Abstract
Non-selective inhibitors of cyclic nucleotide phosphodiesterase (PDE) block allergen-induced contraction of passively sensitized human airways in vitro by a dual mechanism involving a direct relaxant effect on smooth muscle and inhibition of histamine and cysteinyl leukotriene (LT) release from airways. We investigated the effects of non-selective PDE inhibitors and selective inhibitors of PDE3 and PDE4 in order to determine the involvement of PDE isoenzymes in the suppression of allergic bronchoconstriction.
Macroscopically normal airways from 76 patients were sensitized with IgE-rich sera (>250 u ml−1) containing specific antibodies against allergen (Dermatophagoides farinae). Contractile responses of bronchial rings were assessed using standard organ bath techniques.
Passive sensitization caused increased contractile responses to allergen, histamine and LTC4. Non-selective PDE inhibitors (theophylline, 3-isobutyl-1-methylxanthine [IBMX]), a PDE3-selective inhibitor (motapizone), PDE4-selective inhibitors (RP73401, rolipram, AWD 12-281) and a mixed PDE3/4 inhibitor (zardaverine) all significantly relaxed inherent bronchial tone at resting tension and to a similar degree. Theophylline, IBMX, zardaverine and the combination of motapizone and RP73401 inhibited the contractile responses to allergen and LTC4. Pre-treatment with motapizone, RP73401, rolipram or the methylxanthine adenosine receptor antagonist, 8-phenyltheophylline, did not significantly decrease responses to either allergen or LTC4.
We conclude that combined inhibition of PDE3 and PDE4, but not selective inhibition of either isoenzyme or antagonism of adenosine receptors, is effective in suppressing allergen-induced contractions of passively sensitized human airways. The relationship between allergen- and LTC4-induced responses suggests that PDE inhibitors with PDE3 and PDE4 selectivity are likely to act in part through inhibition of mediator release and not simply through direct relaxant actions on airway smooth muscle.
Keywords: Phosphodiesterase isoenzymes; phosphodiesterase inhibitors; bronchus, human; bronchoconstriction; passive sensitization; allergen; leukotriene C4
Introduction
Extrinsic bronchial asthma is characterized by increased airway responsiveness to non-specific and specific stimuli, such as histamine, leukotrienes and allergen. Non-selective inhibitors of cyclic nucleotide phosphodiesterase (PDE), such as the methylxanthine, theophylline, have been used in the treatment of bronchial asthma for several decades (Weinberger & Hendeles, 1996) and are included in current guidelines (National Institutes of Health, 1995; 1997). Besides inducing mild bronchodilation (Pauwels et al., 1985; Ward et al., 1993), PDE inhibitors have been shown to reduce airway inflammation (Banner & Page, 1996; Karlsson, 1996) and to be effective against early and late phase allergic asthmatic responses. The mechanisms by which methylxanthines exert these effects appear to involve adenosine receptor antagonism (Karlsson, 1987; Crimi et al., 1989; Coward et al., 1998) and–through elevation of intracellular adenosine 3′:5′-cyclic monophosphate (cyclic AMP) concentrations (Bergstrand, 1980; Hill et al., 1999)–a direct relaxant effect on smooth muscle, as well as inhibition of mediator release from inflammatory cells (Louis et al., 1992).
It is well known, however, that treatment with theophylline can cause considerable side-effects, such as cardiac dysrhythmias and nausea, which are believed to result mainly from non-selective PDE inhibition and to a lesser extent from adenosine receptor antagonism. Since the immunopharmacology of theophylline has been broadly investigated in recent years, the development of novel selective PDE inhibitors with significant anti-inflammatory and bronchospasmolytic properties but an improved side-effect profile has attracted particular interest.
To date, 10 PDE isoenzyme gene families have been identified (Beavo, 1995; Conti et al., 1995; Fisher et al., 1998; Giembycz et al., 1996; Loughney & Ferguson, 1996; Soderling et al., 1998a,1998b; 1999; Torphy, 1998), which differ not only in their physicochemical and biochemical properties but also in their localization in specific organ systems or tissues. Among them, PDE1 through PDE5 are found in human airways. Functional studies with selective PDE inhibitors have suggested a major role for PDE3 and PDE4 isoenzymes in the regulation of airway tone (Rabe et al., 1993). Moreover, PDE4 appears to be the predominant isoenzyme in various inflammatory cells, such as monocytes and monocyte-derived macrophages (Gantner et al., 1997), B lymphocytes (Cooper et al., 1985) and eosinophils (Dent et al., 1994).
Under in vivo as well as in vitro conditions, allergen-induced bronchoconstriction of human airways is believed to result from the formation and release of inflammatory mediators, mainly cysteinyl leukotrienes (Björck et al., 1991; Björck & Dahlén, 1993; Roquet et al., 1997). Owing to the fact that PDE3 and PDE4 are involved in the regulation of airway tone as well as mediator release from inflammatory cells, the aim of our study was to evaluate their specific role in airway responses to allergen. Therefore, we investigated the effects of PDE inhibitors selective for PDE3, PDE4 or PDE3/4 on allergen- and leukotriene C4 (LTC4)-induced contractions in passively sensitized human airways in vitro. For comparison, an adenosine receptor antagonist and non-selective PDE inhibitors were included in the study.
Methods
Tissue preparations
Macroscopically normal airways were obtained from 76 patients undergoing surgery for lung cancer. None were chronically treated with theophylline, β-adrenoceptor agonists, corticosteroids or anticholinergic drugs. Preoperative lung function parameters were generally normal. Serum IgE levels on the day of surgery were determined for all patients. Immediately after resection, peripheral airways (1–4 mm internal diameter) were dissected free of alveolar tissue and cut into rings (2–4 mm length).
Passive sensitization
The sensitizing serum was prepared from whole blood of individuals who demonstrated high total IgE (>250 u ml−1) and specific IgE antibodies (fluorescent immunosorbent test [FAST] class ⩾3) against allergen (Dermatophagoides farinae). Sera were not pooled but were frozen individually in 200–250-μl aliquots until required. Tissues were rotated overnight at room temperature in tubes containing modified Krebs buffer (composition in mM: NaCl 118.4, KCl 4.7, MgSO4 0.6, CaCl2 1.3, KH2PO4 1.2, NaHCO3 25.0, glucose 11.1), in the absence or presence of sensitizing serum (10% vol vol−1). The next morning rings were transferred to 10-ml organ baths containing oxygenated (95% O2, 5% CO2) modified Krebs buffer (pH 7.4, 37°C) and bronchial responses were recorded using isometric force displacement transducers (model FT-03; Stag Instruments Limited, Oxon, U.K.) coupled to a Multitrace 8 chart recorder (Lectromed, Welwyn Garden City, Herts, U.K.).
