Abstract
Neutrophil spontaneous death plays essential roles in neutrophil homeostasis and resolution of inflammation, whereas the underlying molecular mechanisms are still ill-defined. Neutrophils die because of programmed cell death or apoptosis. However, treatment with inhibitor of caspases, which are responsible for the majority of apoptotic cell deaths, does not prevent the spontaneous death of neutrophils. PKB/Akt possesses prosurvival and antiapoptotic activities in a variety of cells. In this study, we show that Akt activity decreases dramatically during the course of neutrophil death. Both phosphatidylinositol 3-kinase and Akt inhibitors enhance neutrophil death. Conditions delaying neutrophil death, such as treatment with granulocyte–macrophage colony-stimulating factor, granulocyte colony-stimulating factor, or IFN-γ, restore Akt activity. Finally, we demonstrate that neutrophils depleted of PTEN, a phosphatidylinositol 3′-phosphatase that negatively regulates Akt activity, live much longer than WT neutrophils. Thus, we establish Akt deactivation as a causal mediator of neutrophil spontaneous death.
Keywords: apoptosis, PTEN
Neutrophils are the most abundant cell type among circulating white blood cells and constitute the first line of host defense against invading pathogens (bacteria, fungi, viruses, etc.) (1–3). These cells are terminally differentiated and usually have a very short lifespan (1–4 days in tissues). They die via spontaneous programmed cell death (apoptosis). The daily turnover of human neutrophils is ≈0.8–1.6 × 109 cells per kg of body weight. Properly regulated death program is essential for neutrophil homeostasis. Augmented neutrophil death leads to a decrease of neutrophil counts in the blood (neutropenia), which will increase the chance of contracting a bacterial or fungal infection. On the other hand, delayed neutrophil death elevates neutrophil counts in the blood (neutrophilia), which often is associated with pathological conditions such as bacterial infection, myeloid leukemia, and acute myocardial infarction. Programmed neutrophil death is also an essential cellular event for maintaining neutrophil numbers in the sites of infection and inflammation. Neutrophils are recruited to the infected tissues to engulf, kill, and digest invading microorganisms. However, the enzymes and reactive oxygen species released by neutrophils also can damage the surrounding tissues. Thus, the death program in neutrophils need to be well controlled to provide a nice balance between their immune functions and their safe clearance. Delayed clearance of neutrophils in inflamed tissues causes unwanted and exaggerated tissue inflammation (4–7).
PtdIns(3,4,5)P3/Akt signaling pathway possesses prosurvival and antiapoptotic activities in a variety of cell types. PtdIns(3,4,5)P3 (phosphatidylinositol 3,4,5-trisphosphate) contains two hydrophobic fatty acids and, therefore, are mainly localized on the plasma membrane (8). PtdIns(3,4,5)P3 exerts its function by mediating protein translocation via binding to their pleckstrin homolog-domains (9, 10). PKB/Akt, a serine/threonine protein kinase with oncogenic and antiapoptotic activities, is one of the major downstream factors of PtdIns(3,4,5)P3 (11, 12). Akt contains apleckstrin homolog domain, which specifically binds PtdIns(3,4,5)P3. The PtdIns(3,4,5)P3-mediated membrane translocation of Akt is essential for its phosphorylation and activation. Activated Akt, in turn, phosphorylates a variety of proteins, including several associated with cell survival/death pathways such as BAD, Forkhead, ASK1, and NF-κB, leading to diminished apoptotic cell death (12, 13). PtdIns(3,4,5)P3 level on the plasma membrane is regulated by phosphatidylinositol 3-kinases (PI3K) (12, 14–16) and the tumor suppressor PTEN (Phosphatase and tensin homologue deleted on chromosome 10), a phosphatidylinositol 3′-phosphatase that converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2 (17, 18).
Recently, we demonstrated that deactivation of PtdIns(3,4,5)P3/Akt signal characterizes both caspase-dependent and -independent cell death (19). In the present study, we investigated the contribution of PI3K-Akt pathway in neutrophil spontaneous death. We demonstrate that PtdIns(3,4,5)P3/Akt signal is deactivated significantly during neutrophil death. Inhibition of PtdIns(3,4,5)P3/Akt signal further promotes neutrophil death. Moreover, augmentation of PtdIns(3,4,5)P3/Akt signal by depleting PTEN prevents neutrophil spontaneous death. Thus, we establish Akt deactivation as a causal mediator in neutrophil spontaneous death.
Results
Treatment with Caspase Inhibitor Does Not Prevent the Spontaneous Death of Neutrophils.
