Abstract
In many microorganisms, the putative orthologs of the Escherichia coli ygbB gene are tightly linked or fused to putative orthologs of ygbP, which has been shown earlier to be involved in terpenoid biosynthesis. The ygbB gene of E. coli was expressed in a recombinant E. coli strain and was shown to direct the synthesis of a soluble, 17-kDa polypeptide. The recombinant protein was found to convert 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate into 2C-methyl-d-erythritol 2,4-cyclodiphosphate and CMP. The structure of the reaction product was established by NMR spectroscopy using 13C-labeled substrate samples. The enzyme-catalyzed reaction requires Mn2+ or Mg2+ but no other cofactors. Radioactivity from [2-14C]2C-methyl-d-erythritol 2,4-cyclodiphosphate was diverted efficiently to carotenoids by isolated chromoplasts from Capsicum annuum and, thus, was established as an intermediate in the deoxyxylulose phosphate pathway of isoprenoid biosynthesis. YgbB protein also was found to convert 4-diphosphocytidyl-2C-methyl-d-erythritol into 2C-methyl-d-erythritol 3,4-cyclophosphate. This compound does not serve as substrate for the formation of carotenoids by isolated chromoplasts and is assumed to be an in vitro product without metabolic relevance.
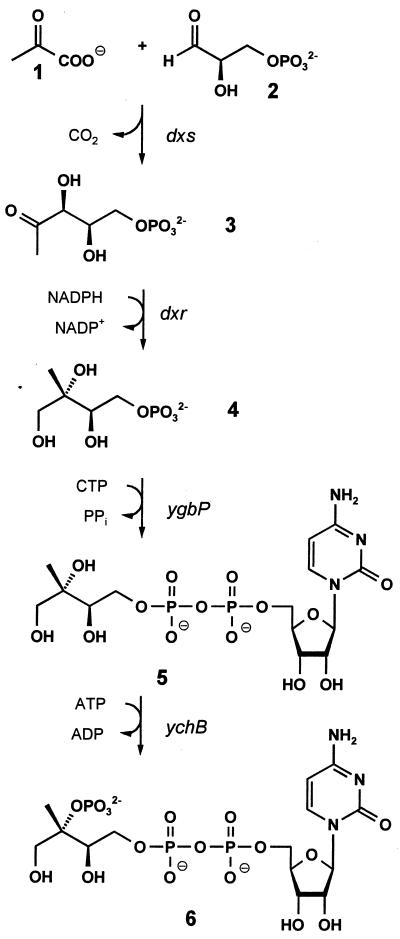
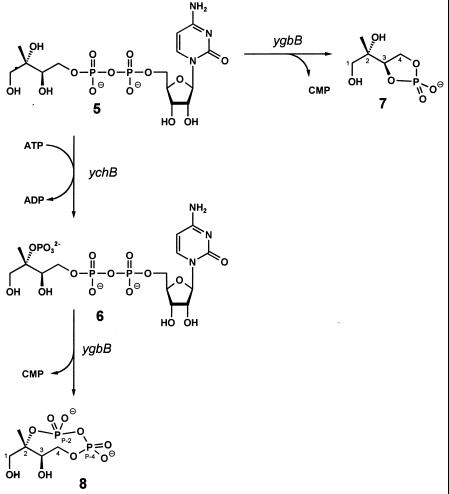
Seminal studies performed independently in the research groups of Arigoni and Rohmer established the existence of a nonmevalonate pathway for the biosynthesis of isoprenoid precursors in certain bacteria and in protozoa and plants (for review, see refs. 1 and 2). 1-Deoxy-d-xylulose serves as an efficient precursor of terpenoids via this alternative pathway (3–8), and its 5-phosphate (3) was shown to be formed from glyceraldehyde 3-phosphate (1) and pyruvate (2) (3, 9, 10) by an enzyme specified by the dxs gene of Escherichia coli (11, 12) and its plant orthologs (13, 14) (Fig. 1). 1-Deoxy-d-xylulose 5-phosphate was shown to be converted into the branched chain polyol 2C-methyl-d-erythritol 4-phosphate (4) by reductoisomerases specified by dxr genes of E. coli, Mentha piperita, Arabidopsis thaliana, and Plasmodium falciparum (17–20). More recently, we found that 2C-methyl-d-erythritol 4-phosphate can be converted into 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (6) via 4-diphosphocytidyl-2C-methyl-d-erythritol by the sequential action of enzymes specified by the ygbP and ychB genes of E. coli (15, 16). This paper shows that YgbB protein converts 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate into the cyclic 2,4-diphosphate of 2C-methyl-d-erythritol.