Tension measurements
Tissues were equilibrated for at least 60 min at a resting tension of about 400 mg before a single concentration (1 μM) of the β-adrenoceptor agonist isoprenaline was applied to determine the amount of inherent tone. After full recovery of the tissues, histamine concentration-effect curves (10 nM–300 μM) were performed. Contractile responses as measured in mg weight were recorded.
After washing and re-equilibration to stable tension, sensitized tissues were pre-treated for 30 min with one of the drugs listed in Table 1 and changes in bronchial tone were measured. Thereafter, drug effects on allergen concentration-effect curves (0.03–30 u ml−1) were assessed and compared to responses in untreated, passively sensitized control tissues from the same patient. Similarly, effects of the highest concentration of each drug on LTC4 concentration-effect curves (3 pM–0.3 μM) were evaluated. All concentration-effect curves were constructed in a cumulative manner, using incremental concentrations spaced at half log10 intervals. To investigate the effect of selective PDE inhibition on pre-contracted bronchi, in some experiments motapizone (1 μM) and RP73401 (300 nM) were added after completion of the allergen concentration-effect curves. At the end of the experiments, tissues were exposed to a single concentration of carbachol (0.1 mM) to ensure that the absence of contractile responses was not the result of deterioration of a bronchial ring. Finally, tissue wet weights were determined at the end of the experiments.
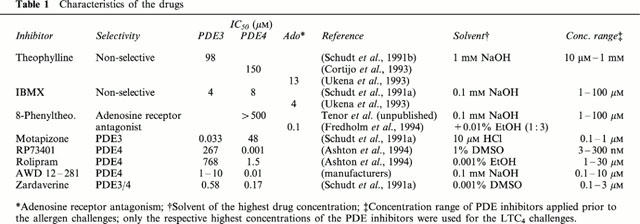
Table 1.
Characteristics of the drugs
Measurements and analysis of results
All responses were recorded as absolute changes in isometric tension. The traces were evaluated manually. The potency of histamine and LTC4 was calculated from concentration-effect curves by non-linear curve-fitting using the Prism® program (GraphPad™ Software, San Diego, CA, U.S.A.) for each individual tissue and expressed as pD2. Histamine, leukotriene and allergen concentration-effect curves were compared for the different conditions (passively sensitized versus non-sensitized, drug pre-treatment versus vehicle controls) using repeated-measures analysis of variance (ANOVA), with the different conditions as between-group factor and histamine, leukotriene and allergen concentrations as within-group factor. In testing for statistically significant differences between curves, the between-group effect (level) and its interaction with the within-group effect (slope) were taken into account. To compare the effects of an individual drug on leukotriene- versus allergen-induced contractions, responses after drug treatment were expressed as per cent inhibition with respect to the respective vehicle control and compared at those allergen and leukotriene concentrations that induced approximately 75% of the maximal response to histamine. These data were compared using the unpaired, two-tailed t-test. For the purpose of data comparison between passively sensitized and non-sensitized tissues (resting tension, inherent tone, wet weights, maximal contraction and pD2) the paired t-test was used. All values quoted are mean±s.e.mean. The level of statistical significance was defined as P⩽0.05.
Materials
Isoprenaline, histamine, carbachol, theophylline, 3-isobutyl-1-methylxanthine (IBMX) and 8-phenyltheophylline were obtained from Sigma Chemical Company (Deisenhofen, Germany). Motapizone, RP73401, rolipram and zardaverine were kindly provided by Byk Gulden (Konstanz, Germany); AWD 12-281 was obtained from ASTA Medica (Dresden, Germany). The allergen (D. farinae) was obtained from Allergopharma KG (Reinbek, Germany); LTC4 was purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.).
Isoprenaline, histamine and carbachol were dissolved in distilled water. LTC4 was dissolved in Hanks balanced salts solution containing 1% (wt vol−1) bovine serum albumin. Allergen was diluted in normal saline. For the respective solvents of the drugs see Table 1.
Results
Baseline characteristics of the bronchial rings
The mean (±s.e.mean) wet weight of the passively sensitized bronchial rings was 11.2±0.56 mg, mean resting tension was 425±11 mg weight and mean inherent tone (i.e. the magnitude of relaxation after a single dose of isoprenaline) was 241±14 mg weight.
Effect of passive sensitization on responses to histamine, LTC4 and allergen
Histamine and LTC4 caused concentration-dependent contractions in both sensitized and non-sensitized bronchial ring preparations. In accordance with previously published data (Schmidt et al., 1999), passive sensitization led to a significant increase in responsiveness to histamine and LTC4 as compared to non-sensitized paired control tissues from the same patient (ANOVA P<0.001 for both stimuli; Table 2). D. farinae caused concentration-dependent contractions in sensitized tissues but not in non-sensitized control tissues, as indicated by the difference in maximal contraction (Figure 1, Table 2). The respective solvents of the drugs (Table 1) did not significantly alter responses to LTC4 or allergen in sensitized bronchial rings.
Table 2.
Mean values (±s.e.mean) of parameters characterizing the concentration-effect curves
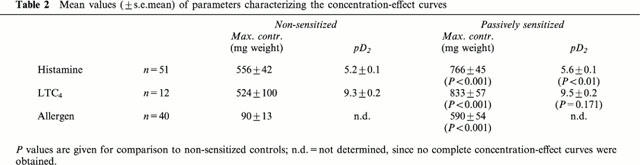
Figure 1.
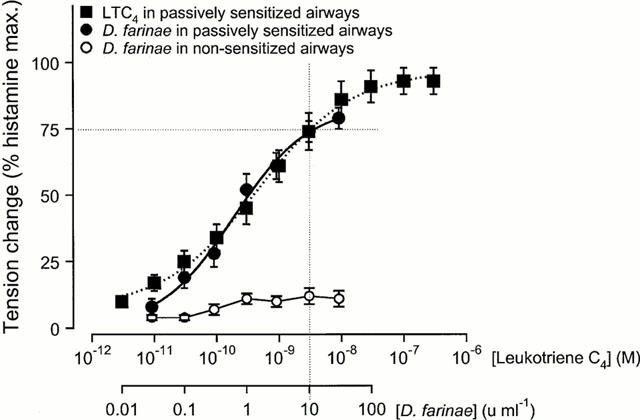
Concentration-effect curves to leukotriene C4 in passively sensitized and to allergen (Dermatophagoides farinae) in sensitized and non-sensitized human airways. Contractile responses to both spasmogens are expressed as per cent of the maximal responses to histamine (per cent hist. max.) in the same airway preparations. Since concentrations of 3 nM LTC4 and 10 u ml−1 D. farinae caused contractile responses of similar magnitude (approx. 75% of the maximal contraction to histamine), the effects of PDE inhibitors on allergen- and LTC4-induced contractile responses were evaluated and compared at these concentrations of spasmogens.
