Introduction
Protease activated receptors (PARs) represent a novel class of seven transmembrane domain G-protein coupled receptors activated by proteolytic cleavage. Up to now, four members of this class of receptors have been identified, PAR1 to PAR4 (Hollenberg, 1999). The mechanism for activation of PARs involves a proteolytic unmasking of an N-terminal sequence that acts as a tethered ligand. In the absence of proteolysis, PAR1, PAR2 and PAR4, but not PAR3, can be activated by synthetic peptides (PAR-activated peptides, PAR-APs) ranging between five and 14 aminoacids, whose sequence mimics the tethered ligand (Dery et al., 1998).
The thrombin receptor PAR1 was the first PAR to be discovered and cloned (Vu et al., 1991). Successively, a second member of PAR family was identified by Nystedt (1994) who cloned a mouse genomic DNA sequence encoding a proteolitically activated receptor, related to but with a different sequence from the thrombin receptor PAR1. The receptor was termed PAR2 and it was found to be activated by trypsin that cleaves the receptor within the sequence N34SKGR/SLIGRLETQP48 of its extracellular -NH2 terminus, exposing a tethered ligand, SLIGRLETQP, that binds the cleaved receptor (Figure 1). Synthetic peptides mimicking the tethered ligand sequence, SLIGRLETQ . , can activate the receptor even in the absence of proteolysis. The site for the receptor proteolytic cleavage contains the sequence SLIGRL and SLIGKV, murine and human respectively (Nystedt et al., 1994).
Figure 1.
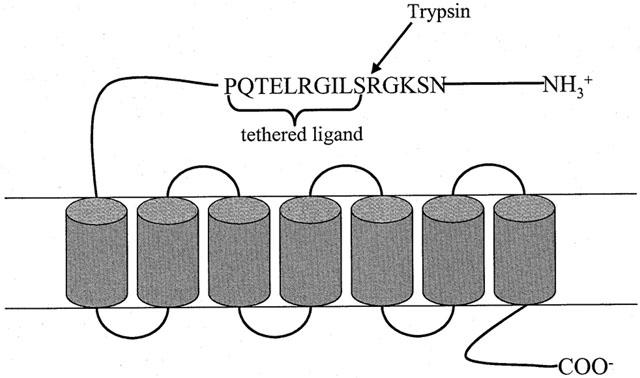
Schematic representation of PAR2 activation by trypsin. PAR2 is activated upon trypsin cleavage within the sequence N34SKGR/SLIGRLETQP48 of its extracellular -NH2 terminus. This event unmasks a tethered ligand, SLIGRLETQP, that binds the cleaved receptor activating it. Synthetic peptides mimicking the tethered ligand sequence, SLIGRLETQ …, can activate the receptor even in absence of proteolysis.
The physiological ligand of PAR2 has not as yet been identified. However, it has been shown that besides trypsin, the receptor may be activated by tryptase (Molino et al., 1997) and factor Xa (Fox et al., 1997), but it is resistant to activation by thrombin, even at concentrations as high as 100 nM.
PAR2 mRNA has been found in several human and animal tissues, including kidney, stomach, pancreas, liver, colon and small intestine (Nystedt et al., 1994). Furthermore, by using immunohistochemical techniques, PAR2 has been localized in almost all human organs; in particular, a strong immunoreactivity has been detected in endothelial and epithelial cells, and in smooth muscle of both vascular and non vascular origin (D'andrea et al., 1998).
To date, the physiological and/or pathological role of PAR2 is unknown. The presence of PAR2 on highly vascularized organs strongly suggests a role for the receptor in the control of vascular reactivity (D'andrea et al., 1998). This hypothesis is supported by a large amount of work that has been produced, since its discovery and cloning, giving evidence for the ability of PAR2 in regulating vascular function.
On the basis of the present knowledge, the aim of this review is to recapitulate the cardiovascular functions of PAR2.
PAR2 on vascular endothelial cells
The finding that PAR2 is highly expressed on endothelial cells is not surprising, considering that a large amount of proteases circulate in the blood. Most of them circulate in their proteolitically inactive form of zymogens, becoming activated following injury. Trypsin, tryptase, factor Xa can all activate PAR2 on endothelial cells and induce cellular effects involving either tissue degradation or regeneration processes.
Evidence that PAR2 is expressed on both endothelium and vascular smooth muscle has suggested a role for this receptor in the control of vascular reactivity. A functional role for PAR2 on vascular endothelial cells was first described by Al-Ani et al. (1995), who found that the peptide mimicking the murine tethered ligand sequence, SLIGRL-NH2, and trypsin caused an endothelium-dependent relaxation of rat thoracic aorta. The effect of PAR2-activating peptide (PAR2-AP) was inhibited by L-NAME, implying the involvement of nitric oxide. At the same time, by RT – PCR analysis, receptor expression in the rat aortic tissue was also demonstrated. Subsequently, several studies have been performed to investigate: (i) whether PAR2 on vascular tissues was functional; (ii) whether vascular effects triggered by peptides activating PAR2 were due to activation of another receptor alone or in addition to PAR2 and (iii) whether thrombin could also activate the receptor. All these questions have now been addressed and it has been clearly shown that peptides activating PAR2, as well as trypsin, cause endothelium-dependent relaxation of rat thoracic aorta, as previously demonstrated (Al-Ani et al., 1995; Hollenberg et al., 1996; Magazine et al., 1996; Saiffedine et al., 1996). The effect is likely due to the activation of PAR2 per se, since several antagonists, such as atropine, phenoxybenzamine, ritanserine, propranolol, do not modify the response. In addition, structure activity relationship studies have shown that among peptides of different length, the hexapeptide SLIGRL is the most active in causing the endothelium dependent relaxation of rat aorta, and this activity is linked to the presence of the leucine and arginine residue at positions 2 and 5 respectively (Hollenberg et al., 1996).
