It had been known for some time that tumour masses which had become contaminated by a bacterial infection would on occasion regress and disappear. It was thought that the bacteria were releasing a factor which would make the tumour necrotic and whither. This factor was termed tumour necrosis factor (TNF) (Old, 1985). It was not until more recent advances in immunology that it became clear that antigens from the bacterial invader (notably lipopolysaccharide LPS) were causing the release of the patient's own TNF which could cause the tumour regression. The hunt was on to isolate this TNF which could be used as a magic therapy to control cancer cell growth and persistence, plus enhance the academic understanding of the ways by which a cell could die. It was discovered that TNF and lymphotoxin (LT) were products from macrophages and lymphocytes that were capable of lysing many cell types including some tumour cells (Carswell et al., 1975; Granger et al., 1969). TNF was also found to be identical to the protein cachectin, which was known to be involved in the fever and muscle wastage seen in cancer patients (Beutler et al., 1985). Hence, the role TNF played in a range of physiological actions was important and the hunt was on to identify TNF and related molecules such as LT. Modern techniques have since allowed the isolation, characterization and cloning of the genes for TNF which is structurally related to LT plus an expanding family of TNF-like ligands (Table 1). These cytokine molecules include ligands such as Fas, CD40 and RANK which cause wide-ranging long-term cellular activities in cells such as differentiation, proliferation or death. Evolution has created this TNF superfamily of cytokines to control and manipulate the immune system, modulating processes such as haematopoiesis, antibody production, or short- and long-term immunity. It is only through its quirky tumour-killing characteristic that TNF cytokine was first identified and may still hold the key to effective tumour therapy. As the majority of information has been gained about TNF and it is the archetypal cytokine of the superfamily, displaying the greatest range of cellular actions, this review will focus on the molecular aspects and biological role of TNF signalling.
Table 1.
TNF ligand superfamily
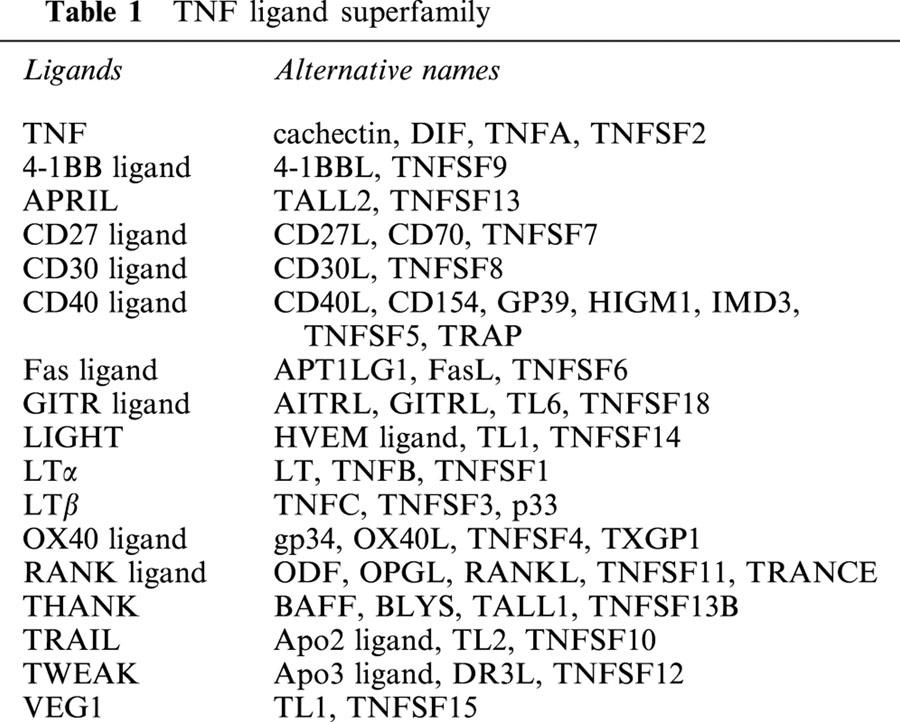
TNF ligand
Biochemically isolated in 1984, TNF has since been found to be a pleiotropic agent produced mostly by activated macrophages and monocytes, but also by many other cell types including B lymphocytes, T lymphocytes and fibroblasts. It is expressed as a 26 kDa transmembrane protein that can be cleaved by the metalloprotease TNF-α-converting enzyme (TACE) to release a 17 kDa soluble TNF form (Idriss & Naismith, 2000). TNF has also referred to as TNFα, cachectin or differentiation inducing factor (DIF) (Aggarwal, 2000). Its superfamily of nearly 20 different homologues including TNF-β (lymphotoxin-α), lymphotoxin-β, and other more specific ligands such as RANKL and TRAIL which are involved more particularly in bone tissue and lymphocyte processes. All the TNF receptor superfamily of ligands are thought form non-covalently-bound homo-trimers which are capable of becoming a secreted form (Figure 1). These TNF ligand are primarily designed for cell – cell contact transfer of signalling information between neighbouring cells, but cleavage into their soluble forms may allow more dispersed cytokine effects. TNF as well as causing necrotic cell death may also cause apoptotic cell death, cellular proliferation, differentiation, inflammation, tumourigenesis, and viral replication (Figure 2). TNF's primary role is in the regulation of immune cells, but is known to be heavily involved in pathogenic disorders such as rheumatoid arthritis, asthma, septic shock, irritable bowel disorder, haemorrhagic fever and cachexia (Locksley et al., 2001).
Figure 1.
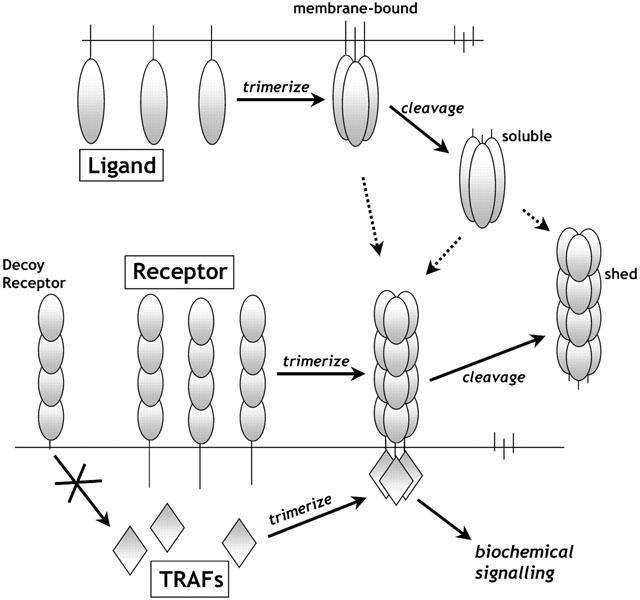
TNF superfamily ligand-receptor interactions.
Figure 2.
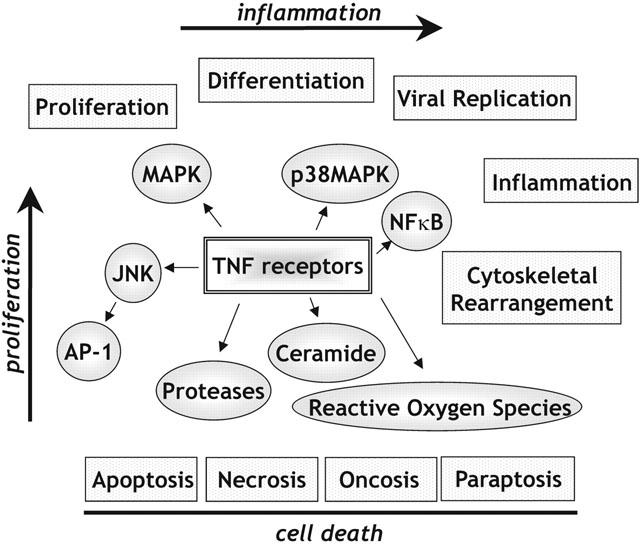
TNF receptor-mediated cellular responses.
TNF receptors
Just as modern molecular techniques have identified a superfamily of TNF-like cytokine ligands, their receptor molecules consist of a superfamily of proteins that can be activated by one or more ligands (Table 2). TNF receptors are an family of proteins that consist of, to date, at least 27 members characterized by their repeated cysteine-rich extracellular sequence homology, and include LT receptor, Fas, CD40, the low affinity nerve growth factor receptor, TRAIL receptors, RANK and death or decoy receptors (Darnay & Aggarwal, 1999). Many of these members go by multiple names and are activated by specific ligands, however TNF only has the ability to bind two of these receptors which are also activated by LTα.
Table 2.
TNF receptor superfamily
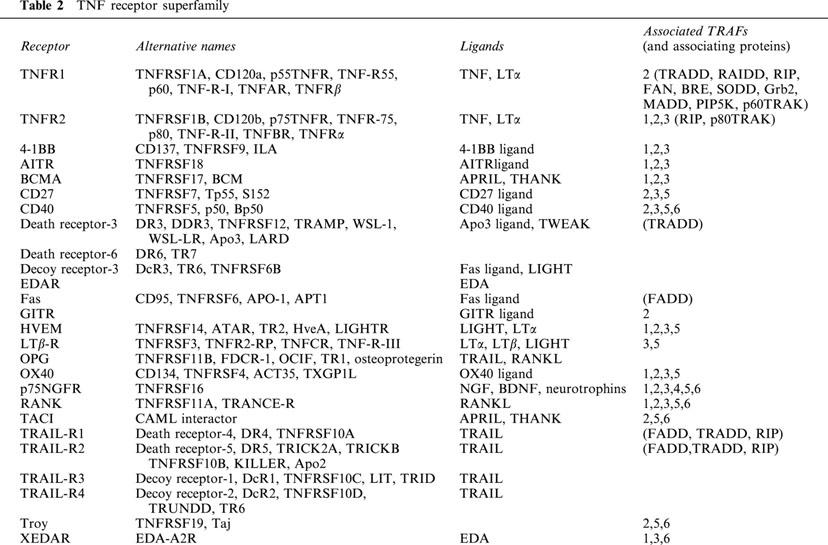
TNF ligand achieves all its different cellular and pathological effects by its binding to either the TNFR1 or TNFR2 receptor subtype. They are single transmembrane glycoproteins with 28% homology mostly in their extracellular domain with both containing four tandemly repeated cysteine rich motifs. Their intracellular sequences are largely unrelated with almost no homology between each other, and early work suggested delineation of their signalling functions (Grell et al., 1994). They contain several motifs with known functional significance. Both TNFR1 and TNFR2 contain an extracellular pre-ligand-binding assembly domain (PLAD) domain (distinct from ligand binding regions) that pre-complexes receptors and encourages them to trimerize particularly upon activation by TNF ligand (Chan et al., 2000). TNFR1 contains a death domain (DD) motif of approximately 80 amino acids in length towards the carboxyl-end of the receptor and is critical in the death-inducing activity of TNFR1 (Tartaglia et al., 1993a). The death domain is present on a number of associating proteins and related molecules that are primarily involved in signalling for cell death. One such molecule is the silencer of death domain protein SODD, which binds to the DD in TNFR1 and prevents other DD-interacting proteins from taking hold (Jiang et al., 1999). SODD dissociates from the TNFR1 DD upon TNF stimulation allowing other activator proteins to access the DD receptor module. TNFR1 also contains an intracellular sequence that is known to bind the adaptor protein FAN, a key stimulatory element in the activation of neutral sphingomyelinase (Adamklages et al., 1998a; Adam et al., 1996) which catalyses the degradation of sphingolipids into smaller ceramide-containing molecules which are key signalling intermediates (Kolesnick & Kronke, 1998). Moreover, a stress responsive 44 kDa protein expressed highly in brain and reproductive organs, BRE, specifically interacts with the juxtamembrane region of TNFR1 (Gu et al., 1998). BRE, like SODD, acts to inhibit and dampen TNFR1-stimulated signalling.
TNFR1
More information is known about TNFR1 than TNFR2. This is due to a number of factors including differences in affinity and efficacy of its ligands. The affinity of TNF ligand for TNFR1 receptor varies depending on the study from approximately 100 pM on native receptor (Tsujimoto et al., 1985; Kull et al., 1985; Vanostade et al., 1993), to estimations on cloned receptors from 100 – 600 pM (Schall et al., 1990; Hohmann et al., 1990; Pennica et al., 1992a; Loetscher et al., 1993; Grell et al., 1993; Moosmayer et al., 1994). Another study on native receptor indicated that soluble TNF, as is used experimentally, rapidly binds to TNFR1 with high affinity (Kd of 19 pM) and a slow dissociation from the receptor once bound (t1/2=33 min), a process which efficiently activates the receptor (Grell et al., 1998b). Such kinetics of ligand association are different from TNFR2 association (see below). Stimulation of TNFR1 leads to its internalization with inhibition of its long-term actions (Higuchi & Aggarwal, 1994) (Figure 3). Phosphorylation of TNFR1 occurs at a consensus MAPK site on its cytoplasmic domain or through tyrosine phosphorylation (Darnay & Aggarwal, 1997; Cottin et al., 1999), although it is not fully understood how these phosphorylations control receptor processing. TNFR1 is expressed on the cell surface but large amounts are found localized at the perinuclear-Golgi complex, as is TRADD, but which only associates with TNFR1 once at the plasmamembrane (Jones et al., 1999; Ledgerwood et al., 1999).
Figure 3.
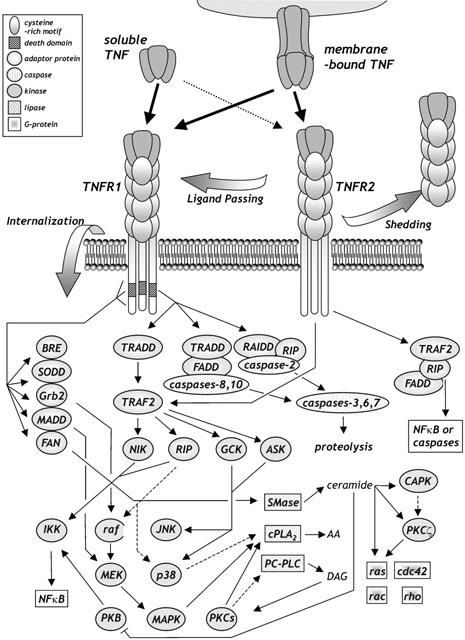
Major signalling pathways modulated by TNF receptor subtypes.
TNFR2
TNFR2 does not contain a DD motif but still recruits adaptor proteins including TRAF2. TNFR2 is thought to be able to signal apoptosis directly (Heller et al., 1992; Declercq et al., 1995) or through a so-called ‘ligand-passing' mechanism by which TNFR2's greater affinity and half-life of TNF binding, holds ligand, increases the local TNF concentration in the vicinity of TNFR1 receptors which accept TNF ligand from TNFR2 and are themselves activated, signalling the TNFR1 apoptotic machinery (Tartaglia et al., 1993b). Others believe that additionally, TNFR2 signals for cell death through its cytoplasmic domain to induce mTNF expression, which then signals apoptosis via TNFR1 (Vercammen et al., 1995; Lazdins et al., 1997; Haas et al., 1999; Grell et al., 1999; Weiss et al., 1998). The kinetics of TNFR2 activation by TNF are different from TNFR1 (Vandenabeele et al., 1995). The dissociation kinetics of TNF from native TNFR2 is approximately 20 – 30 fold faster than from TNFR1 (Grell et al., 1998b), with workers finding the affinity of TNF for TNFR2 significantly greater (Tartaglia et al., 1993b) or less (Grell et al., 1998b) than the ligand's affinity for TNFR1. It is not clear how the binding characteristics of membrane-bound TNF at TNFR1 and TNFR2 compare to soluble TNF. Slight structural changes in the TNF sequence can lead to dramatic changes in its binding characteristics to TNF receptors. For example, murine TNF activates mouse TNFR1 and TNFR2 equally well, whereas human TNF acts on mouse TNFR1 but does not bind mouse TNFR2 (Lewis et al., 1991). Such observations led to studies in which mutant proteins (‘muteins') of soluble TNF were developed that displayed reduced affinity towards TNFRs compared to wild-type TNF, however these muteins showed marked selectivity between TNFR1 and TNFR2 (Table 3) helping to uncover the role of each TNFR (Loetscher et al., 1993; Vanostade et al., 1993).
Table 3.
Pharmacological modulation of TNF responsive signalling pathways

TNFR2 was fully cloned after TNFR1 and its structural and functional characterization is less well understood. The main reason for the relative lack of signalling information about TNFR2 is that, generally, it is not efficiently activated in vitro. It was assumed that recombinant 17 kDa soluble TNF (as is provided commercially) was an efficient activator of TNFR2. However, it was uncovered that the membrane-bound 26 kDa form of TNF (mTNF) was greater than soluble TNF is activating TNFR2 (Grell et al., 1995) leading to qualitatively different responses and new insight into TNFR2 function (Decoster et al., 1995). TNFR1 is activated equally well by soluble and mTNF. TNF ligand acts in the immune system whereby it would activate TNFRs through cell – cell interactions. As such, most of the TNF effects in vivo may be mediated by mTNF (TNFR1=TNFR2 activation) rather than soluble TNF (TNFR1>TNFR2 activation). As such, the physiological role of TNFR2 may be underestimated by most TNF research conducted in the laboratory which uses soluble TNF as the stimuli. Soluble TNF acts similar to a partial agonist on TNFR2 in that it binds to the receptor, but is not highly efficacious and efficient in its activation. Such limitations of stimulation are overcome by the use of agonistic antibodies capable of efficiently stimulating TNFRs (Grell et al., 1993; Tartaglia et al., 1991; Leeuwenberg et al., 1995; Paleolog et al., 1994; Wajant et al., 2000; Borset et al., 1996; Haridas et al., 1998; Jupp et al., 2001), although how these functionally relate to natural forms of TNF can only be assumed.
