Abstract
Stress is a risk factor in psychiatric illnesses such as schizophrenia. The aim of the present study was to investigate the effect of different circulating levels of the adrenal steroid corticosterone (CORT) on locomotor hyperactivity and prepulse inhibition of acoustic startle, two behavioural animal models of aspects of schizophrenia.
Male C57BL/6J mice (n=10 per group) were anaesthetised with isoflurane and sham-operated or adrenalectomised (ADX). ADX mice were implanted with 50 mg pellets consisting of 100% cholesterol, or 2, 10 or 50 mg of CORT mixed with cholesterol. CORT pellet implantation dose dependently increased plasma CORT levels 3 weeks after surgery. Starting 1 week after surgery, mice were tested for prepulse inhibition after injection of saline or 5 mg kg−1 of haloperidol.
In intact mice and in mice implanted with 10 mg of CORT, haloperidol treatment significantly increased prepulse inhibition (average values from 38 – 42 to 52%). Similar results were observed when testing the mice for amphetamine-induced locomotor hyperactivity (5 mg kg−1). In contrast, there was no significant effect of haloperidol in mice implanted either with cholesterol or 2 or 50 mg of CORT.
These results in behavioural animal models of schizophrenia suggest an important role of the stress hormone CORT in modulating dopaminergic activity in this illness.
Keywords: Corticosterone, dopamine, prepulse inhibition, locomotor hyperactivity, mice
Introduction
Stress and stress hormones modulate brain dopaminergic activity (Roth et al., 1988; Lindley et al., 1999; Pani et al., 2000). For example, stress and corticosterone (CORT) treatment caused increased dopamine release in the frontal cortex (Claustre et al., 1986). Adrenalectomy reduced the locomotor hyperactivity response to amphetamine treatment in rats and this effect could be reversed in a dose-dependent way by chronic administration of CORT (Cador et al., 1993). Adrenalectomy reduced the density of dopamine D1 and D2 receptors in a number of brain regions, such as the posterior caudate nucleus and substantia nigra, but not nucleus accumbens (Biron et al., 1992). Dexamethasone treatment reversed these changes.
The proposed interaction of glucocorticoids with central mesolimbic dopaminergic activity has raised considerable interest in a possible role of these hormones in drug sensitisation and abuse (Deroche et al., 1995; Piazza & LeMoal, 1997). Less is known on the involvement of stress hormones in schizophrenia, despite the realization that stress may precipitate symptoms in patients suffering from this disease (Nuechterlein et al., 1994; Benes, 1997; Gispen-de Wied, 2000). In addition, stress early in development may cause permanent neurodevelopmental changes that increase the likelihood of developing schizophrenia (Walker & Diforio, 1997).
In the present study, we investigated the effect of adrenalectomy and supplementation with different levels of CORT on two animal behavioural models with relevance to schizophrenia. Given the increasing importance of genetically modified mouse models in neuropsychopharmacological studies (Henry et al., 1999; Ralph et al., 1999), we chose to use mice for the present experiments. Rats and mice have similar behavioural responses to some drug treatments, including amphetamine and haloperidol, but differ markedly in response to other treatments, such as serotonergic drugs (Geyer, 1999).
Prepulse inhibition is an operational measure of sensorimotor gating that is deficient in patients with schizophrenia (Braff et al., 1992; Kumari et al., 2000). Prepulse inhibition of acoustic startle refers to the inhibition of a startle response to a sudden, loud sound stimulus by a low-intensity prestimulus (Geyer & Swerdlow, 1998). Repeated exposure of rodents to startle stimuli induces progressively smaller startle responses, a process known as habituation and reflecting a form of simple learning. Habituation of startle is also deficient in patients with schizophrenia (Braff et al., 1992). In rats, prepulse inhibition and startle habituation can be disrupted by dopaminergic stimulation with apomorphine or amphetamine (Geyer & Markou, 1995). These effects are mediated by dopaminergic innervation and receptors in the nucleus accumbens (Swerdlow et al., 1990; Geyer & Markou, 1995). Chronic treatment with CORT was shown to induce a decrease of prepulse inhibition in C3H mice (Stevens et al., 2001).
Several mouse strains, including C57BL/6J mice, show relatively low prepulse inhibition (Bullock et al., 1997; Varty et al., 2001). In addition, while dopaminergic stimulation reduces prepulse inhibition in mice, this effect appears to be smaller than in rats (Dulawa & Geyer, 1996; Geyer, 1999). Recently, it was noted that in mice, treatment with dopamine receptors antagonists causes a significant increase in prepulse inhibition (Ouagazzal et al., 2001). Therefore, in the present study, we decided to use the increase in prepulse inhibition induced by administration of the dopamine receptor antagonist haloperidol as an index of central dopaminergic activity.
Another widely used model for aspects of schizophrenia is the locomotor hyperactivity induced by psychostimulants such as amphetamine (Geyer & Markou, 1995). The effect of amphetamine on locomotor activity in rats depends on intact dopaminergic innervation of the nucleus accumbens (Creese & Iversen, 1975; Kelly et al., 1975). Mice of the C57BL/6J strain also show increased locomotor activity to an amphetamine challenge and lesions of the nucleus accumbens block this effect (Teitelbaum et al., 1979). Parallel to the locomotor hyperactivity, amphetamine induced a significant increase in dopamine release in the nucleus accumbens in C57BL/6J mice (Zocchi et al., 1998). Also in humans, amphetamine causes a marked release of dopamine in the ventral striatum and the extent of this increase is similarly correlated with the behavioural effects of the drug (Drevets et al., 2001). In patients with schizophrenia, the effects of amphetamine on dopamine release are significantly enhanced (Laruelle et al., 1996).