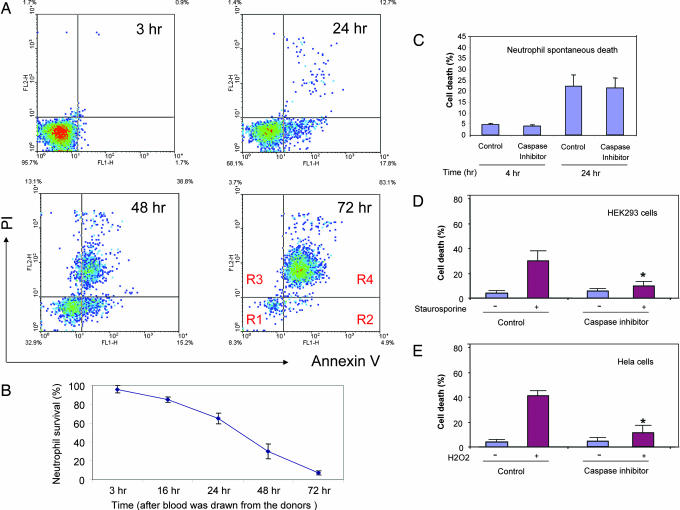
In this study, we used a well established in vitro system to explore neutrophil spontaneous death. Human primary neutrophils were prepared from freshly collected human-citrated whole blood by using a Ficoll-Hypaque solution. After 21 h of culturing, many neutrophils manifest clear morphological signs of apoptosis such as cell shrinkage and nuclear condensation (Fig. 7, which is published as supporting information on the PNAS web site). The number of neutrophils undergoing spontaneous death was quantified by using FACS analysis. In the FACS assays, we use Annexin V, an anticoagulant protein that has high affinity and selectivity for phosphatidylserine, to detect phosphatidylserine exteriorization and propidium iodide (PI), a membrane impermeable dye, to monitor cell membrane integrity (Fig. 7). The phosphatidylserine exposure was evident in neutrophils by 12 h, and the level increased to 35 ± 4% at 24 h. We detected a concomitant increase in the apoptotic (Fig. 1A, R2) and necrotic (Fig. 1A, R3 and R4) populations at 48 h, suggesting that at later time points many of the apoptotic cells have proceeded into secondary necrosis (Fig. 1 A and B).
Fig. 1.
Neutrophil spontaneous death. (A) Human neutrophils were cultured in RPMI medium 1640 containing 10% heat-inactivated FBS at a density of 2 × 106 cells per ml. Apoptotic cells were detected by annexin V-FITC staining and propidium iodide (PI) staining. Ten thousand cells were collected and analyzed by using the CellQuest software (Becton Dickinson). Similar results were obtained in three separate healthy donors. Region R1, viable cell; Region R2, early apoptotic cells; Region R3 and R4, late apoptotic cells and necrotic cells. (B) Time course of neutrophil spontaneous death (R2–R4). All values represent mean ± SD of three separate experiments. (C) The broad-spectrum caspase inhibitor zVAD-fmk does not suppress neutrophil spontaneous death. Human primary neutrophils were isolated and cultured in the presence of 10 μM zVAD-fmk. The cell death was analyzed by FACS as described in A. (D) zVAD-fmk blocks staurosporine-induced HEK293 cell death. The cell death was induced with 1 μM staurosporine. zVAD-fmk (10 μM) was added 1 h before drug treatments. Toxicity was assessed 12 h after the treatment by using a TUNEL assay. Cell death was determined as the ratio of dead-to-total cell number and quantified by counting 1,000 cells. (E) zVAD-fmk blocks H2O2-induced HeLa cell death. The cell death was induced with 1 mM H2O2, and zVAD-fmk (10 μM) was added 1 h before the treatments. Cell death was determined as described above. ∗, P < 0.01 vs. caspase inhibitor untreated apoptotic cells.
Using this system, we investigated the role of caspases in neutrophil spontaneous death. As reported in ref. 20, the broad-spectrum caspase inhibitor zVAD-fmk blocked caspase-dependent apoptosis, such as staurosporine-induced death of HEK293 cells and H2O2-elicited death of HeLa cells, whereas the same drug did not suppress spontaneous neutrophil death at all (Fig. 1 C–E).
Akt Signaling Pathway Is Down-Regulated During Neutrophil Spontaneous Death.
