Abstract
The modulation of large conductance Ca2+-activated K+ (BKCa) channels by the nitric oxide (NO·) donor, S-nitroso-L-cysteine (NOCys) and three sulfhydryl-specific methanethiosulfonate (MTS) reagents, positively charged 2-aminoethyl MTS hydrobromide (MTSEA C3H9NO2S2HBr) and [2-(trimethylammonium) ethyl MTS bromide (MTSET C6H16NO2S2Br), and negatively charged sodium (2-sulfonatoethyl) MTS (MTSES C3H7O5S3Na) were compared in excised inside-out membrane patches of the guinea-pig taenia caeca.
In membrane patches bathed in a low Ca2+ (15 nM) high K+ physiological salt solution, 1–3 BKCa channels opened with a low probability (N.Po) of 0.019±0.011 at 0 mV. N.Po readily increased with membrane depolarization, raised Ca2+ concentration or upon the addition of NOCys (10 μM for 2–5 min) such that 5–15 open BKCa channels were evident.
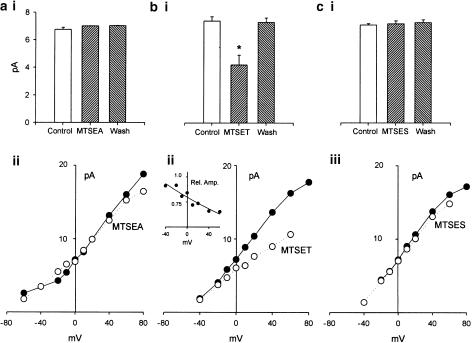
MTSEA (2.5 mM) decreased, while MTSES (2.5 mM) increased N.Po (at 0 mV) and the number of open BKCa channels at positive potentials. These changes in channel activity remained after a prolonged washout of these two MTS reagents.
MTSET (2.5 mM) increased N.Po (at 0 mV) and voltage-dependently decreased BKCa current amplitudes in a manner readily reversed upon washout.
Pre-exposure of excised membrane patches to MTSES or N-ethylmaleimide (NEM 1 mM), a specific alkylating agent of cysteine sulfhydryls, but not MTSEA or MTSET, prevented the excitatory actions of NOCys (10 μM).
It was concluded that NOCys-evoked increases in BKCa channel activity occur via the S-nitrosylation of cysteine residues within basic regions of the channel α subunit that have an accessible water interface.
Keywords: Nitric oxide, large conductance Ca2+-activated K+ channels, methanethiosulfonate reagents, patch clamping, cysteine residues, smooth muscle
Introduction
Large conductance Ca2+-activated K+ (BKCa) channels are abundant in most smooth muscles and are generally thought to be involved in controlling the electrical excitability of the membrane potential that regulates voltage-activated Ca2+ entry and muscle contraction. Although the gating of smooth muscle and neuronal BKCa channels is under the control of the membrane potential, the internal concentration of free Ca2+ and the phosphorylation state of the channel protein, both native (Bolotina et al., 1994; Park et al., 1995; Thuringer & Findlay, 1997; Mistry & Garland, 1998; Ahern et al., 1999; Gong et al., 2000; Lang et al., 2000) and cloned α subunits (DiChiara & Reinhart, 1997; Jeong et al., 2001; Tang et al., 2001; Chung et al., 2002) of BKCa channels can be directly modulated by the cell redox potential. Oxidation of sulfhydryl (thiol or RSH)-containing cysteine residues, particularly in the presence of trace amounts of metal ions, leads to the formation of disulfides (R-S-S-R) that are physiologically reduced back to thiols by endogenous redox agents such as glutathione. It is likely that there are multiple sites of redox modulation as oxidizing agents have been reported to both increase (Park et al., 1995; Thuringer & Findlay, 1997) and decrease (Park et al., 1995; DiChiara & Reinhart, 1997; Wang et al., 1997; Tang et al., 2001) the probability of the opening of BKCa channels. Moreover, these sites of redox modulation are on cysteine residues, as N-ethylmaleimide (NEM), a specific alkylating agent of free sulfhydryls on cysteine residues, prevents the action of the oxidizing agents, thimerosal and diamide (Wang et al., 1997). These reactive cysteine groups are not on the extracellular surface or within the pore of the BKCa channel since membrane-impermeant oxidizing agents are only effective when applied to the cytosolic surface of excised membrane patches and neither the oxidation nor the reduction of the BKCa channel protein has any affect on the unit conductance of the BKCa channels (Wang et al., 1997; Lang et al., 2000).
Nitric oxide (NO·) or reactive nitrogen species formed by NO· released from intestinal inhibitory motor nerves and vascular endothelial cells have been demonstrated in vitro to increase the opening of large (BKCa), intermediate and small conductance Ca2+-activated K+ channels (Bolotina et al., 1994; Koh et al., 1995). Applied NO· donors increase the opening of native and cloned smooth muscle BKCa (Koh et al., 1995; Fukao et al., 1999) and TREK-1 (Koh et al., 2001) channels indirectly via mechanisms involving the activation of cytosolic guanylyl cyclases and channel phosphorylation. However, a direct NO· donor activation of BKCa channels in excised membrane patches of vascular and intestinal smooth muscle in the presence of specific guanylyl cyclase blockers such as 1H-[1,2,4] oxidiazolo [4,3-a] quinoxalin-1-one (ODQ) has also been demonstrated (Bolotina et al., 1994; Mistry & Garland, 1998; Lang et al., 2000). This activation has been suggested to involve the binding of NO·, or an oxidized derivative, NO+ or N2O3, to thiols to form S-nitrosothiols (RSNO) that may well lead to disulfide formation if another reduced thiol is in close proximity (Abderrahmane et al., 1998; Lang et al., 2000).
The purpose of this study was to examine pharmacologically which cysteine residues within the BKCa channel protein(s) in excised membrane patches of the guinea-pig taenia caeca are likely candidates for S-nitrosylation by the NO· donor, S-nitroso-L-cysteine (NOCys). We have used three methanethiosulfonate (MTS) reagents, 2-aminoethyl MTS hydrobromide (MTSEA), [2-(triethylammonium) ethyl] MTS bromide (MTSET) and sodium (2-sulfonatoethyl) MTS (MTSES), which specifically convert sulfhydryls to disulfides in manner that is only reversible upon exposure to thiols such as dithiothreitol (DTT) (Akabas et al., 1992; Kuner et al., 1996; Karlin & Akabas, 1998). We have examined whether the irreversible alkanethiolation of cysteine thiols by MTS reagents can also interrupt the excitatory actions of NOCys. Any action of these MTS reagents on the gating of smooth muscle BKCa channels or the excitatory effects of NOCys has been interpreted in terms of accessibility to cysteine residues with respect to the volume and net charge of the surrounding water-accessible protein surface (Lang & Harvey, 2002). We have assumed that membrane-permeable MTSEA will have the greatest access to cysteine thiols within both hydrophobic and hydrophilic regions of the subunits of the BKCa channel protein. In contrast, MTSET and MTSES will have fairly equal access to water-facing cysteines of the BKCa channel, being restricted only by the charge of the neighboring protein region. Some of these results have been published previously in brief (Lang & Harvey, 2002).