Thus, we expected treatment with haloperidol to cause an increase in prepulse inhibition and treatment with amphetamine to cause an increase in locomotor activity in mice. Owing to the proposed interaction of CORT with central dopaminergic activity (see above), we tested the hypothesis that the effects of haloperidol and amphetamine would only be observed with certain circulating levels of CORT in these mice. Our results suggest that CORT markedly influences central dopaminergic regulation of behaviour. While this effect was similarly apparent for prepulse inhibition, startle amplitude and startle habituation, and locomotor activity, there were, however, quantitative differences in the effect of CORT substitution.
Methods
We used 50 male C57BL/6J mice obtained from the breeding colony of the Department of Microbiology, University of Melbourne. This breeding colony was sourced from the breeding colony of the Animal Resources Centre, Perth, Australia. The animals were housed 2–5 per plastic cage and given standard pellet food and water ad libitum.
All procedures were approved by the Animal Experimentation Ethics Committee of the University of Melbourne.
Surgery
The mice were 7–9 weeks of age (body weight 21– 29g) at the time of surgery and were randomly assigned to one of the five surgery groups. The animals were anaesthetised with an isoflurane/oxygen breathing mixture. A dorsal incision was made in the skin and lateral incisions in the muscle wall to access the adrenals. Except for sham-operated, adrenal-intact mice, all mice had their adrenals removed. In addition, adrenalectomised (ADX) mice were subcutaneously implanted with a solid 50 mg pellet containing either 100% cholesterol, 4% CORT and 96% cholesterol (2 mg CORT group), 20% CORT and 80% cholesterol (10 mg CORT group) or 100% CORT (50 mg CORT group). The mice were given a subcutaneous injection of 5 mg kg−1 of the anti-inflammatory analgesic Carprofen before being returned to their home boxes to recover from anaesthesia. Experiments commenced 1 week after surgery.
Prepulse inhibition
Mice were first tested for startle amplitude, startle habituation and prepulse inhibition of acoustic startle. We used a four-unit automated startle system (SR-Lab, San Diego Instruments, San Diego, CA, U.S.A.), which both delivers startle stimuli and records responses.
Mice were placed in Perspex cylinders (3.8 cm diameter) on a motion-sensitive platform and were allowed to acclimate for 5 min. Throughout the session, the startle system delivered a constant background white noise of 70 dB intensity. The prepulse inhibition session started and ended with a block of ten 40 ms 115 dB pulse-alone trials. In between those blocks, 80 further trials were delivered with an average, but variable, intertrial interval of 25 s. These trials consisted randomly of 20 pulse-alone trials or prepulse trials. Prepulse trials consisted of a 115 dB pulse-alone startle stimulus preceded by 100 ms by a 20 ms prepulse of either 2, 4, 8, 12 or 16 dB over the 70 dB background (i.e. 72, 74, 78, 82 and 86 dB prepulses). There were 10 each of these prepulse trials. Startle habituation was assessed by using the two blocks of 10 pulse-alone trials at the start and end of the session (blocks 1 and 4) in combination with the 20 pulse-alone trials used to calculate prepulse inhibition (blocks 2 and 3). Prepulse inhibition was calculated as the difference between startle responses after a prepulse trial and those after the middle 20 pulse-alone trials, expressed as a percentage of the responses obtained from the pulse-alone trials.
All mice were tested for startle and prepulse inhibition twice, after random injection of either saline (10 ml kg−1 intraperitoneally) or haloperidol (5 mg kg−1). In order to prepare the haloperidol solution, we used a commercially available preparation (Serenace, Searle) that was diluted with saline to the appropriate concentration.
Locomotor activity
In the third week after surgery, mice were tested for locomotor activity and the response to injection with either saline or amphetamine. The animals were placed individually in plastic boxes (l × w × h: 39 × 28 × 12 cm3) and allowed to explore and habituate to the new environment for 60 min. They were then injected intraperitoneally with either saline (10 ml kg−1) or D-amphetamine (Sigma, 5 mg kg−1) and observed for another 60 min. At 3 – 4 days after the first locomotor test, the mice were similarly tested with the reverse treatment.
Behaviour of the mice was recorded on video and analysed using the Ethovision video tracking system (Noldus, The Netherlands). This system provided data for total distance moved and average velocity of movements.
Radioimmunoassay
After the behavioural experiments, all mice were decapitated between 0900 and 1200 and trunk blood was collected in heparinised tubes. Serum levels of CORT were measured using a commercial radioimmunoassay kit for rat and mouse serum (ICN Biomedical, Sydney, Australia). The crossreactivity of the antibody used in this kit was reported to be 100% for CORT, 0.34% for deoxy-CORT, 0.1% for testosterone, and <0.1% for other steroid hormones such as oestradiol, progesterone, or cholesterol.
Data analysis
All data are expressed as mean±standard error of the mean (s.e.m.). Differences between groups and between responses after saline treatment and haloperidol or amphetamine treatment were compared using analysis of variance (ANOVA) with repeated measures where appropriate.
Data for prepulse inhibition, startle amplitude, and startle habituation from intact mice and ADX mice were compared with group (sham, adrenalectomy) as a between-group factor and treatment (saline, haloperidol) as a within-animal factor. Similarly, data from ADX mice and from mice implanted with either a 2, 10 or 50 mg pellet were analysed using CORT group as a between-group factor and treatment (saline, haloperidol) as a within-animal factor. For prepulse inhibition data, prepulse intensity was an additional within-animal factor. For startle habituation, startle pulse block was an additional within-animal factor. One-way ANOVAs were used to further analyse the haloperidol effect in each group separately.