Although PAR2 is not activated by thrombin, a striking similarity has been observed between the two receptors. PAR1-APs, such as PAR2-APs, causes endothelium dependent relaxation of rat aorta that involves nitric oxide release (Muramutsu et al., 1992; Hollenberg et al., 1996; Magazine et al., 1996). Structure activity relationship studies have shown that for both receptors, PAR1 and PAR2, activating peptides with a free carboxyl group are less active than their carboxyamide derivatives (Hollenberg et al., 1996). However, in contrast to PAR1-AP, PAR2-AP fails to cause contraction of endothelium denuded aorta, although it is expressed on vascular smooth muscle (Magazine et al., 1996; Saiffedine et al., 1996). Moreover, there is no cross-desensitization between the two receptors; however, peptides activating PAR1 also activate PAR2 (Blackhart et al., 1996). This implicates that PAR2 might be activated by the sequence of the cleaved receptor PAR1. Although Blackhart et al. (1996) considered physiologically irrelevant this cross-reactivity, since the tethered ligand would be insufficient for an intramolecular interaction, Chen et al. (1994) have shown that intramolecular signalling by the thrombin receptor tethered ligand can occur. It is feasible that the two receptors are very closely located on endothelial cell plasma membrane. In this respect, a cross-talk between the two receptors, PAR1 and PAR2, on HUVEC has been demonstrated; it has been observed that a trans-activation of PAR2 by cleaved PAR1 contributes to the endothelial response to thrombin (O'brien et al., 2000). This result agrees with previous evidence for a functionally coupling between PAR1 and PAR2 receptors on HUVEC, given by Mirza et al. (1996), and it is not surprising considering that a cross-talk among G proteins coupled receptors has already been demonstrated (Djellas et al., 1998). Thus, it remains to be established whether PAR2 contributes to modulate thrombin vascular effects. This hypothesis could justify the increased response to thrombin when compared to the response obtained with agonist peptides, both in vitro and in vivo assays, as probably due to PAR1 activation and PAR2 trans-activation by the cleaved PAR1, that cannot occur with the use of the agonist peptides. It will be possible to discriminate between these two receptors activation only when PAR2 selective antagonists will be available. What is now clearly ascertained is that PAR2 is present on both endothelial and vascular smooth muscle cells. On endothelium, its activation causes a relaxation that involves nitric oxide. The release of nitric oxide from rat aortic rings stimulated with PAR2-AP (SLIGRL) has been demonstrated and also a role for endothelin-1 through Etb receptor activation has been shown (Magazine et al., 1996). Both trypsin and the peptide activating PAR2 (SLIGRL) also cause an endothelium-dependent relaxation of porcine coronary artery (Table 1). The effect of trypsin is clearly dependent upon its proteolytic activity since it is inhibited by soybean trypsin inhibitor (Hwa et al., 1996). However, relaxation induced by PAR2 activation is only partially inhibited by L-NAME, suggesting that it is dependent upon a NO-dependent and NO-independent mechanism (Hwa et al., 1996). More recently it has been shown that the NO-independent component of vasorelaxation induced by SLIGRL on porcine isolated coronary artery might be due to a hyperpolarizing factor, likely endothelium derived hyperpolarizing factor (EDHF) (Hamilton & Cocks, 2000). Besides large conduit vessels, such as thoracic aorta, PAR2 activation has also shown an endothelium-dependent vasorelaxant effect on small arteries, such as rat femoral artery and vein (Emilsson et al., 1997; Roy et al., 1998); rat renal, mesenteric and pulmonary arteries, (Roy et al., 1998), and on mouse renal arteries (Moffatt & Cocks, 1998) suggesting a role in controlling peripheral resistance.
Table 1.
