Abstract
Current work has shown the importance of spinal cyclooxygenase (COX) products in facilitatory processes leading to tissue injury induced hyperalgesia. This cascade must originate with free arachidonic acid (AA) released by the activity of spinal phospholipase A2's (PLA2). In the present work, we studied the role of PLA2's in spinal sensitization.
We first demonstrate the presence of constitutive mRNA in the spinal cord for PLA2 Groups IB, IIA, IIC, IVA, V and VI by reverse transcription–polymerase chain reaction (RT–PCR) and sequencing. Using quantitative-PCR, we found that Group IVA cPLA2 and Group VI iPLA2 are the predominant PLA2 messages in the spinal cord. Western blotting and activity assays specific for Group IVA cPLA2 and Group VI iPLA2 verified the presence of these enzymes. PLA2 activity in spinal cord homogenates was suppressed by methyl arachidonyl fluorophosphonate (MAFP) and arachidonyl trifluoromethylketone (AACOCF3), mixed inhibitors of Group IVA cPLA2 and Group VI iPLA2 as well as by bromoenol lactone (BEL), a Group VI iPLA2 inhibitor. The spinal expression of PLA2 mRNA or protein was not altered in the face of peripheral inflammation. Secondly, we showed that intrathecal (i.t.) administration of MAFP and AACOCF3, but not BEL, dose-dependently prevented thermal hyperalgesia induced by intraplantar carrageenan as well as formalin-induced flinching. Finally, i.t. injection of AACOCF3, at antihyperalgesic doses, decreased the release of prostaglandin E2 (PGE2) into spinal dialysate evoked by i.t. NMDA, while i.t. injection of BEL had no effect.
Taken together, this work points to a role for constitutive Group IVA cPLA2 in spinal nociceptive processing.
Keywords: Arachidonic acid, carrageenan, formalin, prostaglandin E2, hyperalgesia, inflammation, intrathecal, pain, PLA2, spinal cord
Introduction
Tissue injury and inflammation results in a heightened sensitivity to subsequent noxious input (hyperalgesia). Prostanoids play an important role in this augmented processing, both by an action at the peripheral site of injury where they sensitize afferent terminals, and in the spinal cord where they increase evoked excitability. Studies of spinal facilitation have revealed a complex biochemical cascade that is initiated by activation of high threshold C-fibers. Acute activation of NMDA and neurokinin-1 (NK-1) receptors by glutamate and substance P released from high threshold afferents initiates the release of prostaglandins (Yaksh et al., 1999). These prostaglandins can act presynaptically through prostaglandin receptors located on afferent terminals to facilitate neurotransmitter release (Nicol et al., 1992; Hingtgen et al., 1995; Vasko, 1995) and postsynaptically to excite directly dorsal horn neurons (Baba et al., 2001). The functional importance of spinal prostaglandins in this process has been shown in behavioral models of injury- and inflammation-induced hyperalgesia, where inhibition of spinal COX normalize the otherwise enhanced pain sensitivity (Yaksh, 1982; Malmberg & Yaksh, 1992; Yaksh et al., 1998; Samad et al., 2001; Yamamoto & Nozaki-Taguchi, 2002).
Generation of prostaglandins is mediated by the activation of the PLA2-COX cascade. PLA2 activity serves to release arachidonic acid (AA), which can then be processed by COX-1 or COX-2 to generate prostaglandins (Kujubu et al., 1991; Capper & Marshall, 2001). In addition to supplying substrate for the COX pathway, AA liberated by PLA2 may also play additional roles in augmenting nociception. For example, AA potentiates NMDA receptor currents and thus amplifies glutamate-mediated increases in intracellular calcium concentration by binding to sites on the NMDA receptor, or by modifying the receptor's lipid environment (Miller et al., 1992). In addition, isoprostanes, a novel class of eicosanoids, are primarily formed by peroxidation of AA in a non-COX-dependent manner. Isoprostanes act to sensitize rat sensory neurons, thereby reducing mechanical and thermal withdrawal thresholds (Evans et al., 2000). Owing to the multiple mechanisms by which AA itself can contribute to spinal sensitization, PLA2 inhibition may exert antihyperalgesic actions, which are distinguishable from those exerted by the inactivation of COX.
To date, 14 different groups of PLA2's have been described, which can be classified as secreted (Groups IA, IB, IIA, IIB, IIC, IID, IIE, IIF, III, V, IX, X, XII, XIII, and XIV), Ca2+-dependent cytosolic (c)PLA2 (Groups IVA, IVB and IVC), and Ca2+-independent (i) PLA2 (Groups VIA-1, VIA-2 and VIB as well as VII and VIII) (Balsinde et al., 2002). The aim of this work was to study the role of spinal PLA2's in pain processing. We focused on two of the three subclasses of PLA2's, Group IVA cPLA2 and Group VI iPLA2. The role of the Group IVA cPLA2 in AA release and subsequent prostaglandin production is well established. Group IVA cPLA2 has a strong preference for AA at the sn-2 position of phospholipids (Diez et al., 1992) and this observed in vitro preference has been shown to have physiological significance in the release of AA and production of prostaglandins in vivo (Roshak et al., 1994; Naraba et al., 1998). The Group VI iPLA2 has no significant fatty acid specificity and its main function is believed to be in membrane remodeling (Balsinde et al., 1997), although some reports implicate its involvement in AA release (Akiba et al., 1998; Ramanadham et al., 1999) and indirectly in leukotriene synthesis (Larsson Forsell et al., 1998). This report will present data supporting a role for spinal PLA2 in centrally mediated hyperalgesia.
Methods
Animals
Experiments were carried out according to protocols approved by the Institutional Animal Care Committee of University of California, San Diego, CA, U.S.A. Male Holtzman Sprague–Dawley rats (250–350 g; Harlan Industries, Madison, WI, U.S.A.) were housed pairwise in cages and maintained on a 12-h light/dark cycle with free access to food and water at all times.
Intrathecal (i.t.) catheter implantation
For i.t. drug delivery, chronic lumbar catheters were implanted in rats under isoflurane anesthesia according to a modification of the procedure described by Yaksh (Yaksh & Rudy, 1976). A polyethylene catheter (stretched PE-10) was inserted through an incision in the atlanto-occipital membrane and advanced caudally to the rostral edge of the lumbar enlargement. Studies involving rats with chronic i.t. catheters were carried out 5 days after implantation. Rats were housed individually after implantation under the same conditions described above. Exclusion criteria were (i) presence of any neurological sequel, (ii) 10% weight loss after implantation, or (iii) catheter occlusion.
Induction of inflammation and assessment of hyperalgesia
To induce a state of local inflammation, 2 mg of carrageenan (Sigma, St Louis, MO, U.S.A.; 100 μl of 2% solution (w v−1) in physiological saline) was injected subcutaneously into the plantar surface of the left hind paw. To assess the thermally evoked paw-withdrawal response, a commercially available device modeled after that described by Hargreaves et al. (1988) was used (Dirig & Yaksh, 1995; Dirig et al., 1997). The device consists of a glass surface (maintained at 25°C) on which the rats were placed individually in Plexiglas cubicles (9 × 22 × 25 cm3). The thermal nociceptive stimulus originates from a focused projection bulb positioned below the glass surface. The stimulus is delivered separately to either hind paw of each test subject with the aid of an angled mirror mounted on the stimulus source. A timer is actuated with the light source, and latency was defined as the time required for the paw to show a brisk withdrawal as detected by photodiode motion sensors that stopped the timer and terminated the stimulus. Basal paw withdrawal latencies (PWL) were assessed at time (T)=−15 min. At T=−10 min, the animals received i.t. vehicle or drug, and at T=0, the carrageenan was injected intraplantarly. Withdrawal latencies were then assessed at T=60, 90, 120, 150, 180, and 240 min and expressed as the mean PWL of the left and right paws at each time point. The data were also presented as hyperalgesic index (HI). The HI is a calculation, which defines the magnitude of carrageenan-induced sensitization. It represents the area under the time effect curve after stimulation in which the ‘percent reduction from baseline (e.g. precarrageenan) response latency' is plotted versus time. The resulting metric is % change × min. The formula for calculating the percent change is: (base line latency−post drug latency) × 100 (base line latency)−1, where latency is expressed in seconds. Increasing values show increasing hyperalgesia.