Relationship among histamine, LTC4 and allergen contraction-effect curves
Maximal contractions of non-sensitized and sensitized bronchial rings in response to LTC4 were of the same magnitude as maximal contractions to histamine (Figure 1, Table 2). However, on average, LTC4 was 9500 fold more potent than histamine in non-sensitized and 7500 fold more potent in passively sensitized tissues (Table 2).
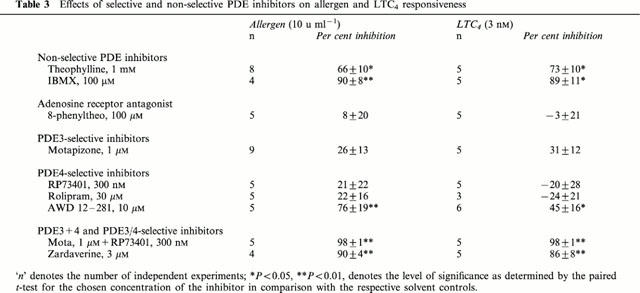
Maximal contractions of sensitized bronchial rings to allergen were on average 80% of the maximal responses to histamine (Figure 1, Table 2). Since concentrations of 3 nM LTC4 and 10 u ml−1 D. farinae caused contractile responses of similar magnitude (approximately 75% of the maximal contraction to histamine; Figure 1), the effects of PDE inhibitors on allergen- and LTC4-induced contractile responses were evaluated and compared at these concentrations of spasmogens (Table 3).
Table 3.
Effects of selective and non-selective PDE inhibitors on allergen and LTC4 responsiveness
Effect of the PDE inhibitors on inherent tone
PDE inhibitors decreased resting tension in concentration dependent manner within the indicated concentration range (Table 1). The highest concentrations of the non-selective PDE inhibitors theophylline and IBMX, as well as the selective PDE3 inhibitor motapizone, the PDE4 selective inhibitors RP73401, rolipram and AWD 12-281, the combination of motapizone and RP73401 and the PDE3/4 inhibitor zardaverine, significantly relaxed bronchial rings compared to the respective solvent controls (P<0.05, for each; Figure 2). In contrast, the adenosine receptor antagonist, 8-phenyltheophylline, had no significant effect as compared to the solvent control. However, when relaxation was compared among all of these drugs by ANOVA, there was no significant difference in their relaxant effects.
Figure 2.
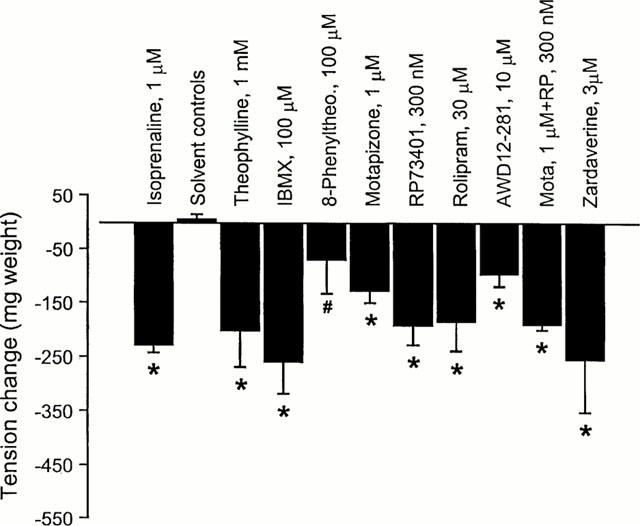
Effect of the selective and non-selective PDE inhibitors on the inherent tone in passively sensitized human airways. Non-selective PDE inhibition (theophylline, IBMX) as well as the selective PDE3 (motapizone), PDE4 (RP73401, rolipram, AWD 12-281), and PDE3/4 inhibition (motapizone+RP73401, zardaverine) significantly relaxed bronchial rings compared to the respective solvent controls, while adenosine receptor antagonism (8-phenyltheophylline) had no significant effect. Responses of the PDE inhibitors were not significantly different from the effect of isoprenaline, whereas 8-phenyltheophylline was significantly less effective. Overall there was no significant difference between the relaxing effects of the different drugs including 8-PT (ANOVA). *P<0.05 in comparison with the solvent control, #P<0.05 in comparison with isoprenaline.
Effect of the PDE inhibitors on allergen-induced contractions
The non-selective PDE inhibitors theophylline and IBMX inhibited the contractile responses to allergen in concentration dependent manner (Figure 3a,b). The concentration-effect curves were shifted to the right with a concomitant reduction of the maximal allergen responses.
Figure 3.
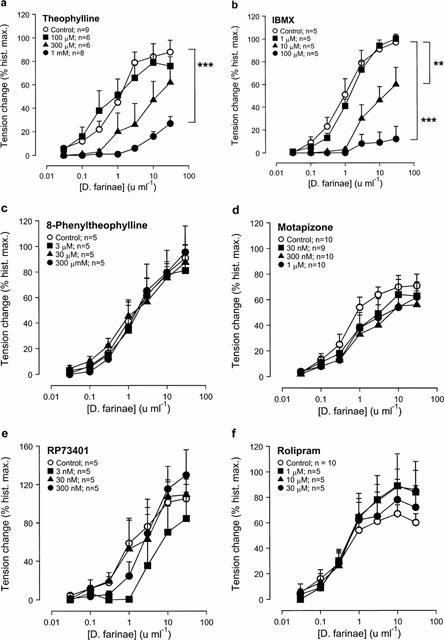
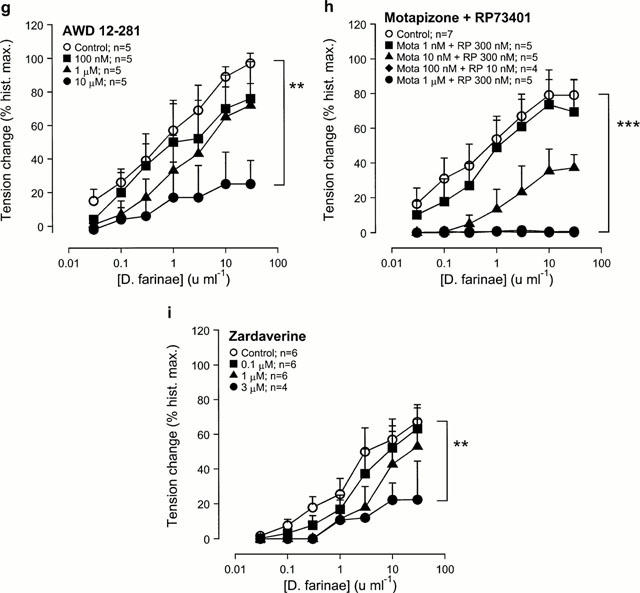
Effect of the selective and non-selective PDE inhibitors on allergen-induced contraction in passively sensitized human airways. Compared to sensitized controls, allergen-induced contractions were reduced in concentration dependent manner by pre-treatment with the non-selective PDE inhibitors theophylline (a) and IBMX (b), the PDE4-selective inhibitor AWD 12-281 (g), the combination of a PDE3- (motapizone) and PDE4-selective (RP73401) inhibitor (Mota+RP) (h), and the PDE3/4-selective inhibitor zardaverine (i). Pretreatment with the adenosine receptor antagonist 8-phenyltheophylline (c), the PDE3-selective inhibitor motapizone (d), the PDE4-selective inhibitors RP73401 (e) and rolipram (f) did not affect allergen-induced contractions. Tension changes are expressed as percent of the maximal responses to histamine in the same tissues. **P<0.01 and ***P<0.001, denote the comparison of whole concentration-effect curves by ANOVA.