Figure 1.
The deoxyxylulose phosphate pathway of isoprenoid biosynthesis (1, 2, 15, 16).
Experimental Procedures
Materials.
Oligonucleotides were custom-synthesized by MWG Biotec, Ebersberg, Germany. The preparation of 13C- and 14C-labeled samples of 4-diphosphocytidyl-2C-methyl-d-erythritol, 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate, and 2C-methyl-d-erythritol 4-phosphate will be described elsewhere.
Enzymes.
The preparation of recombinant YchB protein of E. coli has been described earlier (16).
Construction of a Hyperexpression Strain.
The ygbB gene of E. coli was amplified by PCR, using the oligonucleotides shown in Table 1 as primers and chromosomal E. coli DNA as template. The amplificate was digested with BamHI and PstI and then was ligated into the pQE30 plasmid (Qiagen), which had been treated with the same enzymes. The reaction mixture was electrotransformed into the E. coli strain XL1-Blue (21). The resulting E. coli strain is designated XL1-pQEygbB.
Table 1.
Oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| ygbBvo | 5′-GAGAAGGATCCATGCGAATTGGACACGGTTTTGACG-3′ |
| ygbBhi | 5′-TATTATCTGCAGCCTTGCGGTTTACCGTGGAGG-3′ |
Purification of Recombinant YgbB Protein.
E. coli XL1-pQEygbB cells (2 g) were suspended in 30 ml of 100 mM Tris hydrochloride (pH 8.0) containing 20 mM imidazole hydrochloride and 500 mM NaCl (standard buffer). Cell extract was prepared as described earlier (16) and was applied to a column of Ni2+-chelating Sepharose FF (2 × 8 cm; flow rate, 3 ml/min) that had been equilibrated with standard buffer. The column was washed with 100 ml of standard buffer and developed with a linear gradient of 20–500 mM imidazole in standard buffer (total volume, 300 ml). Fractions were combined and dialyzed overnight against 100 mM Tris hydrochloride (pH 8.0). The solution was concentrated by ultrafiltration and applied to a Superdex 75 HR 26/60 column (flow rate, 3 ml/min) that had been equilibrated with 100 mM NaCl in standard buffer. YgbB protein was eluted at 150 ml.
Assay of YgbB Protein.
Assay mixtures containing 100 mM Tris hydrochloride (pH 8.0), 5 mM MnCl2, 5 mM DTT, 2 μg of protein, and 11.4 μM [2-14C]diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (17.5 μCi/μmol) or 11.4 μM [2-14C]diphosphocytidyl-2C-methyl-d-erythritol (17.5 μCi/μmol) in a total volume of 50 μl were incubated at 37°C for 10 min. Aliquots (40 μl) were applied to TLC plates (Polygram Sil N-HR; Macherey & Nagel) that were developed in n-propanol/ethyl acetate/H2O (6:1:3, vol/vol). Radioactivity was monitored by a PhosphorImager (Storm 860; Molecular Dynamics). The Rf values of 2C-methyl-d-erythritol 2,4-cyclodiphosphate and 2C-methyl-d-erythritol 3,4-cyclophosphate were 0.4 and 0.5, respectively.
Enzymatic Formation of 2C-Methyl-d-Erythritol 2,4-Cyclodiphosphate.
A mixture containing 100 mM Tris hydrochloride (pH 8.0), 5 mM MgCl2, 5 mM ATP, 5 mM DTT, 2 μCi [2-14C]4-diphosphocytidyl-2C-methylerythritol (117 μCi/mmol), 5 mM 4-diphosphocytidyl-2C-methylerythritol labeled with 13C as indicated, and 0.1 mg of recombinant YchB protein from E. coli (16) in a total volume of 4 ml was incubated at 37°C. The reaction was monitored by 31P NMR. After 2 h, 0.3 mg of recombinant YgbB protein from E. coli was added, and the mixture was incubated at 37°C for 1 h. The solution was passed through a Nanosep 10K membrane (Pall Gelman, Rondorf, Germany) and applied to an HPLC column of Nucleosil 10SB (4.6 × 250 mm; Macherey & Nagel). The column was developed with 0.1 M ammonium formate in 40% methanol (vol/vol). The effluent was monitored by refractometry and scintillation counting. The retention volumes of CMP and 2C-methyl-d-erythritol 2,4-cyclodiphosphate were 14 ml and 34 ml, respectively. Fractions were collected and lyophilized.