Methods
Single-cell preparation
Guinea-pigs were killed by stunning and exsanguination, the cecum was exposed through an abdominal incision and one of the taenia caeca dissected free. All experiments were carried using procedures approved by the Physiological Department Animal Ethics Committee at Monash University. Single smooth muscle myocytes were enzymatically dispersed from 5 mm strips of guinea-pig taenia caeca in a low-Ca2+ (50 μM) physiological salt solution (PSS) (see below) with added collagenase (0.5 mg ml−1, Worthington, Sciman), trypsin inhibitor (0.5 mg ml−1, Sigma) and bovine serum albumin (2 mg ml−1, Sigma) at 37°C (see Lang et al., 2000). Single cells were either stored at 4°C or plated onto the glass base of a recording chamber mounted (0.1–0.2 ml) on an inverted microscope (Olympus CK2). Cells were allowed 5–10 min to settle and then perfused (3 ml min−1) for 2–5 min with a normal-Ca2+ (1.5 mM) containing PSS (see below) prior to commencing patch-clamp experiments.
Solutions
Cells were dispersed in a low-Ca2+ PSS containing (mM): NaCl 126, KCl 6, sodium N-2- hydroxyethylpiperazine-N′-2-ethanesulphonic acid (Na-HEPES) 10, MgCl2 1, D-glucose 10, EGTA 0.3. and CaCl2 50 μM. The pH of the solution was adjusted to 7.4 with 5 M NaOH. Following dispersion, the Ca2+ concentration was increased to 1.5 mM before cell storage or experimentation. The patch pipette usually contained normal Ca2+ (1.5 mM) PSS, the presence or absence of this concentration Ca2+ in the pipette solution had no effect on the NO·-dependent activation of the BKCa channels (Lang & Watson, 1998; Lang et al., 2000). After the formation of a seal (>2 GΩ) between the pipette and the smooth muscle membrane, cells were bathed in a high K+, low Ca2+ PSS containing (mM): Kcl 130, Na-HEPES 10, MgCl2 1, D-glucose 10, EGTA 0.3 and CaCl2 50 μM, which gives a computed free Ca2+ concentration of 15 nM (CABUFFER). The solution was adjusted to 7.4 with 5 M KOH. Inside-out patches were then excised into this high K+ PSS, establishing a near-physiological Ca2+ and K+ (6 mM [K]o 1.5 mM [Ca]o: 130 mM [K]i 15 nM [Ca]i) gradient across the membrane patch. All recordings were made at room temperature (22–25°C).
Single channel recording and analysis
Patch pipettes were drawn from glass capillary tubing (1.5–1. 8 mm) (Clarke Electromedical Inst.) on a micropipette puller (Sachs-Flaming PC-84, Sutter Instruments) and pipette tips were heat polished (MF-84 Narishige). Pipette resistances ranged between 4 and 10 MΩ. Command potentials were delivered and channel currents recorded using an Axopatch 200 amplifier (3-dB cutoff frequency 1 kHz, 4-pole Bessel filter) (Axon Instruments). Patch potentials and channel currents were stored on videotape via a Digital Data Recorder VR10B (Instrutech Corp.) and later digitized (at 2 kHz) onto a computer hard disc for analysis using a DigiData 1200 Interface (Axon Instruments) and pClamp 6 software (Axon Instruments). Channel openings were recorded for 30–120 s at holding patch potentials between −80 and +80 mV. Patches were also subjected to a ramped depolarization (between −80 and +80 mV over 1 s) from a holding potential between 0 and +40 mV. These ramp-evoked channel currents (I) were then plotted against the patch potential (V) to give ‘instantaneous' current–voltage (I–V) plots, which were used to follow any time-dependent changes in BKCa channel activity.
BKCa channel current levels at each holding potential were first plotted as an all-points amplitude histogram. Closed and opened levels were fitted with single Gaussian distributions (using a simplex least-squares algorithm), the difference between the mean of each Gaussian giving an estimate of the amplitude (I) (pA) of the BKCa channel currents at each patch potential (V) (mV). The area under each fitted Gaussian was used to calculate the relative probability of the patch current being at each current level (PL) by dividing the summed time spent at each level by the total observation time. The averaged open probability for N channels (N.Po) was therefore
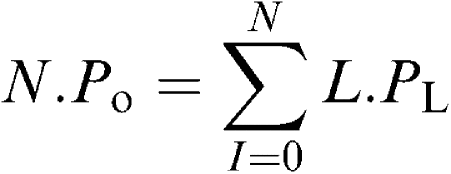 |
To establish the Ca2+ sensitivity of the our BKCa channels after exposure to NEM (1 mM for 5 min), average currents (I) arising from the opening of multiple BKCa channels were recorded in excised macropatches using a repetitive (× 10) multi-stepped depolarization protocol to potentials between −60 and +90 mV from a holding potential of −80 mV. Averaged current (I), conductance (g=I/(V−ER)) (using a BKCa channel reversal potential (ER) of −70 mV) (Lang et al., 2000) and normalized conductance (g/gMax) values were calculated and plotted against V. Activation curves were fitted with Boltzmann equations to obtain a measure of the voltage of half-maximal activation (V0.5) and the slope, K. The maximum conductance (between +60 and +90 mV) of the individual macropatches bathed in 15, 150 and 300 nM Ca2+ was normalized as a fraction of the conductance obtained in 1.5 μM Ca2+ (y), plotted against the Ca2+ concentration (x) and fitted to the Hill equation
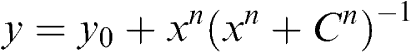 |
where C is the half-maximal concentration of Ca2+ and n is the Hill coefficient.
Data were expressed as the mean±s.e.m N indicates the number of experiments. Statistical significance was determined using Student's t-test, P<0.05 was considered to be significantly different.
Drugs
Stock solutions of S-nitroso-L-cysteine (NOCys) (100 mM) were prepared as previously described (Lang et al., 2000). N-ethylmaleimide (NEM) and the methanethiosulfonate (MTS) reagents, 2-aminoethyl MTS hydrobromide (MTSEA), [2-(triethylammonium)ethyl] MTS bromide (MTSET) and sodium (2-sulfonatoethyl)MTS (MTSES) (Toronto Research Chemicals Inc.) were dissolved in distilled water just prior to experimentation and stored at 4°C. The infusion of the vehicle for NOCys had no effect on BKCa channel activity (Lang et al., 2000). All stock solutions were diluted with PSS and applied as a bolus (1–100 μl) to the bath solution (volume of 0.2 ml); the concentrations indicated are the calculated final concentrations.
Exposures to the MTS reagents were mostly performed at 0 mV and for only relatively short periods (2–5 min) to maximize the reaction of the charged MTS reagents with ionized thiols (–S−) on cysteines at a water-accessible surface. This protocol would also minimize any influences of the electrical field on the permeation of the MTS reagents as well as any nonspecific interactions of these MTS reagents with other unionized thiols on imbedded or lipid-accessible cysteines (Karlin & Akabas, 1998).
