Abstract
Why do bacteria have shape? Is morphology valuable or just a trivial secondary characteristic? Why should bacteria have one shape instead of another? Three broad considerations suggest that bacterial shapes are not accidental but are biologically important: cells adopt uniform morphologies from among a wide variety of possibilities, some cells modify their shape as conditions demand, and morphology can be tracked through evolutionary lineages. All of these imply that shape is a selectable feature that aids survival. The aim of this review is to spell out the physical, environmental, and biological forces that favor different bacterial morphologies and which, therefore, contribute to natural selection. Specifically, cell shape is driven by eight general considerations: nutrient access, cell division and segregation, attachment to surfaces, passive dispersal, active motility, polar differentiation, the need to escape predators, and the advantages of cellular differentiation. Bacteria respond to these forces by performing a type of calculus, integrating over a number of environmental and behavioral factors to produce a size and shape that are optimal for the circumstances in which they live. Just as we are beginning to answer how bacteria create their shapes, it seems reasonable and essential that we expand our efforts to understand why they do so.
INTRODUCTION
It's not in the open we feel comforted but in the shadows. … We can't feel at home with the infinite sky above and around us. Space must be cut off, shaped, defined, for us to inhabit. From cradle to coffin, it's enclosure that defines us.
—Robert Morgan (221)
To be brutally honest, few people care that bacteria have different shapes. Which is a shame, because the bacteria seem to care very much. A simple way to verify this is to take a leisurely stroll through Bergey's Manual of Determinative Bacteriology (133) or The Prokaryotes (65, 313), pausing to admire the surprising and bewildering riot of shapes, sizes, and aggregates, some of which are illustrated in Fig. 1. There are cells that look like lemons, teardrops, or oblong spheroids; some are bent, curved, flat sided, triangular, bean shaped, or helical; others are rounded, squared, pointed, curved, or tapered. One is a flat square, and another is a slim, coin-like circular disk. The prosthecate bacteria radiate extensions that create star-like constellations or bulbous whiskers, all of which, though seemingly irregular, replicate faithfully. Other organisms grow as branched or unbranched filaments, live in sheathed or unsheathed chains, or aggregate in primitive or highly organized multicellular composites. The sizes of individual cells range over at least six orders of magnitude. And yet, amazingly, this short inventory barely begins to catalogue the known forms. As Zinder and Dworkin point out, our dogmatic fixation on rods, cocci, and spirals has “obscured the spectrum of enormous morphological diversity manifested by the bacteria” (380).
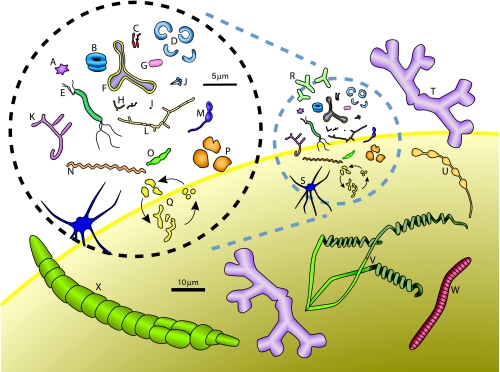
FIG. 1.
Variety of prokaryotic shapes. This collage of different cells, unless otherwise stated, is constructed from descriptions and illustrations given by Starr et al. (313) or by Zinder and Dworkin (380). The cells are drawn to scale. Those in the dashed black circle are drawn relative to the 5-μm line. These same cells are included in smaller form in the dashed blue circle to compare their sizes to those of larger bacteria, which are drawn relative to the 10-μm line. (A) Stella strain IFAM1312 (380); (B) Microcyclus (a genus since renamed Ancylobacter) flavus (367); (C) Bifidobacterium bifidum; (D) Clostridium cocleatum; (E) Aquaspirillum autotrophicum; (F) Pyroditium abyssi (380); (G) Escherichia coli; (H) Bifidobacterium sp.; (I) transverse section of ratoon stunt-associated bacterium; (J) Planctomyces sp. (133); (K) Nocardia opaca; (L) Chain of ratoon stunt-associated bacteria; (M) Caulobacter sp. (380); (N) Spirochaeta halophila; (O) Prosthecobacter fusiformis; (P) Methanogenium cariaci; (Q) Arthrobacter globiformis growth cycle; (R) gram-negative Alphaproteobacteria from marine sponges (240); (S) Ancalomicrobium sp. (380); (T) Nevskia ramosa (133); (U) Rhodomicrobium vanniellii; (V) Streptomyces sp.; (W) Caryophanon latum; (X) Calothrix sp. The yellow-lined background orb represents a slice of the giant bacterium Thiomargarita namibiensis (290), which is represented to scale with the other organisms.
But even those who appreciate the breadth of the shape universe still tend to think of microorganisms as smallish bags into which are stuffed the really important things in life: genetics and biochemistry. The former ensures identical progeny and the latter assimilates nutrients to create and maintain their descendants. That these essential innards are packaged in different shapes and sizes seems of little consequence. And yet, most bacteria doggedly continue to create bodies of defined size and shape. Is this meaningful, or is it all just a fortuitous accident?
The Best Arguments
Our era emphasizes, rightly, the molecular transactions that enable cells to grow, adapt and divide. Might cell shape also contribute something to this cycle, apart from its obvious role as a container? Three considerations suggest that it does. The first is the existence of variety with uniformity: that is, the wide variety of shapes among microbial genera and species, coupled with a near-rigid uniformity of shape within species. Variety hints that organisms adapted a trait to cope with diverse environmental niches or conditions; uniformity implies that there is a functional advantage to individual expressions of that trait. By these measures, a bacterium takes its shape as seriously as does any invertebrate, reptile, or mammal.
The second indication that form is an important physiological character is the fact that bacteria actively modify their shapes. Some changes are temporary (moving from one growth phase to another, responding to nutritional alterations, or passing through a host), some are repetitive (dimorphic or pleomorphic life cycles), and some accompany the development of specialized cells or structures (spores, heterocysts, swarmers, and elaborate multicellular assemblies). Such transitions are under explicit genetic and biochemical control, which is a compelling argument that shape is a significant element in these physiological adaptations.
The third argument entails the evolutionary progression of cell shape. Early on, Woese et al. concluded that the coccoid bacteria were spread across phylogenetic units and should be considered as degenerate forms of more complicated bacterial shapes (311, 366). More recently, Siefert and Fox (303) mapped the basic shapes onto the prokaryotic phylogenetic tree and concluded that bacterial morphology exhibits a definite historical trend, most likely beginning with a filamentous or rod-shaped cell. Certain shapes, morphological cycles, or developmental strategies are confined to particular branches of the tree, and, contrary to the widespread misconception that the first cells had to be spheroidal, coccoid cells are a near-dead-end shape that arose independently numerous times (303). Using different phylogenetic tools, two other groups arrived at similar conclusions. Gupta (111) proposed a map of prokaryotic evolution based on the distribution of DNA insertions and deletions, and Tamames et al. (324) generated an analogous tree by cataloguing gene order in a chromosomal segment devoted to septation. All these analyses indicate that morphology is significant, that it can be charted on an evolutionary scale, and that the earliest cells were probably rods or filaments, with cocci being derived and degenerate forms. Although the results of these approaches do not coincide at every point, the principal conclusions are virtually identical, lending credence to the idea that bacterial morphology is as important a selectable trait as any other biochemical adaptation.
The Perfect Example
Every good argument is improved by a good example, and the best example of how valuable bacterial shape must be is supplied by Caulobacter crescentus. A slightly curved cell that elongates into a full-fledged spiral filament in stationary phase, C. crescentus relies on a single protein to create a distinctive shape (10). In the absence of the protein crescentin, C. crescentus grows perfectly well but as straight rods and filaments instead of vibrioids and spirals (10). An analogous case is provided by Borrelia burgdorferi, in which periplasmic flagella impart to the cells a flat-wave shape (223). The latter example is just shy of perfect because flagellar mutants of B. burgdorferi lose motility as well as cell shape (223), whereas the C. crescentus mutants exhibit no negative phenotype in the laboratory (10). Because only a single missense mutation separates them from their normal shapes, Caulobacter and Borrelia teeter on the genetic precipice of becoming nothing more than rods. That they retain their forms implies that a strong selective advantage keeps them spiral or wave-like. Since a specific morphology serves these bacteria so well, it seems likely that shape will benefit other bacteria, even though we do not yet know all the advantages that entails.
The Perfect Experiment
Aside from the practical, intuitive and theoretical arguments for a connection between cell shape and biological utility, it would be nice to have clear experimental evidence on which to ground our conjectures. The most believable experiments change one variable so that the results can be attributed to a single cause. Unfortunately, approaching this ideal has been virtually impossible in studying bacterial morphology. So far, every mutation or treatment that alters cell shape may affect, directly or indirectly, some other physiological trait, which complicates how we interpret the results. Happily, a recent experiment achieves the elusive scientific standard in a surprising way.
Using nanofabrication techniques, Takeuchi et al. created micrometer-sized agarose moldings in which they trapped and grew Escherichia coli (322). By altering the contours of these traps, they forced cells to grow in a variety of shapes that persisted when the bacteria were released (322). Unexpectedly, the motility of these cells changes according to their gross morphology. Cells that are short crescents move in a straight line, as do helical cells with a long spiral pitch, whereas cells coiled like tightly wound springs move in tight circles, “going nowhere” (Fig. 2) (322). Note that the individual cells differed from one another exclusively in their overt morphology, because their shapes were imposed physically and not genetically or biochemically. Every biological facet of these cells except shape is equivalent, a feat accomplished by no other experimental system to date. The results prove that cells change their three-dimensional motions in extraordinary ways simply by adopting one shape over another, hinting that other shape-dependent behaviors await discovery.
FIG. 2.

Effect of artificially imposed cell shape on motility of Escherichia coli. E. coli filaments were forced into defined shapes by growing the cells in preformed cavities (322). The cells pictured here are genetically and biochemically identical except for differences in helical pitch or curvature. Time-lapse microscopy captured the positions of motile cells as they swam in the indicated directions (straight arrows), moving with a rotary motion (circular arrows) over a few seconds (as indicated by the numbers). (A) Crescent-shaped cell swimming in a straight line. (B) Tightly wound spiral-shaped cell swimming in a counterclockwise circle. (C) Relaxed spiral-shaped cell swimming in a straight line. The cell in panel C was derived from those represented in panel B by incubating the cells outside the original growth chambers for 2 hours. (Reprinted with permission from reference 322. Copyright 2005 American Chemical Society.)
The Imperfect Science
Although the preceding arguments justify the conclusion that shape is important and subject to natural selection, we must remember that evolution is a historical pursuit, and we should be careful to assign functions for morphological traits only when these are supported by specific experimental evidence. Shape, like any biological characteristic, falls into one of three categories. First, the trait may be selective, meaning that it directly and significantly contributes to survival in the face of evolutionary pressure. Second, the trait may be secondary, meaning that it is not important in and of itself but is a by-product that accompanies another feature that is selective. Third, the trait may be superfluous, meaning that it is neutral with respect to survival and its presence is accidental, just one among a number of equivalent states in which a cell could exist. Determining whether a characteristic is selective, secondary, or superfluous can be difficult, and unraveling the answers is a particularly knotty problem in the case of bacterial morphology.
To Protect and To Serve
Bacteria want what all other organisms want: to grow, they need to eat; to reproduce, they need to divide; if things are good where they are, they want to stay; if things are better somewhere else, they want to move; if threatened, they need to escape; and if the world around them changes, they must change. These are the basics of life: accessing nutrients, partitioning material to progeny, attaching, dispersing, escaping predators, and differentiating. Bacterial shape contributes at least some measure of survival value in response to the pressures imposed by these circumstances (Tables 1 and 2), and the ensuing sections of this review will examine how each of these fundamental forces influences cell shape.
TABLE 1.
Selective forces, bacterial shapes, and possible rationales
| Selective force | Shape example | Possible rationale |
|---|---|---|
| Nutrient limitation | Smaller cells | Greater surface-to-volume ratio |
| Filaments | Increased total surface area | |
| Prosthecae | Increased total surface area | |
| Extremorphic | Storage capacity of giant cells | |
| Pleomorphic | ? | |
| Cell division | Geometric symmetry | Equal segregation to daughters |
| Uniform width | Cell division apparatus | |
| Attachment | Rods | Cell-to-cell, fluid shear |
| Filaments | Resistance to fluid shear | |
| Prosthecae | Elevate in aqueous environment | |
| Miscellaneous | Biofilms | |
| Passive dispersal | Small cells | Effect of Brownian motion |
| Cells of various widths | Different flotation requirements | |
| Small cells | Flow through geological strata | |
| Larger cells | Entrapped in geological strata | |
| Active motility | Larger rods | Effect of Brownian motion |
| Medium rods | Efficiency of general motility | |
| Rods of various widths | Chemotaxis, different gradients | |
| Rods of various lengths | Motility near solid surfaces | |
| Helical rods | Motility in viscous solutions | |
| Rods or filaments | Gliding by slime extrusion | |
| Rods or cocci | Pilus-directed twitching | |
| Polar differentiation | Rods or filaments | Stable multiprotein complexes |
| Predation | Smaller cells | Escape predator contact/capture |
| Larger cells | Too large to capture or digest | |
| Filaments | Too large to capture or digest | |
| Prosthecae | Too large to capture or digest | |
| Helical rods | Escape predator internalization | |
| Differentiation | Rod to coccus | Slow-growth conditions |
| Rod to filament | Low-nutrient conditions | |
| Bifids (Y shapes) | More polar-localized complexes | |
| Swarm cells | Increased motility, attachment | |
| Filamentation | Defense during pathogenesis | |
| Miscellaneous | Multicellular adaptations externally imposed (?) |
TABLE 2.
Bacterial shapes and possible selective forces
| Shape | Possible selective forces |
|---|---|
| Symmetrical | Cell division apparatus |
| Equipartition to daughter cells | |
| Various widths | Nutrient availability |
| Flotation requirements | |
| Efficient chemotaxis in gradients | |
| Small size | Nutrient limitation |
| Passive dispersal | |
| Sieving through geological strata | |
| Protistan predation | |
| Larger rods | Reduced dispersal |
| Fluid shear stress | |
| Efficient motility | |
| Motility near surfaces | |
| Swarm cell differentiation | |
| Protistan predation | |
| Filamentation | Nutrient limitation |
| Fluid shear stress | |
| Stability to washing out of soil | |
| Gliding (slime extrusion) motility | |
| Swarm cell differentiation | |
| Protistan predation | |
| Immune system | |
| Multiorganism symbiosis | |
| Prosthecate cells | Nutrient limitation |
| Attachment | |
| Protistan predation | |
| Helical/spiral | Motility in viscous environments |
| cells | Motility near surfaces |
| Protistan predation | |
| Bifids (Y shapes) | Polar-localized protein complexes |
| Symbiosis requirements |
NUTRIENT ACCESS
The unalterable fact is that diffusion is a prime factor for bacterial life and that the wall, by determining shape, will dictate diffusion efficiency.
—T. J. Beveridge (19)
Bacteria have to eat, and diffusion is the fundamental physical factor that determines how well they do so. Cells may secrete molecules to scavenge chemicals in short supply, and those that are motile may move to where nutrients are more highly concentrated, but, however they cope, in the end virtually all prokaryotes rely entirely on diffusion to bring needed compounds to their surfaces and to mix nutrients and macromolecules in their cytoplasm. This dependence on the laws of diffusion exerts a powerful constraint on cell size and may also influence shape. Of course, bacterial size spans an enormous range, from the tiny Pelagibacter ubique (enclosing the miniscule volume of 0.01 μm3) (266) to the gargantuan Thiomargarita namibiensis and Epulopiscium fishelsoni (with internal volumes 108 to 1010 times greater) (8, 291, 292), demonstrating that diffusion alone does not dictate overall cell dimensions. Also, bacteria sharing the same niche may have vastly different shapes, indicating that the nutritional environment does not, by itself, specify shape. Nonetheless, bacterial morphology must conform to, and be circumscribed by, the general physical principles of nutrient access. It is therefore pertinent to know these limitations and the boundaries they impose.
For greater depth and incisive descriptions about how diffusion affects prokaryotic size, interested readers should consult four superb reviews (19, 170, 227, 292). In particular, the article by Schulz and Jørgensen provides a comprehensive, in-depth introduction to the subject (292), and the report of the National Research Council Space Studies Board has the most far-reaching discussions regarding physical and theoretical restraints on cell size (227).
Why Are Prokaryotic Cells Small?
Theoretical limits.
Koch observes that the lower boundary of prokaryotic cell size is that which is “large enough to house the total amount of needed stuff” (170). That is, the cell must have sufficient room to include all the nucleic acids, proteins, molecular complexes, and other gear required for survival and proliferation. By calculating the amount of space required to house this “needed stuff,” the lowest theoretical size for a free-living prokaryotic cell is estimated to be a sphere of 250 to 300 nm in diameter (227). This is very close to the size of the smallest bacteria observed in oligotrophic oceanic environments, these cells being tiny rods or coccoidal cells from 300 to 500 nm in diameter (44, 227, 266).
Surface-to-volume ratio.
The typical argument for prokaryotes being small is that the rate for transporting nutrients into a cell is a function of the amount of exposed surface area (19, 170, 292). However, it is not surface area per se that is important but the fact that the cell can insert greater numbers of nutrient transport complexes, which in turn deliver nutrients to the cytoplasm (170).
Thus, reliance on diffusion creates the strong tendency to form smaller cells, which increases the surface-to-volume ratio and decreases the amount of cytoplasm that has to be supported by any one transporter (19, 170, 292).
The diffusion sphere.
A cell's nutritional problem is complicated by the existence of a “diffusion sphere” (292) or “Reynolds envelope” (19) that adds to the cell's effective dimensions and forms a diffusion barrier around the cell. The diffusion sphere can be thought of as a thin layer of external liquid attached to, surrounding, and traveling with a bacterium and through which nutrients and waste products must pass (15, 19, 263). The existence and dimensions of this sphere are not affected by even the most turbulent conditions in natural waters (292). Because of this, the edges of the diffusion layer can be considered to be the surface area in contact with the undiluted nutrient concentrations in the external medium. The shape of this area is similar to that of the cell itself if the cell is a perfectly symmetrical sphere or smooth rod. However, the diffusion layer of a spiral cell has less “spiral” character than the cell body because parts of the diffusion sphere overlap. This means that distinctly shaped diffusion envelopes may surround cells of different shapes, potentially affecting their access to nutrients. For example, if a smooth straight rod and a thin spiral cell have equivalent diffusion spheres, the spiral cell might import more nutrients because it has more cell surface area into which it can insert transporters (Fig. 3A). The effects of alternate diffusion barriers are hypothetical, however, as I am not aware of calculations that address the consequences of spheres produced by cells of different morphologies.
FIG. 3.
Contributions of shape to nutrient acquisition. (A) Approximately equal diffusion spheres may enclose cells of different shapes. (B) Bacteria may respond to nutrient deprivation by filamentation, which increases their total surface area without an appreciable increase in the surface-to-volume ratio. (C) Prosthecate cells may respond to nutrient deprivation by elongating their thin prosthecae, which increases their total surface area while decreasing their surface-to-volume ratio.
Intracell mixing.
Not only does diffusion affect the absolute size of a cell by determining the rate at which it comes into contact with external nutrients, but diffusion also affects cell size by limiting the rates at which proteins and nutrients contact one another within the cell cytoplasm. Beveridge calculated that a 50-kDa protein in a typical rod-shaped cell (∼0.8 μm by 4.8 μm) will take about 0.5 s to migrate from one side wall to the cell center (a distance of 0.4 μm) or will require about 5 s to migrate from pole to pole (19). Schulz and Jørgensen calculated relatively similar “traffic times,” which describe how long it takes for any two molecules to meet one another (292). Schulz and Jørgensen also calculated the “mixing time” for a 1-μm-diameter coccus and found that a small molecule takes only about 1 millisecond to appear with equal probability anywhere in cell, whereas a larger protein takes about 10 milliseconds (292). These times will change with cells of different sizes and might eventually limit particular biochemical reactions at some combination of size and shape.
How Diffusion Affects Cell Shape
If diffusion and nutrient extraction were the pivotal determinants of cell size and shape, the most efficient nutrient-gathering shape should maximize the surface-to-volume ratio. Therefore, if a cell is going to be spherical, it would be best to be the smallest sphere possible, because decreasing size increases the surface-to-volume ratio (i.e., the volume decreases faster than does the area that can service it with nutrients). However, because “spherical cells have the worst possible shape for efficient substrate uptake” (292), one would think that nature would favor rod-shaped cells because their surface-to-volume ratios are higher than those of cocci with the same volumes (19). In addition, a rod-shaped cell that elongates without increasing its width does not change its surface-to-volume ratio very much. Both features increase linearly so that the ratio between the two changes very little, which may explain why so many bacteria produce filaments in response to changes in the nutritional environment (see below). These advantages of filamentation may be among the fundamental reasons that cells maintain a constant diameter.
The trouble is that if maximizing the surface-to-area ratio were the single guiding principle governing prokaryotic morphology, then a thin, flat, disk-like cell would seem to be the best alternative (63). However, with the exception of the archaeal halobacteria (26, 35, 349, 350), there are few really flat bacteria (63). The major reason may be that the surface area provided by flat cells is not significantly greater than that of thin filamentous cells (349), and a rod-shaped cell imparts an abundance of additional benefits (discussed below). Of course, molecular considerations may also constrain the synthesis of walls with flat shapes.
Contrasting examples.
At the smallest end of the free-living bacteria, the SAR11 clade of marine Alphaproteobacteria constitute up to 25% of all ocean microbes (50% in some surface waters) and 12% of the marine prokaryotic biomass (93, 222, 266). Of these, Pelagibacter ubique has the smallest genome (93) and grows as tiny, slightly curved rods (vibrioid), with newly divided cells measuring ∼0.2 μm by 0.4 μm and having an estimated cell volume of ∼0.01 μm3 (44, 266). Because the cell is extremely thin, the surface-to-volume ratio is very high, which seems to be the rule for oligotrophic (low-nutrient milieu) organisms. Cells with such dimensions fit the model in which natural selection optimizes the surface-to-volume ratio to provide appropriate transport rates in low-nutrient conditions (93). So far, this is consistent with the idea that diffusion plays a powerful role in shaping these cells. But herein lies a conundrum. Although P. ubique is one of the most successful and numerous life forms on the planet, a cell whose size we can explain because it has a tiny volume and large surface-to-volume ratio and whose dimensions we believe to be optimized for nutrient acquisition, even so we cannot explain why P. ubique is vibrioid. There are (as yet) no obvious reasons why the cells should be curved rods. Viewed from the point of view of diffusion alone, straight rods should do just as well. Curiously, many marine microorganisms are vibrioid, with the most notable examples being members of the genus Vibrio or of freshwater genera such as Caulobacter. The reasons probably stem from forces other than diffusion considerations.
At the other end of the spectrum is the giant endosymbiont Epulopiscium fishelsoni, averaging ∼40 μm in width and ∼250 μm in length but reaching 80 μm in diameter and up to 600 μm in length (8). The salient point is that this biovolume does not surround an empty vacuole; instead, the internal volume is made up of true cytoplasm. Thus, these cells really are large; they are not just a collection of thin bacteria masquerading as a large cell. Each E. fishelsoni cell has a volume ∼106 times greater than that of a single E. coli cell, maintains a cytoplasm-to-genome ratio about ∼20 times greater than that of E. coli, and contains ∼37,000 to 40,000 genome equivalents (J. Mendell, personal communication). Especially important is that each unit of surface area supports a cytoplasmic volume ∼200 to 400 times greater than that supported by the surface of P. ubique (Table 3). Though the differences are great, the physics of diffusion must still apply. E. fishelsoni seems to moderate its size disadvantages in three ways: the organism lives in a nutrient-rich environment (the surgeonfish gut), the inner membrane contains many invaginations, and the DNA is located in a narrow band around the inside of this membrane (J. Mendell, personal communication). These features increase nutrient availability by increasing the effective surface-to-volume ratio. Nonetheless, the existence of this behemoth highlights our inability to predict, from physical principles alone, the size, let alone the shape, of individual prokaryotes.
TABLE 3.
Surface-to-volume ratios of bacteria of different sizes and shapes
| Organism | Diam (μm) | Length (μm) | Surface area (μm2)a | Vol (μm3)a | Surface/vol ratio (μm2/μm3) | Pu ratiob |
|---|---|---|---|---|---|---|
| P. ubique | 0.2 | 0.5 | 0.31 | 0.014 | 22 | 1 |
| Cocci | 1 | 3.14 | 0.52 | 6 | 3.7 | |
| 2 | 12.56 | 4.2 | 3 | 7.3 | ||
| 3 | 28.26 | 14.13 | 2 | 11 | ||
| Rods | ||||||
| E. coli | 1 | 2 | 6.28 | 1.3 | 4.8 | 4.6 |
| 1 | 8 | 25.12 | 6.02 | 4.2 | 5.3 | |
| E. fishelsoni | 40 | 250 | 31,400 | 3 × 105 | 0.10 | 220 |
| 80 | 600 | 151,000 | 3 × 106 | 0.05 | 440 |
Calculations for symmetrical, spherical cocci: surface area = 4πr2; volume = 1.33πr3. Calculations for rods, assumed to be capped by two equal and symmetrical hemispherical ends: surface area = 4πr2 + 2πrl; volume = 1.33πr3 + πr2l.
The “Pu ratio” is a multiplication factor that describes how much more volume one unit of cell surface area must support compared to the same unit of surface area in P. ubique.
Conclusions.
If diffusion were the single major constraint on cell size and shape, then cells should either be thin and flat or have numerous long and thin appendages (292). The fact that flat and appendaged cells exist means that no physical reason prevents their formation. And the fact that prokaryotes have a host of other morphologies and a huge size range means that diffusion and surface area concerns cannot be the sole factors driving cell shape, even though these forces are obviously fundamental. Though shape may make only slight differences in the rates at which diffusion brings nutrients to a cell, shape definitely makes a difference in a cell's ability to come into contact with nutrients. Specific shapes may give cells greater access to nutrients or, more precisely, easier access to locales of high nutrient concentrations, after which diffusion can run its course.
Morphological Variation
Environmental microbiologists have long appreciated that bacterial morphology varies with growth rate and nutritional conditions. Unfortunately, in almost no case do we know if shape per se is beneficial, because few experiments have addressed the question. Nonetheless, something important seems to be happening, because numerous bacteria routinely alter their morphology in response to the types and concentrations of external compounds.
Variation with growth rate.
In the classic work of Schaechter et al., Salmonella enterica serovar Typhimurium produced cells that were wider when incubated in rich medium than when grown in minimal medium, and slowly growing cells were shorter than those growing more rapidly (287). Similarly, rapidly growing cells of E. coli B/r are wider than slowly growing cells, with cells having a generation time of 22 min being significantly wider (∼1 μm) than cells having a generation time of 72 min (∼0.5 μm) (226). However, not all strains respond the same way. For example, E. coli B/r becomes more elongated at higher growth rates, but E. coli B/r H266 becomes more rounded (226). A more permanent effect of growth rate on cell shape is suggested by evolutionary experiments by Lenski and Mongold, who identified a measurable shape change in E. coli during a 10,000-generation experiment (190). The change was simple, i.e., an increase in length and width leading to a doubling of cell volume, but was adaptive and heritable (190), verifying in practice that a slight shape change is correlated with the ability to outgrow competitors.
The upshot of these and other experiments is that bacterial morphology is not set in stone; i.e., the size and shape of an individual cell do not have predetermined, permanent dimensions. Instead, although the overall shape may be constrained (e.g., to be rod-like), a cell's length and width may change in response to growth conditions (228).
Filamentation with nutritional status.
Perhaps the most frequent shape change due to nutritional stress is filamentation, triggered by a limitation in the availability of one or more nutrients. For example, in the absence of phosphate, cysteine, or glutathione, Actinomyces israelii grows as branched or filamentous rods, and adding back these compounds returns the cells to a regular rod-like morphology (251). When limited for biotin, Arthrobacter globiformis forms abnormally large, branched rods of variable size (365), as do other isolates when starved for manganese (56, 89). An analogous magnesium deficiency inhibits cell division and produces nonbranching filamentation in Clostridium welchii (355, 356), and in nutrient-poor conditions Pseudomonas aeruginosa, Pseudomonas putida, and Pseudomonas fluorescens elongate into long slim cells, unlike the short rods observed in liquid medium (302, 314). The simplest explanation for these responses is that, when the environment demands it, many bacteria can accelerate or delay cell division and septation, thereby creating shorter or longer cells, respectively.
Why do this? First, as noted above, elongating increases a cell's uptake-proficient surface without changing its surface-to-volume ratio appreciably (Fig. 3B). This may be reason enough for cells in suspension. Second, filamentation may benefit cells attached to a surface, not because elongation increases the total surface area but because it increases that specific surface area in direct contact with the solid medium (314). Steinberger et al. calculated that a perfectly spherical coccus contacts a planar solid with ∼17% of the cell's surface, and a rod twice as long makes contact with 20% of its surface (314). For a rod whose length is 7 times the sphere's diameter the contact surface increases to 23%, but a rod 10 times as long increases its contact area to only ∼24%, and further elongation has little additional effect (314). Thus, a rod seven times as long as a coccus increases its surface contact by ∼40%, which should be sufficient to favor rod-shaped cells if surface contact is the principal source of nutrients. Finally, filamentation may allow cells to access nutrients that would otherwise be out of reach for mechanical reasons, by increasing the possibility that part of the filament will contact a nutrient-rich zone and funnel compounds to the rest of the cell's biomass.
Nutritionally deficient streptococci.
In 1961, Frenkel and Hirsch isolated a streptococcus that grew with a range of unusual morphologies (80). When grown in nutrient-limiting conditions, these isolates had thickened cell walls and often grew as true filaments instead of as cocci (283). These were first described as “nutritionally variant streptococci” (283) but are now known as “nutritionally deficient streptococci” (NDS) (28, 42). When visualized by electron microscopy, 14 NDS strains were observed to be shape variable, having thickened cell walls and improper septation (29). At first thought to be variants of normal viridans streptococci, the organisms were later assigned to two new Streptococcus species, Streptococcus defectivus and S. adjacens (283), and still later were identified by 16S RNA analysis to be in a new genus altogether, Abiotrophia (159), along with a third new species, Abiotrophia elegans (272). Since their discovery, NDS strains have been isolated from diverse clinical sources (28, 42), even though they are difficult to identify because of their bizarre morphologies, which include rods and filaments with irregularly spaced bulbous swellings (28).
The shape changes of the NDS represent yet another response to nutritional status. The morphological aberrations of NDS can be manipulated by altering the vitamin B6 concentration: lower concentrations induce more rod-like, filamentous, bulging, and aberrant morphologies (42). In fact, most NDS revert to the classical coccoid form when supplied with appropriate nutrients (cysteine, thiols, or vitamin B6) (28, 42). The filamentous cells have incomplete septa (42), perhaps because vitamin B6 is required to convert l-alanine to d-alanine for peptidoglycan synthesis (283). In any case, the NDS represent yet another example of bacteria responding to nutrient deprivation by controlled filamentation.
True to form?
The behavior of NDS organisms raises an intriguing possibility. Some bacteria we know as pleomorphic exhibit these morphologies because they are deprived of essential nutrients during culture in vitro, yet they have uniform shapes in the presence of a required nutrient. It may be that some of the shapes with which we are most familiar are artifacts of our culturing methods, in somewhat the same sense that other organisms are said to be “nonculturable.” On the other hand, perhaps the ability to adopt aberrant shapes is useful for bacteria in their natural habitats. In any case, nutritionally dependent cell shape variations should provoke us to report the natural, in vivo shapes of the organisms we study and to ask if bacterial shape accommodates itself to a cell's nutritional status or other aspects of its surroundings.
Prosthecae as Nutrient Whiskers?