Enzymatic Formation of 2C-Methyl-d-Erythritol 3,4-Cyclophosphate.
A mixture containing 100 mM Tris hydrochloride (pH 8.0), 10 mM MnCl2, 20 mM [2,2-methyl-13C2]4-diphosphocytidyl-2C-methyl-d-erythritol, 0.14 μCi of [2-14C]4-diphosphocytidyl-2C-methyl-d-erythritol (14.6 μCi/mmol), and 0.3 mg of purified recombinant YgbB protein from E. coli in a total volume of 0.5 ml was incubated for 2 h. The solution was passed through a Nanosep 10K membrane. The enzyme product was purified by HPLC, using a column of Nucleosil 10SB (4.6 × 250 mm) and 50 mM ammonium formate in 40% methanol (vol/vol) as eluent (flow rate, 1 ml/min). The effluent was monitored by refractometry and by scintillation counting. Fractions (retention volume, 10 ml) were collected and lyophilized.
NMR Spectroscopy.
NMR spectra were recorded by using an AVANCE DRX500 or AC 250 spectrometer from Bruker (Billerica, MA). CMP, 2C-methyl-d-erythritol 2,4-cyclodiphosphate, and 2C-methyl-d-erythritol 3,4-cyclophosphate were measured in D2O as solvent.
Preparation of Chromoplasts and Incorporation Assays.
Chromoplasts of C. annuum were isolated as described previously (16). Incorporation experiments with [2-14C]2C-methyl-d-erythritol 2,4-cyclodiphosphate and [2-14C]2C-methyl-d-erythritol 3,4-cyclophosphate into carotenoids were performed as described previously (16).
Results
The ygbP gene specifying 4-diphosphocytidyl-2C-methyl-d-erythritol synthase is tightly linked to the unannotated reading frame ygbB on the E. coli chromosome (15, 22), and the two genes are likely to be cotranscribed. Putative orthologs of ygbP and ygbB also were found in the database in adjacent positions on the chromosomes of 21 other eubacteria (Table 2), and 6 eubacteria were found to contain genes assumed to specify bifunctional proteins with an N-terminal ygbP domain and a C-terminal ygbB domain (Table 2). These observations suggested that YgbB protein is involved in the deoxyxylulose phosphate pathway of terpenoid biosynthesis. This hypothesis was supported further by the fact that putative orthologs of dxr, dxs, ygbP, ychB, and ygbB are found in all completely sequenced genomes of microorganisms using the nonmevalonate pathway and are absent in all completely sequenced genomes of microorganisms using the mevalonate pathway with the exception of Pyrococcus horikoshii genome, which contains a putative ortholog of ygbP (15, 16).
Table 2.
Accession or contig numbers of ygbP and ygbB in various organisms
| Bacteria | Accession no.* |
|---|---|
| With bifunctional genes | |
| Campylobacter jejuni | Cj.seq |
| Caulobacter crescentus | gcc_1641, gcc_574 |
| Helicobacter pylori | gb AE001474 |
| Rhodobacter capsulatus | emb X72382 |
| Treponema pallidum | gb AE001227 |
| Zymomonas mobilis | gb AF176314 |
| With adjacent genes | |
| Actinobacillus actinomycetemcomitans | Contig704 |
| Bacillus subtilis | emb Z99101 |
| Bordetella pertussis | Contig657 |
| Clostridium acetobutylicum | AE001437 |
| Clostridium difficile | Contig392 |
| E. coli | gb AE000358 |
| Haemophilus influenzae | gb U32750 |
| Klebsiella pneumoniae | Contig1719 |
| Mycobacterium avium | 5759 |
| Mycobacterium bovis | Contig950 |
| Mycobacterium tuberculosis | emb Z92774 |
| Neisseria gonorrhoeae | Contig181 |
| Neisseria meningitidis | Contig3 |
| Pasteurella multocida | Contig556 |
| Pseudomonas aeruginosa | Contig52 |
| Salmonella paratyphi | SPA.0.2916 |
| Salmonella typhi | Contig404 |
| Salmonella typhimurium | Contig997 |
| Shewanella putrefaciens | gsp_845 |
| Thiobacillus ferrooxidans | 949 |
| Vibrio cholerae | asm938 |
| Yersinia pestis | Contig730 |
| With unlinked genes | ygbP | ygbB |
|---|---|---|
| Aquifex aeolicus | gb AE000734 | gb AE000715 |
| Chlamydia pneumoniae | gb AE001642 | gb AE001639 |
| Chlamydia trachomatis | gb AE001320 | gb AE001317 |
| Chlorobium tepidum | gct_38 | gct_41 |
| Corynebacterium diphtheriae | Contig519 | Contig402 |
| Deinococcus radiodurans | 8896 | 8835 |
| Enterococcus faecalis | gef_6311 | gef_6177 |
| Porphyromonas gingivalis | 1209 | 1207 |
| Synechocystis sp. | dbj D990914 | dbj D90906 |
| Thermotoga maritima | gb AE001792 | gb AE001738 |
| Eukaryotes with unlinked genes | ||
| A. thaliana | gb AC004136 | |
| P. falciparum | 1D_M9Fe7.plt | gb AE001394 |
*Accession and contig numbers as of December 6, 1999.