In contrast, even the highest concentrations of the methylxanthine adenosine receptor antagonist 8-phenyltheophylline (Figure 3c), the PDE3-selective inhibitor motapizone (Figure 3d) and the PDE4-selective inhibitors RP73401 (Figure 3e) and rolipram (Figure 3f) had no significant inhibitory effect on the contractile responses to allergen. However, the novel PDE4-selective inhibitor AWD 12-281 inhibited allergen-induced bronchoconstriction in a concentration dependent manner (Figure 3g); the highest concentration significantly reduced allergen responses.
Allergen-induced bronchoconstriction was inhibited in a concentration dependent manner by combining motapizone and RP73401, selective PDE3 and PDE4 inhibitors, respectively (Figure 3h), and by 3 μM of the combined PDE3/4 selective inhibitor zardaverine (Figure 3i). The concentration-effect curves were shifted to the right with a reduction of the maximal responses, while the highest concentrations of motapizone+RP73401 completely abolished allergen-induced bronchoconstriction (Figure 3h).
Effect of the PDE inhibitors on LTC4-induced contractions
Theophylline (Figure 4a), IBMX (Figure 4b), motapizone+RP73401 (Figure 4h) and zardaverine (Figure 4i) shifted the concentration-effect curves to the right with a reduction of the maximal response. The selective PDE4 inhibitor AWD 12-281 (Figure 4g) caused a rightward shift of the concentration-effect curve without reducing the maximal contractile response to LTC4. Pre-treatment with 8-phenyltheophylline (Figure 4c), motapizone (Figure 4d), RP73401 (Figure 4e) or rolipram (Figure 4f) did not affect LTC4-induced contractions. Table 3 summarizes and compares the described effects of the different agents on allergen- and LTC4-induced bronchoconstriction at submaximal concentrations of the spasmogens (10 u ml−1 allergen, 3 nM LTC4) that caused similar contractions in control tissues, i.e. approx. 75% of the maximal response to histamine (see Figure 1).
Figure 4.
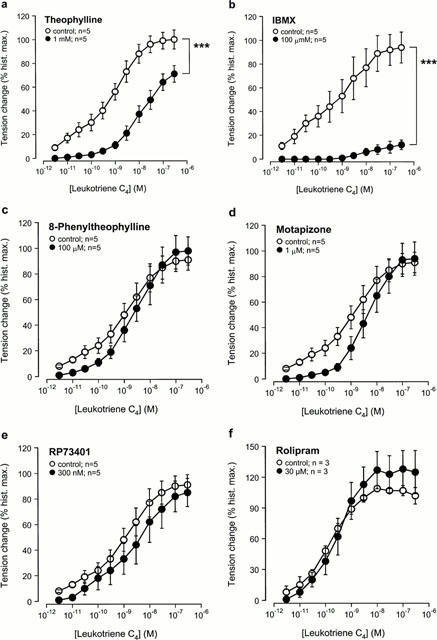
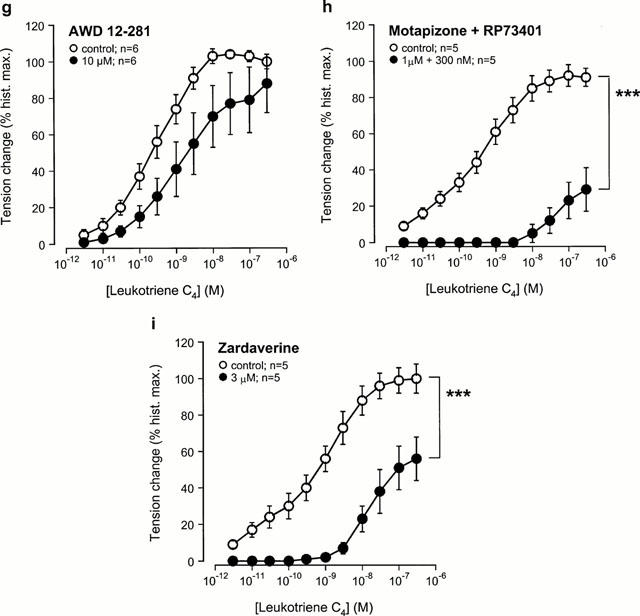
Effect of the selective and non-selective PDE inhibitors on leukotriene (LT) C4-induced contraction in passively sensitized human airways. Compared to sensitized controls, LTC4-induced contractions were reduced by pre-treatment with the non-selective PDE inhibitors theophylline (a) and IBMX (b), the PDE4-selective inhibitor AWD 12-281 (g), the combination of a PDE3-(motapizone) and PDE4-selective (RP 73401) inhibitor (Mota+RP73401) (h), and the PDE3/4-selective inhibitor zardaverine (i). Pretreatment with the adenosine receptor antagonist 8-phenyltheophylline (c), the PDE3-selective inhibitor motapizone (d), the PDE4-selective inhibitors RP73401 (e) and rolipram (f) did not affect LTC4-induced contractions. Tension changes are expressed as per cent of the maximal responses to histamine in the same tissues. **P<0.01 and ***P<0.001, denote the comparison of whole concentration-effect curves by ANOVA.
Effect of selective PDE inhibitors on allergen-precontracted bronchi
The combination of the PDE3-selective inhibitor motapizone (1 μM) and the PDE4-selective inhibitor RP73401 (300 nM) completely relaxed allergen-induced bronchial tone in passively sensitized airways pre-contracted with 30 u ml−1 D. farinae (n=5). The selective inhibition of PDE3 by motapizone reduced induced tone by 76±6%, inhibition of PDE4 by RP73401 reduced tone by 74±7% (n=4). In either case the remaining tone was completely relaxed by addition of the other inhibitor (Figure 5).