If cells can gain an advantage by elongating to increase their surface area without changing their surface-to-volume ratio, then they may benefit even more by elongating while increasing this ratio. The easiest way to accomplish this is for a cell to extrude thin appendages called prosthecae, which have a diameter less than that of the original cell body and therefore contain very little cytoplasm (Fig. 3C) (33, 61). The most intensively studied prosthecate bacterium, Caulobacter crescentus, has one prosthecate stalk with a sticky holdfast at its far end (61), and related organisms elaborate multiple appendages (61, 65, 133, 255). Prosthecae represent an extreme example of the control of cell diameter, and some may represent the minimum diameter available to a cylindrical cell. C. crescentus prosthecae are ∼100 to 150 nm in diameter, and the width of the central pore is only ∼10 to 20 nm (253). Because of this tight squeeze, it is not surprising that the internal channel is mostly free of cytoplasmic proteins (137), which means that the cell surface can be extended substantially with only a miniscule increase in cell volume.
Prosthecae increase the surface area available for nutrient absorption in a nutrient-poor environment because the stalk can collect nutrients and direct them, by diffusion, into the cell body (220, 228, 253). This idea arose from the observation that decreasing phosphate concentrations provoke the growth of longer prosthecae in Caulobacter, Asticcacaulis, Hyphomicrobium, and Rhodomicrobium (254, 255). When grown in limiting phosphate, the stalks of Caulobacter and Rhodomicrobium elongate from their usual length of ∼1 to 3 μm to as much as 20 μm (33, 61, 97, 289). The response is under direct genetic control, because Caulobacter mutants produce elongated stalks even in the presence of sufficient phosphate (33, 97). These mutations map to the pst genes responsible for high-affinity phosphate transport, which strengthens the link between phosphate uptake and regulation of stalk growth (97). Thus, the longer the stalk, the more easily the cell can access exogenous phosphate, which suggests that the stalk plays a prominent and perhaps specialized role in phosphate uptake (254). Further strengthening this supposition is the behavior of Ancalomicrobium, which adopts several morphological types depending on the prevailing nutritional conditions. When nutrient concentrations are high, the cells are spherical or rod shaped; at intermediate concentrations, the cells are knobby rods; and at low nutrient concentrations, the rod-like cells have multiple protruding filamentous branches (61). Since, unlike Caulobacter, Ancalomicrobium does not use its prosthecae for attachment, these length changes are probably related directly to the need for increased surface area for nutrient transport.
Surprisingly, in light of the surfeit of indirect evidence, specific experimental support for the proposition that prosthecae function in phosphate transport has been hard to come by. The basic problem is to show that the required transporters exist in the stalk and are active. The prosthecae of Asticcacaulis biprosthecum can actively transport all 20 amino acids (323) and contain a glucose uptake system (185, 257), but the accumulated glucose is not metabolized, leaving the usefulness of the transport system in question (257). C. crescentus stalks contain mostly outer membrane and periplasmic nutrient binding proteins but have a deficit of cytoplasmic proteins (137, 346), which is “consistent with the hypothesis that the stalk plays a role in nutrient uptake” (137). These stalks do, in fact, import phosphate-ester into the periplasm and hydrolyze it (346). Calculations indicate that long stalks can import material at a higher rate per unit volume than can filamentous cells of the same length, meaning that stalk formation can supply more nutrients per unit of cell mass (346). So far, these data represent the best experimental support for the idea that stalk elongation enhances nutrient accumulation and does so more efficiently than classical cell filamentation.
Improving nutrient uptake is only one potential function for the stalks of prosthecate bacteria, some of which attach themselves to solid substrates by means of adhesins at the tips of their appendages (33, 253, 255). Immobilized prosthecae may orient cells in a flowing liquid and expose them to bulk nutrients, they may reduce overall buoyancy and orient cells near air-water interfaces, or they may elevate the cell body so that daughter cells are dispersed more readily (see “DISPERSAL” below) (253, 255, 346). An interesting question is whether phosphate limitation is created by the competition for nutrients among neighboring cells in a biofilm. A pack of competing cells might effectively lower the effective concentration of phosphate or other nutrients available to any single cell, triggering prosthecate bacteria to elongate their stalks so that the cells rise above the mass of competing biofilm into a less competitive environment (253, 255, 346). In addition, prosthecae may decrease the settling time of cells in the water column (see “DISPERSAL” below). Thus, prosthecae may enhance a cell's access to nutrients in several ways and can be considered one of the morphological strategies for nutrient acquisition.
Filaments and Blimps
Another way that cellular morphology may serve a nutritional function is to help bacteria access nutrients that would otherwise be completely out of reach. For example, the sulfur bacteria oxidize sulfide and reduce nitrate, two compounds that rarely coexist in marine environments (291). Nitrate accumulates in water lying directly on top of the ocean sediment, while sulfide is located several centimeters below (290). This spatial separation poses a challenge for organisms that obtain energy by coupling these reactions, and bacteria have devised two morphological strategies for dealing with this situation (291).
The giant sulfur-oxidizing bacteria Thioplaca, Beggiatoa, and Thiomargarita spp. store sulfur as inclusion bodies in a thin layer of cytoplasm surrounding an enormous central vacuole in which they store nitrate (290). To get to these compounds, Beggiatoa and Thioplaca cells form filaments (290). Thioplaca cells adhere to one another in mucus sheaths that are inserted several centimeters into the sediment, and the cells access both nutrients by shuttling up and down (290). Beggiatoa filaments grow only in thin horizontal zones in the sediment where sufficient concentrations of the two compounds overlap (290). A second strategy is exemplified by Thiomargarita, which forms chains of spherical cells, each of which averages 100 to 300 μm in diameter, with some reaching 750 μm (0.75 mm!) (291). The cells are trapped and buried in sediments and are therefore cut off from nitrate but are in contact with sulfide (290). Every so often, after weeks or months, the sediments are resuspended by eruptions of methane or by other means. While resuspended, the cells come into contact with nitrate, which they accumulate in the voluminous central vacuole to tide them over when they inevitably settle back to the sea floor and are reburied (290). In effect, Thiomargarita is a blimp, rising and falling through different strata, collecting and storing electron donors and acceptors against periods of starvation (291). The huge size and balloon-like vacuole of Thiomargarita are morphological adaptations that permit this unique lifestyle.
Miscellaneous Shape Effects
The halotolerant archaea exhibit a curious range of unusual shapes, including triangular cells (144), square cells such as those of Haloarcula quadrata (242), and flat, wafer-shaped cells such as those of Walsby's square archeon (recently named Haloquadratum walsbyi) (26). First described by Walsby (350) and recently isolated and grown in pure culture (26, 35), individual members of these square, flat cells are about 2 to 5 μm wide and 0.1 to 0.5 μm thick (26). However, they are most frequently encountered in thin mats measuring up to 40 μm by 40 μm, arranged as though they were sheets of postage stamps (26). Floating parallel to the water's surface, these thin cellular mats present a broad and contiguous surface area for exposure to sunlight (26). This arrangement maximizes both buoyancy and the total light-gathering area (349).
Other cell shapes may give their owners flexibility in coping with dramatic changes in osmotic pressure. Javor et al. described box-shaped halophilic archaea shaped like irregular rectangles, squares, trapezoids, or triangles and others that are flat, round, or ovoid (144). They argued that “in their natural environment these cells are more likely than most other prokaryotes to experience abrupt large increases in internal osmotic pressure when rain or high tides dilute the salt ponds” and hypothesized that these “flat shapes and relatively soft cell walls allow a large increase in their internal volume with a relatively small change in their cell envelope shape” (144). The idea has not been tested (as far as I know), but the tendency of certain shapes to deform without lysing may represent another morphological adaptation to environments dominated by diffusion and osmotic pressure.
Summary
One of the most demanding physical constraints bacteria must deal with is their dependence on diffusion-mediated nutrient import. For the most part this means that bacteria are small (within certain ranges) or at least that the cytoplasmic parts of the cell are relatively thin. Fluxes in nutrient availability or growth state may be met with morphological changes, such as filamenting or extruding prosthecae, both of which increase the surface area available for nutrient import without increasing the surface-to-volume ratio. These latter responses are under genetic and physiological control, indicating that bacteria can manipulate morphology to their advantage. Other, more specialized shapes are available to bacteria to cope with the nutritional requirements of unusual environmental niches.
CELL DIVISION AND SEGREGATION
Shape Uniformity
Most bacteria maintain a uniform and symmetrical profile as opposed to growing as a collection of cells with random or irregular shapes. Whatever their overall morphology, cells appear to have at least one bilateral geometric symmetry, either perfect or roughly so. Before we address why cells have particular shapes, we need to ask why most of these shapes are symmetrical in the first place.
Perhaps the most important reason to maintain a uniform morphology is so that chromosomes and cytoplasmic material can be partitioned equally between daughter cells at division (Fig. 4A) (69). The chromosome is most important, but the allocation of near-equal amounts of cytoplasm is also vital. Although the intrinsic variability of distribution ensures that individual daughter cells will never be perfectly equivalent to one another, extrinsic factors also play a role, and the cell can minimize some of these (279, 310). Towards this end, a regular shape would seem to be the best way to ensure that an equal amount of “stuff” is allocated to each daughter, because a symmetrical cell can be halved accurately by mechanisms that measure length or volume (69, 124). In an irregular cell, misplaced septation might leave one cell with both chromosomes or with more than its fair share of other components. In this regard, the actual shape itself would not be important; instead, segregation-driven selection would favor a cell with bilateral twofold symmetry. This requirement for equitable segregation may be the strongest selective pressure for shape uniformity.
FIG. 4.
How division and segregation help maintain geometrically uniform cell shapes. (A) Geometric uniformity simplifies equipartition of material into daughter cells during cell division. (B) In a wild-type bacillus, the cell division protein FtsZ forms a ring (the Z ring) that encircles the midpoint of the rod-shaped cell and initiates division. (C) A spherical cell derived from the cell in panel B may not be able to form a complete Z ring around the increased circumference of the cell's midpoint.
If morphology affects chromosomal segregation, then shape mutants should exhibit chromosome partition defects. Hiraga et al. found just such a correlation in E. coli when they devised a genetic screen to identify segregation mutants by looking for strains that produced abnormally high numbers of anucleate cells (126). Fewer than 0.03% of wild-type E. coli cells are anucleate, but Hiraga et al. isolated mutants that produced anucleate cells at rates of 0.5 to 3.0% (126). One of their mutant classes was composed of spherical cells, suggesting a link between improper shape and defective segregation (126). Using the same screening technique, Ogura et al. isolated temperature-sensitive, spherical mutants caused by a defect in penicillin binding protein 2 (PBP 2), a protein required for creating the normal rod shape in E. coli (237). Consistent with this result is the fact that amdinocillin, an antibiotic that specifically inhibits PBP 2, also provokes production of anucleate cells at a high rate (139). In both cases, the spherical cells have chromosome partition defects. Mutants of Bacillus subtilis also illustrate the consequences of not having a uniform shape. B. subtilis lacking PBPs 2a and H (proteins involved in synthesizing the cell wall) form incomplete, haphazardly placed septa (357). The cells grow as irregularly sized spheres instead of rods, and accurate chromosome segregation is reduced substantially (357).
In complementary work, the anucleate cell screen was used to isolate the antibacterial compound A22, which forces rod-shaped cells to grow as spheres (139). When so treated, E. coli produces a higher percentage of anucleate cells (2.4%) than is present in wild-type rods (0.03%), typical of a segregation defect (139). The mechanism of action of A22 is not via PBP 2 inhibition (139) but by inhibition of the MreB protein (94). In both A22-treated cells and PBP 2 mutants, anucleate cells are smaller than normal and are probably created by asymmetric cell division (139). Consistent with this interpretation, chromosome segregation is impaired in mreB mutants of E. coli (175). Whereas wild-type cells faithfully segregate equal numbers of chromosomes to each rod-shaped daughter, MreB mutants are spheroidal and partition their chromosomes randomly so that some newborn cells contain no chromosomes at all (175). Thus, cell shape does seem to affect symmetrical cell division and chromosomal segregation (139).
There is a caveat to interpreting the above results. Although a uniform shape appears to be important for chromosomal segregation, MreB may play a more direct role in segregation beyond its role in maintaining a cell's rod shape. Expressing certain missense mutants of MreB in E. coli disturbs chromosomal segregation even though the cells retain their rod shapes, leading Kruse et al. to conclude that “it is not the shape of the spherical cells per se that causes the chromosome segregation defect” (175). Likewise, PBP 2 mutants may perturb segregation by affecting MreB activity. It may be impossible to disentangle these two considerations (shape change versus impaired partitioning), because the two may be intimately intertwined. Even so, cell shape is clearly an important contributor, either directly or indirectly, in determining proper segregation.
The Cell Cycle Resists Shape Changes
Once a particular shape is adopted, bacteria have a vested interest in keeping it; and the major incentive for doing so is to maintain a consistent relationship between cytoplasmic volume and surface area so that cell cycle events can be coordinated properly. This is most easily visualized by considering the septation event that creates two daughter cells (Fig. 4B and C). At the center line where division will occur, the linear circumference of a cylindrical cell will be less than that of a sphere enclosing the same volume. In such a case, the concentrations of essential division proteins will not change, but the surface area over which they must act will be greater in the sphere. The amounts of these proteins, if optimized for the dimensions of a rod, might not be sufficient to initiate or complete normal septation and division in a coccus (Fig. 4C). Likewise, if the diameter of a cylindrical cell is not constant along its entire length, a potential division site may require more proteins than are available in a given cell volume. Thus, limited concentrations of division proteins will dictate that the cell maintain a specific and constant diameter.
A good example of this principle is E. coli, in which concentrations of the requisite division proteins are carefully balanced for its normal rod shape. In almost all eubacteria cell division is regulated by the FtsZ protein, which polymerizes to form a physical ring around the girth of a cell at the site where septation will occur (Fig. 4B) (69, 201). In E. coli, successful cell division depends on a constant and critical concentration of FtsZ combined with the proper proportions of Z-ring-stabilizing and -destabilizing proteins (275, 282). Significantly, small changes in the concentrations of FtsZ or other essential division proteins disrupt cell growth (see references cited in reference 57). Thus, division is inhibited if FtsZ is underproduced, extra divisions occur if the protein is overproduced (193, 353), and no division occurs if FtsZ levels are adequate but the FtsZ/FtsA ratio is incorrect (57). These facts prompted Dewar and Dorazi to conclude that “even small fluctuations in the levels of essential cell division proteins can severely disrupt cell growth” (57).
Several E. coli mutants provide examples of how shape may affect this aspect of cell division. Mutants lacking some of the penicillin binding proteins deviate only slightly from wild-type shape during growth, but they eventually stop dividing and continue to grow in length and girth until they lyse (230, 338; unpublished results). This is consistent with an inability to produce enough septation proteins to accommodate their increased cell diameter. Additional verification is provided by E. coli strains lacking PBP 2, which grow as ever-enlarging spheres (342). In these balloon-like cells, septal Z rings either never form or, if they begin to form, do not proceed completely around the cell circumference (342). Such mutants may be rescued by overproducing the proteins FtsA, FtsZ, and FtsQ (342). The easiest explanation is that the problems created by a larger cell circumference are overcome by expressing the septal ring proteins in sufficient numbers so they can polymerize to create a complete, septation-proficient Z ring (342).
Summary
Uniform cell shapes are favored by the need to segregate material equally between daughter cells. Furthermore, bacteria apparently optimize the absolute numbers of division proteins to those amounts required to encircle a cell of a particular diameter. Once a cell adapts its internal protein concentrations to the conditions set by a cell's morphological dimensions, further shape and volume alterations will be resisted. Similar considerations probably apply for other morphologies, so that producing viable mutants with different shapes may require manipulating the division apparatus as well. The principle of symmetrical segregation seems so strong an influence that it may be more important to explain the existence of asymmetries than to explain the symmetries of cell shape.
ATTACHMENT
People who enjoy jigsaw puzzles will understand instinctively why cell shape is important in organizing the interactions between bacteria and objects in their environment. Just as two adjacent puzzle pieces interlock, different bacterial morphologies may help stabilize the physical and chemical forces acting between a cell and an adjoining surface. Even the simplest shapes differ in their potential interactions. Cocci contact a flat surface with a single small area, rod-shaped bacteria touch the same surface with a linear set of points that run along the cell's length, and filamentous organisms multiply these contacts with a greater linear surface and can wrap themselves around neighboring particles to become enmeshed with the substrate or with one another. All these interactions are aided and abetted by the presence of neighboring bacteria.
Physicochemical Considerations
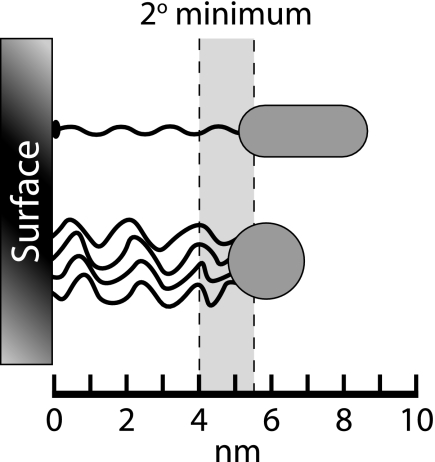
Cell shape influences attachment because bacteria adhere to solid surfaces by van der Waals and electrostatic forces (269, 335, 336). The small distances over which these forces operate dictate that only a tiny fraction of a cell's surface (<0.1%) is in direct atomic contact with an adjoining surface (assuming that each surface is perfectly smooth and without projections) (335). This circumstance derives from the geometry of the bacterial surface (highly curved) coupled with the ∼5-nm range over which the secondary Gibbs free energy minimum allows reversible binding (269, 335, 336) (Fig. 5). Without other aid, bacteria cannot cross this barrier to reach the primary minimum that would give the strongest binding (269).
FIG. 5.
Energetics of cell attachment to a surface. Cells stop within a certain distance of a surface because of electrostatic repulsion, where they may be retained within the Gibbs energy “secondary-minimum” zone (shaded area). The specific minima are shown for one species of Corynebacterium approaching a glass surface in a solution with 0.1 M ionic strength (269). The exact location of the secondary energy minimum will vary from 4 to 10 nm, depending on the nature of the surface and the bulk ionic conditions. Cells may initiate direct physical contact with the surface across the energy barrier by using pili (long thin fiber on upper cell) or by secreting polymeric capsular materials (thin fibers on lower cell). (Adapted and redrawn from reference 270, copyright 1996, with permission from American Urological Association.)
Bacteria increase the strength of their attachment to a surface in several ways. First, cells can increase the numbers of attachment points by placing long-chain molecules on their surface so that the exposed polymers act as tiny grappling hooks, reaching into the Gibbs minimum attachment range and effectively increasing the percentage of the cell's surface contributing to attachment (Fig. 5). This can be done independently of a cell's shape. Second, further advantages can be realized by altering cell shape to amplify the total number of contacts. A coccus could do this by growing as a rod, or a rod could elongate into a filament. Third, attachment strength increases if the target surface is not uniformly smooth or flat. A rough surface reduces near-surface shear forces that negatively affect the initial phases of bacterial adhesion (336). At the same time, an uneven surface allows bacterial cells to settle into molecular grooves or canyons, which increases the number of possible cell-to-surface contacts (Fig. 6B). In just this way, initial processes in biofilm formation may rely more on the “roughness” of the material than on the exact chemical nature of the surface (336).
FIG. 6.
Examples of physical considerations affecting attachment of cells to surfaces. (A) Rod-shaped cells can contact a surface with a larger amount of their cell body than can cocci. (B) Shear flow from moving liquid (arrows) may align rod-shaped cells parallel to the flow, so that cell width is the major dimension that is directly affected by the shearing force. If a coccus and a rod present the same face to the oncoming liquid, the rod should be more difficult to remove because it has more connections to the surface. (C) Individual, curved Simonsiella cells are connected to one another to form a distinctive filamentous shape. The organism binds to epithelial cells in the oral cavities of mammals, with the attachment being mediated by the concave face of the cell filament.
Shear Forces
Like a swimmer hanging on to a tree branch in the middle of a fast river, bacteria on a surface may be buffeted by a current of flowing liquid. The shear stresses of such a flow can remove bacterial cells from glass capillaries and other surfaces (261). Bacteria must counteract this force if they are to attach in the first place and remain attached after initial contact. The magnitude of the shearing stress and the likelihood of a particle becoming detached depend on the velocity of the liquid and on the diameter of the attached particle, with detachment being favored by higher flow rates and larger particle diameters (Fig. 6) (234). Another threat is abrasion from interparticle collisions—i.e., the glancing blows of suspended particles moving in the flow—which also depends on the area presented by the attached cell (234). Other factors may ameliorate these hazards. For example, on a rough or porous surface a cell may nestle into a furrow or behind a microscopic embankment (Fig. 6B). This would expose less of the cell's surface to liquid flow, thus reducing exposure to shear forces and particle bombardment, and surface anomalies might redirect the current's force, creating areas of relative calm (234).
Although individual planktonic cells cannot influence the characteristics of the surface to which they attach, they can optimize their shape to combat shear forces in two ways: by decreasing the magnitude of shear and by increasing the number of physical contacts with the external surface. The strength of the parallel component of surface shear determines if a cell remains attached or is removed, and the magnitude of this parallel force is proportional to the square of the radius of the particle (261, 336). Larger particles are affected more strongly than are smaller particles because more surface area is exposed to direct flow as diameter increases (Fig. 6B) (261, 336). A coccus, because of its spherical symmetry, exposes the same surface area to oncoming fluid flow no matter how the cell is attached. However, a rod-shaped cell can orient itself so that it is broadside to the flow or so that only the face of one pole is facing the onrushing current. Therefore, for cells of equal mass, rod-shaped cells should be able to withstand greater shear forces if they align themselves lengthwise to the direction of current. This exposes a smaller circular surface area to liquid flow while allowing adherence along the length of the cell. Few experiments address this subject directly in bacteria, but the results are consistent with these considerations. When grown under high shear force, E. coli elongates without a significant change in its diameter and is more likely to grow in chains (66). Both responses increase the surface area available for attachment while keeping constant the cross-sectional area that is susceptible to shear forces. A different response is exhibited by B. subtilis. In a high-shear environment cells of this organism are smaller by about half in each dimension, which reduces total shear because the organism's cross-sectional area decreases to one-fourth its original value but its length decreases by only half, so that its attachment-to-shear ratio doubles (284). Finally, cells may enhance the number of contacts by growing as filaments or in chains, intertwining with surface elements to resist detachment. Overall, therefore, rods and filamentous cells should have an advantage in environments with sizeable shear. Of course, as with every other physiological trade-off, these arguments presuppose that all other considerations are equal, which may not be the case. There are other options for improving attachment, and shape may not always be the dominant factor. For example, in Acinetobacter the coccal phase attaches to surfaces more firmly than does the rod phase (142). The point here is that shape represents an additional tool in the cell's arsenal of attachment strategies.
Another possible stabilizing strategy would be to interact with many other cells while attaching. Additional points of horizontal attachment to cells on all sides would increase the number of attachment points and at the same time decrease the effect of shear stress on individual bacteria. An extreme version of this strategy is practiced by leaf cells in Arabidopsis (82). These polymorphic cells interdigitate with one another exactly like puzzle pieces to withstand being dislodged by wind- and water-derived shear forces (82). The crowding effect should be similar for bacterial cells and may help explain the prevalence of environmental biofilms (see below).
The genus Simonsiella provides a fascinating example of a morphological adaptation for surface attachment to a familiar niche. Simonsiella spp. are filamentous, aerobic bacteria that are part of the natural oral flora of many mammals (123). Eight or more daughter cells are attached to one another to form short filaments, but the dimensions of each cell are unusual. When measured with respect to length of the filament, the cells are short and flat (0.5 to 1.3 μm) but quite wide (1.9 to 6.4 μm) (123). Each Simonsiella cell is slightly curved over its width, and the concatenation of these curved cells creates a ribbon-shaped filament that is bent so that one side is concave and the other side convex (Fig. 6C) (123). This morphology seems to be functional and important, because Simonsiella attaches to oral epithelial cells only with the ventral, concave side of the filament (123). Here we see what appears to be a notable surface-maximizing strategy: a large bacterial surface molded into a shape that may accommodate itself to the membranes of its eukaryotic host cells.
If attachment strength increases when bacteria maximize their surface-to-surface contacts, why do we not find many more flat or wafer-shaped bacteria? Such cells could lay flat on a surface, creating a situation with the highest number of contact points combined with the lowest possible exposure to shear forces, but we know of only a few flat prokaryotes (mostly archaea) (144, 242, 349). Perhaps flat cells are at a disadvantage in the early stages of surface attachment because, like leaves, they tumble and catch current flow with their full square sides. The cells may not be able to make an initial contact strong enough to exceed the shear forces imposed on the unattached portions of the cell. However, once attached, such cells would be expected to hang on tightly, so these considerations alone do not explain the absence of flat shapes in cells living in less turbulent environments.
Finally, bacteria need not always combat the shear forces associated with fluid flow. In fact, many may positively embrace these forces and use them to their own advantage. Uropathogenic E. coli actually relies on shear forces to increase the avidity of its attachment to a mannose receptor on epithelial cells (328, 329). This trait may be replicated in several other bacteria (328, 329) and may be affected by cell shape. At the very least, these observations underscore the fact that bacteria detect and adapt to shear forces.
Poles Apart: Polar Localization
In many rod-shaped bacteria, adhesins (attachment proteins) are located specifically at the cell poles (31, 41, 72, 127, 184, 195). In fact, over 90% of E. coli cells adhere to polystyrene particles by one pole, suggesting that localized domains mediate the interactions (145). If, as implied above, rods are favored because the attachment strength is increased by the multiplication of contacts along the length of the cell, then why would cells restrict attachment proteins to their poles?
One possible answer is that adhesion progresses in two stages: an initial approach, governed by electrostatic repulsion and van der Waals attraction, and a stabilization phase, in which cells firm up their attachment by creating secondary interactions (3, 261, 269, 270, 336). The bacterial exterior is negatively charged, and cells usually approach other negatively charged surfaces, which means that the two repel one another. For example, at ∼15 nm from mammalian epithelial cells there is little repulsion between bacteria and the surface, but somewhere between 5 and 10 nm from the surface there is maximum repulsion around a stable point called the “secondary minimum” (Fig. 5) (269, 270). Only if bacteria can get past this boundary can the electrostatic, van der Waals, and protein-specified attractive forces hold the bacterial cell in place (270). So, bacteria use long-range surface-grabbing devices that extend from the position of maximum repulsion to the surface. These are generally fimbriae (pili) in gram-negative organisms and carbohydrate polymers in gram-positive organisms (270).
To minimize the charge repulsion between two negatively charged surfaces, bacteria might approach a surface with the smaller face of one of their poles, initiate attachment, and either remain attached only at the pole or else align the rest of cell in a second step (3, 31, 72, 261). To obtain experimental support for such a scenario, Powell and Slater measured the shear stresses required to remove bacterial cells from glass capillaries (261). Increased fluid flow reduced or eliminated the establishment phase of bacterial attachment, but if they waited to impose a shear flow until after the cells had time to create secondary contacts, the cells were much more difficult to remove (261). More recently, the phenomenon has been visualized by microscopy, showing that E. coli attaches to a surface first by its pole and only later by lateral interactions that are more permanent (3). This two-stage attachment, i.e., poles first and lateral connections second, may be general to many bacteria. Pseudomonas aeruginosa binds to filaments of the fungus Candida albicans, with the initial contact being made by type IV pili at one pole of the bacterium (127), and the sticky holdfast molecules of prosthecate bacteria are located at the tip end of one of their appendages (61, 65, 253, 255). Many gliding bacteria express pili at one pole (195), the fimbriated pole of Thiothrix nivea initiates attachment (184), and attachment of Mycoplasma pneumoniae to glass surfaces may be easier for an elongated cell because the negative charge repulsion is reduced by approaching tip first (72). These behaviors are consistent with an advantage for concentrating adhesin molecules at the tip ends of cellular structures (31).
Cell-Cell Interactions
This cell that had split, end to end
Said, “I was quite upset and then,
I didn't know who
I should turn to.
I was beside myself, my friend. —A. Willis (364)
Safety in numbers.
Bacteria adhere not only to inanimate surfaces but also to neighboring cells, and the most basic cell-to-cell interaction is between two daughters. Time-lapse microscopy of isolated E. coli cells growing on an agar surface reveals that the two daughter cells slip and grow towards one another so that the resulting four daughters end up side by side (Fig. 7) (59, 296). Similar four-cell arrays occur during the growth of Vibrio cholerae and Agrobacterium tumefaciens (see references cited in reference 296), and even swarming myxobacteria stay in side-by-side contact with one another (348). An early conclusion was that growth of each cell was polar and unidirectional (59), but later work proved that growth of the peptidoglycan wall is disperse (34, 43, 52, 368). The explanation for the appearance of directionality is cell-to-cell attachment. When different cells are close enough, their (unrelated) daughters may touch and align with one another instead of with the two original mother cells (296). Thus, the alignment process must be driven by cell-to-cell contact and not by mechanical considerations (296).
FIG. 7.
Cell-to-cell attachments in the formation of daughter cell tetrads and microcolonies. E. coli CS315 cells were inoculated onto a rich agar medium in a microscope chamber and photographed at time intervals while being incubated at 37°C. Incubation time increases from left to right in 10-min increments. This strain lacks penicillin binding proteins 4, 5, and 7 and produces many misshapen cells (50). (A) Cells with the normal rod shape grow and divide, after which the two daughter cells slip and grow along one another's sides to form a typical four-daughter cell tetrad. Subsequent growth of such a microcolony is typified by continued close contact among the cells (M. Larson and K. D. Young, unpublished data). This behavior has been well established by other investigators (59, 296). (B) Cells where at least one daughter is misshapen do not form closely knit daughter tetrads, and the resulting microcolonies often have numerous gaps because of the irregularly shaped cells (M. Larson and K. D. Young, unpublished data).
These and other self-associations must be important. Flocculation of E. coli in broth is caused by an autoaggregating outer membrane adhesin called antigen 43 which is required for biofilm formation (47, 168, 288). Antigen 43 mutant cells do not cluster together on plastic surfaces and are more susceptible to killing by hydrogen peroxide, perhaps because wild-type cells are protected from exposure when surrounded by others (47, 288). This self-association continues as one cell grows into a microcolony. Shapiro concluded that “the standard rule for E. coli microcolonies is to maximize cell-to-cell contact” and observed that disordered cells “align themselves during the first two hours of growth” (295). The tendency to align parallel with one another occurs even between the cells of different microcolonies as they collide (296). Furthermore, cell morphology changes in predictable ways in older colonies (295, 296), forming demarcated zones filled with “cells of distinct sizes, shapes, and patterns of multicellular arrangement,” including cells shaped as cocci, ovoids, bacilli, and filaments (294). Thus, the overall impression is one of directed morphological organization.
Antisocial shapes.
What does all this have to do with cell shape? The artist M. C. Escher exploited intricate geometric relationships to create works filled with complex shapes that fit together perfectly to cover two-dimensional surfaces. The concept is that of “tiling,” where objects having identical shape are positioned so they fill an area with no empty space between any of the pieces. In a similar vein, it may be advantageous for bacteria to self-associate and completely fill an area, leaving as few open gaps as possible. One can imagine that uniform rod-shaped cells would more easily align with one another than would cells with some other shapes (although other regularly shaped cells can also produce side-by-side arrangements [123]). There is evidence this is important. Proteus mirabilis forms swarming-proficient side-by-side cell arrays but cannot do so if mutated so that it grows as curved cells instead of as straight rods (see “DIFFERENTIATION” below) (122). As another example, many E. coli mutants lacking multiple penicillin binding proteins no longer form uniform rods but grow imperfectly, with bumps and uneven diameters (50, 229, 230). Time-lapse movies of such cells forming microcolonies show that cells with even slightly aberrant shapes do not align properly, and the final colonies are filled with gaps (Fig. 7B and unpublished results).
Biofilms: where no one stands alone.
If cell shape helps bacteria grow in cohesive groups, then the question becomes, “Why do cells form closely knit colonies at all?” There are at least three general advantages of such associations. First, members of a community with multiple attachments to one another may reduce detachment by shear forces. Bacteria attached vertically to a solid substrate and laterally to one another mimic a flat sheet. Shear forces that might dislodge a single cell may not be great enough to detach the entire group, and cells that did happen to be lost could be replaced by division. Second, cells may huddle together for protection from exposure to external chemicals or antibiotics (288) or from phagocytosis by protozoa or immune cells (203, 206). Third, the entire group may benefit from cooperative nutritional capacities, although any individual bacterium may have to make do with less exposure to nutritive fluids. These and other topics can be accessed through recent reviews (48, 165, 243, 330, 354).