TNFR signalling mechanisms: TRAFs and other adaptor proteins
Both TNFR1 and TNFR2 possess sequences that are capable of binding intracellular adaptor proteins that link TNF receptor stimulation to activation of many signalling processes. These TNF receptor-associating factors (TRAFs) and adaptors are what transduce the TNF signal from the biochemically inert receptors to dramatic changes of the signalling molecules within target cells (Wajant et al., 2001). TRAF molecules all contain a RING finger and zinc finger motifs in their N-terminal, with their C-terminal regions possessing a TRAF domain sequence. To date six mammalian TRAF proteins have been identified. The first TRAFs to be uncovered, TRAF1 and TRAF2, were discovered by their ability to directly interact with the cytoplasmic domain of TNFR2 (Rothe et al., 1994). Work by the same group also identified the apoptotic adaptor proteins c-IAPI and c-IAP2 that bind to TNF receptor via a TRAF1/TRAF2 heterocomplex (Rothe et al., 1995a). Since then, it is now thought that mostly TRAF2 interacts with TNFR2 directly, with TRAF1 interacting indirectly and TRAF3 also able to associate (Table 2). TRAF2 is recruited to TNFR1 indirectly through a specific interaction with the protein TNF receptor-associated death domain (TRADD), a 34 kDa cytosolic adaptor protein that directly binds to TNFR1 through its own death domain sequence (Hsu et al., 1995). TRADD recruits the downstream signalling adaptor molecules FADD (Fas-associated death domain) and RIP (receptor interacting protein). RIP originally identified as a Fas-associating molecule (Stanger et al., 1995) also interacts with TNF receptors (Hsu et al., 1996a; Liu et al., 1996). RIP contains a kinase sequence, but its role as a kinase enzyme is unclear at present. FADD contains a death effector domain (DED) sequence (Chinnaiyan et al., 1995), that interacts with the DED domain in caspase-8 (also known as FLICE or MACH) and a number of other DED-containing molecules that regulate cell death mechanisms (Muzio et al., 1996). Another DD-containing molecule RAIDD is recruited to TNFR1 and interacts with RIP and caspase-2, to allow its activation. RIP and FADD are also thought capable under certain conditions to be able to indirectly bind to TNFR2 via TRAF2 (Pimentel-Muinos & Seed, 1999).
TRAF2 is also capable of interacting with downstream signalling molecules such as NF-κB-inducing kinase (NIK) which is a member of the serine/threonine mitogen-activated protein kinase (MAPK) kinase (MEK) kinase (MEKK) family (Liu et al., 1996; Malinin et al., 1997). NIK phosphorylates its target at serine 176, an enzyme named inhibitor of κB (IκB) kinase (IKK) which is implicated in the activation of NF-κB transcription factor involved in transcriptional responses to stress and anti-apoptotic cellular action (Ling et al., 1998). NIK is also capable of interacting with TRAFs 1, 3, 5 and 6 (Wajant et al., 2001). One of the genes under the transcriptional control of NF-κB is the cellular inhibitor of apoptosis protein 2 (c-IAP2) which binds to TRAF2 and is capable of blocking caspase-8 activation and apoptosis. Similarly, A20 is an 80 kDa inducible protein that binds TRAF2 and is anti-apoptotic (Song et al., 1996). RIP and NIK are not the only kinases to interact with TRAF2, apoptosis-stimulating kinase (ASK1) (Nishitoh et al., 1998), germinal centre kinase (GCK) (Yuasa et al., 1998; Shi & Kehrl, 1997) and MEKK1 (Baud et al., 1999), have all been implicated in the activation of p38MAPK and c-Jun N-terminal kinase (JNK) stress kinases, as well as AP-1 and NF-κB transcriptional activation processes (Figure 3).
The activation of the MAPK family of kinase enzymes can be achieved by TNF receptor interaction with the factor associated with neutral sphingomyelinase activation (FAN) adaptor protein (Adam et al., 1996). FAN is responsible for neutral sphingomyelinase-mediated generation of ceramide-containing sphingolipids (Adamklages et al., 1998a) which are capable of activating ceramide-activated protein kinases (CAPK) (Mathias et al., 1991; Dressler et al., 1992) (also known as kinase suppresor of ras (Zhang et al., 1997) which activates Raf kinase (Yao et al., 1995), an upstream activator of the MEK and MAPK serine/threonine kinase family (Mathias et al., 1998). Interestingly, TNF receptors have been found to interact with the Grb2 adaptor and son of sevenless (SOS) exchange factor (Hildt & Oess, 1999; Adam et al., 1996). Activated Grb2 binds through its SH3 domain to a motif within the TNF receptor, allowing this activated complex to stimulate c-Raf-1 kinase. Another recently identified protein that interacts with FADD through a DED domain is the FLICE-like inhibitory protein (FLIP (Irmler et al., 1997; Thome et al., 1997) which blocks caspase-8 recruitment and activation. FLIP interacts with TRAFs and RIP to switch signalling pathways through more anti-apoptotic pathways such as NF-κB and Raf-1 kinase, resulting in marked MAPK activation (Kataoka et al., 2000). The death domain within TNFR1 is also able to bind MADD adaptor protein, which contains a DD sequence. MADD activates MAPK when overexpressed in cultured mammalian cells (Schievella et al., 1997; Kataoka et al., 2000; Brinkman et al., 1999). A 59 kDa TNFR2-associated serine/threonine kinase p80TRAK has been described that is TNF-stimulated (Darnay et al., 1994). p80TRAK binds to a 44 amino acid site in TNFR2 cytoplasmic domain in a similar fashion to casein kinase (Darnay et al., 1997).
Lipases
Some of the earliest TNF signalling research revealed the activation of several lipase activities (Dayer et al., 1985; Marquet et al., 1987; Beyaert et al., 1987; Clark et al., 1987; Godfrey et al., 1987). TNF receptors activated phosphatidylcholine-specific phospholipase C (PC – PLC) (Schutze et al., 1991; Wiegmann et al., 1992). PC – PLC stimulation by TNFR1 activates a downstream acidic membrane-bound sphingomyelinase activity. Stimulation of PC – PLC activity degrades phosphatidylcholine into choline (a molecule with no known signalling function) and diacylglycerol, the physiological activator of most protein kinase C (PKC) isoforms. Other reports have suggested TNF ligand stimulates phospholipase D (PLD) creating phosphatidic acid (Devalck et al., 1998; Kang et al., 1998), that may be converted to DAG by phosphatidic acid phosphohydrolase. Others have also implied TNF-stimulated phosphatidylinositol-specific phospholipase C (PI – PLC, generating inositol-1,4,5-trisphosphate and diacylglycerol second messengers) activity (Beyaert et al., 1993).
Much interest has centred on the ability of TNF to stimulate sphingomyelinase of which there are two types, neutral and acidic (Kolesnick & Kronke, 1998). These sphingomyelinases stimulate the breakdown of membrane sphingolipids into ceramide which may then be converted into other ceramide-containing lipids such as sphingosine and sphingosine-1-phosphate (S-1-P) (Hannun, 1994). TNF was the first stimuli found to induce ceramide generation (Okazaki et al., 1989; Mathias et al., 1991; Kim et al., 1991; Dressler et al., 1992). TNFR1 is responsible for the stimulation of membrane-associated neutral sphingomyelinase (Wiegmann et al., 1992, 1994), and via FAN adaptor protein, the stimulation of neutral sphingomyelinase (Adam et al., 1996). Exogenous ceramide is a highly specific yet destructive chemical in most cells with rapid stimulation of apoptotic cell death processes. These apoptosis-signalling processes include the sequential activation of Bad (Basu et al., 1998), then activation of CAPK which stimulates raf kinase (Yao et al., 1995) and MAPK activity (Raines et al., 1993; Bird et al., 1994). TNF-induced ceramide production may also stimulates the stress-activated kinase JNK (Coroneos et al., 1996). Sphingolipids are able to modulate PKC isoforms. For example, human leukaemia cells treated with ceramide or TNF caused the rapid translocation to the cytosol of PKC-δ and PKC-ε (Sawai et al., 1997). Similarly, in L929 rat fibroblasts, ceramide and TNF blocked PKC-α activity and translocation to the plasmamembrane (Lee et al., 2000). TNF-stimulated ceramide generation and apoptosis in HL-60 leukaemia cells was found to be crucially dependent on PKC-β (Laouar et al., 1999), while ceramide has also been found to bind directly to the atypical isoform PKC-ζ which can activate ras, raf, MEK, MAPK and NF-κB transcription (Diazmeco et al., 1994); Lozano et al., 1994; Berra et al., 1995; Conway et al., 2000). TNF-stimulated sphingolipids are also capable of being converted into sphingosine-1-phosphate (S-1-P) which has intracellular actions including raising [Ca2+]i from calcium stores and stimulating MAPK activity (Pyne & Pyne, 2000). As well as its intracellular actions, S-1-P is able to diffuse from the cell and act in an autocrine manner by stimulating endothelial differentiation gene (EDG) cell surface receptors (EDG1R – EDG8R), a family of heterotrimeric G-proteins receptors (Pyne & Pyne, 2000).
Another lipase strongly stimulated by TNF receptors is the 110 kDa hormone-sensitive, Ca2+-dependent cytosolic phospholipase A2 (cPLA2) (Clark et al., 1991). cPLA2 is responsible for the liberation of arachidonic acid from the sn-2 position of mainly phosphatidylcholine. The arachidonic acid again acts in an autocrine fashion and within its own cell of generation, to be converted into prostaglandins and leukotrienes to stimulate eicosanoid-sensing receptors. Moreover, these eicosanoids are responsible for the generation of oxygen radical-containing lipids and reactive oxygen species (ROS) that disrupt mitochondrial integrity and set in motion cell death mechanisms indicated above (Chang et al., 1992). Protective cellular enzymes such as superoxide dismutase counteract these disruptive ROS molecules (Wong et al., 1989; Wong & Goeddel, 1988). TNF receptor stimulation of MAPK and PKC isoforms leads to the phosphorylation and activation of cPLA2 at specific residues (Nemenoff et al., 1993; Lin & Chen, 1998) (most notably serine 505 in the case of MAPK (Lin et al., 1993) resulting in a rapid liberation of arachidonic acid. Cytokine receptor activation of cPLA2 through p38MAPK has also been implicated (Kramer et al., 1996) at serine residue 727, which leads to activation of the lipase (Borschhaubold et al., 1997, 1998), but which may be a route of cPLA2 phosphorylation and activation restricted to platelets. A secondary mechanism of TNF-stimulated cPLA2 gene induction also occurs, leading to the increased expression of cPLA2 protein and activity which is sensitive to inhibition by protein synthesis blockers and glucocorticoids (Hoeck et al., 1993). It has been shown in several cell types that cPLA2 protein and activity is crucial for TNF-mediated cell death (Hayakawa et al., 1993; Wu et al., 1998a, 1998b; Devalck et al., 1998; Suffys et al., 1991; Jayadev et al., 1997). The mechanism of cPLA2 stimulation by TNF is thought to be TNFR1-specific as only this receptor has shown the ability to activate the kinases involved in its phosphorylation (Boone et al., 1998; Jupp et al., 2001; McFarlane et al., 2001) with TNFR2 having a role in the regulation of cPLA2 expression (MacEwan, 1996). Additionally, activated cPLA2 has been shown to be the cleaved by caspases but the relative activity of the cleaved cPLA2 fragments is not clear as this aspect of the reports are contradictory (Luschen et al., 1998; Adamklages et al., 1998b; Atsumi et al., 1998; Wissing et al., 1997; Voelkeljohnson et al., 1995).
Kinases and phosphatases
As mentioned above, through activation of PC – PLC, TNF receptors are capable of diacylglycerol generation and subsequent PKC activation (Bermudez & Young, 1987; Schutze et al., 1990). There exist at least 12 forms of PKC with differential activation characteristics and distinct tissue distribution, and some of these isoforms have been shown to be TNFR-stimulated. For example, PKC-α activation by TNF can occur in L929 fibroblasts, through an indirect activation process involving ceramide production (Lee et al., 2000). A variant of the HL60 human myeloid leukaemia cell line, HL525, that are deficient in PKC-β are resistant to TNF-induced apoptosis which can be reinstated by re-establishing PKC-β protein expression (Laouar et al., 1999). PKC-δ is a substrate for TNF-stimulated caspase-dependent cleavage and has also been shown to cause the serine phosphorylation of TNFR1 protein itself (Kilpatrick et al., 2000). The atypical PKCs-ζ, -λ, and -ι have also been shown to be regulated by more complex TNF-stimulated mechanisms that may involve lipid intermediates (Muller et al., 1995; Sanz et al., 1999; Bonizzi et al., 1999) and as mentioned above, in particular PKC-ζ directly bind TNF-generated ceramide to cause the subsequent activation of ras, raf, MEK, MAPK and NF-κB.
Much work has been concerned with the activation by TNF receptors of the extracellular signal-regulated protein kinase (ERK) superfamily of kinases, responsible for much of a cells reaction to a variety of mitogenic stimuli and stress responses (Paul et al., 1997). These enzymes are characterized by their activation process of dual phosphorylation on threonine and tyrosine residues in their sequences, with the motif Thr-X-Tyr, where X can be Glu for MAPK members, Gly for p38MAPK or Pro for the c-Jun N-terminal kinase (JNK) family of stress-responsive kinases (Fiers et al., 1996). All three of these ERK families are readily stimulated by TNF. Upstream kinases (MEKs) control their activation, which are themselves under the control of MEK kinases (MEKKs). They have a range of known substrates activated in an ERK family-selective manner, including transcription factors and downstream kinases (Davis, 1999). The first members of the ERK family to be identified, p42 and p44MAPK, are transiently activated by TNF through MEK-1 and MEK-2 phosphorylation (Vanlint et al., 1992; Minden et al., 1994) and one report in fibroblasts from TNFR-deficient mice indicate both receptor subtypes are capable of MAPK stimulation (Kalb et al., 1996) but more recent work has shown MAPK activation occurs through the TNFR1 receptor only (Jupp et al., 2001). Several pharmacological inhibitors of these MEKs such as PD98059 and U0126 have helped reveal a role for p42 and p44MAPK activation in TNF modulation of cell death (Chang, 2000; Rao, 2001; Cuvillier et al., 1996).
P38MAPK and JNK kinases are termed stress kinases as they are transiently activated by a range of stress stimuli including cytokines, heat- or osmotic-shock and UV irradiation. Upstream kinases responsible for p38MAPK activation include MEK-3 and MEK-6 (Enslen et al., 1998). The pharmacological tools SB203580 and SE239063 are relatively specific inhibitors of p38MAPK and have shown the role of p38MAPK in many TNF-induced cellular responses (Barone et al., 2001). One of the first reports to implicate p38MAPK in TNF signalling conclusively showed the role of the kinase in TNF-stimulated phosphorylation of heat-shock protein hsp27 and induction of NF-κB activity and interleukin-6 (Beyaert et al., 1996). Indeed, many of the actions of p38MAPK are considered proinflammatory although the role of p38MAPK in cell death and survival is not absolutely clear (Xia et al., 1995; Eliopoulos et al., 1999; Cross et al., 2000). The JNK family of ERKs are a highly active arm of TNF receptor signalling mechanisms. JNK is activated mainly by MEK-4 (SEK-1), but also by MEK-7 (Davis, 1999). Both TNFR1 and TNFR2 are proficient activators of JNK (Weiss et al., 1998); Haridas et al., 1998; Jupp et al., 2001) perhaps through different pathways, but partially involving TRAF2. Unfortunately, widely-available JNK pathway inhibitor exists but the use of tools such as CEP-1347, or a dominant-negative form of MEK-4 have revealed an important role for JNK in TNF-mediated cellular processes including a probable role in apoptosis (Bozyczko-Coyne et al., 2001; Xia et al., 1995; Liu et al., 1996; Verheij et al., 1996; Doman et al., 1999; Helms et al., 2001).
TNF induction of inflammatory mediators and processes are critical steps in many pathological disorders including rheumatoid arthritis These inflammatory processes under the control of several promoter gene sequences but particularly the stimulation of NF-κB transcription factor (Aggarwal, 2000). The activation of NF-κB is controlled by its association with IκBα, whose degradation is controlled by its phosphorylation on serine 176 by NIK (Ling et al., 1998) and which is activated by the TNF stimulus (Malinin et al., 1997). Several of the TNF receptor-associating molecules, including TRAF2 (Hsu et al., 1996b) and RIP (Stanger et al., 1995; Liu et al., 1996), have been implicated as upstream NF-κB activators. TRAF2 was the first of these entities identified to activate NF-κB, with dominant-negative forms of TRAF2 (but not TRAF1 or TRAF3) able to block TNF-induced NF-κB activity (Rothe et al., 1995b). TRAF2 associated with NIK and was suggested to be crucial to NF-κB activation, however embryonic fibroblasts from TRAF2-deficient knockout mice were still capable of TNF-induced NF-κB activation, but not JNK activation (Lee et al., 1997; Yeh et al., 1997). RIP-deficient mice on the other hand, were incapable of TNF-stimulated NF-κB activity, with JNK activation and apoptosis unaffected (Kelliher et al., 1998; Stanger et al., 1995). It appeared that RIP, but not TRAF2, was required for TNF-stimulated NF-κB activation, with TRAF2 needed for IKK recruitment and RIP being responsible for IKK activation (Devin et al., 2000). In a wide range of cell types, that both TNFR1 and TNFR2 are capable of NF-κB activation (Kruppa et al., 1992; Laegreid et al., 1994; Rothe et al., 1994; Weiss et al., 1997; Haridas et al., 1998; Amrani et al., 1999; McFarlane et al., 2001). Recently, RIP has thought to have a possible switching capability where it binds to both TNFRs and regulates their caspase or NF-κB-signalling (Kelliher et al., 1998; Pimentel-Muinos & Seed, 1999). The role of NIK in NF-κB activation processes has been brought into question, as unexpectedly normal activation of NF-κB by TNF was still present in NIK-deficient mice (Yin et al., 2001). Furthermore, a transactivation process of NF-κB stimulation has been identified whereby phosphorylation of IκB (or other signalling components of its activation machinery) results in its activation not necessarily with any characteristic proteolytic degradation (Nasuhara et al., 1999; Vandenberghe et al., 1998; Johannes et al., 1998). Thus, TNF-induced NF-κB activation processes probably occurs through multiple signalling pathways.