Results
Effects of NOCys
Single BKCa channel openings were seldom recorded at potentials <0 mV in excised membrane patches exposed to the physiological K+ gradient ([K]o 6 mM: [K]i 130 mM) and a cytosolic Ca2+ concentration of 15 nM. In one set of 11 experiments, BKCa channel currents at 0 mV were 7.4±0.3 pA (range 6.6–8.3 pA) in amplitude and opened with a probability (N.Po) of 0.019±0.011 (range 0.001–0.053). Both current amplitude and N.Po of these BKCa channels increased with either maintained or ramped (Figure 1bi) depolarization of the membrane potential to positive potentials such that three to five simultaneously opening channels were evident (Lang & Watson, 1998; Lang et al., 2000).
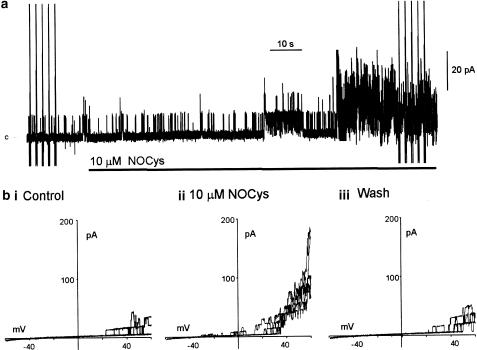
Figure 1.
NOCys activation of BKCa channels in excised patches of the guinea-pig taenia ceca. (a) Addition of NOCys (10 μM) to the cytosolic surface increased BKCa channel activity (at 0 mV) in membrane patches bathed in a near-physiological K+ gradient, c denotes the closed level. (b) comparison of BKCa openings evoked by ramped depolarizations (three ramp depolarizations between −60 and +60 mV every 10 s) in the absence (bi) and 2 (bii) and 5 min (biii) after the addition of NOCys (10 μM) to the bathing solution, holding potential 0 mV.
The application of the relatively unstable S-nitrosothiol compound, S-nitroso-L-cysteine (NOCys 10 μM, with a half-life of 80 s in PSS) to the cytosolic surface of the excised membrane patches increased BKCa channel activity after a delay of about 60 s (Figure 1a) (Lang et al., 2000). In nine experiments, N.Po (at 0 mV) was 0.024±0.006 (range 0.001–0.068) in control PSS and 0.585±0.308 (range 0.043–0.912) (P<0.05, paired t-test) after 2–5 min exposure to 10 μM NOCys. After the washout of NOCys, N.Po values slowly returned to near-control values over 15–20 min (Lang & Watson, 1998; Lang et al., 2000).
The voltage-dependent effects of NOCys were examined using three to five ramped depolarizations applied 2 s apart before (Figure 1a, bi), during (Figure 1a, bii) and after (Figure 1biii) the peak response to NOCys (10 μM). During these ramp depolarizations, the number of active channels opening simultaneously at positive potentials increased 2–10 fold in the presence of NOCys (10 μM). Finally, more BKCa channel openings were evident at potentials negative of 0 mV as might be expected with a substantial shift to the left of these ‘activation' curves (Figure 1bii) (Lang et al., 2000).
Effect of NEM
N-ethylmaleimide (NEM) is a specific alkylating agent of free sulfhydryl groups on cysteine residues that should prevent any NOCys-evoked formation of S-nitrosothiols (RSNO) or any disulfide-bonding formation with vicinal RSNOs or cysteine thiols. NEM (1 mM for 5 min) applied to the cytosolic surface of excised patches decreased BKCa channel opening at +20 mV (Figure 2a) and at positive potentials during ramped depolarizations to +100 mV (Figure 2bi ii), significantly decreasing N.Po (at 20 mV) from 0.0283±0.0067 in control PSS to 0.0096±0.0025 (P<0.05, n=11) (Figure 2a) (Lang et al., 2000). Figure 2 also illustrates that a pre-exposure to NEM (1 mM for 5 min) prevented the NOCys (10 μM)-evoked increase in BKCa channel opening at +20 mV (Figure 2a) or during ramped depolarizations (Figure 2biii); the NOCys-induced increase in N.Po after an exposure (5 min) to and washout (10 min) of NEM (1 mM) being 172±78% (P>0.05, n=6) of the N.Po recorded after the washout of the NEM (Lang et al., 2000). This increase in N.Po can be compared with the 1968-fold increase of N.Po induced by NOCys (10 μM) applied to control membrane patches (see above).
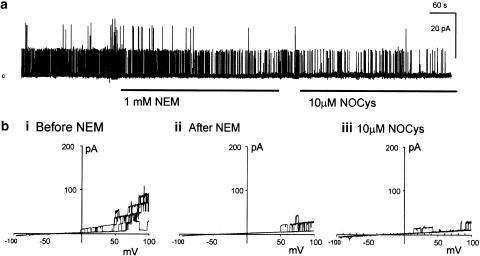
Figure 2.
Alkylation of cysteine thiols with N-ethylmaleimide (NEM) prevented the excitatory action of NOCys. (a) Application of NEM (1 mM) to the cytosolic surface irreversibly decreased BKCa channel activity at 20 mV. The subsequent application of NOCys (10 μM) had little effect on BKCa channel activity. (b) comparison of BKCa openings evoked by ramped depolarizations (three ramp depolarizations between −100 and +100 mV every 10 s) in the absence (bi) and 5 min (bii) after the exposure to NEM (1 mM), holding potential 0 mV. (biii) Pre-exposure to NEM (1 mM for 5 min) prevented the excitatory actions of NOCys (10 μM) on ramped evoked BKCa channel openings.
The effects of NEM exposure on BKCa channel activation were examined in more detail in seven experiments. In 15 nM Ca2+ PSS, averaged macropatch currents at all potentials positive to 0 mV were reduced after a 5 min exposure to and 5 min washout of NEM (1 mM) (Figure 3A, Ba, Ca). When currents recorded before (Figure 3Ca, D, hollow squares) and after (Figure 3Ca, D filled squares) exposure to NEM (1 mM for 5 min) were expressed as normalized conductance, V0.5 was not significantly altered by NEM (62.8±1.1 mV in control 15 nM Ca2+ PSS and 62.03±3.7 mV after NEM exposure, respectively; P>0.05, n=6). However, the slope K was significantly increased (11.8±1.5 mV in control 15 nM Ca2+ PSS and 16.9±1.5 mV after NEM, respectively; P<0.05, n=6) (Figure 3C,D).
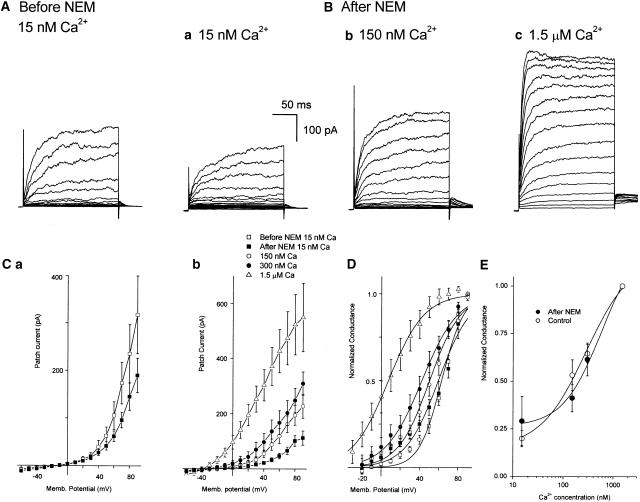
Figure 3.