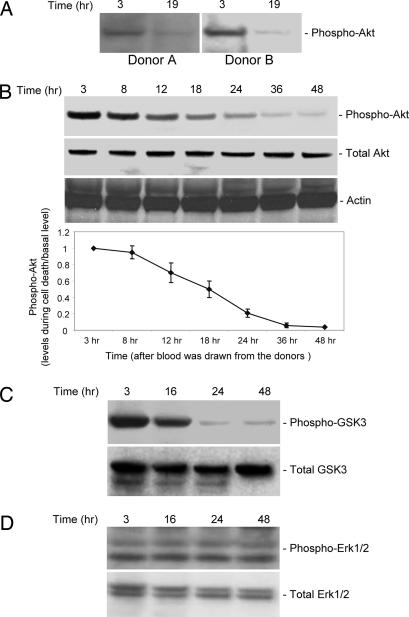
We recently showed that deactivation of PtdIns(3,4,5)P3/Akt signal characterizes both caspase-dependent and -independent cell death. To investigate whether the PtdIns(3,4,5)P3/Akt survival pathway is involved in neutrophil spontaneous death, we examined the activation of this signal in neutrophil spontaneous death. Akt is recruited onto the plasma membrane through its specific binding to PtdIns(3,4,5)P3. Only the Akt molecules on the plasma membrane can be phosphorylated and activated by two phosphatidylinositol-dependent protein kinases and get activated, thus Akt phosphorylation has been used widely as an indicator of Akt activation (12, 14, 15, 19, 21–23). Our results show that during the course of neutrophil death, levels of phospho-Akt decrease dramatically, whereas levels of total Akt do not change (Fig. 2A). This result was detected in all of the blood donors we examined (>10 donors). The level of phospho-Akt declines by more than one-half in only 15 h in culture (Fig. 2B). We next measured the Akt activity directly. We used glycogen synthase kinase-3β (GSK-3β), a substrate of Akt (24, 25), as a marker for in vivo Akt activation. Levels of phospho-GSK-3β decline during neutrophil death, with a time course similar to the decline in levels of phospho-Akt. By contrast, total GSK-3β levels do not change (Fig. 2C). Noticeably, the Akt activity started to decrease before any apoptotic morphology changes were detected, indicating that Akt deactivation might be a causal mediator of neutrophil death. The decreases in phospho-Akt are selective. We measured activity of MAPK p44/42 (Erk1/2) pathway (Fig. 2D). During neutrophil spontaneous death, we detect no alteration in any of these phosphokinases 3–48 h after blood was drawn from the donors.
Fig. 2.
Akt activity is down-regulated during neutrophil spontaneous death. (A) The level of phosphorylation of endogenous Akt decreases during neutrophil spontaneous death. Neutrophils were cultured as described in Fig. 1. Protein extracts were resolved on SDS/PAGE. Total and phosphorylated Akt were detected by Western blot with anti-Akt and anti-phospho-Akt (Ser-473) antibodies (Cell Signaling Technology, Beverly, MA), respectively. Representative results of >10 donors are shown. (B) Time course of Akt deactivation. Relative amounts of phosphorylated Akt were quantified by using NIH Image software. All samples were normalized to the amount of total Akt. “Basal signal” refers to the level of phospho-Akt at time “3 hr.” Data presented are the means (± SD) of three independent experiments. (C) Akt protein kinase activity decreases during neutrophil spontaneous death. Neutrophils were cultured as described in Fig. 1 and lysed at each indicated time point. Protein extracts were resolved on SDS/PAGE and immunoblotted with indicated antibodies. GSK-3β is one of the targets of Akt, and its phosphorylation was detected by using phospho-GSK-3β (Ser-9) antibody (Cell Signaling Technology). All values represent mean ± SD of three separate experiments. (D) MAPK p44/42 (Erk1/2) activity is not altered during neutrophil spontaneous death. Erk1/2 activity was determined by Western blotting analysis with phospho-Eek1/2 antibody (Cell Signaling Technology).
Inhibition of PtdIns(3,4,5)P3/Akt Signaling Promotes Neutrophil Death.
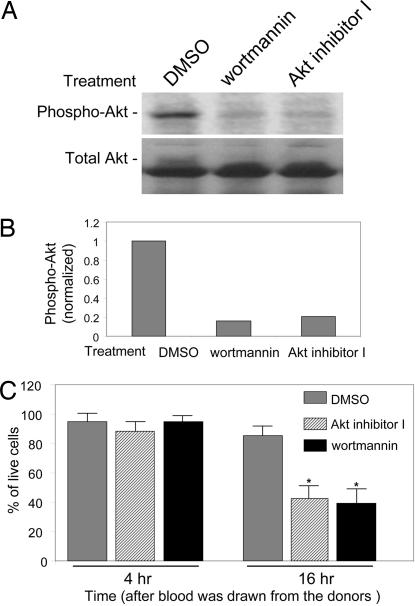
To test the hypothesis that deactivation of PtdIns(3,4,5)P3/Akt signaling is a causal mediator of neutrophil spontaneous death, we examined whether inhibition of this pathway can promote further neutrophil death. We used a newly developed Akt inhibitor, Akt inhibitor I, to suppress the PtdIns(3,4,5)P3/Akt signaling in neutrophils. Moreover, because PI3K is upstream of Akt and inhibition of PI3K is associated with deactivation of Akt, we also treated neutrophil cultures with the PI3K inhibitor wortmannin. Treatment with these drugs markedly deactivates Akt without altering total Akt levels (Fig. 3A and B). Both drugs promoted neutrophil death as monitored by FACS analysis (Fig. 3C).
