after glucose administration before (open symbols) and after the intervention and observation period (closed symbols). (For other details see Hildebrandt et al. 2004.)
(c) Muscle function as a measure (surrogate parameter) of ageing and frailty
Ageing in humans is a complex process involving the generalized impairment of physiological functions, a decreased ability to respond to a wide range of stress, the increased risk of age-associated diseases, and the increased likelihood of death (Kirkwood 1996). Loss of muscle function can be measured with good precision and reproducibility and has been shown to be strongly correlated with age-related probability of death (Guralnik et al. 1994; Rantanen et al. 2000). It is, therefore, one of the best single surrogate parameters of ageing. Loss of skeletal muscle mass and muscle function (wasting) is a common finding in old age and also found in persons over 100 years who had the privilege to avoid other ageing-related conditions. Loss of muscle functions is typically associated with compromised physical and social functions, loss of independence, and psychological stress. Eventually, the wasting process leads to organ failure (Buchner & Wagner 1992) and is, therefore, often considered as a cause of death.
Regular physical activity throughout life is the only method known to increase life span in humans (Blair et al. 1989; Sandvik et al. 1993, 1995). Physical exercise has been shown to improve skeletal muscle function but is not always effective in old age (Fiatarone et al. 1994; Tinetti et al. 1994; Chandler & Hadley 1996; Buchner et al. 1997; Hauer et al. 2001). A placebo-controlled double-blind study on frail elderly patients has shown that treatment with N-acetylcysteine doubled the increase in knee extensor strength during a six week programme of physical exercise and slowed the subsequent decline during a six week follow-up period (figure 14; Hauer et al. 2003). Similarly, treatment with a cysteine-rich undenatured whey protein significantly increased peak power and 30 s work capacity in a whole leg isokinetic cycle test if compared with placebo-treated control subjects in a randomized double-blind study of young healthy subjects (figure 11; Lands et al. 1999). Moreover, treatment with N-acetylcysteine has been found to ameliorate the loss of body cell mass in cancer patients which also reflects a loss of skeletal muscle mass (Hack et al. 1998). Studies of both elderly subjects and cancer patients have shown that the ageing- and disease-related decrease in body cell mass is significantly correlated with the oxidative shift in the plasma thiol/disulfide redox status (Hack et al. 1998).
Figure 14.
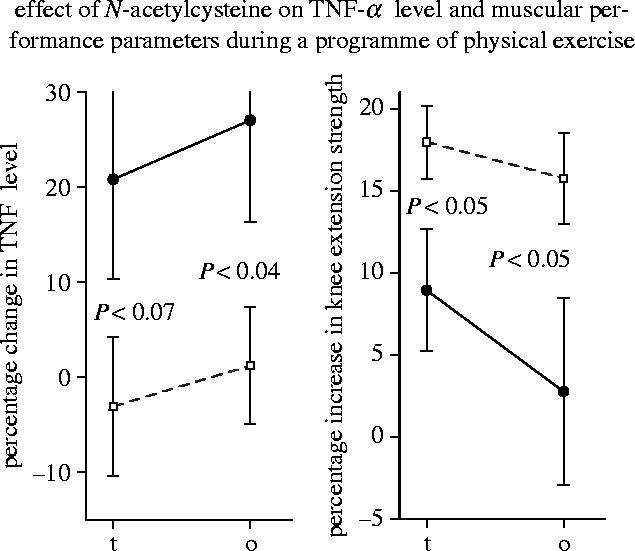
Effect of N-acetylcysteine on TNF-α level and muscular performance during a programme of physical exercise. The data indicate the relative increase (%) in plasma TNF-α level and knee extension strength during a six week treatment and exercise programme (t), and during the total 12–13 week observation period (o). Filled circles, placebo group; open squares, N-acetylcysteine-treated group (see Hauer et al. 2003).
(d) The body cell mass/body fat ratio
In any dietary regimen, it is extraordinarily difficult to maintain muscle mass without gaining weight and increasing body fat. If a diet is adjusted to maintain a constant body weight, skeletal muscle mass will eventually decline, and body fat will increase correspondingly. In view of this problem, it was encouraging to see that supplementation with either N-acetylcysteine (Kinscherf et al. 1996; Hildebrandt et al. 2004) or with a cysteine-rich undenatured whey protein isolate (Lands et al. 1999) caused a relative decrease in body fat versus body cell mass. Similarly, treatment of rats with whey protein during a five week exercise programme caused an increase in lean body mass and a decrease in relative fat mass if compared with rats with a control diet (Bouthegourd et al. 2002). These effects are tentatively explained by the differential expression of the creatine kinase system in skeletal muscle and fat tissues (see §3b). However, a study on the effects of whey protein hydrolysate suggested that the effect may either be lost by hydrolysis or may fail to work in overweight subjects (Demling & DeSanti 2000). In spite of these encouraging results, more systematic studies of the muscle/fat ratio are needed.
(e) TNF-α
Cytokines such as TNF-α are proteins with a hormone-like function. Ageing is associated with increased circulating levels of certain inflammatory cytokines including TNF-α and has, therefore, been interpreted by some authors as a low-grade inflammatory condition (Bruunsgaard et al. 2001; Bruunsgaard et al. 2003). High plasma TNF-α levels are correlated with morbidity, mortality, Alzheimer's disease, atherosclerosis, and decreased muscle mass and muscle strength in elderly subjects (Fillit et al. 1991; Bruunsgaard et al. 1999, 2000; Visser et al. 2002, 2003). In combination with interferon-γ (IFN-γ) TNF-α activates the trancription factor NF-κB that downregulates MyoD, a transcription factor essential for repairing damaged muscle tissue (Guttridge et al. 2000; McKinnell & Rudnicki 2004). In mouse muscles and myotubes, TNF-α in combination with IFN-γ was shown to reduce myosin expression (Acharyya et al. 2004). In centenarians a high plasma TNF-α level has been found to be associated with dementia (Bruunsgaard et al. 1999). TNF-α and the TNF-α-inducible transcription factor NF-κB have also been implicated in the development of inflammation-associated cancer (Greten et al. 2004; Pikarsky et al. 2004). It was, therefore, alarming to see that a programme of physical exercise which was intended to improve the condition of the frail elderly subjects actually raised the plasma TNF-α level (Hauer et al. 2003). Importantly, this increase in TNF-α concentration was completely prevented by cysteine supplementation (figure 14). A similar increase in plasma TNF-α concentration was also seen after physical exercise in healthy young subjects (Vassilakopoulos et al. 2003). This increase was also prevented by cysteine supplementation and antioxidant vitamins A, C and E. A significant decrease in the serum level of TNF-α and other proinflammatory cytokines was also observed after treatment of cachectic cancer patients with a cysteine derivative in combination with other antioxidants (Mantovani et al. 2004).
The effect of cysteine supplementation on TNF-α levels in these independent studies is tentatively explained by the fact that the TNF-α gene is under control of the redox-responsive transcription factor NF-κB and upregulated under oxidative conditions (Verhasselt et al. 1998; reviewed in Dröge 2002a,b,c; see §1). That N-acetylcysteine suppresses NF-κB activation has been documented in patients with sepsis (Paterson et al. 2003).