Effects mediated by PAR2 on endothelial
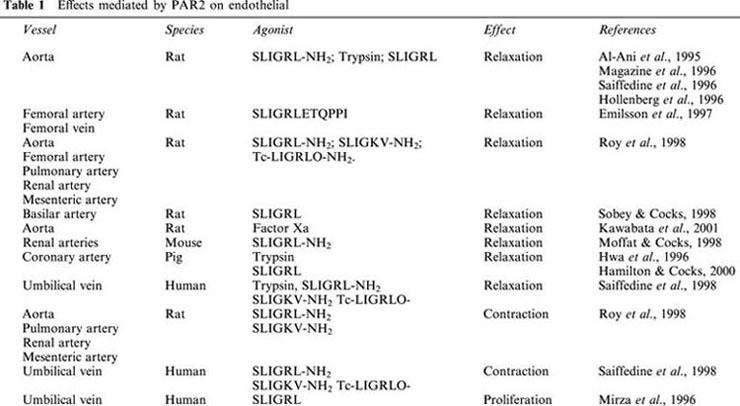
In any vascular district examined, the relaxant effect is dependent upon the presence of a functional endothelium, and it is abolished by inhibition of nitric oxide synthase. In the absence of endothelium, neither contraction nor relaxation has been observed, apart from a small contractile effect on femoral arteries without endothelium achieved only by a high concentration (100 μM) of PAR2-agonist peptide (Emilsson et al., 1997), and a contractile effect on mouse isolated renal artery (Moffatt & Cocks, 1998) described in the next section. Conversely, an endothelium-dependent vasoconstriction has been observed following PAR2 activation on renal, mesenteric, pulmonary arteries and on the aorta, that is independent of endothelin, angiotensin, nor-adrenaline or arachidonic acid metabolites (Roy et al., 1998). However, this contractile effect of PAR2-AP has been postulated to be dependent upon the activation of another receptor for several reasons. First, the peptide SLIGRL-NH2 caused contraction even in a preparation desensitized to the contractile effect of trypsin; second, peptides SLIGRL-NH2 and SLIGKV-NH2, rat and human sequence respectively, were equipotent to cause contraction, while showed different potencies in the relaxation assay, being SLIGKV-NH2 1/5 as potent as SLIGRL-NH2. Finally, the PAR2-AP derivative, transcynnamoil-LIGRLO-NH2 (Tc-LIGRLO-NH2), that is known to be a very selective PAR2 agonist, did not cause contraction, while it was equally active with SLIGRL-NH2 in the relaxation assay (Roy et al., 1998). However, to date, no PAR2 subtypes have been described. Functional PAR2 has also been demonstrated on cerebral arteries. Indeed, a nitric oxide-dependent relaxation of rat basilar artery has been demonstrated in vivo (Sobey & Cocks, 1998). Interestingly, the effect of PAR2 activation on cerebral vasculature is preserved in spontaneous hypertensive rats (SHR), despite the impaired nitric oxide production in this model of hypertension. This has suggested a protective role for the receptor in the cerebral circulation during chronic hypertension (Sobey & Cocks, 1998; Sobey et al., 1999). This finding further supports the hypothesis that besides nitric oxide other mechanisms might mediate PAR2 effects.
While on animal vascular tissues the relaxant effect of PAR2 has been extensively characterized, even though still much work is needed to identify the signal transduction mechanism involved, the effect of PAR2 on human tissues is still controversial. A functional PAR2 on human vascular endothelial cells (HUVEC) has been demonstrated, and its activation causes cell proliferation and elevation of intracellular calcium, a signal known to activate NOS (Mirza et al., 1996). A study performed by using a ‘sandwich' assay between aorta and human umbilical vein (HUV) has shown that while both trypsin and PAR2-AP cause nitric oxide dependent vasorelaxation, only PAR2-AP stimulates the release of an endothelium-derived contracturing factor from the same tissue, that is not identifiable with nor-adrenaline, angiotensin nor with endothelin (Saiffedine et al., 1998). This result agrees with data obtained on rat tissues (Roy et al., 1998) and both imply the existence of a novel PAR or of a PAR2 subtype, whose activation is responsible for the release of an endothelium derived contracturing factor (EDCF).
Human coronary arteries with endothelium have been shown to relax in response to thrombin and trypsin, but not to the human sequence SLIGKV-NH2 or the mouse SLIGRL-NH2, in contrast to porcine coronary arteries that respond to all stimuli (Hamilton & Cocks, 2000). On the basis of this finding, it has been postulated that human coronary arteries present only PAR1 that can be activated by both thrombin and trypsin, but not PAR2.
Inflammatory stimuli, such as TNFα, IL-1 or LPS, cause an over-expression of PAR2 on cultured HUVEC (Nystedt et al., 1996). Similarly, we have demonstrated that animals challenged with bacterial endotoxin present an increased expression of PAR2 on endothelium and smooth muscle cells, from both jugular vein and carotid artery (Cicala et al., 1999). Recently, it has been shown an increased PAR2 expression on human coronary artery following exposure to inflammatory stimuli (Hamilton et al., 2001). This finding, together with the finding of a proliferative effect of PAR2 on HUVEC, given previously (Mirza et al., 1996), suggests that this receptor could play a role in inflammation, in particular in the proliferative/reparative processes.
The role of PAR2 on endothelial cells has not yet been clearly established. However, all data accumulated up to now suggest that it modulates vascular reactivity (Table 1). It remains to be clarified whether its main role is under physiological or pathological conditions. In order to address this question, it is necessary to identify the physiological ligand of PAR2.
PAR2 on vascular smooth muscles
Previously described studies have shown a vasorelaxant endothelium-dependent effect following PAR2 activation, but no increase in vascular tension has been observed on tissues without endothelium, or in L-NAME pre-treated tissues, as described for PAR1 activation (Al-Ani et al., 1995; Magazine et al., 1996; Saiffedine et al., 1996). However, trypsin, that activates the receptor, has been shown to contract endothelium-denuded rabbit aorta (Komuro et al., 1997). In some cases, this discrepancy between PAR2 and trypsin effect has led to suggest the involvement of other PARs or of a PAR2 subtype.