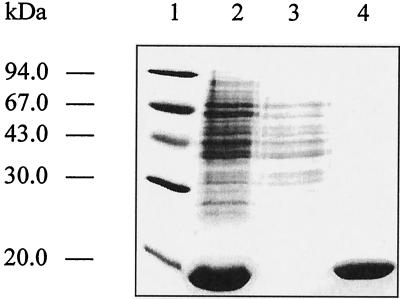
The ygbB gene of E. coli was expressed in a recombinant E. coli host harboring the plasmid pQEygbB, which directs the synthesis of a modified YgbB protein with six consecutive histidine residues at the N terminus. Cell extracts of the recombinant E. coli cells contained large amounts of a 17-kDa polypeptide (about 30% of soluble protein), which was purified to apparent homogeneity by nickel-chelate chromatography (Fig. 2). N-terminal Edman degradation confirmed the predicted amino acid sequence (data not shown).
Figure 2.
SDS/PAGE. Lanes: 1, molecular mass markers; 2, crude cell extract of the recombinant E. coli strain XL1-pQEygbB; 3, flow-through fraction of Ni2+-chelating Sepharose chromatography; 4, eluted fraction of YgbB after Ni2+-chelating Sepharose chromatography.
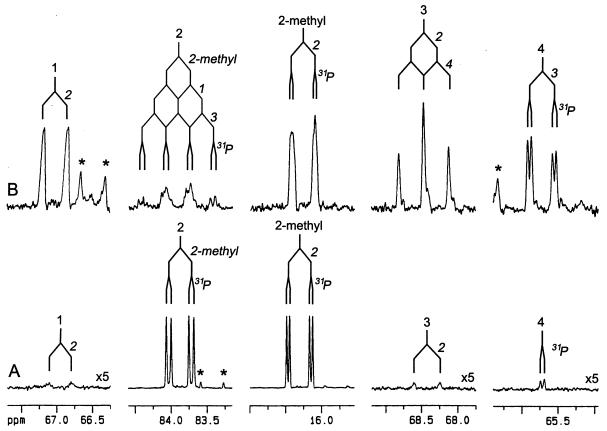
Incubation of purified recombinant YgbB protein with 4-diphosphocytidyl-2C-methyl-d-erythritol (5, Fig. 1) and its 2-phosphate (6) in the presence of Mn2+ or Mg2+ afforded products with Rf values of 0.4 and 0.5, respectively, when analyzed by TLC as described in Experimental Procedures. To determine the structure of the enzyme products, we prepared [1,3,4-13C]-, [2,2-methyl-13C2]-, and [1,2,2-methyl,3,4-13C5]-labeled samples of 4-diphosphocytidyl-2C-methyl-d-erythritol (5) and its respective 2-phosphate (6) by using recombinant enzymes of the deoxyxylulose phosphate pathway as catalysts. The 13C labels of these samples afforded enhanced sensitivity and selectivity in enzyme assays monitored by 13C NMR (Fig. 3). The sensitivity enhancement is illustrated in Fig. 3A for the 13C NMR signals of the product obtained from [2,2-methyl-13C2]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate. The signals with low intensity reflect carbon atoms with natural 13C abundance. The detected signal splittings (Fig. 3) were due to 13C13C and 13C31P couplings, as indicated. The 13C-labeling approach also facilitated the signal assignment via analysis of the 13C13C and 13C1H spin networks by using two-dimensional homo- and heterocorrelation NMR experiments.