(f) Plasma albumin level
Albumin reaches plasma concentrations of more than 600 μM and is, therefore, an important regulator of the oncoosmotic pressure of the blood plasma and needed to prevent oedema (reviewed in Hack et al. 1998). As albumin contains a free thiol group at Cys34, it is potentially oxidized to a mixed disulfide. Albumin is, therefore, also a quantitatively important redox buffer of the blood (Rothschild et al. 1988; Halliwell & Gutteridge 1990; Hack et al. 1998). Although albumin is primarily an extracellular molecule, it is taken up to a certain extent by most cell types either through endocytosis or pinocytosis and is subsequently catabolized by lysosomal degradation (Peters 1985). It thereby contributes to the maintenance of the intracellular glutathione level (Cantin et al. 2000). A marked decrease in plasma albumin levels is seen in elderly subjects and practically all catabolic conditions, including cancer cachexia and HIV infection. In studies on healthy elderly subjects, a low plasma albumin level was correlated with a low 10 year survival rate (Shibata et al. 1991) and loss of skeletal muscle mass (Baumgartner et al. 1996). Among patients with wasting syndrome, the plasma albumin level was found to be a strong predictor for survival (Rothschild et al. 1988).
A placebo-controlled trial on HIV-infected patients (Breitkreutz et al. 2000a,b) and an unblinded study on cancer patients (Hack et al. 1998) showed that cysteine supplementation increases the (otherwise low) plasma albumin levels at least in these conditions. As albumin exists in the plasma in both the reduced and the oxidized (i.e. mixed disulfide) form, and as the oxidized forms of albumin have a higher catabolic rate (Kuwata et al. 1994), the effect of cysteine supplementation is tentatively explained by the conversion of oxidized albumin into its more stable reduced form.
(g) Immune functions
Patients infected with HIV or hepatitis B virus (HBV) typically show a massive decrease in lymphocyte glutathione levels and a massive loss of natural killer (NK) cell activity and other immune functions (Eck et al. 1989; Ullum et al. 1995; Watanabe et al. 2000; Azzoni et al. 2002). It has also been shown that the absolute number of NK cells and the NK cell activity per cell decreases with age (Rukavina et al. 1998). NK cells play an important role in the protection against viral or bacterial infections (Bukowski et al. 1985; Rager-Zisman et al. 1987; Scalzo 2002; Vankayalapati et al. 2004; Warfield et al. 2004), and in the suppression of tumours (Kim et al. 2000; Tahir et al. 2001). NK cells are exquisitely sensitive against oxidative stress (Hansson et al. 1996; Kono et al. 1996; Furuke et al. 1999). The expression of the Fas ligand which is important for the ability of NK cells to kill their target cells is strongly suppressed by small amounts of hydrogen peroxide and can be upregulated by N-acetylcysteine (Furuke et al. 1999).
A significant improvement of immune functions has been seen in HIV patients after treatmeant with N-acetylcysteine (table 2; Breitkreutz et al. 2000b) and in hepatitis B patients after treatment with whey protein (Watanabe et al. 2000). Cysteine supplementation caused in either case an increase in NK cell activity and certain T cell functions as indicated by the increase in antigen-induced T cell proliferation or serum interleukin-2 concentrations. In another study on healthy human subjects, the median intracellular glutathione level of 25 ng mg−1 protein was found to correlate with maximum numbers of CD4+ and CD8+ lymphocytes. Treatment with N-acetylcysteine was found to increase the CD4+T cell count in persons with low but not high baseline concentrations of glutathione (Kinscherf et al. 1994). The immune enhancing effect of whey protein has been confirmed in a series of animal studies (Bounous & Kongshaven 1982; Bounous et al. 1983; Parker & Goodrum 1990; Wong & Watson 1995; Low et al. 2003). It is, therefore, tempting to speculate that the age-related decline in immune functions in humans may also be ameliorated by cysteine supplementation. This remains to be shown. Prophylactic cysteine supplementation may help healthy subjects to prevent the decrease in lymphocyte glutathione levels in upcoming virus infections (see above).
Table 2.
Effects of N-acetylcysteine on immune functions of HIV patients in two different trials.
| study no. | NAC | placebo | |||
|---|---|---|---|---|---|
| baseline | terminal | baseline | terminal | ||
| NK (lytic units per CD3−/16+/56+ cell) | 1 | 0.22±0.06 | 1.34±0.34 | 0.45±0.15 | 0.37±0.26 |
| 2 | 0.33±0.13 | 1.33±0.56 | 0.52±0.19 | 0.47±0.11 | |
| S.I. TET | 1 | 25±14 | 150±128 | 43±21 | 37±23 |
| 2 | 40±31 | 190±139 | 46±17 | 31±15 | |
| S.I. PHA | 1 | 747±137 | 1906±355 | 967±292 | 759±131 |
| 2 | 779±164 | 1267±301 | 1022±132 | 906±221 | |
Data from 40 HIV patients with antiretroviral therapy (ART; study 1) and 29 patients without ART (study 2) at baseline examination and after treatment for seven months with N-acetylcysteine (NAC) or placebo. The data (mean±s.e.m.) show three immunological parameters, i.e. natural killer (NK) cell activity and proliferal T cell responses (stimulation index, S.I.) after stimulation with tetanus toxoid antigen (TET), or phytohaemagglutinin (PHA). (For other details see Breitkreutz et al. 2000a,b.)
(h) Physiological signals from oxygen sensors
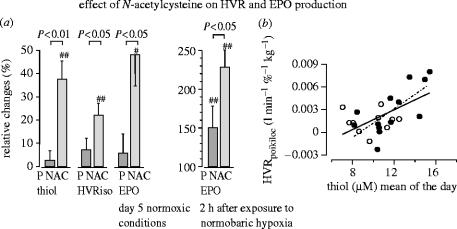
N-acetylcysteine treatment has also been shown to increase the hypoxic ventilatory response and the plasma concentration of erythropoietin (figure 15; Hildebrandt et al. 2002a), indicating that the set points of the corresponding oxygen sensors are altered by changes in cysteine availability. The plasma concentration of erythropoietin is controlled by the redox-responsive transcription factor HIF-1.
Figure 15.
Effect of N-acetylcysteine on hypoxic ventilatory response (HVR) and erythropoietin (Epo) production. (a) Relative changes during medication (N-acetylcysteine, NAC) between baseline and terminal examination expressed as percentage of baseline values. The data show the changes in plasma thiol concentrations, HVR under isocapnic conditions, EPO concentration under nomoxic conditions, and EPO 2 h after exposure to prolonged normobaric hypoxia. (b) Shows the correlation between the poikilocapnic HVR with the corresponding plasma thiol level. Each point represents one subject (filled circle, N-acetylcysteine-treated; open circle, placebo group). The solid line shows the regression function of the total population (r=0.59, P<0.01). (For other details see Hildebrandt et al. 2002a,b.)