Among human vascular tissues, PAR2 expression has been found predominantly on arterial smooth muscles and less on venous smooth muscles. Conversely, myocardial smooth muscles are negative for PAR2 expression (D'andrea et al., 1998). By measuring changes in cytosolic calcium following stimulation with the agonist peptides (SLIGKV and SLIGRL), Molino et al. (1998) have shown that PAR2 normally expressed on smooth muscle cells from human aorta and coronary arteries is functional, while smooth muscles from saphenous vein do not show any functional receptor. These data demonstrate that there are differences in PAR2 expression between venous and arterial vascular beds, that might implicate a specific role for PAR2 in the modulation of peripheral resistance (Molino et al., 1998).
Despite previous findings, a direct vascular smooth muscle contraction in response to both trypsin and PAR2 ligand (SLIGRL-NH2) has been described on mouse isolated renal arteries (Moffatt & Cocks, 1998). The possibility that the effect is due to PAR1 activation has been ruled out by the finding that tissue desensitization to PAR1 agonist, SFLLRN, does not prevent contractions induced by PAR2 agonist, SLIGRL-NH2. This represented the first evidence for a direct contractile effect of PAR2 on vascular smooth muscles, apart from a small contractile effect triggered by a high concentration of SLIGRLETQPPI on endothelium-denuded rat femoral artery (Emilsson et al., 1997; Table 2).
Table 2.
Effects mediated by PAR2 on vascular smooth muscle

The observation that the increase in intracellular calcium following PAR2 activation on aortic smooth muscles is lower than that caused by PAR1 activation suggests an expression of PAR2 lower than PAR1 on smooth muscles; conversely, on endothelial cells there is no evidence for difference in the expression between these two receptors (Molino et al., 1998). Nonetheless, PAR2 is expressed on smooth muscles from large and small arteries, both in humans and in animals, however, on endothelium-denuded vessel preparations there is neither relaxation nor contraction following its activation, with the only exception of the receptor on mouse renal arteries, as described above (Moffatt & Cocks, 1998). This suggests that the mere presence of the receptor is not a sufficient condition to elicit a response indicated by change in tissue tension, rising the possibility that vascular responses to PAR2 agonists might be due to activation of another receptor alone or in addition to PAR2 (Saiffedine et al., 1996). To address this question, selective PAR2 antagonists should be available. Hence, PAR2 on vascular smooth muscle might mediate different effects, depending on the vascular bed and/or animal species considered. This hypothesis is consistent with a proliferative effect of PAR2 agonist (SLIGKV) and trypsin on human aortic smooth muscle cells (Bono et al., 1997) and also on human endothelial cells (Mirza et al., 1996).
An increased expression of PAR2 has been found in balloon-injured rat carotid artery in association with media smooth muscle damage and proliferating smooth muscle cells of the neointima (Damiano et al., 1999a). Thus, one hypothesis could be that during vascular injury PAR2 presents on smooth muscles could be the effector of protease-induced cell proliferation. This would also be consistent with the presence of mast cells at the site of vascular injury and likely high local protease concentration (Jeziorska et al., 1997).
In agreement with this hypothesis is the well characterized ability of proteases to induce proliferation of several cell types; in some cases, it has been demonstrated that the effect is mediated through PAR2 activation (Akers et al., 2000; Chinni et al., 2000). Another possible candidate to mediate smooth muscle proliferation through PAR2 activation is factor Xa. Indeed, it has been shown that factor Xa stimulates rat and human vascular smooth muscle cell proliferation and the effect seems to be mediated by the effector cell protease receptor-1 (EPR-1) (Gasic et al., 1992; Ko et al., 1996; Herbert et al., 1998). However, evidence that only proteolitically active factor Xa mediates cellular effects, suggests that another receptor, rather than EPR1, is involved, since EPR1 does not contain proteolysis-sensitive sites (Altieri, 1995). Interestingly, an association between EPR1 and PAR2 has recently been demonstrated in mediating factor Xa effects on endothelial cells (Bono et al., 2000).
In vivo vascular responses to PAR2 activation
Vascular effects due to PAR2 activation have also been shown in vivo, by using PAR2-APs (Table 3). Intravenous injection of PAR2 agonist, SLIGRLETQPPI, into rats causes hypotension, rapid in onset and independent of kidney function, since it is not affected by functional nephrectomy and it is likely due to a peripheral effect. The hypotension is partially inhibited by L-NAME, suggesting that, as well as in vitro, nitric oxide is involved (Emilsson et al., 1997).
Table 3.
In vivo cardiovascular effects due to PAR2 activation

A hypotension following intravenous injection of PAR2 agonist (SLIGRL) has also been demonstrated in mice (Cheung et al., 1998). Also in this case, the hypotensive effect of the peptide activating PAR2 is likely due to a peripheral effect of the peptide, rather than secondary to an effect on heart, as demonstrated by the absence of bradycardia associated to hypotension (Cheung et al., 1998).
We have recently shown that hypotension induced by SLIGRL in rats is due to an NO-dependent and NO-independent mechanism, the latter increases when the vascular tone increases, confirming the hypothesis of a direct effect on vessels (Cicala et al., 2001). Similarly, in vitro data have shown that PAR2 activation on porcine coronary artery causes NO-dependent and NO-independent, EDHF-like, relaxation (Hamilton & Cocks, 2000).