Figure 5.
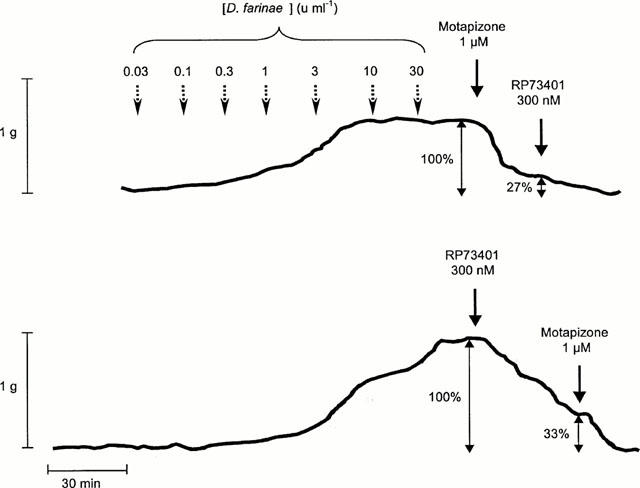
Original traces of allergen concentration-effect curves (D. farinae, range: 0.03–30 u ml−1). The bronchospasmolytic effects of (a) motapizone followed by RP73401 and (b) RP73401 followed by motapizone were recorded. In either case a complete relaxation of the allergen-precontracted bronchial ring was achieved.
Discussion
The present study demonstrates that combined inhibition of PDE3 and PDE4, but not selective inhibition of the individual isoenzymes, is effective in suppressing allergen-induced contractions of passively sensitized human airways. The combination of PDE3 and PDE4 inhibition was as effective as the non-selective inhibition by theophylline in suppressing allergen responses, while adenosine antagonism had no effect in preventing bronchoconstriction.
The in vitro model of passively sensitized human airways, i.e. the incubation of isolated airways with IgE-rich serum obtained from atopic individuals, closely mimics features of bronchial hyperresponsiveness as observed in patients with extrinsic bronchial asthma. On one hand, these features comprise non-specific hyperresponsiveness to stimuli, such as histamine and leukotrienes, that can be observed in subjects with asthma in vivo (O'Hickey et al., 1988) as well as in passively sensitized airways in vitro (Watson et al., 1997), again confirmed by the present study. On the other hand, isolated sensitized airways demonstrate specific hyperresponsiveness to allergen that is mediated through the release of inflammatory mediators, mainly cysteinyl leukotrienes, under in vivo (Björck et al., 1991) as well as in vitro conditions (Björck et al., 1991; Björck & Dahlén, 1993; Roquet et al., 1997).
In patients with asthma, early and late phase allergic responses can be inhibited by theophylline through mechanisms which might involve its ability to elicit bronchodilation (Pauwels et al., 1985) but also immunomodulatory and anti-inflammatory activity (Banner & Page, 1996). Since treatment with theophylline is associated with considerable side-effects, we aimed to investigate whether its beneficial effects are dependent on the non-specific inhibition of PDEs and/or adenosine receptor antagonism–both of which mechanisms are believed to be responsible for the unwanted effects–or whether the benefits could be attributed to the inhibition of certain PDE isoenzymes.
Our data demonstrate that allergen-induced contraction of passively sensitized human airways in vitro was effectively suppressed only by the simultaneous inhibition of PDE3 and PDE4 through the use of the non-selective inhibitors theophylline and IBMX, the PDE3/4 selective inhibitor zardaverine or the combination of a selective PDE3 and PDE4 inhibitor (motapizone+RP73401). Remarkably, neither the inhibition of the individual PDE3 isoenzyme by motapizone nor of PDE4 by RP73401 or rolipram was sufficient to alter allergen responses significantly, nor did the mathematical addition of the individual effects of these isoenzyme inhibitors result in a significant inhibitory effect on allergen responses.
Surprisingly–and at first sight in contrast to the PDE4-selective inhibitors rolipram and RP73401–the novel PDE4 inhibitor AWD 12-281 significantly reduced the bronchospasmogenic effect of allergen. It is conceivable that this inhibition of allergen-induced bronchoconstriction by AWD 12-281 is caused by a different mode of action as compared to the other PDE4 inhibitors tested. However, the exact site of interaction with the PDE is not known so far and, therefore, this assumption could be only based on speculation. More likely AWD 12-281 exhibits bronchoprotective effects through a loss of its PDE4 selectivity at higher concentrations, thereby gaining additional activity against PDE3 (Table 1). This latter possibility would be in line with our findings that a simultaneous inhibition of PDE3 and PDE4 is necessary to significantly decrease allergen responses in passively sensitized human airways.
Until a few years ago it was believed that PDE inhibitors affect airway function primarily through relaxation of airway smooth muscle resulting from cyclic AMP elevation and subsequent phosphorylation of muscle regulatory proteins and attenuation of cellular Ca2+ concentrations. However, in our study all PDE inhibitors demonstrated comparable bronchorelaxant effects. Even selective inhibition of PDE3 (motapizone) or PDE4 (RP73401, rolipram), which had no effect on allergen-induced contractions, decreased resting tension of passively sensitized bronchial rings by a similar magnitude to theophylline. This lack of a correlation between the bronchorelaxant and bronchoprotective effects of the PDE inhibitors renders it unlikely that the observed protection against allergen-induced responses resulted exclusively from a prior decrease in bronchial tone through smooth muscle relaxation. Our findings are in line with the clinical observation that, in patients with asthma, allergen-induced bronchoconstriction as well as bronchial responsiveness to methacholine and histamine are effectively reduced by theophylline, while baseline lung function is only weakly affected (Magnussen et al., 1986; 1987; Ward et al., 1993). Taken together, these findings reinforce the idea that bronchorelaxant and bronchoprotective effects of PDE inhibitors are not necessarily linked and might involve mechanisms other than direct effects on airway smooth muscle.
Furthermore, it has been suggested that methylxanthines such as theophylline and IBMX might exhibit their effects in part through adenosine receptor antagonism (Karlsson, 1987; Coward et al., 1998; Crimi et al., 1989). However, in the present study the adenosine receptor antagonist, 8-phenyltheophylline, had no effect on bronchial tone or allergen responses in passively sensitized preparations. This finding suggests that adenosine receptor antagonism is unlikely to be an important mechanism through which methylxanthines relax bronchial tone and protect against allergen-induced bronchoconstriction in passively sensitized human airways.