(i) Cell culture studies
Results from these clinical and animal studies were further supported by an even larger series of studies in cell cultures, showing that relatively small changes in the intracellular glutathione level were associated with significant changes in immune functions, changes in intracellular signalling processes, induction of a postmitotic phenotype (cellular senescence), mitochondrial DNA damage, and apoptosis in fibroblasts (reviewed in Dröge & Holm 1997; Dröge 2002a).
(j) Key message of section 4
A series of recent clinical studies and complementary laboratory studies strongly suggest that cysteine supplementation on top of the normal protein diet has clear benefits with respect to several parameters relevant to ageing.
5. Conclusions
(a) Redox signalling and oxidative stress
Superoxide radicals and hydrogen peroxide play an important role as regulatory mediators in physiological signalling processes. Oxidative stress occurs if either the production of ROS is abnormally increased or the antioxidant concentration is decreased. Oxidative stress can lead to tissue damage and to the dysregulation of redox-sensitive signalling pathways.
At least some of the critical redox-responsive signalling proteins contain redox-sensitive cysteine moieties which activate or inactivate their regulatory function upon oxidation into a mixed disulfide. This conversion into disulfide formation is typically mediated by ROS as the signalling molecules proper, but inherently, it is also facilitated by an oxidative shift in the thiol/disulfide redox status of the microenvironment. An oxidative shift in redox status may thus also lead to dysregulation.
(b) Multiple benefits of cysteine supplementation
Several lines of evidence indicate that oxidative stress increases with age. These changes include a decrease in glutathione levels and/or a shift in redox status. To ameliorate oxidative stress and to improve the oxidant/antioxidant balance, antioxidative vitamins E, C and β-carotene (provitamin A) are widely used as dietary supplements. Surprisingly little attention has been given to the dietary intake of cysteine which is needed for the biosynthesis of glutathione, the quantitatively most important antioxidant and radical scavenger. Recent clinical studies have actually shown that cysteine supplementation on top of the normal protein diet has clear benefits with respect to several parameters relevant to ageing, suggesting that the age-related decrease in glutathione plays indeed a causative role in various ageing related degenerative processes. For the sake of brevity, this review has mainly focused on skeletal muscle functions, body fat, immunological reactivity, circulating TNF-α levels, and plasma albumin concentrations (see table 1). Cysteine supplementation has shown significant effects on all of these parameters in several independent studies.
As the quality of life in old age is severely compromised by the loss of skeletal muscle function, and given that muscle function can be quantitatively measured with good precision and reproducibility, loss of muscle function is taken as the best surrogate parameter of ageing. Cysteine supplementation mediated a significant increase in skeletal muscle functions in two independent placebo-controlled trials.
TNF-α is one of the hormone-like factors (cytokines) implicated in the loss of muscle mass and muscle function under catabolic conditions. In addition, high plasma TNF-α levels have been associated with a number of ageing-related degenerative processes. Cysteine supplementation was found to prevent the increase in plasma TNF-α concentration after physical exercise in two independent trials.
Elderly subjects and patients with practically all types of catabolic conditions typically show a conspicuous decrease in the plasma albumin level, and albumin plays an important role in the maintenance of the oncoosmotic pressure of blood that is needed to avoid oedema. Cysteine supplementation has been shown to increase the mean plasma albumin level in two independent clinical studies.
The best documented effects of cysteine supplementation on immunological functions in humans have been found in patients infected with a virus such as HIV and HBV. As both types of virus infection are typically associated with compromised immune functions and an exceptionally strong decrease in lymphocyte glutathione levels, it remains to be determined whether the immune enhancing activity of cysteine supplementation may be limited to these types of stress conditions.
Taken together, the association between oxidative stress resistance and life span in a series of animal studies (see §3a), the beneficial effects of cysteine supplementation on various parameters relevant to the ageing process (§4), and the age-related changes in the cysteine and glutathione status (§2b) strongly support the hypothesis that these oxidative changes may contribute to the ageing process. At least some of the effects of cysteine supplementation are best explained by the interpretation that this treatment ameliorates the age-related oxidative stress and the resulting dysregulation of redox-sensitive signalling cascades (§1). The redox-sensitive insulin receptor signalling cascade (§3b)is just one prominent example.
(c) Balanced modulation of the insulin receptor signalling cascade
A large body of evidence suggests that silencing of the insulin receptor signalling cascade is needed for optimal activation of SIRT1 activity, autophagy, and FOXO transcription factor activity, i.e. a diverse set of activities that were all found to have an impact on life span at least in mutant strains of worms, flies and mice. A well-balanced cysteine supplementation accompanied by a delicately balanced supply of creatine and guided by occasional measurements of fasting glucose levels and glucose tolerance tests appears to be a method suitable for humans to decrease insulin receptor signalling in the fasted state without compromising the clearance of glucose and other nutrients in the postprandial state.
Whether downregulation of insulin receptor signalling accounts for some of the beneficial effects cysteine supplementation described above remains to be shown. At least some of the beneficial effects are obviously based on redox mechanisms which do not involve insulin receptor signalling.
(d) The concept of cysteine as a ‘paravitamin’
It is now becoming increasingly clear that cysteine and its derivatives play a role similar to that of vitamins. Unlike vitamins, cysteine serves as a building unit of proteins and as a source of energy. But like the antioxidative vitamins, cysteine and its derivatives play a role in the oxidant/antioxidant balance and indirectly in the regulation of metabolic processes. One may, therefore, define cysteine or a cysteine-delivering substance as a ‘paravitamin’, i.e. a substance resembling a vitamin.
A deficit of any particular vitamin gives rise to typical symptoms which are reversed by supplementation of that vitamin. The various beneficial effects of cysteine supplementation on top of the normal diet suggest by analogy: (i) that there is an ageing-related deficit in body cysteine and glutathione reservoirs and (ii) that a deficit in cysteine leads to a decrease in muscle function, a decrease in immune function, a decrease in plasma albumin concentration, and an increase in TNF-α concentration. As all these changes are hallmarks of the ageing process, it is concluded that the age-related decrease in body cysteine and/or glutathione level may be a major driving force for multiple ageing-related degenerative processes.
(e) Everybody is likely to experience a cysteine deficiency sooner or later
As everybody beyond the fifth decade of life will experience sooner or later a decrease in muscle function, a decrease in immune function, a decrease in plasma albumin concentration, and/or an increase in TNF-α concentration, it is hypothesized that practically everybody experiences sooner or later an ageing-related deficit in the body cysteine and glutathione reservoirs that warrants cysteine supplementation. This hypothesis implies that ageing may be postponed and frailty be avoided to some extent by supplementation of the ‘paravitamin’ cysteine. It is emphasized, however, that several details still require more systematic investigation. Although substantial negative side effects have not been observed in previous studies on cysteine supplementation, it is felt that the treatment protocols ought to be further improved to achieve maximum safety and efficacy over long periods of time. Properly done, cysteine supplementation can reasonably be expected to improve the quality of life in old age. With the availability of novel cysteine delivery systems with minimum amounts of calories, which are superior to any of the naturally available cysteine sources, it is conceivable that even the maximum human life span may be increased beyond the previous limit.