Figure 3.
13C NMR signals of 2C-methyl-d-erythritol 2,4-cyclodiphosphate obtained from the reaction of recombinant YgbB protein with [2,2-methyl-13C2]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (A) or [1,2,2-methyl,3,4-13C5]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (B). 13C13C couplings and 13C31P couplings are indicated. *, Impurities.
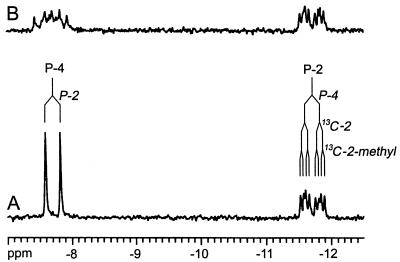
NMR data of the product obtained from the reaction of YgbB protein with 13C-labeled 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate (6) are summarized in Table 3. The 13C13C and 13C1H networks were gleaned by INADEQUATE and heteronuclear multiple quantum correlation spectroscopy, thus establishing a methylerythritol motif. 31P NMR spectra showed two 31P NMR signals at −7.7 ppm and −11.7 ppm (Fig. 4). Each of the signals was characterized by 31P31P coupling (coupling constant, 23.6 Hz), reflecting a diphosphate motif. 31P13C coupling of one phosphorous atom to the 2-methyl carbon and the absence of 31P1H coupling for the 31P NMR signal at −11.66 ppm (Figs. 3 and 4), in conjunction with the 13C NMR chemical shift for C-2 of the 2C-methyl-d-erythritol moiety (83.9 ppm) as compared with the respective chemical shift for C-2 of 4-diphosphocytidyl-2C-methyl-d-erythritol (73.8 ppm) (15) clearly indicated the connection of the diphosphate motif to C-2 (Table 3). These data established the structure as 2C-methyl-d-erythritol 2,4-cyclodiphosphate (8, Fig. 5), which is supported further by the coupling pattern of C-1, C-3, and C-4 (Fig. 3 and Table 3). This compound had been identified earlier by NMR spectroscopy as a metabolite in some bacteria (23–25). Our NMR data are in excellent agreement with those published in the literature.
Table 3.
NMR data of products from reaction with YgbB protein
| Position | Chemical shifts,
ppm
|
Coupling constants, Hz
|
|||||
|---|---|---|---|---|---|---|---|
| 1H* | 13C† | 31P‡ | JHH | JPC | JPP | JCC | |
| 2C-methyl-d-erythritol 2,4-cyclodiphosphate (8) | |||||||
| 1 | 3.51 (d, 1) | 66.95 | 12.2 (/1§) | 41.8 (2)¶∥ | |||
| 1§ | 3.66 (d, 1) | 12.2 (1) | |||||
| 2 | 83.87 | 8.4 (P-2)∥ | 39.8 (2-Me)¶∥ | ||||
| 2-Me | 1.31 (s, 3) | 16.30 | 5.3 (P-2)∥ | 39.8 (2)¶∥ | |||
| 3 | 4.01 (m, 1) | 68.42 | ND | ND | 46.0 (2)¶ | ||
| 4 | 4.02 (m, 1) | 65.72 | ND | 6.6 (P-4)** | 42.7 (3)¶ | ||
| 4§ | 4.07 (m, 1) | ND | |||||
| P-4 | −7.65 | 23.6 (P-2) | |||||
| P-2 | −11.66 | 8.5 (2)∥, 5.3 (2-Me)∥ | 23.6 (P-4) | ||||
| 2C-methyl-d-erythritol 3,4-cyclomonophosphate (7) | |||||||
| 1 | 3.38 (d, 1) | 65.64 | 12.0 (1§) | ||||
| 1§ | 3.47 (d, 1) | 12.0 (1) | |||||
| 2 | 73.02 | 6.5∥ | |||||
| 2-Me | 1.09 (s, 3) | 17.73 | |||||
| 3 | 4.15 (m, 1) | 77.61 | 1.7∥ | ||||
| 4 | 4.18 (m, 1) | 64.96 | 1.1∥ | ||||
| 4§ | 4.34 (ddd, 1) | 11.2 (4), 3.8, 7.2 (3) | |||||
| P | 21.67 (s) | ||||||
Referenced to external trimethylsilylpropane sulfonate. The multiplicities and the relative integral values of 1H NMR signals of an unlabeled sample are indicated in parentheses.