A similar statistical analysis design was used for the data for amphetamine-induced locomotor hyperactivity. Analyses compared the distance moved in the 60 min before and after saline injection and before and after amphetamine injection with these treatments as within-animal factors. One-way ANOVAs were used to further analyse the amphetamine effect in each group separately.
Serum CORT values were analysed with one-way ANOVA and Bonferroni-corrected t-test. In all cases, differences were considered significant if P<0.05.
Results
Body weights and serum CORT levels
There were no significant body weight differences between the groups at the time of surgery or at the end of the experiment 3 weeks later (not shown). However, unlike the small increase in body weight found during the experimental period in sham-operated controls and in ADX mice implanted with a cholesterol pellet or a 2 mg CORT pellet, body weight tended to decrease slightly in mice implanted with a 10 or 50 mg CORT pellet.
As expected, serum CORT levels were markedly lower in ADX mice implanted with a cholesterol pellet (4.4±0.9 μg dl−1) than in sham-operated controls (10.3±1.5 μg dl−1). Implantation of a 10 mg (11.2±0.8 μg dl−1), but not a 2 mg CORT pellet (3.6±0.8 μg dl−1), raised serum levels to those seen in sham-operated mice. In contrast, in ADX mice implanted with a 50 mg CORT pellet, serum levels were significantly increased (27.5±2.2 μg dl−1) compared to those seen in sham-operated mice. These values are similar to previously published values (Jacobson, 1999; Birt et al., 2001; Le Cudennec et al., 2002; Tjurmina et al., 2002; Vrang et al., 2002).
Prepulse inhibition
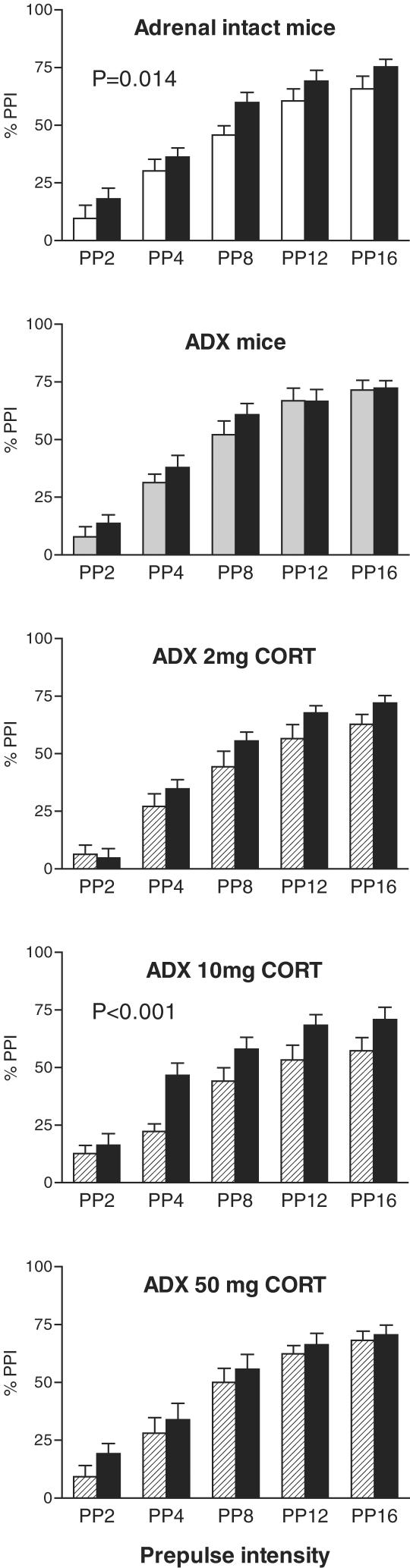
When analysing data from intact mice and ADX mice (Figure 1), we found no main effect of group; however, there was a significant overall effect of haloperidol treatment (F(1,17)=5.5, P=0.031) and a trend towards a group × treatment interaction (F(1,17)=3.0, P=0.102). While there was a marked main effect of prepulse intensity (F(4,68)=129.7, P<0.001), there were no interactions of prepulse intensity with either group or treatment. In sham-operated, adrenal-intact mice, haloperidol treatment caused a significant increase in prepulse inhibition that was independent of the level of prepulse intensity (Figure 1, main effect of haloperidol treatment, F(1,9)=9.3, P=0.014). Average prepulse inhibition increased by 22% after haloperidol injection in this group. In contrast to intact mice, there was no significant effect of haloperidol treatment in ADX mice implanted with a cholesterol pellet (Figure 1).
Figure 1.
Prepulse inhibition of acoustic startle in C57BL/6J mice that were either adrenal-intact (top panel), ADX, or ADX and implanted with a 2, 10 or 50 mg CORT pellet (ADX 2 mg CORT, ADX 10 mg CORT and ADX 50 mg CORT, respectively). The mice were injected with saline (white bars and hatched bars) or 5 mg kg−1 of haloperidol (black bars) 10 min before the start of the prepulse inhibition session. There were 10 mice per group. Haloperidol treatment increased prepulse inhibition in intact and ADX 10 mg CORT mice only. For further details and statistical comparisons, see text.