Formalin-induced flinching
Flinching was assessed by an automated detection system (Yaksh et al., 2001b). A soft metal band (10 mm wide and 27 mm long, shaped into a C, and weighing ∼0.5 g) is placed on the hind paw of the animal being tested. Animals are allowed to acclimate in individual Plexiglas chambers for 1 h before being moved to a test chamber. Just before the animal's placement into the test chamber, it is briefly restrained in a cloth towel, and 5% formalin (in volumes of 50 μl physiological saline) is injected into the dorsal side of the banded paw. Data collection are initiated after the animal is placed inside the test chamber. Pain behavior was quantified by counting the incidences of spontaneous flinching or shaking of the injected paw. The flinches were counted for 1-min periods for 60 min. Two phases of spontaneous flinching of the injected paw were observed after formalin injection and defined as phase 1 (0–9 min) and phase 2 (10–60 min). For analysis, the total flinches for the phase 1 and phase 2 are calculated for each animal and these data are used for statistical comparison with a paired t-test or one-way ANOVA as appropriate.
Drugs and delivery
To examine the effects of drugs on carrageenan-induced hyperalgesia and flinching behavior, rats received i.t. or intraperitoneal (i.p.) injections of the drug. I.t. injections were carried out in rats that had been previously implanted with chronic i.t. catheters (see above) using drug volumes of 10 μl, followed by a 10-μl flush using vehicle. I.p. drugs were delivered in volumes of 0.5 ml kg−1. AACOCF3, MAFP, and BEL (Cayman Chemical, Ann Arbor, MI, U.S.A.) were dissolved in DMSO (Sigma, St Louis, MO, U.S.A.). The maximum tolerable i.t. dose was determined in a pilot dose–range study. AACOCF3 and MAFP were well tolerated up to the highest soluble dose (200 and 300 μg, respectively). I.t. injection of 30 μg of BEL was well tolerated, while 100 μg caused motor dysfunction (catatonia) that resolved after 30 min and 300 μg was lethal. I.t., but not i.p., injection of vehicle gave rise to a transient (<10 s) reaction with mild signs of agitation
I.t. dialysis and PGE2 assay
To assess the release of PGE2 from the lumbar i.t. space of the unanesthetized rat, animals were prepared with indwelling triple lumen dialysis probes. In brief, each probe consisted of a triple lumen length of polyethylene tubing. The two outer lumens were connected by a loop of dialysis tubing. The middle lumen was used for local drug delivery. The probe was implanted as described above for the i.t. catheter and externalized on the back of the neck. Details of this probe system and its validation are provided elsewhere (Malmberg & Yaksh, 1995; Marsala et al., 1995; Yaksh et al., 2001a). Dialysis experiments were conducted in unanesthetized rats 3 days after the implant. A syringe pump (Harvard, Natick, MA, U.S.A.) was connected and dialysis tubing was perfused with artificial cerebrospinal fluid (ACSF) at a rate of 10 μl min−1. The ACSF contained (mM) 151.1 Na+, 2.6 K+, 0.9 Mg2+, 1.3 Ca2+, 122.7 Cl−, 21.0 HCO3, and 2.5 HPO4, and it was bubbled with 95% O2/5% CO2 before each experiment to adjust the final pH to 7.2. The efflux (20 min per fraction) was collected in an automatic fraction collector (Eicom, Kyoto, Japan) at 4°C. Two baseline samples were collected following a 30-min washout, and an additional three fractions after i.t. injection of NMDA (0.6 μg) through the central lumen of the dialysis catheter. AACOCF3, MAFP, and BEL were delivered i.t. 20 min prior to NMDA in a solution of 5% Cremophor El and 5% DMSO in saline. The concentration of PGE2 in spinal dialysate was measured by ELISA using a commecialy available kit (Assay Designs 90001, Assay Designs, Ann Arbor, MI, U.S.A.). The antibody is selective for PGE2 with less than 2.0% crossreactivity to PGF1α, PGF2α, 6-ketoPGF1α, PGA2, or PGB2, but crossreacts with PGE1 and PGE3.
Tissue preparation
Prior to tissue harvest, the rats were deeply anesthetized (isoflurane anesthesia; 5% for induction and 2% for maintenance in 50% O2), and after decapitation, the spinal cords were ejected from the spinal column by a saline-filled syringe. The lumbar part of the spinal cord was frozen on dry ice and stored at −70°C. Frozen spinal cords were pulverized using a BioPulverizor (Biospec Products, Bartlesville, OK, U.S.A.) prechilled on dry ice. Pulverized tissue was then transferred to a microcentrifuge tube and mixed with 750 μl lysis buffer: 10 mM Hepes (pH 7.5), 1 mM EDTA, and 0.34 M sucrose. Mammalian protease inhibitor cocktail (20 μl) (Sigma, St Louis, MO, U.S.A.) was added immediately. Samples were vortexed and sonicated until homogeneous, and then centrifuged at 16,000 × g, 4°C, 40 min. Supernatants were transferred to a fresh Eppendorf tube and the pellet was discarded.
Western blot
The protein concentration of the supernatant was determined by Bradford assay using BSA as the standard. Protein (30 μg) was run on a NuPAGE 4–12% Bis–Tris gel (Invitrogen, Carlsbad, CA, U.S.A.) under denaturing conditions, and then transferred to nitrocellulose. Protein was detected using an anti-human iPLA2 antibody (a gift from Genetics Institute, Cambridge, MA, U.S.A.) or an anti-cPLA2 antibody (Cell Signaling Technology, Beverly, MA, U.S.A.). Each blot was then stripped (Re-Blot Antibody Striping Solution, Chemicon, Temecula, CA, U.S.A.) and reprobed with an anti-β-actin antibody (Sigma, St Louis, MO, U.S.A.) as a loading control.
cDNA preparation
mRNA was extracted from lumbar spinal cord homogenates of nontreated rats or from rats either 2 or 4 h following hind paw injection with carrageenan using the TRIzol method (Gibco BRL, now Invitrogen, Carlsbad, CA, U.S.A.) and contaminating DNA was eliminated by DNA-free treatment (Ambion, Austin, TX, U.S.A.) following the manufacturer's instructions. The concentration of the resulting DNA-free RNA was determined spectrophotometrically. cDNA was prepared using M-MLV reverse transcriptase (Gibco BRL, now Invitrogen, Carlsbad, CA, U.S.A.) and oligodT as the primer. Following cDNA preparation, the samples were incubated with RNase H for 20 min at 37°C to remove RNA.
PLA2 gene fragment cloning
PLA2 gene fragments were generated by polymerase chain reaction (PCR) using the primers listed in Table 1 below. The resulting fragments were purified from an agarose gel and cloned into the pCR-Blunt II-TOPO vector (Invitrogen, Carlsbad, CA, U.S.A.). Inserts were sequenced to verify their identity and vector concentration was determined spectrophotometrically.
Table 1.