N-ethylmaleimide (NEM, 1 mM for 5 min) does not affect BKCa channel sensitivity to Ca2+. Typical macroscopic currents (A,B) and current–voltage relationships (C) for BKCa before (A, Ca hollow squares) and 5 min after (Ba, Ca,b filled squares) an exposure to NEM (1 mM for 5 min). Each current trace represents the average of 10 steps to test potentials every 10 mV between −60 and +90 mV from a holding potential of −80 mV. After an NEM exposure, the macroscopic BKCa currents (Ba–c) and resulting I–V relationships (Cb) were concentration-dependently increased by the concentration of Ca2+ bathing the cytosolic surface of the patch. Each data point represents the average of six to seven experiments. (D) Normalizing individual experiments to the maximum conductance reveals that NEM caused a significant increase in K (11.8±1.5 mV in control 15 nM Ca2+ PSS and 16.9±1.5 mV after NEM, respectively; P<0.05, n=6) without significantly altering V0.5 (62.8±1.1 mV in control 15 nM Ca2+ PSS and 62.03±3.7 mV after NEM exposure, respectively; P>0.05, n=6). Raising the cytosolic Ca2+ concentrations also shifted the Botzlmann (activation) curves fitted to the averaged data (n=6–7) in a negative direction such that V0.5 and K were 59.5 and 17 mV, 50.4 and 16.4 mV, 42.3 and 18.8 mV, and 7.4 and 18.7 mV in 15, 150, 300 nM and 1.5 μM Ca2+ PSS, respectively. (E) Characteristics of Ca2+-dependent activation of BKCa channel currents either in control conditions or after an exposure to NEM. Maximal conductance recorded at test potentials between +60 and +90 mV from either control patches (hollow circles) (n=3–6) or patches previously exposed to NEM (1 mM for 5 min) (filled circles) (n=3–6) were normalized as a fraction of the conductance recorded in 1.5 μM Ca2+ PSS, averaged and plotted against Ca2+ concentration. Plots were fitted with the Hill equation to give a half-maximal activation (C) of 277 nM and a Hill coefficient (n) of 0.97 in control, and 576 nM and 1.12, respectively, after NEM exposure.
In Figure 3Cb, a 5 min exposure to and 5 min washout of NEM (1 mM) did not prevent the increase in the macropatch current evoked by either membrane depolarization (Figure 3Cb) or when the Ca2+ concentration in the PSS bathing the cytosolic surface was raised from 15 (Figure 3Cb, filled squares) to 150 nM (Figure 3Cb, hollow circles), 300 nM (Figure 3Cb, filled circles) or 1.5 μM (Figure 3Cb, hollow triangles). When macropatch currents were expressed as normalized conductance it was apparent that there was a concentration-dependent leftward shift of V0.5 with Ca2+ concentration, such that V0.5 obtained from the fitted Boltzmann curves to the averaged data in Figure 3D was 59.5, 50.4, 42.3 and 7.4 in 15, 150, 300 nM and 1.5 μM Ca2+ PSS, respectively. Raising the cytosolic Ca2+ concentration had little effect on K, being 17, 16.4, 18.8 and 18.7 mV in 15, 150, 300 nM and 1.5 μM Ca2+ PSS, respectively. Finally, a clear dependence of channel activation on Ca2+ concentration was obtained when the maximum conductance (between +60 and +90 mV) of the individual macropatches bathed in 15, 150 and 300 nM Ca2+ was normalized as a fraction of the conductance obtained in 1.5 μM Ca2+, averaged and plotted against the log of the Ca2+ concentration (Figure 3E, filled circles). This plot is not significantly different from that obtained when three control patches, not exposed to NEM, were bathed in the four concentrations of Ca2+ (Figure 3E, hollow circles). When fitted with the Hill equation, the half-maximal activation (C) of these BKCa channels before and after an exposure to NEM were 277 and 576 nM Ca2+, respectively; the Hill coefficients were 0.97 and 1.12, respectively.
Effect of MTSEA
Although positively charged, MTSEA has been demonstrated to cross cell membranes suggesting that this MTS reagent readily changes from a protonated (membrane impermeant) to a deprotonated (membrane permeable) form (Karlin & Akabas, 1998). We have assumed that MTSEA will have access to cysteine residues that are present in water-accessible regions not surrounded by basic amino acids and as well as cysteine residues in the hydrophobic regions of the BKCa channel protein.
The application of MTSEA (2.5 mM for 5 min) to the cytosolic surface of excised patches rapidly reduced BKCa channel openings recorded at 0 mV (Figure 4a) or during ramped depolarizations (Figure 4ci-ii). In 13 experiments, N.Po (at 0 mV) was reduced to 68±14% (P<0.05 paired t-test) of control after 2 min exposure to MTSEA (2.5 mM). This inhibitory effect of MTSEA was irreversible, even after 2–5 min washout of the MTSEA-containing PSS, involving multiple exchanges of the bath volume (Figure 4b).
Figure 4.
Positively charged but membrane permeable MTS reagent, MTSEA decreased BKCa channel activity but did not prevent the excitatory actions of NOCys. Application of MTSEA (2.5 mM for 5 min), to the cytosolic surface decreased BKCa channel activity (N.Po) recorded at either 0 mV (a, b) or during ramped depolarizations between −80 and +80 mV (ci, ii). This reduction in N.Po remained upon washout of the MTSEA (b). The subsequent application of NOCys (10 μM) still increased N.Po at 0 mV (a, b) as well as the number of channel openings evoked by a ramp depolarization (ciii). The significant effects of all agents were established using Student's paired t-tests. *denotes P< 0.05.
The possibility that MTSEA might be permeating through the excised patch membrane and reacting with cysteine groups facing the extracellular surface of the channel protein was eliminated by observing that the MTSEA-induced decrease in N.Po still occurred when the pipette solution contained L-cysteine (10 mM) (n=4). Under these conditions, it would be expected that any MTSEA reaching the extracellular surface of the BKCa channel protein(s) would be bound by the excess L-cysteine in the pipette saline.
In three experiments, the amplitude of the BKCa channel currents were recorded at various potentials between –60 and +80 mV in the presence (Figure 8aii filled circles) and absence (Figure 8aii hollow circles) of MTSEA (2.5 mM). BKCa channel current amplitudes at all potentials were not affected by MTSEA. At 0 mV, the averaged channel current amplitude was 7±0.2 pA in the presence of MTSEA, and 6.8±0.2 and 7.03±0.01 pA, respectively, prior to and after the exposure to MTSEA (n=13, P>0.05 paired t-test) (Figure 8ai).
Figure 8.