Protein kinase B (Akt) is a more recently discovered kinase that is recruited to the plasmamembrane by phosphatidylinositol-3,4,5-trisphophate (PtdIns(3,4,5)P3) generated by phosphoinositide 3-kinases (PI3Ks) (Vanhaesebroeck & Alessi, 2000). PKB activation is completed by its phosphorylation by 3′-phosphoinositide-dependent kinase 1 (PDK1). PKB modulates signalling molecules controlling insulin actions as well as components thought to be important in regulating cell survival responses, including Bad, caspase-9, IKK, raf and ERK activities (Scheid & Duronio, 1998; Vanhaesebroeck & Alessi, 2000). Destruction of PKB by caspases allows inhibition of its survival signal (Widmann et al., 1998), with inhibition of PKB activity also achieved by ceramide lipids allowing apoptosis to occur (Zhou et al., 1998; Schubert et al., 2000). It was uncovered that PKB plays a role in TNF-induced stimulation of anti-apoptotic NF-κB activity with the activation of NIK dependent on PKB which phosphorylates IKKα at threonine 23 (Ozes et al., 1999). In addition another recently discovered kinase phosphatidylinositol-4-phophate 5-kinase (PIP5K-IIβ) responsible for the generation of phosphatidylinositiol-4,5-bisphosphate (substrate for PI-PLC-mediated inositol-1,4,5-trisphosphate and diacylglycerol second messengers) was found to interact with and be stimulated by TNFR1, but not TNFR2 (Castellino et al., 1997).
As well as TNF stimulating the production of ceramide to stimulate CAPK (see above), ceramide also has a role in the activation of ceramide-activated protein phosphatase (CAPP) of the type 2A (Dobrowsky et al., 1993). CAPP was found to be important in TNF-induced c-myc down-regulation as well as the dephosphorylation of c-Jun in TNF-stimulated A431 human epithelial cells (Reyes et al., 1996). Other reports have also indicated the possible involvement of serine/threonine (Totpal et al., 1992; Barber et al., 1995) or tyrosine-specific (Guo et al., 2000; Mishra et al., 1994) phosphatases in the regulation of cellular TNF responses. In particular, tyrosine phosphatases may be involved in the regulation of NF-κB activation processes that are controlled by TNF stimulation (Singh & Aggarwal, 1995; Dhawan et al., 1997). Thus serine/threonine and tyrosine phosphorylations or dephosphorylations are an aspect of TNFR signalling mechanisms.
Caspases: the key to death?
Members of the TNFR superfamily have the ability to cause proliferation, differentiation, cell death, or be blockers of apoptosis (Inoue et al., 2000). TNFR1 and TNFR2 are unique in that they can cause either a proliferative response or a cytotoxic response dependent on the cell type or its configuration (Figure 2). What the precise molecular switch is which defines whether activating a TNFR will lead to proliferative or destructive outcome is a matter for intense investigation. Certainly, the activation of destructive protease cascades will play a crucial part in this switching by TNFRs.
Cysteine-aspartate-directed proteases (caspases) are a group of at least 14 different enzyme forms, which orchestrate induction of most types of apoptotic cell death. As mentioned above, caspase-8 is part of the TNFR1 death-inducing signalling complex (DISC) by virtue of its DED domain allowing its interaction with the DD-containing TRADD/FADD machinery (Locksley et al., 2001). Caspases-2, -8, -9 and -10 are known as apoptotic initiator caspases that lie upstream of executioner procaspases-3, -6 and -7 which exist in a latent form until activated by its initiator through a process of cleavage, oligomerisation and autoactivation (Denecker et al., 2001). Caspase-1 is the enzyme that processes the proteolytic maturation of pro-interleukin-1β, and together with caspase-11 these enzymes are predominantly responsible for cytokine processing. Less is known about the function of caspases-4, -5, -12, -13 and -14. Evidence suggests a crucial role of caspases-3, -8 and -10 in TNF-induced apoptotic mechanisms with some viral products, such as CrmA cowpox modifier protein, blocking specific caspase forms and showing their absolute importance in TNF-mediated apoptotic (Hsu et al., 1995; Tewari et al., 1995), but not necrotic (Vercammen et al., 1998a, 1998b) cell death. Much of the importance of caspase members in cell death has arisen from the use of cell-permeable pharmacological inhibitors of caspases, such as zVAD-FMK (Table 3) which have shown dramatic effects at blocking ligand-induced cell death (Kinloch et al., 1999). As with all pharmacological data, however, the claimed specificity of these agents does not match up to their actual inhibitions at the concentrations they are used at experimentally (Schotte et al., 1999), and as such, much of the precise role of each caspase family member remains uncertain. Transgenic mice deficient in caspases-1, -2, -3 and -9 showed TNF-induced apoptotic death that was largely unaffected, indicating multiple caspase pathways that may be commissioned by activated TNF receptors in each particular cell type (Kuida et al., 1995; 1996; 1998; Zheng et al., 1999). Much of the present thinking implicates only TNFR1 in its ability to activate caspase proteases (Figure 3), however, recent evidence investigating the variable ability of RIP adaptor protein (Holler et al., 2000) (itself a target for caspase-mediated degradation (Lin et al., 1999) to associate with TRAF2 and TNFR1 or TNFR2 suggests that cellular conditions may favour the switching of TNFR2 between anti-apoptotic NF-κB activation signalling and death induction through caspase mechanisms (Kelliher et al., 1998; Pimentel-Muinos & Seed, 1999).
Caspases initiate cell suicide by the controlled destruction of the cell's own repair mechanisms (Cohen, 1997). Mitochondria sense apoptotic signals via a family of Bcl-2 proteins that either inhibit (Bcl-2, Bcl-xL) or promote (Bax, Bad, Bak, Bik, Bid) apoptosis (Chao & Korsmeyer, 1998). Caspase-8-mediated cleavage of Bid produces a truncated form (tBid) that translocates from the cytosol to the mitochondria. This caspase-mediated Bid activation step leads to reduced mitochondrial membrane potential and the release of cytochrome c, which binds the adaptor protein Apaf-1, a 130 kDa protein that contains an N-terminal caspase recruitment domain (CARD). With dATP/ATP and cytochrome c acting as cofactors Apaf-1 self-oligomerizes and binds procaspase-9 to form the apoptosome complex, which activates the executioner caspases-3 and -7 then other caspases to mediate apoptotic proteolysis of key repair and housekeeping proteins (Screaton & Xu, 2000). For example, caspase-dependent proteolysis cleaves and activates PKC-δ, and inactivates polyadenosine ribosyl polymerase (PARP) repair enzyme as well as the proteolytic activation of caspase-activated DNAses (CADs) that destroy the genome by excising genes leading to the characteristic DNA fragmentation and laddering associated with apoptotic death (Rich et al., 2000). Recent work on the Ca2+-sensitive calpain group of proteases and cathepsins and granzymes has shown these destructive enzymes are brought into play during cell death (Squier & Cohen, 1996; Johnson, 2000; Utz & Anderson, 2000). They may be more important in forms of death associated with Ca2+ overload and excitotoxicity (Pornares et al., 1998a, 1998b), but their role in general TNF signalling has been implicated (Diaz & Bourguignon, 2000; Han et al., 1999).
A role for G-proteins in TNFR signalling?
Although not one of the seven transmembrane G-protein-coupled class of receptors, TNF receptors can influence heterotrimeric G-protein activities. Bacterial toxin experiments in osteoblasts, breast cancer cells and hepatocytes implicated pertussis toxin-sensitive G-proteins in the sensitivity of these cells to TNF treatment (Branellec et al., 1992; Yanaga et al., 1992; Hernandezmunoz et al., 1997). In neutrophils, the involvement of the Giα class of G-proteins has been implicated in TNF responses (McLeish et al., 1996). TNF priming of leukocytes also leads to regulation of G-protein activity and levels, particularly of the Gi2α class (Scherzer et al., 1997), whereas in airway smooth muscle cells, TNF treatment could lead to the regulation of Gi2α and Gi3α levels. TNF was recently found to selectively regulate the degradation of Gqα/11α class of G-protein in HeLa and L929 cell models of cytotoxicity (Pollock et al., 2000). Proteins that modulate G-protein function, such as GSP2 (G-protein pathway suppressor) and RGS16 (regulator of G-protein signalling), have also been found to be regulated by TNF activation (Fong et al., 2000; Jin et al., 1997).
Monomeric small G-proteins such as ras probably play a role in TNF signalling. For example, inhibition of ras activity by rap1 a tumour suppressor gene or rasN17 dominant-negative mutant, blocked TNF-induced apoptosis in fibroblasts (Trent et al., 1996). TNFR1 binds Grb2 adaptor protein which co-ordinates ras activation to stimulate the raf/MEK/MAPK signalling axis (Hildt & Oess, 1999). TNF-generated ceramides are capable of binding to and activating ras protein (Muller et al., 1998). Indeed, CAPK is a regulator of ras (Zhang et al., 1997) and is necessary for TNF stimulation of MAPK in intestinal epithelial cells (Yan & Polk, 2001). TNF has also recently been shown to stimulate cdc42 in fibroblasts (Puls et al., 1999) or Rac & cdc42 (Min & Pober, 1997) and rho (Petrache et al., 2001) monomeric G-protein pathway in endothelial cells. Such TNF-induced changes in endothelial cell cytoskeletal structures have also been reported by others to involve Rac, cdc42 and p21 rho monomeric G-protein (Wojciak-Stothard et al., 1998). In airway smooth muscle cells, TNF stimulates a rho-activation pathway that is involved in myosin light chain phosphorylation and contractile sensitivity (Hunter et al., 2001). Activation by TNF of transcription factors such as NF-κB and c-fos serum response element, have been shown to involve monomeric G-proteins such as rho, Rac and cdc42 (Perona et al., 1997; Kim et al., 1999). Thus, many aspects of TNFR signalling are probably significantly regulated by monomeric as well as heterotrimeric G-proteins.
A role for Ca2+ in TNFR signalling?
Inositol phosphates and Ca2+ were reported to by important in TNF-stimulated cellular responses (Beyaert et al., 1993; Denecker et al., 1997). TNF was shown to inhibit inositol phosphate action and cellular Ca2+ handling processes in a variety of cell types (Reithmann & Werdan, 1994; Yorek et al., 1999; Rosado et al., 2001), with a possible regulation of Ca2+-mobilizing InsP3 receptor subtypes by caspase-dependent processes (Diaz & Bourguignon, 2000). In cardiac myocytes, TNF regulated Ca2+ mobilization (Bick et al., 1997) and long-term potentiation (a Ca2+ handling phenomenon) was inhibited by TNF treatment (Cunningham et al., 1996). TNF is thought to have a primary role in the onset of asthmatic conditions (Thomas, 2001). In airway smooth muscle, TNF enhances Ca2+ mobilization in a process that is thought to be crucial to the airway hyper-responsiveness (Amrani et al., 1995). TNFR1 is the receptor isoform responsible for the observed hyper-responsiveness (Amrani et al., 1996) which also signals for MAPK and NF-κB activities (Amrani et al., 2000; McFarlane et al., 2001). It was uncovered that TNF was rapidly stimulating a novel pathway resulting in the enhanced phosphorylation of myosin light chain20, resulting in greater contractile force at the same [Ca2+]i (Parris et al., 1999). Prolonged TNF stimulation has itself been shown to raise [Ca2+]i in L929 fibroblasts which undergo TNF-induced necrosis (Kong et al., 1997), however these Ca2+-raising effects of TNF are not evident in a range of cell types which undergo TNF-induced cell death (Pollock et al., 2000; McFarlane et al., 2000). Interestingly in sensory neurones, TNF has the ability to stimulate rapid transient waves of Ca2+ mobilization (Pollock et al., 2002). Although similar to InsP3-induced release of intracellular Ca2+ stores, these TNF-induced Ca2+ spikes are probably through ryanodine-sensitive stores, brought about by S-1-P converted from ceramide and sphingomyelinase activity.
Physiological role of TNFRs
TNF is also known as cachectin because of its primary role in the muscle wasting disorder cachexia (Beutler et al., 1985). It is now becoming apparent that TNF plays an important role in metabolic disorders including type II diabetes mellitus (Saltiel, 2001). Early work on this topic revealed TNF interfered with insulin signalling mechanisms, by inhibiting the tyrosine kinase activities of the insulin receptor and serine phosphorylation of the insulin receptor substrate 1 (IRS-1) (Hotamisligil et al., 1993; 1994). It has since been shown that TNFR1 isoform plays the major role in the TNF-mediated insulin resistance (Sethi et al., 2000) which occurs in a variety of lipid-handling tissues, suggesting anti-TNF therapy or blocking TNFR1 activity may be helpful in diabetes (Uysal et al., 1997). The exact signalling mechanism for these insulin-modulating effects of TNF are not fully clear but have been proposed to include PLC-γ, PKC-ζ, PKB and the STAT5 transcription factor that controls interferon-stimulated gene activity (Storz et al., 1998; Ravichandran et al., 2001; Ermakova et al., 1999).
TNFR2 is readily cleaved by the metalloprotease TACE into its soluble shed form which is still capable of TNF binding, rapidly altering the number of functional TNFR2 receptors that can signal their proliferative or apoptotic actions (Pennica et al., 1992b; Porteu & Hieblot, 1994; Higuchi & Aggarwal, 1994). Both TNFRs protein expression levels are also regulated by a number of physiological or signalling mechanisms (Vandenabeele et al., 1995). Although regulation of TNFR protein expression is not restricted purely to TNFR2 (Manna & Aggarwal, 1998), generally the more restrictive tissue distribution of TNFR2 and the flexible TNFR2 protein regulation suggest a physiological role for TNFR2 regulation in modulating TNF-responsiveness. TNFRs form homotrimers upon activation by TNF without the assembly of receptor heterotrimers (Moosmayer et al., 1994), however the TNFR1 : TNFR2 protein ratio has been found to be important in the way a cell predetermines its TNF response (Medvedev et al., 1996; Declercq et al., 1998; Baxter et al., 1999). Thus, largely unmodulated TNFR1 expression coupled to changeable TNFR2 levels in cells, alters the TNFR1 : TNFR2 ratio and controls the functional outcome to TNF of that cell, thus effectively altering the cellular and physiological responses that the same cytokine elicits.
Transgenic mice deficient in TNFR1 have greater sensitivity to infection by Listeria monocytogenes but are resistant to TNF or interleukin-1-mediated in vivo lethality, plus were resistant to models of endotoxic shock induced by lipopolysaccharide and D-galactosamine (Pfeffer et al., 1993; Rothe et al., 1993). TNFR1 has also been shown to control early graph versus host disease (Speiser et al., 1997). It has been noted that cleaved soluble TNFR1 is found in the sera of healthy patients, with higher levels in patients suffering leukaemia (Digel et al., 1992) with these shed forms of TNFRs may play a possible role in arthritis. Deletion of TNFR2 in transgenic mice has uncovered that this receptor subtype is important in low dose TNF-induced lethality (Erickson et al., 1994). In addition to a role in thymocyte proliferation (Grell et al., 1998a), TNFR2 plays an important role in models of cerebral malaria and microvascular endothelial cell damage (Lucas et al., 1997a, 1997b; 1998). Langerhans cell migration was depressed in mice lacking TNFR2 (Wang et al., 1996), whereas TNFR2 plays a critical role in multiorgan inflammation (Douni & Kollias, 1998). Experimental hepatitis involves both TNFRs (Kusters et al., 1997) and TNFR2 was seen to have a minor role in Mycobacterium bovus (BCG) immunity in knock-out mice (Jacobs et al., 2000). Clearly, TNFR2 has a role in certain tissues and diseased states, but the validity of direct comparisons between TNFR-null transgenic mice and normal cells and tissues which ubiquitously express TNFRs at altering TNFR1:TNFR2 ratios, has to be considered when analysing the physiological role of TNFR1 and TNFR2.
Other members of the TNF ligand and receptor superfamilies have, in most cases, only recently been identified, and as such their full physiological role has still to be fully appreciated (Locksley et al., 2001). Nonetheless, many diseased states or transgenic studies have indicated a wide range of physiological responsibilities for these ligands and receptors. For example many of these ligands and receptors contribute critically to the adaptive immune response, co-ordinating the direction and magnitude of any immunological reaction. The receptors Fas, CD40 and OX40 have a crucial role in T cell responses including activation-induced apoptosis, whereas RANK receptor or RANKL are expressed selectively of CD4+ precursor cells and contribute towards haemotopoiesis and peripheral or mesenteric lymph node maturation (Dougall et al., 1999). Moreover, lack of functional RANK and RANKL cause osteoporosis as monocyte differentiation into osteoclasts is defunct. Likewise RANK and highly related receptors such as OPG control bone formation and integrity resulting in various bone density disorders (Wuyts et al., 2001; Kim et al., 2000; Li et al., 2000; Hughes et al., 2000; Kong et al., 1999). Neural development and hair follicle and sweat gland formation require the action of p75NGFR and other receptors including XEDAR and Troy also play a role in such tissue development (Locksley et al., 2001). Thus, TNF ligand and receptor superfamilies play and important function in a diverse range of physiological activities, hence further characterization and understanding of the signalling controlled by these receptors will hopefully lead to the development of pharmacological tools as therapies for a multitude of human disorders.