Primers and probes employed
| Gene | Primer or probe | Sequence (5′–3′) | [Primer] or [probe] (nM) |
|---|---|---|---|
| IB | F Primer | CTCCAAGGTCCCCTACAACA | 500 |
| R Primer | GAAGTGGGGTGACAGCCTAA | 500 | |
| IIA | F Primer | TGAACAAGAAGCCATACCACCAT | 900 |
| R Primer | AGGAGGACCTTCATGCTGTCA | 900 | |
| Probe | CCCATCCAAGAGAGC | 250 | |
| IIC | F Primer | CTCCACCCTACCCAGGTACA | 500 |
| R Primer | AGCCTCTGGCATTGGTAGAA | 500 | |
| IVA | F Primer | GACTTTTCTGCAAGGCCAAG | 300 |
| R Primer | CTTCAATCCTTCCCGATCAA | 300 | |
| V | F Primer | CCATCCGGACCCAGTCCTAT | 300 |
| R Primer | CTTCCGGTCACAAGCACAAA | 300 | |
| Probe | TGCGAACACGACTCCTTCTGTCCAG | 250 | |
| VI | F Primer | GCCTTCGCAGGTATCAAAAG | 500 |
| R Primer | GGGAATCTGGTGAAAGTCCA | 500 | |
| GAPDH | F Primer | ATGACTCTACCCACGGCAAG | 300 |
| R Primer | GATCTCGCTCCTGGAAGATG | 300 |
Quantitative-PCR (Q-PCR)
Gene-specific primers were designed using the Primer3 program (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3.cgi/) or Primer Express 1.0 (Applied Biosystems, Foster City, CA, U.S.A.). Primers were designed to encompass introns in the genomic sequence. SYBR green PCR master mix (Applied Biosystems, Foster City, CA, U.S.A.) was used to generate single amplicons for all genes tested except for PLA2 Groups IIA and V. For these genes, probes were required to increase the specificity of the PCR reaction. FAM probes were designed for both PLA2 Groups IIA and V and Taqman PCR master mix (Applied Biosystems, Foster City, CA, U.S.A.) was used to generate the product. Primer concentration and PCR conditions were optimized as described in the Applied Biosystems user bulletin (ABI Part #4303859B) and are listed below. For each gene, PCR product was also amplified from total rat RNA (Ambion, Austin, TX, U.S.A.) to verify that the primers and PCR conditions were working. Standard thermocycling conditions were used: 2 min at 50°C UNG activation (for Taqman chemistry), 10 min at 95°C for polymerase activation, 40 cycles of 15 s at 95°C, and 1 min at 60°C. Q-PCR results were analyzed by the standard curve method using cDNA from untreated rat spinal cord to generate the standard curve. In the case of absolute quantitation, TOPO vectors (Invitrogen, Carlsbad, CA, U.S.A.) containing PLA2 gene fragment inserts were used to generate the standard curve. Total cDNA (10 ng) was analyzed for each rat sample. GAPDH was used as the internal standard and acts as a loading control. Analysis by absolute quantitation indicates no significant change in GAPDH levels between control and treated animals. Dissociation curves were generated following each Q-PCR run to verify the amplification of a single amplicon. In the case of PLA2 Groups IIA and V where probes were utilized, the PCR products were analyzed by agarose gel to verify the production of a single amplicon. PCR products were purified from gels and sequenced to confirm their identity. Primers, probes, and PCR conditions are listed in Table 1. Primer and probe concentrations listed are the final concentrations in the assay.
PLA2 activity assays
Following homogenization and centrifugation, the spinal cord supernatants were assayed for Group IV cPLA2 or Group VI iPLA2 activity using the group-specific assays developed in our laboratory (Yang et al., 1999). Radioactive lipids were purchased from Perkin-Elmer (Shelton, CT, U.S.A.). Nonradioactive lipids were purchased from Avanti Polar Lipids (Alabaster, AL, U.S.A.). Briefly, the Group IV cPLA2 assay conditions were: 100 μM lipid PAPC/PIP2 (97/3) doping with 1% 14C-labeled PAPC in 400 μM Triton X-100 mixed micelles, 100 mM Hepes (pH 7.5), 0.08 mM CaCl2, 0.1 mg ml−1 BSA, and 2 mM DTT. Group VI iPLA2 assay conditions were: 100 μM DPPC doping with 1% 14C-labeled DPPC in 400 μM Triton X-100 mixed micelles, 100 mM Hepes (pH 7.5), 5 mM EDTA, and 1 mM ATP. The total volume for each assay is 500 μl: 200 μl lipid, 250 μl assay buffer, and 50 μl sample. In each case, the amount of calcium or EDTA added was adjusted to account for the addition of EDTA in the lysis buffer to give the final concentrations listed above. Lipid preparation: lipid was dried under N2 and lyophilized for at least 1 h to remove all traces of chloroform. Lipid was then resuspended in 100 mM Hepes and Triton X-100 and micelles were created by repeated vortexing and heating in hot water until the solution clarified. Samples were incubated with substrate for 1 h at 40°C in a shaking bath. The assay was then terminated by the addition of 2.5 ml Dole Reagent (isopropyl alcohol : heptane : 0.5 M sulfuric acid 400 : 100 : 20, V : V : V). Silica gel (0.1–0.2 mg) was added to each tube followed by 1.5 ml heptane and 1.5 ml deionized water. Each tube was vortexed 15 s. A measure of 1 ml of the organic phase was removed and passed through a Pasteur pipette filled with silica gel (0.1–0.2 mg). This column was then washed with 1 ml diethyl ether. Scintillation cocktail (5 ml) (Biosafe II, RPI, Mount Prospect, IL, U.S.A.) was then added to the eluent and the radioactivity was determined by scintillation counting. Following analysis, the data for the Group IV cPLA2 assay was adjusted for contaminating iPLA2 activity as described in an earlier paper (Yang et al., 1999). For the inhibitor studies, the same phospholipid and buffer conditions were used as above with the addition of 4 μM (0.8 mol%) inhibitor. All inhibitors were purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). AACOCF3 was incubated with the homogenate for 4 h prior to assay. MAFP and BEL were aliquoted into the lipid substrate immediately before the start of the assay. Prior to testing the effects of inhibitors on spinal cord activity, the inhibitory efficacy and specificity of each inhibitor was tested on purified human Group IVA cPLA2 and Sf9 insect cell lysates known to express iPLA2 (data not shown). As expected, MAFP and AACOCF3 inhibited both enzymes, while BEL was specific for iPLA2.
Statistics
Four to eight rats were included in each group for behavioral assessments. Four to five rats were included in each group for the dialysis experiments. Each time point and bar represents mean±s.e.m. Differences between groups were compared with one-way ANOVA using Statview or Prism statistical software if nothing else is indicated. Three to four rats were included for each activity assay and PCR experiment. Values represent the average±s.d. P-values were determined for the inhibitor assays using Graph Pad's on-line calculator.
Results
RNA message for PLA2 Groups IB, IIA, IIC, IVA, V, and VI is constitutively expressed in the spinal cord
To determine the presence or absence of PLA2 message in the spinal cord, sequence-specific primers were chosen to surround a splice junction for each rat PLA2 gene available in the public database and RT–PCR (reverse transcription–PCR) was performed. Resulting fragments were then purified and sequenced to verify their identity. RNA message was found for PLA2 Groups IB, IIA, IIC, IVA, V, and VI, but not for PLA2 Group X. A positive control of total rat RNA was used to verify that the Group X primers do indeed amplify Group X mRNA.
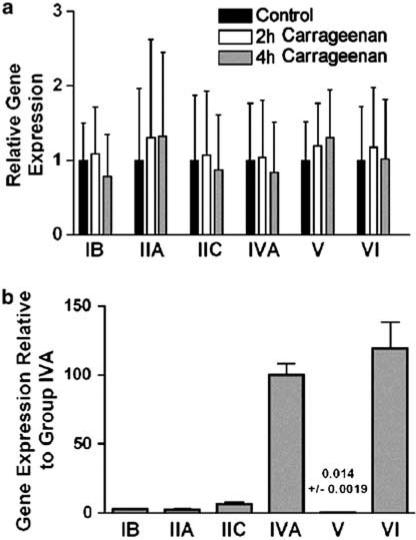
Carrageenan induces a characteristic inflammation and associated thermal hyperalgesia. To test for a possible upregulation in PLA2 message following exposure to carrageenan, Q-PCR was performed utilizing the primers from Table 1 on control rats or rats killed 2 or 4 h following injection of carrageenan to the hind paw. Analysis indicates no significant alteration in abundance of any of the PLA2 gene transcripts following carrageenan injection as compared with control rats (Figure 1a).
Figure 1.
Q-PCR analysis. (a) Expression levels are determined by standard curve method. For each gene, expression in control rat spinal cord is compared to expression in the spinal cord of rats treated with carrageenan hind paw injection either 2 or 4 h after injection. (b) Expression levels are determined by absolute quantitation using vector containing cDNA fragments of the PLA2's for the standard curve. The expression of each gene can then be compared to the others. Each bar represents the average and s.d. of three rats assayed in triplicate.