E. coli provides an instructive example of the importance of cell shape in biofilm formation, because even slightly aberrantly shaped mutants form biofilms less efficiently (86). Deleting a single low-molecular-weight penicillin binding protein, PBP 5, causes cells to grow with nonuniform shape (210), which is enough to interfere with biofilm formation (86). Cell shape is affected more and more as additional PBPs are removed (210, 229, 230), and the efficiency of biofilm formation decreases in step with increasingly aberrant shapes (86). Thus, it seems that morphology is important even in simple one-organism biofilms, perhaps because shape alterations obstruct the customary intercell packing (Fig. 7B).
Other examples of the shape-biofilm interaction include the interaction between Burkholdereia cepacia and Pseudomonas aeruginosa (135). In cystic fibrosis patients, B. cepacia forms a biofilm in conjunction with P. aeruginosa. However, a rodA mutant of B. cepacia forms defective biofilms. The mutant cells are coccoid instead of rod shaped and they form tall, thick, compact aggregates next to uncolonized surface areas instead of spreading uniformly over the surface (135). The molecular reasons for this are unclear, but the shape change parallels the alteration of important cell-to-cell interactions. Also, a Lactococcus lactis mutant lacking the AcmA peptidoglycan hydrolase forms longer chains and less biofilm. Since addition of a small amount of lysozyme reduces cell chain length and restores biofilm formation (214), it is possible that chaining disrupts the normal cell-to-cell contacts between shorter chains and individual cells.
Summary
Bacterial morphology helps optimize interactions between cells and the surfaces to which they attach. Rod shapes may allow cells to attach more readily in environments with shear stress (e.g., in flowing water), perhaps by allowing cells to form uniform mat-like sheets. Filamentous cells have more surface area for long-term attachments and can entwine themselves with porous surfaces. Cocci may have access to small pores in a substrate, creating more attachment sites per cell and concealing themselves from external shear forces. Prosthecate cells may approach and attach to surfaces more easily by placing adhesins on the tips of thin appendages or may insinuate these into pores or crevices in solid substrates. As far as attachment goes, spiral cells would seem to combine the worst traits of cocci (small footprints) and of filaments (more surface area on which shear forces can act). Finally, the ability to form an unbroken carpet of cells may be helpful in biofilms. Cells may need to have a defined or geometrically regular shape so they can interact specifically and uniformly with other cells of their own species or with different organisms.
DISPERSAL
Food and shelter are the great driving forces in life, bacteria not excepted. In seeking these, it is often imperative that organisms be able to move—to a more nutritious environment, away from a toxic one, to a safer refuge—and, once there, to stop. Bacteria move by passive dispersal or active motility, and cell shape influences both mechanisms, helping to determine where bacteria can go and how they get there.
Float Like a Butterfly
Bacteria in aqueous environments search out specific niches: suspended in various strata or throughout the water column, residing at or just below the water's surface, or ensconced near the bottom or in sediments. Controlling cell shape is one way organisms reach and remain at their optimum positions. Dusenbery described the general physical principles governing passive dispersal (63). For cells that disperse by pure diffusion alone, Brownian motion moves spheres more rapidly than disk-shaped cells, and rods disperse slowest of all (63). So, if you are nonmotile but need to go somewhere, your best bet is to be a small coccus. Assuming that cells are more dense than the surrounding liquid, the rankings are the same for sedimentation rates. Spheres sediment faster than disk-shaped cells, and rods sink slowest because of their large axial ratios (63). If the best environment is near the water's surface, then this order suggests that being rod shaped may be the better strategy. Another tactic is adopted by the wafer-like photosynthetic archeon Haloquadratum walsbyi, whose thin, flat sheets float parallel to the water's surface and present the largest possible area to incoming sunlight (26, 163, 349).
Some prokaryotes move beyond these general rules by modifying their morphology in real time, usually involving some form of filamentation. As discussed in “NUTRIENT ACCESS” above, prosthecate bacteria can change their nutritional capacity by extending thin appendages to increase their surface area, but it is a poor trait that serves only one purpose. Because of their ribbon-like surfaces, the appendaged bacteria settle more slowly in a water column and remain suspended by the actions of even the most minor currents (228). In addition, bacteria with long prosthecae can form tangled pellicles that retard sedimentation and favor flotation at air-water interfaces, enabling the cells to persist at the surface for years (220). Stella, which grows as tiny, flattish, star-shaped cells with five or six short prosthecae, floats more easily than its perfectly spherical cousins or can settle horizontally onto surfaces because of the cells' planar morphology. Thus, by moderating the length of their prosthecae, bacteria can slow their sedimentation or, given a sufficient number of intertwined cells, form floating mats to preserve the population's access to oxygen and surface nutrients.
Hovercraft
In a virtuoso balancing act, many cyanobacteria position themselves at defined depths of the water column so they receive just the right amount of light (351). The mechanism is passive: cells receiving a great deal of light produce carbohydrates, which increases the density of each cell so it sinks; cells receiving too little light use up their carbohydrates, which reduces the density of each cell so it floats upward (351). The rates of ascent and descent are sufficiently low that the cells gradually equilibrate to inhabit an optimized depth range (351).
The interesting aspect for our current purpose is that cell size and shape influence the rates of floating and sedimenting. Cells that are too small do not move quickly enough to stay in their optimum light range, and cells that are too large overshoot their optimum range and are buried in the sediment or are ensnared in the mass of organisms floating near the surface (351). To illustrate these effects, a Planktothrix filament 800 μm long and 4 μm wide sinks or rises at about 30% the rate of a filament 8 μm wide; a filament 10 mm long rises 50% faster; and filaments of Limnothrix, which are only 2 μm wide, sink or rise only 10% as fast as the 8-μm-wide Planktothrix (351). At the other extreme, Anabaena filaments, which may be 10 mm long and 16 μm wide, rise so fast that they become trapped at the surface (351). Complicating the calculus of adopting one shape or another are the effects of cell diameter on light absorption. Thinner cells collect light more efficiently but change depth slowly, while wider cells change depth rapidly but absorb light and nutrients less well (351). By optimizing size, shape, and light-harvesting capabilities, species with different morphologies can coexist, each profiting from its own combination of characteristics.
Just as individual cyanobacterial shapes may be honed to match the environmental forces acting on them, so may the “multicellular shapes” of other bacterial populations. Lactococcus lactis subsp. cremoris secretes a hydrolase that cleaves septal peptidoglycan to separate daughter cells (213). In unshaken media, wild-type L. lactis cocci remain suspended but the long chains of a hydrolase mutant sediment to the bottom of the container (213). Interestingly, the opposite occurs when the organism is grown in semisolid media, where individual cocci sediment and chains float (213). Lactobacillus acidophilus cells and chains behave similarly (6). Thus, depending on the environment, sedimentation properties may determine the “best-shape” decision for microaerophilic or anaerobic organisms, and these may manipulate the extent of their chaining to reach desirable niches with respect to oxygenation (213). Cells of any shape can take advantage of these sedimentation strategies to accumulate in different environmental locations: rods and cocci can form chains, individual cells can filament and intertwine, and cocci can divide in multiple planes to form two- or three-dimensional agglomerates.
Subterranean Explorers: Geological Transport
Bacteria live on soil and other surfaces, in liquid, or at interfaces between the two, and their geological and geochemical impact is substantial, critical, and underappreciated (174, 232). Potentially able to live at depths of 3.5 km in the interstices of sandstone or shale, in deep groundwater and aquifers, and in suboceanic sediments, the biomass of these subsurface denizens may rival or exceed that of their aboveground relatives (174, 232). Even though only a few experiments specifically address the effects of cell shape on life in these environments, cellular morphology clearly affects how well prokaryotes drift or swim through these subterranean aqueous channels (363).
An aquifer is not simply an underground lake but resembles a vast sponge-like network of microscopic channels permeating different materials: relatively loose conglomerates such as gravel, sand, silt, and clay or more dense materials such as fractured rock, basalt, or sandstone. Many factors influence how microorganisms percolate through these systems, including cell size, morphology, motility, and surface chemistry (14), so that cells may be “retarded, immobilized, confined to preferential flow paths … or fall prey to protozoa” (120), all depending on the physical and biological characteristics of the specific environment.
Most bacteria collected from deep aquifers are “small cocci or coccoid rods,” which motivated Weiss et al. to perform the first pure study of how shape affects bacterial transport through artificial geological media (363). Prior to this, researchers had mostly ignored the possible effects of cell shape (363). Using gravity filtration, Weiss et al. washed bacteria through 3.5-cm-tall columns of quartz sand particles 0.7 to 0.84 mm in diameter. For 12 of 14 organisms, cells in the effluent were smaller and more spherical than the population loaded onto the columns, and for another strain, the lengths of eluted cells decreased significantly (363). Thus, smaller, coccoid cells moved through the column more rapidly than did larger, rod-like cells, in a chromatographic effect opposite that of biochemical chromatography, where larger particles appear in the void volume. Physical sieving should not contribute to the elution profile, because the mineral grain size (∼700 μm in diameter) was much greater than that of the bacteria (∼1 μm) (363). Physical straining becomes important only if the suspended cells have a diameter at least 5% of the diameter of the particles that make up the material through which the cells are passing (121). Put another way, straining occurs only when the diameter of a suspended particle is greater than 20% of the diameter of the open channels within the porous medium (14, 30). So, a 1-μm-diameter bacterium would have to pass through pores of about 5 μm before true chromatographic sieving would occur (14). Weiss et al. concluded that spheroidal cells move more rapidly through geological material and that rod-shaped cells are retarded compared to smaller cocci, and they hypothesized that rod shapes attach more strongly to quartz sand than do cocci (363).
The seemingly simple conclusion that cocci move more quickly through a sand column than do rods does not reflect what happens in all geological media, nor does it account for all components of natural situations. Harvey and Garabedian measured bacterial transport in an in situ experiment in which bacteria were injected into a sandy aquifer at a depth of 7 to 9 m and then recovered from a well 640 m away (121). Eluted cells were, on average, ∼0.25 μm (50%) longer than the injected population, increasing from ∼0.46 μm to ∼0.7 μm (121). However, the shapes of these eluted cells were not reported, so the population might have been composed of more-spherical cells with a larger diameter. If so, the results would correspond to those of Weiss et al. (363). This interpretation is made more likely by the fact that Harvey and Garabedian saw more bacteria in the small size class (∼0.2 μm) and fewer bacteria in the largest size class (1.2 to 1.6 μm) in transported samples than was predicted by their colloidal filtration model (121). Notwithstanding this shortfall of larger bacteria, the numbers of larger cells recovered did increase with increasing distance from the injection point (380 m versus 640 m), suggesting that at least some of the smaller cells were being selectively removed (trapped?) after traveling over sufficiently long distances (121). Once again, the cells were much too small compared to the diameter of the soil particles for true chromatographic sieving. Differences in rates of movement were likely due to differences in cell-to-particle adhesion, but since the experiment was a true in situ measurement, their diffusion model could not take into account differences in cell surface characteristics, motility, or bacterial loss from predation or lysis (121).
In another case, Becker et al. investigated the dispersal of four organisms through fractured crystalline bedrock: a gram-positive rod (Microbacterium sp., ∼1.4 to 1.9 μm in length), a motile gram-negative rod (Pseudomonas sp., ∼1.5 to 2.2 μm in length), a nonmotile mutant of Pseudomonas, and a gram-positive coccus (Staphylococcus sp., ∼0.5 to 0.8 μm in diameter) (14). Less than 4% of the injected population was recovered from a well 36 m from the injection point, compared to >90% of the predicted amount of deuterated water injected as a diffusion marker, suggesting that most bacteria were trapped or slowed by the intervening material (14). Interestingly, cocci arrived at the withdrawal site slightly before the deuterated water, which may reflect a more classical chromatographic effect in which cells eluted with a “void volume” while deuterated molecules were retarded (14). As in the previously discussed experiments, cocci dispersed faster than all but one rod-shaped bacterium, and gram-negative rods dispersed better than did gram-positive rods of similar size (14). Recovery of the gram-positive coccus was 17 times higher than that of the gram-positive rod, implying that the latter dispersed least quickly due to preferential filtration (14). Here again, chromatographic sieving cannot explain the differences in cell transport, because the mean diameter of fractures between the injecting and withdrawal wells was estimated to be ∼400 μm, significantly greater than the cell sizes (14). Similar to what was found in the work of Harvey and Garabedian (121), recovery of bacterial cocci and inert microspheres was higher for smaller sizes (from 0.2 to 1 μm in diameter), prompting the suspicion that being small and spherical reduces attachment to subsurface minerals (14). Oddly, motile bacteria were recovered less well than their nonmotile isogenic partners (14). This is at first counterintuitive, but is not surprising if motile bacteria explore more volume than do nonmotile cells, which are merely swept along the prevailing paths of underground currents. Another possibility is that motile bacteria may congregate to high densities in tiny dead-end cavities in the bedrock, being drawn together and retained there by chemotactic attractions to one another (244).
Although small bacteria may move best through sand-sized media (363), cells disperse differently through shale versus sand (46). The “pore-throat diameters” (bottlenecks in channels) are smaller in shale (<0.2 μm) than they are in sandstone (up to 13 μm) (174), which means that bacterial dispersion in these materials can be restricted by true sieving in addition to other effects. However, many types of shale are fractured, meaning that there are larger openings through which fluid may flow. Thus, particles can be transported through highly weathered and fractured clay-rich shale more rapidly than through sandy aquifers, where the fluid flow is more widely distributed (46). When Cumbie and McKay injected latex microspheres from 0.05 to 4.25 μm in diameter into fractured shale, 0.5-μm spheres were transported better than the others (46), which is interesting because this approximates the size of many bacteria in these environments. Larger spheres may have been retained by gravitational settling or physical straining, and smaller spheres may have penetrated smaller pores outside the major fractures so that they were removed temporarily from the bulk flow (46). The larger microspheres congregated along fracture surfaces and at fracture intersections, suggesting some type of preferential adhesion or capture (46).
Rock Solid
Many soil bacteria are filamentous, perhaps so they can fix their position in space by latching onto and wrapping around soil particles. The strategy may decrease the likelihood that cells will be washed away by percolating water, an effect that might also be duplicated by elaborating long prosthecae.
A specific example emphasizes this concept and illustrates how organisms manipulate the environment in turn. Kurtz and Netoff found that microorganisms can stabilize rocks against erosion instead of accelerating weathering, which is what we normally envision (178). Although sandstone exposed to wind is abraded and blown away over time, prokaryotes can counteract this process (178). After the wind cuts a groove in sandstone, a succession of cyanobacteria colonize the newly exposed surface: coccoid cells attach first, followed by a secondary community of filamentous organisms that bind neighboring grains to one another and grow to interconnect pores in the stone. These entangled filaments and their accompanying extracellular polysaccharides stabilize surface crusts so they resist wind erosion (178). Other microbes stabilize soils and desert crusts (178), and a filamentous cyanobacterium stabilizes sand dunes by a similar mechanism (252). Kurtz and Netoff speculated that by filling the sandstone pores the microorganisms may also reduce the rock's porosity and thus reduce the amount of water lost to evaporation, which would benefit the microbes and the underlying strata (178). The implications for cell shape are relatively straightforward: filamentous bacteria survive long enough to protect the sandstone and create a stable environment.
Amazingly enough, there is a parallel use for bacteria in materials science. When Shewanella is added to Portland cement, the compressive strength of the material increases by 25% after 28 days, whereas adding the same amount of E. coli has no effect (92). The organisms evidently plug the pores in the cement-sand mixture, perhaps by nucleating the precipitation of thin spicules of calcite (92). Bacillus pasteurii also increases the compressive strength of cement by ∼18%, a phenomenon probably correlated with calcite formation (264). Bacterial cell shape per se is not implicated in these cases. However, since shape affects the distribution of cells in real geological materials, then microorganisms of particular morphologies may influence the overall structure and physical characteristics of more materials that we know of.
Summary
For different geological habitats, the appropriate prokaryotic size and shape are dictated in part by opportunities for dispersal. In bulk liquids the relevant physical forces are those of diffusion, flotation, and sedimentation. Subterranean environments include the additional considerations of flow rates, surface properties, and fracture sizes. Cells relying entirely on passive dispersal can adapt their shapes to optimize the rates at which they move and to choose the locales in which they will accumulate. Although no one rule can be applied to all geological situations, a general guideline seems to be that the smallest cells may be retained in fine crevices, larger cells are retained by preferential attachment, and cells with dimensions in between may be more apt to flow through. Unfortunately, until we can manipulate the morphology of individual organisms, we will have to be content with indirect ways of deducing the effects of geology on cell shape.
MOTILITY
Cells may be able to get where they want to go by passive means, but many circumstances require a more direct approach. The major impediment to bacterial motility is that the bacteria are so very similar to their aqueous environment. The extent of this problem is captured mathematically by the Reynolds number (the ratio of inertial force to viscous force), which is a relative measure of the resistance to movement imposed on a particle by the surrounding medium. Bacteria have an exceedingly low Reynolds number. This means they are not acted on by inertia: if motile cells stop rotating their flagella, the cells stop immediately (within 0.6 μs) and they glide not at all (stopping within 0.1 angstrom) (15, 19, 263). So, if a cell wants to go anywhere fast, it needs to be engineered for speed.
Energetics of Motility
J. G. Mitchell and D. B. Dusenbery each appraised the energy demands that motility and chemotaxis impose on bacteria, and their articles should be consulted for a complete picture of the physical and chemical demands with which cells must contend (63, 217). The mathematical treatment of forces acting on bacteria during motion is complicated, especially given the diversity of parameters to be considered (swimming speed, swimming mode, diameter and length of flagella, number and location of flagella, diameter and length of bacterial cells, cell shape, surface characteristics, medium viscosity, and the presence or absence of chemical gradients). The following discussion summarizes their main conclusions as they apply to bacterial size and shape.
Mitchell considers four modes of motility that may be coupled with chemotaxis: run and tumble (cells actively change their direction of travel, typified by E. coli), run and stop (cells rely on Brownian motion to change their direction, typified by Rhodobacter sphaeroides), run and arc (cells reorient their direction by moving in a short curve, typified by Thiovulum majus), and run and reverse (both a migratory form, in which the reversals are shorter than the runs so that the cells move further along in one direction, and a localization form, in which the reversals are approximately as long as the runs so that cells tend to remain in one area) (217).
Brownian motion.
A major consideration that affects the motility of all bacteria is Brownian motion, the random force of molecular collisions that rotates small bacteria more easily and faster than large bacteria (217). A run-and-stop chemotactic strategy is good for small bacteria because Brownian motion can reorient them to pursue a new, random direction as the cells seek to follow a gradient. Brownian motion reorients larger bacteria more slowly, so these cells benefit by using a run-and-tumble strategy to change directions. However, tumbling requires so much energy that for large bacteria the expenditure becomes too great. Such bacteria do not tumble but adopt the run-and-arc mode, which is the only energetically competitive strategy for very large cells (217). Of course, Brownian motion is a double-edged sword because this buffeting reduces the amount of time that small cells can swim in a straight line, which, in turn, reduces their ability to follow a gradient efficiently. Using external flagella combats this randomization tendency by increasing drag (like a long tail on a kite), stabilizing the direction of movement (217). Bear in mind that these are rules of thumb in that they may be modified by additional considerations such as viscosity of the medium and proximity of cells to surfaces, etc.
Chemotaxis: efficiencies of stalking.
In addition to moving at all, cells often travel in a specific direction by responding to chemical gradients. As an illustration of how important this can be, in oceanic environments some bacteria pursue free-swimming algae by detecting tiny oxygen gradients emanating from these photosynthetic organisms (12). For example, two gram-negative rods, Pseudoalteromonas haloplanktis and Shewanella putrefaciens, detect and chase the motile alga Pavlova lutheri (12). The bacteria swim most slowly (∼126 μm/s) in the absence of algae, faster (∼237 μm/s) in their presence, and fastest (∼445 μm/sec) when actively tracking an algal cell (217). Such chemotactic pursuit is essential in open waters where bacteria have only transient access to point source nutrient supplies (other microorganisms or “marine snow” particles) (22, 217). Of course, motility and chemotaxis impart selective advantages to bacteria in all sorts of environments, thereby creating a corresponding selection for the most energetically favorable bacterial shapes.
There are two types of energy cost during chemotaxis: one for running and tumbling (“the cost of searching for a resource”) and one for moving along a gradient (“the cost of migrating toward that resource”) (217). These costs are different for cells of different diameters, as reflected in the relative values in Table 4. It is clear that even slight changes in diameter dramatically alter the energy cost of chemotactic motility (217). For example, consider a cell with a flagellum 10 times longer than the cell is wide. Compared to a cell 0.4 μm in diameter, ∼5 times more energy is required to move a cell 1.0 μm wide, ∼20 times more to move a cell 1.6 μm wide, and ∼40 times more to move a cell 2.0 μm wide (Table 4) (from Fig. 2B in reference 217). Differences in energy expenditure are biased even more when calculated for a cell moving along a gradient, which requires that the cell maintain a minimum speed to run long enough to detect a gradient and make a decision before being rotated by Brownian motion (J. G. Mitchell, personal communication). Compared to a cell 0.4 μm in diameter and moving along a gradient 100 μm in length, ∼10 times more energy is required to move a cell 1.0 μm wide, and ∼100 times more energy for a cell 2.0 μm wide (from Fig. 3B in reference 217). Cells with smaller diameters are more energy efficient for moving along short chemical gradients, whereas cells with larger diameters are more efficient for moving in longer gradients (Table 5). In fact, for each gradient length there is a specific cell size that expends the least energy.
TABLE 4.
Shape effects on the energetics of bacterial chemotaxis
| Cell diam (μm) | Relative energy required to move cellsa
|
|||
|---|---|---|---|---|
| Chemotaxis search | Chemotaxis along a gradient of length:
|
|||
| 100 μm | 1 mm | 1 cm | ||
| 0.4 | 1 | 1 | 30 | >103 |
| 1.0 | 5 | 10 | 10 | 10 |
| 1.6 | 20 | |||
| 2.0 | 40 | 100 | 100 | 100 |
Energy amounts are expressed relative to the energy required to move a cell of 0.4 μm in diameter and are restated from values graphed in Fig. 2B and 3B in reference 217. The absolute values of the unit amount of energy are different for the chemotaxis search and for chemotaxis along a gradient. The values are rounded and are for comparison purposes only (see reference 217 for the absolute values). Note that cell dimensions in this table are stated as cell diameters, whereas cell radii are reported in the original figures (217).
TABLE 5.
Most efficient cell diameters for chemotaxisa
| Gradient length | Efficient diam (μm) |
|---|---|
| 1 cm | ∼0.9 |
| 1 mm | ∼0.6 |
| 100 μm | ∼0.4 |
| 10 μm | ∼0.3 |
Values are restated from those in reference 217.
Because small differences in diameter produce huge differences in energy cost, the ability to detect and move through chemical gradients may contribute more to a cell's tendency to be rod-like than requirements associated with undirected movement (63, 217). In addition to the above considerations, bacteria measure chemical gradients about 650 times better if the cells are rod shaped, mainly because spherical cells are affected so much more by Brownian motion and rotational diffusion (63). Certainly, though, for cells in any particular environment the most appropriate size and shape will be heavily influenced by the distance to nutrients and the steepness of the gradients leading to their sources. Small cells will be favored in gradients that cover short distances, but wider cells will be more competitive in very long, shallow gradients (217).
Size vise.
The opposing effects of Brownian motion and chemical gradients on the energy efficiency of chemotaxis create what Mitchell calls the size vise (217), meaning that bacterial morphology is squeezed by selective pressures from both ends. For motile bacteria the energy advantages drive large cells to become smaller, but “the sensory and rotational advantages” for following chemical gradients drive cells to become larger, thereby squeezing cell shape into “a narrow optimal size range for a given set of environmental conditions” (217). Note that in any one environment, organisms with various biochemical capabilities will respond to different chemical gradients and may therefore have different shape optima.
Maki et al. examined one facet of this equation by creating motile E. coli filaments 7.5 to 10 μm long that respond to chemical gradients as well as normal-sized E. coli (199). These filamentous cells do not tumble, and therefore they cannot run and tumble. Instead, after moving in a straight line for a while, they stop and then either reverse direction or continue along their original path (199). Filamentous cells move more slowly (11 μm/s, versus 19 μm/s for the wild type), and mutations that provoke normal rods to tumble continuously cause the filaments to stop completely, or, in the authors' vivid words, the mutant filaments stop and “gently thrash about” (199). The point is, if a cell is too long it cannot tumble to search for the most appropriate direction toward a chemoattractant or away from a repellent. Therefore, cells longer than a certain length will be counterselected because they cannot follow a gradient to its source or, if they can, will do so more slowly.
The preceding analyses predict there exists a selective pressure for cells to be rods (so they are more mobile) that is counterbalanced by a selection against cells being too long (since such cells cannot tumble to seek out better directions). The opposing pressures define a window of most efficient motile shapes in any set of environmental conditions, operating as components of the size vice.
Sleds and saucers.
Mitchell's calculations suggest why a cell might prefer to be one diameter as opposed to another, but why might a cell prefer a certain length? By considering the fore-to-aft pressure difference (pressure drag) and the friction of movement through a viscous medium (surface drag), Cooper and Denny calculated the shape that allows a cell to move through water most efficiently (45). For cells of any size, moving through liquid requires the least amount of force when a cell's length is ∼3.7 times its width (45, 63), a ratio that is largely independent of size (45). However, the resisting force at a ratio of 2 is hardly greater, and the force required to move longer and longer cells increases very slowly. Because there is such a broad range of length-to-width ratios near the optimum, long rods (with ratios of 10 or more) can move almost as easily as short rods (45) (but note that Dusenbery's calculations moderate these conclusions somewhat [63]). In addition, it is much easier for an elongated cell to move through liquid than for a sphere of equal mass to do so. The most efficient rod-shaped cell moves ∼5 times more efficiently than can a coccus of the same mass or volume (45, 63). In fact, a rod-shaped cell would have to be about 130 times as long as it was wide before resistance to swimming would be equal to that of the much smaller spherical cell having the volume of the original rod (45). Thus, although a coccus can be motile, it has to work much harder to go anywhere than if it were a rod.
How do these calculations compare with the dimensions of actual cells? An average newborn E. coli cell has a length-to-width ratio of ∼2.5, and the average for a population in all stages of growth is ∼3.9 (45). Therefore, E. coli falls squarely in the shape realm that provides the least resistance to movement. In fact, of 218 free-living genera, 21% are spherical, most of the rest are rod-like, and only 10% of motile forms are coccoid (63), reinforcing the notion that motility favors rod shapes. The mean length-to-width ratio of the rod group is 2.82, very near that of newborn E. coli cell, and the distribution of ratios is almost symmetrical around this number, with only four genera having ratios greater than 32 (63). Thus, in the real world, bacterial rods of all sizes adopt shapes that closely approach the optimum design for efficient motility (63).
The fact that rods are the best shape for a motile bacterium will not surprise those who rode snow sleds as children. Although you can hurtle down a hill in a plate-shaped saucer or in a rod-shaped sled, you quickly learn that you can steer a sled but are at fate's mercy in a saucer. It is the same with bacteria: if they want to go somewhere specific, they should be slightly elongated, but if they just want to go, not caring too much where they end up or when, then being a motile sphere is satisfactory.
Side Effects: Motility Near Surfaces
Bacteria don't worry about driving lanes or right of way … or do they? The preceding calculations and most experiments on bacterial motility are performed under aqueous conditions where the cells have room to roam and few barriers. However, newer work suggests that bacteria moving near solid surfaces behave differently than we might have imagined. In fact, it turns out that some bacteria have a penchant for driving on the right side of the road.
When smooth-swimming (nontumbling) E. coli cells are forced to swim through a thin and narrow fluid-filled passage, the cells almost always swim straight ahead while hugging the right-hand side of the channel (58). (Wild-type cells have no side preference, evidently because their run-and-tumble strategy breaks up the pattern.) The reason for this behavior is because bacteria swim differently near a surface (81). In agreement with theory (265), E. coli swims in repeating circles just above and parallel to the plane of a large flat surface (81). Why? The anticlockwise motion of the flagella drives the cells smoothly forward, but the resulting torque causes the cell body to rotate clockwise (186). The interactions of these opposing forces in the thin zone above a flat expanse causes the cells to swim clockwise in a circle (58, 186), and the cells linger in the zone because they tend to swim into the surface (186). Left to itself, E. coli can circle for more than a minute over a smooth surface with no boundaries (81). Most importantly, experiments and calculations indicate that cell length affects the size of the circle: short cells move in smaller circles than do longer cells (186).
This behavior is not unique to E. coli. When the curved cells of Vibrio alginolyticus swim near a planar surface, forward-moving cells run in a straight line but backward-moving cells run in a curved arc or in circles (176, 197). What distinguishes the behavior of forward- and backward-running cells is unknown (197). Once again the surface is “down” relative to the bacterial cell, but in this case, V. alginolyticus swims in large counterclockwise turns, opposite those of E. coli (197), probably because in backward-moving cells the single flagellum leads and pulls the cells. Alternatively, since V. alginolyticus is slightly curved (as are many marine vibrioids) and forms a right-handed helix (11), topology might dictate cellular motion near surfaces.
Bacteria not only behave differently near surfaces, but they also behave differently near different surfaces. A bacterium in a large volume of liquid feels little or no “up” or “down.” However, the cells do discriminate between and gravitate towards surfaces of appropriate composition. For example, E. coli swims on the right-hand side of a channel when the bottom surface is coated with agar, but when the top coverglass is coated, the cells swim preferentially on the left-hand side (from the point of view of the microscope above). E. coli, then, behaves as if the coated surface is “down” and so swims to the right relative to that surface. The cells exhibit no preference if the floor and ceiling of the channels have the same composition. Also, the nature of the surface must matter, because E. coli prefers to swim near an agar surface rather than one of oxidized poly(dimethylsiloxane) (58).
What are the mechanisms at work here? Surfaces do not influence bacterial motility by short-range hydrophobic or electrostatic interactions, because adding different surfactants does not change the cellular behaviors (58, 197). The main mechanism is the rotation that the flagella impart to the cell body (186), but there also seems to be a unique hydrodynamic environment near a porous agar surface that reduces resistance to bacterial movement (58, 197). This is consistent with the supposition that a moving sphere encounters less hydrodynamic drag when close to a porous surface than when near a solid surface (21). This zone must be thin, because the swimming preference of E. coli disappears if the height of the channel is more than 10 μm (58), which is evidently the critical area in which these bacterium-to-surface interactions take place (16, 21, 81, 176, 197).
The above discussion highlights the fact that the forces acting on bacteria because they need to be motile are augmented by near-surface forces and interactions we can describe only vaguely. Magariyama et al. go so far as to conclude that “important factors have been overlooked” in trying to explain these effects (197). So, although we normally think about motility in free-floating bacteria, in many cases motility and cell shape may be tuned to optimize motion near biologically relevant surfaces. Since we do not know the exact nature of all the forces acting on cells in the thin volume of liquid near a surface (81, 186), we cannot know exactly how different environments affect the optimum sizes and shapes that cells need to assume. We will not know more until these near-surface forces are described thoroughly and incorporated into the equations of motion used to model bacterial shape.
What benefit might these behaviors impart to bacteria swimming near surfaces? Most likely, by matching their mechanisms of movement with the unique hydrodynamic properties surrounding solid materials, bacteria increase their residence time in zones close to surfaces from which nutrients are diffusing. A special example of this would be that of V. alginolyticus. At first glance, motility in the open ocean proper would not seem very useful because any attractants would be diluted far below satisfactory concentrations (197). In fact, this fact makes motility near a surface a most important characteristic (197). The combination of straight forward runs and curved backward runs means that V. alginolyticus can explore the entire surface area of a solid better than if the bacterium used a run-and-tumble strategy that might send the cells away from a precious, and often rare, solid surface nutrient source (197).
Motility versus Viscosity
Thicker than water.