Fig. 3.
Deactivation of PtdIns(3,4,5)P3/Akt signaling by Akt or PI3K inhibitors promotes neutrophil death. (A) Deactivation of Akt by PI3K or Akt inhibitors. Freshly prepared neutrophils (3 h after blood was drawn from healthy donors) were treated with 50 nM wortmannin or 10 μM Akt inhibitor I (Calbiochem, San Diego, CA) for 1 h. Akt activity (phosphorylation) was assessed by Western blotting analysis as described above. (B) Relative amounts of phosphorylated Akt in A were quantified by using NIH Image software. All inhibitors markedly decreased Akt activity. (C) Cell viability was assessed by FACS analysis as described in Fig. 1. Inhibitors were added at 3 h after blood was drawn from the donors. At least three separate experiments were carried out with a minimum of 100,000 cells counted per data point. Cell viability was determined as the ratio of live-to-total cell number. The results are the means of three independent experiments. Bars indicate mean ± SD. ∗, P < 0.001 versus untreated cells by Student's t test.
Factors Preventing Neutrophil Spontaneous Death Restore Akt Kinase Activity.
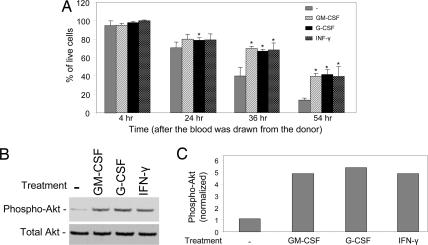
If Akt deactivation mediates neutrophil spontaneous cell death, then treatments that prevent neutrophil death should inhibit the deactivation. Many extracellular factors, such as granulocyte–macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and IFN-γ (LPS), have been shown to block neutrophil spontaneous death (4–7, 26, 27). We have confirmed the antineutrophil death effect of these reagents in our experimental system (Fig. 4A). In addition, we showed that these factors also can prevent deactivation of Akt during neutrophil death with no alterations in total Akt levels (Fig. 4 B and C). These results are consistent with our hypothesis that Akt deactivation is an essential causal mediator in neutrophil spontaneous death.
Fig. 4.
Conditions preventing neutrophil spontaneous death restore Akt kinase activity. (A) Granulocyte–macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), and IFN-γ rescue neutrophils from spontaneous death. Human primary neutrophils were cultured in the presence of 20 ng/ml recombinant human GM-CSF (PromoCell, Heidelberg, Germany), 20 ng/ml recombinant human G-CSF (PromoCell), or 1,500 units/ml IFN-γ (Sigma, St. Louis, MO). Cell death was analyzed as described in Fig. 1 at each indicated time point. (B) Total and phosphorylated Akt were detected by Western blotting analysis as described in Fig. 2. Neutrophils cultured for 21 h (24 h after the blood was drawn from the donor) were used in this experiment. (C) Relative amounts of phosphorylated Akt in B were quantified by using NIH Image software.
Augmentation of PtdIns(3,4,5)P3/Akt Signal by PTEN Depletion Prevents Neutrophil Spontaneous Death.
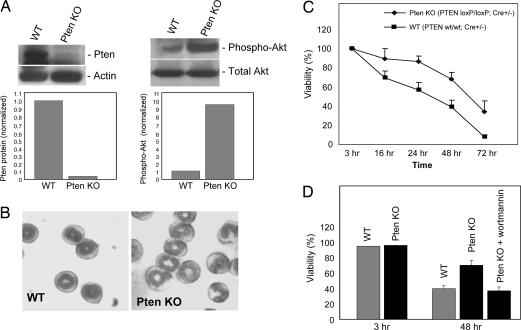
If Akt deactivation mediates neutrophil spontaneous death, then elevating this pathway should diminish neutrophil death. To test this idea, we tried to augment PtdIns(3,4,5)P3/Akt signaling by disrupting PTEN gene. The tumor suppressor PTEN is a phosphatidylinositol 3′-phosphatase that converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2. Depletion of this lipid phosphatase leads to accumulation of PtdIns(3,4,5)P3 on the plasma membrane and, thus, elevation of PtdIns(3,4,5)P3/Akt signaling. To examine the effect of elevated PtdIns(3,4,5)P3/Akt signaling on neutrophil death, we isolated neutrophils from PTEN knockout mice. Because of the early embryonic lethality of conventional Pten−/− mice (28), we used a conditional Pten knockout mouse, in which two loxP sequences were inserted on either side of the exon 5 of PTEN encoding the phosphatase domain (Fig. 8, which is published as supporting information on the PNAS web site). We then crossed this mouse with a myeloid-specific Cre line, in which the Cre recombinase gene was inserted into the endogenous M lysozyme locus (Fig. 8). Mice that are homozygous for this allele are viable, fertile, normal in size, and do not display any gross physical or behavioral abnormalities (data not shown). The expression of WT PTEN protein is completely abolished in neutrophils isolated from either Cre+/−;PTEN loxP/loxP or Cre+/+;PTEN loxP/loxP mice (Fig. 5A and data not shown). Thus, Cre-mediated deletion of the loxP-flanked PTEN gene in myeloid cells is highly efficient. The amount of Cre recombinase expressed from only one copy of the Cre gene is enough to initiate PTEN deletion (Fig. 5A).