The relative expression levels of the six PLA2 genes were determined using the absolute quantitation method of Q-PCR (Figure 1b). The gene fragments generated by PCR were cloned into the TOPO vector. The resulting circular DNA could then be quantitated by spectrophotometry to determine the copy number. These vectors containing PLA2 fragments were then serially diluted to have between 10,000 and 10 copies and Q-PCR was again performed on control rat samples using these vectors to generate the standard curves. As can be seen in Figure 1b, Group IVA cPLA2 and Group VI iPLA2 are the predominant PLA2 messages found in rat spinal cord. Accordingly, we focused our attention on these two PLA2's.
Group IVA cPLA2 and Group VI iPLA2 protein levels are stable through the onset of hyperalgesia
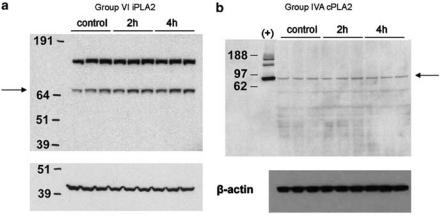
Analysis by Western blot also indicates a stable level of both Group IVA cPLA2 and Group VI iPLA2 protein in control versus treated animals (Figure 2). Group VI iPLA2 has been reported to run at 80 kDa on SDS–PAGE (Wolf & Gross, 1996). A heavier band was also observed (Figure 2a) and is likely the dimerized enzyme. Group VI iPLA2 is known to form such tight binding oligomers (Ackermann et al., 1994). Group IVA cPLA2 has been reported to run at 100 kDa (Sharp et al., 1991). Interestingly, in our gel system both the positive control (purified human Group IVA cPLA2) and Group IVA cPLA2 in the spinal cord samples ran nearer the known molecular weight of 85 kDa. In addition to the time points shown here (Figure 1), protein levels for both enzymes were determined to be stable at 1 and 8 h as well (data not shown).
Figure 2.
Western blots of Group VI iPLA2 and Group IVA cPLA2 protein expressed in rat lumbar spinal cord. (a) Top: α-Human Group VI iPLA2 antibody; bottom: β-actin loading control. Lane (1) molecular weight marker (numbers indicate molecular masses in kDa), lanes (2–4) control rat, lanes (5–7) 2 h carrageenan, and lanes(8–10) 4 h carrageenan. (b) Top: α-Group IVA cPLA2 antibody; bottom: β-actin loading control. Lane (1) molecular weight marker (numbers indicate molecular masses in kDa), lane (2) (+) purified human Group IVA cPLA2, lanes (3–5) control rat, lanes (6–8) 2 h carrageenan, and lanes (9–11) 4 h carrageenan.
Group IVA cPLA2 and Group VI iPLA2 are active in the spinal cord
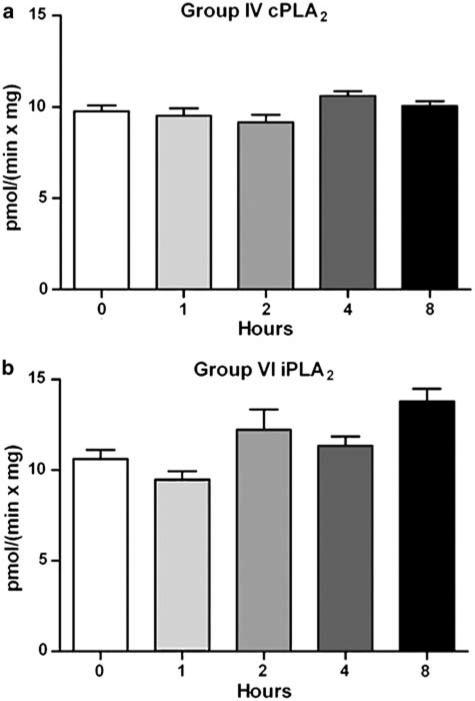
Having found evidence that PLA2 message and protein is present in the spinal cord tissue, we next tested the spinal cord for PLA2 activity using group specific assays that can differentiate Group IVA cPLA2 from Group VI iPLA2 (Yang et al., 1999). Lumbar spinal homogenates were assayed from control rats or carrageenan-treated rats killed 1, 2, 4, or 8 h after hind paw injection. Both Group IVA cPLA2 and Group VI iPLA2 showed significant activity in the spinal cord homogenates, but no measurable change in activity was observed following carrageenan treatment (Figure 3).
Figure 3.
PLA2 activity of rat lumbar spinal cord homogenate was assayed for 1 h as described in the Methods section. Constitutive enzyme activity was observed following carrageenan injection to the hind paw over an 8 h time course in comparison with control. (a) Group IVA cPLA2 activity assay: PAPC/PIP2 (97/3) Triton X-100 mixed micelles. (b) Group VI iPLA2 activity assay: DPPC Triton X-100 mixed micelles. Each bar represents the average and s.d. of four rats assayed in triplicate.
In vitro inhibition of Group IVA cPLA2 and Group VI iPLA2 activity
Three commercially available PLA2 inhibitors were tested for their ability to decrease spinal PLA2 activity. AACOCF3 and MAFP inhibit both the Group IVA cPLA2 as well as the Group VI iPLA2, while BEL is specifically a Group VI iPLA2 inhibitor. Spinal homogenates from untreated rats were assayed for Group IVA cPLA2 and Group VI iPLA2 activity in the presence of the inhibitors (Figure 4). In order to prevent nonspecific effects due to high surface concentrations, the inhibitors were tested at 4 μM or 0.8 mol% total substrate surface. Preincubation of AACOCF3 with enzyme prior to assaying has been shown to increase its inhibitory strength 10-fold (Ghomashchi et al., 1999); therefore, homogenates were preincubated with AACOCF3 for 4 h prior to assaying. BEL and MAFP were both presented with the substrate. MAFP and AACOCF3 inhibited both spinal Group IVA cPLA2 and Group VI iPLA2 activity, while BEL inhibited only Group VI iPLA2 activity as was expected.
Figure 4.
Inhibition of spinal PLA2 activity in vitro. Rat lumbar spinal cord homogenates were assayed for 1 h for (a) Group IVA cPLA2 activity, or (b) Group VI iPLA2 activity in the presence of 0.8 mol% inhibitor (presented with substrate). Each bar represents the average and s.d. of three rats assayed in duplicate and (*) represents P<0.009 versus control conditions.
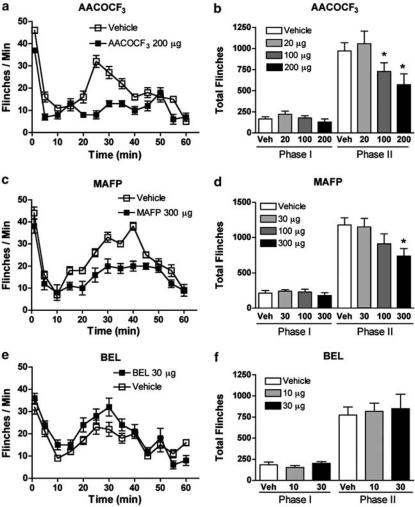
I.t. administration of PLA2 inhibitors suppresses formalin-induced flinching
Injection of formalin into the dorsum of the right hind paw evokes a biphasic appearance of flinching (Figure 5). I.t. pretreatment with AACOCF3 and MAFP, but not the iPLA2-specific inhibitor BEL, resulted in a dose-dependent reduction of the formalin-induced flinching (Figure 5a, c, and e). While the drugs did not significantly affect phase 1, a statistically significant reduction was seen in phase 2 for animals receiving i.t. AACOCF3 or i.t. MAFP (Figure 5b and d), but not IT BEL (Figure 5f), when compared to the group that received i.t. vehicle.
Figure 5.
Flinching behavior plotted versus time following injection of formalin into the dorsal side of the right hind paw of rats pretreated (−10 min) with i.t. vehicle (open squares) or (a) i.t. AACOCF3 (200 μg, closed squares), (c) i.t. MAFP (300 μg, closed squares), or (e) i.t. BEL (30 μg, closed squares). Cumulative number of flinches during phase 1 (0–9 min, total number) or phase 2 (10–60 min, total number) observed after different doses of (b) i.t. AACOCF3, (d) i.t. MAFP, or (f) i.t. BEL. Each time point and bar represents the average and s.e.m. for six to eight rats and (*) represents P<0.05 versus vehicle-treated formalin-injected group.