†Referenced to external trimethylsilylpropane sulfonate.
‡Referenced to external phosphoric acid (85%, vol/vol).
§Indicates one of diastereotopic hydrogens bonded to the respective index carbon.
¶From the reaction with [1,2,2-methyl,3,4-13C5] 4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate.
∥From the reaction with [2,2-methyl-13C2]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate.
**From the reaction with [1,3,4-13C1]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate.
Figure 4.
31P NMR spectrum of [2,2-methyl-13C2]2C-methyl-d-erythritol 2,4-cyclodiphosphate. (A) 1H-decoupled. (B) Without 1H-decoupling.
Figure 5.
Reactions catalyzed by YgbB protein.
The second reaction product obtained from 6 and YgbB protein was characterized as CMP by HPLC and by 31P and 1H NMR spectroscopy (data not shown). The 31P NMR spectrum of the reaction product obtained by treatment of 4-diphosphocytidyl-2C-methyl-d-erythritol (5) with YgbB protein displayed a singlet signal at 21.7 ppm, suggesting a pentacyclic monophosphate structure (Table 3). Analysis of the 1H and 13C NMR signature (Table 3) identified the compound as 2C-methyl-d-erythritol 3,4-cyclophosphate (7, Fig. 5).
Feeding experiments in intact chromoplasts of C. annuum using [2-14C]2C-methyl-d-erythritol 2,4-cyclodiphosphate (specific activity, 17.5 μCi/mmol) as substrate resulted in incorporation of 55% of the proffered radioactivity into the carotenoid fraction (data not shown). The addition of 2C-methyl-d-erythritol 4-phosphate did not diminish the incorporation of radioactivity. On the other hand, [2-14C]2C-methyl-d-erythritol 3,4-cyclophosphate (specific activity, 17.5 μCi/mmol) was not incorporated into terpenoids by chromoplasts of C. annuum.
Separate application of [2-14C]1-deoxy-d-xylulose 5-phosphate, [2-14C]2C-methyl-d-erythritol 4-phosphate, [2-14C]4-diphosphocytidyl-2C-methyl-d-erythritol, and [2-14C]4-diphosphocytidyl-2C-methyl-d-erythritol 2-phosphate to stroma of C. annuum chromoplasts gave incorporation into a compound that was identified unequivocally as 2C-methyl-d-erythritol 2,4-cyclodiphosphate by NMR analysis (data not shown); the incorporation rates achieved for the above-mentioned precursors were 50%, 55%, 100%, and 100%, respectively.
Discussion
YgbB protein of E. coli can use the respective products of YgbP protein (5) or YchB protein (6) as substrates affording the cyclic monophosphate 7 and the cyclic diphosphate 8, respectively. Studies with plant chromoplasts showed that only the latter compound can be converted into carotenoids. We conclude that YgbP, YchB, and YgbB proteins act sequentially in the nonmevalonate terpenoid biosynthetic pathway both in bacteria and higher plants.
The cyclic diphosphate 8 has been isolated earlier from cultures of bacteria exposed to oxidative stress (23–25) and had been interpreted tentatively as a dead-end product derived from the deoxyxylulose phosphate pathway. In contrast to this hypothesis, our findings indicate that 2C-methyl-d-erythritol 2,4-cyclophosphate is a genuine intermediate of the deoxyxylulose phosphate pathway.
Putative ygbP and ygbB orthologs are closely linked or even fused in many microorganisms that use the deoxyxylulose phosphate pathway (Table 2) (15). In each case, the ygbP gene or domain is located upstream from the ygbB gene or domain.
Plasmodium falciparum has unlinked putative orthologs of ygbP and ygbB. A putative ortholog of ygbP was also found in A. thaliana (Table 2).
2C-methyl-d-erythritol 3,4-cyclomonophosphate (7) produced by YgbB protein from 4-diphosphocytidyl-2C-methyl-d-erythritol appears as an in vitro product without biosynthetic relevance. The compound cannot serve as substrate for any other known enzyme of the deoxyxylulose phosphate pathway and is not utilized by isolated chromoplasts.
Acknowledgments
We thank A. Werner and F. Wendling for help with the preparation of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 369), the Fonds der Chemischen Industrie, and the Hans-Fischer-Gesellschaft.