Fig. 5.
Augmentation of PtdIns(3,4,5)P3/Akt signal by PTEN depletion delays neutrophil spontaneous death. (A) Western blot analysis of neutrophil proteins. Mouse bone marrow was isolated from the femur and the tibia of 10-week-old mice. PTEN protein was detected with a specific anti-PTEN antibody (Cell Signaling Technology). Akt activation was monitored by its phosphorylation as described above. (B) Neutrophil morphology examined by Wright–Giemsa staining. (C) The death of neutrophils isolated from bone marrow. Mouse neutrophils were isolated from bone marrow and cultured for indicated times as described in Methods. The percentage of live cells was calculated as described in Fig. 1. The results are the means (±SD) of at least three independent experiments. (D) The delayed death of PTEN-null neutrophils can be reversed by the PI3K inhibitor wortmannin. Freshly prepared mouse neutrophils were treated with 50 nM wortmannin, and the cell death was examined at each indicated time point as described above.
In neutrophils depleted of the PTEN gene, PtdIns(3,4,5)P3/Akt signaling, monitored by Akt phosphorylation, is dramatically enhanced (Fig. 5A). This result is consistent with the role of PTEN as a PtdIns(3,4,5)P3 phosphatase. PTEN-null neutrophils differentiate normally, and neutrophil count in peripheral blood is the same between PTEN knockout and WT mice (Fig. 5B and data not shown). We measured the cell death rate of neutrophils directly isolated from the bone marrow. We found that Pten-null neutrophils live much longer than WT neutrophils, and this effect was observed at all time points examined. Only 5% of WT neutrophils could live>72 h in the culture, whereas ≈40% PTEN-null neutrophils were detected healthy under the same condition (Fig. 5C). Delayed death of PTEN-null neutrophils can be reversed by treatment with the PI3K inhibitor wortmannin, suggesting this delayed neutrophil death is directly mediated by PtdIns(3,4,5)P3 signaling (Fig. 5D). The same result was obtained when in vitro-generated neutrophils were used (Fig. 6). More than 85% of cells in WT granulocyte colonies died at day 22, whereas only <10% of PTEN-null neutrophils went to apoptosis.
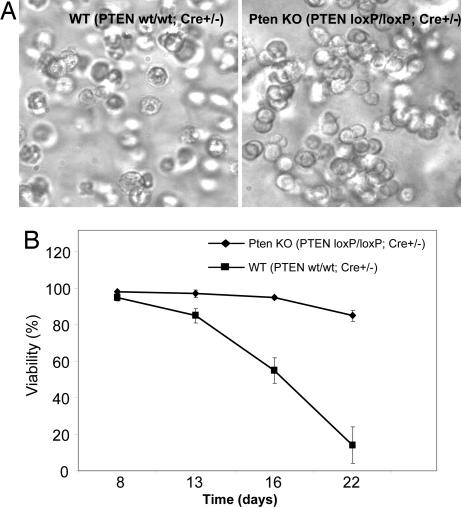
Fig. 6.
Spontaneous death of in vitro-generated neutrophils. Mouse bone marrow was isolated from the femur and the tibia as described in Fig. 5. The granulocyte colonies were generated by using a methylcellulose-base semisolid medium as described in Methods. Neutrophil death was analyzed after cells were cultured for the indicated number of days. (A) Phase-contrast photomicrograph of in vitro-generated WT and PTEN-null neutrophils (day 22). (B) Cell viability was determined as the ratio of live-to-total cell number in the granulocyte colonies. The assay was conducted as described in Fig. 1. The results are the means (±SD) of at least three independent experiments.