Bacteria have enough trouble being small and moving through water, but their problems are magnified when attempting to move through media of higher viscosity. This is precisely where many bacteria live: mired in mucus, bound up in biofilms, and snarled in polymers secreted by bacteria, algae, and animal tissues (158, 198). These environments are more demanding and are, it seems, best navigated by bacteria with helical or spiral shapes (325). The elongated, helical cells of Vibrio alginolyticus adhere to mucus on the skin and gills of fish, suggesting that the spiral form enables the bacterium to move through the mucus to colonize the underlying epithelium (11). Campylobacter jejuni and Helicobacter pylori move through gastrointestinal mucus and high-viscosity media, probably benefiting from their spiral shapes (74, 271, 321). Mucispirillum schaedleri, a newly described spiral species representing ∼27% of all bacteria isolated from the gastrointestinal tracts of rodents, probably does the same (271). All of this leads many to conclude that spiral cells are perfectly suited for motility in highly viscous environments (11, 74, 158, 198, 271, 300, 321, 325).
One good turn: helical motility.
The experimental evidence for the above supposition began when Kaiser and Doetsch demonstrated that spiral-shaped leptospires move faster in viscous fluids than in less viscous media (158). The cells' translational motion and speed peaked in media having a viscosity of 300 cP, whereas the overall motility and speed of rod-shaped flagellated bacteria peaked at 2 to 5 cP and then decreased (158). At the optimal viscosity, a greater percentage of the spirochete population was motile (increasing sixfold, from 10% of the population to 60%), and the speed of individual cells increased twofold (from 15 to 30 μm/s) (158).
Simple rod-shaped cells behave differently. Remember that bacteria have a low Reynolds number, so that to be motile at all they must actively propel themselves even in media of low viscosity. At high viscosities, most rods (e.g., E. coli, Salmonella spp., and Pseudomonas aeruginosa) are immobilized or move at only 20 to 30% of their original velocities (9, 74, 300), while the helical cells of Vibrio alginolyticus, for example, still move quite rapidly (9). Apparently, the energy required for movement in these conditions exceeds the flagellar force that rod-shaped cells can generate. The kinetics of swimming inhibition are also different. In contrast to the behavior of rods, the velocity of helical Campylobacter jejuni decreases more slowly with increasing viscosity. In addition, at a viscosity where the velocity of C. jejuni rises to 40% of its original value, rods can swim at only 10 to 15% of their original speed (300). This “secondary peak” at a higher viscosity is typical for many spirochetes (300). Not only does C. jejuni move faster at higher viscosities, but, unlike rod-shaped bacteria, almost every cell in the population remains motile (74). Finally, in a medium mimicking the viscosity of intestinal mucus, C. jejuni is obviously more spiral shaped, moves faster, tumbles less, and travels for longer times and distances in a straight line (74, 321). All indications are that being spiral is at the root of these behaviors.
The polymer maze.
What is it about the physical nature of viscous fluids that motivates cells to adopt a spiral shape? First, viscosity per se is not necessarily the determining factor. Gel-like solutions of viscous molecules form a quasirigid fibrous-like network through which bacteria have to move (17). In solutions of unbranched polymers the viscosity “felt” by a microscopic particle is that of the solvent, which is low (17). Thus, a highly viscous solution of these materials may behave like a molecular sieve, in which the less viscous solute forms “channels” between individual polymers. These solutions are so porous that they do not change the sedimentation or diffusion of bacterial cells very much, and bacteria can swim as easily through these as through water as long as the cells are thin enough (17). Berg and Turner noted that a solution with an “apparent viscosity thousands of times greater than that of a solvent” may have little effect on the diffusion of small particles (17). Thus, how much the environment impedes bacterial movement is measured not directly by the bulk viscosity of the medium but by the characteristics of the solute channels.
To explain the fact that bacteria speed up in more-viscous solutions (17, 198), Magariyama and Kudo applied a modified resistive force theory to the physics of the situation and predicted that long, thin, helical shapes produce the best swimming speeds in a viscous polymer-filled medium (198). Instead of swimming freely through these solutions, helical bacteria “move without slip, like a corkscrew through a cork” (17). In this case, the length of any one cell is not a liability but an advantage, in that rotation of the helical cell body and its interaction with the polymers create the force that propels bacteria forward (158). Rotational movement translates into straight-line movement. Therefore, in a sufficiently viscous medium, the diameter of the solute channels between polymers affects the optimum diameter of helical bacteria; i.e., higher viscosities select for thinner cells, as is the case for many spirochetes.
Because bacteria encounter media of different bulk viscosities, it may be that cellular diameter is determined (or at least strongly affected) by the diameter of the solute channels. From the point of view of a bacterium, much of the microscopic world is a sieve, a biochemical sizing column, constructed of a maze of thin channels. Diameter would be important because cells have to fit through these channels, and length would be important because longer rods or straight filaments might not navigate the many twists and turns in the highly convoluted channels. The length-to-width ratio for any cell would reflect some optimum combination to deal with these factors, and the combination of helicity and thinness is an adaptation for motion in a viscous environment.
Motility and Polarity
In addition to the physics of moving efficiently through liquids, the propulsion mechanisms for bacterial motility may necessitate that cell shape be polarized—that is, be elongated so that one end is differentiated from the other, giving cells a “fore” and an “aft” as they move. Flagella push or pull cells through liquid, and, as discussed above, hydrodynamic considerations decree that rods and spirals are best suited for this purpose. But bacteria may also move by “gliding,” originally defined as “translocation in the direction of the long axis of the bacterium while in contact with a surface” (268, 371). Here too, a polarized cell shape is advantageous for moving in a defined direction, and in fact, most cells that move this way are rod shaped.
Gliding bacteria use either of two mechanisms. Bacteria that move by “twitching motility” extend pili from one end of the cell, attach to a surface via the tips of the pili, retract the pili, and then repeat the process, thereby pulling the cell body forward as you might extend and retract your arms to climb a ladder (36, 202). Long before the actual mechanism was discovered, it was known that bacteria moving this way had pili at one pole and not at the other (195), a prerequisite for moving in one direction. Cells without polarity can move by twitching, but a polarized cell shape improves the directionality.
The second mechanism (now termed true gliding to distinguish it from twitching) is the forceful expulsion of a jet of extracellular slime that moves cells by physically thrusting them forward (130, 132, 371). The slime is ejected from multiple junctional pore complexes, which are barrel-like pores angled 30 to 40° relative to the cell's axis and located at the cell poles or at septa (130, 132, 371). Cells control their direction of movement by extruding slime from one end at a time, and they may reverse direction by switching extrusion to the other end (155, 157, 370). Some cyanobacterial gliding cells rotate around their long axis (like a helical screw) while moving forward, as the slime is directed along helical protein channels wrapped around their outer surface (129-131, 208, 371). A similar mechanism is exhibited in some noncyanobacteria (183) and the myxobacteria (155, 370, 371). Directed movement by such a mechanism requires polar differentiation and a particular cell shape, most often elongated and rod-like.
Similar to the physical method of gliding, a few bacteria push themselves forward in intracellular environments by assembling insoluble actin polymers at one of their poles. Rod-shaped cells can more easily direct the overall direction of motion by polymerizing actin at one end, an ability lost in mutants with abnormal septation and polar differentiation (250).
Summary
To propel themselves forward, bacteria must overcome liquid viscosities that normally arrest them in their tracks. Furthermore, to go anywhere in particular they must detect and follow chemical gradients of various lengths and strengths, and many cells may travel in close proximity to solid surfaces. The physics and energetics for dealing with these conditions strongly favor rod-shaped bacteria, and environmental circumstances will dictate the most efficient combinations of cellular dimensions. Bacteria that move by twitching or gliding when in direct contact with solid surfaces are often rod shaped, though spherical cells may use twitching motility. In contrast, a spiral or helical morphology seems best suited for navigating through solutions of high viscosity.
POLAR DIFFERENTIATION
Except for the perfect sphere, all other bacterial shapes display some sort of geometric asymmetry—a polarity, either locally or taken as a whole—along which different parts of the cell may be organized. Rod-shaped cells have poles, discoidal cells have a top and an edge, prosthecate cells (even if symmetrical) have points and sides, and even square or cuboidal cells have apices. This means that most prokaryotes have a choice about where to place proteins and other macromolecular structures within their cells. We have already seen advantages that cells may derive by deviating from the perfect sphere, but it is fitting to review them briefly with regard to polarity.
Why should adhesins, pili or other attachment complexes be localized to the cell poles? The first reason may be to minimize the charge repulsion between two negatively charged surfaces as they approach one another. Cells can approach a surface, initiate attachment, and then either remain attached by one pole or fasten themselves lengthwise (see “ATTACHMENT” above). Another advantage of attaching to a surface via one pole is that the cell body remains exposed to the surrounding aqueous milieu (see “NUTRIENT ACCESS” above) (61, 253, 346). But the benefits can entail more than this. For example, members of the genera Thiothrix and Caulobacter, which have holdfasts at one end, stick to one another to form rosettes (33, 184). Such an arrangement leaves one end of each cell or chain of cells exposed to the environment, free to take up nutrients or to entrap passing prokaryotes (218). These flocculent bacterial masses might also confer valuable settling, floating, or dispersion characteristics (see “DISPERSAL” above) (220, 228) or may reduce predation by protists (see “PREDATION” below). A third reason to have a shape that imparts a definite polarity is to give the cell directionality, primarily for the efficient motility of rods (see “MOTILITY” above).
Sequestration
Organisms can also exploit polarity by sequestering molecules into clusters and domains and then organizing these so that some are separated while others are joined into durable groups. Polarity allows cells to do this more easily by creating identifiable, distinguishable parts of the cell.
Just as the exteriors of most bacteria are morphologically asymmetric, their interiors are also nonuniform. It is now clear that prokaryotes arrange much of their internal structure as microdomains, many of which are associated with specific positions in the cell envelope. For example, various membrane components form heterogeneous microdomains enriched for individual lipids or lipid combinations (62, 173, 215, 216, 337). These differences are especially conspicuous at the cell poles (215, 216). A second, parallel microheterogeneity is apparent in the protein content of membrane vesicles isolated from E. coli (140, 141, 189). Once again, the effect is most clearly associated with the poles, reflected as a nonhomogeneous distribution of proteins in E. coli minicells (representing mostly polar domains) as opposed to normal rods (representing all membrane domains) (180).
The third component of the cell envelope, the cell wall, is also differentiated. Peptidoglycan comprising the poles is, in some way, different from that in the lateral walls (52, 53, 55). This polar peptidoglycan is “inert” in that it is neither degraded and recycled nor diluted by addition of new material (43, 52, 171). Interestingly, this same stability extends to proteins and lipopolysaccharides in the outer membranes of the poles of gram-negative bacteria (51, 91, 235) and to proteins on the surfaces of gram-positive organisms (239). These inhomogeneities among lipids, proteins, and peptidoglycan indicate that bacterial poles differ from the rest of the cell, which makes them prime candidates for hosting specialized functional elements.
A rapidly growing number of components are relegated to these privileged polar sites (143, 194, 297). The list includes chemotaxis proteins, parts of the chromosomal segregation apparatus, type IV pili, slime nozzles in gliding bacteria, two-component signaling proteins, septation regulators, flagella, actin polymerization nucleators, autotransporters, type II secretion proteins, a type III secretion system, a type IV apparatus, an RNA helicase, and a bacterial reverse transcriptase (38, 67, 143, 147, 194, 293, 297, 340, 374, 378). The spheroidal cells of Streptococcus pyogenes have a single microdomain for protein secretion (277, 278), which might serve as the analogue of a defined pole in rod-shaped bacteria. Even long, single-celled bacterial filaments exhibit internal asymmetries associated with places where poles should have been created. For example, methyl-accepting chemotaxis proteins localize at intervals corresponding to uncompleted septa in E. coli (199), and the ActA protein congregates at future division sites in filaments of Listeria monocytogenes (250). All these behaviors identify an underlying heterogeneity in the cell envelope, one that is defined by and enhanced by the existence of polarities in cell shape.
The benefits of sequestration affect the internal operations of the cell, especially those that involve attachment to or traverse of materials through the cell envelope. During normal growth and expansion of the cell wall in most rod-shaped bacteria, material is inserted in a relatively disperse manner over the whole surface (52-54, 212, 368). This means that embedded proteins or complex structures that were once close neighbors might become separated as new growth forces them apart. Therefore, if interactions between neighboring components are essential or contribute to biochemical efficiencies, it would be advantageous to confine these to a less lively area.
Inert cell poles seem to provide just the sort of stable, long-lived environment where multicomponent complexes can be assembled without fear of being separated by growth of the cell wall. For example, numerous bacteria localize their chemotactic receptors at one or both poles, which points to an evolutionarily conserved strategy of localization (90, 196, 309). What is the advantage? Colocalization of these and related components optimizes chemical interactions among components of the chemotactic signaling system (308), thereby reducing the potentially disturbing effects of chemical gradients (334). Although these proteins might colocalize anywhere, their interactions may be more durable when they are placed together at the poles.
As another example, the apparatus required to transfer DNA into and out of bacterial cells may benefit from being confined to the poles. In E. coli and B. subtilis, chromosomal transfer during cell division or sporulation is mediated by specialized proteins localized specifically at the site of septation, which will eventually become new poles (68, 69, 362). In Streptomyces, plasmid DNA is transferred from one organism to another through Tra protein pores located only at the tips of growing poles (108). In Agrobacterium tumefaciens, the T-pili that initiate conjugation and the VirD4 protein required for DNA transfer are located only at cell poles (177, 179). Finally, several competence and recombination proteins localize to the cell poles in B. subtilis, making this the preferred site for DNA binding and uptake (112, 164). What does all this mean? The simplest explanation is that the poles provide an appropriate, stable environment that facilitates interactions among the pores, pili, and proteins required for attachment, DNA transfer, and processing.
Separation
The preceding extols the benefits of sequestering molecules to confined locales. However, the opposite may also be advantageous. That is, an asymmetric shape allows the cell to separate proteins or complexes from one another. Thus, similar complexes might perform different functions in different environments, or the cell may benefit if similar complexes were prevented from interacting. For example, Rhodobacter sphaeroides synthesizes two sets of chemoreceptors that the cell sequesters, one to the poles and the other to the middle of the lateral wall (344, 345). The molecular components of these two sets do not interchange, implying that functional separation may be aided by spatial segregation in the envelope.
Localized force.
The filamentous cells of the bacterium Streptomyces and the fungus Aspergillus grow by elongating only at their tip ends (104), utilizing at least one protein that localizes specifically to that site (78, 341). This apical growth creates a concentrated pressure force by which filamentous cells can burrow into neighboring materials (103, 262, 341). Thus, in some cases, filamentation and polar differentiation may be adaptations for tunneling into niches and semisolid environments that would be inaccessible by other means.
Aging.
The poles of dividing cells are not the same age: one is brand new, the product of a recent division event, and the other may be many generations old. Recently, an old question—“Do bacteria age?”—was answered in the (mostly) affirmative (2, 315, 317, 369). Older C. crescentus stalked cells produce progeny at reduced rates (2), and E. coli cells with older poles grow slightly more slowly than do cells with younger poles (317), though questions regarding the latter conclusion remain (369). The situation could be interpreted as a general disadvantage, since the cells are not immortal. On the other hand, it could be turned to the cells' advantage. For instance, damaged proteins or nucleic acids or other cumulative structural defects that cannot be recycled or jettisoned might be segregated differentially towards the older end of the cell. At division, one daughter cell would be virtually new, while the “older” daughter would carry away the spoiled material (315, 317). The long-term benefit may be that the renovated cell produces more fit progeny than would two slightly damaged cells. Since bacteria localize many cellular components to the poles, it is not too much of a stretch to imagine this “wastebasket” scenario, made possible by optimizing a suitable cell shape.
Summary
A cell may adopt an asymmetric shape for many reasons, including the ability to localize proteins and complexes to circumscribed areas. Currently, we recognize the poles as the most obvious sites at which the latter process occurs, probably because our prokaryotic sampling is so heavily tilted towards rod-shaped experimental subjects. But there is no reason why cellular differentiation should be restricted to the poles. Bacteria may exploit the polarities and geometric discontinuities of any cell shape, for purposes of which we are at present only dimly aware.
PREDATION
Predation is not generally recognized by microbiologists as an important selective agent.
—C. Matz and S. Kjelleberg (206)
The evolutionary pressure of protozoans preying on bacteria has been called “one of the oldest interactions between the prokaryotic and eukaryotic worlds” (246) and “an exciting frontier for microbial ecology and evolution” (206). The relationship has been heralded as a critical force that “promoted major transitions in bacterial evolution” (206), shaped “the phenotypic and taxonomic composition of bacterial communities” (150), is “at least partly responsible for the diversity of present bacteria” (23), and may have “shaped microbial evolution as profoundly as oxygenic photosynthesis” (246). In light of these exclamations, why are many of us ignorant about how predation affects bacterial physiology and morphology in particular? It is a curious blind spot, considering that a specific subset of predatory relationships, under the heading “pathogenesis,” has been studied for 150 years. In this case, the predators are “the immune system” and the bacterial responses are “virulence factors.” Familiar concepts from this field can be applied easily to the environment at large.
So far we have focused on “bottom-up” control of bacterial biomass and shape, that is, how bacteria adapt to meet their nutritional needs. In contrast, predation is about “top-down” control by actively hostile organisms. Thingstad grouped these ideas into three evolutionary forces (326). First, nutrient restrictions limit the total amount of biomass that can accumulate; second, predation by protozoa affects prokaryotic size and the distribution of microbial groups; and third, predation by viruses influences bacterial speciation (326). The second force is the main subject of this section, since more is known about how it affects prokaryotic cell shape.
Protistan Grazing (Bacterivory)
Bacterivory (protozoans feeding on bacteria) and its effects on general ecology have been examined in several recent reviews (114, 150, 246, 298, 299). Here, I briefly introduce some of the leading players and the pressures they apply to cell shape.
The main categories of protozoan predators and their principal feeding styles are listed in Tables 6 and 7 (23, 24, 73, 246). Of particular import are the feeding mechanisms used to seek and capture prey, because these are the factors bacteria must elude to avoid being consumed. Most bacterivory is performed by phagotrophic flagellates less than 10 μm in size and by other heterotrophic nanoflagellates (HNFs) (150, 236, 246). Describing the explicit feeding mechanisms of these groups is outside the scope of this review, but consider as one example the feeding stages of an interception-feeding flagellate (150). The protozoan encounters bacteria at a rate that is affected by the size and abundance of the prokaryote, a beating flagellum creates a water current that draws the prey inwards, the flagellum folds over the bacterium and pins it to the body of flagellate, and the bacterial cell is enveloped into a food vacuole where it is digested (150). Another common mechanism is that used by filter-feeding protozoa, which trap bacteria by straining water through a sieving apparatus (23, 24, 150, 246). A more comprehensive description of a variety of feeding mechanisms is given by Fenchel (73).
TABLE 6.
Predators and preya
| Predator | Prey |
|---|---|
| Suspension feeders | Suspended bacteria |
| Raptorial feeders | Bacteria on surfaces |
| Grasping feeders | Attached bacteria |
TABLE 7.
Feeding mechanismsa
| Mechanism | Action |
|---|---|
| Filter feeding | Transports water through a filter or sieve |
| Sedimentation | Allows prey to settle into a capture device |
| Interception | Capture by predator-induced current or motility and phagocytosis |
| Raptorial | Predator crawls and ingests prey through pharynx or by pseudopods |
| Pallium | Prey engulfed (e.g., by extrusion of feeding membrane) |
| Myzocytosis | Punctures prey and “sucks out” cytoplasm and contents |
The impact of this grazing pressure is significant, but exact numbers are difficult to pin down. The HNFs are present in aqueous environments at anywhere from 100 to 10,000 per ml, and each can imbibe “∼105 times their own volume of water every hour” (246). At this rate, the entire water column may be filtered through HNFs every day (73). The percentage of phytoplankton (including bacteria) grazed per day is estimated at anywhere from 25 to 100% (298), with actual measurements from 27 oceanic locations ranging from 3 to 131% (299). In geological transport experiments, predation by flagellates reduces bacterial recovery between 60 and 97% (167). Overall, the best guess for bacterial mortality in the open ocean is approximately 50% from grazing by protists and 50% from lysis due to bacteriophage (83, 318). Complicating all predictions is the fact that predatory nanoflagellates and ciliates are themselves preyed upon by metazooplankton, which affects the extent of bacterial removal (150, 360). In summary, the severity of the bacterivory selective pressure depends on a complex array of feeding styles and predator combinations. Nonetheless, existing measurements do demonstrate that the pressure is strong and continuous.
As in any evolutionary contest, bacteria are not defenseless. In particular, they exhibit a high degree of “morphological plasticity” that helps protect them from predation (246). Because of the way protozoa handle their prey, bacterial capture is affected by their size and irregularities in their shape. Oversized, filamentous, or prosthecate bacteria may be too large to ingest. Exceedingly tiny cells may elude capture. High-speed motility, tenacious attachment to surfaces, and formation of biofilms and multicellular conglomerates may also reduce predation (150, 246).
Selection for Altered Cell Dimensions
Goldilocks and the bimodal effect.
In the fairy tale about Goldilocks and the three bears, a young girl constantly finds herself faced with three choices. In every case (chair size, food portions, and beds), one choice is too large or too hard, one choice is too small or too soft, but the third choice is always “just right.” Protists face the same choices when grazing, and the results are similar: quite often, bacteria too large or too small are rejected in favor of a “just-right” range of intermediate-sized cells (114, 150, 206, 246, 248, 249).
Hydrodynamic calculations indicate that bacteria smaller than 0.5 μm in diameter encounter grazing protists four to six times less often than do larger cells (∼1 μm), and filamentous cells or cells with diameters greater than 3 μm are often too large for protists to ingest (204, 206, 246). Therefore, cells in the intermediate size range are consumed more rapidly, very often creating a “bimodal effect” that selects for the very large or the very small (Fig. 8) (246). The boundaries are not sharp, of course, and the specific effects vary with the size ratio between predator and prey. Pernthaler et al. grouped susceptible bacteria into four rough size classes: those <0.4 μm were not grazed well, those between 0.4 and 1.6 μm were “grazing vulnerable,” those between 1.6 and 2.4 μm were “grazing suppressed,” and cells greater than 2.4 μm were “grazing resistant” (248). In fact, no bacterium is entirely safe; even larger filaments are grazed to some degree by some predators (246, 375). The acceptable size ranges of prey vary quite widely depending on the specific protozoan, as compiled and described by Fenchel (73).
FIG. 8.
Protozoal predation often produces a bimodal pressure on bacterial cell size. (Top) Bacterial cell sizes in the absence of protozoal predation. (Bottom) Bacterial cell sizes after protozoal predation. In the latter case, cells of intermediate size have been removed by grazing, leaving increased numbers of smaller and larger bacteria. (Adapted and redrawn with permission from Nature Reviews Microbiology [246] copyright Macmillan Magazines Ltd.)
The long of it: selection for filaments.
Some predators feed mainly on smaller cells, so a favorite bacterial solution is to increase cell length. For example, when 12 different rod-shaped bacteria were used as prey, the nanoflagellate Spumella contacted the longer cells more frequently but ingested them less efficiently, so that larger bacteria accumulated in the presence of this protist (204). Cyclidium glaucoma, a scuticociliate capable of high grazing rates on small prey, also selects for larger cells (259). These selections for longer cells can be quite dramatic, as in the case of elongated, hyperflagellated swarm cells that move en masse over solid surfaces (see “DIFFERENTIATION” below). Since the ciliate Tetrahymena feasts on short bacteria but cannot consume cells longer than 15 μm, the longer swarm cells of Serratia liquefaciens resist predation by this protist (7). After being grazed by Tetrahymena for only 5 h, intermediate-sized bacteria (2 to 10 μm long) decrease from 46% to 0% of the population, cells longer than 30 μm increase from 4% to 50%, and 90% of the population consists of cells longer than 20 μm (7).
Because feeding mechanics differ among the protozoa, different bacterial responses are elicited depending on the predator and prey combinations. Bodo saltans, a heterotrophic nanoflagellate, ingests cells of less than 3 μm in diameter (259). Its presence results in the survival of grazing-resistant filamentous cells (247, 259, 304) or of bacteria with increased growth rates so that the population can simply outrun predation (247). Individual rod-shaped cells longer than 6 μm are too large for the filter-feeding zooplankton Daphnia magna, and grazing by this predator provokes accumulation of at least five different types of bacterial filaments: long rods, curved and S-shaped cells, threads, filaments with pointed ends, and cells in chains (151). Ciliated protozoa of the genus Cyclidium have difficulty feeding on bacterial cells longer than 4 to 5 μm (301), so when these protozoa are fed a 1.5-μm-long gram-negative rod, 7% of the surviving bacterial population is 12 to 20 μm long (301). Interestingly, these filaments lack septa, as though cell division is inhibited temporarily, and when Cyclidium is removed the elongated cells are replaced by cells of normal length (301). This suggests that bacteria may filament reversibly to meet such challenges. Finally, cell filaments up to 14 μm long can be grazed by the mixotrophic nanoflagellate Ochromonas (113, 259, 375), but at only one-half to one-fourth the rate of shorter cells (375). Bacteria that survive Ochromonas predation are small, filamentous, or stellate chains of up to 900 rod-shaped cells (113). Once again, the changes are not permanent; the bacterial population returns to its original size distribution when the predator is removed (113).
An intriguing observation is that members of the commonly studied Enterobacteriaceae respond in a similar way to predation by protozoa. Acanthomoeba polyphaga ingests Salmonella enterica serovar Typhimurium, and, at a very low rate, the bacterium grows in the contractile vacuoles of the amoeba (88). The intracellular bacteria grow as filaments, with cell lengths increasing from 2 μm to 18 μm and reaching upwards of 75 to 150 or 500 μm after several days (88). The cells continue to grow as filaments even after being released from the amoeba, and they often form small microcolonies of filamentous cells (88). Analogous behavior is observed when E. coli is ingested (88). These reactions parallel those of urinary tract pathogenic strains of E. coli, which will be discussed at more length in “DIFFERENTIATION” below.
The short of it: selection for small cells.
The opposite side of the bimodal effect is the selection for cells too small to be captured by particular protists. For example, the cladoceran predator Bosmina longirostris inhales bacteria through a filter with a mesh size of 0.43 to 0.97 μm. Bacteria equal to or exceeding these lengths are captured most efficiently, but smaller coccoid cells escape, so that prolonged grazing reduces the average size of the survivors (331). Rods in the process of cell division and spirillum-shaped bacteria are captured twice as well as single cells, indicating that bacterial size selection is due to physical capture by the filtering apparatus and not by surface properties of the prey (331). The effect is not confined to filter feeders, because grazing by the mixotrophic flagellate Ochromonas also selects for cells of less than 0.9 μm in diameter (259).
If small bacteria survive more frequently when grazed by protists, then the largest advantage should go to the smallest bacteria. Evidence suggests that this is true for the “ultramicrobacteria,” defined as bacteria with a cell volume of less than 0.1 μm3 (246). For example, an ultramicro-sized species of Actinobacteria (0.2 μm by 0.5 μm) is so vibrioid and curved that individual cells may form C shapes or even closed circles (115). This tiny bacterium is grazed at extremely low rates by Ochromonas and related flagellates (25, 115), suggesting that environmental pressure may select for this whole group (115, 246).
Wide load: selection for increased diameter.
When exposed to continuous cultivation with Cyclidium glaucoma, a filter-feeding ciliate, surviving bacteria exhibit increased diameter instead of length, and no filaments or microcolonies appear (258). This unusual response implies that there is more than one method to combat protistan grazing and that cells can alter their diameters in response to such pressure.
Selection for Altered Shape
Prosthecate bacteria.
If being larger or longer protects bacteria against predation, then it is reasonable to imagine that cells with elongated prosthecae reap the same advantages with less expenditure of cell mass. In fact, a common response to protistan grazing is selection for large or complex prosthecate bacteria (150), probably because, like cell filaments, prosthecae interfere with the efficiency of the protozoal feeding apparatus.
Prosthecate cells represent up to 10% of the total bacteria in pulp mill lagoons, including species of Ancalomicrobium, Caulobacter, Prosthecobacter, Prosthecomicrobium, Stella, Hyphomicrobium, and Hyphomonas (312). The population is probably dominated by Caulobacter species, which may represent 64 to 93% of all the prosthecate bacteria (312). Although predation was not directly identified as a selective force in that work, later results suggest that it is probably a major factor in maintaining these population levels. When a mixed bacterial culture is subjected to grazing by microflagellates, the population of Ancalomicrobium, a bacterium with multiple and extremely long prosthecae (Fig. 1S), grows to more than 106 cells/ml (20). Prior to grazing, these organisms were “too scarce to be counted accurately” (20), indicating that selective removal of bacteria with other morphologies favored this new shape. The surviving Ancalomicrobium cells grow as single cells with arm-to-arm lengths of ∼4.5 μm or in chains 10 to 15 μm long (20). Here, then, is an example of the confluence of two forces favoring the same cell shape: nutrient access (discussed above) and resistance to predation.
Helices and spirals.
Becoming larger is not the only effective defense mechanism against predation, as was apparent in the first work to definitively examine the morphological effects of protistan grazing (109). Güde investigated how natural bacterial populations responded to the presence of the flagellated protist Bodo in sludge and in defined cultures (109, 114). Initially, single-celled bacteria of the Cytophaga group dominated the cultures, but after 5 days of grazing by Bodo these were replaced by spiral-shaped cells, filamentous bacteria longer than 100 μm, bacterial flocs (aggregates), and suspended microcolonies of curved rods in a capsular matrix (109). The spiral cells were grazing-resistant species of Microcyclus (now Ancylobacter) (367) which, in chemostat experiments, outlasted Cytophaga when exposed to grazing pressure (109).
An unusual but similar phenomenon is exhibited by the cyanobacterium Arthrospira, which grows as helical trichomes, i.e., long filaments of individual cells encased and aligned within a common tubular structure (224). When exposed to protistan grazing, two helical Arthrospira trichomes began the experiment growing as right-handed spiral trichomes. However, at the end of the experiments these had changed so that left-handed spiral filaments dominated (224). In fact, while grazing, the ciliate predator rotates to follow the Arthrospira spiral during feeding (224) and turns on its axis while sucking in up to six complete bacterial coils (A. Belay, personal communication). Also, the helical pitch of the spirals seems to matter, because no grazing is observed if the Arthrospira trichomes are tightly coiled (A. Belay, personal communication). These are curious and provocative findings, suggesting that spiral morphology may play a defensive role towards predator feeding. Thus, Arthrospira may reduce its susceptibility to predation by altering its spiral pitch to inhibit some natural geometric feature of the protist's ingestion apparatus.
Selection for Multicellular Complexes
Cells in biofilms or microcolonies are often more resistant to predation (203, 206, 207). For example, as mentioned above, swarm cells of Serratia liquefaciens resist predation by Tetrahymena (7). Because the normal-sized cells that first contact a surfac are most susceptible (203), elongating swarm cells may help protect against protistan grazing until the biofilm matures (7). Therefore, if particular cell shapes encourage biofilm formation (see “ATTACHMENT” above), then predation, by selecting for biofilms, may indirectly select for cells with certain shapes.
As mentioned in passing in several of the preceding examples, predation also selects for bacteria that grow as chains, as microcolonies, or in large aggregates or flocs (88, 109, 113, 151). In addition to these examples, when preyed on by the amoeba Acanthamoeba castellanii, Bacillus cells grow in large aggregates that resist phagocytosis (49). Also, a unique multicellular prokaryotic organization has recently been described in which 10 to 17 cells form a hollow spherical ball, which, among other things, may also protect the organism against predation (162). Of course, predation is only one of several factors that may prompt cells to aggregate into cellular clusters.