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF230738).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040554697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040554697
References
- 1.Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk M, Bacher A. Chem Biol. 1998;5:R221–R233. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- 2.Rohmer M. In: Comprehensive Natural Products Chemistry. Barton D, Nakanishi K, editors. Vol. 2. Oxford: Pergamon; 1999. pp. 45–68. [Google Scholar]
- 3.Broers S T J. Ph.D. thesis. Eidgenössische Technische Hochschule Zürich; 1994. [Google Scholar]
- 4.Arigoni D, Sagner S, Latzel C, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1997;94:10600–10605. doi: 10.1073/pnas.94.20.10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwender J, Zeidler J, Gröner R, Müller C, Focke M, Braun S, Lichtenthaler F W, Lichtenthaler H K. FEBS Lett. 1997;414:129–134. doi: 10.1016/s0014-5793(97)01002-8. [DOI] [PubMed] [Google Scholar]
- 6.Piel J, Donath J, Bandemer K, Boland W. Angew Chem Int Ed Engl. 1998;37:2478–2481. doi: 10.1002/(SICI)1521-3773(19981002)37:18<2478::AID-ANIE2478>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Rosa Putra S, Lois L-M, Campos N, Boronat A, Rohmer M. Tetrahedron Lett. 1998;29:23–26. [Google Scholar]
- 8.Giner J-L, Jaun B, Arigoni D. J. Chem. Soc. Chem. Commun. 1998. 1857–1858. [Google Scholar]
- 9.Schwarz M K. Ph.D. thesis. Eidgenössische Technische Hochschule Zürich; 1994. [Google Scholar]
- 10.Rohmer H, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 11.Sprenger G A, Schörken U, Wiegert T, Grolle S, deGraaf A A, Taylor S V, Begley T P, Bringer-Meyer S, Sahm H. Proc Natl Acad Sci USA. 1997;94:12857–12862. doi: 10.1073/pnas.94.24.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lois L M, Campos N, Putra S R, Danielsen K, Rohmer M, Boronat A. Proc Natl Acad Sci USA. 1997;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange B M, Wildung M R, McCaskill D, Croteau R. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouvier F, d'Harlingue A, Suire C, Backhaus R A, Camara B. Plant Physiol. 1998;117:1423–1431. doi: 10.1104/pp.117.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohdich F, Wungsintaweekul J, Fellermeier M, Sagner S, Herz S, Kis K, Eisenreich W, Bacher A, Zenk M H. Proc Natl Acad Sci USA. 1999;96:11758–11763. doi: 10.1073/pnas.96.21.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lüttgen H, Rohdich F, Herz S, Wungsintaweekul J, Hecht S, Schuhr C A, Fellermeier M, Sagner S, Zenk M H, Bacher A, et al. Proc Natl Acad Sci USA. 2000;97:1062–1067. doi: 10.1073/pnas.97.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi S, Kuzuyama T, Watanabe H, Seto H. Proc Natl Acad Sci USA. 1998;95:9879–9884. doi: 10.1073/pnas.95.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lange B M, Croteau R. Arch Biochem Biophys. 1999;365:170–174. doi: 10.1006/abbi.1999.1168. [DOI] [PubMed] [Google Scholar]
- 19.Schwender J, Müller C, Zeidler J, Lichtenthaler H K. FEBS Lett. 1999;455:140–144. doi: 10.1016/s0014-5793(99)00849-2. [DOI] [PubMed] [Google Scholar]
- 20.Jomaa H, Wiesner J, Sanderbrand S, Altinicicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler H K, et al. Science. 1999;285:1573–1576. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 21.Bullock W O, Fernandez J M, Short J M. BioTechniques. 1987;5:376–379. [Google Scholar]
- 22.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, et al. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 23.Turner D, Santos H, Fareleira P, Pacheco I, LeGall Y, Xavier A V. Biochem J. 1992;285:387–390. doi: 10.1042/bj2850387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrovsky D, Kharatian E, Dubrovsky T, Ogrel O, Shipanova I, Sibeldina L. Biofactors. 1992;4:63–68. [PubMed] [Google Scholar]
- 25.Ostrovsky D, Diomina G, Lysak E, Matveeva E, Ogrel O, Trutko S. Arch Microbiol. 1998;171:69–72. doi: 10.1007/s002030050680. [DOI] [PubMed] [Google Scholar]