In the myeloid-specific Cre line, the Cre recombinase gene was inserted into the lysozyme locus and, therefore, the endogenous lysozyme gene is disrupted. To ascertain that the phenotype we observed is caused by PTEN disruption instead of lysozyme deletion, we examined the neutrophils isolated from Cre+/+; PTEN wt/wt mice, which express WT PTEN but contain two mutated lysozyme alleles. These cells die at a similar rate as WT neutrophils, suggesting that depletion of lysozyme does not affect neutrophil spontaneous death. Furthermore, the diminished cell death of PTEN-null neutrophils could be reversed by treatment with PI3K or Akt inhibitors, suggesting that this effect is directly caused by elevated Akt activity in PTEN-null neutrophils (data not shown). In fact, we routinely use only Cre heterozygous mice in our assay (Fig. 5 and 6). Thus, effects caused by lysozyme depletion will be minimized.
Discussion
Neutrophil spontaneous death shares many features of classical apoptosis, such as cell body shrinkage, exteriorization of phosphatidylserine from inner to outer leaflet of plasma membrane, mitochondria depolarization, nuclear condensation, and DNA fragmentation (27). Most apoptosis is a caspase-dependent process. Caspase activation during neutrophil death has been well documented (4–7, 27). However, although caspase activity is essential for ligand (e.g., TNFα)-induced apoptosis of neutrophil, the broad-spectrum caspase inhibitor zVAD-fmk or Boc-D-fmk could not suppress constitutive spontaneous neutrophil death (20). Pongracz et al. (29) reported that Caspase 3 inhibitor DEVD-fmk could partially inhibit neutrophil death. In their experimental system, neutrophil apoptosis rate was much higher than the neutrophil spontaneous death rate, reaching 70% in 24 h. Treatment with DEVD-fmk reduced it to the spontaneous death rate (35–40%), suggesting that the neutrophil death they investigated might be a combination of spontaneous death and cytokine-induced death.
The molecular mechanism governing this caspase-independent neutrophil spontaneous death is still largely unknown. The susceptibility of neutrophils to apoptosis appears to depend on the balance between proapoptotic and survival (antiapoptotic) signals. Cell death can be triggered by augmenting proapoptotic signals or attenuating survival signals. In recent years, the activation of proapoptotic pathways in neutrophil spontaneous death has been studied extensively, and several important players such as BAD, reactive oxygen species, and p38MAPK have been identified (4–7). Nevertheless, the contribution of the deactivation of survival signals to neutrophil death has not yet been investigated. In this study, we reported that the deactivation of a well known survival signal, PI3K-Akt pathway, plays a causal role in neutrophil spontaneous death. Augmentation of PtdIns(3,4,5)P3/Akt signal by PTEN depletion significantly prevents neutrophil spontaneous death, thus opening a previously undescribed avenue for intervening in neutrophil death. This study also advances our knowledge of the molecular mechanism of cell death (particularly the caspase-independent apoptosis) in general and helps us to understand death signaling in other types of cells, such as hematopoietic stem cells, monocytes/macrophages, lymphocytes, and leukemia cells.
The upstream deactivators of Akt remain to be elucidated. The best established activator of Akt is PtdIns(3,4,5)P3 generated by PI3K (12, 15). Conceivably, deactivation of PI3K is responsible for Akt's deactivation. PtdIns(3,4,5)P3 level also can be regulated by the tumor suppressor PTEN and SHIP (SH2-containing inositol 5′-phosphatase), which converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively (18, 30). PTEN or/and SHIP might get activated during neutrophil spontaneous death, leading to down-regulation of Akt. Similar with what is discovered in the PTEN-null neutrophils, Gardai et al. (31) reported that the half-life of neutrophils depleted of SHIP also was dramatically increased. Akt activation relies on its membrane translocation mediated by its specific association with PtdIns(3,4,5)P3 on the plasma membrane. Recently, we demonstrated that two inositol phosphates, InsP7 and Ins(1,3,4,5)P4, compete for Akt-pleckstrin homolog domain binding with PtdIns(3,4,5)P3, providing another level of regulation for Akt membrane translocation and activation (32). Thus, Akt deactivation also could be a result of elevated InsP7 or Ins(1,3,4,5)P4 level.
Multicellular organisms defend themselves against invading pathogens (bacteria, fungi, and viruses) by mounting both innate and adaptive immune responses. Neutrophil accumulation at sites of infection is essential for host defense. However, exaggerated accumulation could be responsible for pathogenesis of many acute and chronic inflammatory diseases, such as pneumonia, asthma, multiple sclerosis, and rheumatoid arthritis. There is a consensus that the rate of neutrophil spontaneous death can determine the number of neutrophils at the sites of inflammation and, therefore, are important in the regulation of inflammation. Results presented in this paper provide a previously undescribed strategy and target for the treatment of a variety of infectious and inflammatory diseases associated with delayed or enhanced neutrophil death.