When analysing data from ADX mice and mice implanted with a 2 mg CORT pellet, there were no significant differences or interactions except for the expected main effect of prepulse intensity (F(4,68)=167.3, P<0.001). In contrast, when analysing data from ADX mice and mice implanted with a 10 mg CORT pellet, while there was no main effect of group, there was a significant main effect of haloperidol treatment (F(1,17)=13.6, P=0.002) and a group × treatment interaction (F(1,17)=9.1, P=0.008). Furthermore, there were the expected main effect of prepulse intensity (F(4,68)=111.1, P<0.001) and a prepulse × treatment × group interaction (F(4,68)=2.9, P<0.027). In mice implanted with a 10 mg CORT pellet, haloperidol injection caused a significant increase in prepulse inhibition (Figure 1, F(1,9)=31.8, P<0.001). Average prepulse inhibition increased by 37% after haloperidol injection in this group (Figure 1). When analysing data from ADX mice and mice implanted with a 50 mg CORT pellet, there were no significant differences or interactions except for the expected main effect of prepulse intensity (F(4,68)=142.6, P<0.001).
Startle and startle habituation
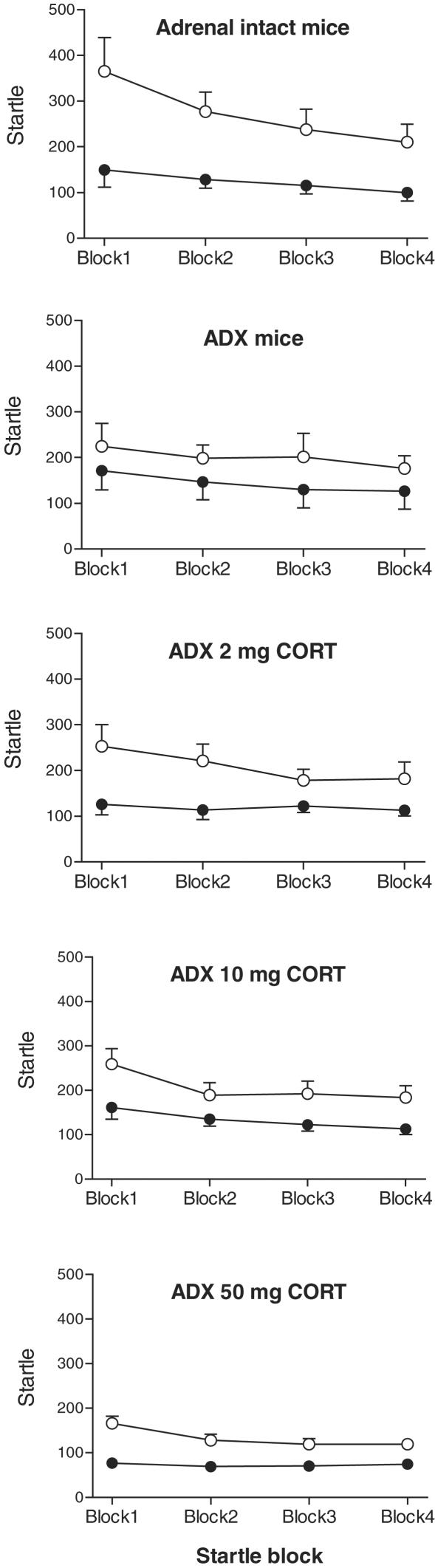
In response to pulse-alone stimuli, all mice showed robust startle responses that habituated with repeated exposure (Figure 2). When analysing data from intact mice and ADX mice, we found no main effect of group; however, there was a significant overall effect of haloperidol treatment (F(1,17)=18.8, P<0.001) and a trend towards a group × treatment interaction (F(1,17)=3.0, P=0.101). While there was marked habituation of the startle response (main effect of pulse block, F(3,51)=9.3, P<0.001), there were no significant differences between intact mice and ADX mice in the overall startle level or startle habituation (Figure 2). In sham-operated, adrenal-intact mice, haloperidol treatment caused a significant decrease in startle amplitude (F(1,9)=17.5, P=0.002, average responses reduced by 55%) and in startle habituation (treatment × block interaction, F(3,27)=4.1, P=0.016). In ADX mice, the effect of haloperidol on startle amplitude failed to reach significance (F(1,8)=3.7, P=0.090) and there was also no significant effect of haloperidol treatment on startle habituation (Figure 2).
Figure 2.
Startle amplitude and startle habituation in C57BL/6J mice that were either adrenal-intact (top panel), adrenalectomised (ADX), or ADX and implanted with a 2, 10 or 50 mg CORT pellet (ADX 2 mg CORT, ADX 10 mg CORT and ADX 50 mg CORT, respectively). The mice were injected with saline (open circles) or 5 mg kg−1 of haloperidol (black circles) 10 min before the start of the prepulse inhibition session. There were 10 mice per group. Haloperidol treatment significantly reduced startle amplitude in all groups except ADX mice. Haloperidol treatment reduced startle habituation in adrenal intact mice and in ADX 50 mg CORT mice only. For further details and statistical comparisons, see text.
When analysing data from ADX mice and mice implanted with a 2 mg CORT pellet, we found no main effect of group; however, there was a significant overall effect of haloperidol treatment (F(1,17)=17.9, P<0.001). While there was marked habituation of the startle response (main effect of pulse block, F(3,51)=8.4, P<0.001), there were no significant group differences in the overall startle level or startle habituation (Figure 2). In mice implanted with a 2 mg CORT pellet, haloperidol injection caused a significant decrease in startle amplitude (F(1,9)=28.5, P<0.001, 41% decrease). When analysing data from ADX mice and mice implanted with a 10 mg CORT pellet, we found no main effect of group; however, there was a significant overall effect of haloperidol treatment (F(1,17)=13.5, P=0.002). While there was again marked habituation of the startle response (main effect of pulse block, F(3,51)=16.5, P<0.001), there were no significant group differences in the overall startle level or startle habituation (Figure 2). In mice implanted with a 10 mg CORT pellet, haloperidol injection caused a significant decrease in startle amplitude (F(1,9)=14.1, P=0.004, 36% decrease). When analysing data from ADX mice and mice implanted with a 50 mg CORT pellet, we found that overall startle levels tended to be decreased (F(1,17)=4.0, P=0.063). However, while there was the expected startle habituation (main effect of block, F(3,51)=9.8, P<0.001), there were no main effects of haloperidol treatment or interactions. In mice implanted with a 50 mg CORT pellet, haloperidol injection caused a significant decrease in startle amplitude (F(1,9)=41.1, P<0.001, 45% decrease) and a significant reduction of startle habituation (treatment × block interaction, F(3,27)=3.5, P=0.029).