Acknowledgments
The dedicated assistance of Mrs I. Fryson in the preparation of the manuscript is gratefully acknowledged.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Acharyya S, Ladner K.J, Nelsen L.L, Damrauer J, Reiser P.J, Swoap S, Guttridge D.C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 2004;114:370–378. doi: 10.1172/JCI20174. 10.1172/JCI200420174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arivazhagan P, Ramanathan K, Panneerselvam C. Effect of dl-alpha-lipoic acid on mitochondrial enzymes in aged rats. Chem. Biol. Interact. 2001;138:189–198. doi: 10.1016/s0009-2797(01)00268-x. 10.1016/S0009-2797(01)00268-X [DOI] [PubMed] [Google Scholar]
- Azzoni L, Papasavvas E, Chehimi J, Kostman J.R, Mounzer K, Ondercin J, Perussia B, Montaner L.J. Sustained impairment of IFN-γ secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 2002;168:5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- Balkan J, Kanbagli O, Mehmetcik G, Mutlu-Turkoglu U, Aykac-Toker G, Uysal M. Increased lipid peroxidation in serum and low-density lipoproteins associated with aging in humans. Int. J. Vitam. Nutr. Res. 2002;72:315–320. doi: 10.1024/0300-9831.72.5.315. [DOI] [PubMed] [Google Scholar]
- Barford D, Jia Z, Tonks N.K. Protein tyrosine phosphatases take off. Nat. Struct. Biol. 1995;2:1043–1053. doi: 10.1038/nsb1295-1043. 10.1038/nsb1295-1043 [DOI] [PubMed] [Google Scholar]
- Barrett W.C, DeGnore J.P, Keng Y.-F, Zhang Z.-Y, Yim M.B, Chock P.B. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J. Biol. Chem. 1999a;274:34 543–34 546. doi: 10.1074/jbc.274.49.34543. 10.1074/jbc.274.49.34543 [DOI] [PubMed] [Google Scholar]
- Barrett W.C, DeGnore J.P, Konig S, Fales H.M, Keng Y.-F, Zhang Z.-Y, Yim M.B, Chock P.B. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry. 1999b;38:6699–6705. doi: 10.1021/bi990240v. 10.1021/bi990240v [DOI] [PubMed] [Google Scholar]
- Bartfay W.J, Davis M.T, Medves J.M, Lugowski S. Milk whey protein decreases oxygen free radical production in a murine model of chronic iron-overload cardiomyopathy. Can. J. Cardiol. 2003;19:1163–1168. [PubMed] [Google Scholar]
- Bates C.J, Manssor M.A, Gregory J, Pentieva K, Prentice A. Correlates of plasma homocysteine, cysteine and cysteinyl-glycine in respondents in the British National Diet and Nutrition Survey of Young People Aged 4–18 years, and a comparison with the Survey of People Aged 65 Years and Over. Br. J. Nutr. 2002;87:71–79. doi: 10.1079/bjn2001479. 10.1079/BJN2001479 [DOI] [PubMed] [Google Scholar]
- Baumgartner R.N, Koehler K.M, Romero L, Garry P.J. Serum albumin is associated with skeletal muscle in elderly men and women. Am. J. Clin. Nutr. 1996;64:552–558. doi: 10.1093/ajcn/64.4.552. [DOI] [PubMed] [Google Scholar]
- Beckman K.B, Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Blair S.N, Kohl H.W, III, Paffenbarger R.S, Jr, Clark D.G, Cooper K.H, Gibbons L.W. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J. Am. Med. Assoc. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. 10.1001/jama.262.17.2395 [DOI] [PubMed] [Google Scholar]
- Blair S.N, Kohl H.W, III, Barlow C.E, Paffenbarger R.S, Jr, Gibbons L.W, Macera C.A. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. J. Am. Med. Assoc. 1995;273:1093–1098. 10.1001/jama.273.14.1093 [PubMed] [Google Scholar]
- Blüher M, Kahn B.B, Kahn C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. 10.1126/science.1078223 [DOI] [PubMed] [Google Scholar]
- Bois P.R.J, Grosveld G.C. FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. EMBO J. 2003;22:1147–1157. doi: 10.1093/emboj/cdg116. 10.1093/emboj/cdg116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounous G, Gold P. The biological activity of undenatured dietary whey proteins: role of glutathione. Clin. Invest. Med. 1991;14:296–309. [PubMed] [Google Scholar]
- Bounous G, Kongshaven P.A. Influence of dietary proteins on the immune system of mice. J. Nutr. 1982;112:1747–1755. doi: 10.1093/jn/112.9.1747. [DOI] [PubMed] [Google Scholar]
- Bounous G, Letourneau L, Kongshaven P.A. Influence of dietary proteins on the immune system of mice. J. Nutr. 1983;113:1415–1421. doi: 10.1093/jn/113.7.1415. [DOI] [PubMed] [Google Scholar]
- Bouthegourd J.-C.J, Roseau S.M, Makarios-Lahham L, Leruyet P.M, Tomé D.G, Even P.C. A preexercise α-lactalbumin-enriched whey protein meal preserves lipid oxidation and decreases adiposity in rats. Am. J. Physiol. Endocrinol. Metab. 2002;283:E565–E572. doi: 10.1152/ajpendo.00132.2002. [DOI] [PubMed] [Google Scholar]
- Brattström L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J. Intern. Med. 1994;236:633–641. doi: 10.1111/j.1365-2796.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Breitkreutz R, Holm S, Pittak N, Beichert M, Babylon A, Yodoi J, Dröge W. Massive loss of sulfur in HIV infection. AIDS Res. Hum. Retroviruses. 2000a;16:203–209. doi: 10.1089/088922200309296. 10.1089/088922200309296 [DOI] [PubMed] [Google Scholar]
- Breitkreutz R, et al. Imrovement of immune functions in HIV infection by sulfur supplementation–two randomized trials. J. Mol. Med. 2000b;78:55–62. doi: 10.1007/s001099900073. 10.1007/s001090050382 [DOI] [PubMed] [Google Scholar]
- Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Fork-head transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. 10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen A.N, Skinhoj P, Pedersen B.K. A high plasma concentration of TNF-a is associated with dementia in centenarians. J. Gerontol. Med. Sci. 1999;54A:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Skinhøj P, Pedersen A.N, Schroll M, Pedersen B.K. Ageing, TNF-α and atherosclerosis. Clin. Exp. Immunol. 2000;21:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. 10.1046/j.1365-2249.2000.01281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen B.K. Aging and proinflammatory cytokines. Curr. Opin. Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. 10.1097/00062752-200105000-00001 [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J.V.B, Pedersen B.K, Jeune B. Elevated levels of tumor necrosis factor-α and mortality in centenarians. Am. J. Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. 10.1016/S0002-9343(03)00329-2 [DOI] [PubMed] [Google Scholar]
- Buchner D.M, Wagner E.H. Preventing frail health. Clin. Geriatr. Med. 1992;8:1–17. [PubMed] [Google Scholar]
- Buchner D.M, Cress M.E, DeLateur B.J, Esselman P.C, Margherita A.J, Price R, Wagner E.H. The effect of strength and endurance on gait, balance, fall risk, and health services use in community-living older adults. J. Gerontol. A Biol. Sci. Med. Sci. 1997;52A:M218–M224. doi: 10.1093/gerona/52a.4.m218. [DOI] [PubMed] [Google Scholar]