On the other hand, inherent tone of isolated human airways is believed to result mainly from the spontaneous release of cysteinyl leukotrienes and histamine from resident inflammatory cells such as mast cells and also eosinophils in the airway wall (Peters et al., 1984; Schleimer et al., 1986; Hay et al., 1993; Ellis & Undem, 1994). The combination of antagonists of CysLT and H1 histamine receptors was as effective as isoprenaline in relaxing human airways in vitro (Ellis & Undem, 1994). As pre-treatment with β-agonists does not modify concentration effect-curves of LTC4 (Gorenne et al., 1995), it could be assumed that cyclic AMP-elevating drugs such as PDE inhibitors and β-adrenoceptor agonists might exhibit their effects on basal bronchial tone at least in part through the inhibition of endogenous mediator release.
Independently of their bronchorelaxant capacity, PDE inhibitors are also believed to exhibit their effects on allergen-induced bronchoconstriction through inhibition of the formation and release of inflammatory mediators, mainly cysteinyl leukotrienes. If this were the case, PDE inhibitors that protect against allergen should be significantly less effective against contractions induced by these mediators. Our study, however, showed that LTC4-induced bronchoconstriction was effectively reduced by the same PDE inhibitors–or their combination–that inhibited allergen responses (Table 3). LTC4- and allergen-induced contractions were reduced to a similar degree by the simultaneous inhibition of PDE3 and PDE4 through the use of the non-selective inhibitors theophylline and IBMX, the PDE3/4 selective inhibitor zardaverine, AWD 12-281 or the combination of a selective PDE3 (motapizone) and PDE4 inhibitor (RP73401).
Furthermore, it is noteworthy that allergen-precontracted airways were significantly relaxed by the individual inhibition of PDE3 or PDE4, while their combination caused a complete decrease in bronchial tone to the level prior to the addition of allergen. The completeness of the relaxation was dependent on the presence of both selective inhibitors but was not altered if one of them had been added already prior to the induction of contraction by allergen. These findings suggest that PDE3 and PDE4 co-regulate cyclic AMP content in human airway smooth muscle. Other studies support this hypothesis, as either a combination of PDE3 and PDE4 inhibitors or dual PDE3/4 inhibitors produce a much greater bronchospasmolytic effect in carbachol-precontracted airway preparations than individual isoenzyme-selective agents alone (de Boer et al., 1992; Torphy et al., 1993).
The relationship between the effects of PDE inhibitors on isolated airways under various conditions–i.e. resting tension versus allergen and leukotriene responsiveness and allergen-induced tone–suggests that different mechanisms might be involved. While the bronchoprotective and bronchospasmolytic effect of PDE inhibitors with selectivity for PDE3 and PDE4 (non-selective, PDE3-selective+PDE4-selective, PDE3/4-selective inhibitors) appear to result mainly from a direct effect on airway smooth muscle, their effect on resting tension is likely to be primarily mediated through the inhibition of mediator release from inflammatory cells within the airway wall. Experiments on human lung mast cells have shown that mast cells contain PDE3 and PDE4 (Tenor & Schudt, 1996; Weston et al., 1997), and that both PDE3 and PDE4 inhibitors are effective in reducing antigen-driven mediator release from these cells (Louis et al., 1992; Anderson & Peachell, 1994). This might form one explanation for the similar effects of all PDE inhibitors tested in this study, including the PDE3-selective inhibitor motapizone and the PDE4-selective inhibitors RP73401 and rolipram, on resting tension. Taken together, these findings suggest that PDE inhibitors' mode of action comprises effects on smooth muscle as well as inflammatory cells and that the relative contributions of the two mechanisms might vary depending on the conditions.
Whether these findings hold true for the clinical use of selective PDE inhibitors remains to be shown. First clinical studies on the effect of the PDE3-selective inhibitor olprinone and the PDE4-selective inhibitor SB 207499, demonstrated very variable and mild effects on baseline lung function, comparable with those effects observed after theophylline (Magnussen et al., 1986; Myou et al., 1999; Torphy et al., 1999). Importantly, neither study with the selective PDE inhibitors–the PDE3 inhibitor was inhaled, the PDE4 inhibitor was administered orally–showed any significant adverse effects of the drugs. On the basis of our findings it could be assumed that selective PDE inhibitors, although demonstrating similar effects on baseline lung function, could affect bronchial obstruction and airway inflammation to a different degree. However, to evaluate the characteristic effects of PDE3- and PDE4-selective inhibitors, future clinical studies with these novel drugs and/or their combinations might have to focus on possible anti-inflammatory and bronchoprotective effects as study endpoints rather than looking exclusively at changes in baseline lung function parameters.
The present in vitro data suggest that phosphodiesterase inhibitors with a combined selectivity for PDE3 and PDE4 are likely to be effective drugs for the treatment of bronchial asthma, whereas the development of highly selective drugs might have no additional advantages and rather lack effectiveness. PDE inhibitors with combined selectivity for PDE3 and PDE4 appear to exert their effects on human airways in vitro by a dual mechanism involving direct relaxing effects on airway smooth muscle and inhibition of mediator formation and/or release from inflammatory cells. However, their relative importance and implication remain to be demonstrated in clinical studies that look not only on the effects on baseline lung function but also on inflammatory parameters and bronchial responsiveness. Those studies would show whether the selective phosphodiesterase inhibitors are effective in the treatment of obstructive airway diseases and on the other demonstrate an improved side effect profile in comparison with theophylline.
Acknowledgments
This study was jointly supported by Glaxo Wellcome, U.K., Byk Gulden, Germany, and ASTA Medica, Germany and the State of Saxony, project no. 4236, Germany.