In previous experiments, performed either in mice or rats, PAR2 activation has been shown to induce only a hypotensive response, without causing any hypertensive effect (Emilsson et al., 1997; Cheung et al., 1998; Cicala et al., 1999). However, we have recently shown that hypotension is followed by a hypertensive phase due to a sympathetic reflex and modulated by basal nitric oxide. Indeed, hypertension induced by PAR2 agonist (SLIGRL) is abolished in ganglion-blocked animals and restored after treatment with L-NAME (Cicala et al., 2001). Most likely, this hypertensive response to peptide injection was not evidenced in previous studies, since the time span of the experimental protocol was not long enough. A reflex hypertension is consistent with the hypothesis of a peripheral action of PAR2-AP and also with a reflex increase in heart rate observed in rats following injection of PAR2-AP (Emilsson et al., 1997), although differences among different species have been observed.
Hypotension induced by PAR2 activation is quite sustained, lasting about 2 – 3 min. The effect of L-NAME, only affecting the endurance of hypotension induced by PAR2-AP, has been confirmed by all the studies performed in vivo (Emilsson et al., 1997; Cheung et al., 1998; Cicala et al., 2001). This has suggested that other mechanisms besides nitric oxide release account for the vasorelaxant effect of peptides activating PAR2.
The specificity of vascular response to PAR2 receptor activation has been confirmed by experiments performed on PAR2- and PAR1-deficient mice. Hypotensive response to PAR2-AP, SLIGRL, is absent in PAR2-deficient mice. Conversely, in the same animals, response to PAR1 activation is accentuated. This suggests that PAR2 might modulate thrombin vascular effects (Damiano et al., 1999b) and further supports the hypothesis of PAR2 trans-activation by cleaved PAR1, described above (O'brien et al., 2000).
The physiological ligand of PAR2 is not known. The receptor is activated by trypsin, tryptase and factor Xa, and it could mediate local and/or systemic vascular effects of these proteases at the site of an injury. A hypotensive effect of factor Xa has been demonstrated (Papapetropoulos et al., 1998) and it has been postulated the involvement of EPR1. However, recently it has been shown that activation of endothelial cells by factor Xa is related to sequential activation of EPR1 and PAR2 (Bono et al., 2000) and that PAR2 is involved in factor Xa-induced vasorelaxation of rat thoracic aorta (Kawabata et al., 2001). Thus, PAR2 might be the effector of hypotension induced by factor Xa. Furthermore, PAR2 can be activated by factor VIIa in the presence of tissue factor (TF) and factor X (Camerer et al., 2000). Likely, TF/factor VIIa complex activates factor X into the proteolitically active form that, in turn, activates PAR2. A previous work by the same authors (Camerer et al., 1996) showed that only proteolitically active factor VII and factor X elicit a calcium response in kidney cells. Thus, it is feasible that a proteolysis-sensitive receptor is involved, most likely PAR2. Very recently it has been demonstrated that factor Xa in the complex with TF/factor VIIa efficiently activates PAR2 (Riewald & Ruf, 2001). All these findings point toward a role for PAR2 in mediating upstream coagulation protease effects.
Hypotensive effect of PAR2 agonist, SLIGKV-NH2, and trypsin is increased in rats injected with bacterial endotoxin; this effect is coupled to an increased PAR2 expression on endothelium and smooth muscles from either jugular vein and carotid artery (Cicala et al., 1999). This finding is in agreement with in vitro data showing an increased expression of PAR2 on endothelial cells due to inflammatory stimuli (Nystedt et al., 1996; Hamilton et al., 2001). An increased expression of PAR2 has been also demonstrated in the rat heart following ischaemia reperfusion injury (Napoli et al., 2000; Table 4).
Table 4.
Cardiovascular diseases associated to an over-expression of PAR2

The physiological role of PAR2 has not been defined, as well as it has not been established whether the receptor is pre-posed for the control of local and systemic changes in blood pressure under physiological conditions or only in pathological states. Evidence that PAR2 is physiologically expressed on the vascular tissues suggests that it normally participates in the control of vascular homeostasis. However, its ligand is not known and possible candidates, such as trypsin, tryptase or factor Xa, do not normally circulate in the blood at concentrations able to activate the receptor and it is unlikely that they are pre-posed to activation of such an ubiquitous receptor. On the other hand, the finding that the receptor is over-expressed on tissues stimulated with inflammatory cytokines, either in vitro and in vivo (Nystedt et al., 1996; Cicala et al., 1999; Hamilton et al., 2001), strongly suggests that PAR2 might participate in the well established link between inflammation and coagulation as sensor of coagulation proteases (Cicala & Cirino, 1998; Cirino et al., 2000).
Conclusions
On the basis of experimental evidence accumulated since its discovering and cloning, it is possible to delineate a cardiovascular profile for PAR2. The receptor is highly expressed on vascular tissues and mediates both vascular contractility and proliferation, suggesting that it participates to the physiological control of vascular homeostasis. On the other hand, PAR2 is also over-expressed in pathological conditions suggesting a role in the defence mechanisms activated following vascular injury. In this respect it might be considered as a sensor of coagulation proteases during inflammation.