Summary of the effects of MTS reagents on the I–V properties of BKCa channels in excised patches of the guinea-pig taenia caeca. BKCa current amplitudes were measured either a 0 mV (ai, n=13; bi, n=11; ci, n=7) or at every 10 mV between −60 and +80 mV in control saline (filled circles), in the presence of MTSEA (aii, n=3), MTSET (bii, n=5) or MTSES (cii, n=3) and after washout of the MTS reagents (ai, bi, ci). MTSEA and MTSES did not significantly alter BKCa current amplitudes at 0 mV (ai, ci) or any other potential (aii, cii). In contrast, MTSET significantly reduced channel amplitudes at 0 mV (bi) compared to control amplitudes, as well as the slope of the I–V plot between –40 and +80 mV (bii). bii Inset illustrates the current amplitudes in MSET expressed as a fraction of the control channel amplitudes (Rel. Amp.) plotted against V. The continuous line through the data points represents an exponential function, fitted by least squares: f=0.798 exp(−0.004x).
Pre-exposure of membrane patches to MTSEA did not prevent the 418-fold increase in N.Po evoked in the presence of NOCys (10 μM) (Figure 4). N.Po in the presence of NOCys (10 μM) applied after an exposure (2 min) and washout (2–5 min) of MTSEA, was 0.83±0.22 compared to the value of N.Po (0.005±0.002) (n=10, P<0.05) obtained following the washout of the MTS reagent (Figure 4b).
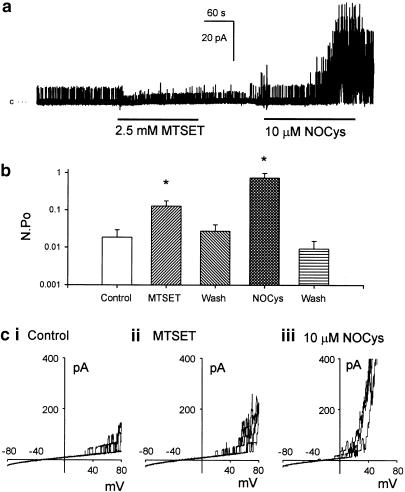
Effect of MTSET
Permanently positively charged and therefore membrane-impermeant MTSET would be expected to have access to cysteine residues within non-basic surface regions of the channel protein with a water interface. A 2 min exposure to MTSET (2.5 mM) significantly increased N.Po (at 0 mV) 22-fold (n=10, P<0.05) above control values (Figure 5a,b), as well as the number of BKCa channel openings evoked during a ramped depolarization (Figure 5c). However, MTSET also decreased BKCa channel amplitudes at 0 mV to 4.2±0.7 pA (n=11) (compared with 7.4±0.3 pA in control P<0.05 paired t-test) (Figure 8bi). In Figure 8bii, the averaged I–V plots (n=5) obtained in the presence (Figure 8bii, hollow circles) or absence (Figure 8bii, filled circles) of MTSET have been compared. It can be seen that the slope of the I–V plot in the presence of MTSET is markedly reduced such that the slope conductance (at 0 mV) of these BKCa channels was 155 pS in control PSS and 95 pS in MTSET-containing PSS. In addition, the relative amplitude of these BKCa channel currents in MTSET, expressed as a fraction of their respective control amplitudes (Figure 8bii, inset), decreased at more positive potentials suggesting a voltage-dependent blockade of current flow at positive potentials.
Figure 5.
Positively charged MTS reagent MTSET increased BKCa channel activity (at 0 mV) in a manner reversed upon washout. MTSET did not prevent the excitatory actions of NOCys. Application of MTSET (2.5 mM for 5 min) significantly increased N.Po recorded at 0 mV (a, b) as well as the BKCa channel openings at positive potentials (ci, ii). This increased in N.Po was abolished upon washout of MTSET (b). After an exposure to MTSET, NOCys (10 μM) still significantly increased BKCa channel openings at 0 mV (a, b) and positive potentials (ciii).
These MTSET-evoked changes in BKCa channel amplitude and N.Po were not maintained upon washout of the MTS reagent. Both N.Po (Figure 5b) and channel current amplitudes (Figure 8bi) returning to near-control values upon washout. This rapid reversibility of the effects of MTSET suggests that the observed effects of MTSET were nonspecific and not involving the conversion of cysteine sulfhydryls to a disulfide, which is presumably only reversible upon the addition of a thiol such as DTT. Moreover, the pre-exposure of membrane patches to MTSET did not prevent the 92-fold increase (n=10, P<0.5) in N.Po (0.742±0.238) evoked by NOCys (10 μM) (Figure 5a) applied after the washout of MTSET (N.Po of 0.0279±0.013) (Figure 5a, b).
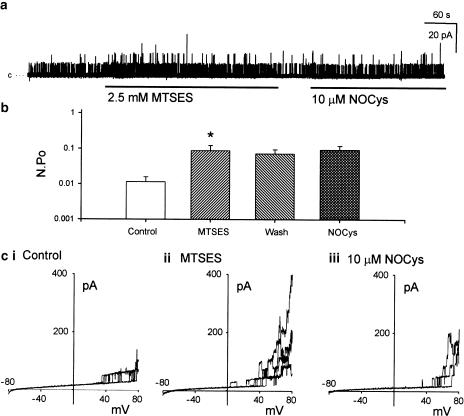
Effect of MTSES
Exposure of the cytosolic surface of excised membrane patches to negatively charged MTSES (2.5 mM for 5 min) evoked a rapid increase in BKCa channel activity at 0 mV (Figure 6a) and during ramped depolarizations to positive potentials (Figure 6ci, ii) such that N.Po (at 0 mV) in the presence of MTSET was significantly increased 11-fold above control (n=7, P<0.05 paired t-test) (Figure 6b). In contrast to MTSET, the amplitude of these BKCa channel currents either at 0 mV (Figure8ci) (n=7) or measured at various potentials between −40 and +80 mV (Figure 8cii) (n=3) were not significantly affected by MTSES.
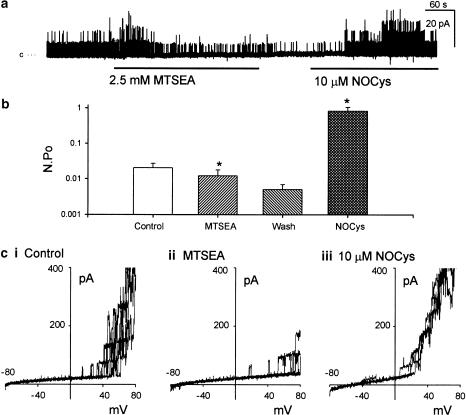
Figure 6.
Negatively charged MTS reagent MTSES, irreversibly increased BKCa channel activity (at 0 mV) and prevented the excitatory actions of NOCys. Application of MTSES (2.5 mM for 5 min) significantly increased N.Po recorded at 0 mV (a, b) as well as the BKCa channel openings evoked at positive potentials (ci, ii). This increase in N.Po remained upon washout of MTSES (b). Pre-exposure to MTSES prevented any further NOCys (10 μM)-evoked increases of N.Po at 0 mV (a, b) or at positive potentials (ciii).
In contrast to MTSEA and MTSET, previously exposing excised membrane patches to MTSES (2.5 mM for 2 min) prevented the further activation of BKCa channels by NOCys (10 μM) (Figure 6a). It can be seen in Figure 6b that the MTSES-evoked increase in N.Po was still evident after a 2-min washout of the MTS reagent. Moreover, the subsequent application of NOCys (10 μM) did not evoke a further increase in N.Po, being only 147±39% (n=6, P>0.05 paired t-test) of the N.Po values recorded during the washout period after the exposure to MTSES (Figure 6b).