Therapeutic implications
Clearly TNF and its related ligands and receptors play a wide ranging role in a multitude of cellular and physiological acts. These roles have recently translated into a new generation of therapies for several common human disorders (Kollias et al., 1999). TNF itself has recently been tested as a tumour killing agent in the clinic on perfused isolated limbs to treat soft tissue sarcomas and melanomas (Couriel et al., 2000; Moore et al., 1999). Work is progressing to use lower doses of TNF ligands to minimize toxic side effects on healthy tissue (van der veen et al., 2000). The most notable successes in controlling TNF's effects have been with the use of anti-TNF therapies to treat patients suffering from rheumatoid arthritis (Taylor, 2001; Feldmann & Maini, 2001) or Crohn's disease and severe irritable bowel syndrome (MacDonald et al., 2000; McDermott, 2001; van assche & Rutgeerts, 2000). These profitable multi-billion dollar ventures have shown the significance of TNF in human diseases, and the importance of the development of pharmacological tools to modulate cytokine activities.
Other research at an earlier developmental stage has shown a fundamental role for TNF in diseased states such as asthma and chronic obstructive pulmonary disorder (COPD) (Thomas, 2001), septic shock (Waage et al., 1987; Schluter & Deckert, 2000), meningitis (Schluter & Deckert, 2000), and even chronic heart failure (Bolger & Anker, 2000; Ferrari, 1999). TNF is also thought to be important in inflammatory diseases including malaria (Odeh, 2001) or lupus (Kontoyiannis & Kollias, 2000), and neural demyelinating conditions such as encephalomyelitis and multiple sclerosis (Probert et al., 2000; Kassiotis & Kollias, 2001). Osteoclast formation and actions are heavily controlled by the TNF ligands RANKL and OPG, which may be the new generation of cytokines used to treat bone diseases such as osteoporosis and Paget's disease (Horowitz et al., 2001). It is hoped that future development of drugs which modulate TNF ligand actions, or small molecular weight antagonists of the signalling pathways specifically controlled by TNF receptors, will lead to new and exciting strategies for the therapeutic intervention in a wider range of human diseases.
Abbreviations
- Apaf
apoptosis protease activation factor
- CAD
caspase-activated DNAse
- caspase
cysteine aspartate-directed protease
- CAPK
ceramide-activated protein kinase
- CARD
caspase recruitment domain
- c-IAP
inhibitor of cellular apoptosis
- cPLA2
cytosolic phospholipase A2
- DcR
decoy receptor
- DD
death domain
- DED
death effector domain
- DIF
differentiation inducing factor
- DISC
death-inducing signalling complex
- DR
death receptor
- EDG
endothelial differentiation gene
- FADD
Fas-associated DD
- FAN
factor associated with neutral sphingomyelinase activation
- FLICE
FADD-like interleukin-converting enzyme
- FLIP
FLICE-like inhibitory protein
- IκB
inhibitor of κB
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- LT
lymphotoxin
- MADD
MAPK-activating DD protein
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- MEKK
MEK kinase
- mTNF
membrane-bound TNF
- NIK
NF-κB-inducing kinase
- NF-κB
nuclear factor-κB
- PARP
polyadenosine ribosyl polymerase
- RIP
receptor-interacting protein
- PKA
cyclic AMP-dependent protein kinase
- PKB
Akt/protein kinase B
- PKC
protein kinase C
- PLAD
pre-ligand assembly domain
- ROS
reactive oxygen species
- S-1-P
sphingosine-1-phosphate
- SAPK
stress-activated protein kinase
- SODD
silencer of DD
- TACE
TNF-α converting enzyme
- TNF
tumour necrosis factor-α
- TNFR
TNF receptor
- TNFR1
type I 55 kDa TNFR
- TNFR2
type II 75 kDa TNFR
- TNFSF
TNF superfamily nomenclature
- TNFRSF
TNFR superfamily nomenclature
- TRADD
TNFR-associated DD
- TRAF
TNFR-associating factor
- TRAK
TNFR-associated kinase
References
- ADAM D., ADAMKLAGES S., KRONKE M. Identification of p55 tumor necrosis factor receptor-associated proteins that couple to signaling pathways not initiated by the death domain. J. Inflamm. 1996;47:61–66. [PubMed] [Google Scholar]
- ADAM D., RUFF A., STRELOW A., WIEGMANN K., KRONKE M. Induction of stress-activated protein kinases c-Jun N-terminal kinases by the p55 tumour necrosis factor receptor does not require sphingomyelinases. Biochem. J. 1998;333:343–350. doi: 10.1042/bj3330343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADAMKLAGES S., SCHWANDNER R., ADAM D., KREDER D., BERNARDO K., KRONKE M. Distinct adapter proteins mediate acid versus neutral sphingomyelinase activation through the p55 receptor for tumor necrosis factor. J. Leukoc. Biol. 1998a;63:678–682. doi: 10.1002/jlb.63.6.678. [DOI] [PubMed] [Google Scholar]
- ADAMKLAGES S., SCHWANDNER R., LUSCHEN S., USSAT S., KREDER D., KRONKE M. Caspase-mediated inhibition of human cytosolic phospholipase A2 during apoptosis. J. Immunol. 1998b;161:5687–5694. [PubMed] [Google Scholar]
- AGGARWAL B.B. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-κB. Ann. Rheum. Dis. 2000;59:6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMRANI Y., LAZAAR A.L., HOFFMAN R., AMIN K., OUSMER S., PANETTIERI R.A. Activation of p55 tumor necrosis factor-α receptor-1 coupled to tumor necrosis factor receptor-associated factor 2 stimulates intercellular adhesion molecule-1 expression by modulating a thapsigargin-sensitive pathway in human tracheal smooth muscle cells. Mol. Pharmacol. 2000;58:237–245. doi: 10.1124/mol.58.1.237. [DOI] [PubMed] [Google Scholar]
- AMRANI Y., LAZAAR A.L., PANETTIERI R.A. Up-regulation of ICAM-1 by cytokines in human tracheal smooth muscle cells involves an NF-κB-dependent signaling pathway that is only partially sensitive to dexamethasone. J. Immunol. 1999;163:2128–2134. [PubMed] [Google Scholar]
- AMRANI Y., MARTINET N., BRONNER C. Potentiation by tumor-necrosis-factor-α of calcium signals induced by bradykinin and carbachol in human tracheal smooth-muscle cells. Br. J. Pharmacol. 1995;114:4–5. doi: 10.1111/j.1476-5381.1995.tb14896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMRANI Y., PANETTIERI R.A., FROSSARD N., BRONNER C. Activation of the TNF α-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 1996;15:55–63. doi: 10.1165/ajrcmb.15.1.8679222. [DOI] [PubMed] [Google Scholar]
- ATSUMI G., TAJIMA M., HADANO A., NAKATANI Y., MURAKAMI M., KUDO I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2 which undergoes proteolytic inactivation. J. Biol. Chem. 1998;273:13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- BAR-SAGI D., HALL A. Ras and rho GTPases: a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- BARBER S.A., PERERA P.Y., MCNALLY R., VOGEL S.N. The serine threonine phosphatase inhibitor, calyculin-a, inhibits and dissociates macrophage responses to lipopolysaccharide. J. Immunol. 1995;155:1404–1410. [PubMed] [Google Scholar]
- BARONE F.C., IRVING E.A., RAY A.M., LEE J.C., KASSIS S., KUMAR S., BADGER A.M., LEGOS J.J., ERHARDT J.A., OHLSTEIN E.H., HUNTER A.J., HARRISON D.C., PHILPOTT K., SMITH B.R., ADAMS J.L., PARSONS A.A. Inhibition of p38 mitogen-activated protein kinase provides neuroprotection in cerebral focal ischemia. Med. Res. Rev. 2001;21:129–145. doi: 10.1002/1098-1128(200103)21:2<129::aid-med1003>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- BASU S., BAYOUMY S., ZHANG Y., LOZANO J., KOLESNICK R. Bad enables ceramide to signal apoptosis via ras and raf-1. J. Biol. Chem. 1998;273:30419–30426. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- BAUD V., LIU Z.-G., BENNETT B., SUZUKI N., XIA Y., KARIN M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER G.T., KUO R.C., JUPP O.J., VANDENABEELE P., MACEWAN D.J. Tumor necrosis factor-α mediates both apoptotic cell death and cell proliferation in a human hematopoietic cell line dependent on mitotic activity and receptor subtype expression. J. Biol. Chem. 1999;274:9539–9547. doi: 10.1074/jbc.274.14.9539. [DOI] [PubMed] [Google Scholar]
- BERMUDEZ L.E., YOUNG L.S. Tumor-necrosis-factor (TNF) activates macrophages (MO) to kill mycobacterium-avium complex (MAC) by a protein-kinase C-(PKC)-independent pathway. J. Leukoc. Biol. 1987;42:598–599. [Google Scholar]
- BERRA E., DIAZMECO M.T., LOZANO J., FRUTOS S., MUNICIO M.M., SANCHEZ P., SANZ L., MOSCAT J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C ζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEUTLER B., GREENWALD D., HULMES J.D., CHANG M., PAN Y.C.E., MATHISON J., ULEVITCH R., CERAMI A. Identity of tumor necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- BEYAERT R., CUENDA A., VANDENBERGHE W., PLAISANCE S., LEE J.C., HAEGEMAN G., COHEN P., FIERS W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis in response to tumour necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- BEYAERT R., HEYNINCK K., DEVALCK D., BOEYKENS F., VANROY F., FIERS W. Enhancement of tumor-necrosis-factor cytotoxicity by lithium-chloride is associated with increased inositol phosphate accumulation. J. Immunol. 1993;151:291–300. [PubMed] [Google Scholar]
- BEYAERT R., SUFFYS P., VANROY F., FIERS W. Inhibition of TNF cytotoxicity by protease inhibitors. Immunobiology. 1987;175:3. [Google Scholar]
- BICK R.J., LIAO J.P., KING T.W., LEMAISTRE A., MCMILLIN J.B., BUJA L.M. Temporal effects of cytokines on neonatal cardiac myocyte Ca2+ transients and adenylate cyclase activity. Am. J. Physiol.-Heart Circ. Physiol. 1997;41:H1937–H1944. doi: 10.1152/ajpheart.1997.272.4.H1937. [DOI] [PubMed] [Google Scholar]
- BIRD T.A., KYRIAKIS J.M., TYSHLER L., GAYLE M., MILNE A., VIRCA G.D. Interleukin-1 activates p54 mitogen-activated protein (MAP) kinase kinase stress-activated protein-kinase by a pathway that is independent of p21ras, raf-1, and MAP kinase kinase. J. Biol. Chem. 1994;269:31836–31844. [PubMed] [Google Scholar]
- BODIE S.L., FORD I., GREAVES M., NIXON G.F. Thrombin-induced activation of rhoA in platelet shape change. Biochem. Biophys. Res. Commun. 2001;287:71–76. doi: 10.1006/bbrc.2001.5547. [DOI] [PubMed] [Google Scholar]
- BOLGER A.P., ANKER S.D. Tumour necrosis factor in chronic heart failure – a peripheral view on pathogenesis, clinical manifestations and therapeutic implications. Drugs. 2000;60:1245–1257. doi: 10.2165/00003495-200060060-00002. [DOI] [PubMed] [Google Scholar]
- BONIZZI G., PIETTE J., SCHOONBROODT S., MERVILLE M.P., BOURS V. Role of the protein kinase C λ/ι isoform in nuclear factor-κB activation by interleukin-1 β or tumor necrosis factor-α: cell type specificities. Biochem. Pharmacol. 1999;57:713–720. doi: 10.1016/s0006-2952(98)00353-0. [DOI] [PubMed] [Google Scholar]
- BOONE E., VANDEVOORDE V., DEWILDE G., HAEGEMAN G. Activation of p42/p44 mitogen-activated protein kinases (MAPK) and p38 MAPK by tumor necrosis factor (TNF) is mediated through the death domain of the 55-kDa TNF receptor. FEBS Lett. 1998;441:275–280. doi: 10.1016/s0014-5793(98)01567-1. [DOI] [PubMed] [Google Scholar]
- BORSCHHAUBOLD A.G., BARTOLI F., ASSELIN J., DUDLER T., KRAMER R.M., APITZCASTRO R., WATSON S.P., GELB M.H. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J. Biol. Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- BORSCHHAUBOLD A.G., KRAMER R.M., WATSON S.P. Phosphorylation and activation of cytosolic phospholipase A2 by 38-kDa mitogen-activated protein kinase in collagen-stimulated human platelets. Eur. J. Biochem. 1997;245:751–759. doi: 10.1111/j.1432-1033.1997.t01-1-00751.x. [DOI] [PubMed] [Google Scholar]
- BORSET M., MEDVEDEV A.E., SUNDAN A., ESPEVIK T. The role of the two TNF receptors in proliferation, NF-κB activation and discrimination between TNF and LTα signalling in the human myeloma cell line OH-2. Cytokine. 1996;8:430–438. doi: 10.1006/cyto.1996.0059. [DOI] [PubMed] [Google Scholar]
- BOZYCZKO-COYNE D., O'KANE T.M., WU Z.L., DOBRZANSKI P., MURTHY S., VAUGHT J.L., SCOTT R.W. CEP-1347/KT-7515, An inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with a β-induced cortical neuron apoptosis. J. Neurochem. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- BRANELLEC D., DECREMOUX P., BARREAU P., CALVO F., CHOUAIB S. Tumor necrosis factor-mediated cell-lysis in vitro – relationship to camp accumulation and guanine nucleotide-binding proteins. Eur. J. Immunol. 1992;22:963–967. doi: 10.1002/eji.1830220413. [DOI] [PubMed] [Google Scholar]
- BRINKMAN B.M.N., TELLIEZ J.B., SCHIEVELLA A.R., LIN L.L., GOLDFELD A.E. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2 and p38 mitogen-activated protein kinase-dependent TNF-α gene expression. J. Biol. Chem. 1999;274:30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- CARSWELL E.A., OLD L.J., KASSEL R.L., GREEN S., FIORE N., WILLIAMSON B. An endotoxin-induced serum factor that causes necrosis of tumours. Proc. Natl. Acad. Sci. U.S.A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTELLINO A.M., PARKER G.J., BORONENKOV I.V., ANDERSON R.A., CHAO M.V. A novel interaction between the juxtamembrane region of the p55 tumor necrosis factor receptor and phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem. 1997;272:5861–5870. doi: 10.1074/jbc.272.9.5861. [DOI] [PubMed] [Google Scholar]
- CHAN F.K.M., CHUN H.J., ZHENG L.X., SIEGEL R.M., BUI K.L., LENARDO M.J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- CHANG D.J., RINGOLD G.M., HELLER R.A. Cell killing and induction of manganous superoxide-dismutase by tumor-necrosis-factor-α is mediated by lipoxygenase metabolities of arachidonic-acid. Biochem. Biophys. Res. Commun. 1992;188:538–546. doi: 10.1016/0006-291x(92)91089-9. [DOI] [PubMed] [Google Scholar]
- CHANG N.S. TGF-β-induced matrix proteins inhibit p42/44 MAPK and JNK activation and suppress TNF-mediated i κ b α degradation and NF-κB nuclear translocation in L929 fibroblasts. Biochem. Biophys. Res. Commun. 2000;267:194–200. doi: 10.1006/bbrc.1999.1909. [DOI] [PubMed] [Google Scholar]
- CHAO D.T., KORSMEYER S.J. Bcl-2 family: regulators of cell death. Ann. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- CHINNAIYAN A.M., O'ROURKE K., TEWARI M., DIXIT V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- CLARK J.D., LIN L.L., KRIZ R.W., RAMESHA C.S., SULTZMAN L.A., LIN A.Y., MILONA N., KNOPF J.L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+ – dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- CLARK M.A., CHEN M.J., BOMALASKI J.S. Tumor-necrosis-factor induces phospholipase-A2 activity and the synthesis of a phospholipase-A2 activating protein (PLAP) in endothelial-cells. Fed. Proc. 1987;46:1946. doi: 10.1042/bj2500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY A.M., PYNE N.J., PYNE S. Ceramide-dependent regulation of p42/p44 mitogen-activated protein kinase and c-Jun N-terminal-directed protein kinase in cultured airway smooth muscle cells. Cell. Signal. 2000;12:737–743. doi: 10.1016/s0898-6568(00)00119-4. [DOI] [PubMed] [Google Scholar]
- CORONEOS E., WANG Y.Z., PANUSKA J.R., TEMPLETON D.J., KESTER M. Sphingolipid metabolites differentially regulate extracellular signal-regulated kinase and stress-activated protein kinase Cascades. Biochem. J. 1996;316:13–17. doi: 10.1042/bj3160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTIN V., VAN LINDEN A., RICHES D.W.H. Phosphorylation of tumor necrosis factor receptor CD120a (p55) by p42(MAPK/ERK2) induces changes in its subcellular localization. J. Biol. Chem. 1999;274:32975–32987. doi: 10.1074/jbc.274.46.32975. [DOI] [PubMed] [Google Scholar]
- COURIEL D.R., HICKS K., GIRALT S., CHAMPLIN R.E. Role of tumor necrosis factor-α inhibition with infliximAb in cancer therapy and hematopoietic stem cell transplantation. Curr. Opin. Oncol. 2000;12:582–587. doi: 10.1097/00001622-200011000-00011. [DOI] [PubMed] [Google Scholar]