On the endothelial cells, PAR2 mainly mediates relaxation both in large and in small arteries, suggesting a role in the control of systemic and local haemodynamics. It remains to be elucidated the mechanism at the basis of this effect, since nitric oxide seems to be only partially involved.
Less clear appears the role of PAR2 on vascular smooth muscles. Indeed, although largely expressed, its activation does not cause change in tension in any vascular tissue examined, but in mouse renal arteries, as described by Moffatt & Cocks (1998). Nonetheless, on vascular smooth muscle PAR2 has been proven to be functional; thus, it remains to be established whether PAR2 on smooth muscle is functionally associated to another receptor, likely another G-protein coupled receptor, or whether its main effect is to induce smooth muscle proliferation, especially under pathological conditions.
Proteases are involved in a wide array of processes, they represent a cross link between coagulation and inflammation and their levels strongly increase following vascular injury, either systemically or locally, at site of an injury. There is much evidence that protease inhibitors, such as aprotinin (Bone et al., 1992) and ulinastatin (Okano et al., 1994; Ohnishi et al., 1985), have a beneficial effect on systemic inflammatory diseases, such as sepsis. The discovery of PARs, and in particular of PAR2, has shed a new light on proteases and on their cellular effects. Evidence that PAR2 is also activated by TF/factor VIIa demonstrates that even upstream coagulation proteases mediate cellular effects.
To unravel the role of PAR2 in the cardiovascular system still much work is needed. This review was aimed to outline that all data obtained since its discovery join to one point: PAR2 in the vascular system modulates vascular contractility and vascular cell proliferation, both under physiological and pathological conditions. Likely, in a future it will be impossible to study mechanisms involved in cardiovascular diseases without taking in account PAR2.
Abbreviations
- EDHF
endothelium derived hyperpolarizing factor
- EPR1
effector cell protease receptor 1
- HUVEC
human umbilical vein endothelial cells
- IL-1
interleukin 1
- L-NAME
Nω-L-arginine methyl ester
- LPS
lipopolysaccharide
- PARs
protease activated receptors
- PAR2-AP
PAR2-activated peptide
- TNFα
tumour necrosis factor α
References
- AKERS I.A., PARSONS M., HILL M.R., HOLLENBERG M.D., SANJAR S., LAURENT G.J., MCANULTY R.J. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- AL-ANI B., SAIFFEDINE M., HOLLENBERG M.D. Detection of functional receptors for the proteinase-activated receptor-2-activating polypeptide, SLIGRL-NH2, in rat vascular and gastric smooth muscle. Can. J. Physiol. Pharmacol. 1995;73:1203–1207. doi: 10.1139/y95-172. [DOI] [PubMed] [Google Scholar]
- ALTIERI D.C. Xa receptor EPR-1. FASEB J. 1995;9:860–865. doi: 10.1096/fasebj.9.10.7615156. [DOI] [PubMed] [Google Scholar]
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- BONE R.C. Modulators of coagulation. A critical appraisal of their role in sepsis. Arch. Intern. Med. 1992;152:1381–1389. doi: 10.1001/archinte.152.7.1381. [DOI] [PubMed] [Google Scholar]
- BONO F., LAMARCHE I., HERBERT J.M. Induction of vascular smooth muscle cell growth by selective activation of the proteinase activated receptor-2 (PAR-2) Biochem. Biophys. Res. Commun. 1997;241:762–764. doi: 10.1006/bbrc.1997.7847. [DOI] [PubMed] [Google Scholar]
- BONO F., SCHAEFFER P., HERAULT J.P., MICHAUX C., NESTOR A.L., GUILLEMONT J.C., HERBERT J.M. Factor Xa activates endothelial cells by a receptor cascade between EPR1 and PAR2. Arterioscler. Thromb. Vasc. Biol. 2000;20:E107–E112. doi: 10.1161/01.atv.20.11.e107. [DOI] [PubMed] [Google Scholar]
- CAMERER E., ROTTINGEN J.A., IVERSEN J.G., PRYDZ H. Coagulation factor VII and X induce Ca2+ oscillations in madine-darby canine kidney cells only when proteolitically active. J. Biol. Chem. 1996;271:29034–29042. doi: 10.1074/jbc.271.46.29034. [DOI] [PubMed] [Google Scholar]
- CAMERER E., HUANG W., COUGLIN S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J., ISHII M., WANG L., ISHII K., COUGHLIN S.R. Thrombin receptor activation. Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J. Biol. Chem. 1994;269:16041–16045. [PubMed] [Google Scholar]
- CHEUNG W., ANDRADE-GORDON P., DERIAN C.K., DAMIANO B.P. Receptor activating peptides distinguish thrombin receptor (PAR1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can. J. Physiol. Pharmacol. 1998;76:16–25. doi: 10.1139/cjpp-76-1-16. [DOI] [PubMed] [Google Scholar]
- CHINNI C., DE NIESE M.R., JENKINS A.L., PIKE R.N., BOTTOMLEY S.P., MACKIE E.J. Protease-activated receptor-2 mediates proliferative responses in skeletal myoblasts. J. Cell. Sci. 2000;24:4427–4433. doi: 10.1242/jcs.113.24.4427. [DOI] [PubMed] [Google Scholar]