Neutrophil spontaneous death also plays essential roles in neutrophil homeostasis. We examined whether disruption of PTEN can increase peripheral blood neutrophil count due to delayed spontaneous death but did not detect any alteration. Peripheral blood neutrophil count is decided by multiple cellular processes, such as cytokine-elicited mobilization from bone marrow, spontaneous death, transmigration from blood to tissues, and clearance by phagocytic cells. Conceivably, depletion of PTEN can affect processes other than spontaneous death, and these effects are able to overcome the effect elicited by delayed neutrophil death, leading to unaltered peripheral blood neutrophil count.
Methods
Human Primary Neutrophils.
We isolated human primary neutrophils from discarded white blood cell filters (WBF2 filter; Pall Corporation, East Hills, NY), which were provided by the Blood Bank Lab at Children's Hospital Boston. Neutrophils are purified by using a standard protocol. Briefly, erythrocytes were sedimented by adding an equal volume of dextran/saline solution (3% dextran T-500 in 0.9% NaCl) at room temperature for 25 min. The erythrocyte-depleted supernatants then were layered on Lymphocyte Separation Medium (1.077 g/ml Ficoll-Hypaque solution; Voigt Global Distribution LLC, Kansas City, MO) and centrifuged at 400 × g at room temperature for 20 min. Contaminated erythrocytes in the neutrophil pellets were lysed after a brief (<30 seconds) treatment with 0.2% NaCl. Neutrophils then were resuspended in RPMI medium 1640 containing 10% heat-inactivated FBS at a density of 4 × 106 cells per ml and maintained at 37°C. The purity of neutrophils was >97% as determined by both Wright–Giemsa staining and FACS analysis with CD15 antibody (data not shown). We routinely obtained ≈1–3 × 108 neutrophils from one filter (450 ml of blood from a healthy donor). We compared the neutrophils that we collected through filter with those obtained by vein puncture and stored in anticoagulant testing tubs and found that the filtration method does not impair neutrophil function (e.g., chemotaxis and the time course of cell death). All blood was drawn from healthy blood donors. All protocols were approved by the Children's Hospital Institutional Review Board and were subjected to annual review.
Mouse Neutrophils.
The conditional Pten knockout mouse (PTEN loxP/loxP) and the myeloid-specific Cre mouse were purchased from The Jackson Laboratory (Bar Harbor, ME). Mouse genotyping was performed by using a standard protocol provided by The Jackson Laboratory. Mouse neutrophils were prepared from bone marrow, which was isolated from the femur and the tibia of 10-week-old mice. Bone marrow neutrophils are separated by centrifugation over a three-layer Percoll gradient (78%/69%/52%). We routinely obtain 7–8 million cells from one mouse, and >90% of them are morphologically mature neutrophils (donut-shape, segmented nucleus). All animal manipulations were conducted in accordance with the Animal Welfare Guidelines of Children's Hospital and were monitored by the Children's Hospital Animal Care and Use Committee. To generate mature neutrophils in vitro, 4 million total bone marrow cells were cultured in semisolid METHOCULT GF M3534 medium (StemCell Tech, Vancouver, BC, Canada) in which only monocytes and granulocyte colonies can be formed. We can routinely obtain 40–60 granulocyte colonies from one plate. Neutrophil death in these colonies was analyzed as described below.
FACS Analysis of Neutrophil Spontaneous Death.
Neutrophils were cultured for an indicated time and stained by using an Annexin V Detection Kit (Caltag Laboratories, Burlingame, CA) following a protocol provided by the manufacturer. FACS was performed by using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with a 488-nm argon laser. Ten thousand cells were collected and analyzed by using the CellQuest software (Becton Dickinson).
Cell Lines and Cell Death Assays.
HeLa cells, a human cervical carcinoma-derived cell line, and HEK293 cells, a kidney tumor cell line, were maintained in DMEM with 10% FBS, 2 mM l-glutamine, and 100 units of penicillin–streptomycin at 37°C with 5% CO2 atmosphere in a humidified incubator. Cell death was induced by the addition of 1 mM H2O2 or 1 μM staurosporine. Toxicity was assayed 12–14 h after drug exposure by microscopic examination with computer-assisted cell counting. For staining of dead cells by TUNEL assay, cells were fixed in 4% paraformaldehyde/PBS and then stained by using a TUNEL Assay Kit by following protocols provided by the manufacturer (Molecular Probes, Eugene, OR). The broad-spectrum caspase inhibitor, zVAD-fmk (10 μM), was added 1 h before drug treatments. Cell death was determined as the ratio of dead (TUNEL-positive)-to-total cell number and quantified by counting 1,000 cells. Western blot analysis was conduced as described in ref. 33.