Abbreviations
- FAST
fluorescent allergosorbent test
- IBMX
3-isobutyl-1-methylxanthine
- IgE
immunoglobulin E
- LTC4
leukotriene C4
- PDE
phosphodiesterase
References
- ANDERSON N., PEACHELL P.T.Effect of isoenzyme-selective inhibitors of phosphodiesterase (PDE) on human lung mast cells (HLMC) FASEB J. 19948A239[Abstract] [Google Scholar]
- ASHTON M.J., COOK D.C., FENTON G., KARLSSON J.-A., PALFREYMAN M.N., RAEBURN D., RATCLIFFE A.J., SOUNESS J.E., THURAIRAITNAM S., VICKER N. Selective type IV phosphodiesterase inhibitors as anti-asthmatic agents: the synthesis and biological activities of 3-cyclopentyloxy-4-methoxybenzamides and analogues. J. Med. Chem. 1994;37:1696–1703. doi: 10.1021/jm00037a021. [DOI] [PubMed] [Google Scholar]
- BANNER K.H., PAGE C.P. Anti-inflammatory effects of theophylline and selective phosphodiesterase inhibitors. Clin. Exp. Allergy. 1996;26:2–9. doi: 10.1111/j.1365-2222.1996.tb01136.x. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BERGSTRAND H. Phosphodiesterase inhibition and theophylline. Eur. J. Respir. Dis. 1980;109:37–44. [PubMed] [Google Scholar]
- BJÖRCK T., DAHLÉN S.-E. Leukotrienes and histamine mediate IgE-dependent contractions of human bronchi: pharmacological evidence obtained with tissues from asthmatic and nonasthmatic subjects. Pulm. Pharmacol. 1993;6:87–96. doi: 10.1006/pulp.1993.1012. [DOI] [PubMed] [Google Scholar]
- BJÖRCK T., HARADA Y., DAHLÉN B., ZETTERSTRÖM O., JOHANSSON G., RODRIGUEZ L., HEDQVIST P., DAHLÉN S.-E. Further evidence that leukotrienes are the major mediators of allergic constriction in human bronchi. Adv. Prostaglandin Thromboxane Leukotr. Res. 1991;21:429–432. [PubMed] [Google Scholar]
- CONTI M., NÉMOZ G., SETTE C., VICINI E. Recent progress in understanding the hormonal regulation of phosphodiesterases. Endocr. Rev. 1995;16:370–389. doi: 10.1210/edrv-16-3-370. [DOI] [PubMed] [Google Scholar]
- COOPER K.D., KANG K., CHAN S.C., HANIFIN J.M. Phosphodiesterase inhibition by Ro 20-1724 reduces hyper-IgE synthesis by atopic dermatitis cells in vitro. J. Invest. Dermatol. 1985;84:477–482. doi: 10.1111/1523-1747.ep12272486. [DOI] [PubMed] [Google Scholar]
- CORTIJO J., BOU J., BELETA J., CARDELÚS I., LLENAS J., MORCILLO E., GRISTWOOD R.W. Investigation into the role of phosphodiesterase IV in bronchorelaxation, including studies with human bronchus. Br. J. Pharmacol. 1993;108:562–568. doi: 10.1111/j.1476-5381.1993.tb12841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWARD W., SAGARA H., CHURCH M.K. Asthma, adenosine, mast cells and theophylline. Clin. Exp. Allergy. 1998;28:42–46. [PubMed] [Google Scholar]
- CRIMI N., POLOSA R., MISTRETTA A. Role of adenosine in the pathogenesis of bronchial asthma. Recenti Prog. Med. 1989;80:372–376. [PubMed] [Google Scholar]
- DE BOER J., PHILPOTT A.J., VAN AMSTERDAM G.M., SHAHID M., ZAAGSMA J., NICHOLSON C.D. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br. J. Pharmacol. 1992;106:1028–1034. doi: 10.1111/j.1476-5381.1992.tb14451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENT G., GIEMBYCZ M.A., EVANS P.M., RABE K.F., BARNES P.J. Suppression of human eosinophil respiratory burst and cyclic AMP hydrolysis by inhibitors of type IV phosphodiesterase: interaction with the beta adrenoceptor agonist albuterol. J. Pharmacol. Exp. Ther. 1994;271:1167–1174. [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Role of cysteinyl-leukotrienes and histamine in mediating intrinsic tone in isolated human bronchi. Am. J. Respir. Crit. Care Med. 1994;149:118–122. doi: 10.1164/ajrccm.149.1.8111568. [DOI] [PubMed] [Google Scholar]
- FISHER D.A., SMITH J.F., PILLAR J.S., ST. DENIS S.H., CHENG J.B. Isolation and characterization of PDE8A, a novel human cAMP-specific phosphodiesterase. Biochem. Biophys. Res. Commun. 1998;246:570–577. doi: 10.1006/bbrc.1998.8684. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GANTNER F., KUPFERSCHMIDT R., SCHUDT C., WENDEL A., HATZELMANN A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumour necrosis factor-α release by PDE inhibitors. Br. J. Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., CORRIGAN C.J., SEYBOLD J., NEWTON R., BARNES P.J. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and the biosynthesis of interleukin-2. Br. J. Pharmacol. 1996;118:1945–1958. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORENNE I., LABAT C., NOREL X., DEMONTPREVILLE V., GUILLET M.-C., CAVERO I., BRINK C. Effects of β2-adrenoceptor agonists on anti-IgE-induced contraction and smooth muscle reactivity in human airways. Br. J. Pharmacol. 1995;114:935–940. doi: 10.1111/j.1476-5381.1995.tb13294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY D.W.P., HUBBARD W.C., UNDEM B.J. Endothelin-induced contraction and mediator release in human bronchus. Br. J. Pharmacol. 1993;110:392–398. doi: 10.1111/j.1476-5381.1993.tb13822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL G.E., ANDERSON J.L., WHITTEN C.W. Ketamine inhibits agonist-induced cAMP accumulation increase in human airway smooth muscle cells. Can. J. Anaesth. 1999;46:1172–1177. doi: 10.1007/BF03015528. [DOI] [PubMed] [Google Scholar]
- KARLSSON J.-A. Purines and sensory neuropeptides in human asthma. Bull. Eur. Physiopathol. Respir. 1987;23:95s–101s. [PubMed] [Google Scholar]
- KARLSSON J.-A.Theophylline: anti-inflammatory effects Pulmonary and Critical Care Pharmacology and Therapeutics 1996New York: McGraw-Hill; 609–620.ed. Leff A.R. pp [Google Scholar]
- LOUGHNEY K., FERGUSON K.Identification and quantification of PDE isoenzymes and subtypes by molecular biological methods Phosphodiesterase inhibitors 1996London: Academic Press; 1–19.ed. Schudt, C., Dent, G. & Rabe, K.F. pp [Google Scholar]
- LOUIS R., BURY T., CORHAY J.L., RADERMECKER M. LY18665, a phosphodiesterase inhibitor, inhibits histamine release from human basophils, lung and skin fragments. Int. J. Immunopharmacol. 1992;14:191–194. doi: 10.1016/0192-0561(92)90030-o. [DOI] [PubMed] [Google Scholar]