- CICALA C., CIRINO G. Linkage between inflammation and coagulation: an update on the molecular basis of the cross talk. Life Sci. 1998;62:1817–1824. doi: 10.1016/s0024-3205(97)01167-3. [DOI] [PubMed] [Google Scholar]
- CICALA C., PINTO A., BUCCI M., SORRENTINO R., WALKER B., HARRIOT P., CRUCHLEY A., KAPAS S., HOWELLS G., CIRINO G. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circ. 1999;99:2590–2597. doi: 10.1161/01.cir.99.19.2590. [DOI] [PubMed] [Google Scholar]
- CICALA C., MORELLO S., SANTAGADA V., CALIENDO G., SORRENTINO L., CIRINO G. Pharmacological dissection of vascular effects caused by activation of protease-activated receptor 1 and 2 in anesthetized rats. FASEB J. 2001;15:1433–1435. doi: 10.1096/fj.00-0633fje. [DOI] [PubMed] [Google Scholar]
- CIRINO G., NAPOLI C., BUCCI M., CICALA C. Inflammation-coagulation network: are serine protease receptors the knot. Trends Pharmacol. Sci. 2000;21:170–172. doi: 10.1016/s0165-6147(00)01469-3. [DOI] [PubMed] [Google Scholar]
- DAMIANO B.P., D'ANDREA M.R., DE GARAVILLA L., CHEUNG W.M., ANDRADE-GORDON P. Increased expression of protease activated receptor-2 (PAR-2) in balloon-injured rat carotid artery. Thromb. Hemost. 1999a;81:808–814. [PubMed] [Google Scholar]
- DAMIANO B.P., CHEUNG W.M., SANTULLI R.J., FUNG-LEUNG W.P., NGO K., YE R.D., DARROW A.L., DERIAN C.K., DE GARAVILLA L., ANDRADE-GORDON P. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J. Pharmacol. Exp. Ther. 1999b;288:671–678. [PubMed] [Google Scholar]
- D'ANDREA M.R., DERIAN C.K., LETURCQ D., BAKER S.M., BRUNMARK A., LING P., DARROW A.L., SANTULLI R.J., BRASS L.F., ANDRADE GORDON P. Characterization of protease activated receptor-2 immunoreactivity in normal human tissues. J. Hist. Cytoch. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- DJELLAS Y., ANTONAKIS K., LE BRETON G.C. A molecular mechanism for signaling between seven transmembrane receptors: evidence for a redistribution of G proteins. Proc. Natl. Acad. Sci. U.S.A. 1998;95:10944–10948. doi: 10.1073/pnas.95.18.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signalling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- EMILSSON K., WAHLESTEDT C., SUN M., NYSTEDT S., OWMAN C., SUNDELIN J. Vascular effects of proteinase activated receptor 2 agonist peptide. J. Vasc. Res. 1997;34:267–272. doi: 10.1159/000159233. [DOI] [PubMed] [Google Scholar]
- FOX M.T., HARRIOTT P., WALKER B., STONE S.R. Identification of potential activators of proteinase activated receptor-2. FEBS Lett. 1997;417:267–269. doi: 10.1016/s0014-5793(97)01298-2. [DOI] [PubMed] [Google Scholar]
- GASIC G.P., ARENAS C.P., GASIC T.B., GASIC G.J. Coagulation factor X, Xa, and protein S as potent mitogens of cultured aortic smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2317–2320. doi: 10.1073/pnas.89.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON J.R., COCKS T.M. Heterogenous mechanisms of endothelium-dependent relaxation for thrombin and peptide activators of protease-activated receptor-1 in porcine isolated coronary artery. Br. J. Pharmacol. 2000;130:181–188. doi: 10.1038/sj.bjp.0703146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON J.R., FRAUMAN A.G., COCKS T.M. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ. Res. 2001;89:92–98. doi: 10.1161/hh1301.092661. [DOI] [PubMed] [Google Scholar]
- HERBERT J., BONO F., HERAULT J., AVRIL C., DOL F., MARES A., SCHAEFFER P. Effector protease receptor 1 mediates the mitogenic activity of factor Xa for vascular smooth muscle cells in vitro and in vivo. J. Clin. Invest. 1998;101:993–1000. doi: 10.1172/JCI1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFFEDINE M., AL-ANI B. Proteinase activated receptor-2 in rat aorta: structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- HOLLENBERG M.D. Protease-activated receptors: PAR4 and counting: how long is the course. Trends Pharmacol. Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- HWA J.J., GHIBAUDI L., WILLIAMS P., CHINTALA M., ZHANG R., CHATTERJEE M., SYBERTZ E. Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Cir. Res. 1996;78:581–588. doi: 10.1161/01.res.78.4.581. [DOI] [PubMed] [Google Scholar]
- JEZIORSKA M., MCCOLLUM C., WOODLEY D.E. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J. Pathol. 1997;182:115–155. doi: 10.1002/(SICI)1096-9896(199705)182:1<115::AID-PATH806>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NAKAYA Y., KAWAY K., NISHIKAWA H., KAWAO N. Factor Xa-evoked relaxation in rat aorta: involvement of PAR2. Biochem. Biophys. Res. Commun. 2001;282:432–435. doi: 10.1006/bbrc.2001.4597. [DOI] [PubMed] [Google Scholar]