Size Isn't Everything
Size and shape, though probably the predominant determinants of bacterial ingestion by protists, are not the only factors that regulate predation. Bacteria capable of high-speed motility sometimes escape grazing better than their nonmotile or slower cousins (150, 205, 206, 246). In particular, the smallest, fastest bacteria escape predation more frequently than slightly larger, slower cells (205), which may help explain why marine bacteria are generally small and highly motile. This means that predation pressure should favor the survival of rod-shaped bacteria, since this is the fastest form (see “MOTILITY” above). In addition, predation may affect a cell's overall strategy of movement. Motile bacteria make more contact with the protist Spumella but often manage to escape before being ingested (204). The reason may be because instead of moving by the run-and-tumble strategy these bacteria move by the run-and-reverse strategy, which may allow them to beat a hasty retreat before being trapped (204). This will also favor rod-shaped cells.
Some protists discriminate between cells for chemical reasons, indicating that grazing selection does not operate solely on the basis of size or shape but is also affected by bacterial surface properties (150). For example, protists evidently prefer gram-negative instead of gram-positive bacteria. Gram-positive cells are consumed at much lower rates than are gram-negative cells (138, 246), and heterotrophic nanoflagellates actively avoid grazing on gram-positive actinobacteria (246). The most likely reason is that it takes significantly longer to digest a gram-positive cell than a gram-negative one (99, 138, 246). Usually only one or a few bacteria are ingested, so longer digestion times put the predator at a disadvantage because it cannot handle more prey until the previously ingested material is consumed or expelled. Consistent with these ideas, protistan grazing results in the accumulation of gram-positive bacteria (246), though gram-negative bacteria are not eliminated completely because predation is only one of many selective forces. It is not yet clear whether both types of bacteria can produce the same morphologies. Therefore, in addition to its effect on bacterial size, predation may affect cell shape indirectly by selecting for bacteria having specific types of walls.
Phage Effects
Posch et al. surmise that “feeding on bacteria by flagellates and ciliates perhaps represents the oldest predator-prey interaction we can study in nature” (258), but others speculate that prior to protistan grazing “viruses were probably the main predators of cells in the prokaryotic world” (361). What is fairly certain is that the two predators exert different selective pressures. Protists are mostly omnivorous, meaning that they devour all bacteria in a certain size range, so protistan predation seems to limit the total abundance of bacteria (246). On the other hand, viruses usually have a narrow host range and are therefore more likely to affect overall prokaryotic diversity (246).
Bergh et al. first reported the extremely high concentrations of bacteriophage in aquatic environments, up to 2.5 × 108 virus particles per milliliter, leading them to conclude that “virus infection may be an important factor in the ecological control of microorganisms” (18). Since then, the contribution of virus-mediated lysis of marine bacteria has been estimated to be approximately equal to the bacterial mortality contributed by protistan grazing (83), although at high bacterial concentrations death by phage infection may prevail (360). Also, bacterial populations may experience different effects at different depths in the water column. In one eutrophic lake, approximately three times as many bacteria were killed by flagellate grazing than by phage infection in the epilimnion (the upper 5 m, where the water is oxygenated and warm) (358). However, in the hypolimnion (depths below 10 m, where the water is anoxic and cold), viral lysis of bacteria was estimated to be ∼10 times greater than that produced by grazing (358). Taking into account many similar studies, Suttle concluded that the best estimate of the effect of virus-mediated bacterial mortality is that 20 to 40% of marine bacteria are lysed each day, a value thought to be roughly equivalent to that of grazing by zooplankton (318). However, these and all previous estimates leave room for doubt, because the approaches for estimating virus-mediated microbial mortality “suffer from poorly constrained assumptions” that leave our knowledge “not much further ahead than a decade ago” (318).
Two recent reviews summarize the status of virus contributions to ocean ecology, including their effects on bacterial diversity and evolution (318, 361). These do not address specifically how virus pressure might affect bacterial cell shape, but they do include interesting discussions on the contributions of transduction and lysogeny to bacterial speciation (318, 361). The most clear-cut view of the effects on aquatic bacteria is that viruses “kill the winner” (326). That is, bacteriophages infect the most numerous bacterial species (because they are the dominant target) and thereby reduce these populations (326). This situation creates an evolutionary pressure for producing and maintaining the existence of many different bacterial species or strains as opposed to allowing one to gain a monopoly. Thus, marine phages create a pressure to diversify (326).
Granted that phages have a significant impact on prokaryotic numbers and seem to drive speciation and diversity, do viruses have any effect on bacterial cell shape per se? The major effect seems to be that phages tend to select for reduced bacterial size (359). In surveying bacterial populations in lake waters, Weinbauer and Höfle found that the percentage of small bacteria in a population increases when phages are present (359). In addition, phages come into contact with, attach to, and lyse larger cells more frequently than smaller cells, so that smaller cells are infected at a rate of only 23 to 26% of that of larger cells (359). Small size also spreads bacterial biomass among more individuals, so if one cell is infected and dies, the loss in overall biomass is smaller than it would be if each cell were larger. This will be especially true for populations of filamentous organisms, which will lose more biomass per infection than will smaller cells. These considerations led Weinbauer and Höfle to predict that “viral lysis might be one of the mechanisms keeping cell size small in natural systems” (359). Therefore, phage pressure appears to operate in concert with low-nutrient environments and protistan predation to enhance the selective forces favoring small bacterial sizes.
Predatory Prokaryotes
The final predator class is composed of other bacteria. Free-living prokaryotes prey on bacteria by at least three major mechanisms. Epibiotic predators (e.g., Vampirococcus) attach to and remain at the surface of their targets and suck the cytoplasm from the host; periplasmic predators (e.g., Bdellovibrio) burrow into the periplasm of gram-negative bacteria, extract the intracellular nutrients, and leave after lysing the cells; and cytoplasmic predators (e.g., Daptobacter) penetrate all layers of the gram-negative envelope to grow and divide in the host's cytoplasm (110). The effect of these predators on bacterial size or shape is unknown, with little or no work being done on the subject. However, given the effects that viruses and protozoa exert, this third class of predators may also influence bacterial morphology.
There is one curious reversal of the predator-prey shape interaction. Though bdellovibrios are normally host-dependent predators, host-independent mutants that grow slowly in rich media in the absence of prey can be isolated. When parasitizing E. coli, these strains exhibit a normal, uniform morphology (either straight rods or vibrioids), but when grown in medium alone, the mutant cells are misshapen, appearing as filaments or as curled or spiral-shaped cells (181; R. E. Sockett, personal communication). Thus, some unknown component of the prey selects for or triggers the generation of uniformly shaped bdellovibrios. So, one way or another, this relationship deserves a more thorough investigation regarding its effects on bacterial morphology.
Transcellular shape attack.
There is an old joke about two guys being chased by a bear. Says one, “We'll never outrun him!” Says the other, “Who cares? I only need to outrun you!” The moral is that it is not necessary to kill your competitor; it may be sufficient to cripple him so you can outrun him. In an analogous vein, if shape imparts selective benefits, then modifying the shapes of other organisms might provide a competitive advantage. For example, as mentioned in “DISPERSAL” above, Lactococcus lactis regulates the extent of its own chaining by elaborating a peptidoglycan hydrolase, the AcmA protein (213). Interestingly, this hydrolase also cleaves the septal peptidoglycan of Streptococcus thermophilus, completely disassembling its cell chains until all that is left are individual cells (213). These S. thermophilus cells are not dead, just lonely. Thus, this cross-reacting enzyme may prevent a potential rival from forming chains, sedimenting, and competing for an anaerobic ecological niche (213). Many organisms synthesize a variety of autolysins that hydrolyze their own peptidoglycan wall. But instead of acting exclusively on the wall of the originating organism, perhaps some autolysins double as secretory factors that alter the gross shape or organization of other bacteria. In an environment where chaining provides a selective advantage, dismantling your competitor could be very useful.
An even more dramatic example of transcellular shape attack is provided by the gram-negative bacteria Pseudomonas aeruginosa and Xanthomonas campestris pv. Campestris. P. aeruginosa binds to filamentous forms of the fungus Candida albicans, and the resulting bacterial biofilm kills the eukaryote (127). What does this have to do with cell shape? First, P. aeruginosa approaches pole first to attach to the fungal filament, suggesting (again) that polar differentiation is important for attachment. Second, C. albicans grows either as a yeast (ovoid) or as a mycelium (short filaments). P. aeruginosa discriminates between the two forms, binding to and killing only filamentous cells (127). In addition, P. aeruginosa secretes a homoserine lactone that inhibits growth of the filamentous form of C. albicans, thereby acting as an external regulator of fungal morphology (128). Similarly, X. campestris secretes a factor that inhibits germ tube formation in C. albicans, which is the first stage in transitioning from the yeast to the hyphal form (352). These responses by C. albicans are probably gambits to avoid becoming prey, a defensive strategy that allows the fungus to continue growing in its yeast form in the presence of certain bacteria (128). In any case, the state (shape) of C. albicans determines whether it is susceptible or resistant to bacterial attack, and this morphology is coregulated by both organisms. Of course, the surface receptor to which P. aeruginosa binds may be associated only with the filamentous form of C. albicans, so shape per se may not be the targeted factor. Nevertheless, this is another example where bacterial activity affects the morphological status of a second organism. The genesis of these surprising and (so far) unexplained relationships is uncertain. Did the bacteria take advantage of a preexisting dimorphism in C. albicans, or did bacterial attack provoke the fungus to adopt its dimorphic lifestyle?
Summary
The selection of filamentous bacteria “seems to be a phylogenetically widespread mechanism for resisting protozoan predation” (151), as is the selection for smaller cells. Although cell length is not an absolute defense against predation, the differences in grazing rates are enough to confer a selective advantage on longer and shorter cells. The mechanism may entail a true and reversible induction of short cells, filaments, or chains, or predation may simply select subpopulations that are already present (150). Bacteria that can extend long prosthecae or coalesce into multicellular complexes also survive protistan grazing. In addition, recent evidence indicates that some bacteria actively control not only their own shapes but also that of other microorganisms. Similar predatory or antagonistic relationships may have eluded our attention. Finally, the nature and extent of the bacterial responses vary with the types and numbers of predators in the environment, including protists, phages, and other prokaryotes (259). Prior to these pressures of predation, perhaps giant microbes once ruled the seas even as dinosaurs once ruled the land!
DIFFERENTIATION
During the early 1900s, microbiologists debated with “an intensity not seen since the arguments over spontaneous generation” about whether bacteria were pleomorphic and had complex life cycles (347). Pleomorphists believed that individual bacteria could change shape dramatically and might adopt any number of elaborate forms; monomorphists believed that each bacterium maintained one unambiguous morphology (347). Much of what the pleomorphists believed was, indeed, untrue, such as the idea that bacteria were part of the life cycles of some fungi (347). Similar now-outlandish claims caused most pleomorphic ideas to give way to the mindset that “apart from minor variation, each bacterial cell is derived from a cell of practically the same size and shape … with only occasional, slight variation” (347). However, oddly enough, the pleomorphists can now claim several victories. Individual bacteria can and do change shape, and some display a morphological progression during their life cycles. Many of these alterations are under explicit genetic control and are associated with important physiological phenotypes. But in most cases, we, the new and more relaxed pleomorphists, still do not know what stimulates these changes or why they are useful. This section resurrects a subset of the pleomorphic viewpoint—that bacteria change shape, often at will and in definite patterns—and addresses the question of why they do.
Asymmetric Division
Although most Alphaproteobacteria lead unremarkable lives without conspicuous morphological variation, Caulobacter crescentus routinely divides so that its two daughter cells are differentiated: one stalked and sessile, the other flagellated and motile (33). However, Hallez et al. demonstrated that at least three other bacteria, i.e., Brucella abortus, Sinorhizobium meliloti, and Agrobacterium tumefaciens, tend to divide asymmetrically, giving rise to daughter cells of unequal length in a process they believe may represent a bona fide, widespread, primitive form of differentiation (116). Since the CtrA regulatory system that controls cell division in Caulobacter is conserved among the Alphaproteobacteria, Hallez et al. speculated that the system may control asymmetric division in other members of the group (116). If dividing asymmetrically confers a survival benefit, then these results suggest that exceedingly small shape differences are significant.
Stationary Phase
Rod to coccus.
One of the simplest bacterial differentiation events is the transition from growing as a rod-shaped cell during exponential phase to growth as a coccus upon entry into stationary phase. The phenomenon is quite prevalent. E. coli becomes smaller and more spheroidal in stationary phase (142, 182), Arthrobacter transitions to cocci at growth rates characteristic for each species (192), Acinetobacter undergoes a two-stage rod-to-coccus differentiation (142), and Rhodococcus equi grows in early exponential phase as rods or Y-shaped bifids but becomes coccoid with prolonged incubation (84, 100). These transitions appear to be due to reductive division (continuing division in the absence of an increase in cell mass) (100, 142) and are not accidental but are under explicit genetic and physiological control. In E. coli, the change is regulated by the BolA protein and the RpoS sigma factor, and mutants lacking these proteins remain rod shaped in all growth phases (142, 182, 285). Similarly, three mutants of Arthrobacter crystallopoietes exhibit defects in the normal rod-to-sphere transition: one grows only as spheres, another only as short rods, and the third as branched rods (1).
The fact that this morphological transition is widespread and under active genetic control suggests that bacteria realize advantages in being coccoid (142). Cells may be responding to a change in their nutritional status, because stationary cells diluted into a high-nutrient medium return to rod shape whereas cells diluted into a low-nutrient medium remain coccoid (142). Consistent with such an interpretation is the fact that the morphology of Arthrobacter can be manipulated by altering its growth rate in glucose-limited chemostat cultures (192). Specific physiological changes may accompany the coccoid form even if shape per se is not responsible for the characteristics. For example, in Acinetobacter, stationary-phase cocci attach firmly to surfaces, but exponential-phase rods attach less well and detach more often (142). Finally, becoming coccoid may make the cell a smaller target, reducing the likelihood that any single bacterium is inactivated by chemical or physical assault. For a given biomass, a population of smaller cells means that environmental challenges are distributed over more individuals, increasing the possibility that some will survive. Unfortunately, especially for such a common phenomenon, the exact reasons for making these shape changes remain ill defined for any particular organism.
Rod to filament.
Because reality is messy (or at least confusing, from our point of view), the opposite transition also occurs: instead of becoming smaller, some bacteria filament in stationary phase. The most dramatic example is Caulobacter crescentus, whose cells transform from short curved rods to helical filaments of up to 30 μm in length after several days in stationary phase (373). Thiomicrospira thyasirae, a gram-negative bacterium inhabiting the gills of a marine bivalve, is pleomorphic, growing as a combination of straight, bent, and spiral cells of various lengths (372). In early exponential phase the cells are straight or slightly bent rods from 1 to 2.3 μm long, but in stationary phase the cells elongate into spirals from 5 to 15 μm in length (372). Similarly, in exponential phase Campylobacter jejuni grows as spiral cells that elongate into straight filaments, which are four times as long in late stationary phase (107, 327). The latter forms coexist with residual coccoid cells (107, 119, 187, 233, 327). Whether this elongation phenomenon is more prevalent in spiral and vibrioid bacteria remains to be seen.
Bifids: Two Heads Are Better than One
“Bifid” is the general term for a Y-shaped cell that has club-like protrusions or very short branches at one or both poles (13). The morphology is quite common, occurring regularly or in response to environmental cues in many bacteria. This curious differentiation appears to be more than accidental because it is a part of the normal life cycle of several organisms (as discussed below with regard to the symbiotic nitrogen-fixing rhizobia) and also because the forms can be induced by specific treatments.
From 1917 to 1920, E. C. Hort reported that several colon bacteria reproduced “by budding and by producing Y-shaped and large aberrant forms” (347). These were probably members of what we now know as the bifidobacteria, a class of gram-positive bacteria found especially in the human gastrointestinal tract and vagina and which are noted for the prevalence of bifid forms. In fact, the genus Bifidobacterium is marked by a pronounced pleomorphism within and among its species (13). One strain grows as an interwoven filamentous mat on top of the agar surface, with the mat being composed of filaments, short rods, curved rods, bifids, and branched rods (13). Other species have even more curious, subtle modifications, such as B. bifidum, whose poles are abnormal in that they are not semihemispherical but instead taper smoothly to a rounded point (like the “sharper” end of chicken egg) (13). Other species, such as B. longum, produce bifids but also divide asymmetrically so that one daughter is a rod and the other coccoidal (13).
Although bifid forms are observed most often in gram-positive bacteria, they also occur in gram-negative organisms. A member of the Alphaproteobacteria isolated from marine sponges, and whose closest relative is Roseibium denhamense, is one of these rarities—producing numerous cells with true and distinct Y-shaped branches and others with bulbous ends (240). Also, an aerobic anoxygenic phototrophic bacterium is quite pleomorphic, growing as cocci, rods, and branched cells that have clearly differentiated Y-shaped ends (377).
The bifid morphology is more than a curiosity, because, at least in a few cases where the question has been asked, formation of the structure is under genetic and physiological control. For example, Bradyrhizobium japonicum, one of the rhizobia, undergoes a natural morphological change to bifid-shaped bacteroids within plant cells (see discussion below). The relevant point here is that adding succinate or fumarate to in vitro cultures induces a differentiation event in which ∼90% of B. japonicum cells swell and branch (267). The response is substrate dependent and regulated, because strains lacking succinate dehydrogenase do not progress through the cycle (267). In an analogous reaction, in the presence of certain univalent cations or sodium salts, Lactobacillus bifidus produces a prolific number of bifids and branched cells (172). Finally, several mutants of E. coli form multiple bifid-shaped cells (4, 50, 230, 376). All these examples indicate that bacteria exercise some control over bifid formation, implying that the morphology is more than accidental.
It seems such a minor alteration, this creation of “two heads” at one pole, but so far its purpose remains perplexing. The characteristics of a bifid should differ very little from those of a rod, except for the fact that one end is bifurcated. Exactly why is this advantageous? Earlier, I summarized evidence that contemporary prokaryotes are actually quite balkanized in the way that proteins are distributed around the cell (see “POLAR DIFFERENTIATION” above). In particular, many multisubunit, transenvelope, or trans-cell wall complexes localize to the cell poles, perhaps because the peptidoglycan in these areas is inert and provides a stable environment for complicated structures. So, if environmental circumstances require that cells have more of these structures, perhaps the only way a cell can increase their number is by increasing the number of poles (or polar material) per cell. The bifid form fits the situation perfectly because it carries an extra pole with but little increase in cell mass. As far as I know, no data support this supposition. But now that we know some of the proteins and structures that localize to the poles, perhaps we may finally address the question experimentally.
Swarming: in Serried Ranks Assembled
Cells may swim alone, but some prefer to swim in large, coordinated groups called swarms. Swarming is a well-studied morphological differentiation in which cells transform from short vegetative rods into “multinucleate, aseptate, profusely flagellated, highly elongated cells” that quickly migrate across surfaces (117). The swarmers are usually 5 to 20 μm long but may reach 200 to 300 μm (79, 117). The phenomenon can be pictured as a herd of individual cells that align themselves with one another along their long axes to form broad, two-dimensional rafts that move by coordinating the flagellar motions of the entire group (79, 122). Whereas normal-sized individual cells move in a relatively random walk, tightly packed and elongated swarm cells migrate farther and faster and in straighter lines. Swarming cells move rapidly over solid surfaces, initiate biofilm formation, and adsorb to and colonize specific environmental niches; the phenomenon is also associated with virulence (5, 118, 122). The behavior is best known and most studied in Proteus mirabilis (79, 122), but several other organisms also undergo this distinctive differentiation process, including E. coli (118), Salmonella enterica serovar Typhimurium (118), Serratia marcescens (241), Vibrio parahaemolyticus (209), Bacillus subtilis (149, 160), and Clostridium (117, 125).
Out of shape.
All swarmers form rafts of side-by-side cells. These are not random cell aggregates but are, in the case of Bacillus subtilis, palisades of four or five cells with their poles “rather precisely aligned” (149). The importance of this arrangement is underscored by the fact that cells unable to form or to join a raft do not swarm. For example, isolated B. subtilis cells are immobile unless they merge into an existing raft (160). The counterexample is that common laboratory strains of B. subtilis are swarming deficient, probably because they cannot form rafts (among other deficiencies) (160). More to the point, isolated cells of lab strains do not elongate and cannot join wild-type motile rafts (160), suggesting that an attachment interaction is missing.
A specific bacterial shape is essential for the creation, survival, and function of swarming-proficient rafts. The best evidence for this comes from the behavior of the Proteus mirabilis ccmA mutant, which differentiates into swarmer cells but cannot swarm (122). The only apparent difference is morphological. The presence of a truncated ccmA gene produces curved, almost C-shaped cells of uneven diameter that cannot align parallel to one another in the closely packed side-by-side arrays required to form multicellular rafts (122). Presently, the ccmA gene is found only in Proteus and the mechanism by which CcmA affects cell shape is unknown, although expressing the protein in E. coli and P. mirabilis causes these organisms to become large ellipsoids (122). Nevertheless, the implication is that only uniformly shaped rod cells can self-associate to form rafts, thus creating a selective pressure for cells to be rod shaped, to be uniform, and to regulate their length.
Shape regulation.
Normally, swarm cells elongate dramatically before they assemble, and the easiest way to elongate is to inhibit the septation stage of cell division. Vibrio parahaemolyticus does exactly this, reducing its rate of septation to become 30-μm rods (209). This differentiation is transient, and the return to normalcy requires the activity of the lonS gene product, which is 81% identical to the E. coli Lon protein (316). The Vibrio LonS protein complements the phenotypes of an E. coli lon mutant, including the ability to degrade the cell division inhibitor SulA (316). Thus, swarmer cell elongation in V. parahaemolyticus requires the production (and later degradation) of a specific cell division inhibitor (316). Partial inhibition of cell division also leads to filamentous swarming cells in B. subtilis (149). In addition, B. subtilis cells grow longer as they are surrounded and move toward the centers of their rafts, implying that the division cycle is regulated differentially depending on the position of cells in the swarm community (149). Here, then, is at least one dimension of cell shape (length) that is under direct developmental control. The combination of inhibitor and proteolysis represents a simple mechanism of shape control, promoting reversible filamentation, and is essential for creating optimum swarming shapes.
Heterocysts
Species of the cyanobacterium Anabaena fix nitrogen by forming heterocysts, differentiated cells that provide an anaerobic environment in which the nitrogenase enzyme can function (96, 211). Usually, Anabaena grows as filaments of identical cells, except for the occasional heterocyst. But in medium lacking nitrate the cell chains are shorter and slightly twisted, the individual vegetative cells vary in size and shape, and abnormal heterocysts appear (188). Thus, the presence of nitrate affects gross cell morphology in an undetermined manner. More interestingly, Anabaena mutants lacking PBP 2 cannot make heterocysts and so cannot fix nitrogen (188). PBP 2 is a class A PBP, a group of enzymes involved in the synthesis and cross-linking of the peptidoglycan cell wall (95, 256). Also, in Anabaena strain PCC7120, a mutation in the hcwA gene inactivates the heterocyst pathway (379). The HcwA protein is a putative N-acetylmuramoyl-l-amidase which removes short peptide side chains from glycan polymers of cell wall peptidoglycan. The existence of these mutants implies that peptidoglycan modification is required for heterocyst maturation (379). The situation begs the question of whether it is the change in cell shape or some other aspect of peptidoglycan structure that is required for heterocyst formation and nitrogen fixation. Perhaps adjusting the cell wall creates or preserves the essential anaerobic conditions within the heterocyst. It is, however, intriguing that a shape change always precedes nitrogen fixation, whether in Anabaena or in the symbiotic rhizobia (discussed below). Sadly, without mutants deficient only in cell shape, we cannot know if the shape changes are selective, secondary, or superfluous.
Pathogenesis-Associated Differentiation
We humans have such a high opinion of ourselves that we have reserved a special name for organisms that grow in or on us: pathogens. Except for the damage we ourselves sustain, this is just another example of the more general phenomenon of organisms fitting themselves to an environmental niche by adaptation and differentiation. Thus, the principles described in the preceding sections apply here as well, with the added dimension that the immune system plays a selective role.
Fungal Pathogenesis and Differentiation
The fungi are renowned for their morphological alterations. For these eukaryotes, no one doubts that there is a distinct coupling of shape with pathogenic potential (37, 103, 231, 273, 276). Because of this, I want to summarize a few characteristics of one organism, the opportunistic pathogen Candida albicans, as an abbreviated example of how microbial shape affects pathogenesis. The idea is that if the fungi can take advantage of morphological variation, then the bacteria may profit from similar strategies.
C. albicans grows as a yeast at normal environmental temperatures and as a filamented, hyphal form at 37°C in a mammalian host. Deletion of a single protein, CaHsl1p, promotes the growth of the hyphal form of C. albicans and reduces virulence (332). On the other hand, the product of the SSN6 gene is required if C. albicans is to form true hyphae, and mutants lacking this gene grow as foreshortened pseudohyphae that are also less virulent (136). Thus, C. albicans actively controls and optimizes its morphology and virulence and does so in response to its external environment (332). One mechanism underlying these structural modifications may be the localization and function of the Tsa1P differentiation protein, which affects cell wall assembly and morphogenesis, perhaps by helping other proteins integrate into the wall (333). C. albicans is not unusual in these respects, because similar genetic and physiological accounts can be given for other pathogenic fungi (103, 169, 276). Although shape changes are clearly associated with virulence, the reduced pathogenicity of any particular mutant cannot yet be attributed to shape alone (231, 276), leading some to conclude that there is still “no unequivocal demonstration” (37) of an explicit cause-and-effect relationship between fungal morphology per se and virulence (103, 276).
What is needed to answer questions about the role of cell shape in pathogenesis is a system that manipulates microbial morphology without altering other physiological parameters. For fungi, this ideal was approached most closely by Saville et al., who controlled the morphological state of C. albicans by using an inducible filamentation suppressor (134, 286). After infection with the yeast form of C. albicans, mice died only if fungal filamentation was triggered by injecting the animals with the inducer doxycycline (286). However, the mice survived if they were infected with the yeast form but without subsequent filamentation, even though the livers of these mice were just as packed with fungal cells as were the livers of those mice that died while harboring the hyphal form (286). Although the morphological transition is obviously important, the question remains as to whether it is essential. Is the difference in virulence caused by the presence of hyphae per se, or is heightened virulence associated with a parallel induction of other factors?
Shape Discrimination by the Immune System
An independent clue that morphology plays an important role in microbial pathogenesis is that the mammalian immune system discriminates among cells with different shapes. For example, dendritic cells can detect the yeast or hyphal form of C. albicans and invoke different immune reactions depending on which is present (60). Perhaps activation of different receptors triggers alternate pathways for internalizing the fungus, or perhaps the host does not detect dimorphism per se but responds to distinctive surface antigens on each morphotype (274). In either case, shape plays a role.
Recently, the importance of morphology with regard to the immune system was highlighted more dramatically in experiments by Champion and Mitragotri (39). Those authors created microscopic polystyrene particles of six different shapes in various sizes, fed them to alveolar macrophages, and found that shape, not size, was the dominant factor in determining whether particles were phagocytized (39). Macrophages cannot know in advance the total size or volume of the particle with which they come into contact. Instead, the cells apparently gauge the local shape of an external entity by creating an actin cap at the point of first contact between particle and phagocyte (39). The angle created between the macrophage membrane and the particle determines whether the cell initiates phagocytosis or simply spreads over the surface of the particle, with phagocytosis ceasing when a critical angle of attachment is exceeded (39). Champion and Mitragotri cautioned against extrapolating these results directly to biological targets, but the implications are profound: particle morphology is sufficiently vital that the immune system has devised means of detecting subtle differences among shapes. The natural conclusion is that what the immune system can detect bacteria may exploit, by adopting those shapes most advantageous to their survival. Some of these possibilities are examined in the next section.
Bacterial Pathogenesis and Differentiation
Numerous bacteria have made the transition from being intracellular parasites of freshwater amoebae or other protozoa and have become intracellular parasites of phagocytic cells of the immune system (106). The tricks and traits accumulated by continuous selection in the wild have been successfully adapted for survival in the mammalian environment (106, 219). One of these characteristics is morphological. In several pathogens, biochemical changes during their developmental pathways are accompanied by alterations in cell shape. As explained above, this does not make shape per se a virulence factor, because the morphological changes may be secondary or superfluous. However, the prevalence of comparable shape changes in diverse organisms suggests that morphology may be important either by itself or because certain shapes enhance specific biochemical functions. A few examples are presented to highlight the far-reaching impact this trait may have.
Uropathogenic Escherichia coli.
The contribution of cell shape to virulence is best exemplified by uropathogenic E. coli, which progresses through a distinct cycle when infecting superficial bladder epithelial cells in mice (153, 225). Infecting cells change from nonmotile rods to cocci to motile rods, and they finally grow as filaments. These latter forms resist phagocytosis by polymorphonuclear leukocytes and thus promote infection, and the eruption of filaments from a previously infected host cell permits bacteria to infect neighboring epithelial cells (153). This reaction is particularly clever. Normally, the host responds to an E. coli infection by exfoliation, i.e., sloughing off infected epithelial cells and removing whole colonies of the pathogen (225). E. coli, in its turn, responds by elongating to as much as 50 μm and exiting the originally infected epithelial cells (225). This strategy allows E. coli to stay in contact with one host cell, enjoying its nutrients and protection, while searching for, finding, and entering nearby cells (225). Thus, this simple shape change, from rod to filament, both protects and disperses. The mechanism is simple, as well. Elongated bacteria have irregular or partial septa along their lengths, implying that control of cell division contributes to maintaining the infection (225). In fact, filamentation requires the activity of the SulA protein, an internal septation inhibitor (152; S. Justice, personal communication). A similar mechanism may be at work in Mycobacterium tuberculosis, which also elongates after being phagocytized (40). Interestingly, the host's Toll-like receptor 4 response triggers E. coli filamentation, suggesting that the bacterium detects and responds to specific host signals (153).
Outside a mammalian host, and like Legionella pneumophila and S. enterica serovar Typhimurium (76, 77), E. coli can grow as an intracellular parasite within amoebae (88). This survival of elongated cells is similar to the way bacteria combat protistan predation (see “PREDATION” above). Because the same strategies should work in the mammalian environment, it is not surprising that bacteria employ them during pathogenesis.
Legionella pneumophila.
L. pneumophila causes a particularly serious opportunistic pneumonia in humans (75, 320) and produces at least two morphologically different forms during the course of entry, growth, and exit from its natural amoeba hosts (280). One form—a stubby, thick-walled, pleomorphic, and highly infectious cell—appears only after prolonged intracellular growth (70, 87, 219). In fact, L. pneumophila is extremely pleomorphic, and many of these shape changes accompany physiological changes during its life cycle (70). The organism adopts at least eight different forms, three in vitro and five in vivo, including rods, cocci, filaments, and a form created by “fragmented” cell septation (70). Because the sequence of morphological changes is conserved during infection, Faulkner and Garduño consider the modifications to be part of a true developmental cycle (70).
Listeria monocytogenes.
L. monocytogenes, a gram-positive, food-borne pathogen, causes life-threatening systemic or localized disease (64, 105, 260) and moves within and between host cells by pushing itself forward on an actin tail that is polymerized from one pole (64). A mutant lacking the p60 protein is less virulent, forms abnormal septa, and is morphologically impaired, growing as short filaments that have a terminal hook (bent pole) and are severely deficient in intracellular motility and intercellular spreading (250). The reason is that, instead of being concentrated at one pole, the ActA protein, which induces actin polymerization, is distributed unevenly around the cell body so that the mutants cannot form a directional actin tail (250). p60 is a putative peptidoglycan hydrolase (250), perhaps an ld-endopeptidase (27), which implicates cell shape, division, or peptidoglycan structure as being important for the motility mechanism. Regardless of the final molecular details, it seems that a uniform rod shape is important for virulence and motility in this organism.
Helicobacter pylori.
When incubated on blood agar plates H. pylori grows as short spiral rods, but when grown in broth the bacterium produces long spirals containing 5 to 20 turns (71). These changes may occur naturally in gastric microenvironments, because corkscrew forms of H. pylori sometimes appear in biopsies (343). The shape alteration is reversible, making it likely that some infections previously considered to be caused by a separate species, Helicobacter heilmannii, are in reality examples of filamentous H. pylori (71). Significantly, H. pylori does not express different immunoreactive proteins after it filaments (343), which suggests that the shape change itself may be important (instead of simply being associated with a new constellation of proteins in the filamentous state).
Campylobacter jejuni.