- CROSS T.G., SCHEEL-TOELLNER D., HENRIQUEZ N.V., DEACON E., SALMON M., LORD J.M. Serine/threonine protein kinases and apoptosis. Exp. Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM A.J., MURRAY C.A., ONEILL L.A.J., LYNCH M.A., OCONNOR J.J. Interleukin-1β (IL-1β) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- CUVILLIER O., PIRIANOV G., KLEUSER B., VANEK P.G., COSO O.A., GUTKIND J.S., SPIEGEL S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- DAI Y., YU C.R., SINGH V., TANG L., WANG Z.L., MCINISTRY R., DENT P., GRANT S. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61:5106–5115. [PubMed] [Google Scholar]
- DARNAY B.G., AGGARWAL B.B. Inhibition of protein tyrosine phosphatases causes phosphorylation of tyrosine-331 in the p60 TNF receptor and inactivates the receptor-associated kinase. FEBS Lett. 1997;410:361–367. doi: 10.1016/s0014-5793(97)00652-2. [DOI] [PubMed] [Google Scholar]
- DARNAY B.G., AGGARWAL B.B. Signal transduction by tumour necrosis factor and tumour necrosis factor related ligands and their receptors. Ann. Rheum. Dis. 1999;58:2–13. doi: 10.1136/ard.58.2008.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARNAY B.G., REDDY S.A.G., AGGARWAL B.B. Physical and functional association of a serine-threonine protein-kinase to the cytoplasmic domain of the p80 form of the human tumor- necrosis-factor receptor in human histiocytic lymphoma U-937 cells. J. Biol. Chem. 1994;269:19687–19690. [PubMed] [Google Scholar]
- DARNAY B.G., SINGH S., AGGARWAL B.B. The p80 TNF receptor-associated kinase (p80TRAK) associates with residues 354-397 of the p80 cytoplasmic domain: similarity to casein kinase. FEBS Lett. 1997;406:101–105. doi: 10.1016/s0014-5793(97)00251-2. [DOI] [PubMed] [Google Scholar]
- DAVIS R.J. Signal transduction by the c-Jun N-terminal kinase. Cell. Responses Stress. 1999. pp. 1–12. [DOI] [PubMed]
- DAYER J.M., BEUTLER B., CERAMI A. Cachectin tumor necrosis factor stimulates collagenase and prostaglandin-E2 production by human synovial-cells and dermal fibroblasts. J. Exp. Med. 1985;162:2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DECLERCQ W., DENECKER G., FIERS W., VANDENABEELE P. Cooperation of both TNF receptors in inducing apoptosis: involvement of the TNF receptor-associated factor binding domain of the TNF receptor 75. J. Immunol. 1998;161:390–399. [PubMed] [Google Scholar]
- DECLERCQ W., VANDENABEELE P., FIERS W. Dimerization of chimeric erythropoietin 75 kDa tumor-necrosis-factor (TNF) receptors transduces TNF signals – necessity for the 75 kDa TNF-Receptor transmembrane domain. Cytokine. 1995;7:701–709. doi: 10.1006/cyto.1995.0082. [DOI] [PubMed] [Google Scholar]
- DECOSTER E., VANHAESEBROECK B., VANDENABEELE P., GROOTEN J., FIERS W. Generation and biological characterization of membrane-bound, uncleavable murine tumor-necrosis-factor. J. Biol. Chem. 1995;270:18473–18478. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- DENECKER G., VANDENABEELE P., GROOTEN J., PENNING L.C., DECLERCQ W., BEYAERT R., BUURMAN W.A., FIERS W. Differential role of calcium in tumour necrosis factor-mediated apoptosis and secretion of granulocyte-macrophage colony-stimulating factor in a T cell hybridoma. Cytokine. 1997;9:631–638. doi: 10.1006/cyto.1997.0218. [DOI] [PubMed] [Google Scholar]
- DENECKER G., VERCAMMEN D., DECLERCQ W., VANDENABEELE P. Apoptotic and necrotic cell death induced by death domain receptors. Cell. Mol. Life Sci. 2001;58:356–370. doi: 10.1007/PL00000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVALCK D., VERCAMMEN D., FIERS W., BEYAERT R. Differential activation of phospholipases during necrosis or apoptosis: a comparative study using tumor necrosis factor and anti- Fas antibodies. J. Cell. Biochem. 1998;71:392–399. [PubMed] [Google Scholar]
- DEVIN A., COOK A., LIN Y., RODRIGUEZ Y., KELLIHER M., LIU Z.G. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- DHAWAN S., SINGH S., AGGARWAL B.B. Induction of endothelial cell surface molecules by tumor necrosis factor is blocked by protein tyrosine phosphatase inhibitors: role of the nuclear transcription factor NF-κB. Eur. J. Immunol. 1997;27:2172–2179. doi: 10.1002/eji.1830270909. [DOI] [PubMed] [Google Scholar]
- DIAZ F., BOURGUIGNON L.Y.W. Selective down-regulation of ip3 receptor subtypes of caspases and calpain during TNF α-induced apoptosis of human T-lymphoma cells. Cell Calcium. 2000;27:315–328. doi: 10.1054/ceca.2000.0126. [DOI] [PubMed] [Google Scholar]
- DIAZMECO M.T., LOZANO J., MUNICIO M.M., BERRA E., FRUTOS S., SANZ L., MOSCAT J. Evidence for the in-vitro and in-vivo interaction of ras with protein-kinase-c-ζ. J. Biol. Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- DIGEL W., PORZSOLT F., SCHMID M., HERRMANN F., LESSLAUER W., BROCKHAUS M. High-levels of circulating soluble receptors for tumor-necrosis-factor in hairy-cell leukemia and type-B chronic lymphocytic-leukemia. J. Clin. Invest. 1992;89:1690–1693. doi: 10.1172/JCI115769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROWSKY R.T., KAMIBAYASHI C., MUMBY M.C., HANNUN Y.A. Ceramide activates heterotrimeric protein phosphatase-2a. J. Biol. Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- DOMAN R.K., PEREZ M., DONATO N.J. JNK and p53 stress signaling cascades are altered in MCF-7 cells resistant to tumor necrosis factor-mediated apoptosis. J. Interferon Cytokine Res. 1999;19:261–269. doi: 10.1089/107999099314199. [DOI] [PubMed] [Google Scholar]
- DOUGALL W.C., GLACCUM M., CHARRIER K., ROHRBACH K., BRASEL K., DE SMEDT T., DARO E., SMITH J., TOMETSKO M.E., MALISZEWSKI C.R., ARMSTRONG A., SHEN V., BAIN S., COSMAN D., ANDERSON D., MORRISSEY P.J., PESCHON J.J., SCHUH J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUNI E., KOLLIAS G. A critical role of the p75 tumor necrosis factor receptor (p75TNF-R) in organ inflammation independent of TNF, lymphotoxin α, or the p55TNF-R. J. Exp. Med. 1998;188:1343–1352. doi: 10.1084/jem.188.7.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESSLER K.A., MATHIAS S., KOLESNICK R.N. Tumor-necrosis-factor-α activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- DRUMMOND M.W., HOLYOAKE T.L. Tyrosine kinase inhibitors in the treatment of chronic myeloid leukaemia: so far so good. Blood Rev. 2001;15:85–95. doi: 10.1054/blre.2001.0152. [DOI] [PubMed] [Google Scholar]
- ELIOPOULOS A.G., GALLAGHER N.J., BLAKE S.M.S., DAWSON C.W., YOUNG L.S. Activation of the p38 mitogen-activated protein kinase pathway by epstein-barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- ENSLEN H., RAINGEAUD J., DAVIS R.J. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J. Biol. Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- ERICKSON S.L., DESAUVAGE F.J., KIKLY K., CARVERMOORE K., PITTSMEEK S., GILLETT N., SHEEHAN K.C.F., SCHREIBER R.D., GOEDDEL D.V., MOORE M.W. Decreased sensitivity to tumor-necrosis-factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- ERMAKOVA N.V., PAULSSEN R.H., FLORHOLMEN J. The effect of TNF-α on cell growth and PLC-γ expression in insulin-secreting β-cells. Diabetologia. 1999;42:529. [Google Scholar]
- ESTROV Z., MANNA S.K., HARRIS D., VAN Q., ESTEY E.H., KANTARJIAN H.M., TALPAZ M., AGGARWAL B.B. Phenylarsine oxide blocks interleukin-1β-induced activation of the nuclear transcription factor NF-κB, inhibits proliferation, and induces apoptosis of actue myelogenous leukemia cells. Blood. 1999;94:2844–2853. [PubMed] [Google Scholar]
- FELDMANN M., MAINI R.N. Anti-TNFα therapy of rheumatoid arthritis: what have we learned. Annu. Rev. Immunol. 2001;19:163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-BOTRAN R. Soluble cytokine receptors: novel immunotherapeutic agents. Expert Opin. Investigational Drugs. 2000;9:497–514. doi: 10.1517/13543784.9.3.497. [DOI] [PubMed] [Google Scholar]
- FERRARI R. The role of TNF in cardiovascular disease. Pharmacol. Res. 1999;40:97–105. doi: 10.1006/phrs.1998.0463. [DOI] [PubMed] [Google Scholar]
- FIERS W., BEYAERT R., BOONE E., CORNELIS S., DECLERCQ W., DECOSTER E., DENECKER G., DEPUYDT B., DEVALCK D., DEWILDE G., GOOSSENS V., GROOTEN J., HAEGEMAN G., HEYNINCK K., PENNING L., PLAISANCE S., VANCOMPERNOLLE K., VANCRIEKINGE W., VANDENABEELE P., VANDENBERGHE W., VANDECRAEN M., VANDEVOORDE V., VERCAMMEN D. TNF-induced intracellular signaling leading to gene induction or to cytotoxicity by necrosis or by apoptosis. J. Inflamm. 1996;47:67–75. [PubMed] [Google Scholar]
- FONG C.W., ZHANG Y., NEO S.Y., LIN S.C. Specific induction of rgs 16 (regulator of G-protein signalling 16) mRNA by protein kinase C in CEM leukaemia cells is mediated via tumour necrosis factor α in a calcium-sensitive manner. Biochem. J. 2000;352:747–753. [PMC free article] [PubMed] [Google Scholar]
- GESCHER A. Staurosporine analogues – pharmacological toys or useful antitumour agents. Crit. Rev. Oncol. Hematol. 2000;34:127–135. doi: 10.1016/s1040-8428(00)00058-5. [DOI] [PubMed] [Google Scholar]
- GODFREY R.W., JOHNSON W.J., HOFFSTEIN S.T. Recombinant tumor-necrosis-factor and interleukin-1 both stimulate human synovial cell arachidonic-acid release and phospholipid-metabolism. Biochem. Biophys. Res. Commun. 1987;142:235–241. doi: 10.1016/0006-291x(87)90476-1. [DOI] [PubMed] [Google Scholar]
- GONG M.C., IIZUKA K., NIXON G., BROWNE J.P., HALL A., ECCLESTON J.F., SUGAI M., KOBAYASHI S., SOMLYO A.V., SOMLYO A.P. Role of guanine nucleotide-binding proteins ras-family or trimeric proteins or both in Ca2+ sensitization of smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANGER G.A., SHACK S.J., WILLIAMS T.W., KOLB W.P. Lymphocyte in vitro cytotoxicity: specific release of lymphotoxin-like materials from tuberculin-sensitive lymphoid cells. Nature. 1969;221:1155–1157. doi: 10.1038/2211155a0. [DOI] [PubMed] [Google Scholar]
- GRELL M., BECKE F.M., WAJANT H., MANNEL D.N., SCHEURICH P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur. J. Immunol. 1998a;28:257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- GRELL M., DOUNI E., WAJANT H., LOHDEN M., CLAUSS M., MAXEINER B., GEORGOPOULOS S., LESSLAUER W., KOLLIAS G., PFIZENMAIER K., SCHEURICH P. The transmembrane form of tumor-necrosis-factor is the prime activating ligand of the 80 kDa tumor-necrosis-factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- GRELL M., SCHEURICH P., MEAGER A., PFIZENMAIER K. TR60 and TR80 tumor-necrosis-factor (TNF)-receptors can independently mediate cytolysis. Lymphokine Cytokine Res. 1993;12:143–148. [PubMed] [Google Scholar]
- GRELL M., WAJANT H., ZIMMERMANN G., SCHEURICH P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc. Natl. Acad. Sci. U.S.A. 1998b;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRELL M., ZIMMERMANN G., GOTTFRIED E., CHEN C.M., GRUNWALD U., HUANG D.C.S., LEE Y.H.W., DURKOP H., ENGELMANN H., SCHEURICH P., WAJANT H., STRASSER A. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. EMBO J. 1999;18:3034–3043. doi: 10.1093/emboj/18.11.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRELL M., ZIMMERMANN G., HULSER D., PFIZENMAIER K., SCHEURICH P. TNF receptors TR60 and TR80 can mediate apoptosis via induction of distinct signal pathways. J. Immunol. 1994;153:1963–1972. [PubMed] [Google Scholar]
- GU C.H., CASTELLINO A., CHAN J.Y.H., CHAO M.V. BRE: a modulator of TNF-α action. FASEB J. 1998;12:1101–1108. doi: 10.1096/fasebj.12.12.1101. [DOI] [PubMed] [Google Scholar]
- GUO D.Q., WU L.W., DUNBAR J.D., OZES O.N., MAYO L.D., KESSLER K.M., GUSTIN J.A., BAERWALD M.R., JAFFE E.A., WARREN R.S., DONNER D.B. Tumor necrosis factor employs a protein-tyrosine phosphatase to inhibit activation of KDR and vascular endothelial cell growth factor-induced endothelial cell proliferation. J. Biol. Chem. 2000;275:11216–11221. doi: 10.1074/jbc.275.15.11216. [DOI] [PubMed] [Google Scholar]
- HAAS E., GRELL M., WAJANT H., SCHEURICH P. Continuous autotropic signaling by membrane-expressed tumor necrosis factor. J. Biol. Chem. 1999;274:18107–18112. doi: 10.1074/jbc.274.25.18107. [DOI] [PubMed] [Google Scholar]
- HAN Y.Q., WEINMAN S., BOLDOGH I., WALKER R.K., BRASIER A.R. Tumor necrosis factor-α-inducible IκBα proteolysis mediated by cytosolic m-calpain – a mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-κB activation. J. Biol. Chem. 1999;274:787–794. doi: 10.1074/jbc.274.2.787. [DOI] [PubMed] [Google Scholar]
- HANNUN Y.A. The sphinogomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- HARIDAS V., DARNAY B.G., NATARAJAN K., HELLER R., AGGARWAL B.B. Overexpression of the p80 TNF receptor leads to TNF-dependent apoptosis, nuclear factor-κB activation, and c-Jun kinase activation. J. Immunol. 1998;160:3152–3162. [PubMed] [Google Scholar]
- HAYAKAWA M., ISHIDA N., TAKEUCHI K., SHIBAMOTO S., HORI T., OKU N., ITO F., TSUJIMOTO M. Arachidonic acid-selective cytosolic phospholipase-A2 is crucial in the cytotoxic action of tumor-necrosis-factor. J. Biol. Chem. 1993;268:11290–11295. [PubMed] [Google Scholar]
- HELLER R.A., SONG K., FAN N., CHANG D.J. The p70 tumor-necrosis-factor receptor mediates cytotoxicity. Cell. 1992;70:47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- HELMS M.J., MOHAMED A.A.A., MACEWAN D.J. Modulated kinase activities in cells undergoing tumour necrosis factor-induced apoptotic cell death. FEBS Lett. 2001;505:68–74. doi: 10.1016/s0014-5793(01)02779-x. [DOI] [PubMed] [Google Scholar]
- HERNANDEZMUNOZ I., DELATORRE P., SANCHEZALCAZAR J.A., GARCIA I., SANTIAGO E., MUNOZYAGUE M., SOLISHERRUZO J.A. Tumor necrosis factor α inhibits collagen α1i gene expression in rat hepatic stellate cells through a g protein. Gastroenterol. 1997;113:625–640. doi: 10.1053/gast.1997.v113.pm9247485. [DOI] [PubMed] [Google Scholar]
- HIGUCHI M., AGGARWAL B.B. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J. Immunol. 1994;152:3550–3558. [PubMed] [Google Scholar]
- HILDT E., OESS S. Identification of Grb2 as a novel binding partner of tumor necrosis factor (TNF) receptor 1. J. Exp. Med. 1999;189:1707–1714. doi: 10.1084/jem.189.11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOECK W.G., RAMESHA C.S., CHANG D.J., FAN N., HELLER R.A. Cytoplasmic phospholipase-A2 activity and gene-expression are stimulated by tumor-necrosis-factor – dexamethasone blocks the induced synthesis. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4475–4479. doi: 10.1073/pnas.90.10.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHMANN H.P., BROCKHAUS M., BAEUERLE P.A., REMY R., KOLBECK R., VAN LOON A.P. Expression of the types a and b tumour necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-κB. TNF α is not needed for induction of a biological effect via TNF receptors. J. Biol. Chem. 1990;265:22409–22417. [PubMed] [Google Scholar]
- HOLLER N., ZARU R., MICHEAU O., THOME M., ATTINGER A., VALITUTTI S., BODMER J.L., SCHNEIDER P., SEED B., TSCHOPP J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- HOROWITZ M.C., XI Y.G., WILSON K., KACENA M.A. Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev. 2001;12:9–18. doi: 10.1016/s1359-6101(00)00030-7. [DOI] [PubMed] [Google Scholar]