- MAGNUSSEN H., JÖRRES R.A., HARTMANN V. Bronchodilator effects of theophylline preparations and aerosol fenoterol in stable asthma. Chest. 1986;90:722–725. doi: 10.1378/chest.90.5.722. [DOI] [PubMed] [Google Scholar]
- MAGNUSSEN H., REUSS G., JÖRRES R.A. Theophylline has a dose-related effect on the airway response to inhaled histamine and methacholine in asthmatics. Am. Rev. Respir. Dis. 1987;136:1163–1167. doi: 10.1164/ajrccm/136.5.1163. [DOI] [PubMed] [Google Scholar]
- MYOU S., FUJIMURA M., KAMIO Y., ISHIURA Y., TACHIBANA H., HIROSE T., HASHIMOTO T., MATSUDA T. Bronchodilator effect of inhaled olprinone, a phosphodiesterase 3 inhibitor, in asthmatic patients. Am. J. Respi. Crit. Care Med. 1999;160:817–820. doi: 10.1164/ajrccm.160.3.9812065. [DOI] [PubMed] [Google Scholar]
- NATIONAL INSTITUTES OF HEALTH, NHLBI Global strategy for asthma management and prevention 199595–3659.WHO/NHLBI workshop report. Publication No. 1995
- NATIONAL INSTITUTES OF HEALTH Guidelines for the diagnoses and management of asthma 199797–4051.Expert Panel Report 2. Publication No. 1997
- O'HICKEY S.P., ARM J.P., REES P.J., SPUR B.W., LEE T.H. The relative responsiveness to inhaled leukotriene E4, methacholine and histamine in normal and asthmatic subjects. Eur. Respir. J. 1988;1:913–917. [PubMed] [Google Scholar]
- PAUWELS R., VAN RENTERGHEM D., VAN DER STRAETEN M., JOHANNESSON N., PERSSON C.G.A. The effect of theophylline and enprofylline on allergen-induced bronchoconstriction. J. Allergy Clin. Immunol. 1985;76:583–553. doi: 10.1016/0091-6749(85)90779-1. [DOI] [PubMed] [Google Scholar]
- PETERS S.P., MACGLASHAN D.W. , Jr, SCHULMAN E.S., SCHLEIMER R.P., HAYES E.C., ROKACH J., ADKINSON N.F., Jr, LICHTENSTEIN L.M. Arachidonic acid metabolism in purified human lung mast cells. J. Immunol. 1984;132:1972–1979. [PubMed] [Google Scholar]
- RABE K.F., TENOR H., DENT G., SCHUDT C., LIEBIG S., MAGNUSSEN H. Phosphodiesterase isozymes modulating inherent tone in human airways: identification and characterization. Am. J. Physiol. 1993;264:L458–L464. doi: 10.1152/ajplung.1993.264.5.L458. [DOI] [PubMed] [Google Scholar]
- ROQUET A., DAHLEN B., KUMLIN M., IHRE E., ANSTREN G., BINKS S., DAHLEN S.E. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am. J. Respir. Crit. Care Med. 1997;155:1856–1863. doi: 10.1164/ajrccm.155.6.9196086. [DOI] [PubMed] [Google Scholar]
- SCHLEIMER R.P., MACGLASHAN D.W., Jr, PETERS S.P., PINCKARD R.N., ADKINSON N.F. , Jr, LICHTENSTEIN L.M. Characterization of inflammatory mediator release from purified human lung mast cells. Am. Rev. Respir. Dis. 1986;133:614–617. doi: 10.1164/arrd.1986.133.4.614. [DOI] [PubMed] [Google Scholar]
- SCHMIDT D., RÜHLMANN E., BRANSCHEID D., MAGNUSSEN H., RABE K.F. Passive sensitization of human airways increases responsiveness to leukotriene C4. Eur. Respir. J. 1999;14:315–319. doi: 10.1034/j.1399-3003.1999.14b13.x. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., ELTZE M., KILIAN U., BEUME R. Zardaverine: A cyclic AMP specific PDE III/IV inhibitor. Agents Actions Suppl. 1991a;34:379–402. [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., FORDERKUNZ S., HATZELMANN A., ULLRICH V. Influence of selective phosphodiesterase inhibitors on human neutrophil function and levels of cAMP and Cai. Naunyn Schmiedebergs Arch. Pharmacol. 1991b;344:682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BAYUGA S.J., BEAVO J.A. Cloning and characterization of a cAMP-specific cyclic nucleotide phosphodiesterase. Proc. Natl. Acad. Sci. U.S.A. 1998a;95:8991–8996. doi: 10.1073/pnas.95.15.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SODERLING S.H., BAYUGA S.J., BEAVO J.A. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J. Biol. Chem. 1998b;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BAYUGA S.J., BEAVO J.A. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7071–7076. doi: 10.1073/pnas.96.12.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENOR H., SCHUDT C.Analysis of PDE isoenzyme profiles in cells and tissues by pharmacological methods Phosphodiesterase Inhibitors 1996London: Academic Press; 21–40.ed. Schudt, C., Dent, G. & Rabe, K.F. pp [Google Scholar]
- TORPHY T.J. Molecular targets for novel anti-asthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., BARNETTE M.S., UNDERWOOD D.C., GRISWOLD D.E., CHRISTENSEN S.B., MURDOCH R.D., NIEMAN R.B., COMPTON C.H. Ariflo™ (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from the concept to the clinic. Pulm. Pharmacol. Ther. 1999;12:131–135. doi: 10.1006/pupt.1999.0181. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., UNDEM B.J., CIESLINSKI L.B., LUTTMANN M.A., REEVES M.L., HAY D.W.P. Identification, characterization and functional role of phosphodiesterase isozymes in human airway smooth muscle. J. Pharmacol. Exp. Ther. 1993;265:1213–1223. [PubMed] [Google Scholar]
- UKENA D., SCHUDT C., SYBRECHT G.W. Adenosine receptor-blocking xanthines as inhibitors of phosphodiesterase isozymes. Biochem. Pharmacol. 1993;45:847–851. doi: 10.1016/0006-2952(93)90168-v. [DOI] [PubMed] [Google Scholar]
- WARD A.J., MCKENNIFF M., EVANS J.M., PAGE C.P., COSTELLO J.F. Theophylline: an immunomodulatory role in asthma. Am. Rev. Respir. Dis. 1993;147:518–523. doi: 10.1164/ajrccm/147.3.518. [DOI] [PubMed] [Google Scholar]
- WATSON N., BODTKE K., COLEMAN R.A., DENT G., MORTON B.E., RÜHLMANN E., MAGNUSSEN H., RABE K.F. Role of IgE in hyperresponsiveness induced by passive sensitization of human airways. Am. J. Respir. Crit. Care Med. 1997;155:839–844. doi: 10.1164/ajrccm.155.3.9117014. [DOI] [PubMed] [Google Scholar]
- WEINBERGER M., HENDELES L. Theophylline in asthma. N. Engl. J. Med. 1996;334:1380–1388. doi: 10.1056/NEJM199605233342107. [DOI] [PubMed] [Google Scholar]
- WESTON M.C., ANDERSON N., PEACHELL P.T. Effects of phosphodiesterase inhibitors on human lung mast cell and basophil function. Br. J. Pharmacol. 1997;121:287–295. doi: 10.1038/sj.bjp.0701115. [DOI] [PMC free article] [PubMed] [Google Scholar]