Locomotor activity
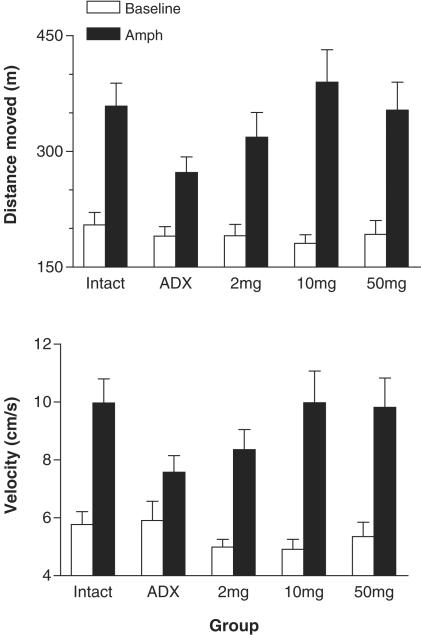
There were no significant differences between any of the surgery groups in preinjection baseline locomotor activity, whether expressed as distance moved or velocity of movements. In all surgery groups, amphetamine treatment significantly increased the distance moved (P<0.01) and the velocity of movements (P<0.01; however, P=0.033 in ADX mice implanted with a cholesterol pellet). Combined ANOVAs revealed that the increase in distance moved induced by amphetamine was significantly reduced in ADX mice implanted with a cholesterol pellet compared to intact, sham-operated controls (interaction of treatment and group, F(1,17)=5.4, P=0.032) and to ADX mice implanted with a 10 mg CORT pellet (F(1,17)=8.4, P=0.010) (Figure 3). Similarly, the effect of amphetamine on velocity of movements was significantly smaller in ADX mice implanted with a cholesterol pellet compared to intact mice (F(1,17)=6.7, P=0.019) and to ADX mice implanted with a 10 mg CORT pellet (F(1,17)=7.6, P=0.014) (Figure 3).
Figure 3.
Locomotor activity of C57BL/6J mice that were either adrenal-intact, ADX, or ADX and implanted with a 2, 10 or 50 mg CORT pellet (2, 10 and 50 mg, respectively). Total distance moved (top panel) and velocity of movements (bottom panel) were measured before (open bars) and after injection of 5 mg kg−1 of amphetamine (black bars). There were 10 mice per group. Amphetamine treatment caused significant locomotor hyperactivity in all groups, but the effect was significantly smaller in ADX mice than in intact mice. For further details and statistical comparisons, see text.
Discussion
In this study, we showed that in adrenal-intact mice, haloperidol treatment caused an increase in prepulse inhibition and amphetamine treatment caused an increase in locomotor activity. In ADX mice, haloperidol treatment had no effect on prepulse inhibition and the amphetamine effect was reduced. In mice that were implanted with a 10 mg CORT pellet, but not a 2 or 50 mg pellet, haloperidol treatment also caused an increase in prepulse inhibition. The amphetamine-induced locomotor hyperactivity was significantly greater in these CORT-treated mice than in ADX mice.
Haloperidol treatment significantly reduced the amplitude of startle responses in adrenal-intact mice, and in ADX mice implanted with a 2, 10 or 50 mg CORT pellet. An effect of haloperidol on startle habituation was only observed in adrenal-intact mice and in mice implanted with a 50 mg CORT pellet. The interaction of circulating CORT levels with prepulse inhibition, startle amplitude, and startle habituation thus appears to show a different dose – response relationship. While the different behaviours thus appear differentially sensitive to CORT replacement, in all cases the effect of adrenalectomy was not seen after treatment with this hormone. This suggests that the effects of adrenalectomy were not likely mediated by catecholamine release from the adrenal medulla or because of the loss of other adrenal steroids such as aldosterone. It is possible that the effect of CORT in the brain was indirect, that is, mediated by other factors, such as corticotrophin-releasing factor (CRF). Indeed, it was shown recently that in rats that were administered CRF (Conti et al., 2002) or in mice that overexpressed CRF (Dirks et al., 2003), prepulse inhibition was diminished.
Adrenalectomy or CORT treatment did not significantly alter either baseline startle, startle habituation or prepulse inhibition of startle. Similarly, baseline locomotor activity or locomotor activity after saline injection was not significantly different between groups. Previously, Stevens et al. (2001) observed that in ADX mice implanted with a CORT pellet, baseline startle amplitudes and prepulse inhibition were reduced. The difference between this study and ours is unclear, but could be related to the different mouse line (C3H) used by Stevens et al. (2001) compared to our C57BL/6J mice. In our study, only when a dopaminergic challenge was used, that is, haloperidol or amphetamine, were different responses seen in ADX mice compared to intact mice, or CORT-treated mice. This suggests that the effect of CORT is mediated by central dopaminergic activity. Thus, we hypothesise that adrenalectomy leads to reduced baseline dopaminergic activity in the brain areas involved in modulating startle and prepulse inhibition. The effect of haloperidol on prepulse inhibition in intact mice is likely to be mediated by the blockade of endogenous dopamine receptor stimulation, which normally suppresses this mechanism. Thus, haloperidol treatment blocks this suppression and allows prepulse inhibition to be increased. After adrenalectomy, this endogenous dopaminergic tone is reduced, either at the level of release, turnover or receptor density, and thus there is little effect of haloperidol on prepulse inhibition. With CORT substitution, dopaminergic tone is returned to normal and the haloperidol effect is restored. In this way, the effect of haloperidol on prepulse inhibition represents an indirect measure of endogenous dopaminergic tone. The nature of this effect remains to be determined. One possibility could be that adrenalectomy reduced dopamine D1 and/or D2 receptor density (Biron et al., 1992), and thus reduced the number of receptors that haloperidol or dopamine (released by amphetamine) can act upon. Replacement with CORT would then restore the normal number of dopamine D2 receptors. In addition, at high doses at least, CORT has been shown to reduce dopamine metabolism, thus allowing for enhanced extracellular dopamine levels (Lindley et al., 1999).