- KO F.N., YANG Y.C., HUANG S.C., OU J.T. Coagulation factor Xa stimulates platelet-derived growth factor release and mitogenesis in cultured vascular smooth muscle cells of rat. J. Clin. Invest. 1996;98:1493–1501. doi: 10.1172/JCI118938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMURO T., MIWA S., OKAMOTO Y., ENOKI T., NINOMIYA H., ZHANG X.F., UEMURA Y., KIKUCHI H., MASAKI T. The involvement of a novel mechanism distinct from the thrombin receptor in the vasocontraction induced by trypsin. Br. J. Pharmacol. 1997;120:851–856. doi: 10.1038/sj.bjp.0701003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGAZINE H.I., KING J.M., SRIVASTAVA K.D. Protease activated receptors modulate aortic vascular tone. Int. J. Cardiov. 1996;53:S75–S80. doi: 10.1016/0167-5273(96)02569-7. [DOI] [PubMed] [Google Scholar]
- MIRZA H., YATSULA V., BAHOU W.F. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J. Clin. Invest. 1996;97:1705–1714. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOFFATT J.D., COCKS T.M. Endothelium-dependent and independent responses to protease-activated receptor-2 (PAR-2) activation in mouse isolated renal arteries. Br. J. Pharmacol. 1998;125:591–594. doi: 10.1038/sj.bjp.0702157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J., SCHECHTER N., WOOLKALIS M., BROASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- MOLINO M., RAGHUNATAH P.N., KUO A., AHUJA M., HOXIE J.A., BRASS L.F., BARNATHAN E.S. Differential expression of functional protease-activated-receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:825–832. doi: 10.1161/01.atv.18.5.825. [DOI] [PubMed] [Google Scholar]
- MURAMUTSU I., LANIYONU A.A., MOORE G.J., HOLLENBERG M.D. Vascular actions of thrombin receptor peptide. Can. J. Physiol. Pharmacol. 1992;70:996–1003. doi: 10.1139/y92-137. [DOI] [PubMed] [Google Scholar]
- NAPOLI C., CICALA C., WALLACE J.L., DE NIGRIS F., SANTAGADA V., CALIENDO G., FRANCONI F., IGNARRO L.J., CIRINO G. Protease-activated receptor-2 modulates myocardial ischemia-reperfusion injury in the rat heart. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3678–3683. doi: 10.1073/pnas.97.7.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., RAMAKRISHNAN V., SUNDELIN J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. 1996;21:14010–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J.P., PREVOS N., MOLINO M., HOLLINGER M.K., WOOLKALIS M.J., WOULFE D.S., BRASS L.F. Thrombin responses in human endothelial cells. J. Biol. Chem. 2000;275:13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- OHNISHI H., SUZUKI K., NIHO T., ITO C., YAMAGUCHI K. Protective effects of urinary trypsin inhibitor in experimental shock. Jpn. J. Pharmacol. 1985;39:137–144. doi: 10.1254/jjp.39.137. [DOI] [PubMed] [Google Scholar]
- OKANO S., TAGAWA M., URAKAWA N., OGAWA R. A therapeutic effect of ulinastatin on endotoxin-induced shock in dogs-comparison with methylprednisolone. J. Vet. Med. Sci. 1994;56:645–649. doi: 10.1292/jvms.56.645. [DOI] [PubMed] [Google Scholar]
- PAPAPETROPOULOS A., PICCARDONI P., CIRINO G., BUCCI M., SORRENTINO R., CICALA C., JOHNSON K., ZACHARIOU V., SESSA W.C., ALTIERI D.C. Hypotension and inflammatory cytokine gene expression triggered by factor Xa-nitric oxide signaling. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4738–4742. doi: 10.1073/pnas.95.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIEWALD M., RUF W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc. Natl. Acad. Sci. U.S.A. 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY S., SAIFFEDINE M., LOUTZENISHER R., TRIGGLE C.R., HOLLENBERG M.D. Dual endothelium-dependent vascular activities of proteinase-activated receptor-2-activating peptides: evidence for receptor heterogeneity. Br. J. Pharmacol. 1998;123:1434–1440. doi: 10.1038/sj.bjp.0701726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFFEDINE M., AL-ANI B., CHENG C.H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFFEDINE M., ROY S.S., AL-ANI B., TRIGGLE C.R., HOLLENBERG M.D. Endothelium-dependent contractile actions of proteinase-activated receptor-2 activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. Br. J. Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOBEY C.G., COCKS T.M. Activation of protease activated receptor-2 (PAR-2) elicits nitric oxide-dependent dilatation of the basilar artery in vivo. Stroke. 1998;29:1439–1444. doi: 10.1161/01.str.29.7.1439. [DOI] [PubMed] [Google Scholar]
- SOBEY C.G., MOFFATT J.D., COCKS T.M. Evidence for selective effects of chronic hypertension on cerebral artery vasodilatation to protease activated receptor-2 activation. Stroke. 1999;30:1933–1941. doi: 10.1161/01.str.30.9.1933. [DOI] [PubMed] [Google Scholar]
- VU T.K.H., HUNG D.T., WHEATON V.I., COUGLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]