- HOTAMISLIGIL G.S., MURRAY D.L., CHOY L.N., SPIEGELMAN B.M. Tumor-necrosis-factor-α inhibits signaling from the insulin-receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTAMISLIGIL G.S., SHARGILL N.S., SPIEGELMAN B.M. Adipose expression of tumor-necrosis-factor-α-direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- HSU H.L., HUANG J.N., SHU H.B., BAICHWAL V., GOEDDEL D.V. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity. 1996a;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- HSU H.L., SHU H.B., PAN M.G., GOEDDEL D.V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996b;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- HSU H.L., XIONG J., GOEDDEL D.V. The TNF receptor 1-associated protein TRADD signals cell-death and NF-κ-B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- HUGHES A.E., RALSTON S.H., MARKEN J., BELL C., MACPHERSON H., WALLACE R.G.H., VAN HUL W., WHYTE M.P., NAKATSUKA K., HOVY L., ANDERSON D.M. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nature Genet. 2000;24:45–48. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- HUNTER I., COBBAN H.J., MACEWAN D.J., NIXON G.F. Tumour necrosis factor-α increases the Ca2+ sensitivity of myosin light chain20 phosphorylation in cultured airway smooth muscle cells via a rho-activated pathway. FASEB J. 2001;15:A163. [Google Scholar]
- IDRISS H.T., NAISMITH J.H. TNF α and the TNF receptor superfamily: structure-function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- INOUE J., ISHIDA T., TSUKAMOTO N., KOBAYASHI N., NAITO A., AZUMA S., YAMAMOTO T. Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp. Cell Res. 2000;254:14–24. doi: 10.1006/excr.1999.4733. [DOI] [PubMed] [Google Scholar]
- IRMLER M., THOME M., HAHNE M., SCHNEIDER P., HOFMANN B., STEINER V., BODMER J.L., SCHROTER M., BURNS K., MATTMANN C., RIMOLDI D., FRENCH L.E., TSCHOPP J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- JACOBS M., BROWN N., ALLIE N., CHETTY K., RYFFEL B. Tumor necrosis factor receptor 2 plays a minor role for mycobacterial immunity. Pathobiology. 2000;68:68–75. doi: 10.1159/000028116. [DOI] [PubMed] [Google Scholar]
- JAYADEV S., HAYTER H.L., ANDRIEU N., GAMARD C.J., LIU B., BALU R., HAYAKAWA M., ITO F., HANNUN Y.A. Phospholipase A2 is necessary for tumor necrosis factor α-induced ceramide generation in L929 cells. J. Biol. Chem. 1997;272:17196–17203. doi: 10.1074/jbc.272.27.17196. [DOI] [PubMed] [Google Scholar]
- JIANG Y.P., WORONICZ J.D., LIU W., GOEDDEL D.V. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- JIN D.Y., TERAMOTO H., GIAM C.Z., CHUN R.F., GUTKIND J.S., JEANG K.T. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor α. J. Biol. Chem. 1997;272:25816–25823. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- JOHANNES F.J., HORN J., LINK G., HAAS E., SIEMIENSKI K., WAJANT H., PFIZENMAIER K. Protein kinase C μ downregulation of tumor-necrosis-factor-induced apoptosis correlates with enhanced expression of nuclear-factor-κB-dependent protective genes. Eur. J. Biochem. 1998;257:47–54. doi: 10.1046/j.1432-1327.1998.2570047.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON D.E. Noncaspase proteases in apoptosis. Leukemia. 2000;14:1695–1703. doi: 10.1038/sj.leu.2401879. [DOI] [PubMed] [Google Scholar]
- JONES S.J., LEDGERWOOD E.C., PRINS J.B., GALBRAITH J., JOHNSON D.R., POBER J.S., BRADLEY J.R. TNF recruits TRADD to the plasma membrane but not the trans-golgi network, the principal subcellular location of TNF-R1. J. Immunol. 1999;162:1042–1048. [PubMed] [Google Scholar]
- JUPP O.J., MCFARLANE S.M., ANDERSON H.M., LITTLEJOHN A.F., MCKAY R.H., VANDENABEELE P., MACEWAN D.J. Type II tumor necrosis factor-α receptor (TNFR2) activates c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) or p38MAPK pathways. Biochem. J. 2001;359:525–535. doi: 10.1042/0264-6021:3590525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALB A., BLUETHMANN H., MOORE M.W., LESSLAUER W. Tumor necrosis factor receptors (TNFR) in mouse fibroblasts deficient in TNFR1 or TNFR2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J. Biol. Chem. 1996;271:28097–28104. doi: 10.1074/jbc.271.45.28097. [DOI] [PubMed] [Google Scholar]
- KANG J.H., SHIN I., HAN J.S. Changes of phospholipase d activity in TNF-α and anti- Fas/apo1 monoclonal antibody induced apoptosis in HL-60 and A20 cells. Exp. Mol. Med. 1998;30:21–27. doi: 10.1038/emm.1998.3. [DOI] [PubMed] [Google Scholar]
- KASSIOTIS G., KOLLIAS G. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 2001;193:427–434. doi: 10.1084/jem.193.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATAOKA T., BUDD R.C., HOLLER N., THOME M., MARTINON F., IRMLER M., BURNS K., HAHNE M., KENNEDY N., KOVACSOVICS M., TSCHOPP J. The caspase-8 inhibitor FLIP promotes activation of NF-κB and ERK signaling pathways. Curr. Biol. 2000;10:640–648. doi: 10.1016/s0960-9822(00)00512-1. [DOI] [PubMed] [Google Scholar]
- KELLIHER M.A., GRIMM S., ISHIDA Y., KUO F., STANGER B.Z., LEDER P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- KILPATRICK L.E., SONG Y.H., ROSSI M.W., KORCHAK H.M. Serine phosphorylation of p60 tumor necrosis factor receptor by PKC-δ in TNF-α-activated neutrophils. Am. J. Physiol.-Cell Physiol. 2000;279:C2011–C2018. doi: 10.1152/ajpcell.2000.279.6.C2011. [DOI] [PubMed] [Google Scholar]
- KIM B.C., LEE M.N., KIM J.Y., LEE S.S., CHANG J.D., KIM S.S., LEE S.Y., KIM J.H. Roles of phosphatidylinositol 3-kinase and rac in the nuclear signaling by tumor necrosis factor-α in rat-2 fibroblasts. J. Biol. Chem. 1999;274:24372–24377. doi: 10.1074/jbc.274.34.24372. [DOI] [PubMed] [Google Scholar]
- KIM M.Y., LINARDIC C., OBEID L., HANNUN Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor-necrosis-factor-α and γ-interferon – specific role in cell-differentiation. J. Biol. Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- KIM N., ODGREN P.R., KIM D.K., MARKS S.C., CHOI Y.W. Diverse roles of the tumor necrosis factor family member trance in skeletal physiology revealed by trance deficiency and partial rescue by a lymphocyte-expressed trance transgene. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10905–10910. doi: 10.1073/pnas.200294797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINLOCH R.A., TREHERNE M., FURNESS M., HAJIMOHAMADREZA I. The pharmacology of apoptosis. Trends Pharmacol. Sci. 1999;20:35–42. doi: 10.1016/s0165-6147(98)01277-2. [DOI] [PubMed] [Google Scholar]
- KOLESNICK R.N., KRONKE M. Regulation of ceramide production and apoptosis. Ann. Rev. Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- KOLLIAS G., DOUNI E., KASSIOTIS G., KONTOYIANNIS D. The function of tumour necrosis factor and receptors in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann. Rheum. Dis. 1999;58:32–39. doi: 10.1136/ard.58.2008.i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG S.K., FUNG K.P., CHOY Y.M., LEE C.Y. Slow increase in intranuclear and cytosolic free calcium concentrations in L929 cells is important in tumour necrosis factor- α-mediated cell death. Oncology. 1997;54:55–62. doi: 10.1159/000227662. [DOI] [PubMed] [Google Scholar]
- KONG Y.Y., FEIGE U., SAROSI I., BOLON B., TAFURI A., MORONY S., CAPPARELLI C., LI J., ELLIOTT R., MCCABE S., WONG T., CAMPAGNUOLO G., MORAN E., BOGOCH E.R., VAN G., NGUYEN L.T., OHASHI P.S., LACEY D.L., FISH E., BOYLE W.J., PENNINGER J.M. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- KONTOYIANNIS D., KOLLIAS G. Accelerated autoimmunity and lupus nephritis in NZB mice with an engineered heterozygous deficiency in tumor necrosis factor. Eur. J. Immunol. 2000;30:2038–2047. doi: 10.1002/1521-4141(200007)30:7<2038::AID-IMMU2038>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- KRAMER R.M., ROBERTS E.F., UM S.L., BORSCHHAUBOLD A.G., WATSON S.P., FISHER M.J., JAKUBOWSKI J.A. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets-evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- KRUPPA G., THOMA B., MACHLEIDT T., WIEGMANN K., KRONKE M. Inhibition of tumor-necrosis-factor (TNF)-mediated NF-κ-B activation by selective blockade of the human 55-kDa TNF receptor. J. Immunol. 1992;148:3152–3157. [PubMed] [Google Scholar]
- KUIDA K., HAYDAR T.F., KUAN C.Y., GU Y., TAYA C., KARASUYAMA H., SU M.S.S., RAKIC P., FLAVELL R.A. Reduced apoptosis and cytochrome C-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- KUIDA K., LIPPKE J.A., KU G., HARDING M.W., LIVINGSTON D.J., SU M.S.S., FLAVELL R.A. Altered cytokine export and apoptosis in mice deficient in interleukin-1-β converting-enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- KUIDA K., ZHENG T.S., NA S.Q., KUAN C.Y., YANG D., KARASUYAMA H., RAKIC P., FLAVELL R.A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- KUJIME K., HASHIMOTO S., GIN Y., SHIMIZU K., HORIE T. p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase regulate rantes production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 2000;164:3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- KULL F.C., JACOBS S., CUATRECASAS P. Cellular receptor for i-125-labeled tumor necrosis factor-specific binding, affinity labeling, and relationship to sensitivity. Proc. Natl. Acad. Sci. U.S.A. 1985;82:5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSTERS S., TIEGS G., ALEXOPOULOU L., PASPARAKIS M., DOUNI E., KUNSTLE G., BLUETHMANN H., WENDEL A., PFIZENMAIER K., KOLLIAS G., GRELL M. In vivo evidence for a functional role of both tumor necrosis factor (TNF) receptors and transmembrane TNF in experimental hepatitis. Eur. J. Immunol. 1997;27:2870–2875. doi: 10.1002/eji.1830271119. [DOI] [PubMed] [Google Scholar]
- LAEGREID A., MEDVEDEV A., NONSTAD U., BOMBARA M.P., RANGES G., SUNDAN A., ESPEVIK T. Tumor-necrosis-factor receptor p75 mediates cell-specific activation of nuclear factor κ-B and induction of human cytomegalovirus enhancer. J. Biol. Chem. 1994;269:7785–7791. [PubMed] [Google Scholar]
- LAOUAR A., GLESNE D., HUBERMAN E. Involvement of protein kinase C-β and ceramide in tumor necrosis factor-α-induced but not Fas-induced apoptosis of human myeloid leukemia cells. J. Biol. Chem. 1999;274:23526–23534. doi: 10.1074/jbc.274.33.23526. [DOI] [PubMed] [Google Scholar]
- LAZDINS J.K., GRELL M., WALKER M.R., WOODSCOOK K., SCHEURICH P., PFIZENMAIER K. Membrane tumor necrosis factor (TNF) induced cooperative signaling of TNFR60 and TNFR80 favors induction of cell death rather than virus production in HIV-infected T cells. J. Exp. Med. 1997;185:81–90. doi: 10.1084/jem.185.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDGERWOOD E.C., POBER J.S., BRADLEY J.R. Recent advances in the molecular basis of TNF signal transduction. Lab. Invest. 1999;79:1041–1050. [PubMed] [Google Scholar]
- LEE J.I., BURCKART G.J. Nuclear factor κB: important transcription factor and therapeutic target. J. Clin. Pharmacol. 1998;38:981–993. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- LEE J.Y., HANNUN Y.A., OBEID L.M. Functional dichotomy of protein kinase C (PKC) in tumor necrosis factor-α (TNF-α) signal transduction in L929 cells-translocation and inactivation of PKC by TNF-α. J. Biol. Chem. 2000;275:29290–29298. doi: 10.1074/jbc.M000170200. [DOI] [PubMed] [Google Scholar]
- LEE S.Y., REICHLIN A., SANTANA A., SOKOL K.A., NUSSENZWEIG M.C., CHOI Y. TRAF2 is essential for JNK but not NF-κB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- LEEUWENBERG J.F.M., VANTITS L.J.H., JEUNHOMME T.M.A.A., BUURMAN W.A. Evidence for exclusive role in signaling of tumor-necrosis-factor p55 receptor and a potentiating function of p75 receptor on human endothelial-cells. Cytokine. 1995;7:457–462. doi: 10.1006/cyto.1995.0062. [DOI] [PubMed] [Google Scholar]
- LEWIS M., TARTAGLIA L.A., LEE A., BENNETT G.L., RICE G.C., WONG G.H.W., CHEN E.Y., GOEDDEL D.V. Cloning and expression of cDNAs for two distinct murine tumor-necrosis- factor receptors demonstrate one receptor is species-specific. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J., SAROSI I., YAN X.Q., MORONY S., CAPPARELLI C., TAN H.L., MCCABE S., ELLIOTT R., SCULLY S., VAN G., KAUFMAN S., JUAN S.C., SUN Y., TARPLEY J., MARTIN L., CHRISTENSEN K., MCCABE J., KOSTENUIK P., HSU H., FLETCHER F., DUNSTAN C.R., LACEY D.L., BOYLE W.J. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN L.L., WARTMANN M., LIN A.Y., KNOPF J.L., SETH A., DAVIS R.J. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- LIN W.-W., CHEN B.C. Distinct PKC isoforms mediate the activation of cPLA2 and adenylyl cyclase by phorbol ester in RAW264.7 macrophages. Br. J. Pharmacol. 1998;125:1601–1609. doi: 10.1038/sj.bjp.0702219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN Y., DEVIN A., RODRIGUEZ Y., LIU Z.G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING L., CAO Z.D., GOEDDEL D.V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc. Natl. Acad. Sci. U.S.A. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Z.G., HSU H.L., GOEDDEL D.V., KARIN M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- LOCKSLEY R.M., KILLEEN N., LENARDO M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- LOETSCHER H., STUEBER D., BANNER D., MACKAY F., LESSLAUER W. Human tumor-necrosis-factor-α (TNF-α) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J. Biol. Chem. 1993;268:26350–26357. [PubMed] [Google Scholar]
- LOZANO J., BERRA E., MUNICIO M.M., DIAZMECO M.T., DOMINGUEZ I., SANZ L., MOSCAT J. Protein-kinase-c-ζ isoform is critical for κB-dependent promoter activation by sphingomyelinase. J. Biol. Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- LUCAS R., GARCIA I., DONATI Y.R.A., HRIBAR M., MANDRIOTA S.J., GIROUD C., BUURMAN W.A., FRANSEN L., SUTER P.M., NUNEZ G., PEPPER M.S., GRAU G.E. Both TNF receptors are required for direct TNF-mediated cytotoxicity in microvascular endothelial cells. Eur. J. Immunol. 1998;28:3577–3586. doi: 10.1002/(SICI)1521-4141(199811)28:11<3577::AID-IMMU3577>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- LUCAS R., JUILLARD P., DECOSTER E., REDARD M., BURGER D., DONATI Y., GIROUD C., MONSOHINARD C., DEKESEL T., BUURMAN W.A., MOORE M.W., DAYER J.M., FIERS W., BLUETHMANN H., GRAU G.E. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur. J. Immunol. 1997a;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- LUCAS R., LOU J.N., JUILLARD P., MOORE M., BLUETHMANN H., GRAU G.E. Respective role of TNF receptors in the development of experimental cerebral malaria. J. Neuroimmunol. 1997b;72:143–148. doi: 10.1016/s0165-5728(96)00185-3. [DOI] [PubMed] [Google Scholar]
- LUSCHEN S., USSAT S., KRONKE M., ADAMKLAGES S. Cleavage of human cytosolic phospholipase A2 by caspase-1 (ICE) and caspase-8 (FLICE) Biochem. Biophys. Res. Commun. 1998;253:92–98. doi: 10.1006/bbrc.1998.9754. [DOI] [PubMed] [Google Scholar]
- MACDONALD T.T., MONTELEONE G., PENDER S.L.F. Recent developments in the immunology of inflammatory bowel disease. Scand. J. Immunol. 2000;51:2–9. doi: 10.1046/j.1365-3083.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- MACEWAN D.J. Elevated cPLA2 levels as a mechanism by which the p70 TNF and p75 NGF receptors enhance apoptosis. FEBS Lett. 1996;379:77–81. doi: 10.1016/0014-5793(95)01495-0. [DOI] [PubMed] [Google Scholar]
- MALININ N.L., BOLDIN M.P., KOVALENKO A.V., WALLACH D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- MANNA S.K., AGGARWAL B.B. Interleukin-4 down-regulates both forms of tumor necrosis factor receptor and receptor-mediated apoptosis, NF-κB, AP-1, and c-Jun N-terminal kinase-comparison with interleukin-13. J. Biol. Chem. 1998;273:33333–33341. doi: 10.1074/jbc.273.50.33333. [DOI] [PubMed] [Google Scholar]
- MANNA S.K., AGGARWAL B.B. Immunosuppressive leflunomide metabolite (A77 1726) blocks TNF-dependent nuclear factor-κB activation and gene expression. J. Immunol. 1999;162:2095–2102. [PubMed] [Google Scholar]
- MANNA S.K., MUKHOPADHYAY A., VAN N.T., AGGARWAL B.B. Silymarin suppresses TNF-induced activation of NF-κB, c-Jun N-terminal kinase, and apoptosis. J. Immunol. 1999;163:6800–6809. [PubMed] [Google Scholar]
- MARONEY A.C., FINN J.P., CONNORS T.J., DURKIN J.T., ANGELES T., GESSNER G., XU Z.H., MEYER S.L., SAVAGE M.J., GREENE L.A., SCOTT R.W., VAUGHT J.L. CEP-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J. Biol. Chem. 2001;276:25302–25308. doi: 10.1074/jbc.M011601200. [DOI] [PubMed] [Google Scholar]