The location of an interaction of CORT with dopaminergic activity is at present unknown, but could be the nucleus accumbens, which has been implicated in the regulation of prepulse inhibition (Swerdlow et al., 1990,1996). Similar models could be used to explain the effect of adrenalectomy and CORT on the haloperidol-induced reduction of startle and startle habituation (Koch, 1999). However, as noted above, startle amplitude and startle habituation are not equally sensitive to CORT replacement when compared to prepulse inhibition. The reason for this differential sensitivity is unclear and could indicate that brain circuits involved in modulating startle amplitude, startle habituation, and prepulse inhibition are different (Koch, 1999). As discussed recently, prepulse inhibition is independent of the level of startle in mice (Geyer et al., 2002).
Amphetamine-induced locomotor hyperactivity is apparently also under CORT modulation, as adrenalectomy reduced and CORT replacement re-instated this effect. Similar interactions have been reported before in rats (Cador et al., 1993; Piazza et al., 1996a; Barrot et al., 2000). The effect of amphetamine is dependent on intact dopaminergic innervation of the nucleus accumbens in rats (Creese & Iversen, 1975; Kelly et al., 1975) and in mice of the C57BL/6J strain (Teitelbaum et al., 1979; Zocchi et al., 1998). This could indicate that the effects of CORT on prepulse inhibition and on amphetamine-induced locomotor hyperactivity are mediated by the same central substrate; however, the differential sensitivity to CORT would suggest that additional mechanisms are also involved.
While haloperidol treatment caused a significant increase in prepulse inhibition in ADX mice implanted with 10 mg of CORT, there was no significant haloperidol effect if the animals had been substituted with 50 mg of the steroid. Close inspection of the data reveals that this loss of haloperidol effect is associated with a slight but nonsignificant increase of baseline prepulse inhibition. It is unlikely that such a small shift alone could explain the loss of an effect of haloperidol. Prepulse inhibition is by no means maximum, and particularly at low prepulse intensities a large increase in prepulse inhibition could still be possible. We rather think that the high dose of CORT triggers a second type of effect on prepulse inhibition. It is well known that moderate circulating levels of CORT predominantly activate mineralocorticoid receptors in the brain (Reul & De Kloet, 1985; De Kloet et al., 1998). The mineralocorticoid receptor is discretely localized in the brain, particularly in the hippocampus and septum, and acts as a ‘permissive' receptor, enabling several brain neurotransmitter systems to operate optimally (Reul & De Kloet, 1985; De Kloet et al., 1998). Thus, loss of activation of this receptor after adrenalectomy reduces normal dopaminergic tone, as evidenced by the reduced effects of haloperidol and amphetamine. Substitution with the 10 mg dose of CORT appears to restore this mechanism. In contrast, when high levels of CORT are circulating, glucocorticoid receptors as well as mineralocorticoid receptors are activated in the brain (Reul & De Kloet, 1985; De Kloet et al., 1998). Glucocorticoid receptors show a widespread, ubiquitous distribution in the brain (Reul & De Kloet, 1985,1986), and are involved in termination of the stress response (De Kloet et al., 1998; Van den Buuse et al., 2002). Thus, as discussed above, in our mice this could lead to a reduction of dopaminergic tone similar to that after adrenalectomy. Indeed, the slight enhancement of baseline prepulse inhibition seen in this group of mice would be consistent with this. The ‘bell-shaped' dose – response curve of CORT on prepulse inhibition was not apparent on amphetamine-induced locomotor activity or on startle amplitude and startle habituation. It will be important to further assess the role of mineralocorticoid receptors vs glucocorticoid receptors in these behaviours, for example, by substituting the mice with glucocorticoid receptor agonists, such as dexamethasone, rather than CORT, which acts on both receptors. These studies are currently ongoing.
Previous studies have focused on several aspects of interaction of glucocorticoid hormones with central dopaminergic activity. Such studies have implicated these hormones in mechanisms of drug addiction (Piazza & Le Moal, 1996b), depression (Meyer et al., 2001) and schizophrenia (Gispen-de Wied, 2000). The present study shows that CORT is likely to be involved in the regulation of startle and prepulse inhibition. As prepulse inhibition is widely regarded as an excellent model of sensorimotor gating (Geyer & Markou, 1995; Swerdlow & Geyer, 1998), which is disrupted in patients with schizophrenia (Braff & Geyer, 1990; Geyer & Markou, 1995), our results could have implications for a possible role of CORT (cortisol in humans) in sensorimotor gating and symptoms of schizophrenia. Further studies are needed to ascertain the site in the brain where CORT and dopaminergic activity interact.