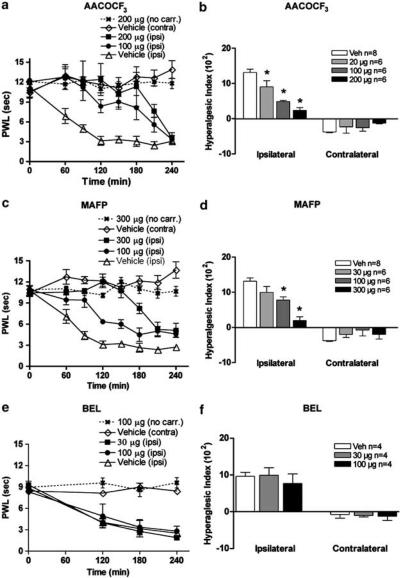
I.t. PLA2 inhibitors attenuated carrageenan-induced thermal hyperalgesia
Baseline latencies were assessed for all animals before injection of carrageenan and the average time to response was 11.1±0.4 s for the left hind paw (ipsilateral) and 10.6±0.7 s for the right hind paw (contralateral). After carrageenan injection into the plantar side of the left hind paw, a reduction in time to paw withdrawal was detected. The withdrawal latency time decreased to 3.2±0.7 s at 120 min after carrageenan injection (Figure 6a). There was no change of withdrawal time for either of the two control groups receiving i.t. saline (data not shown) or i.t. vehicle (Figure 6). Pretreatment with either i.t. AACOCF3 or i.t. MAFP resulted in a potent dose-dependent prevention of carrageenan-induced thermal hyperalgesia (Figure 6a and c). Importantly, there were no changes in the response latency of the uninflamed paw of rats that received carrageenan, or in the control group that received i.t. PLA2 inhibitor, but not paw carrageenan, even at the highest doses of either drug, indicating that the drugs are acting as antihyperalgesic agents rather than as analgesics (Figure 6a, c, and e). A statistically significant reduction in the HI occurred upon administration of all doses of i.t. AACOCF3 or the two highest doses of i.t. MAFP in comparison with rats that received vehicle control (Figure 6b and d). Owing to limitation in solubility, the maximum dose given i.t. differs between AACOCF3 and MAFP. Pretreatment with i.t. BEL did not prevent carrageenan-induced thermal hyperalgesia (Figure 6e and f). The higher dose (100 μg) caused motor dysfunction in the form of catatonia, lasting for 30 min. These animals were tested first at 120 min after carrageenan injection to allow recovery of motor function. Owing to limitations in tolerability, 30 μg was given i.t. in all other studies involving BEL. A test of spontaneous movement indicated that the compounds at the highest doses employed in analgesia assessment had no apparent effect on normal motor function at the doses used (Table 2).
Figure 6.
PWL plotted versus time after injection of carrageenan into plantar face of left hind paw of rats pretreated (−10 min) with i.t. (a) AACOCF3, (c) MAFP, and (e) BEL. PWL for the inflamed paw (ipsi) and noninflamed paw (contra) are shown. The control groups received i.t. vehicle followed by paw carrageenan or i.t. drug but no carrageenan. HI calculated over time from T=0 to T=180 min after different doses of i.t. (b) AACOCF3, (d) MAFP, and (f) BEL. The hyperalgesic index (H1) represents the area under the time effectcurve after stimulation in which the ‘percent reduction from baseline (e.g. precarrageenan) response latency ‘ is plotted versus time. The resulting metric is % change × min. The formula for calculating the percent change is: (base line latency−postdrug latency) × 100 (base line latency)−1, where latency is expressed in seconds. Increasing values show increasing hyperalgesia. Each time point and bar represents the average and s.e.m. and (*) represents P<0.05 versus vehicle-treated carrageenan-injected group (ipsilateral paw values).
Table 2.
Effect of PLA2 inhibitors on spontaneous movementa
| Number of counts | n | |
|---|---|---|
| Saline | 367±45 | 6 |
| Vehicle | 306±36 | 6 |
| AACOCF3 200 μg | 298±41 | 6 |
| MAFP 300 μg | 311±40 | 6 |
| BEL 100 μg | 212±32 | 6 |
| BEL 30 μg | 354±39 | 6 |
Spontaneous movement during 60 min following i.t. injection of saline, vehicle, or PLA2 inhibitors. Movement was quantified using the automated flinch counting device with the difference that no formalin was injected into the paw. Animals were acclimatized to the device and measurements started 10 min after i.t. administration of vehicle or drug.
To confirm that the antihyperalgesic effect of the i.t. delivered PLA2 inhibitors was due to spinal actions and not peripheral actions following redistribution from spinal to peripheral sites, the highest dose of AACOCF3 (200 μg) that was given i.t. was given i.p. As shown in Figure 7a, no effect on the carrageenan-induced thermal hyperalgesia was seen following i.p. administration of 200 μg AACOCF3. Additionally, the effect of i.t. injection of AACOCF3 on carrageenan-induced paw edema was assessed by measurement of paw height. The height of the ipsilateral paw increased significantly over the course of the experiment. The paw height peaked at 4–6 h (Figure 7b) and was back at baseline after 72 h (data not shown). The contralateral paw did not show any signs of height increase and the PLA2 inhibitor treatment did not have a statistically significant effect on the height of the carrageenan-injected paw (Figure 7b).
Figure 7.
(a) Thermal escape latency (PWL) plotted versus time after injection of carrageenan into plantar face of left hind paw of rats pretreated (−10 min) with i.p. AACOCF3 (200 μg 0.5 ml−1, contralateral paw, closed squares; ipsilateral paw, closed triangles). Each time point represents the average and s.e.m. for four rats. (b) Paw height of carrageenan-injected (ipsi) and nontreated (contra) hind paws measured at different time points. The control group received i.t. vehicle and the other group received i.t. AACOCF3 (200 μg) 10 min prior to carrageenan injection. Each time point represents the average and s.e.m. for six to eight rats.
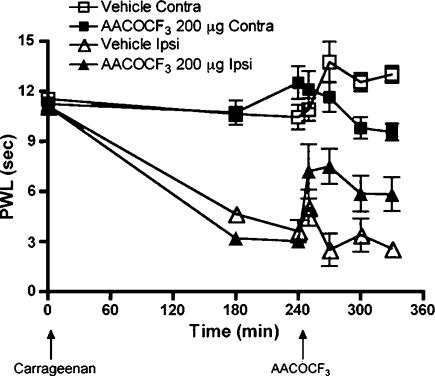
Post-treatment with i.t. PLA2 inhibitor partially reversed carrageenan-induced hyperalgesia
To examine the effect of the PLA2 inhibitors on a state where thermal hyperalgesia is already established, AACOCF3 was administered i.t. 240 min after the carrageenan injection into the paw. In this study, we noted that in comparison to pretreatment that fully prevented the onset on thermal hyperalgesia, post-treatment only partially reversed the hyperalgesia (Figure 8). I.t. administration of vehicle at the same time point showed no antihyperalgesic effect (Figure 8). There is a slight increase in contralateral PWL 210 and 240 min after carrageenan injection (Figures 6a, c and 8). This is a phenomenon we frequently observe in untreated or vehicle receiving animals, and it is most likely due to the dramatic change in sensitivity in the ipsilateral paw. The rat appears reluctant to shift in its bodyweight to the inflamed paw. Accordingly, there is a slight increase in the uninjured PWL as the PWL time on the ipsilateral side decreases.
Figure 8.
Thermal escape latency (PWL) plotted versus time after injection of carrageenan into plantar face of left hind paw of rats post-treated (+240 min) with i.t. AACOCF3 (200 μg, contralateral paw, closed squares; ipsilateral paw, closed triangles) or with i.t. vehicle (contralateral paw, open squares; ipsilateral paw, open triangles). Each time point represents the average and s.e.m. for six to eight rats.