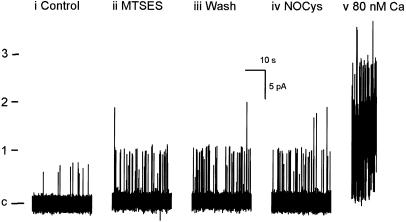
Finally, this exposure to MTSES did not prevent the increase in N.Po (at +20 mV) evoked by either membrane depolarization (Figure 6c) or when the cytosolic Ca2+ concentration was raised from 15 to 80 nM (n=3) (Figure 7).
Figure 7.
Comparing the effects of NOCys (10 μM for 2 min) and raising the cytosolic Ca2+ concentration from 15 to 80 nM on BKCa channel activity (at 0 mV) after exposure and washout of MTSES (2.5 mM for 5 min). c denotes the closed level, 1–3 denote number of superimposed open channels.
Discussion
BKCa channels exist as a tetrameric complex of two subunits: the pore-forming α subunit and the regulatory β subunit. The primary sequence of the α subunit obtained from different tissues is almost identical being encoded by a single gene, KCNMA1, previously termed ‘slowpoke' or ‘dslo' in Drosophila (Butler et al., 1993; Pallanck & Ganetzky, 1994; McCobb et al., 1995; Vogalis et al., 1996; Meera et al., 1997; Tanaka et al., 1997; Vergara et al., 1998; Jiang et al., 1999; Wallner et al., 1999; Ha et al., 2000). In contrast, β subunits are encoded by four genes (KCNMB1-4) (Tanaka et al., 1997; Jiang et al., 1999; Orio et al., 2002). The minimum molecular structure required to create a functional BKCa channel is a tetramer recombination of the α subunit. In addition to containing the six membrane-spanning domains (S1–S6), the S4 voltage sensor and the pore domain between S5 and S6, which identifies this channel as a members of the S4 superfamily of voltage-gated K+ channels (KV), the α subunit also contains a seventh transmembrane domain (S0) that locates the amino terminus in the extracellular domain. Additional hydrophobic cytoplasmic-located segments (S7–S10) are located within the long carboxy-terminal domain. A tetramerization domain (BK-T1) has been located in the hydrophilic region between S6 and S7, while Ca2+ binding has been located to a region (‘calcium bowl') between S9 and S10, which is highly conserved in all cloned BKCa channels (Orio et al., 2002). However, replacing all of the negatively charged aspartate residues within the calcium bowl with uncharged asparagine only reduced Ca2+ binding and its associated Hill coefficient by approximately 50% (Bian et al., 2001), suggesting that there are multiple sites in the carboxyl terminal involved in Ca2+ binding and channel activation. Alternate splicing at two to four sites between S8 and S10 of the α subunit changes the kinetics and Ca2+ sensitivity of expressed α subunit channels. However, larger changes in channel kinetics are generally created when α subunits are co-expressed with β subunits (Orio et al., 2002).
β subunits (B1–B4) consist of two transmembrane segments connected by an extracellular ‘loop' so that both termini are in the cytoplasmic domain (Knaus et al., 1994; Meera et al., 1996; Vogalis et al., 1996; Orio et al., 2002). B1 is highly expressed in smooth muscle, while B2 is expressed preferentially in brain and chromaffin cells, B3 is found in the testis, pancreas and spleen and B4 in the brain (Wallner et al., 1996; Wallner et al., 1999; Orio et al., 2002). The β subunit increases the sensitivity of the α subunit to Ca2+, particularly at high [Ca]i (McCobb et al., 1995; McManus et al., 1995; Vogalis et al., 1996; Jiang et al., 1999) and modifies charybdotoxin binding and the α subunit selectivity for K+ ions (McManus et al., 1995). The presence of the β subunit is also necessary for the extracellular binding and activation of BKCa channels by 17-β estradiol (Valverde et al., 1999). Targeted genetic deletion of smooth muscle B1 in mice increases arterial tone and blood pressure, as well as modulates bladder action potential discharge and contraction (Brenner et al., 2000; Petkov et al., 2001).
The specific oxidation of sulfur-containing side chains within methionine residues in cloned human slo (hslo) channels expressed in HEK 293 cells with chloramine-T increases channel opening in a manner partially reversed by the enzyme peptide methionine sulfoxide reductase plus DTT (Hoshi & Heinemann, 2001; Tang et al., 2001). In contrast, the specific oxidation of cysteine thiols with NEM decreases N.Po (Figure 2) in excised membrane patches of the guinea-pig taenia ceca (Lang et al., 2000), which could be arising from the decrease in the BKCa channel sensitivity to voltage, evident by the small but significant increase in K (Figure 3C, D). However, exposure to NEM did not affect the sensitivity of the BKCa channel activation to raising the cytosolic concentration of Ca2+ (Figure 3). These results are consistent with the NEM-evoked decrease in BKCa channel activity in excised patches from the rabbit aorta (Bolotina et al., 1994) and horse trachea (Wang et al., 1997), and the NEM-evoked reduction of the whole-cell current of HEK 293 cells expressing rat slo (rslo) channels (Jeong et al., 2001). In contrast, NEM has also been demonstrated to increase BKCa channel activity in excised patches pulled from nerve terminals of the rat posterior pituitary (Ahern et al., 1999) or after isolation from bovine airways and incorporation into a lipid bilayer (Abderrahmane et al., 1998).
The alkylation of cysteine thiols by NEM should prevent any NO· donor-evoked S-nitrosylation, or the conversion of neighboring thiols to a disulfide. Cytosolically applied NEM inhibited both the NOCys activation of BKCa channels in excised patches from the guinea-pig taenia ceca (Figure 2) (Lang et al., 2000) and the NO· activation of BKCa channel gating in pituitary nerve terminals (Ahern et al., 1999). However, NEM does not prevent the increase in rslo channel activity evoked by the NO· donor, S-nitroso-N-acetylpenicillamine (SNAP) (Jeong et al., 2001). These variable effects of NEM on control and NO·-evoked BKCa and slo channel activity may well be arising from differences in the amino-acid sequence and therefore the microenvironment of the native BKCa and the rslo channels under study. In particular, there may well be a number of different spliced variants of the α subunit of the BKCa channel being expressed in the smooth muscle cells of the taenia ceca. These differing spliced variants could be combining in varying proportions with a number of differing spliced variants of the β subunit to form a variety of BKCa channels, which could explain the increases and decreases in channel gating observed in the presence of oxidizing agents and NEM, respectively. However, in the present experiments, it is clear that cysteine residues lying on the cytosolic surface of the BKCa channel protein(s) are the sites of NEM action.
Although positively charged, MTSEA has been reported to cross cell membranes and lipid bilayers (Karlin & Akabas, 1998). Thus, we have assumed that MTSEA will have the greatest access to the cysteine residues of the α subunit both within the hydrophobic regions and hydrophilic regions. In our experiments, MTSEA evoked a significant decrease in BKCa channel opening that remained upon washout, suggesting an irreversible binding of MTSEA to cysteine residues (Figure 4) (Tang et al., 2001).