Abbreviations
- ADX
adrenalectomised
- ANOVA
analysis of variance
- CORT
corticosterone
- CRF
corticotrophin-releasing factor
- s.e.m.
standard error of the mean
References
- BARROT M., MARINELLI M., ABROUS D.N., ROUGE-PONT F., LE MOAL M., PIAZZA P.V. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur. J. Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- BENES F.M. The role of stress and dopamine – GABA interactions in the vulnerability for schizophrenia. J. Psychiatric Res. 1997;31:257–275. doi: 10.1016/s0022-3956(96)00044-1. [DOI] [PubMed] [Google Scholar]
- BIRON D., DAUPHIN C., DI PAOLO T. Effects of adrenalectomy and glucocorticoids on rat brain dopamine receptors. Neuroendocrinology. 1992;55:468–476. doi: 10.1159/000126158. [DOI] [PubMed] [Google Scholar]
- BIRT D.F., DUYSEN E., WANG W., YAKTINE A. Corticosterone supplementation reduced selective protein kinase C isoform expression in the epidermis of adrenalectomised mice. Cancer Epidemiol. Biomarkers Prev. 2001;10:679–685. [PubMed] [Google Scholar]
- BRAFF D.L., GEYER M.A. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch. Gen. Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- BRAFF D.L., GRILLON C., GEYER M.A. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- BULLOCK A.E., SLOBE B.S., VÁZQUEZ V., COLLINS A.C. Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav. Neurosci. 1997;111:1353–1360. doi: 10.1037//0735-7044.111.6.1353. [DOI] [PubMed] [Google Scholar]
- CADOR M., DULLUC J., MORMÈDE P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993;56:981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- CLAUSTRE Y., RIVY J.P., DENNIS T., SCATTON B. Pharmacological studies on stress-induced increase in frontal cortex dopamine metabolism in the rat. J. Pharmacol. Exp. Ther. 1986;238:693–700. [PubMed] [Google Scholar]
- CONTI L.H., MURRY J.D., RUIZ M.A., PRINTZ M.P. Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology. 2002;161:296–303. doi: 10.1007/s00213-002-1025-2. [DOI] [PubMed] [Google Scholar]
- CREESE I., IVERSEN S.D. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- DE KLOET E.R., VREUGDENHIL E., OITZL M.S., JOELS M. Brain corticosteroid receptor balance in health and disease. Endocrine Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DEROCHE V., MARINELLI M., MACCARI S., Le MOAL M., SIMON H., PIAZZA P.V. Stress-induced sensitization and glucocorticoids. 1. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J. Neurosci. 1995;15:7181–7188. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIRKS A., GROENINK L., WESTPHAL K.G., OLIVIER J.D., VERDOUW P.M., VAN DER GUGTEN J., GEYER M.A., OLIVIER B. Reversal of startle gating deficits in transgenic mice overexpressing corticotropin-releasing factor by antipsychotic drugs. Neuropsychopharmacology. 2003;28:1790–1798. doi: 10.1038/sj.npp.1300256. [DOI] [PubMed] [Google Scholar]
- DREVETS W.C., GAUTIER C., PRICE J.C., KUPFER D.J., KINAHAN P.E., GRACE A.A., PRICE J.L., MATHIS C.A. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol. Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- DULAWA S.C., GEYER M.A. Psychopharmacology of prepulse inhibition in mice. Chin. J. Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- GEYER M.A. Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology. 1999;147:11–13. doi: 10.1007/s002130051130. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., MARKOU A.Animal models of psychiatric disorders Psychopharmacology: The Fourth Generation of Progress 1995New York: Raven Press; 787–798.ed. Bloom, F.E. & Kupfer, D.J [Google Scholar]
- GEYER M.A., MCILWAIN K.L., PAYLOR R. Mouse genetic models for prepulse inhibition: an early review. Mol. Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- GEYER M.A., SWERDLOW N.R.Measurement of startle response, prepulse inhibition, and habituation Current Protocols in Neuroscience 1998New York: Wiley; 8.7.1–8.ed. Crawley, J.N. & Skolnick, P [DOI] [PubMed] [Google Scholar]
- GISPEN-DE WIED C.C. Stress in schizophrenia: an integrative view. Eur. J. Pharmacol. 2000;405:375–384. doi: 10.1016/s0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- HENRY S.A., DULAWA S.C., CONQUET F., GEYER M.A. Severe disruption of prepulse inhibition in mice lacking mGluR5. Soc. Neurosci. Abstr. 1999;25:449. doi: 10.1111/j.1460-9568.2003.03073.x. [DOI] [PubMed] [Google Scholar]
- JACOBSON L. Glucocorticoid replacement, but not corticotropin-releasing hormone deficiency, prevents adrenalectomy-induced anorexia in mice. Endocrinology. 1999;140:310–317. doi: 10.1210/endo.140.1.6416. [DOI] [PubMed] [Google Scholar]
- KELLY P.H., SEVIOUR P.W., IVERSEN S.D. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- KOCH M. The neurobiology of startle. Prog. Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- KUMARI V., SONI W., MATHEW V.M., SHARMA T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch. Gen. Psychiatry. 2000;57:609–614. [Google Scholar]
- LARUELLE M., ABI-DARGHAM A., Van DYCK C.H., GIL R., D'SOUZA C.D., ERDOS J., MCCANCE E., ROSENBLATT W., FINGADO C., ZOGHBI S.S., BALDWIN R.M., SEIBYL J.P., KRYSTAL J.H., CHARNEY D.S., INNIS R. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE CUDENNEC C., NAUDIN B., DO REGO J.-C., COSTENTIN J. Nociceptin/orphanin FQ and related peptides reduce the increase in plasma corticosterone elicited in mice by an intracerebroventricular injection. Life Sci. 2002;72:163–171. doi: 10.1016/s0024-3205(02)02218-x. [DOI] [PubMed] [Google Scholar]