C. jejuni adopts variable forms: spirals, S shapes, commas, doughnut shapes, and dimpled cells (233). However, a “highly contentious” (327) disagreement exists over whether these shapes represent transitional or differentiated forms arising in response to environmental stress (107) or whether they are merely “old, inactive and degenerate” forms on their way to cell death (119, 187, 233, 327). Needless to say, their participation in virulence is under debate.
Conclusions.
In the fungi, there is a tight association between morphology and virulence and between morphology and the expression of specific proteins. In these organisms, cell shape is either a virulence factor in its own right or else provides the appropriate setting for the expression and activity of more classic virulence factors. That morphology contributes directly to virulence is implied by the ability of the vertebrate immune response to distinguish among microscopic shapes. As for the bacteria themselves, shape is definitely important during the infectious cycle of uropathogenic E. coli, and morphological changes attend infections caused by other pathogens. Though little information is available for these latter organisms, some data imply that cell shape itself plays a role in virulence. Nevertheless, what the field needs are experiments that can disentangle shape effects from other physiological adaptations.
Bacteroids: Plant-Microbe Symbiosis
In nature, bacteria do not live in isolated colonies. Instead, they consort with a variety of microorganisms and surfaces, each likely to impose its own requirements and selective forces. Cell shape appears to be an integral parameter in some of these multicell associations.
Rhizobia infect the roots of certain leguminous plants, forming nodules filled with differentiated bacterial cells (bacteroids) that fix nitrogen (98, 200, 238, 245, 319). As they develop, and depending on the bacterium-plant combination, bacteroids assume diverse shapes in a fairly linear set of stages: they may enlarge up to four to seven times their normal size, grow as distorted Y-shaped cells, branch repeatedly, or be pear shaped, swollen, rounded, or perfectly spherical (238, 319). In alfalfa, Rhizobium meliloti undergoes a stepwise differentiation into five types of bacteroids, progressing from rod-shaped cells to elongated rods to multiple pleomorphic forms (200, 339). Even greater polymorphism occurs in other bacterium-plant systems (102, 200). Although many of these disparate shapes have been known since the hand-drawn figures produced by Beijerinck in 1888 (238) and although the morphological alterations correlate with the development of nitrogen fixation (339), the two phenomena have yet to be connected in a definite cause-and-effect sequence. Nonetheless, some data strongly imply that morphological change may play an important role in these processes.
First, the size, shape, ultrastructure, and number of bacteroids within nodules are determined in large part by the plant being infected (200, 319). Different bacteria may exhibit the same morphology within nodules of a given host, and one bacterium may take on different, plant-specified morphologies in different hosts (200, 319). For example, one set of bacteroids are rod shaped when hosted by Vigna mungo but grow as large cocci when hosted by Arachis hypogaea (319). Similarly, one species of Rhizobium forms rod-shaped bacteroids in Phaseolus angularis but spherical bacteroids in A. hypogaea (319). Also, plant signals from Rauwolfia induce miscellaneous shapes in the cyanobacterium Nostoc muscorum, including giant cells (9- to 24-μm-diameter spheres), minicells (0.7- to 1.5-μm-diameter cocci), and protoplasts with defective cell walls (101). In the case of Rauwolfia, it is not clear if these are true morphological stages or if they are secondary features associated with tissue invasion. However, the shape changes do correlate with cyanobacterial survival (101). In any case, a substantial portion of bacteroid development is triggered and directed by chemicals from the host plant, which therefore control bacterial morphology and/or cell division (146, 200, 238, 319). If bacterial shape is important enough that the host plant devotes resources to modifying it, then shape may be a significant element in the overall process.
A second evocative body of evidence is that interfering with bacterial cell wall synthesis affects bacteroid development, function, and metabolic exchange with the host (146, 319). Mutants resistant to antibiotics that inhibit cell wall synthesis are often unable to exit infection threads or form differentiated bacteroids (319). The fact that these mutants do not respond to specific plant signals suggests that a link may exist between morphological change and the successful completion of bacteroid development. There is also the question of why so many bacteroids are bifid shaped. Perhaps interactions between plant and bacterium are enhanced by protein complexes localized only to the bacterial poles, such as dedicated secretion portals or import gateways (see “Bifids: Two Heads Are Better than One” above). This question, at least, could be approached by determining the locations of important symbiotic factors.
Contemporary rhizobia fix approximately 50% of all nitrogen on earth, and prior to human agriculture this value may have been greater than 90% (85). If morphological changes are critical to the life cycle of these organisms and to nitrogen fixation by plants, then bacterial shape may be truly vital to life on the planet.
Unusual Symbioses
The rhizobia represent the best, most deeply understood symbiotic relationship. But there are many others, some of which may also rely on morphological traits of the microbial partners.
In a sulfidic marsh near Bavaria, Germany, a rod-shaped, filament-forming bacterium related to Thiothrix unzii attaches to surfaces via a holdfast at one end (184, 218). The novelty is that this organism traps an archaeal coccus within spheres of intertwined filaments, as though the cocci were embedded in balls of twine (218, 281). Alternating sets of these archaea-containing globules and connecting Thiothrix filaments form a microscopic “string of pearls,” and the entire assemblage extends up to 15 cm into a flowing stream of water (281). Both organisms are exposed to nutrients, both service one another metabolically by generating a local sulfur cycle (218), and the archaea cells are not washed away. The importance of shape here is not complicated; the filamentous nature of the bacterium retains the coccus in close proximity and extends the coupled pair into nutrient-rich water.
The cyanobacterium Phormidium is a common component of microbial mats in the North Sea. Individual cells are slightly rod-like, measuring ∼3 μm in diameter and ∼4 to 4.5 μm in length, but these are arranged in filamentous trichomes of up to hundreds of micrometers long (32). Eventually, a few trichomes intermix and assemble into a circle, which attracts other trichomes that weave themselves into the group to create the shell of a spherical ball that reaches a diameter of 1 to 3 mm (32). This hollow sphere traps diatoms and bacteria which grow and divide in the inner cavity, and the whole symbiotic collection exists for as long as 10 weeks before the sphere decays and releases the captives (32). The entire process is unique, including the coordinated movements of the initial trichome-trichome interactions and the resulting three-dimensional structure (32). The point here is that the rod-and-filament shape is necessary to construct the spherically woven skin of the complex.
Multicellular Interactions
If morphology is important in interactions between bacteria and their symbiotic partners, then it is reasonable to expect that cell shape should be important in other multicellular relationships. Some relatively simple connections between daughter cells and among organisms in biofilms have been discussed above (see “Cell-Cell Interactions” in “ATTACHMENT”), but more-complex relationships also occur.
Fruiting bodies.
Bacterial shape plays a unique role in cell-to-cell signaling in Myxococcus xanthus. When starved or exposed to certain chemicals, M. xanthus cells aggregate into dramatic, macroscopic, multicellular fruiting bodies, within which some cells differentiate from rods into spherical, heat-resistant spores (191). One of the principle proteins in this process is “C-signal,” whose functions have been reviewed in detail elsewhere (148, 154-156, 306, 307). At intermediate concentrations C-signal increases the gliding motility of M. xanthus and reduces the frequency of stops and reversals, and at high concentrations C-signal induces sporulation (155, 156, 306).
Unlike soluble quorum-sensing molecules, C-signal remains on the cell surface so that direct cell-to-cell contact is required if cells are to detect one another (155). The surprise is that not all contacts are equal: C-signaling occurs primarily between pairs of cells that experience a pole-to-pole encounter (155, 156, 166). This explains why motility is essential for fruiting body formation; it is the way that cells generate the appropriate end-to-end contacts (155, 156, 348). When M. xanthus concentrations reach high enough levels, motile cells run into one another more frequently and those involved in productive collisions begin to align side by side and end to end, incorporating new cells and forming long parallel arrays (307, 348). As the process continues, productive pole-to-pole C-signaling increases and drives the population to differentiate (307).
To test the idea that specific geometric contacts between M. xanthus cells are required, Kim and Kaiser forced nonmotile mutants to align end to end or side to side by growing the cells in microscopic grooves etched into an agar surface (166). This physical intervention restored sporulation levels to 16% of that observed in wild-type cells (166), whereas random packing of nonmotile mutants increased sporulation to only 1% of wild-type levels (166). Thus, sporulation was rescued by manipulating cell position, suggesting that specific end-to-end contacts are crucial for transmitting the C-signal from donor to recipient (166).
Passing C-signal via pole-to-pole contact tells cells they are in the proper geometric alignment (156) and allows them to “decode their spatial position during morphogenesis” (307). The fact that physical pole-to-pole communication is necessary for C-signal transmission implies that the rod shape of M. xanthus is integral to distinguishing its geometrical position.
Interlocking shapes: a puzzlement.
One intriguing prospect is that adjoining cells might manipulate one another's shape so that the two fit more perfectly. Perhaps cells require specific shapes to fit into or create multicellular complexes; a potential example is cells in biofilms, where multiple cells interact in close proximity. An example of just such an interaction occurs in epidermal cells of the eukaryote Arabidopsis, where adjacent cells interdigitate to form a jigsaw puzzle of interconnecting cell shapes (82). One cell promotes the outgrowth of a lobe in a neighboring cell, which, in turn, inhibits outgrowth of a lobe from the first cell, resulting in interlocking sections (82). The overall effect is to physically strengthen the epidermis to resist wind and rain (82).
It is not too outlandish to suppose that similar morphological interactions might occur between prokaryotic cells or between cells and bits of their environment. As described in the introduction (see “The Perfect Experiment” above), Takeuchi et al. forced E. coli to grow in semipermanent and specific shapes by trapping the cells in tiny agarose chambers (322). As they grew, each cell adopted the shape of its compartment, constrained by simple mechanical considerations (322). That this can be done at all means that multiple shapes are available to E. coli, some of which may be subject to its surroundings, though such shapes are lost over time when the cells are released and cultured. A second implication is that cell shape is plastic, meaning that individual bacteria may bend and curve and twist around or through neighboring surfaces, some of which may include other cells. Time-lapse microscopic observations of E. coli microcolonies composed of shape-defective mutants reveal that some cells are indeed deformed by physical interactions with adjacent cells (Fig. 7B and unpublished results). Therefore, it is possible that neighboring prokaryotes influence one another's morphologies but that such interactions have escaped our notice because we study shape in solitary, planktonic cells.
One hint that such shape-defining interactions occur in nature is provided by the recent discovery of an unidentified gram-negative magnetotactic prokaryote whose cells have a peculiar shape and multicellular arrangement (162). Fourteen to 21 cells arrange themselves in a single-cell layer to form a hollow ball, in which each cell is in direct contact both with the outside milieu and with a completely separate inner cavity (161, 162). To create this spherical assembly, each individual cell is roughly conical, having its larger end oriented towards the outside of the sphere and tapering smaller towards the inside (Fig. 9) (162). The length of a single cell is ∼3 μm (from the outside to the inside of the sphere), and its diameter is ∼2 μm at the outer edge but only ∼0.5 μm on the inside. The multicellular sphere itself is 7 to 8 μm in diameter, enclosing a hollow center of ∼1 μm (162). The important point here is that “the final shape of one cell is the result of the arrangement of its neighbors” (161). Keim et al. imagined two reasons why cells might form such an unprecedented multicellular prokaryotic conglomerate. The cells may benefit by maintaining the contents of the internal compartment separate from the outside environment, or perhaps this large complex protects against predation by protists (162). The possibilities are not mutually exclusive, and neither has been tested. In any case, the existence of these unusually shaped cells and their curious organization underscore the likelihood that we are a long way from understanding all the selective factors experienced by the prokaryotic world.
FIG. 9.
Influence of multicellularity on individual cell shapes. Cell shapes in a spherical, multicellular, magnetotactic bacterium (161, 162) are shown. Individual cells are pyramid or cone shaped so that they fit together to form a sphere with a small hollow interior. The shape of each cell is influenced by physical interactions with its neighbors. Bar, 1 μm. (Reprinted from reference 161 with permission from Elsevier.)
Summary
In 1928, Henrici observed that critical physiological modifications may accompany changes in bacterial morphology (347). What he could not say, and what we still do not know in most instances, is whether these morphological alterations merely accompany the physiological adjustments or whether shape change per se plays a fundamental role. The morphological changes in stationary phase, the creation of Y-shaped bifids, and the morphological stages during the differentiation of some soil bacteria, pathogens, and multicellular assemblies are all examples in which cell shape is implicated but not yet proven to be important. More convincing evidence exists for the importance of cell shape in swarm cell rafts and in bladder infections by E. coli. Finally, a possible and (until recently) completely fanciful possibility is that the shape of some cells may be imposed externally by the immediate environment—that is, by microscopic “growth chambers” embedded in solid or semisolid substrates.
THE SHAPE OF THINGS TO COME
We shape our buildings: thereafter they shape us.
—Sir Winston Churchill (305)
To a large extent, the merits of asking questions about prokaryotic cell shape have been obscure. One reason is that a great deal of information is spread among many researchers whose interest in morphology is secondary to other pursuits. Cell shape per se is rarely the immediate subject of experimentation, and many morphological observations are relegated to the recesses of Results or Discussion sections that are devoted to other, more primary topics. This often makes it difficult to dig out data that address the significance of bacterial shape. Therefore, one purpose of this review has been to assemble this material and present it to a wide audience in the hopes of publicizing the diversity and importance of bacterial morphology. Another, parallel purpose has been to build on these observations to make the case that the investigation of prokaryotic morphology deserves to be considered a legitimate scientific discipline. A productive discipline addresses a fundamental question about the world, develops explanatory and predictive powers, may have practical applications, and is sufficiently intriguing that it draws the attention of a body of interested researchers. By these guidelines, the investigation of bacterial shape should indeed be considered worthy of study in its own right.
First, the subject of morphology addresses a fundamental biological question, the significance of which has been decided by the organisms themselves. Bacteria adopt and maintain uniform shapes from among a large number of possible forms, some organisms modify their morphologies to meet different environmental conditions, and the trait has an evolutionary history. Thus, cell shape is heritable, genetically malleable, and adaptive—all the characteristics of an important biological theme—and represents a set of tools and capabilities with which bacteria cope with the world around them. Therefore, it should be well worth the effort to discover the reasons that lie behind individual morphologies and to determine the mechanisms by which cells create and maintain these shapes.
Second, a viable scientific discipline should have explanatory power; that is, it should describe what is and strive to explain how it came to be. In this regard, the most fundamental question to be answered is, “Why does a bacterium have one particular shape rather than another?” The bulk of this review has been devoted to addressing and organizing this aspect of the field. The relevant explanations belong to a suite of factors that can be arranged into broad categories, encompassing the cell's most basic needs. In some cases one or more of these can be invoked to explain the morphology of an individual bacterium, but usually the most challenging step is to determine the combination of forces and responses that produce a specific phenotype.
Using the tools and data at hand, we can outline a few general guidelines about cell shape, many of which are listed in Tables 1 and 2 and discussed in previous sections. The strongest conclusion may be that cells tend to be symmetric, a trend driven by the requirement that the genome and cytoplasm be partitioned equally between daughter cells. Because this is fundamental to perpetuating the organism, it becomes exceedingly important to understand the mechanisms that generate and preserve this feature. In fact, an intriguing corollary is that the absence of symmetry may signify more than its presence. Asymmetry may signal the existence of genetic or physiological mechanisms that differ from our favorite (and symmetric) model organisms, or it may indicate the existence of a strong selection against the usual symmetrical solutions. Another general guideline is that the rod shape confers upon cells several practical capabilities, which may explain why this morphology dominates many environments. The shape increases a cell's surface-to-volume ratio, a factor that is particularly important in low-nutrient environments. Underlying physical principles probably favor rods when motility is advantageous and perhaps where surface attachments must be optimized. Being a rod may also be the best choice if a cell requires filamentation as a simple, readily available response to environmental stress. Finally, the rod shape may be most efficient for distributing cellular components to different (polar) locations.
Clearly, though, the most daunting task before us is to evaluate the relative contributions of the different selective forces so as to explain the shapes of known bacteria. I am not at all sure that such evaluations can be made with confidence yet, given the paucity and narrowness of current data. However, as an example of the form such evaluations may take, consider the explanations for the shapes of oceanic bacteria. That these bacteria are predominantly tiny rods or vibrioids is consistent with selective forces that favor small and highly motile cells that must also remain in the vicinity of solid surfaces or move through viscous materials. However, although these conclusions are consistent with what we know, we probably do not understand all the relevant forces at work, and it is almost certain that there exist different morphological solutions of equal or similar fitness for any particular environment.
Unfortunately, the types of shapes we can rationalize are outnumbered by those for which we have little or no explanation. The number of examples is approximately equal to the number of differently shaped cells. Why is Stella star shaped and relatively flat? Do the multiple prosthecate arms of Ancalomicrobium resist water convection by increasing drag? Are the promiscuous shapes of some bacteria the result of unknown nutritional requirements? Why is the morphology of bifidobacteria not uniform? What does seem clear is that most of the vagaries of cell shape surpass our current explicatory powers. The situation begs that more questions be asked and more possibilities be tested. Even so, current knowledge provides us with enough descriptive power that it is reasonable to expect that our explanatory prowess will become more robust as research progresses.
The third aspect of a mature scientific discipline is that it is predictive. Regrettably, at present we can predict bacterial shapes only in the most general terms, and even these will be highly provisional. One approach is to reason backwards, beginning with cellular morphology and imagining the selective forces that have created a specific shape. The question is, “Knowing a cell's shape, what can we infer about the environment or habits of the organism?” Not surprisingly, the complexities of each situation and the level of our current understanding limit what we can conclude. This is made clear by the list in Table 2, which outlines some of the shapes bacteria may adopt and some of the forces that select for those shapes. But these environmental pressures are only potential influences, factors to be considered and tested instead of foregone conclusions or definitive predictions. Except for unusual cases, it is almost certain that no single parameter will determine the morphology of a particular bacterium. Instead, each shape will be a weighting, a summation or integration of numerous forces acting on cells, whose final shapes will be governed by competing demands within their environmental niches. Because of the many ways that such forces can combine to select any particular shape, predictions are bound to be imperfect or incomplete, and more than one solution may exist. What such a list does provide is a starting point for fleshing out possibilities for further experimentation.
Besides the question about what shape can tell us about the environment, we have the complementary concern: “Why are there differently shaped cells in the same environment?” If environmental forces influence morphology, why doesn't one shape predominate in each niche, and why can't we predict what that shape might be? One answer is that cells remedy their deficiencies in many ways, modifying what they already possess so they can compete with other organisms. For example, cocci may mimic the advantages of being rod-like or filamentous by sticking together to form chains of unseparated cells. Another answer is that even within a shared habitat bacteria occupy different microenvironments, each with its own constellation of pressures. Yet a third possibility is that microorganisms are subjected to such fast, furious, and frequent environmental change that they must be able to respond more quickly and with a greater breadth of morphological options than can their more elaborate multicellular eukaryotic cousins. Even so, with further experimentation, we should be able to predict in general terms how biological populations will be affected by changes in one or more of these competing forces (e.g., see Table 1). If we observe something other than what we would expect, then several things may be true: there may be something we don't know about the environment, something we don't know about the forces that select cell shape, or something we don't know about how cells can respond. Pursuing the answers to any of these will drive our understanding forward.
A fourth characteristic of a scientific discipline is that it frequently produces practical applications. But what does knowing more about bacterial morphology have to offer? For one thing, those who are interested in releasing genetically engineered bacteria need to take into account every pressure with which these organisms must cope. The planned survival (or planned disappearance) of cells in the wild will probably depend on their shapes as well as on their biochemical abilities, so it will be useful to know what effects cell shape may have. Another area of concern is our ability to predict the effects on microbial populations when we modify the environment, because subtle changes in the shape landscape may alter previously stable ecological balances. If we don't know how morphology affects survival, these kinds of effects will be forever beneath our awareness. Finally, learning how bacteria manipulate their morphologies should produce new insights that may be incorporated into nanotechnological schemes to create and propagate materials with artificially designed shapes.
Regardless of the intricacies, cell shape can be used to guide the formation and testing of biological hypotheses, allowing us to establish trends and possibilities. Thus, the most pertinent contribution of the present review may be to point out the variety of possibilities that should be considered for experimental confirmation. We will know we have a handle on the science of cell shape when we can do three things: first, when we can evaluate individual shapes in terms of general selective forces, infer information about the organisms, and list possible influences to be tested by experiment; second, when we can consider an environment or environmental changes and predict which bacterial shapes are most likely to be present and how the population will change; and third, when we have some idea of how cell shape may influence the future evolutionary trajectory of a cell.
What We Need
Finally, what needs to be done to create a more independent and viable field of study?
A shape atlas.
First, it would be useful to have a comprehensive survey and catalogue of cell shapes. How many different shapes are there? What are the variations? Can these be organized into comprehensible families? What morphologies predominate in different environments? Perhaps the community can build an electronic resource to display this vast array. We cannot explain what we do not know about, so we must become more aware of the range of prokaryotic morphologies.
Unanswered questions.
We need to address a plethora of unanswered questions that complete the phrase “How does cell shape affect ________?” (Fill in the blank.) The answers will expand our understanding of the importance of morphology.
Techniques.
The field especially needs new and more-rapid techniques to observe, categorize, and analyze bacterial morphology. In particular, we need to be able to manipulate shape apart from other biochemical changes. At present, the most applicable tools include nanotechnological manipulations or the study of bacteria with and without the protein crescentin (though the latter might have as-yet-unknown biochemical effects). It would also be of tremendous help if genetic selections could be devised, instead of relying on tedious and labor-intensive visual screens for isolating and studying shape mutants.
Ecology.
We need to know the ecological ramifications of altering bacterial shape. Are there shape successions? When the environment changes, do cells with similar shapes repopulate the niche, or can other mixes arise? Are there rules for change and survival when an organism is placed in an already-populated environment? What is the effect on organisms higher in the eukaryotic food chain?
Evolution.
We need to know more about the evolutionary lineages of morphology. Can we trace shape changes through known evolutionary histories? When do we see convergent versus divergent evolution? Are the different shape-making mechanisms variations on a single theme, or have alternate mechanisms been invented?
Mechanisms.
For both fundamental and technical reasons, we need a deeper understanding of the mechanisms by which cells create and maintain their shapes.
Going Forward
If we find biochemical evidence that a cell expresses β-galactosidase, we infer that the organism's natural environment contains lactose (or did at one time). We cannot approach this level of certainty by reasoning from cell shape. Perhaps, in time, we will come closer to this ideal. The two major impediments to doing so are (i) our relative ignorance of the selective value of bacterial shape, a subject that this review attempts to open for increased deliberation and debate, and (ii) our relatively embryonic understanding of the mechanisms that control bacterial morphology. Eventually, maturation of these two areas should allow us to manipulate cell shape specifically and rationally, making it possible to perform rigorous experiments to discover how important shape really is for bacterial growth and survival.
Acknowledgments
I appreciate very much the helpful discussions and communication of relevant research results from the following individuals: Amha Belay, Terry Beveridge, Jens Boenigk, Yves Brun, Tom Fenchel, Sheryl Justice, Carsten Matz, Jennifer Mendell, Jim Mitchell, Jakob Pernthaler, Ev Sherr, and Douglas Weibel. In addition, I deeply thank the journal reviewers of this work, who provided exceptionally valuable feedback. I owe special thanks to Miguel de Pedro for reading the entire manuscript and to John Lee for preparing the graphics.
This work was supported by grant R01-GM061019 from the National Institutes of Health.
REFERENCES
- 1.Achberger, E. C., and P. E. Kolenbrander. 1978. Isolation and characterization of morphogenetic mutants of Arthrobacter crystallopoietes. J. Bacteriol. 135:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann, M., S. C. Stearns, and U. Jenal. 2003. Senescence in a bacterium with asymmetric division. Science 300:1920. [DOI] [PubMed] [Google Scholar]
- 3.Agladze, K., X. Wang, and T. Romeo. 2005. Spatial periodicity of Escherichia coli K-12 biofilm microstructure initiates during a reversible, polar attachment phase of development and requires the polysaccharide adhesin PGA. J. Bacteriol. 187:8237-8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Åkerlund, T., K. Nordström, and R. Bernander. 1993. Branched Escherichia coli cells. Mol. Microbiol. 10:849-858. [DOI] [PubMed] [Google Scholar]
- 5.Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. [DOI] [PubMed] [Google Scholar]
- 6.Altermann, E., L. B. Buck, R. Cano, and T. R. Klaenhammer. 2004. Identification and phenotypic characterization of the cell-division protein CdpA. Gene 342:189-197. [DOI] [PubMed] [Google Scholar]
- 7.Ammendola, A., O. Geisenberger, J. B. Andersen, M. Givskov, K. H. Schleifer, and L. Eberl. 1998. Serratia liquefaciens swarm cells exhibit enhanced resistance to predation by Tetrahymena sp. FEMS Microbiol. Lett. 164:69-75. [DOI] [PubMed] [Google Scholar]
- 8.Angert, E. R., K. Clements, and N. Pace. 1993. The largest bacterium. Nature 362:239-241. [DOI] [PubMed] [Google Scholar]
- 9.Atsumi, T., Y. Maekawa, T. Yamada, I. Kawagishi, Y. Imae, and M. Homma. 1996. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J. Bacteriol. 178:5024-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ausmees, N., J. R. Kuhn, and C. Jacobs-Wagner. 2003. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell 115:705-713. [DOI] [PubMed] [Google Scholar]
- 11.Balebona, M. C., M. J. Andreu, M. A. Bordas, I. Zorrilla, M. A. Morinigo, and J. J. Borrego. 1998. Pathogenicity of Vibrio alginolyticus for cultured gilt-head sea bream (Sparus aurata L.). Appl. Environ. Microbiol. 64:4269-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbara, G. M., and J. G. Mitchell. 2003. Bacterial tracking of motile algae. FEMS Microbiol. Ecol. 44:79-87. [DOI] [PubMed] [Google Scholar]
- 13.Bauer, H., E. Sigarlakie, and J. C. Faure. 1975. Scanning and transmission electron microscopy of three strains of Bifidobacterium. Can. J. Microbiol. 21:1305-1316. [DOI] [PubMed] [Google Scholar]
- 14.Becker, M. W., D. W. Metge, S. A. Collins, A. M. Shapiro, and R. W. Harvey. 2003. Bacterial transport experiments in fractured crystalline bedrock. Ground Water 41:682-689. [DOI] [PubMed] [Google Scholar]
- 15.Berg, H. C., and E. M. Purcell. 1977. Physics of chemoreception. Biophys. J. 20:193-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg, H. C., and L. Turner. 1990. Chemotaxis of bacteria in glass capillary arrays. Biophys. J. 58:919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg, H. C., and L. Turner. 1979. Movement of microorganisms in viscous environments. Nature 278:349-351. [DOI] [PubMed] [Google Scholar]
- 18.Bergh, O., K. Y. Borsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 19.Beveridge, T. J. 1988. The bacterial surface: general considerations towards design and function. Can. J. Microbiol. 34:363-372. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi, M. 1989. Unusual bloom of star-like prosthecate bacteria and filaments as a consequence of grazing pressure. Microb. Ecol. 17:137-141. [DOI] [PubMed] [Google Scholar]
- 21.Biondi, S. A., J. A. Quinn, and H. Goldfine. 1998. Random motility of swimming bacteria in restricted geometries. AICHE J. 44:1923-1929. [Google Scholar]
- 22.Blackburn, N., T. Fenchel, and J. Mitchell. 1998. Microscale nutrient patches in planktonic habitats shown by chemotactic bacteria. Science 282:2254-2256. [DOI] [PubMed] [Google Scholar]
- 23.Boenigk, J., and H. Arndt. 2002. Bacterivory by heterotrophic flagellates: community structure and feeding strategies. Antonie Leeuwenhoek 81:465-480. [DOI] [PubMed] [Google Scholar]
- 24.Boenigk, J., and H. Arndt. 2000. Comparative studies on the feeding behavior of two heterotrophic nanoflagellates: the filter-feeding choanoflagellate Monosiga ovata and the raptorial-feeding kinetoplastid Rhynchomonas nasuta. Aquat. Microb. Ecol. 22:243-249. [Google Scholar]
- 25.Boenigk, J., P. Stadler, A. Wiedlroither, and M. W. Hahn. 2004. Strain-specific differences in the grazing sensitivities of closely related ultramicrobacteria affiliated with the Polynucleobacter cluster. Appl. Environ. Microbiol. 70:5787-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolhuis, H., E. M. Poele, and F. Rodriguez-Valera. 2004. Isolation and cultivation of Walsby's square archaeon. Environ. Microbiol. 6:1287-1291. [DOI] [PubMed] [Google Scholar]
- 27.Boneca, I. G. 2005. The role of peptidoglycan in pathogenesis. Curr. Opin. Microbiol. 8:46-53. [DOI] [PubMed] [Google Scholar]
- 28.Bottone, E. J., C. A. Thomas, D. Lindquist, and J. M. Janda. 1995. Difficulties encountered in identification of a nutritionally deficient streptococcus on the basis of its failure to revert to streptococcal morphology. J. Clin. Microbiol. 33:1022-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouvet, A., A. Ryter, C. Frehel, and J. F. Acar. 1980. Nutritionally deficient streptococci: electron microscopic study of 14 strains isolated in bacterial endocarditis. Ann. Microbiol. (Paris). 131B:101-120. [PubMed] [Google Scholar]
- 30.Bouwer, H. 1984. Elements of soil science and groundwater hydrology, p. 9-38. In G. Bitton and C. P. Gerba (ed.), Groundwater pollution microbiology. Wiley, New York, N.Y.
- 31.Bredt, W., J. Feldner, I. Kahane, and S. Razin. 1983. Mycoplasma attachment to solid surfaces: a review. Yale J. Biol. Med. 56:653-656. [PMC free article] [PubMed] [Google Scholar]
- 32.Brehm, U., W. E. Krumbein, and K. A. Palinska. 2003. Microbial spheres: a novel cyanobacterial-diatom symbiosis. Naturwissenschaften 90:136-140. [DOI] [PubMed] [Google Scholar]
- 33.Brun, Y. V., and R. Janakiraman. 2000. The dimorphic life cycle of Caulobacter and stalked bacteria, p. 297-317. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 34.Burman, L. G., J. Raichler, and J. T. Park. 1983. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J. Bacteriol. 155:983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns, D. G., H. M. Camakaris, P. H. Janssen, and M. L. Dyall-Smith. 2004. Cultivation of Walsby's square haloarchaeon. FEMS Microbiol. Lett. 238:469-473. [DOI] [PubMed] [Google Scholar]
- 36.Burrows, L. L. 2005. Weapons of mass retraction. Mol. Microbiol. 57:878-888. [DOI] [PubMed] [Google Scholar]
- 37.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 38.Chakravortty, D., M. Rohde, L. Jager, J. Deiwick, and M. Hensel. 2005. Formation of a novel surface structure encoded by Salmonella pathogenicity island 2. EMBO J. 24:2043-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Champion, J. A., and S. Mitragotri. 2006. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 103:4930-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chauhan, A., M. V. Madiraju, M. Fol, H. Lofton, E. Maloney, R. Reynolds, and M. Rajagopalan. 2006. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J. Bacteriol. 188:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang, P., M. Habash, and L. L. Burrows. 2005. Disparate subcellular localization patterns of Pseudomonas aeruginosa type IV pilus ATPases involved in twitching motility. J. Bacteriol. 187:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark, R. B., R. E. Gordon, E. J. Bottone, and M. Reitano. 1983. Morphological aberrations of nutritionally deficient streptococci: association with pyridoxal (vitamin B6) concentration and potential role in antibiotic resistance. Infect. Immun. 42:414-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke-Sturman, A. J., A. R. Archibald, I. C. Hancock, C. R. Harwood, T. Merad, and J. A. Hobot. 1989. Cell wall assembly in Bacillus subtilis: partial conservation of polar wall material and the effect of growth conditions on the pattern of incorporation of new material at the polar caps. J. Gen. Microbiol. 135:657-665. [DOI] [PubMed] [Google Scholar]
- 44.Connon, S. A., and S. J. Giovannoni. 2002. High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl. Environ. Microbiol. 68:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper, S., and M. W. Denny. 1997. A conjecture on the relationship of bacterial shape to motility in rod-shaped bacteria. FEMS Microbiol. Lett. 148:227-231. [Google Scholar]
- 46.Cumbie, D., and L. McKay. 1999. Influence of diameter on particle transport in a fractured shale saprolite. J. Contam. Hydrol. 37:139-157. [Google Scholar]
- 47.Danese, P. N., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 48.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeLeo, P. C., and P. Baveye. 1997. Factors affecting protozoan predation of bacteria clogging laboratory aquifer microcosms. Geomicrobiol. J. 14:127. [Google Scholar]
- 50.Denome, S. A., P. K. Elf, T. A. Henderson, D. E. Nelson, and K. D. Young. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J. Bacteriol. 181:3981-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Pedro, M. A., C. G. Grunfelder, and H. Schwarz. 2004. Restricted mobility of cell surface proteins in the polar regions of Escherichia coli. J. Bacteriol. 186:2594-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Pedro, M. A., J. C. Quintela, J.-V. Höltje, and H. Schwarz. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Pedro, M. A., H. Schwarz, and A. L. Koch. 2003. Patchiness of murein insertion into the sidewall of Escherichia coli. Microbiology 149:1753-1761. [DOI] [PubMed] [Google Scholar]
- 54.de Pedro, M. A., and U. Schwarz. 1981. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc. Natl. Acad. Sci. USA 78:5856-5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Pedro, M. A., K. D. Young, J. V. Holtje, and H. Schwarz. 2003. Branching of Escherichia coli cells arises from multiple sites of inert peptidoglycan. J. Bacteriol. 185:1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deutch, C. E., and G. S. Perera. 1992. Myceloid cell formation in Arthrobacter globiformis during osmotic stress. J. Appl. Bacteriol. 72:493-499. [DOI] [PubMed] [Google Scholar]
- 57.Dewar, S. J., and R. Dorazi. 2000. Control of division gene expression in Escherichia coli. FEMS Microbiol. Lett. 187:1-7. [DOI] [PubMed] [Google Scholar]
- 58.DiLuzio, W. R., L. Turner, M. Mayer, P. Garstecki, D. B. Weibel, H. C. Berg, and G. M. Whitesides. 2005. Escherichia coli swim on the right-hand side. Nature 435:1271-1274. [DOI] [PubMed] [Google Scholar]
- 59.Donachie, W. D., and K. J. Begg. 1970. Growth of the bacterial cell. Nature 227:1220-1224. [DOI] [PubMed] [Google Scholar]
- 60.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dow, C., and R. Whittenbury. 1979. Prosthecate bacteria, p. 139-165. In J. Parish (ed.), Developmental biology of prokaryotes. Blackwell Scientific Publications, Oxford, United Kingdom.