- Bukowski J.F, Warner J.F, Dennert G, Welsh R.M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J. Exp. Med. 1985;161:40–52. doi: 10.1084/jem.161.1.40. 10.1084/jem.161.1.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakatay U, Telci A, Kayali R, Tekeli F, Akçay T, Sivas A. Relation of aging with oxidative protein damage prameters in the rat skeletal muscle. Clin. Biochem. 2003;36:51–55. doi: 10.1016/s0009-9120(02)00407-1. 10.1016/S0009-9120(02)00407-1 [DOI] [PubMed] [Google Scholar]
- Cantin A.M, Paquette B, Richter M, Larivée P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am. J. Respir. Crit. Care Med. 2000;162:1539–1546. doi: 10.1164/ajrccm.162.4.9910106. [DOI] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. 10.1006/dbio.2002.0780 [DOI] [PubMed] [Google Scholar]
- Chandler J.M, Hadley E.C. Exercise to improve physiologic and functional performance in old age. Clin. Geriatr. Med. 1996;12:761–784. [PubMed] [Google Scholar]
- De Cabo R, Cabello R, Rios M, López-Lluch G, Ingram D.K, Lane M.A, Navas P. Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp. Gerontol. 2004;39:297–304. doi: 10.1016/j.exger.2003.12.003. 10.1016/j.exger.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Demling R.H, DeSanti L. Effect of a hypocaloric diet, increased protein intake and resistance training on lean mass gains and fat mass loss in overweight police officers. Ann. Nutr. Metab. 2000;44:21–29. doi: 10.1159/000012817. 10.1159/000012817 [DOI] [PubMed] [Google Scholar]
- Denu J.M, Tanner K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. 10.1021/bi973035t [DOI] [PubMed] [Google Scholar]
- Dorman J.B, Albinder B, Shroyer T, Kenyon C. The age-1 and daf-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics. 1995;141:1399–1406. doi: 10.1093/genetics/141.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W, Holm E. Role of cysteine and glutathione in HIV infection and other diseases associated with muscle wasting and immunological dysfunction. FASEB J. 1997;11:1077–1089. doi: 10.1096/fasebj.11.13.9367343. [DOI] [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002a;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dröge W. Aging-related changes in the thiol/disulfide redox state: implications for the use of thiol antioxidants. Exp. Gerontol. 2002b;37:1333–1345. doi: 10.1016/s0531-5565(02)00175-4. 10.1016/S0531-5565(02)00175-4 [DOI] [PubMed] [Google Scholar]
- Dröge W. The plasma redox state and ageing. Ageing Res. Rev. 2002c;1:257–278. doi: 10.1016/s1568-1637(01)00008-3. [DOI] [PubMed] [Google Scholar]
- Dröge W. Autophagy and aging—importance of amino acid levels. Mech. Ageing Dev. 2003;125:161–168. doi: 10.1016/j.mad.2003.12.003. 10.1016/j.mad.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Eck H.-P, Gmünder H, Hartmann M, Petzoldt D, Daniel V, Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1 infected patients. Biol. Chem. Hoppe-Seyler. 1989;370:101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- Erden-Inal M, Sunal E, Kanbak G. Age-related chnages in the glutathione redox system. Cell Biochem. Funct. 2002;20:61–66. doi: 10.1002/cbf.937. 10.1002/cbf.937 [DOI] [PubMed] [Google Scholar]
- Fiatarone M.A, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N. Engl. J. Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. 10.1056/NEJM199406233302501 [DOI] [PubMed] [Google Scholar]
- Fillit H, Ding W.H, Buee L, et al. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci. Lett. 1991;129:318–320. doi: 10.1016/0304-3940(91)90490-k. 10.1016/0304-3940(91)90490-K [DOI] [PubMed] [Google Scholar]
- Fraga C.G, Shigenaga M.K, Park J.W, Degan P, Ames B.N. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl Acad. Sci. USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Meydani S.N, Blumberg J.B. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech. Ageing Dev. 1987;38:107–117. doi: 10.1016/0047-6374(87)90071-6. 10.1016/0047-6374(87)90071-6 [DOI] [PubMed] [Google Scholar]
- Furuke K, Shiraishi M, Mostowski H.S, Bloom E.T. Fas ligand induction in human NK cells is regulated by redox through a calcineurin-nuclear factors of activated T cell-dependent pathway. J. Immunol. 1999;162:1988–1993. [PubMed] [Google Scholar]
- Galter D, Mihm S, Dröge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur. J. Biochem. 1994;221:639–648. doi: 10.1111/j.1432-1033.1994.tb18776.x. 10.1111/j.1432-1033.1994.tb18776.x [DOI] [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. 10.1038/sj.onc.1207521 [DOI] [PubMed] [Google Scholar]
- Greten F.R, Eckmann L, Greten T.F, Park J.M, Li Z.-W, Egan L.J, Kagnoff M.F, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. 10.1016/j.cell.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. 10.1038/35041700 [DOI] [PubMed] [Google Scholar]
- Guralnik J.M, Simonsick E.M, Ferucci L, Glynn R.J, Berkman L.F, Blazer D.G, Scherr P.A, Wallace R.B. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guttridge D.C, Mayo M.W, Madrid L.V, Wang C.Y, Baldwin A.S., Jr NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. 10.1126/science.289.5488.2363 [DOI] [PubMed] [Google Scholar]
- Hack V, Breitkreutz R, Kinscherf R, Röhrer H, Bärtsch P, Taut F, Benner A, Dröge W. The redox state as a correlate of senescence and wasting and as a target for therapeutic intervention. Blood. 1998;92:59–67. [PubMed] [Google Scholar]
- Haj F.G, Verveer P.J, Squire A, Neel B.G, Bastiaens P.I. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. 10.1126/science.1067566 [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge M.C. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. 10.1016/0003-9861(90)90510-6 [DOI] [PubMed] [Google Scholar]
- Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. J. Immunol. 1996;156:42–47. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc. Natl Acad. Sci. USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer K, Rost B, Rütschle K, Opitz H, Specht N, Bärtsch P, Oster P, Schlierf G. Exercise training for rehabilitation and secondary prevention of falls in geriatric patients with a history of injurious falls. J. Am. Geriatr. Soc. 2001;49:10–20. doi: 10.1046/j.1532-5415.2001.49004.x. 10.1046/j.1532-5415.2001.49004.x [DOI] [PubMed] [Google Scholar]
- Hauer K, Hildebrandt W, Sehl Y, Edler L, Oster P, Dröge W. Improvement in muscular performance and decrease in tumor necrosis factor level in old age after antioxidant treatment. J. Mol. Med. 2003;81:118–125. doi: 10.1007/s00109-002-0406-7. [DOI] [PubMed] [Google Scholar]
- Hehner S.P, Breitkreutz R, Shubinsky G, Unsoeld H, Schulze-Osthoff K, Schmitz M.L, Dröge W. Enhancement of T cell receptor signaling by a mild oxidative shift in the intracellular thiol pool. J. Immunol. 2000;165:4319–4328. doi: 10.4049/jimmunol.165.8.4319. [DOI] [PubMed] [Google Scholar]
- Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. 10.1126/science.1082358 [DOI] [PubMed] [Google Scholar]
- Hernanz A, Fernández-Vivancos E, Montiel C, Vazquez J.J, Arnalich F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000;67:1317–1324. doi: 10.1016/s0024-3205(00)00722-0. 10.1016/S0024-3205(00)00722-0 [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A, De Rosa R.S, Dubs J.G, Roederer M, Anderson M.T, Ela S.W, Deresinski S.C, Herzenberg L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl Acad. Sci. USA. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. 10.1073/pnas.94.5.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt W, Alexander S, Bärtsch P, Dröge W. Effect of N-acetylcysteine on the hypoxic ventilatory response and erythropoietin productoin: linkage between plasma thiol redox state and O2-chemosensitivity. Blood. 2002a;99:1552–1555. doi: 10.1182/blood.v99.5.1552. 10.1182/blood.V99.5.1552 [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, Kinscherf R, Hauer K, Holm E, Dröge W. Plasma cysteine concentration and redox state in ageing and physical exercise. Mech. Ageing Dev. 2002b;123:1269–1281. doi: 10.1016/s0047-6374(02)00013-1. 10.1016/S0047-6374(02)00013-1 [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, et al. Effect of thiol antioxidant on body fat and insulin reactivity. J. Mol. Med. 2004;82:336–344. doi: 10.1007/s00109-004-0532-5. 10.1007/s00109-004-0532-5 [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hribal M.L, Nakae J, Kitamura T, Shutter J.R, Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J. Cell Biol. 2003;162:535–541. doi: 10.1083/jcb.200212107. 10.1083/jcb.200212107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inal M.E, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta. 2001;305:75–80. doi: 10.1016/s0009-8981(00)00422-8. 10.1016/S0009-8981(00)00422-8 [DOI] [PubMed] [Google Scholar]
- Jacob N, Bruckert E, Giral P, Foglietti M.J, Turpin G. Cysteine is a cardiovascular risk factor in hyperlipidemic patients. Atherosclerosis. 1999;146:53–59. doi: 10.1016/s0021-9150(99)00128-8. 10.1016/S0021-9150(99)00128-8 [DOI] [PubMed] [Google Scholar]
- Jones D.P, Carlson J.L, Mody V.C, Jr, Cai J, Lynn M.J, Sternberg P., Jr Redox state of glutathione in human plasma. Free Radic. Biol. Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. 10.1016/S0891-5849(99)00275-0 [DOI] [PubMed] [Google Scholar]
- Kasapoglu M, Ozben T. Alterations of antioxidant enzymes and oxidative stress markers in aging. Exp. Gerontol. 2001;36:209–220. doi: 10.1016/s0531-5565(00)00198-4. 10.1016/S0531-5565(00)00198-4 [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtlang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. 10.1038/366461a0 [DOI] [PubMed] [Google Scholar]
- Kim S, Iizuka K, Aguila H.L, Weissman I.L, Yokoyama W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. PNAS. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. 10.1073/pnas.050588297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K.D, Tissenbaum H.A, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapuase in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. 10.1126/science.277.5328.942 [DOI] [PubMed] [Google Scholar]
- Kinscherf R, Fischbach T, Mihm S, Roth S, Hohenhaus-Sievert E, Weiss C, Edler L, Bärtsch P, Dröge W. Effect of glutathione depletion and oral N-acetyl-cysteine treatment on CD4+ and CD8+ cells. FASEB J. 1994;8:448–451. [PubMed] [Google Scholar]
- Kinscherf R, Hack V, Fischbach T, Friedmann B, Weiss C, Edler L, Bärtsch P, Dröge W. Low plasma glutamine in combination with high glutamate levels indicate risk for loss of body cell mass in healthy individuals: the effect of N-acetyl-cysteine. J. Mol. Med. 1996;74:393–400. doi: 10.1007/BF00210633. 10.1007/s001090050040 [DOI] [PubMed] [Google Scholar]
- Kirkwood T.B.L. Human senescence. BioEssays. 1996;18:1009–1016. doi: 10.1002/bies.950181211. 10.1002/bies.950181211 [DOI] [PubMed] [Google Scholar]
- Klionsky D.J, Emr S.D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Slazar-Onfray F, Petersson M, Hansson J, Masucci G, Wasserman K, Nakazawa T, Anderson P, Kiessling R. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell and natural killer cell-mediated cytotoxicity. Eur. J. Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- Kops G.J.P.L, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. 10.1038/nature01036 [DOI] [PubMed] [Google Scholar]
- Kuge S, Jones N. YAP-1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994;13:655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran S, Deepak B, Naveen B, Panneerselvam C. Effects of levocarnitine on mitochondrial antioxidant systems and oxidative stress in aged rats. Drugs R&D. 2003;4:141–147. doi: 10.2165/00126839-200304030-00001. [DOI] [PubMed] [Google Scholar]
- Kuwata K, Era S, Sogami M. The kinetic studies on the intracellular SH, S–S exchange reaction of bovine mercaptalbumin. Biochim. Biophys. Acta. 1994;1205:317–324. doi: 10.1016/0167-4838(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Lands L.C, Grey V.L, Smountas A.A. Effect of supplementation with a cysteine donor on muscular performance. J. Appl. Physiol. 1999;87:1381–1385. doi: 10.1152/jappl.1999.87.4.1381. [DOI] [PubMed] [Google Scholar]
- Larsen P.L, Albert P.S, Riddle D.I. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.K, Klopp R.G, Weindruch R, Prolla T.A. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. 10.1126/science.285.5432.1390 [DOI] [PubMed] [Google Scholar]
- Lee S.-R, Yang K.-S, Kwon J, Lee C, Jeong W, Rhee S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002;277:20 336–20 342. doi: 10.1074/jbc.M111899200. 10.1074/jbc.M111899200 [DOI] [PubMed] [Google Scholar]
- Lenton K.J, Therriault H, Cantin A.M, Fulop T, Payette H, Wagner J.R. Direct correlation of glutathione and ascorbate and their dependence on age and season in human lymphocytes. Am. J. Clin. Nutr. 2000;71:1194–1200. doi: 10.1093/ajcn/71.5.1194. [DOI] [PubMed] [Google Scholar]
- Levine R.L, Stadtman E.R. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. 10.1016/S0531-5565(01)00135-8 [DOI] [PubMed] [Google Scholar]
- Lin Y.-J, Seroude L, Benzer S. Extended life-span and stress resistance in the drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. 10.1126/science.282.5390.943 [DOI] [PubMed] [Google Scholar]
- Longo V.D, Finch C.E. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. 10.1126/science.1077991 [DOI] [PubMed] [Google Scholar]
- Low P.P.L, Rutherfurd K.J, Gill H.S, Cross M.L. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int. Immunopharmacol. 2003;3:393–401. doi: 10.1016/S1567-5769(02)00297-7. 10.1016/S1567-5769(02)00297-7 [DOI] [PubMed] [Google Scholar]