Evoked spinal prostaglandin release is decreased by PLA2 inhibition
It has previously been shown that direct activation of the NMDA receptor evoke increase of PGE2 concentrations in cerebrospinal fluid as well as thermal hyperalgesia that is prevented by inhibition of spinal COX-2 (Malmberg & Yaksh, 1992; Yaksh et al., 2001a; Koetzner et al., 2004). Baseline release of PGE2 was determined after a washout period of 30 min. In the absence of pretreatment, baseline dialysate concentrations were determined to be 120±27 fmol 100 μl−1 perfusate (N=12) and in the presence of pretreatment 134±23 fm 100 μl−1 perfusate (N=18). I.t. injection of NMDA (0.6 μg) resulted in a statistically significant increase in PGE2 concentrations in spinal dialysate (Figure 9a–d). Pretreatment with AACOCF3 (200 μg i.t. 20 min prior to NMDA) attenuated i.t. NMDA-evoked PGE2 release (Figure 9a) in fraction F1 and F2 as compared to baseline release (Figure 9a). In Figure 9b, the data are expressed as PGE2 release per minute during the baseline phase (−40 to 0 min) in comparison to the stimulated phase (0–60 min). I.t. NMDA evoked a statistically significant release (unpaired t-test P=0.001) and pretreatment with AACOCF3 prevented this increase in PGE2 release (unpaired t-test P=0.001). Pretreatment with BEL (30 μg i.t. 20 min prior to NMDA) had no effect on NMDA-evoked PGE2 release (Figure 9c). In Figure 9d, where the data are expressed as PGE2 release per minute, the data demonstrate that i.t. NMDA evoked a statistically significant release also in the presence of BEL (unpaired t-test P=0.002). Injection of i.t. vehicle or i.t. vehicle prior to i.t. NMDA did not affect PGE2 release (data not shown). Efforts to study the effects of MAFP on NMDA-evoked PGE2 release were not successful because, unlike AACOCF3 and BEL, the antibody used in the PGE2 ELISA recognizes MAFP.
Figure 9.
PGE2 concentration (fmol 100 μl−1) measured in cerebrospinal fluid collected by in vivo spinal dialysis of conscious rats before and after i.t. injection of NMDA (0.6 μg). Spinal dialysate is removed and assayed for PGE2 by ELISA at baseline (b, average of two 20 min fractions collected prior to i.t. injection of NMDA) and F1: 0–20 min, F2: 20–40 min, and F3: 40–60 min after NMDA injection. Rats received (a and c) i.t. NMDA (0.6 μg) alone (black bars), and (a) i.t. AACOCF3 (200 μg) or (c) i.t. BEL (30 μg) 20 min prior to i.t. NMDA (gray bars). In (b) and (d), the data are expressed as PGE2 release (fmol 100 μl−1) per minute during baseline (−40 to 0 min) and following NMDA injection (0–60 min) with or without pretreatment with PLA2 inhibitor. I.t. injection of vehicle prior to i.t. NMDA or i.t. injection of vehicle alone did not affect PGE2 release (data not shown). Each bar represents the average and s.e.m. for four to five rats per group and (*) represents P<0.5 versus baseline.
Effect of i.t. injection of PLA2 inhibitors on spontaneous movement
While no evidence of motor dysfunction was noted at the highest doses employed in analgesic studies, we sought to further assess if the antihyperalgesic actions might be attributed to a suppressed behavioral function. Groups of rats received i.t. injections of AACOCF3 (200 μg), MAFP (300 μg), BEL (30 μg), or vehicle, and their spontaneous activity was assessed using the flinch-counting device in the absence of paw formalin injection. The total number of counts generated over 60 min after injection showed no statistically significant difference in comparison to the vehicle-treated group. I.t. injection of higher doses of BEL (100 μg), however, resulted in a significant reduction of spontaneous activity. Therefore, only the lower dose of BEL was considered in these studies.
Discussion
Hydrolysis of phospholipids by PLA2's releases AA, which is processed by COX enzymes to produce prostaglandins. This leads to increased spinal facilitation through increased afferent neurotransmitter release and dorsal horn depolarization. Additionally, the release of AA can facilitate nociceptive processing through NMDA receptor potentiation and it has been speculated that non-COX-dependent isoprostane production may also be involved. While many groups have studied the role of the COX enzymes in this process, the role of spinal PLA2 in the facilitation of spinal nociceptive processing has not been well considered. Here, we illustrate the important role PLA2 plays in spinally mediated hyperalgesia based on three principal lines of evidence: (i) spinal presence of active PLA2 enzymes, (ii) the ability of spinally delivered PLA2 inhibitors to decrease pain behavior, and (iii) the ability of behaviorally effective doses of PLA2 inhibitor to reduce the release of a downstream product of PLA2 activation. These studies were carried out using two established models of hyperalgesia: carrageenan- and formalin-induced hypersensitivity. There already exists considerable evidence that the PLA2-COX pathway is involved in these models making them a good starting point for the further elucidation of the contribution of individual PLA2's to pathological pain.
Using Q-PCR, we have shown that mRNA for six PLA2 enzymes, Groups IB, IIA, IIC, IVA, V, and VI, is present in the spinal cord. Groups IVA and VI are the most highly expressed of the PLA2's, showing over a 16-fold expression difference in comparison to the Groups IB, IIA, or IIC sPLA2. Of the sPLA2's, Group V shows the lowest expression having 200-fold less message than Groups IB, IIA, or IIC sPLA2 and fully four orders of magnitude less message than Group IVA cPLA2 or Group VI iPLA2. As Group IVA cPLA2 and VI iPLA2 have the highest expression in the spinal cord, we focused our initial investigation on these two cytosolic enzymes.
Comparison of each PLA2 gene to itself in control versus carrageenan-injected animals indicates that none of the PLA2's are transcriptionally upregulated following the onset of hyperalgesia, a finding consistent with a previous report of Samad et al. (2001). As is observed in the behavioral models, the onset of hyperalgesia occurs as soon as 15 min following injury. This suggests that the proteins involved in the initial onset of hyperalgesia must be present at the time of injury as this is not sufficient time to allow for transcription and translation of new enzyme. Although Group IVA cPLA2 has been shown to be upregulated by proinflammatory molecules in rheumatoid synovial fibroblast cells (Roshak et al., 1996) and human monocytes (Roshak et al., 1994), the immediate regulation of Group IVA cPLA2 activity directly following agonist exposure is believed to be through translocation of the enzyme from the cytosol to the membrane subsequent to increases in cytosolic calcium levels (Channon & Leslie, 1990; Clark et al., 1995; Glover et al., 1995; Gijon et al., 1999) rather than through increases in mRNA transcription. Similarly, in astrocytes, Group IVA cPLA2 is required for the production of prostaglandins, but is not transcriptionally upregulated following exposure to cytokines (Sun et al., 2004). In the present study, because the spinal cord tissue was homogenized prior to the activity assay, our data do not indicate how much protein was actively hydrolyzing phospholipid at the cell membrane in the intact spinal cord at the time the animal was killed, but rather is simply a measure of total active PLA2 in the tissue. Our data demonstrate that active Group IVA and Group VI PLA2 enzyme is present in the spinal cord before tissue injury and that the enzymes maintain their activity through the full onset of hyperalgesia.
In two separate models of hyperalgesia, we showed that i.t. administration of MAFP and AACOCF3 (each of which inhibit both Group IVA cPLA2 and Group VI iPLA2) results in a potent, dose-dependent suppression of hyperalgesia. I.t. administration of the Group VI iPLA2-specific inhibitor BEL, however, had no effect on either model. This suggests that Group VI iPLA2 is not involved in the mediation of spinal hyperalgesia. We note that an alternate interpretation is that Group IVA cPLA2 and Group VI iPLA2 are acting in concert to mediate the release of prostaglandins and subsequent hyperalgesia. Until the advent of a selective Group IVA cPLA2 inhibitor, we cannot completely disregard this possibility. However, in agreement with our work, multiple studies focused at the cellular level implicate a role for Group IVA cPLA2 and not Group VI iPLA2 in AA release (Balsinde et al., 1995; Balsinde & Dennis, 1996b; Dieter et al., 2002).