- 62.Dowhan, W., E. Mileykovskaya, and M. Bogdanov. 2004. Diversity and versatility of lipid-protein interactions revealed by molecular genetic approaches. Biochim. Biophys. Acta 1666:19-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dusenbery, D. B. 1998. Fitness landscapes for effects of shape on chemotaxis and other behaviors of bacteria. J. Bacteriol. 180:5978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587-610. [DOI] [PubMed] [Google Scholar]
- 65.Dworkin, M., et al. (ed.). 2005. The prokaryotes: an evolving electronic resource for the microbiological community, release 3.19, 3rd ed. Springer-Verlag, New York, N.Y. [Online.] http://link.springer-ny.com/link/service/books/10125/.
- 66.Edwards, N., S. Beeton, A. T. Bull, and J. C. Merchuk. 1989. A novel device for the assessment of shear effects on suspended microbial cultures. Appl. Microbiol. Biotechnol. 30:190-195. [Google Scholar]
- 67.El-Fahmawi, B., and G. W. Owttrim. 2003. Polar-biased localization of the cold stress-induced RNA helicase, CrhC, in the cyanobacterium Anabaena sp. strain PCC 7120. Mol. Microbiol. 50:1439-1448. [DOI] [PubMed] [Google Scholar]
- 68.Errington, J., J. Bath, and L. J. Wu. 2001. DNA transport in bacteria. Nat. Rev. Mol. Cell Biol. 2:538-545. [DOI] [PubMed] [Google Scholar]
- 69.Errington, J., R. A. Daniel, and D. J. Scheffers. 2003. Cytokinesis in bacteria. Microbiol. Mol. Biol. Rev. 67:52-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faulkner, G., and R. A. Garduño. 2002. Ultrastructural analysis of differentiation in Legionella pneumophila. J. Bacteriol. 184:7025-7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fawcett, P. T., K. M. Gibney, and K. M. Vinette. 1999. Helicobacter pylori can be induced to assume the morphology of Helicobacter heilmannii. J. Clin. Microbiol. 37:1045-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldner, J., W. Bredt, and I. Kahane. 1983. Influence of cell shape and surface charge on attachment of Mycoplasma pneumoniae to glass surfaces. J. Bacteriol. 153:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fenchel, T. 1986. Protozoan filter feeding. Prog. Protistol. 1:65-113. [Google Scholar]
- 74.Ferrero, R. L., and A. Lee. 1988. Motility of Campylobacter jejuni in a viscous environment: comparison with conventional rod-shaped bacteria. J. Gen. Microbiol. 134:53-59. [DOI] [PubMed] [Google Scholar]
- 75.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finlay, B. B., and S. Falkow. 1989. Salmonella as an intracellular parasite. Mol. Microbiol. 3:1833-1841. [DOI] [PubMed] [Google Scholar]
- 78.Flärdh, K. 2003. Essential role of DivIVA in polar growth and morphogenesis in Streptomyces coelicolor A3(2). Mol. Microbiol. 49:1523-1536. [DOI] [PubMed] [Google Scholar]
- 79.Fraser, G. M., R. B. Furness, and C. Hughes. 2000. Swarming migration by Proteus and related bacteria, p. 381-401. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 80.Frenkel, A., and W. Hirsch. 1961. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature 191:728-730. [DOI] [PubMed] [Google Scholar]
- 81.Frymier, P. D., R. M. Ford, H. C. Berg, and P. T. Cummings. 1995. Three-dimensional tracking of motile bacteria near a solid planar surface. Proc. Natl. Acad. Sci. USA 92:6195-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu, Y., Y. Gu, Z. Zheng, G. Wasteneys, and Z. Yang. 2005. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120:687-700. [DOI] [PubMed] [Google Scholar]
- 83.Fuhrman, J. A., and R. T. Noble. 1995. Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol. Oceanogr. 40:1236-1242. [Google Scholar]
- 84.Fuhrmann, C., I. Soedarmanto, and C. Lammler. 1997. Studies on the rod-coccus life cycle of Rhodococcus equi. Zentbl. Veterinarmed. B 44:287-294. [DOI] [PubMed] [Google Scholar]
- 85.Gage, D. J. 2004. Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68:280-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallant, C. V., C. Daniels, J. M. Leung, A. S. Ghosh, K. D. Young, L. P. Kotra, and L. L. Burrows. 2005. Common β-lactamases inhibit bacterial biofilm formation. Mol. Microbiol. 58:1012-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garduño, R. A., E. Garduño, M. Hiltz, and P. S. Hoffman. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect. Immun. 70:6273-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaze, W. H., N. Burroughs, M. P. Gallagher, and E. M. Wellington. 2003. Interactions between Salmonella typhimurium and Acanthamoeba polyphaga, and observation of a new mode of intracellular growth within contractile vacuoles. Microb. Ecol. 46:358-369. [DOI] [PubMed] [Google Scholar]
- 89.Germida, J. J., and L. E. Casida, Jr. 1980. Myceloid growth of Arthrobacter globiformis and other Arthrobacter species. J. Bacteriol. 144:1152-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gestwicki, J. E., A. C. Lamanna, R. M. Harshey, L. L. McCarter, L. L. Kiessling, and J. Adler. 2000. Evolutionary conservation of methyl-accepting chemotaxis protein location in Bacteria and Archaea. J. Bacteriol. 182:6499-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghosh, A. S., and K. D. Young. 2005. Helical disposition of proteins and lipopolysaccharide in the outer membrane of Escherichia coli. J. Bacteriol. 187:1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghosh, P., S. Mandal, B. D. Chattopadhyay, and S. Pal. 2005. Use of microorganism to improve the strength of cement mortar. Cement Concrete Res. 35:1980-1983. [Google Scholar]
- 93.Giovannoni, S. J., H. J. Tripp, S. Givan, M. Podar, K. L. Vergin, D. Baptista, L. Bibbs, J. Eads, T. H. Richardson, M. Noordewier, M. S. Rappé, J. M. Short, J. C. Carrington, and E. J. Mathur. 2005. Genome streamlining in a cosmopolitan oceanic bacterium. Science 309:1242-1245. [DOI] [PubMed] [Google Scholar]
- 94.Gitai, Z., N. A. Dye, A. Reisenauer, M. Wachi, and L. Shapiro. 2005. MreB actin-mediated segregation of a specific region of a bacterial chromosome. Cell 120:329-341. [DOI] [PubMed] [Google Scholar]
- 95.Goffin, C., and J.-M. Ghuysen. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golden, J. W., and H. S. Yoon. 2003. Heterocyst development in Anabaena. Curr. Opin. Microbiol. 6:557-563. [DOI] [PubMed] [Google Scholar]
- 97.Gonin, M., E. M. Quardokus, D. O'Donnol, J. Maddock, and Y. V. Brun. 2000. Regulation of stalk elongation by phosphate in Caulobacter crescentus. J. Bacteriol. 182:337-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gonzalez, J. E., and M. M. Marketon. 2003. Quorum sensing in nitrogen-fixing rhizobia. Microbiol. Mol. Biol. Rev. 67:574-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.González, J. M., J. Iriberri, L. Egea, and I. Barcina. 1990. Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl. Environ. Microbiol. 56:1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goodfellow, M. 1989. Genus Rhodococcus Zopf 1891, p. 2362-2371. In S. T. Williams, M. E. Sharpe, and J. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 4. Williams and Wilkins, Baltimore, Md. [Google Scholar]
- 101.Gorelova, O. A. 2001. Surface ultrastructure of the heteromorphic cells of Nostoc muscorum CALU 304 in a mixed culture with the Rauwolfia callus tissue. Microbiology 70:285-294. [Google Scholar]
- 102.Gourret, J. P., and H. Fernandez-Arias. 1974. Ultrastructural and cytochemical studies of bacteroid differentiation of Rhizobium trifolii Dangeard in the nodules of Trifolium repens L. Can. J. Microbiol. 20:1169-1181. [PubMed] [Google Scholar]
- 103.Gow, N. A., A. J. Brown, and F. C. Odds. 2002. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 5:366-371. [DOI] [PubMed] [Google Scholar]
- 104.Gray, D. I., G. W. Gooday, and J. I. Prosser. 1990. Apical hyphal extension in Streptomyces coelicolor A3(2). J. Gen. Microbiol. 136:1077-1084. [DOI] [PubMed] [Google Scholar]
- 105.Gray, M. L., and A. H. Killinger. 1966. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 30:309-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Griffiths, P. L. 1993. Morphological changes of Campylobacter jejuni growing in liquid culture. Lett. Appl. Microbiol. 17:152-155. [DOI] [PubMed] [Google Scholar]
- 108.Grohmann, E., G. Muth, and M. Espinosa. 2003. Conjugative plasmid transfer in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67:277-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Güde, H. 1979. Grazing by protozoa as selection factor for activated sludge bacteria. Microb. Ecol. 5:225-237. [DOI] [PubMed] [Google Scholar]
- 110.Guerrero, R., C. Pedros-Alio, I. Esteve, J. Mas, D. Chase, and L. Margulis. 1986. Predatory prokaryotes: predation and primary consumption evolved in bacteria. Proc. Natl. Acad. Sci. USA 83:2138-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gupta, R. S. 2000. The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol. Rev. 24:367-402. [DOI] [PubMed] [Google Scholar]
- 112.Hahn, J., B. Maier, B. J. Haijema, M. Sheetz, and D. Dubnau. 2005. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell 122:59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hahn, M. W., and M. G. Hofle. 1999. Flagellate predation on a bacterial model community: interplay of size-selective grazing, specific bacterial cell size, and bacterial community composition. Appl. Environ. Microbiol. 65:4863-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hahn, M. W., and M. G. Hofle. 2001. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 35:113-121. [DOI] [PubMed] [Google Scholar]
- 115.Hahn, M. W., H. Lunsdorf, Q. Wu, M. Schauer, M. G. Hofle, J. Boenigk, and P. Stadler. 2003. Isolation of novel ultramicrobacteria classified as actinobacteria from five freshwater habitats in Europe and Asia. Appl. Environ. Microbiol. 69:1442-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hallez, R., A. F. Bellefontaine, J. J. Letesson, and X. De Bolle. 2004. Morphological and functional asymmetry in α-proteobacteria. Trends Microbiol. 12:361-365. [DOI] [PubMed] [Google Scholar]
- 117.Harshey, R. M. 1994. Bees aren't the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13:389-394. [DOI] [PubMed] [Google Scholar]
- 118.Harshey, R. M., and T. Matsuyama. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. USA 91:8631-8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harvey, P., and S. Leach. 1998. Analysis of coccal cell formation by Campylobacter jejuni using continuous culture techniques, and the importance of oxidative stress. J. Appl. Microbiol. 85:398-404. [DOI] [PubMed] [Google Scholar]
- 120.Harvey, R. W. 1997. Microorganisms as tracers in groundwater injection and recovery experiments: a review. FEMS Microbiol. Rev. 20:461-472. [DOI] [PubMed] [Google Scholar]
- 121.Harvey, R. W., and S. Garabedian. 1991. Use of colloid filtration theory in modeling movement of bacteria through a contaminated sandy aquifer. Environ. Sci. Technol. 25:178-185. [Google Scholar]
- 122.Hay, N. A., D. J. Tipper, D. Gygi, and C. Hughes. 1999. A novel membrane protein influencing cell shape and multicellular swarming of Proteus mirabilis. J. Bacteriol. 181:2008-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hedlund, B. P., and D. A. Kuhn. 2005. The genera Simonsiella and Alysiella. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, release 3.19, 3rd ed. Springer-Verlag, New York, N.Y. [Online.] http://link.springer-ny.com/link/service/books/10125/.
- 124.Helmstetter, C. E., and A. C. Leonard. 1990. Involvement of cell shape in the replication and segregation of chromosomes in Escherichia coli. Res. Microbiol. 141:30-39. [DOI] [PubMed] [Google Scholar]
- 125.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hiraga, S., H. Niki, T. Ogura, C. Ichinose, H. Mori, B. Ezaki, and A. Jaffe. 1989. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J. Bacteriol. 171:1496-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hogan, D. A., and R. Kolter. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229-2232. [DOI] [PubMed] [Google Scholar]
- 128.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 129.Hoiczyk, E., and W. Baumeister. 1995. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 177:2387-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hoiczyk, E., and W. Baumeister. 1998. The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr. Biol. 8:1161-1168. [DOI] [PubMed] [Google Scholar]
- 131.Hoiczyk, E., and W. Baumeister. 1997. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol. Microbiol. 26:699-708. [DOI] [PubMed] [Google Scholar]
- 132.Hoiczyk, E., and A. Hansel. 2000. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J. Bacteriol. 182:1191-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Holt, J. G., N. R. Krieg, P. H. A. Sneath, J. T. Staley, and S. T. Williams (ed.). 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 134.Holzman, D. 2003. C. albicans virulence attributed to yeast-to-filament shift. ASM News 69:587-588. [Google Scholar]
- 135.Huber, B., K. Riedel, M. Kothe, M. Givskov, S. Molin, and L. Eberl. 2002. Genetic analysis of functions involved in the late stages of biofilm development in Burkholderia cepacia H111. Mol. Microbiol. 46:411-426. [DOI] [PubMed] [Google Scholar]
- 136.Hwang, C. S., J. H. Oh, W. K. Huh, H. S. Yim, and S. O. Kang. 2003. Ssn6, an important factor of morphological conversion and virulence in Candida albicans. Mol. Microbiol. 47:1029-1043. [DOI] [PubMed] [Google Scholar]
- 137.Ireland, M. M., J. A. Karty, E. M. Quardokus, J. P. Reilly, and Y. V. Brun. 2002. Proteomic analysis of the Caulobacter crescentus stalk indicates competence for nutrient uptake. Mol. Microbiol. 45:1029-1041. [DOI] [PubMed] [Google Scholar]
- 138.Iriberri, J., I. Azua, A. Labirua-Iturburu, I. Artolozaga, and I. Barcina. 1994. Differential elimination of enteric bacteria by protists in a freshwater system. J. Appl. Bacteriol. 77:476-483. [DOI] [PubMed] [Google Scholar]
- 139.Iwai, N., K. Nagai, and M. Wachi. 2002. Novel S-benzylisothiourea compound that induces spherical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicillin-binding protein 2. Biosci. Biotechnol. Biochem. 66:2658-2662. [DOI] [PubMed] [Google Scholar]
- 140.Jacoby, G. H., and K. D. Young. 1990. Heterogeneity among membrane vesicles of Escherichia coli: effects of production and fractionation techniques. Anal. Biochem. 184:48-54. [DOI] [PubMed] [Google Scholar]
- 141.Jacoby, G. H., and K. D. Young. 1988. Unequal distribution of penicillin-binding proteins among inner membrane vesicles of Escherichia coli. J. Bacteriol. 170:3660-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.James, G. A., D. R. Korber, D. E. Caldwell, and J. W. Costerton. 1995. Digital image analysis of growth and starvation responses of a surface-colonizing Acinetobacter sp. J. Bacteriol. 177:907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janakiraman, A., and M. B. Goldberg. 2004. Recent advances on the development of bacterial poles. Trends Microbiol. 12:518-525. [DOI] [PubMed] [Google Scholar]
- 144.Javor, B., C. Requadt, and W. Stoeckenius. 1982. Box-shaped halophilic bacteria. J. Bacteriol. 151:1532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones, J. F., J. D. Feick, D. Imoudu, N. Chukwumah, M. Vigeant, and D. Velegol. 2003. Oriented adhesion of Escherichia coli to polystyrene particles. Appl. Environ. Microbiol. 69:6515-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jordan, D. C. 1962. The bacteroids of the genus Rhizobium. Bacteriol. Rev. 26:119-141. [PMC free article] [PubMed] [Google Scholar]
- 147.Judd, P. K., R. B. Kumar, and A. Das. 2005. Spatial location and requirements for the assembly of the Agrobacterium tumefaciens type IV secretion apparatus. Proc. Natl. Acad. Sci. USA 102:11498-11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 97:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Julkowska, D., M. Obuchowski, I. B. Holland, and S. J. Seror. 2004. Branched swarming patterns on a synthetic medium formed by wild-type Bacillus subtilis strain 3610: detection of different cellular morphologies and constellations of cells as the complex architecture develops. Microbiology 150:1839-1849. [DOI] [PubMed] [Google Scholar]
- 150.Jürgens, K., and C. Matz. 2002. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Leeuwenhoek 81:413-434. [DOI] [PubMed] [Google Scholar]
- 151.Jürgens, K., J. Pernthaler, S. Schalla, and R. Amann. 1999. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl. Environ. Microbiol. 65:1241-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Justice, S. S., J. Garcia-Lara, and L. I. Rothfield. 2000. Cell division inhibitors SulA and MinC/MinD block septum formation at different steps in the assembly of the Escherichia coli division machinery. Mol. Microbiol. 37:410-423. [DOI] [PubMed] [Google Scholar]
- 153.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaiser, D. 2001. Building a multicellular organism. Annu. Rev. Genet. 35:103-123. [DOI] [PubMed] [Google Scholar]
- 155.Kaiser, D. 2003. Coupling cell movement to multicellular development in myxobacteria. Nat. Rev. Microbiol. 1:45-54. [DOI] [PubMed] [Google Scholar]
- 156.Kaiser, D. 2004. Signaling in myxobacteria. Annu. Rev. Microbiol. 58:75-98. [DOI] [PubMed] [Google Scholar]
- 157.Kaiser, D., and R. Yu. 2005. Reversing cell polarity: evidence and hypothesis. Curr. Opin. Microbiol. 8:216-221. [DOI] [PubMed] [Google Scholar]
- 158.Kaiser, G. E., and R. N. Doetsch. 1975. Enhanced translational motion of Leptospira in viscous environments. Nature 255:656-657. [DOI] [PubMed] [Google Scholar]
- 159.Kawamura, Y., X. G. Hou, F. Sultana, S. Liu, H. Yamamoto, and T. Ezaki. 1995. Transfer of Streptococcus adjacens and Streptococcus defectivus to Abiotrophia gen. nov. as Abiotrophia adiacens comb. nov. and Abiotrophia defectiva comb. nov., respectively. Int. J. Syst. Bacteriol. 45:798-803. [DOI] [PubMed] [Google Scholar]
- 160.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 161.Keim, C. N., F. Abreu, U. Lins, H. Lins de Barros, and M. Farina. 2004. Cell organization and ultrastructure of a magnetotactic multicellular organism. J. Struct. Biol. 145:254-262. [DOI] [PubMed] [Google Scholar]
- 162.Keim, C. N., J. L. Martins, F. Abreu, A. S. Rosado, H. L. de Barros, R. Borojevic, U. Lins, and M. Farina. 2004. Multicellular life cycle of magnetotactic prokaryotes. FEMS Microbiol. Lett. 240:203-208. [DOI] [PubMed] [Google Scholar]
- 163.Kessel, M., and Y. Cohen. 1982. Ultrastructure of square bacteria from a brine pool in southern Sinai. J. Bacteriol. 150:851-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kidane, D., and P. L. Graumann. 2005. Intracellular protein and DNA dynamics in competent Bacillus subtilis cells. Cell 122:73-84. [DOI] [PubMed] [Google Scholar]
- 165.Kierek-Pearson, K., and E. Karatan. 2005. Biofilm development in bacteria. Adv. Appl. Microbiol. 57:79-111. [DOI] [PubMed] [Google Scholar]
- 166.Kim, S. K., and D. Kaiser. 1990. Cell alignment required in differentiation of Myxococcus xanthus. Science 249:926-928. [DOI] [PubMed] [Google Scholar]
- 167.Kinner, N. E., R. W. Harvey, and M. Kazmierkiewicz-Tabaka. 1997. Effect of flagellates on free-living bacterial abundance in an organically contaminated aquifer. FEMS Microbiol. Rev. 20:249-259. [DOI] [PubMed] [Google Scholar]
- 168.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 169.Klose, J., M. M. de Sa, and J. W. Kronstad. 2004. Lipid-induced filamentous growth in Ustilago maydis. Mol. Microbiol. 52:823-835. [DOI] [PubMed] [Google Scholar]
- 170.Koch, A. L. 1996. What size should a bacterium be? A question of scale. Annu. Rev. Microbiol. 50:317-348. [DOI] [PubMed] [Google Scholar]
- 171.Koch, A. L., and C. L. Woldringh. 1994. The metobolic inertness of the pole wall of a Gram-negative rod. J. Theor. Biol. 171:415-425. [Google Scholar]
- 172.Kojima, M., S. Suda, S. Hotta, and K. Hamada. 1968. Induction of pleomorphism in Lactobacillus bifidus. J. Bacteriol. 95:710-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koppelman, C. M., T. Den Blaauwen, M. C. Duursma, R. M. Heeren, and N. Nanninga. 2001. Escherichia coli minicell membranes are enriched in cardiolipin. J. Bacteriol. 183:6144-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krumholz, L. 2000. Microbial communities in the deep subsurface. Hydrogeol. J. 8:4-10. [Google Scholar]
- 175.Kruse, T., J. Moller-Jensen, A. Lobner-Olesen, and K. Gerdes. 2003. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 22:5283-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kudo, S., N. Imai, M. Nishitoba, S. Sugiyama, and Y. Magariyama. 2005. Asymmetric swimming pattern of Vibrio alginolyticus cells with single polar flagella. FEMS Microbiol. Lett. 242:221-225. [DOI] [PubMed] [Google Scholar]
- 177.Kumar, R. B., and A. Das. 2002. Polar location and functional domains of the Agrobacterium tumefaciens DNA transfer protein VirD4. Mol. Microbiol. 43:1523-1532. [DOI] [PubMed] [Google Scholar]
- 178.Kurtz, H. D., and D. I. Netoff. 2001. Stabilization of friable sandstone surfaces in a desiccating, wind-abraded environment of south-central Utah by rock surface microorganisms. J. Arid Environ. 48:89-100. [Google Scholar]
- 179.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705-3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lai, E. M., U. Nair, N. D. Phadke, and J. R. Maddock. 2004. Proteomic screening and identification of differentially distributed membrane proteins in Escherichia coli. Mol. Microbiol. 52:1029-1044. [DOI] [PubMed] [Google Scholar]
- 181.Lambert, C., K. J. Evans, R. Till, L. Hobley, M. Capeness, S. Rendulic, S. C. Schuster, S. Aizawa, and R. E. Sockett. 2006. Characterizing the flagellar filament and the role of motility in bacterial prey-penetration by Bdellovibrio bacteriovorus. Mol. Microbiol. 60:274-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lange, R., and R. Hengge-Aronis. 1991. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor σS. J. Bacteriol. 173:4474-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Larkin, J. M., and M. C. Henk. 1996. Filamentous sulfide-oxidizing bacteria at hydrocarbon seeps of the Gulf of Mexico. Microsc. Res. Tech. 33:23-31. [DOI] [PubMed] [Google Scholar]
- 184.Larkin, J. M., and R. Nelson. 1987. Mechanism of attachment of swarm cells of Thiothrix nivea. J. Bacteriol. 169:5877-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Larson, R. J., and J. L. Pate. 1976. Glucose transport in isolated prosthecae of Asticcacaulis biprosthecum. J. Bacteriol. 126:282-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lauga, E., W. R. Diluzio, G. M. Whitesides, and H. A. Stone. 2006. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90:400-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lázaro, B., J. Cárcamo, A. Audícana, I. Perales, and A. Fernández-Astorga. 1999. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 65:4677-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lázaro, S., F. Fernandez-Piñas, E. Fernández-Valiente, A. Blanco-Rivero, and F. Leganés. 2001. pbpB, a gene coding for a putative penicillin-binding protein, is required for aerobic nitrogen fixation in the cyanobacterium Anabaena sp. strain PCC7120. J. Bacteriol. 183:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leidenix, M. J., G. H. Jacoby, T. A. Henderson, and K. D. Young. 1989. Separation of Escherichia coli penicillin-binding proteins into different membrane vesicles by agarose electrophoresis and sizing chromatography. J. Bacteriol. 171:5680-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lenski, R. E., and J. A. Mongold. 2000. Cell size, shape, and fitness in evolving populations of bacteria, p. 221-235. In J. H. Brown and G. B. West (ed.), Scaling in biology. Oxford University Press, Oxford, United Kingdom.
- 191.Licking, E., L. Gorski, and D. Kaiser. 2000. A common step for changing cell shape in fruiting body and starvation-independent sporulation of Myxococcus xanthus. J. Bacteriol. 182:3553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luscombe, B., and T. Gray. 1974. Characteristics of Arthrobacter grown in continuous culture. J. Gen. Microbiol. 82:213-222. [Google Scholar]
- 193.Lutkenhaus, J., and S. G. Addinall. 1997. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 66:93-116. [DOI] [PubMed] [Google Scholar]
- 194.Lybarger, S. R., and J. R. Maddock. 2001. Polarity in action: asymmetric protein localization in bacteria. J. Bacteriol. 183:3261-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.MacRae, T. H., W. J. Dobson, and H. D. McCurdy. 1977. Fimbriation in gliding bacteria. Can. J. Microbiol. 23:1096-1108. [DOI] [PubMed] [Google Scholar]
- 196.Maddock, J. R., and L. Shapiro. 1993. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259:1717-1723. [DOI] [PubMed] [Google Scholar]
- 197.Magariyama, Y., M. Ichiba, K. Nakata, K. Baba, T. Ohtani, S. Kudo, and T. Goto. 2005. Difference in bacterial motion between forward and backward swimming caused by the wall effect. Biophys. J. 88:3648-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Magariyama, Y., and S. Kudo. 2002. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 83:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Maki, N., J. E. Gestwicki, E. M. Lake, L. L. Kiessling, and J. Adler. 2000. Motility and chemotaxis of filamentous cells of Escherichia coli. J. Bacteriol. 182:4337-4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Margolin, W. 2000. Differentiation of free-living rhizobia into endosymbiotic bacteroids, p. 441-466. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 201.Margolin, W. 2000. Themes and variations in prokaryotic cell division. FEMS Microbiol. Rev. 24:531-548. [DOI] [PubMed] [Google Scholar]
- 202.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 203.Matz, C., T. Bergfeld, S. A. Rice, and S. Kjelleberg. 2004. Microcolonies, quorum sensing and cytotoxicity determine the survival of Pseudomonas aeruginosa biofilms exposed to protozoan grazing. Environ. Microbiol. 6:218-226. [DOI] [PubMed] [Google Scholar]
- 204.Matz, C., J. Boenigk, H. Arndt, and K. Jurgens. 2002. Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellate Spumella sp. Aquat. Microb. Ecol. 27:137-148. [Google Scholar]
- 205.Matz, C., and K. Jurgens. 2005. High motility reduces grazing mortality of planktonic bacteria. Appl. Environ. Microbiol. 71:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Matz, C., and S. Kjelleberg. 2005. Off the hook—how bacteria survive protozoan grazing. Trends Microbiol. 13:302-307. [DOI] [PubMed] [Google Scholar]
- 207.Matz, C., D. McDougald, A. M. Moreno, P. Y. Yung, F. H. Yildiz, and S. Kjelleberg. 2005. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 102:16819-16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.McBride, M. J. 2001. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 55:49-75. [DOI] [PubMed] [Google Scholar]
- 209.McCarter, L. 1999. The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1:51-57. [PubMed] [Google Scholar]
- 210.Meberg, B. M., A. L. Paulson, R. Priyadarshini, and K. D. Young. 2004. Endopeptidase penicillin-binding proteins 4 and 7 play auxiliary roles in determining uniform morphology of Escherichia coli. J. Bacteriol. 186:8326-8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meeks, J. C., and J. Elhai. 2002. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 66:94-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Merad, T., A. R. Archibald, I. C. Hancock, C. R. Harwood, and J. A. Hobot. 1989. Cell wall assembly in Bacillus subtilis: visualization of old and new wall material by electron microscopic examination of samples stained selectively for teichoic acid and teichuronic acid. J. Gen. Microbiol. 135:645-655. [DOI] [PubMed] [Google Scholar]
- 213.Mercier, C., E. Domakova, J. Tremblay, and S. Kulakauskas. 2000. Effects of a muramidase on a mixed bacterial community. FEMS Microbiol. Lett. 187:47-52. [DOI] [PubMed] [Google Scholar]
- 214.Mercier, C., C. Durrieu, R. Briandet, E. Domakova, J. Tremblay, G. Buist, and S. Kulakauskas. 2002. Positive role of peptidoglycan breaks in lactococcal biofilm formation. Mol. Microbiol. 46:235-243. [DOI] [PubMed] [Google Scholar]
- 215.Mileykovskaya, E., and W. Dowhan. 2005. Role of membrane lipids in bacterial division-site selection. Curr. Opin. Microbiol. 8:135-142. [DOI] [PubMed] [Google Scholar]
- 216.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182:1172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mitchell, J. G. 2002. The energetics and scaling of search strategies in bacteria. Am. Nat. 160:727-740. [DOI] [PubMed] [Google Scholar]
- 218.Moissl, C., C. Rudolph, and R. Huber. 2002. Natural communities of novel archaea and bacteria with a string-of-pearls-like morphology: molecular analysis of the bacterial partners. Appl. Environ. Microbiol. 68:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Molofsky, A. B., and M. S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29-40. [DOI] [PubMed] [Google Scholar]
- 220.Morgan, P., and C. S. Dow. 1985. Environmental control of cell-type expression in prosthecate bacteria, p. 131-169. In M. Fletcher and G. D. Floodgate (ed.), Bacteria in their natural environments. Academic Press Inc., London, United Kingdom.