- LINDLEY S.E., BENGOECHEA T.G., SCHATZBERG A.F., WONG D.L. Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology. 1999;21:399–407. doi: 10.1016/S0893-133X(98)00103-1. [DOI] [PubMed] [Google Scholar]
- MEYER S.E., CHROUSOS G.P., GOLD P.W. Major depression and the stress system: a life span perspective. Dev. Psychopathol. 2001;13:565–580. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- NUECHTERLEIN K.H., DAWSON M.E., VENTURA J., GITLIN M., SUBOTNIK K.L., SNYDER K.S., MINTZ J., BARTZOKIS G. The vulnerability/stress model of schizophrenic relapse: a longitudinal study. Acta Psychiatr. Scand. 1994;382:58–64. doi: 10.1111/j.1600-0447.1994.tb05867.x. [DOI] [PubMed] [Google Scholar]
- OUAGAZZAL A.-M., JENCK F., MOREAU J.-L. Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity. Psychopharmacology. 2001;156:273–283. doi: 10.1007/s002130100763. [DOI] [PubMed] [Google Scholar]
- PANI L., PORCELLA A., GESSA G.L. The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- PIAZZA P.V., BARROT M., ROUGE-PONT F., MARINELLI M., MACCARI S., ABROUS D.N., SIMON H., LE MOAL M. Suppression of glucocorticoid secretion and antipsychotic drugs have similar effects on the mesolimbic dopaminergic transmission. Proc. Natl. Acad. Sci. U.S.A. 1996a;93:15445–15450. doi: 10.1073/pnas.93.26.15445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIAZZA P.V., LE MOAL M. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu. Rev. Pharmacol. Toxicol. 1996b;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- PIAZZA P.V., LEMOAL M. Glucocorticoids as a biological substrate of reward: physiological pathophysiological implications. Brain Res. Rev. 1997;25:359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- RALPH R.J., VARTY G.B., KELLY M.A., WANG Y.M., CARON M.G., RUBINSTEIN M., GRANDY D.K., LOW M.J., GEYER M.A. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J. Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUL J.M.H.M., DE KLOET E.R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- REUL J.M.H.M., DE KLOET E.R. Anatomical resolution of two types of corticosterone receptor sites in rat brain with invitro autoradiography and computerized image analysis. J. Steroid Biochem. 1986;1:269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- ROTH R.H., TAM S.Y., IDA Y., YANG J.X., DEUTCH A.Y. Stress and the mesocorticolimbic dopamine systems. Ann. NY Acad. Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- STEVENS K.E., BULLOCK A.E., COLLINS A.C. Chronic corticosterone treatment alters sensory gating in C3H mice. Pharmacol. Biochem. Behav. 2001;69:359–366. doi: 10.1016/s0091-3057(01)00523-8. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., BRAFF D.L., BAKSHI V.P., GEYER M.A.An animal model of sensorimotor gating deficits in schizophrenia predicts antipsychotic drug action Antipsychotics 1996Berlin: Springer; ed. Csernansky, J.G [Google Scholar]
- SWERDLOW N.R., GEYER M.A. Using an animal model of deficient sensorimotor gating to study the pathophysiology and new treatments of schizophrenia. Schizophrenia Bull. 1998;24:285–301. doi: 10.1093/oxfordjournals.schbul.a033326. [DOI] [PubMed] [Google Scholar]
- SWERDLOW N.R., MANSBACH R.S., GEYER M.A., PULVIRENTI L., KOOB G.F., BRAFF D.L. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology. 1990;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- TEITELBAUM H., GIAMMATTEO P., MICKLEY G.A. Differential effects of localized lesions of n. accumbens on morphine- and amphetamine-induced locomotor hyperactivity in the C57BL/6J mouse. J. Comp. Physiol. Psychol. 1979;93:745–751. doi: 10.1037/h0077605. [DOI] [PubMed] [Google Scholar]
- TJURMINA O.A., ARMANDO I., SAAVEDRA J.M., GOLDSTEIN D.S., MURPHY D.L. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- VAN DEN BUUSE M., VAN ACKER S.A.B.E., FLUTTERT M.J., DE KLOET E.R. Involvement of corticosterone in cardiovascular responses to open-field novelty stress in freely-moving rats. Physiol. Behav. 2002;75:207–215. doi: 10.1016/s0031-9384(01)00642-4. [DOI] [PubMed] [Google Scholar]
- VARTY G.B., WALTERS N., COHEN-WILLIAMS M., CAREY G.J. Comparison of apomorphine, amphetamine and dizolcipine disruptions of prepulse inhibition in inbred and outbred mice strains. Eur. J. Pharmacol. 2001;424:27–36. doi: 10.1016/s0014-2999(01)01115-3. [DOI] [PubMed] [Google Scholar]
- VRANG N., KRISTENSEN P., TANG-CHRISTENSEN M., LARSEN P.J. Effects of leptin on arcuate pro-opiomelanocortin and cocaine-amphetamine-regulated transcript expression are independent of circulating levels of corticosterone. J. Neuroendocrinol. 2002;14:880–886. doi: 10.1046/j.1365-2826.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- WALKER E.F., DIFORIO D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- ZOCCHI A., ORSINI C., CABIB S., PUGLISI-ALLEGRA S. Parallel strain-dependent effect of amphetamine on locomotor activity and dopamine release in the nucleus accumbens: an in vivo study in mice. Neuroscience. 1998;82:521–528. doi: 10.1016/s0306-4522(97)00276-5. [DOI] [PubMed] [Google Scholar]