However, pre-exposure and washout of membrane patches to MTSEA did not prevent the excitatory effects of NOCys. This suggests that the cysteine residues bound by MTSEA are not the targets of S-nitrosylation in the presence of NOCys. Permanently positively charged MTSET would be expected to have access to cysteine residues within all acidic regions of the channel protein with a water interface. However, the reduction in BKCa channel amplitude (Figure 8b) and increase in N.Po (Figure 5) observed in the presence of MTSET were not maintained upon washout. These data suggest that the actions of MTSET do not involve the conversion of cysteine sulfhydryls to a disulfide. In fact, the MTSET-evoked reduction of channel current amplitude appeared similar to the rapid open-channel blockade observed in the presence of high concentrations of tetraethylammonium chloride or procaine applied to the cytosolic surface of smooth muscle BKCa channels (Benham et al., 1985). These observations have not been studied further.
Only MTSES evoked an increase in N.Po that remained after washout. Moreover, only MTSES prevented any further increase in N.Po when NOCys was added (Figure 6). This specificity of MTSES is not based on the size of the MTS reagents used or on the volume of the water interface of the protein surface at which these agents are interacting. The –SO3 group of MTSES is smaller than the –N(CH3) group of MTSET (0.58 nm) but larger than the –NH3 group of MTSEA (0.36 nm). If the size of the head group was the essential factor restricting MTS reagent binding to the cysteines S-nitrosylated by NOCys, then MTSES and MTSET would be expected to have equal access (Akabas et al., 1992). As MTSET did not prevent the excitatory actions of NOCys, we can conclude that the S-nitrosylatable cysteines lie in regions of the channel protein that would select against cations, within basic water-facing regions of the channel protein that would exclude MTSEA and MTSET. The gating of cloned α subunits of the BKCa channels isolated from rat and human brain and expressed in immortal cells lines have been demonstrated to be modulated by NO· donors (Jeong et al., 2001), redox reagents (DiChiara & Reinhart, 1997; Tang et al., 2001; Chung et al., 2002) and NEM (Jeong et al., 2001). These data suggest that it is unlikely that the β subunit plays any role in NOCys- or redox-evoked changes in BKCa channel activity.
There is considerable (>90%) homology in the sequences of the alpha subunit of BKCa channels (alpha-slo) isolated from the mouse and rat brain, dog intestine and human aorta and myometrium. In the published sequences of α subunit of the BKCa channels found in human myometrium (Accession No. AAK91504 or GI 15215553) and aorta (Accession No. AAA92290 or GI 758791) and the dog colon (Accession No. AAA84000, GI 1127824) there are 20–25 cysteine residues in the amino-acid sequence. Only five to six cysteine residues lie within hydrophobic regions leaving 15–20 cysteine residues as potential targets for S-nitrosylation by NOCys or alkylation by NEM. A detailed examination of the sequence of the alpha subunit of the human brain BKCa channel (alpha-hbr1) reveals that there are only two pairs of cysteine residues in the C terminal domain that are separated or surrounded by basic amino acids. The sequences surrounding these cysteines are also highly conserved in all species, except for some minor substitutions of basic amino acids in the rat and mouse. Alternate RNA splicing occurs in alpha-hbr1 at three sites between S8 and S9 and at one site between S9 and S10 in the C-terminal domain. In the human brain, the insertion of a number of alternate exons at these sites has led to the identification of nine constructs (hbr1–hbr9), which can insert one additional cysteine residue (hbr4) or three (hbr2, hbr8, hbr9) to six to nine (hbr3, hbr7) additional basic amino acids, all of which may well have functional consequences on the sensitivity of the channel to redox reagents, Ca2+ or nitrogen reactive species (Tseng-Crank et al., 1994).
It is likely that the BKCa channels in the guinea-pig taenia caeca are equally diverse in their expression, distribution and sensitivity to NO·, which limits the identification of the reactive cysteine residues in the present study. A systematic study involving the expression of α subunit constructs of known molecular structure with single mutations of individual cysteine residues to nonreactive alanine in an immortal cell line and the retesting of the effects of NEM, MTSES and NOCys will be needed before we can identify unequivocally which cysteine thiols are involved in the S-nitrosylation mechanisms examined in the present experiments.
In summary, we have demonstrated that NEM prevents NOCys-evoked increases in BKCa channel (Ahern et al., 1999; Lang et al., 2000) activity, indicating that the S-nitrosylation of cysteine residues of the α subunit plays an important part of NOCys action. As negatively charged MTSES also increased N.Po and prevented the further activation by NOCys, it is likely that the cysteine residues reactive to NOCys lie within basic regions of the α subunit, which exclude positively charged MTSEA and MTSET.
Acknowledgments
This work was supported in part by the National Health & Medical Research Council of Australia.
Abbreviations
- BKCa
large conductance Ca2+-activated K+
- I–V
current-voltage
- DTT
dithiothreitol
- MTS
methanethiosulfonate
- MTSEA
2-aminoethyl MTS hydrobromide
- MTSET
[2-(trimethylammonium) ethyl MTS bromide
- MTSES
sodium (2-sulfonatoethyl) MTS
- Na-HEPES
sodium N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- NOCys
S-nitroso-L-cysteine
- NEM
N-ethylmaleimide
- N.Po
probability of opening
- NO·
nitric oxide
- NO+
nitrosonium
- ODQ
1H-[1,2,4] oxidiazolo [4,3-a] quinoxalin-1-one
- PSS
physiological salt solution
- RSH
sulfhydryl
- RSNO
S-nitrosothiol
- R-S-S-R
disulfide
References
- ABDERRAHMANE A., SALVAIL D., DUMOULIN M., GARON J., CADIEUX A., ROUSSEAU E. Direct activation of KCa channel in airway smooth muscle by nitric oxide: involvement of a nitrothiosylation mechanism. Am. J. Respir. Cell Mol. Biol. 1998;19:485–497. doi: 10.1165/ajrcmb.19.3.2996. [DOI] [PubMed] [Google Scholar]
- AHERN G.P., HSU S.F., JACKSON M.B. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J. Physiol. 1999;520:165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKABAS M.H., STAUFFER D.A., XU M., KARLIN A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J., TAKEWAKI T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch. Eur. J. Physiol. 1985;403:120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- BIAN S., FAVRE I., MOCZYDLOWSKI E. Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4776–4781. doi: 10.1073/pnas.081072398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BRENNER R., PEREZ G.J., BONEV A.D., ECKMAN D.M., KOSEK J.C., WILER S.W., PATTERSON A.J., NELSON M.T, ALDRICH R.W. Vasoregulation by the beta 1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- BUTLER A., TSUNODA S., MCCOBB D.P., WEI A., SALKOFF L. mSlo, a complex mouse gene encoding ‘maxi' calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- CHUNG S., JUNG W., UHM D.Y., HA T.S., PARK C.S. Glutathione potentiates cloned rat brain large conductance Ca2+-activated K+ channels (rslo) Neurosci. Lett. 2002;318:9–12. doi: 10.1016/s0304-3940(01)02435-1. [DOI] [PubMed] [Google Scholar]