- Lucas D.T, Szweda L.I. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc. Natl Acad. Sci. USA. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. 10.1073/pnas.95.2.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G, Madeddu C, Macciò A, Gramignano G, Lusso M.R, Massa E, Astara G, Serpe R. Cancer-related anorexia/cachexia syndrome and oxidative stress: an innovative approach beyond current treatment. Cancer Epidemiol. Biomarkers Prev. 2004;13:1651–1659. [PubMed] [Google Scholar]
- Matthews D.R, Hosker J.P, Rudenski A.S, Naylor B.A, Treacher D.F, Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- McKinnell I.W, Rudnicki M.A. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–910. doi: 10.1016/j.cell.2004.12.007. 10.1016/j.cell.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Meister A, Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. 10.1146/annurev.bi.52.070183.003431 [DOI] [PubMed] [Google Scholar]
- Melendez A, Talloczy Z, Seaman M, Eskelinen E.L, Hall D.H, Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. 10.1126/science.1087782 [DOI] [PubMed] [Google Scholar]
- Middleton N, Jelen P, Bell G. Whole blood and mononuclear cell glutathione response to dietary whey protein supplementation in sedentary and trained male human subjects. Int. J. Food Sci. 2004;55:131–141. doi: 10.1080/096374080410001666504. 10.1080/096374080410001666504 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi P.P, Lanfrancone L, Pelicci P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. 10.1038/46311 [DOI] [PubMed] [Google Scholar]
- Mihm S, Ennen J, Pessara U, Kurth R, Dröge W. Inhibition of HIV-1 replication and NFkB activity by cysteine and cysteine derivatives. AIDS. 1991;5:497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Morris J.Z, Tissenbaum H.A, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. 10.1038/382536a0 [DOI] [PubMed] [Google Scholar]
- Murphy C.T, McCarroll S.A, Bargmann C.I, Fraser A, Kamath R.S, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2004;424:277–284. doi: 10.1038/nature01789. 10.1038/nature01789 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T. Ageing and the mystery at Arles. Nature. 2004;429:149–152. doi: 10.1038/429149a. 10.1038/429149a [DOI] [PubMed] [Google Scholar]
- Nuttall S.L, Martin U, Sinclair A.J, Kendall M.J. Glutathione: in sickness and in health. The Lancet. 1998;351:645–646. doi: 10.1016/s0140-6736(05)78428-2. 10.1016/S0140-6736(05)78428-2 [DOI] [PubMed] [Google Scholar]
- Oldham S, Hafen E. Insulin/IGF and target of rapamycin signlaing: a TOR de force in growth control. Trends Cell Biol. 2003;13:79–85. doi: 10.1016/s0962-8924(02)00042-9. 10.1016/S0962-8924(02)00042-9 [DOI] [PubMed] [Google Scholar]
- Orr W.C, Sohal R.S. Extension of lifespan by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Oubidar M, Boquillon M, Marie C, Bouvier C, Beley A, Bralet J. Effect of intracellular iron loading on lipid peroxidation of brain slices. Free Radic. Biol. Med. 1996;21:763–769. doi: 10.1016/0891-5849(96)00173-6. 10.1016/0891-5849(96)00173-6 [DOI] [PubMed] [Google Scholar]
- Paasche G, Huster D, Reichenbach A. The glutathione content of retinal Muller (glial) cells: the effects of aging and of application of free-radical scavengers. Ophthalmic Res. 1998;30:351–360. doi: 10.1159/000055495. 10.1159/000055495 [DOI] [PubMed] [Google Scholar]
- Pallardó F.V, Asensi M, Garcia de la Asuncion J, Anton V, Lloret A, Sastre J, Vina J. Late onset administration of oral antioxidants prevents age-related loss of motor co-ordination and brain mitochondrial DNA damage. Free Radic. Res. 1999;29:617–623. doi: 10.1080/10715769800300671. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas J.H, Ruvkun G.A. PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N.T, Goodrum K.J. A comparison of casein, lactalbumin, and soy protein effect on the immune response to a T-dependent antigen. Nutr. Res. 1990;10:781–792. [Google Scholar]
- Parkes T.L, Elia A.J, Dickinson D, Hilliker A.J, Boulianne G.L, John P. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998;19:171–174. doi: 10.1038/534. 10.1038/534 [DOI] [PubMed] [Google Scholar]
- Paterson R.L, Galley H.F, Webster N.R. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit. Care Med. 2003;31:2574–2578. doi: 10.1097/01.CCM.0000089945.69588.18. 10.1097/01.CCM.0000089945.69588.18 [DOI] [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv. Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. 10.1038/nature02924 [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B, Quan P.C, Rosner M, Moller J.R, Bloom B.R. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 1987;138:884–888. [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leville S.G, Visser M, Foley D, Masdaki K, Guarlnik J.M. Muscle strength and body mass index as long term predictors for mortality in initially healthy men. J. Gerontol. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- Roth S, Dröge W. Regulation of T cell activation and T cell growth factor (TCGF) production by hydrogen peroxide. Cell. Immunol. 1987;108:417–424. doi: 10.1016/0008-8749(87)90224-3. 10.1016/0008-8749(87)90224-3 [DOI] [PubMed] [Google Scholar]
- Rothschild M.A, Oratz M, Schreiber S.S. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- Rukavina D, Laskarin G, Rubesa G, Strbo N, Bedenicki I, Manestar D, Glavas M, Christmas S.E, Podack E.R. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92:2410–2420. [PubMed] [Google Scholar]
- Sacheck J.M, Ohtsuka A, McLary C, Goldberg A.L. IGF-1 stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. 10.1152/ajpendo.00073.2004 [DOI] [PubMed] [Google Scholar]
- Salmeen A, Andersen J.N, Myers M.P, Meng T.-C, Hinks J.A, Tonks N.K, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. 10.1038/nature01680 [DOI] [PubMed] [Google Scholar]
- Samiec P.S, Drews-Botsch C, Flagg E.W, Kurtz J.C, Sternberg P, Jr, Reed R.L, Jones D.P. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. 10.1016/S0891-5849(97)00286-4 [DOI] [PubMed] [Google Scholar]
- Sandri M, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. 10.1016/S0092-8674(04)00400-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N. Engl. J. Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. 10.1056/NEJM199302253280803 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Senda M, Kim S, Kojima S, Kubodera A. Age-related changes of glutathione content, glucose transport and metabolism, and mitochondrial electron transfer function in mouse brain. Nucl. Med. Biol. 2001;28:25–31. doi: 10.1016/s0969-8051(00)00180-3. 10.1016/S0969-8051(00)00180-3 [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999;274:11 455–11 458. doi: 10.1074/jbc.274.17.11455. 10.1074/jbc.274.17.11455 [DOI] [PubMed] [Google Scholar]