- MARQUET R.L., DEBRUIN R.W.F., FIERS W., JEEKEL J. Indomethacin reduces TNF-toxicity without interfering with its efficacy. Immunobiology. 1987;175:54–55. [Google Scholar]
- MATHIAS S., DRESSLER K.A., KOLESNICK R.N. Characterization of a ceramide-activated protein-kinase-stimulation by tumor-necrosis-factor-α. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIAS S., PENA L.A., KOLESNICK R.N. Signal transduction of stress via ceramide. Biochem. J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUDA S., KOYASU S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- MAYER A.M.S., BRENIC S., GLASER K.B. Pharmacological targeting of signaling pathways in protein kinase C-stimulated superoxide generation in neutrophil-like HL-60 cells: effect of phorbol ester, arachidonic acid and inhibitors of kinase(s), phosphatase(s) and phospholipase A2. J. Pharmacol. Exp. Ther. 1996;279:633–644. [PubMed] [Google Scholar]
- MCDERMOTT M.F. TNF and TNFR biology in health and disease. Cell. Mol. Biol. 2001;47:619–635. [PubMed] [Google Scholar]
- MCFARLANE S.M., ANDERSON H.M., TUCKER S.J., JUPP O.J., MACEWAN D.J. Unmodified calcium concentrations in tumour necrosis factor receptor subtype-mediated apoptotic cell death. Mol. Cell. Biochem. 2000;211:19–26. doi: 10.1023/a:1007189911897. [DOI] [PubMed] [Google Scholar]
- MCFARLANE S.M., JUPP O.J., COBBAN H.J., HUNTER I., ANDERSON H.M., VANDENABEELE P., NIXON G.F., MACEWAN D.J. Stimulation of stress-activated but not mitogen-activated protein kinases by tumour necrosis factor receptor subtypes in airway smooth muscle. Biochem. Pharmacol. 2001;61:749–759. doi: 10.1016/s0006-2952(01)00530-5. [DOI] [PubMed] [Google Scholar]
- MCLEISH K.R., KLEIN J.B., LEDERER E.D., HEAD K.Z., WARD R.A. Azotemia, TNF α, and 1ps prime the human neutrophil oxidative burst by distinct mechanisms. Kidney Int. 1996;50:407–416. doi: 10.1038/ki.1996.330. [DOI] [PubMed] [Google Scholar]
- MEDVEDEV A.E., ESPEVIK T., RANGES G., SUNDAN A. Distinct roles of the two tumor necrosis factor (TNF) receptors in modulating TNF and lymphotoxin α effects. J. Biol. Chem. 1996;271:9778–9784. doi: 10.1074/jbc.271.16.9778. [DOI] [PubMed] [Google Scholar]
- MIN W., POBER J.S. TNF initiates e-selectin transcription in human endothelial cells through parallel TRAF-NF-κB and TRAF-rac/cdc42-JNK-c-Jun/ATF2 pathways. J. Immunol. 1997;159:3508–3518. [PubMed] [Google Scholar]
- MINDEN A., LIN A., MCMAHON M., LANGECARTER C., DERIJARD B., DAVIS R.J., JOHNSON G.L., KARIN M. Differential activation of ERK and JNK mitogen-activated protein-kinases by raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- MISHRA S., MATHUR R., HAMBURGER A.W. Modulation of the cytotoxic activity of tumor-necrosis-factor by protein-tyrosine kinase and protein-tyrosine-phosphatase inhibitors. Lymphokine Cytokine Res. 1994;13:77–83. [PubMed] [Google Scholar]
- MOORE R.J., OWENS D.M., STAMP G., ARNOTT C., BURKE F., EAST N., HOLDSWORTH H., TURNER L., ROLLINS B., PASPARAKIS M., KOLLIAS G., BALKWILL F. Mice deficient in tumor necrosis factor-α are resistant to skin carcinogenesis. Nature Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- MOOSMAYER D., DINKEL A., GERLACH E., HESSABI B., GRELL M., PFIZENMAIER K., SCHEURICH P. Coexpression of the human TNF receptors tr6o and TR80 in insect cells-analysis of receptor complex-formation. Lymphokine Cytokine Res. 1994;13:295–301. [PubMed] [Google Scholar]
- MORINVILLE A., MAYSINGER D., SHAVER A. From vanadis to atropos: vanadium compounds as pharmacological tools in cell death signalling. Trends Pharmacol. Sci. 1998;19:452–460. doi: 10.1016/s0165-6147(98)01257-7. [DOI] [PubMed] [Google Scholar]
- MULLER G., AYOUB M., STORZ P., RENNECKE J., FABBRO D., PFIZENMAIER K. PKC ζ is a molecular switch in signal-transduction of TNF-α, bifunctionally regulated by ceramide and arachidonic-acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER G., STORZ P., BOURTEELE S., DOPPLER H., PFIZENMAIER K., MISCHAK H., PHILIPP A., KAISER C., KOLCH W. Regulation of raf-1 kinase by TNF via its second messenger ceramide and cross-talk with mitogenic signalling. EMBO J. 1998;17:732–742. doi: 10.1093/emboj/17.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAKATA C., KANEKO M., MATSUDA Y., SAITO H., SAITO Y., TANAKA E., TAMAOKI T., MATSUMOTO T., KANER J.C., HUDKINS R.L., ROTELLA D.P., GLICKSMAN M.A., SAPORITO M., CARSWELL S., HAUN F., KNIGHT E., DIONNE C., NEFF N.T., VAUGHT J.L., MALLAMO J.P. Neurotrophic derivatives of K252a. profile of CEP1347/KT7515 and SAR of survival promoting analogs. Abstr. Papers Am. Chemical Soc. 1996;212:211. [Google Scholar]
- MUZIO M., CHINNAIYAN A.M., KISCHKEL F.C., O'ROURKE K., SHEVCHENKO A., NI J., SCAFFIDI C., BRETZ J.D., ZHANG M., GRENTZ R., MANN M., KRAMMER P.H., PETER M.E., DIXIT V.M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/apo-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- NASUHARA Y., ADCOCK I.M., CATLEY M., BARNES P.J., NEWTON R. Differential IκB kinase activation and IκBα degradation by interleukin-1β and tumor necrosis factor-α in human U937 monocytic cells-evidence for additional regulatory steps in κB-dependent transcription. J. Biol. Chem. 1999;274:19965–19972. doi: 10.1074/jbc.274.28.19965. [DOI] [PubMed] [Google Scholar]
- NEMENOFF R.A., WINITZ S., QIAN N.X., VANPUTTEN V., JOHNSON G.L., HEASLEY L.E. Phosphorylation and activation of a high-molecular-weight form of phospholipase-A2 by p42 microtubule-associated protein-2 kinase and protein-kinase-C. J. Biol. Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- NISHITOH H., SAITOH M., MOCHIDA Y., TAKEDA K., NAKANON H., ROTHE M., MIYAZONO K., ICHIJO H. Ask1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 1998;2:1–20. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- ODEH M. The role of tumour necrosis factor-α in the pathogenes of complicated falciparum malaria. Cytokine. 2001;14:11–18. doi: 10.1006/cyto.2001.0845. [DOI] [PubMed] [Google Scholar]
- O'DWYER M.E., DRUKER B.J. Chronic myelogenous leukaemia – new therapeutic principles. J. Intern. Med. 2001;250:3–9. doi: 10.1046/j.1365-2796.2001.00823.x. [DOI] [PubMed] [Google Scholar]
- OKAZAKI T., BELL R.M., HANNUN Y.A. Sphingomyelin turnover induced by vitamin-D3 in HL-60 cells – role in cell-differentiation. J. Biol. Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- OLD L.J. Tumor necrosis factor (TNF) Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- OZES O.N., MAYO L.D., GUSTIN J.A., PFEFFER S.R., PFEFFER L.M., DONNER D.B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- PALEOLOG E.M., DELASALLE S.A.J., BUURMAN W.A., FELDMANN M. Functional activities of receptors for tumor-necrosis-factor-α on human vascular endothelial-cells. Blood. 1994;84:2578–2590. [PubMed] [Google Scholar]
- PARRIS J.R.M., COBBAN H.J., LITTLEJOHN A.F., MACEWAN D.J., NIXON G.F. Tumour necrosis factor-α activates a calcium sensitization pathway in guinea-pig bronchial smooth muscle. J. Physiol. 1999;518:561–569. doi: 10.1111/j.1469-7793.1999.0561p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL A., WILSON S., BELHAM C.M., ROBINSON C.J.M., SCOTT P.H., GOULD G.W., PLEVIN R. Stress-activated protein kinases: activation, regulation and function. Cell. Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- PENNICA D., KOHR W.J., FENDLY B.M., SHIRE S.J., RAAB H.E., BORCHARDT P.E., LEWIS M., GOEDDEL D.V. Characterization of a recombinant extracellular domain of the type-1 tumor-necrosis-factor receptor – evidence for tumor-necrosis-factor-α induced receptor aggregation. Biochemistry. 1992a;31:1134–1141. doi: 10.1021/bi00119a023. [DOI] [PubMed] [Google Scholar]
- PENNICA D., LAM V.T., MIZE N.K., WEBER R.F., LEWIS M., FENDLY B.M., LIPARI M.T., GOEDDEL D.V. Biochemical-properties of the 75-kDa tumor-necrosis-factor receptor – characterization of ligand-binding, internalization, and receptor phosphorylation. J. Biol. Chem. 1992b;267:21172–21178. [PubMed] [Google Scholar]
- PERONA R., MONTANER S., SANIGER L., SANCHEZPEREZ I., BRAVO R., LACAL J.C. Activation of the nuclear factor-κB by rho, cdc42, and rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- PETRACHE I., VERIN A.D., CROW M.T., BIRUKOVA A., LIU F., GARCIA J.G.N. Differential effect of MLC kinase in TNF-α-induced endothelial cell apoptosis and barrier dysfunction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001;280:L1168–L1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- PFEFFER K., MATSUYAMA T., KUNDIG T.M., WAKEHAM A., KISHIHARA K., SHAHINIAN A., WIEGMANN K., OHASHI P.S., KRONKE M., MAK T.W. Mice deficient for the 55 kDa tumor-necrosis-factor receptor are resistant to endotoxic-shock, yet succumb to L-monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- PIMENTEL-MUINOS F.X., SEED B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- POLLOCK J., MCFARLANE S.M., CONNELL M.C., ZEHAVI U., VANDENABEELE P., MACEWAN D.J., SCOTT R.H. TNF receptors simultaneously activate Ca2+ mobilisation and stress kinases in cultured sensory neurones. Neuropharmacology. 2002;42:93–106. doi: 10.1016/s0028-3908(01)00163-0. [DOI] [PubMed] [Google Scholar]
- POLLOCK V.P., LOFTHOUSE E.J., JUPP O.J., GAULD S.B., ANDERSON H.M., MACEWAN D.J. Selective down-regulation of the Gqα/G11α G-protein family in tumour necrosis factor-α induced cell death. Mol. Cell. Biochem. 2000;206:67–74. doi: 10.1023/a:1007066409645. [DOI] [PubMed] [Google Scholar]
- PORNARES M.I., ARES M.P.S., ORRENIUS S. Calcium signalling and the regulation of apoptosis. Toxicol. in vitro. 1998a;12:539–543. doi: 10.1016/s0887-2333(98)00032-0. [DOI] [PubMed] [Google Scholar]
- PORNARES M.I., SAMALI A., ORRENIUS S. Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Diff. 1998b;5:1028–1033. doi: 10.1038/sj.cdd.4400424. [DOI] [PubMed] [Google Scholar]
- PORTEU F., HIEBLOT C. Tumor-necrosis-factor induces a selective shedding of its p75-receptor from human neutrophils. J. Biol. Chem. 1994;269:2834–2840. [PubMed] [Google Scholar]
- PROBERT L., EUGSTER H.P., AKASSOGLOU K., BAUER J., FREI K., LASSMANN H., FONTANA A. TNFR1 signalling is critical for the development of demyelination and the limitation of T-cell responses during immune-mediated CNS disease. Brain. 2000;123:2005–2019. doi: 10.1093/brain/123.10.2005. [DOI] [PubMed] [Google Scholar]
- PULS A., ELIOPOULOS A.G., NOBES C.D., BRIDGES T., YOUNG L.S., HALL A. Activation of the small GTPase cdc42 by the inflammatory cytokines TNF α and IL-1, and by the epstein-barr virus transforming protein lmp 1. J. Cell Sci. 1999;112:2983–2992. doi: 10.1242/jcs.112.17.2983. [DOI] [PubMed] [Google Scholar]
- PYNE S., PYNE N.J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINES M.A., KOLESNICK R.N., GOLDE D.W. Sphingomyelinase and ceramide activate mitogen-activated protein-kinase in myelin HL-60 cells. J. Biol. Chem. 1993;268:14572–14575. [PubMed] [Google Scholar]
- RAO K.M.K. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001;69:3–10. [PubMed] [Google Scholar]
- RAVICHANDRAN L.V., ESPOSITO D.L., CHEN J., QUON M.J. Protein kinase C-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J. Biol. Chem. 2001;276:3543–3549. doi: 10.1074/jbc.M007231200. [DOI] [PubMed] [Google Scholar]
- REITHMANN C., WERDAN K. Tumor-necrosis-factor-α decreases inositol phosphate formation and phosphatidylinositol-bisphosphate (PIP2) synthesis in rat cardiomyocytes. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:175–182. doi: 10.1007/BF00169834. [DOI] [PubMed] [Google Scholar]
- REYES J.G., ROBAYNA I.G., DELGADO P.S., GONZALEZ I.H., AGUIAR J.Q., ROSAS F.E., FANJUL L.F., DEGALARRETA C.M.R. c-Jun is a downstream target for ceramide-activated protein phosphatase in A431 cells. J. Biol. Chem. 1996;271:21375–21380. doi: 10.1074/jbc.271.35.21375. [DOI] [PubMed] [Google Scholar]
- RICH T., ALLEN R.L., WYLLIE A.H. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- ROSADO J.A., ROSENZWEIG I., HARDING S., SAGE S.O. Tumor necrosis factor-α inhibits store-mediated Ca2+ entry in the human hepatocellular carcinoma cell line HEPG2. Am. J. Physiol.-Cell Physiol. 2001;280:C1636–C1644. doi: 10.1152/ajpcell.2001.280.6.C1636. [DOI] [PubMed] [Google Scholar]
- ROTHE J., LESSLAUER W., LOTSCHER H., LANG Y., KOEBEL P., KONTGEN F., ALTHAGE A., ZINKERNAGEL R., STEINMETZ M., BLUETHMANN H. Mice lacking the tumor-necrosis-factor receptor-1 are resistant to TNF-mediated toxicity but highly susceptible to infection by listeria-monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- ROTHE M., PAN M.G., HENZEL W.J., AYRES T.M., GOEDDEL D.V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral-inhibitor of apoptosis proteins. Cell. 1995a;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- ROTHE M., SARMA V., DIXIT V.W., GOEDDEL D.V. TRAF2-mediated activation of NF-κ-B by TNF receptor-2 and CD40. Science. 1995b;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- ROTHE M., WONG S.C., HENZEL W.J., GOEDDEL D.V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor-necrosis-factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- SANZ L., SANCHEZ P., LALLENA M.J., DIAZ-MECO M.T., MOSCAT J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWAI H., OKAZAKI T., TAKEDA Y., TASHIMA M., SAWADA H., OKUMA M., KISHI S., UMEHARA H., DOMAE N. Ceramide-induced translocation of protein kinase C-δ and -ε to the cytosol – implications in apoptosis. J. Biol. Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- SCHALL T.J., LEWIS M., KOLLER K.J., LEE A., RICE G.C., WONG G.H.W., GATANAGA T., GRANGER G.A., LENTZ R., RAAB H., KOHR W.J., GOEDDEL D.V. Molecular-cloning and expression of a receptor for human tumor-necrosis-factor. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- SCHEID M., DURONIO V. Dissociation of cytokine-induced phosphorylation of bad and activation of PKB/Akt: involvement of MEK upstream of bad phosphorylation. Proc. Natl. Acad. Sci. 1998;95:7439–7444. doi: 10.1073/pnas.95.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERZER J.A., LIN Y., MCLEISH K.R., KLEIN J.B. TNF translationally modulates the expression of G protein αi2 subunits in human polymorphonuclear leukocytes. J. Immunol. 1997;158:913–918. [PubMed] [Google Scholar]
- SCHIEVELLA A.R., CHEN J.H., GRAHAM J.R., LIN L.-L. MADD, a novel death domain protein that interacts with the type 1 tumour necrosis factor receptor and activates mitogen-activated protein kinase. J. Biol. Chem. 1997;272:12069–12075. doi: 10.1074/jbc.272.18.12069. [DOI] [PubMed] [Google Scholar]
- SCHLUTER D., DECKERT M. The divergent role of tumor necrosis factor receptors in infectious diseases. Microbes Inf. 2000;2:1285–1292. doi: 10.1016/s1286-4579(00)01282-x. [DOI] [PubMed] [Google Scholar]
- SCHOTTE P., DECLERCQ W., VANHUFFEL S., VANDENABEELE P., BEYAERT R. Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett. 1999;442:117–121. doi: 10.1016/s0014-5793(98)01640-8. [DOI] [PubMed] [Google Scholar]
- SCHUBERT K.M., SCHEID M.P., DURONIO V. Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 2000;275:13330–13335. doi: 10.1074/jbc.275.18.13330. [DOI] [PubMed] [Google Scholar]
- SCHUTZE S., BERKOVIC D., TOMSING O., UNGER C., KRONKE M. Tumor necrosis factor induces rapid production of 1′2′diacylglycerol by a phosphatidylcholine-specific phospholipase C. J. Exp. Med. 1991;174:975–988. doi: 10.1084/jem.174.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUTZE S., NOTTROTT S., PFIZENMAIER K., KRONKE M. Tumor-necrosis-factor signal transduction – cell-type-specific activation and translocation of protein kinase-C. J. Immunol. 1990;144:2604–2608. [PubMed] [Google Scholar]