- DICHIARA T.J., REINHART P.H. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKAO M., MASON H.S., BRITTON F.C., KENYON J.L., HOROWITZ B., KEEF K.D. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J. Biol. Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- GONG L., GAO T.M., HUANG H., TONG Z. Redox modulation of large conductance calcium-activated potassium channels in CA1 pyramidal neurons from adult rat hippocampus. Neurosci. Lett. 2000;286:191–194. doi: 10.1016/s0304-3940(00)01121-6. [DOI] [PubMed] [Google Scholar]
- HA T.S., JEONG S.Y., CHO S.W., JEON H., ROH G.S., CHOI W.S., PARK C.S. Functional characteristics of two BKCa channel variants differentially expressed in rat brain tissues. Eur. J. Biochem. 2000;267:910–918. doi: 10.1046/j.1432-1327.2000.01076.x. [DOI] [PubMed] [Google Scholar]
- HOSHI T., HEINEMANN S. Regulation of cell function by methionine oxidation and reduction. J. Physiol. 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEONG S.Y., HA T.S., PARK C.S., UHM D.Y., CHUNG S. Nitric oxide directly activates large conductance Ca2+-activated K+ channels (rSlo) Mol. Cells. 2001;12:97–102. [PubMed] [Google Scholar]
- JIANG Z., WALLNER M., MEERA P., TORO L. Human and rodent maxi K channel beta-subunit genes: cloning and characterization. Genomics. 1999;55:57–67. doi: 10.1006/geno.1998.5627. [DOI] [PubMed] [Google Scholar]
- KARLIN A., AKABAS M.H. Substituted-cysteine accessibility method. Methods Enzymol. 1998;293:123–145. doi: 10.1016/s0076-6879(98)93011-7. [DOI] [PubMed] [Google Scholar]
- KNAUS H.G., FOLANDER K., GARCIA-CALVO M., GARCIA M.L., KACZOROWSKI G.J., SMITH M., SWANSON R. Primary sequence and immunological characterization of beta-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J. Biol. Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- KOH S.D., CAMPBELL J.D., CARL A., SANDERS K.M. Nitric oxide activates multiple potassium channels in canine colonic smooth muscle. J. Physiol. 1995;489:735–743. doi: 10.1113/jphysiol.1995.sp021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., MONAGHAN K., SERGEANT G.P., RO S., WALKER R.L., SANDERS K.M., HOROWITZ B. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J. Biol. Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- KUNER T., WOLLMUTH L.P., KARLIN A., SEEBURG P.H., SAKMANN B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- LANG R.J., HARVEY J.R. Thiol reagents and nitric oxide modulate the gating of BKCa channels from the guinea-pig taenia caeca. Clin. Exp. Pharmacol. Physiol. 2002;29:944–949. doi: 10.1046/j.1440-1681.2002.03754.x. [DOI] [PubMed] [Google Scholar]
- LANG R.J., HARVEY J.R., MCPHEE G.J., KLEMM M.F. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeca. J. Physiol. 2000;525:363–376. doi: 10.1111/j.1469-7793.2000.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG R.J., WATSON M.J. Effects of nitric oxide donors, S-nitroso-L-cysteine and sodium nitroprusside, on the whole-cell and single channel currents in single myocytes of the guinea-pig proximal colon. Br. J. Pharmacol. 1998;123:505–517. doi: 10.1038/sj.bjp.0701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCOBB D.P., FOWLER N.L., FEATHERSTONE T., LINGLE C.J., SAITO M., KRAUSE J.E., SALKOFF L. A human calcium-activated potassium channel gene expressed in vascular smooth muscle. Am. J. Physiol. 1995;269:H767–H777. doi: 10.1152/ajpheart.1995.269.3.H767. [DOI] [PubMed] [Google Scholar]
- MCMANUS O.B., HELMS L.M., PALLANCK L., GANETZKY B., SWANSON R., LEONARD R.J. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14:645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- MEERA P., WALLNER M., JIANG Z., TORO L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv,cabeta) of maxi K channels. FEBS Lett. 1996;385:127–128. doi: 10.1016/0014-5793(96)83884-1. [DOI] [PubMed] [Google Scholar]
- MEERA P., WALLNER M., SONG M., TORO L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORIO P., ROJAS P., FERREIRA G., LATORRE R. New disguises for an old channel: maxi K channel beta-subunits. NIPS. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- PALLANCK L., GANETZKY B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum. Mol. Genet. 1994;3:1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- PARK M.K., LEE S.H., LEE S.J., HO W.K., EARM Y.E. Different modulation of Ca-activated K channels by the intracellular redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflügers Arch. Eur. J. Physiol. 1995;430:308–314. doi: 10.1007/BF00373904. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BONEV A.D., HEPPNER T.J., BRENNER R., ALDRICH R.W., NELSON M.T. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J. Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA Y., MEERA P., SONG M., KNAUS H.G., TORO L. Molecular constituents of maxi KCa channels in human coronary smooth muscle: predominant alpha+beta subunit complexes. J. Physiol. 1997;502:545–557. doi: 10.1111/j.1469-7793.1997.545bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG X.D., DAGGETT H., HANNER M., GARCIA M.L., MCMANUS O.B., BROT N., WEISSBACH H., HEINEMANN S.H., HOSHI T. Oxidative regulation of large conductance calcium-activated potassium channels. J. Gen. Physiol. 2001;117:253–274. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THURINGER D., FINDLAY I. Contrasting effects of intracellular redox couples on the regulation of maxi-K channels in isolated myocytes from rabbit pulmonary artery. J. Physiol. 1997;500:583–592. doi: 10.1113/jphysiol.1997.sp022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSENG-CRANK J., FOSTER C.D., KRAUSE J.D., MERTZ R., DICHIARA T.J., REINHART P.H. Cloning, expression and distribution of functional Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- VALVERDE M.A., ROJAS P., AMIGO J., COSMELLI D., ORIO P., BAHAMONDE M.I., MANN G.E., VERGARA C., LATORRE R. Acute activation of maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- VERGARA C., LATORRE R., MARRION N.V., ADELMAN J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- VOGALIS F., VINCENT T., QURESHI I., SCHMALZ F., WARD M.W., SANDERS K.M., HOROWITZ B. Cloning and expression of the large-conductance Ca2+-activated K+ channel from colonic smooth muscle. Am. J. Physiol. 1996;271:G629–G639. doi: 10.1152/ajpgi.1996.271.4.G629. [DOI] [PubMed] [Google Scholar]
- WALLNER M., MEERA P., TORO L. Determinant for beta-subunit regulation in high-conductance voltage-activated and Ca2+-sensitive K+ channels: an additional transmembrane region at the N terminus. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14922–14927. doi: 10.1073/pnas.93.25.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLNER M., MEERA P., TORO L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z.W., NARA M., WANG Y.X., KOTLIKOFF M.I. Redox regulation of large conductance Ca2+-activated K+ channels in smooth muscle. J. Gen. Physiol. 1997;110:35–44. doi: 10.1085/jgp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]