- 221.Morgan, R. 1992. Dans les hautes montagnes de Caroline. Story 40:47-55. [Google Scholar]
- 222.Morris, R. M., M. S. Rappé, S. A. Connon, K. L. Vergin, W. A. Siebold, C. A. Carlson, and S. J. Giovannoni. 2002. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420:806-810. [DOI] [PubMed] [Google Scholar]
- 223.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 97:10899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mühling, M., N. Harris, A. Belay, and B. A. Whitton. 2003. Reversal of helix orientation in the cyanobacterium Arthrospira. J. Phycol. 39:360-367. [Google Scholar]
- 225.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nanninga, N. 1988. Growth and form in microorganisms: morphogenesis of Escherichia coli. Can. J. Microbiol. 34:381-389. [DOI] [PubMed] [Google Scholar]
- 227.National Research Council Space Studies Board. 1999. Size limits of very small microorganisms: proceedings of a workshop. National Academic Press, Washington, D.C. [PubMed]
- 228.Neidhardt, F. C., J. L. Ingraham, and M. Schaechter. 1990. Physiology of the bacterial cell: a molecular approach, p. 442-462. Sinauer Associates, Inc., Sunderland, Mass.
- 229.Nelson, D. E., and K. D. Young. 2001. Contributions of PBP 5 and dd-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J. Bacteriol. 183:3055-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nelson, D. E., and K. D. Young. 2000. Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli. J. Bacteriol. 182:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nemecek, J. C., M. Wuthrich, and B. S. Klein. 2006. Global control of dimorphism and virulence in fungi. Science 312:583-588. [DOI] [PubMed] [Google Scholar]
- 232.Newman, D. K., and J. F. Banfield. 2002. Geomicrobiology: how molecular-scale interactions underpin biogeochemical systems. Science 296:1071-1077. [DOI] [PubMed] [Google Scholar]
- 233.Ng, L. K., R. Sherburne, D. E. Taylor, and M. E. Stiles. 1985. Morphological forms and viability of Campylobacter species studied by electron microscopy. J. Bacteriol. 164:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nicolella, C., R. Di Felice, and M. Rovatti. 1996. An experimental model of biofilm detachment in liquid fluidized bed biological reactors. Biotechnol. Bioeng. 51:713-719. [DOI] [PubMed] [Google Scholar]
- 235.Nilsen, T., A. S. Ghosh, M. B. Goldberg, and K. D. Young. 2004. Branching sites and morphological abnormalities behave as ectopic poles in shape-defective Escherichia coli. Mol. Microbiol. 52:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Novarino, G., A. Warren, H. Butler, G. Lambourne, A. Boxshall, J. Bateman, N. E. Kinner, R. W. Harvey, R. A. Mosse, and B. Teltsch. 1997. Protistan communities in aquifers: a review. FEMS Microbiol. Rev. 20:261-275. [DOI] [PubMed] [Google Scholar]
- 237.Ogura, T., P. Bouloc, H. Niki, R. D'Ari, S. Hiraga, and A. Jaffé. 1989. Penicillin-binding protein 2 is essential in wild-type Escherichia coli but not in lov or cya mutants. J. Bacteriol. 171:3025-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Oke, V., and S. R. Long. 1999. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 2:641-646. [DOI] [PubMed] [Google Scholar]
- 239.Olmsted, S. B., S. L. Erlandsen, G. M. Dunny, and C. L. Wells. 1993. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J. Bacteriol. 175:6229-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Olson, J. B., D. K. Harmody, and P. J. McCarthy. 2002. Alpha-proteobacteria cultivated from marine sponges display branching rod morphology. FEMS Microbiol. Lett. 211:169-173. [DOI] [PubMed] [Google Scholar]
- 241.O'Rear, J., L. Alberti, and R. M. Harshey. 1992. Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J. Bacteriol. 174:6125-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Oren, A., A. Ventosa, M. C. Gutierrez, and M. Kamekura. 1999. Haloarcula quadrata sp. nov., a square, motile archaeon isolated from a brine pool in Sinai (Egypt). Int. J. Syst. Bacteriol. 49:1149-1155. [DOI] [PubMed] [Google Scholar]
- 243.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 244.Park, S., P. M. Wolanin, E. A. Yuzbashyan, H. Lin, N. C. Darnton, J. B. Stock, P. Silberzan, and R. Austin. 2003. Influence of topology on bacterial social interaction. Proc. Natl. Acad. Sci. USA 100:13910-13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Patriarca, E. J., R. Tate, S. Ferraioli, and M. Iaccarino. 2004. Organogenesis of legume root nodules. Int. Rev. Cytol. 234:201-262. [DOI] [PubMed] [Google Scholar]
- 246.Pernthaler, J. 2005. Predation on prokaryotes in the water column and its ecological implications. Nat. Rev. Microbiol. 3:537-546. [DOI] [PubMed] [Google Scholar]
- 247.Pernthaler, J., T. Posch, K. Simek, J. Vrba, R. Amann, and R. Psenner. 1997. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl. Environ. Microbiol. 63:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pernthaler, J., B. Sattler, K. Simek, A. Schwarzenbacher, and R. Psenner. 1996. Top-down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat. Microb. Ecol. 10:255-263. [Google Scholar]
- 249.Pfandl, K., T. Posch, and J. Boenigk. 2004. Unexpected effects of prey dimensions and morphologies on the size selective feeding by two bacterivorous flagellates (Ochromonas sp. and Spumella sp.). J. Eukaryot. Microbiol. 51:626-633. [DOI] [PubMed] [Google Scholar]
- 250.Pilgrim, S., A. Kolb-Maurer, I. Gentschev, W. Goebel, and M. Kuhn. 2003. Deletion of the gene encoding p60 in Listeria monocytogenes leads to abnormal cell division and loss of actin-based motility. Infect. Immun. 71:3473-3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pine, L., and C. J. Boone. 1967. Comparative cell wall analyses of morphological forms within the genus Actinomyces. J. Bacteriol. 94:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Pluis, J. 1994. Algal crust formation in the inland dune area, Laarder Wasmeer, the Netherlands. Plant Ecol. 113:41-51. [Google Scholar]
- 253.Poindexter, J. S. 1981. The caulobacters: ubiquitous unusual bacteria. Microbiol. Rev. 45:123-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Poindexter, J. S. 1984. The role of calcium in stalk development and in phosphate acquisition in Caulobacter crescentus. Arch. Microbiol. 138:140-152. [DOI] [PubMed] [Google Scholar]
- 255.Poindexter, J. S. 1984. Role of prostheca development in oligotrophic aquatic bacteria, p. 33-40. In M. J. Klug and C. A. Reddy (ed.), Current perspectives in microbial ecology. ASM Press, Washington, D.C.
- 256.Popham, D. L., and K. D. Young. 2003. Role of penicillin-binding proteins in bacterial cell morphogenesis. Curr. Opin. Microbiol. 6:594-599. [DOI] [PubMed] [Google Scholar]
- 257.Porter, J. S., and J. L. Pate. 1975. Prosthecae of Asticcacaulis biprosthecum: system for the study of membrane transport. J. Bacteriol. 122:976-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Posch, T., J. Jezbera, J. Vrba, K. Simek, J. Pernthaler, S. Andreatta, and B. Sonntag. 2001. Size selective feeding in Cyclidium glaucoma (Ciliophora, Scuticociliatida) and its effects on bacterial community structure: a study from a continuous cultivation system. Microb. Ecol. 42:217-227. [DOI] [PubMed] [Google Scholar]
- 259.Posch, T., K. Simek, J. Vrba, S. Pernthaler, J. Nedoma, B. Sattler, B. Sonntag, and R. Psenner. 1999. Predator-induced changes of bacterial size-structure and productivity studied on an experimental microbial community. Aquat. Microb. Ecol. 18:235-246. [Google Scholar]
- 260.Posfay-Barbe, K. M., and E. R. Wald. 2004. Listeriosis. Pediatr. Rev. 25:151-159. [DOI] [PubMed] [Google Scholar]
- 261.Powell, M., and N. Slater. 1982. Removal rates of bacterial cells from glass surfaces by fluid shear. Biotechnol. Bioeng. 24:2527-2537. [DOI] [PubMed] [Google Scholar]
- 262.Prosser, J. I., and A. J. Tough. 1991. Growth mechanisms and growth kinetics of filamentous microorganisms. Crit. Rev. Biotechnol. 10:253-274. [DOI] [PubMed] [Google Scholar]
- 263.Purcell, E. M. 1977. Life at low Reynolds number. Am. J. Phys. 45:3-10. [Google Scholar]
- 264.Ramachandran, S. K., V. Ramkrishnan, and S. S. Bang. 2001. Remediation of concrete using microorganisms. ACI Mater. J. 98:3-9. [Google Scholar]
- 265.Ramia, M., D. L. Tullock, and N. Phan-Thien. 1993. The role of hydrodynamic interaction in the locomotion of microorganisms. Biophys. J. 65:755-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rappé, M. S., S. A. Connon, K. L. Vergin, and S. J. Giovannoni. 2002. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418:630-633. [DOI] [PubMed] [Google Scholar]
- 267.Reding, H. K., and J. E. Lepo. 1989. Physiological characterization of dicarboxylate-induced pleomorphic forms of Bradyrhizobium japonicum. Appl. Environ. Microbiol. 55:666-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Reichenbach, H. 1981. Taxonomy of the gliding bacteria. Annu. Rev. Microbiol. 35:339-364. [DOI] [PubMed] [Google Scholar]
- 269.Rijnaarts, H. H. M., W. Norde, E. J. Bouwer, J. Lyklema, and A. J. B. Zehnder. 1995. Reversibility and mechanism of bacterial adhesion. Colloids Surf. B 4:5-22. [Google Scholar]
- 270.Roberts, J. A. 1996. Tropism in bacterial infections: urinary tract infections. J. Urol. 156:1552-1559. [PubMed] [Google Scholar]
- 271.Robertson, B. R., J. L. O'Rourke, B. A. Neilan, P. Vandamme, S. L. On, J. G. Fox, and A. Lee. 2005. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int. J. Syst. Evol. Microbiol. 55:1199-1204. [DOI] [PubMed] [Google Scholar]
- 272.Roggenkamp, A., M. Abele-Horn, K. H. Trebesius, U. Tretter, I. B. Autenrieth, and J. Heesemann. 1998. Abiotrophia elegans sp. nov., a possible pathogen in patients with culture-negative endocarditis. J. Clin. Microbiol. 36:100-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Romani, L., F. Bistoni, and P. Puccetti. 2003. Adaptation of Candida albicans to the host environment: the role of morphogenesis in virulence and survival in mammalian hosts. Curr. Opin. Microbiol. 6:338-343. [DOI] [PubMed] [Google Scholar]
- 274.Romani, L., F. Bistoni, and P. Puccetti. 2002. Fungi, dendritic cells and receptors: a host perspective of fungal virulence. Trends Microbiol. 10:508-514. [DOI] [PubMed] [Google Scholar]
- 275.Romberg, L., and P. A. Levin. 2003. Assembly dynamics of the bacterial cell division protein FtsZ: poised at the edge of stability. Annu. Rev. Microbiol. 57:125-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rooney, P. J., and B. S. Klein. 2002. Linking fungal morphogenesis with virulence. Cell Microbiol. 4:127-137. [DOI] [PubMed] [Google Scholar]
- 277.Rosch, J., and M. Caparon. 2004. A microdomain for protein secretion in Gram-positive bacteria. Science 304:1513-1515. [DOI] [PubMed] [Google Scholar]
- 278.Rosch, J. W., and M. G. Caparon. 2005. The ExPortal: an organelle dedicated to the biogenesis of secreted proteins in Streptococcus pyogenes. Mol. Microbiol. 58:959-968. [DOI] [PubMed] [Google Scholar]
- 279.Rosenfeld, N., J. W. Young, U. Alon, P. S. Swain, and M. B. Elowitz. 2005. Gene regulation at the single-cell level. Science 307:1962-1965. [DOI] [PubMed] [Google Scholar]
- 280.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678-689. [PubMed] [Google Scholar]
- 281.Rudolph, C., G. Wanner, and R. Huber. 2001. Natural communities of novel archaea and bacteria growing in cold sulfurous springs with a string-of-pearls-like morphology. Appl. Environ. Microbiol. 67:2336-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rueda, S., M. Vicente, and J. Mingorance. 2003. Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle. J. Bacteriol. 185:3344-3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ruoff, K. L. 1991. Nutritionally variant streptococci. Clin. Microbiol. Rev. 4:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sahoo, S., R. K. Verma, A. K. Suresh, K. K. Rao, J. Bellare, and G. K. Suraishkumar. 2003. Macro-level and genetic-level responses of Bacillus subtilis to shear stress. Biotechnol. Prog. 19:1689-1696. [DOI] [PubMed] [Google Scholar]
- 285.Santos, J. M., P. Freire, M. Vicente, and C. M. Arraiano. 1999. The stationary-phase morphogene bolA from Escherichia coli is induced by stress during early stages of growth. Mol. Microbiol. 32:789-798. [DOI] [PubMed] [Google Scholar]
- 286.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Schaechter, M., O. Maaloe, and N. O. Kjeldgaard. 1958. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J. Gen. Microbiol. 19:592-606. [DOI] [PubMed] [Google Scholar]
- 288.Schembri, M. A., L. Hjerrild, M. Gjermansen, and P. Klemm. 2003. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J. Bacteriol. 185:2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Schmidt, J. M., and R. Y. Stanier. 1966. The development of cellular stalks in bacteria. J. Cell Biol. 28:423-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Schulz, H. 2002. Thiomargarita namibiensis: giant microbe holding its breath. ASM News 68:122-127. [Google Scholar]
- 291.Schulz, H. N., T. Brinkhoff, T. G. Ferdelman, M. H. Marine, A. Teske, and B. B. Jorgensen. 1999. Dense populations of a giant sulfur bacterium in Namibian shelf sediments. Science 284:493-495. [DOI] [PubMed] [Google Scholar]
- 292.Schulz, H. N., and B. B. Jørgensen. 2001. Big bacteria. Annu. Rev. Microbiol. 55:105-137. [DOI] [PubMed] [Google Scholar]
- 293.Scott, M. E., Z. Y. Dossani, and M. Sandkvist. 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc. Natl. Acad. Sci. USA 98:13978-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shapiro, J. A. 1987. Organization of developing Escherichia coli colonies viewed by scanning electron microscopy. J. Bacteriol. 169:142-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shapiro, J. A. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81-104. [DOI] [PubMed] [Google Scholar]
- 296.Shapiro, J. A., and C. Hsu. 1989. Escherichia coli K-12 cell-cell interactions seen by time-lapse video. J. Bacteriol. 171:5963-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Shapiro, L., H. H. McAdams, and R. Losick. 2002. Generating and exploiting polarity in bacteria. Science 298:1942-1946. [DOI] [PubMed] [Google Scholar]
- 298.Sherr, E. B., and B. F. Sherr. 1994. Bacterivory and herbivory: key roles of phagotrophic protists in pelagic food webs. Microb. Ecol. 28:223-235. [DOI] [PubMed] [Google Scholar]
- 299.Sherr, E. B., and B. F. Sherr. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Leeuwenhoek 81:293-308. [DOI] [PubMed] [Google Scholar]
- 300.Shigematsu, M., A. Umeda, S. Fujimoto, and K. Amako. 1998. Spirochaete-like swimming mode of Campylobacter jejuni in a viscous environment. J. Med. Microbiol. 47:521-526. [DOI] [PubMed] [Google Scholar]
- 301.Shikano, A., L. Luckinbill, and Y. Kurihara. 1990. Changes of traits in a bacterial population associated with protozoal predation. Microb. Ecol. 20:75-84. [DOI] [PubMed] [Google Scholar]
- 302.Shim, H., and S. T. Yang. 1999. Biodegradation of benzene, toluene, ethylbenzene, and o-xylene by a coculture of Pseudomonas putida and Pseudomonas fluorescens immobilized in a fibrous-bed bioreactor. J. Biotechnol. 67:99-112. [DOI] [PubMed] [Google Scholar]
- 303.Siefert, J. L., and G. E. Fox. 1998. Phylogenetic mapping of bacterial morphology. Microbiology 144:2803-2808. [DOI] [PubMed] [Google Scholar]
- 304.Simek, K., J. Vrba, J. Pernthaler, T. Posch, P. Hartman, J. Nedoma, and R. Psenner. 1997. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl. Environ. Microbiol. 63:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Simpson, J. B. (ed.). 1988. Simpson's contemporary quotations. Attribution from Time Magazine, 12 September 1960. Houghton Mifflin, Boston, Mass. [Online.] www.bartleby.com/63/.
- 306.Sogaard-Andersen, L. 2004. Cell polarity, intercellular signalling and morphogenetic cell movements in Myxococcus xanthus. Curr. Opin. Microbiol. 7:587-593. [DOI] [PubMed] [Google Scholar]
- 307.Søgaard-Andersen, L., M. Overgaard, S. Lobedanz, E. Ellehauge, L. Jelsbak, and A. A. Rasmussen. 2003. Coupling gene expression and multicellular morphogenesis during fruiting body formation in Myxococcus xanthus. Mol. Microbiol. 48:1-8. [DOI] [PubMed] [Google Scholar]
- 308.Sourjik, V. 2004. Receptor clustering and signal processing in E. coli chemotaxis. Trends Microbiol. 12:569-576. [DOI] [PubMed] [Google Scholar]
- 309.Sourjik, V., and H. C. Berg. 2000. Localization of components of the chemotaxis machinery of Escherichia coli using fluorescent protein fusions. Mol. Microbiol. 37:740-751. [DOI] [PubMed] [Google Scholar]
- 310.Spudich, J. L., and D. E. Koshland, Jr. 1976. Non-genetic individuality: chance in the single cell. Nature 262:467-471. [DOI] [PubMed] [Google Scholar]
- 311.Stackebrandt, E., and C. R. Woese. 1979. A phylogenetic dissection of the family Micrococcaceae. Curr. Microbiol. 2:317-322. [Google Scholar]
- 312.Stanley, P., E. Ordal, and J. T. Staley. 1979. High numbers of prosthecate bacteria in pulp mill waste aeration lagoons. Appl. Environ. Microbiol. 37:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Starr, M. P., H. Stolp, H. G. Trüper, A. Balows, and H. G. Schlegel (ed.). 1981. The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Berlin, Germany.
- 314.Steinberger, R. E., A. R. Allen, H. G. Hansma, and P. A. Holden. 2002. Elongation correlates with nutrient deprivation in Pseudomonas aeruginosa-unsaturated biofilms. Microb. Ecol. 43:416-423. [DOI] [PubMed] [Google Scholar]
- 315.Stephens, C. 2005. Senescence: even bacteria get old. Curr. Biol. 15:R308-R310. [DOI] [PubMed] [Google Scholar]
- 316.Stewart, B. J., J. L. Enos-Berlage, and L. L. McCarter. 1997. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J. Bacteriol. 179:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Stewart, E. J., R. Madden, G. Paul, and F. Taddei. 2005. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 3:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Suttle, C. A. 2005. Viruses in the sea. Nature 437:356-361. [DOI] [PubMed] [Google Scholar]
- 319.Sutton, W. D., C. E. Pankhurst, and A. S. Craig. 1981. The Rhizobium bacteriod state, p. 149-177. In K. L. Giles and A. G. Atherly (ed.), Biology of the Rhizobiaceae. Academic Press, Inc., New York, N.Y.
- 320.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogesesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 321.Szymanski, C. M., M. King, M. Haardt, and G. D. Armstrong. 1995. Campylobacter jejuni motility and invasion of Caco-2 cells. Infect. Immun. 63:4295-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Takeuchi, S., W. R. DiLuzio, D. B. Weibel, and G. M. Whitesides. 2005. Controlling the shape of filamentous cells of Escherichia coli. Nano Lett. 5:1819-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Tam, E., and J. L. Pate. 1985. Amino acid transport by prosthecae of Asticcacaulis biprosthecum: evidence for a broad-range transport system. J. Gen. Microbiol. 131:2687-2699. [DOI] [PubMed] [Google Scholar]
- 324.Tamames, J., M. Gonzalez-Moreno, J. Mingorance, A. Valencia, and M. Vicente. 2001. Bringing gene order into bacterial shape. Trends Genet. 17:124-126. [DOI] [PubMed] [Google Scholar]
- 325.Thar, R., and T. Fenchel. 2005. Survey of motile microaerophilic bacterial morphotypes in the oxygen gradient above a marine sulfidic sediment. Appl. Environ. Microbiol. 71:3682-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Thingstad, T. F. 2000. Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol. Oceanogr. 45:1320-1328. [Google Scholar]
- 327.Thomas, C., D. J. Hill, and M. Mabey. 1999. Morphological changes of synchronized Campylobacter jejuni populations during growth in single phase liquid culture. Lett. Appl. Microbiol. 28:194-198. [DOI] [PubMed] [Google Scholar]
- 328.Thomas, W. E., L. M. Nilsson, M. Forero, E. V. Sokurenko, and V. Vogel. 2004. Shear-dependent ‘stick-and-roll’ adhesion of type 1 fimbriated Escherichia coli. Mol. Microbiol. 53:1545-1557. [DOI] [PubMed] [Google Scholar]
- 329.Thomas, W. E., E. Trintchina, M. Forero, V. Vogel, and E. V. Sokurenko. 2002. Bacterial adhesion to target cells enhanced by shear force. Cell 109:913-923. [DOI] [PubMed] [Google Scholar]
- 330.Tolker-Nielsen, T., and S. Molin. 2000. Spatial organization of microbial biofilm communities. Microb. Ecol. 40:75-84. [DOI] [PubMed] [Google Scholar]
- 331.Tóth, L. G., and K. Kato. 1997. Size-selective grazing of bacteria by Bosmina longirostris—an image-analysis study. J. Plankton Res. 19:1477-1493. [Google Scholar]
- 332.Umeyama, T., A. Kaneko, Y. Nagai, N. Hanaoka, K. Tanabe, Y. Takano, M. Niimi, and Y. Uehara. 2005. Candida albicans protein kinase CaHsl1p regulates cell elongation and virulence. Mol. Microbiol. 55:381-395. [DOI] [PubMed] [Google Scholar]
- 333.Urban, C., X. Xiong, K. Sohn, K. Schroppel, H. Brunner, and S. Rupp. 2005. The moonlighting protein Tsa1p is implicated in oxidative stress response and in cell wall biogenesis in Candida albicans. Mol. Microbiol. 57:1318-1341. [DOI] [PubMed] [Google Scholar]
- 334.Vaknin, A., and H. C. Berg. 2004. Single-cell FRET imaging of phosphatase activity in the Escherichia coli chemotaxis system. Proc. Natl. Acad. Sci. USA 101:17072-17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.van Loosdrecht, M. C., J. Lyklema, W. Norde, and A. J. Zehnder. 1990. Influence of interfaces on microbial activity. Microbiol. Rev. 54:75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.van Loosdrecht, M. C. M., J. Lyklema, W. Norde, and A. J. B. Zehnder. 1989. Bacterial adhesion: a physicochemical approach. Microb. Ecol. 17:1-15. [DOI] [PubMed] [Google Scholar]
- 337.Vanounou, S., A. H. Parola, and I. Fishov. 2003. Phosphatidylethanolamine and phosphatidylglycerol are segregated into different domains in bacterial membrane. A study with pyrene-labelled phospholipids. Mol. Microbiol. 49:1067-1079. [DOI] [PubMed] [Google Scholar]
- 338.Varma, A., and K. D. Young. 2004. FtsZ collaborates with penicillin binding proteins to generate bacterial cell shape in Escherichia coli. J. Bacteriol. 186:6768-6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Vasse, J., F. de Billy, S. Camut, and G. Truchet. 1990. Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172:4295-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Vignon, G., R. Kohler, E. Larquet, S. Giroux, M. C. Prevost, P. Roux, and A. P. Pugsley. 2003. Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides. J. Bacteriol. 185:3416-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Vinck, A., M. Terlou, W. R. Pestman, E. P. Martens, A. F. Ram, C. A. M. J. J. van den Hondel, and H. A. B. Wösten. 2005. Hyphal differentiation in the exploring mycelium of Aspergillus niger. Mol. Microbiol. 58:693-699. [DOI] [PubMed] [Google Scholar]
- 342.Vinella, D., D. Joseleau-Petit, D. Thévenet, P. Bouloc, and R. D'Ari. 1993. Penicillin-binding protein 2 inactivation in Escherichia coli results in cell division inhibition, which is relieved by FtsZ overexpression. J. Bacteriol. 175:6704-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Vinette, K. M., K. M. Gibney, R. Proujansky, and P. T. Fawcett. 2002. Growth of Helicobacter pylori in a long spiral form does not alter expression of immunodominant proteins. BMC Microbiol. 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wadhams, G. H., A. C. Martin, A. V. Warren, and J. P. Armitage. 2005. Requirements for chemotaxis protein localization in Rhodobacter sphaeroides. Mol. Microbiol. 58:895-902. [DOI] [PubMed] [Google Scholar]
- 345.Wadhams, G. H., A. V. Warren, A. C. Martin, and J. P. Armitage. 2003. Targeting of two signal transduction pathways to different regions of the bacterial cell. Mol. Microbiol. 50:763-770. [DOI] [PubMed] [Google Scholar]
- 346.Wagner, J. K., S. Setayeshgar, L. A. Sharon, J. P. Reilly, and Y. V. Brun. 2006. A nutrient uptake role for bacterial cell envelope extensions. Proc. Natl. Acad. Sci. USA 103:11772-11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wainwright, M. 1997. Extreme pleomorphism and the bacterial life cycle: a forgotten controversy. Perspect. Biol. Med. 40:407-414. [Google Scholar]
- 348.Wall, D., and D. Kaiser. 1998. Alignment enhances the cell-to-cell transfer of pilus phenotype. Proc. Natl. Acad. Sci. USA 95:3054-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Walsby, A. E. 2005. Archaea with square cells. Trends Microbiol. 13:193-195. [DOI] [PubMed] [Google Scholar]
- 350.Walsby, A. E. 1980. A square bacterium. Nature 283:69-71. [Google Scholar]
- 351.Walsby, A. E. 2005. Stratification by cyanobacteria in lakes: a dynamic buoyancy model indicates size limitations met by Planktothrix rubescens filaments. New Phytol. 168:365-376. [DOI] [PubMed] [Google Scholar]
- 352.Wang, L. H., Y. He, Y. Gao, J. E. Wu, Y. H. Dong, C. He, S. X. Wang, L. X. Weng, J. L. Xu, L. Tay, R. X. Fang, and L. H. Zhang. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903-912. [DOI] [PubMed] [Google Scholar]
- 353.Ward, J. E., Jr., and J. Lutkenhaus. 1985. Overproduction of FtsZ induces minicell formation in E. coli. Cell 42:941-949. [DOI] [PubMed] [Google Scholar]
- 354.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Webb, M. 1953. Effects of magnesium on cellular division in bacteria. Science 118:607-611. [DOI] [PubMed] [Google Scholar]
- 356.Webb, M. 1948. The influence of magnesium on cell division. I. The growth of Clostridium welchii in complex media deficient in magnesium. J. Gen. Microbiol. 2:275-287. [Google Scholar]
- 357.Wei, Y., T. Havasy, D. C. McPherson, and D. L. Popham. 2003. Rod shape determination by the Bacillus subtilis class B penicillin-binding proteins encoded by pbpA and pbpH. J. Bacteriol. 185:4717-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Weinbauer, M. G., and M. G. Höfle. 1998. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl. Environ. Microbiol. 64:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Weinbauer, M. G., and M. G. Höfle. 1998. Size-specific mortality of lake bacterioplankton by natural virus communities. Aquat. Microb. Ecol. 15:103-113. [Google Scholar]
- 360.Weinbauer, M. G., and P. Peduzzi. 1995. Significance of viruses versus heterotrophic nanoflagellates for controlling bacterial abundance in the northern Adriatic Sea. J. Plankton Res. 17:1851-1856. [Google Scholar]
- 361.Weinbauer, M. G., and F. Rassoulzadegan. 2004. Are viruses driving microbial diversification and diversity? Environ. Microbiol. 6:1-11. [DOI] [PubMed] [Google Scholar]
- 362.Weiss, D. S. 2004. Bacterial cell division and the septal ring. Mol. Microbiol. 54:588-597. [DOI] [PubMed] [Google Scholar]
- 363.Weiss, T. H., A. L. Mills, G. M. Hornberger, and J. S. Herman. 1995. Effect of bacterial cell shape on transport of bacteria in porous media. Environ. Sci. Technol. 29:1737-1740. [DOI] [PubMed] [Google Scholar]
- 364.Willis, A. 1994. This cell that had split. Perspect. Biol. Med. 37:361. [Google Scholar]
- 365.Wills, A. P., and E. C. Chan. 1978. Morphogenetic expression of Arthrobacter globiformis 425 in continuous culture with carbon or biotin limitation. Can. J. Microbiol. 24:28-30. [DOI] [PubMed] [Google Scholar]
- 366.Woese, C. R., P. Blanz, R. B. Hespell, and C. M. Hahn. 1982. Phylogenetic relationships among various helical bacteria. Curr. Microbiol. 7:119-124. [Google Scholar]
- 367.Woese, C. R., S. Maloy, L. Mandelco, and H. D. Raj. 1990. Phylogenetic placement of the Spirosomaceae. Syst. Appl. Microbiol. 13:19-23. [DOI] [PubMed] [Google Scholar]
- 368.Woldringh, C., P. Huls, E. Pas, G. J. Brakenhoff, and N. Nanninga. 1987. Topography of peptidoglycan synthesis during elongation and polar cap formation in a cell division mutant of Escherichia coli MC4100. J. Gen. Microbiol. 133:575-586. [Google Scholar]
- 369.Woldringh, C. L. 2005. Is Escherichia coli getting old? Bioessays 27:770-774. [DOI] [PubMed] [Google Scholar]
- 370.Wolgemuth, C., E. Hoiczyk, D. Kaiser, and G. Oster. 2002. How myxobacteria glide. Curr. Biol. 12:369-377. [DOI] [PubMed] [Google Scholar]
- 371.Wolgemuth, C. W., and G. Oster. 2004. The junctional pore complex and the propulsion of bacterial cells. J. Mol. Microbiol. Biotechnol. 7:72-77. [DOI] [PubMed] [Google Scholar]
- 372.Wood, A. P., and D. P. Kelly. 1993. Reclassification of Thiobacillus thyasiris as Thiomicrospira thyasirae comb. nov., an organism exhibiting pleomorphism in response to environmental conditions. Arch. Microbiol. 159:45-47. [Google Scholar]
- 373.Wortinger, M. A., E. M. Quardokus, and Y. V. Brun. 1998. Morphological adaptation and inhibition of cell division during stationary phase in Caulobacter crescentus. Mol. Microbiol. 29:963-973. [DOI] [PubMed] [Google Scholar]
- 374.Wu, L. J., and J. Errington. 2003. RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol. Microbiol. 49:1463-1475. [DOI] [PubMed] [Google Scholar]
- 375.Wu, Q. L., J. Boenigk, and M. W. Hahn. 2004. Successful predation of filamentous bacteria by a nanoflagellate challenges current models of flagellate bacterivory. Appl. Environ. Microbiol. 70:332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Yu, X. C., and W. Margolin. 2000. Deletion of the min operon results in increased thermosensitivity of an ftsZ84 mutant and abnormal FtsZ ring assembly, placement, and disassembly. J. Bacteriol. 182:6203-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Yurkov, V. V., and J. T. Beatty. 1998. Aerobic anoxygenic phototrophic bacteria. Microbiol. Mol. Biol. Rev. 62:695-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhao, J., and A. M. Lambowitz. 2005. A bacterial group II intron-encoded reverse transcriptase localizes to cellular poles. Proc. Natl. Acad. Sci. USA 102:16133-16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Zhu, J., K. Jäger, T. Black, K. Zarka, O. Koksharova, and C. P. Wolk. 2001. HcwA, an autolysin, is required for heterocyst maturation in Anabaena sp. strain PCC 7120. J. Bacteriol. 183:6841-6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Zinder, S. H., and M. Dworkin. 2001. Morphological and physiological diversity. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, release 3.6, 3rd ed. Springer-Verlag, New York, N.Y. [Online.] www.prokaryotes.com.