An important issue relates to the spinal locus of the observed actions. I.t. administration of AACOCF3 did not decrease paw edema. Furthermore, i.p. administration of the same amount of AACOCF3 as was given i.t. had no effect on the carrageenan-induced thermal hyperalgesia. These joint observations emphasize that the antihyperalgesic effect of i.t. PLA2 inhibition is not due to a change in peripheral inflammation and is not produced by a systemic redistribution of the inhibitors from the spinal cord. Accordingly, the antihyperalgesic effects of the PLA2 inhibitors reported here reflect a role in spinal sensory processing.
The assertion that these i.t. administered inhibitors acted in vivo through inhibition of spinal PLA2's is further strengthened by the demonstration that a behaviorally relevant dose of a PLA2 inhibitor alters a downstream product of PLA2 activation. Spinal administration of NMDA evokes release of prostaglandins, initiating a hyperalgesic state that is independent of peripheral inflammation (Malmberg & Yaksh, 1992). In the present study, i.t. delivery of AACOCF3 was sufficient to attenuate the hyperalgesia observed in both the formalin and carrageenan models. Our analysis of spinal dialysate indicates that i.t. administration of the same dose of AACOCF3 significantly decreased the NMDA-evoked release of spinal PGE2. This evidence provides a linkage between spinal PLA2 activity and prostaglandin production in the spinal cord. The likelihood that this decrease in prostaglandin production is attributable to Group IVA cPLA2 inhibition is further supported by the fact that i.t. administration of the Group VI iPLA2 inhibitor BEL, which did not effect hyperalgesic behavior in either model, did not decrease the NMDA-evoked release of PGE2. Although we attended to study the effects of MAFP on i.t. NMDA-evoked spinal PGE2 release, we were unable to complete this study due to assay interference. Unlike AACOCF3 and BEL, MAFP was recognized by the antibody used in the PGE2 ELISA.
To confirm a role for PLA2 in any physiological system, it is important to verify that the PLA2 inhibitors used actually act on the enzymes in the particular tissues. The in vitro experiments show that the Group IVA cPLA2 and Group VI iPLA2 activity in rat spinal cord is indeed decreased by the inhibitors used for the in vivo carrageenan and formalin studies. Of the three inhibitors studied, BEL is the most specific, having been shown only to inhibit Group VI iPLA2 and phosphatidic acid phosphohydrolase (Balsinde & Dennis, 1996a). At doses where BEL had no effect on motor function, its administration did not decrease the hyperalgesic behavior. MAFP and AACOCF3 both inhibit Group IVA cPLA2 and Group VI iPLA2 activity in spinal cord homogenates. Although these are currently the best commercially available PLA2 inhibitors, neither inhibitor is entirely specific. In addition to PLA2 inhibition, at high doses MAFP has also been reported to inhibit the enzymatic hydrolysis of anandamide (Deutsch et al., 1997) and to act as an agonist of the cannabinoid CB1 receptor (Fernando & Pertwee, 1997; Martin et al., 2000). Therefore, the antihyperalgesic effect of MAFP cannot be uniquely attributed to cPLA2 inhibition with complete confidence. AACOCF3, in addition to its inhibition of Group IVA cPLA2 and Group VI iPLA2, has also been implicated in the inhibition of the production of thromboxane B2, possibly through the COX pathway (Riendeau et al., 1994; Reddy & Herschman, 1997). Although it is possible that the antihyperalgesic behavioral effects observed in this study are due to other non-PLA2-directed actions of MAFP and AACOCF3, the fact that both compounds have a potent, dose-dependent antihyperalgesic effect when injected i.t. exhibit capacity to clearly reduce Group IVA cPLA2 and Group VI iPLA2 activity in spinal homogenates, and that i.t. AACOCF3 decreases NMDA-evoked prostaglandin release is a strong indication that the observed effects are PLA2 mediated.
In summary, we have presented evidence that Group IVA cPLA2 and Group VI iPLA2 are constitutively expressed and are active in the spinal cord, that spinal administration of inhibitors of these enzymes decreases hyperalgesic behavior in two established rat models and, importantly, that inhibition of spinal PLA2 results in the decrease of a downstream molecular product. These studies provide additional support for the role of constitutively expressed PLA2 and COX-2 in post-tissue injury nociceptive processing. We would like to stress that the PLA2 inhibition has the additional importance of abating the potential contributions of PLA2 products that occur upstream of COX and may contribute to the facilitated processing observed in these conditions.
Acknowledgments
This work was supported by National Institute of Health Grants NS 16541 (TLY), GM 20,501 (EAD), and by a United States Department of Education training grant GAANN (KKL). We wish to thank Ray Deems and Ralf Schaloske for their assistance in editing, Ursula Kessen and Iveta Kalcheva for advice on the Q-PCR experiments, Bethany Fitzsimmons for dialysis experiments, and Alan Moore for the prostaglandin E2 assays.
Abbreviations
- AA
arachidonic acid
- AACOCF3
arachidonyl trifluoromethylketone
- BEL
bromoenol lactone
- COX
cyclooxygenase
- i.p.
intraperitoneal
- i.t.
intrathecal
- MAFP
methyl arachidonyl fluorophosphonate
- PLA2
phospholipase A2
References
- ACKERMANN E.J., KEMPNER E.S., DENNIS E.A. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J. Biol. Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- AKIBA S., HAYAMA M., SATO T. Inhibition of Ca2+-independent phospholipase A2 by bromoenol lactone attenuates prostaglandin generation induced by interleukin-1 beta and dibutyryl cAMP in rat mesangial cells. FEBS Lett. 1998;437:225–228. doi: 10.1016/s0014-5793(98)01236-8. [DOI] [PubMed] [Google Scholar]
- BABA H., KOHNO T., MOORE K.A., WOOLF C.J. Direct activation of rat spinal dorsal horn neurons by prostaglandin E2. J. Neurosci. 2001;21:1750–1756. doi: 10.1523/JNEUROSCI.21-05-01750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALSINDE J., BALBOA M.A., DENNIS E.A. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J. Biol. Chem. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- BALSINDE J., BIANCO I.D., ACKERMANN E.J., CONDE-FRIEBOES K., DENNIS E.A. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALSINDE J., DENNIS E.A. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J. Biol. Chem. 1996a;271:31937–31941. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- BALSINDE J., DENNIS E.A. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 1996b;271:6758–6765. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- BALSINDE J., WINSTEAD M.V., DENNIS E.A. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- CAPPER E.A., MARSHALL L.A. Mammalian phospholipases A2: mediators of inflammation, proliferation and apoptosis. Prog. Lipid Res. 2001;40:167–197. doi: 10.1016/s0163-7827(01)00002-9. [DOI] [PubMed] [Google Scholar]
- CHANNON J.Y., LESLIE C.C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J. Biol. Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- CLARK J.D., SCHIEVELLA A.R., NALEFSKI E.A., LIN L.L. Cytosolic phospholipase A2. J. Lipid Mediat. Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., OMEIR R., ARREAZA G., SALEHANI D., PRESTWICH G.D., HUANG Z., HOWLETT A. Methyl arachidonyl fluorophosphonate: a potent irreversible inhibitor of anandamide amidase. Biochem. Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- DIETER P., KOLADA A., KAMIONKA S., SCHADOW A., KASZKIN M. Lipopolysaccharide-induced release of arachidonic acid and prostaglandins in liver macrophages: regulation by Group IV cytosolic phospholipase A2, but not by Group V and Group IIA secretory phospholipase A2. Cell Signal. 2002;14:199–204. doi: 10.1016/s0898-6568(01)00243-1. [DOI] [PubMed] [Google Scholar]
- DIEZ E., LOUIS-FLAMBERG P., HALL R.H., MAYER R.J. Substrate specificities and properties of human phospholipases A2 in a mixed vesicle model. J. Biol. Chem. 1992;267:18342–18348. [PubMed] [Google Scholar]