- SCHWENGER P., ALPERT D., SKOLNIK E.Y., VILCEK J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IκBα phosphorylation and degradation. Mol. Cell. Biol. 1998;18:78–84. doi: 10.1128/mcb.18.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCREATON G., XU X.N. T cell life and death signalling via TNF-Receptor family members. Curr. Opin. Immunol. 2000;12:316–322. doi: 10.1016/s0952-7915(00)00093-5. [DOI] [PubMed] [Google Scholar]
- SETHI J.K., XU H.Y., UYSAL T., WIESBROCK S.M., SCHEJA L., HOTAMISLIGIL G.S. Characterisation of receptor-specific TNF α functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000;469:77–82. doi: 10.1016/s0014-5793(00)01250-3. [DOI] [PubMed] [Google Scholar]
- SHI C.S., KEHRL J.H. Activation of stress-activated protein kinase c-Jun N-terminal kinase, but not NF-κB, by the tumor necrosis factor (TNF) receptor 1 through a TNF receptor-associated factor 2- and germinal center kinase related-dependent pathway. J. Biol. Chem. 1997;272:32102–32107. doi: 10.1074/jbc.272.51.32102. [DOI] [PubMed] [Google Scholar]
- SINGH S., AGGARWAL B.B. Protein-tyrosine-phosphatase inhibitors block tumor necrosis factor-dependent activation of the nuclear transcription factor NF-κ-B. J. Biol. Chem. 1995;270:10631–10639. doi: 10.1074/jbc.270.18.10631. [DOI] [PubMed] [Google Scholar]
- SONG H.Y., ROTHE M., GOEDDEL D.V. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-κB activation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEISER D.E., BACHMANN M.F., FRICK T.W., MCKALLFAIENZA K., GRIFFITHS E., PFEFFER K., MAK T.W., OHASHI P.S. TNF receptor p55 controls early acute graft-versus-host disease. J. Immunol. 1997;158:5185–5190. [PubMed] [Google Scholar]
- SQUIER M.K.T., COHEN J.J. Calpain and cell death. Cell Death Diff. 1996;3:275–283. [PubMed] [Google Scholar]
- STANGER B.Z., LEDER P., LEE T.H., KIM E., SEED B. RIP – a novel protein containing a death domain that interacts with Fas/Apo-1 (CD95) in yeast and causes cell-death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- STORZ P., DOPPLER H., WERNIG A., PFIZENMAIER K., MULLER G. TNF inhibits insulin induced STAT5 activation in differentiated mouse muscle cells PMI28. FEBS Lett. 1998;440:41–45. doi: 10.1016/s0014-5793(98)01421-5. [DOI] [PubMed] [Google Scholar]
- SUFFYS P., BEYAERT R., DEVALCK D., VANHAESEBROECK B., VANROY F., FIERS W. Tumor-necrosis-factor-mediated cytotoxicity is correlated with phospholipase-A2 activity, but not with arachidonic-acid release per- se. Eur. J. Biochem. 1991;195:465–475. doi: 10.1111/j.1432-1033.1991.tb15727.x. [DOI] [PubMed] [Google Scholar]
- TARTAGLIA L.A., AYRES T.M., WONG G.H.W., GOEDDEL D.V. A novel domain within the 55 kDa TNF receptor signals cell-death. Cell. 1993a;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- TARTAGLIA L.A., PENNICA D., GOEDDEL D.V. Ligand passing – the 75-kDa tumor-necrosis-factor (TNF) receptor recruits TNF for signaling by the 55-kDa TNF receptor. J. Biol. Chem. 1993b;268:18542–18548. [PubMed] [Google Scholar]
- TARTAGLIA L.A., WEBER R.F., FIGARI I.S., REYNOLDS C., PALLADINO M.A., GOEDDEL D.V. The two different receptors for tumor-necrosis-factor mediate distinct cellular-responses. Proc. Natl. Acad. Sci. U.S.A. 1991;88:9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P.C. Anti-TNF therapy for rheumatoid arthritis and other inflammatory diseases. Mol. Biotech. 2001;19:153–168. doi: 10.1385/MB:19:2:153. [DOI] [PubMed] [Google Scholar]
- TEWARI M., QUAN L.T., OROURKE K., DESNOYERS S., ZENG Z., BEIDLER D.R., POIRIER G.G., SALVESEN G.S., DIXIT V.M. YAMA/CPP32-β, a mammalian homolog of CED-3, is a CrmA – inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- THOMAS P.S. Tumour necrosis factor-α: the role of this multifunctional cytokine in asthma. Immunol. Cell Biol. 2001;79:132–140. doi: 10.1046/j.1440-1711.2001.00980.x. [DOI] [PubMed] [Google Scholar]
- THOME M., SCHNEIDER P., HOFMANN K., FICKENSCHER H., MEINL E., NEIPEL F., MATTMANN C., BURNS K., BODMER J.L., SCHROTER M., SCAFFIDI C., KRAMMER P.H., PETER M.E., TSCHOPP J. Viral FLICE-inhibitory proteins (FLIPS) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- TOLAN D., CONWAY A.M., STEELE L., PYNE S., PYNE N.J. The identification of DL-threo dihydrosphingosine and sphingosine as novel inhibitors of extracellular signal- regulated kinase signalling in airway smooth muscle. Br. J. Pharmacol. 1996;119:185–186. doi: 10.1111/j.1476-5381.1996.tb15967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOTPAL K., AGARWAL S., AGGARWAL B.B. Phosphatase inhibitors modulate the growth-regulatory effects of human tumor-necrosis-factor on tumor and normal-cells. Cancer Res. 1992;52:2557–2562. [PubMed] [Google Scholar]
- TRENT J.C., MCCONKEY D.J., LOUGHLIN S.M., HARBISON M.T., FERNANDEZ A., ANANTHASWAMY H.N. Ras signaling in tumor necrosis factor-induced apoptosis. EMBO J. 1996;15:4497–4505. [PMC free article] [PubMed] [Google Scholar]
- TSUJIMOTO M., YIP Y.K., VILCEK J. Tumor necrosis factor – specific binding and internalization in sensitive and resistant cells. Proc. Natl. Acad. Sci. U.S.A. 1985;82:7626–7630. doi: 10.1073/pnas.82.22.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTZ P.J., ANDERSON P. Life and death decisions: regulation of apoptosis by proteolysis of signaling molecules. Cell Death Diff. 2000;7:589–602. doi: 10.1038/sj.cdd.4400696. [DOI] [PubMed] [Google Scholar]
- UYSAL K.T., WIESBROCK S.M., MARINO M.W., HOTAMISLIGIL G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- VAN ASSCHE G., RUTGEERTS P. Anti-TNF agents in Crohn's disease. Expert Opin. Investigational Drugs. 2000;9:103–111. doi: 10.1517/13543784.9.1.103. [DOI] [PubMed] [Google Scholar]
- VANDENABEELE P., DECLERCQ W., BEYAERT R., FIERS W. 2 Tumor-necrosis-factor receptors – structure and function. Trends Cell Biol. 1995;5:392–399. doi: 10.1016/s0962-8924(00)89088-1. [DOI] [PubMed] [Google Scholar]
- VANDENBERGHE W., PLAISANCE S., BOONE E., DEBOSSCHER K., SCHMITZ M.L., FIERS W., HAEGEMAN G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor κB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- VAN DER VEEN A.H., TEN HAGEN T.L.M., DE WILT J.H.W., VAN IJKEN M.G.A., EGGERMONT A.M.M. An overview on the use of TNF-α: our experience with regional administration and developments towards new opportunities for systemic application. Anticancer Res. 2000;20:3467–3474. [PubMed] [Google Scholar]
- VANHAESEBROECK B., ALESSI D.R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- VANLINT J., AGOSTINIS P., VANDEVOORDE V., HAEGEMAN G., FIERS W., MERLEVEDE W., VANDENHEEDE J.R. Tumor-necrosis-factor stimulates multiple serine threonine protein-kinases in swiss 3T3 and L929 cells – implication of casein kinase-2 and extracellular signal-regulated kinases in the tumor-necrosis-factor signal transduction pathway. J. Biol. Chem. 1992;267:25916–25921. [PubMed] [Google Scholar]
- VANOSTADE X., VANDENABEELE P., EVERAERDT B., LOETSCHER H., GENTZ R., BROCKHAUS M., LESSLAUER W., TAVERNIER J., BROUCKAERT P., FIERS W. Human TNF mutants with selective activity on the p55 receptor. Nature. 1993;361:266–269. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- VERCAMMEN D., BEYAERT R., DENECKER G., GOOSSENS V., VANLOO G., DECLERCQ W., GROOTEN J., FIERS W., VANDENABEELE P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 1998a;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERCAMMEN D., BROUCKAERT G., DENECKER G., VANDECRAEN M., DECLERCQ W., FIERS W., VANDENABEELE P. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 1998b;188:919–930. doi: 10.1084/jem.188.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERCAMMEN D., VANDENABEELE P., DECLERCQ W., VANDECRAEN M., GROOTEN J., FIERS W. Cytotoxicity in L929 murine fibrosarcoma cells after triggering of transfected human p75 tumor-necrosis-factor (TNF) receptor is mediated by endogenous murine TNF. Cytokine. 1995;7:463–470. doi: 10.1006/cyto.1995.0063. [DOI] [PubMed] [Google Scholar]
- VERHEIJ M., BOSE R., LIN X.H., YAO B., JARVIS W.D., GRANT S., BIRRER M.J., SZABO E., ZON L.I., KYRIAKIS J.M., HAIMOVITZFRIEDMAN A., FUKS Z., KOLESNICK R.N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- VILLA P., KAUFMANN S.H., EARNSHAW W.C. Caspases and caspase inhibitors. Trends Biochem. Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- VOELKELJOHNSON C., ENTINGH A.J., WOLD W.S.M., GOODING L.R., LASTER S.M. Activation of intracellular proteases is an early event in TNF-induced apoptosis. J. Immunol. 1995;154:1707–1716. [PubMed] [Google Scholar]
- WAAGE A., ESPEVIK T., BAKKE O., HALSTENSEN A., BRANDTZAEG P., NISSENMEYER J., LAMVIK J. Detection of TNF in human septicemia. Immunobiology. 1987;175:41. [Google Scholar]
- WAJANT H., HAAS E., SCHWENZER R., MUHLENBECK F., KREUZ S., SCHUBERT G., GRELL M., SMITH C., SCHEURICH P. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD) J. Biol. Chem. 2000;275:24357–24366. doi: 10.1074/jbc.M000811200. [DOI] [PubMed] [Google Scholar]
- WAJANT H., HENKLER F., SCHEURICH P. The TNF-Receptor-associated factor family – scaffold molecules for cytokine receptors, kinases and their regulators. Cell. Signal. 2001;13:389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- WANG B., KONDO S., SHIVJI G.M., FUJISAWA H., MAK T.W., SAUDER D.N. Tumour necrosis factor receptor II (p75) signalling is required for the migration of Langerhans' cells. Immunology. 1996;88:284–288. doi: 10.1111/j.1365-2567.1996.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD C., DRANSFIELD I., CHILVERS E.R., HASLETT C., ROSSI A.G. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol. Sci. 1999;20:503–509. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- WAY K.J., CHOU E., KING G.L. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol. Sci. 2000;21:181–187. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- WEISS T., GRELL M., HESSABI B., BOURTEELE S., MULLER G., SCHEURICH P., WAJANT H. Enhancement of TNF receptor p60-mediated cytotoxicity by TNF receptor p80 – requirement of the TNF receptor-associated factor-2 binding site. J. Immunol. 1997;158:2398–2404. [PubMed] [Google Scholar]
- WEISS T., GRELL M., SIEMIENSKI K., MUHLENBECK F., DURKOP H., PFIZENMAIER K., SCHEURICH P., WAJANT H. TNFR80-dependent enhancement of TNFR60-induced cell death is mediated by TNFR-associated factor 2 and is specific for TNFR60. J. Immunol. 1998;161:3136–3142. [PubMed] [Google Scholar]
- WIDMANN C., GIBSON S., JOHNSON G.L. Caspase-dependent cleavage of signaling proteins during apoptosis – a turn-off mechanism for anti-apoptotic signals. J. Biol. Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- WIEGMANN K., SCHUTZE S., KAMPEN E., HIMMLER A., MACHLEIDT T., KRONKE M. Human 55-kDa receptor for tumor-necrosis-factor coupled to signal transduction cascades. J. Biol. Chem. 1992;267:17997–18001. [PubMed] [Google Scholar]
- WIEGMANN K., SCHUTZE S., MACHLEIDT T., WITTE D., KRONKE M. Functional dichotomy of neutral and acidic sphingomyelinases in tumour necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- WISSING D., MOURITZEN H., EGEBLAD M., POIRIER G.G., JAATTELA M. Involvement of caspase-dependent activation of cytosolic phospholipase A2 in tumor necrosis factor-induced apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5073–5077. doi: 10.1073/pnas.94.10.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOJCIAK-STOTHARD B., ENTWISTLE A., GARG R., RIDLEY A.J. Regulation of TNF-α-induced reorganization of the actin cytoskeleton and cell-cell Junctions by rho, rac, and cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- WONG G.H.W., ELWELL J.H., OBERLEY L.W., GOEDDEL D.V. Manganous superoxide-dismutase is essential for cellular-resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- WONG G.H.W., GOEDDEL D.V. Induction of manganous superoxide-dismutase by tumor necrosis factor – possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- WU Y.L., JIANG X.R., LILLINGTON D.M., ALLEN P.D., NEWLAND A.C., KELSEY S.M. 1,25-Dihydroxyvitamin D-3 protects human leukemic cells from tumor necrosis factor-induced apoptosis via inactivation of cytosolic phospholipase A2. Cancer Res. 1998a;58:633–640. [PubMed] [Google Scholar]
- WU Y.L., JIANG X.R., NEWLAND A.C., KELSEY S.M. Failure to activate cytosolic phospholipase A2 causes TNF resistance in human leukemic cells. J. Immunol. 1998b;160:5929–5935. [PubMed] [Google Scholar]
- WUYTS W., VAN WESENBEECK L., MORALES-PIGA A., RALSTON S., HOCKING L., VANHOENACKER F., WESTHOVENS R., VERBRUGGEN L., ANDERSON D., HUGHES A., VAN HUL W. Evaluation of the role of RANK and OPG genes in Paget's disease of bone. Bone. 2001;28:104–107. doi: 10.1016/s8756-3282(00)00411-7. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- YAN F., POLK D.B. Kinase suppressor of ras is necessary for tumor necrosis factor α activation of extracellular signal-regulated kinase/mitogen-activated protein kinase in intestinal epithelial cells. Cancer Res. 2001;61:963–969. [PubMed] [Google Scholar]
- YANAGA F., ABE M., KOGA T., HIRATA M. Signal transduction by tumor-necrosis-factor-α is mediated through a guanine nucleotide-binding protein in osteoblast-like cell-line, MC3T3-E1. J. Biol. Chem. 1992;267:5114–5121. [PubMed] [Google Scholar]
- YAO B., ZHANG Y.H., DELIKAT S., MATHIAS S., BASU S., KOLESNICK R. Phosphorylation of raf by ceramide-activated protein-kinase. Nature. 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]
- YEH W.C., SHAHINIAN A., SPEISER D., KRAUNUS J., BILLIA F., WAKEHAM A., DELAPOMPA J.L., FERRICK D., HUM B., ISCOVE N., OHASHI P., ROTHE M., GOEDDEL D.V., MAK T.W. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2- deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- YIN L., WU L., WESCHE H., ARTHUR C.D., WHITE J.M., GOEDDEL D.V., SCHREIBER R.D. Defective lymphotoxin-β receptor-induced NF-κB transcriptional activity in NIK-deficient mice. Science. 2001;291:2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- YOREK M., JAIPAUL N., DUNLAP J., BIELEFELDT K. Endothelin-stimulated Ca2+ mobilization by 3T3-L1 adipocytes is suppressed by tumor necrosis factor-α. Arch Biochem Biophys. 1999;361:241–251. doi: 10.1006/abbi.1998.0982. [DOI] [PubMed] [Google Scholar]
- YOUNG P.R. Pharmacological modulation of cytokine action and production through signaling pathways. Cytokine Growth Factor Rev. 1998;9:239–257. doi: 10.1016/s1359-6101(98)00011-2. [DOI] [PubMed] [Google Scholar]
- YUASA T., OHNO S., KEHRL J.H., KYRIAKIS J.M. Tumor necrosis factor signaling to stress-activated protein kinase (SAPK)/Jun NH2-terminal kinase (JNK) and p38 – germinal center kinase couples TRAF2 to mitogen-activated protein kinase/ERK kinase kinase 1 and SAPK while receptor interacting protein associates with a mitogen-activated protein kinase kinase kinase upstream of MKK6 and p38. J. Biol. Chem. 1998;273:22681–22692. doi: 10.1074/jbc.273.35.22681. [DOI] [PubMed] [Google Scholar]
- ZHANG Y.H., YAO B., DELIKAT S., BAYOUMY S., LIN X.H., BASU S., MCGINLEY M., CHANHUI P.Y., LICHENSTEIN H., KOLESNICK R. Kinase suppressor of ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- ZHENG T.S., HUNOT S., KUIDA K., FLAVELL R.A. Caspase knockouts: matters of life and death. Cell Death Diff. 1999;6:1043–1053. doi: 10.1038/sj.cdd.4400593. [DOI] [PubMed] [Google Scholar]
- ZHOU H.L., SUMMERS S.K., BIRNBAUM M.J., PITTMAN R.N. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- ZHOU Q., SALVESEN G.S. Viral caspase inhibitors CrmA and p35. Apoptosis. 2000;322:143–154. doi: 10.1016/s0076-6879(00)22014-4. [DOI] [PubMed] [Google Scholar]