- Scalzo A.A. Successful control of viruses by NK cells—a balance of opposing forces? Trends Microbiol. 2002;10:470–474. doi: 10.1016/s0966-842x(02)02441-1. 10.1016/S0966-842X(02)02441-1 [DOI] [PubMed] [Google Scholar]
- Schmid E, El Benna J, Galter D, Klein G, Dröge W. Redox priming of the insulin receptor b-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J. 1998;12:863–870. doi: 10.1096/fasebj.12.10.863. [DOI] [PubMed] [Google Scholar]
- Schmitt T.L, Hotz-Wagenblatt A, Klein H, Dröge W. Interdependent regulation of insulin receptor kinase activity by ADP and hydrogen peroxide. J. Biol. Chem. 2005;280:3795–3801. doi: 10.1074/jbc.M410352200. 10.1074/jbc.M410352200 [DOI] [PubMed] [Google Scholar]
- Schreck R, Baeuerle P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of NF-κB transcription factor and HIV-1. Trends Cell Biol. 1991;1:39–42. doi: 10.1002/j.1460-2075.1991.tb07761.x. 10.1016/0962-8924(91)90072-H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrofano M.M, et al. The effects of aging and calorie restriction on plasma nutrient levels in male and female emory mice. Mech. Ageing Dev. 1998;105:31–44. doi: 10.1016/s0047-6374(98)00077-3. 10.1016/S0047-6374(98)00077-3 [DOI] [PubMed] [Google Scholar]
- Shao Y, Gao Z, Marks P.A, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. PNAS. 2004;101:18 030–18 035. doi: 10.1073/pnas.0408345102. 10.1073/pnas.0408345102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Haga H, Ueno M, Nagai H, Yasumura S, Koyano W. Longitudinal changes of serum albumin in elderly people living in the community. Age Aging. 1991;20:417–420. doi: 10.1093/ageing/20.6.417. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. 10.1016/S0891-5849(99)00177-X [DOI] [PubMed] [Google Scholar]
- Solomon A, Goldberg A. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J. Biol. Chem. 1996;271:26 690–26 697. doi: 10.1074/jbc.271.43.26690. 10.1074/jbc.271.41.25240 [DOI] [PubMed] [Google Scholar]
- Staal F.J.T, Roederer M, Herzenberg L.A, Herzenberg L.A. Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proc. Natl Acad. Sci. USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt T.N, Drujan D, Clarke B.A, Panaro F, Timofeyva Y, Kline W.O, Gonzalez M, Yancopoulos G.D, Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. 10.1016/S1097-2765(04)00211-4 [DOI] [PubMed] [Google Scholar]
- Tahir S.M.A, Cheng O, Shaulov A, Koezuka Y, Bubley G.J, Wilson S.B, Balk S.P, Exley M.A. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J. Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- Tallóczy Z, Jiang W, Virgin H.W, IV, Leib D.A, Scheuner D, Kaufman R.J, Eskelinen E-.L, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc. Natl Acad. Sci. USA. 2002;99:190–195. doi: 10.1073/pnas.012485299. 10.1073/pnas.012485299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub J, Lau J.F, Ma C, Hahn J.H, Hoque R, Rothblatt J, Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans darf-C and clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. 10.1038/20208 [DOI] [PubMed] [Google Scholar]
- Tinetti M.E, Baker D.J, McAvay G, Claus E.B, Garrett P, Gottschalk M, Koch M.L, Trainor K, Horwitz R.I. A multifactorial intervention to reduce the risk of falling among elderly people in the community. N. Engl. J. Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. 10.1056/NEJM199409293311301 [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith E.C, Greenberg M.E. The many forks in FOXO's road. Sci. STKE. 2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Traverso N, et al. Anti malondialdehyde-adduct immunological response as a possible marker of successful aging. Exp. Gerontol. 2003;38:1129–1135. doi: 10.1016/s0531-5565(03)00188-8. 10.1016/S0531-5565(03)00188-8 [DOI] [PubMed] [Google Scholar]
- Ullum H, Gøtzsche P.C, Victor J, Dickmeiss E, Skinhøj P, Pedersen K. Defective natural immunity: an early manifestation of human immunodeficiency virus infection. J. Exp. Med. 1995;182:789–799. doi: 10.1084/jem.182.3.789. 10.1084/jem.182.3.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Lüscher T.F. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem. Biophys. Res. Commun. 2003;303:483–487. doi: 10.1016/s0006-291x(03)00360-7. 10.1016/S0006-291X(03)00360-7 [DOI] [PubMed] [Google Scholar]
- Vankayalapati R, Klucar P, Wizel B, Weis S.E, Samten B, Safi H, Shams H, Barnes P.F. NK cells regulate CD8+T cell effector function in response to an intracellular pathogen. J. Immunol. 2004;172:130–137. doi: 10.4049/jimmunol.172.1.130. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T, Karatza M.-H, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C. Antioxidants attenuate the plasma cytokine response to exercise in humans. J. Appl. Physiol. 2003;94:1025–1032. doi: 10.1152/japplphysiol.00735.2002. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs A.L, Orosz L, Müller F. Influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. 10.1038/426620a [DOI] [PubMed] [Google Scholar]
- Verhasselt V, Goldman M, Willems F. Oxidative stress up-regulates IL-8 and TNF-α synthesis by human dendritic cells. Eur. J. Immunol. 1998;28:3886–3890. doi: 10.1002/(SICI)1521-4141(199811)28:11<3886::AID-IMMU3886>3.0.CO;2-M. 10.1002/(SICI)1521-4141(199811)28:11%3C3886::AID-IMMU3886%3E3.3.CO;2-D [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe D.R, Goodpaster B.H, Simonsick E.M, Newman A.B, Nevitt M, Harris T.B. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- Warfield K.L, Perkins J.G, Swenson D.L, Deal E.M, Bosio C.M, Aman M.J, Yokoyama W.M, Young H.A, Bavari S. Role of natural killer cells in innate protection against lethal ebola virus infection. J. Exp. Med. 2004;200:169–179. doi: 10.1084/jem.20032141. 10.1084/jem.20032141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, et al. Nutrional therapy of chronic hepatitis by whey protein (non-heated) J. Med. 2000;31:283–302. [PubMed] [Google Scholar]
- Wessells R.J, Fitzgerald E, Cypser J.R, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat. Genet. 2004;36:1275–1281. doi: 10.1038/ng1476. 10.1038/ng1476 [DOI] [PubMed] [Google Scholar]
- Wong C.W, Watson D.L. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 1995;62:359–368. doi: 10.1017/s0022029900031058. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Abe J, Haendeler J, Huang Q, Berk B.C. Src and Cas mediate JNK activation but not ERK1/2 and p38 kinases by reactive oxygen species. J. Biol. Chem. 2000;275:11 706–11 712. doi: 10.1074/jbc.275.16.11706. 10.1074/jbc.275.16.11706 [DOI] [PubMed] [Google Scholar]