- DIRIG D.M., SALAMI A., RATHBUN M.L., OZAKI G.T., YAKSH T.L. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J. Neurosci. Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- DIRIG D.M., YAKSH T.L. Differential right shifts in the dose–response curve for intrathecal morphine and sufentanil as a function of stimulus intensity. Pain. 1995;62:321–328. doi: 10.1016/0304-3959(95)00006-E. [DOI] [PubMed] [Google Scholar]
- EVANS A.R., JUNGER H., SOUTHALL M.D., NICOL G.D., SORKIN L.S., BROOME J.T., BAILEY T.W., VASKO M.R. Isoprostanes, novel eicosanoids that produce nociception and sensitize rat sensory neurons. J. Pharmacol. Exp. Ther. 2000;293:912–920. [PubMed] [Google Scholar]
- FERNANDO S.R., PERTWEE R.G. Evidence that methyl arachidonyl fluorophosphonate is an irreversible cannabinoid receptor antagonist. Br. J. Pharmacol. 1997;121:1716–1720. doi: 10.1038/sj.bjp.0701303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHOMASHCHI F., LOO R., BALSINDE J., BARTOLI F., APITZ-CASTRO R., CLARK J.D., DENNIS E.A., GELB M.H. Trifluoromethyl ketones and methyl fluorophosphonates as inhibitors of group IV and VI phospholipases A2: structure–function studies with vesicle, micelle, and membrane assays. Biochim. Biophys. Acta. 1999;1420:45–56. doi: 10.1016/s0005-2736(99)00056-5. [DOI] [PubMed] [Google Scholar]
- GIJON M.A., SPENCER D.M., KAISER A.L., LESLIE C.C. Role of phosphorylation sites and the C2 domain in regulation of cytosolic phospholipase A2. J. Cell Biol. 1999;145:1219–1232. doi: 10.1083/jcb.145.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOVER S., BAYBURT T., JONAS M., CHI E., GELB M.H. Translocation of the 85-kDa phospholipase A[IMAGE] from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J. Biol. Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- HARGREAVES K., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HINGTGEN C.M., WAITE K.J., VASKO M.R. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3′,5′-cyclic monophosphate transduction cascade. J. Neurosci. 1995;15:5411–5419. doi: 10.1523/JNEUROSCI.15-07-05411.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOETZNER L., HUA X.Y., LAI J., PORRECA F., YAKSH T.L. Nonopioid actions of intrathecal dynorphin evoke spinal excitatory amino acid and prostaglandin E2 release mediated by cyclooxygenase-1 and -2. J. Neurosci. 2004;24:1451–1458. doi: 10.1523/JNEUROSCI.1517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUJUBU D.A., FLETCHER B.S., VARNUM B.C., LIM R.W., HERSCHMAN H.R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 1991;266:12866–12872. [PubMed] [Google Scholar]
- LARSSON FORSELL P.K., RUNARSSON G., IBRAHIM M., BJORKHOLM M., CLAESSON H.E. On the expression of cytosolic calcium-independent phospholipase A2 (88 kDa) in immature and mature myeloid cells and its role in leukotriene synthesis in human granulocytes. FEBS Lett. 1998;434:295–299. doi: 10.1016/s0014-5793(98)00999-5. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J. Neurosci. 1995;15:2768–2776. doi: 10.1523/JNEUROSCI.15-04-02768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSALA M., MALMBERG A.B., YAKSH T.L. The spinal loop dialysis catheter: characterization of use in the unanesthetized rat. J. Neurosci. Methods. 1995;62:43–53. doi: 10.1016/0165-0270(95)00053-4. [DOI] [PubMed] [Google Scholar]
- MARTIN B.R., BELETSKAYA I., PATRICK G., JEFFERSON R., WINCKLER R., DEUTSCH D.G., DI M., V DASSE O., MAHADEVAN A., RAZDAN R.K. Cannabinoid properties of methylfluorophosphonate analogs. J. Pharmacol. Exp. Ther. 2000;294:1209–1218. [PubMed] [Google Scholar]
- MILLER B., SARANTIS M., TRAYNELIS S.F., ATTWELL D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- NARABA H., MURAKAMI M., MATSUMOTO H., SHIMBARA S., UENO A., KUDO I., OH-ISHI S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J. Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- NICOL G.D., KLINGBERG D.K., VASKO M.R. Prostaglandin E2 increases calcium conductance and stimulates release of substance P in avian sensory neurons. J. Neurosci. 1992;12:1917–1927. doi: 10.1523/JNEUROSCI.12-05-01917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMANADHAM S., HSU F.F., BOHRER A., MA Z., TURK J. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J. Biol. Chem. 1999;274:13915–13927. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- REDDY S.T., HERSCHMAN H.R. Prostaglandin synthase-1 and prostaglandin synthase-2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J. Biol. Chem. 1997;272:3231–3237. doi: 10.1074/jbc.272.6.3231. [DOI] [PubMed] [Google Scholar]
- RIENDEAU D., GUAY J., WEECH P.K., LALIBERTE F., YERGEY J., LI C., DESMARAIS S., PERRIER H., LIU S., NICOLL-GRIFFITH D. Arachidonyl trifluoromethyl ketone, a potent inhibitor of 85-kDa phospholipase A2, blocks production of arachidonate and 12-hydroxyeicosatetraenoic acid by calcium ionophore-challenged platelets. J. Biol. Chem. 1994;269:15619–15624. [PubMed] [Google Scholar]
- ROSHAK A., MOCHAN E., MARSHALL L.A. Suppression of human synovial fibroblast 85 kDa phospholipase A2 by antisense reduces interleukin-1 beta induced prostaglandin E2. J. Rheumatol. 1996;23:420–427. [PubMed] [Google Scholar]
- ROSHAK A., SATHE G., MARSHALL L.A. Suppression of monocyte 85-kDa phospholipase A2 by antisense and effects on endotoxin-induced prostaglandin biosynthesis. J. Biol. Chem. 1994;269:25999–26005. [PubMed] [Google Scholar]
- SAMAD T.A., MOORE K.A., SAPIRSTEIN A., BILLET S., ALLCHORNE A., POOLE S., BONVENTRE J.V., WOOLF C.J. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- SHARP J.D., WHITE D.L., CHIOU X.G., GOODSON T., GAMBOA G.C., MCCLURE D., BURGETT S., HOSKINS J., SKATRUD P.L., SPORTSMAN J.R. Molecular cloning and expression of human Ca2+-sensitive cytosolic phospholipase A2. J. Biol. Chem. 1991;266:14850–14853. [PubMed] [Google Scholar]
- SUN G.Y., XU J., JENSEN M.D., SIMONYI A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- VASKO M.R. Prostaglandin-induced neuropeptide release from spinal cord. Prog. Brain Res. 1995;104:367–380. doi: 10.1016/s0079-6123(08)61801-4. [DOI] [PubMed] [Google Scholar]
- WOLF M.J., GROSS R.W. Expression, purification, and kinetic characterization of a recombinant 80-kDa intracellular calcium-independent phospholipase A2. J. Biol. Chem. 1996;271:30879–30885. doi: 10.1074/jbc.271.48.30879. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L.Central and peripheral mechanisms for the analgesic action of acetylsalicylic acid Acetylsalicylic Acid: New Uses for an Old Drug 1982New York: Raven Press; 137–152.eds. Barnet, J.M., Hirsh, J., Mustard, J.F. pp [Google Scholar]
- YAKSH T.L., DIRIG D.M., CONWAY C.M., SVENSSON C., LUO Z.D., ISAKSON P.C. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J. Neurosci. 2001a;21:5847–5853. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKSH T.L., DIRIG D.M., MALMBERG A.B. Mechanism of action of nonsteroidal anti-inflammatory drugs. Cancer Invest. 1998;16:509–527. doi: 10.3109/07357909809011705. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., HUA X.Y., KALCHEVA I., NOZAKI-TAGUCHI N., MARSALA M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7680–7686. doi: 10.1073/pnas.96.14.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAKSH T.L., OZAKI G., MCCUMBER D., RATHBUN M., SVENSSON C., MALKMUS S., YAKSH M.C. An automated flinch detecting system for use in the formalin nociceptive bioassay. J. Appl. Physiol. 2001b;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. An improved method for chronic catheterization of the rat spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO S., NOZAKI-TAGUCHI N. The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth. Analg. 2002;94:962–967. doi: 10.1097/00000539-200204000-00035. [DOI] [PubMed] [Google Scholar]
- YANG H.C., MOSIOR M., JOHNSON C.A., CHEN Y., DENNIS E.A. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal. Biochem. 1999;269:278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]