Statistical Analysis.
Values shown in each figure represent mean ± SD. Statistical significances were calculated with the Student t test. Differences were considered significant for P values <0.005.
Supplementary Material
Acknowledgments
We thank James Campbell, John Manis, and Li Cai for helpful discussions. D.Z. is supported by National Institutes of Health (NIH) Training Grant HL066987. H.L. was supported by NIH Grants NS052200 and GM076084 and a research scholar grant from American Cancer Society.
Abbreviations
- PI3K
phosphatidylinositol 3-kinase
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Baggiolini M. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 2.Wipke BT, Allen PM. J Immunol. 2001;167:1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 3.Davis C, Fischer J, Ley K, Sarembock IJ. J Thromb Haemost. 2003;1:1699–1709. doi: 10.1046/j.1538-7836.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 4.Maianski NA, Maianski AN, Kuijpers TW, Roos D. Acta Haematol. 2004;111:56–66. doi: 10.1159/000074486. [DOI] [PubMed] [Google Scholar]
- 5.Akgul C, Moulding DA, Edwards SW. FEBS Lett. 2001;487:318–322. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 6.Scheel-Toellner D, Wang KQ, Webb PR, Wong SH, Craddock R, Assi LK, Salmon M, Lord JM. Biochem Soc Trans. 2004;32:461–464. doi: 10.1042/BST0320461. [DOI] [PubMed] [Google Scholar]
- 7.Webb PR, Wang KQ, Scheel-Toellner D, Pongracz J, Salmon M, Lord JM. Apoptosis. 2000;5:451–458. doi: 10.1023/a:1009601220552. [DOI] [PubMed] [Google Scholar]
- 8.Hemmings BA. Science. 1997;277:534. doi: 10.1126/science.277.5325.534. [DOI] [PubMed] [Google Scholar]
- 9.Lemmon MA. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 10.Maffucci T, Falasca M. FEBS Lett. 2001;506:173–179. doi: 10.1016/s0014-5793(01)02909-x. [DOI] [PubMed] [Google Scholar]
- 11.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 12.Vivanco I, Sawyers CL. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 13.Brazil DP, Park J, Hemmings BA. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 14.Cantrell DA. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 15.Cantley LC. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers EE, Theibert AB. Int J Dev Neurosci. 2002;20:187–197. doi: 10.1016/s0736-5748(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 17.Maehama T, Dixon JE. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 18.Di Cristofano A, Pandolfi PP. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 19.Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, Donowitz M, Nagata E, Snyder SH. Proc Natl Acad Sci USA. 2003;100:11712–11717. doi: 10.1073/pnas.1634990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowburn AS, White JF, Deighton J, Walmsley SR, Chilvers ER. Blood. 2005;105:2970–2972. doi: 10.1182/blood-2004-07-2870. [DOI] [PubMed] [Google Scholar]
- 21.Brazil DP, Hemmings BA. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 22.Downward J. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 23.Brunet A, Datta SR, Greenberg ME. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 24.Green JB. Cell Cycle. 2004;3:12–14. [PubMed] [Google Scholar]
- 25.Cohen P, Frame S. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 26.Yuan J, Yankner BA. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 27.Hengartner MO, Horvitz HR. Curr Opin Genet Dev. 1994;4:581–586. doi: 10.1016/0959-437x(94)90076-f. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, et al. Development (Cambridge, UK) 2002;129:4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- 29.Pongracz J, Webb P, Wang K, Deacon E, Lunn OJ, Lord JM. J Biol Chem. 1999;274:37329–37334. doi: 10.1074/jbc.274.52.37329. [DOI] [PubMed] [Google Scholar]
- 30.Rauh MJ, Kalesnikoff J, Hughes M, Sly L, Lam V, Krystal G. Biochem Soc Trans. 2003;31:286–291. doi: 10.1042/bst0310286. [DOI] [PubMed] [Google Scholar]
- 31.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadok V, Bratton D, Henson PM. J Biol Chem. 2002;277:5236–5246. doi: 10.1074/jbc.M110005200. [DOI] [PubMed] [Google Scholar]
- 32.Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, Huang Y, Nagata E, Devreotes P, Snyder SH. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 33.Luo HR, Saiardi A, Nagata E, Ye K, Yu H, Jung TS, Luo X, Jain S, Sawa A, Snyder SH. Neuron. 2001;31:439–451. doi: 10.1016/s0896-6273(01)00384-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.