Abstract
The latency-associated nuclear antigen (LANA) of Kaposi's sarcoma-associated herpesvirus (KSHV) is expressed in all KSHV-associated malignancies. LANA is essential for replication and maintenance of the viral episomes during latent infection. However, LANA also has a transcriptional regulatory role and can affect gene expression both positively and negatively. A previously performed yeast two-hybrid screen identified glycogen synthase kinase 3 (GSK-3) as a LANA-interacting protein. Interaction with both GSK-3α and GSK-3β was confirmed in transfected cells with coprecipitation assays. GSK-3β also interacted with the herpesvirus saimiri homolog ORF73. GSK-3β is an intermediate in the Wnt signaling pathway and a negative regulator of β-catenin. In transfected cells, LANA was shown to overcome GSK-3β-mediated degradation of β-catenin. Examination of primary effusion lymphoma (PEL) cells found increased levels of β-catenin relative to KSHV-negative B cells, and this translated into increased activity of a β-catenin-responsive reporter containing Tcf/Lef binding sites. In tetradecanoyl phorbol acetate-treated PEL cells, loss of LANA expression correlated temporally with loss of detectable β-catenin. LANA was found to alter the intracellular distribution of GSK-3β so that nuclear GSK-3β was more readily detectable in the presence of LANA. Mapping experiments with coimmunoprecipitation assays revealed that both N-terminal and C-terminal LANA sequences were required for efficient GSK-3β interaction. LANA mutants that were defective for GSK-3β interaction were unable to mediate GSK-3β relocalization or activate a β-catenin-responsive Tcf-luciferase reporter. This study identified manipulation of GSK-3β activity as a mechanism by which LANA may modify transcriptional activity and contribute to the phenotype of primary effusion lymphoma.
Kaposi's sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8, is a gamma-2 herpesvirus that was initially discovered in association with the endothelial cell malignancy Kaposi's sarcoma and is also associated with the B-cell malignancies primary effusion lymphoma and plasmablastic variant multicentric Castleman's disease (16, 17, 57, 77). In primary effusion lymphoma and in Kaposi's sarcoma lesions, KSHV gene expression is restricted primarily to expression of the viral latency genes (13, 26, 40, 72, 78, 91), and only a small number of cells within the lesions show expression of viral lytic proteins such as viral G protein-coupled receptor, viral interleukin-6, and ORF59 (15, 20, 62). KSHV gene expression is less stringently regulated in multicentric Castleman's disease, in which both latent and lytic proteins have been detected (39, 62). KSHV latency genes are the coordinately expressed latency-associated nuclear antigen (LANA), viral cyclin (v-cyclin), and FAS-associating protein with death domain-like interleukin-1 converting enzyme (FLICE)-inhibitory protein viral FLICE inhibitory protein (v-FLIP) plus the interferon regulatory factor (IRF) homolog LANA2 (also called v-IRF3 or K10.5), which is expressed during latent infection in primary effusion lymphoma and multicentric Castleman's disease but has not been detected in Kaposi's sarcoma (26, 29, 38, 52, 64, 67, 70).
The v-cyclin, v-FLIP, and LANA2 proteins have been shown to contribute to KSHV-associated pathogenesis in ways that are related to modification or constitutive activation of the functions of their cellular homologs. v-cyclin is a human cyclin D2 homolog that binds to and activates the cyclin-dependent kinases CDK4 and CDK6 (18, 33, 49), but the complex, unlike that formed by the cellular cyclin D2, is not susceptible to inhibition by the regulatory proteins p16INK4a, p21CIP1, and p27KIP1 (81). v-cyclin/CDK6 phosphorylates retinoblastoma protein, which results in release from E2F and activation of E2F-responsive S-phase genes (reviewed in reference 56). In addition, v-cyclin/CDK6 phosphorylation of p27KIP1 leads to p27KIP1 degradation and loss of p27KIP1 function in cell cycle arrest (28, 54). v-FLIP, like its cellular homolog, protects cells from Fas-mediated apoptosis (25), but v-FLIP also associates with and activates the IκB kinase complex to constitutively activate NF-κB (51, 79). LANA2 (v-IRF3) has been found to inhibit p53 transcriptional activity in reporter assays and p53-induced apoptosis in transfected SAOS-2 cells (70) and show anti-interferon activity (53).
LANA, which is encoded by ORF73 (41, 67), has no recognizable cellular homolog, and hence the functions of LANA have had to be addressed empirically. The punctate distribution of LANA in the cell nucleus and the colocalization with KSHV genomes on cell chromosomes led to a focus on parallels between LANA and the Epstein-Barr virus (EBV) EBNA-1 protein, which binds to multiple sites within the EBV latency origin of replication, oriP, and is essential for replication and maintenance of the EBV genome during latent infection (48, 68, 89). LANA is necessary and sufficient for maintenance of episomes containing the terminal repeat region of the KSHV genome (5, 23). LANA is also the only viral protein required for short-term replication of terminal repeat-containing plasmids (36).
The carboxy-terminal domain of LANA binds as a dimer (74) to two binding sites in the KSHV terminal repeats (6, 22, 32). LANA associates with mitotic chromosomes (5), and a chromatin binding domain that is necessary for binding of full-length LANA to chromosomes is located within N-terminal amino acids 5 to 22 (63). This domain mediates chromosomal tethering through interaction with the methyl-CpG binding protein MeCP2 (46). The C terminus of LANA, when expressed independently, also associates with chromosomes, indicating that a second chromosome tethering domain exists. This second domain binds to the cellular DEK protein (46). Thus, LANA utilizes two separate cellular protein intermediates to tether the KSHV genomes to chromosomes during mitosis and ensure long-term viral persistence. Maintenance of artificial episomes containing the KSHV terminal repeats can also be achieved when the N-terminal chromatin binding domain is deleted and LANA is expressed as a fusion with the chromatin-associated protein histone H1 (76). Interactions between LANA and histone H1 may also contribute to chromosomal association (23).
In addition to its contribution to latency DNA replication and maintenance, LANA has transcriptional regulatory properties. Experiments targeting LANA to DNA by expressing the protein fused to the DNA binding domain of GAL4 revealed the existence of N-terminal and C-terminal transcriptional repression domains (47, 74). Repression by the N-terminal domain is mediated in part by association with the mSin3 corepressor complex (47). Direct binding of LANA to the terminal repeat binding sites also results in transcriptional repression in reporter assays (32). Repression of p53-responsive promoters is attributed to a physical interaction between LANA and p53 (31). Interactions between LANA and CREB binding protein (CBP) that interfere with CBP histone acetyltransferase activity and between LANA and ATF4/CREB2 (50) may also contribute to transcriptional downregulation.
On the other hand, LANA activates expression from a variety of promoters. Activation of E2F-dependent promoters as a consequence of interaction between LANA and the retinoblastoma promoter is likely to be important in KSHV-induced growth stimulation (66). LANA activates the cellular interleukin-6 promoter through an AP-1 site and activates its own promoter (47, 69) and the telomerase reverse transcriptase promoter (43). Interaction between LANA and RING3 (55) may also have gene-regulatory consequences.
The ways in which LANA modifies cellular transcription are not yet fully understood. We now present evidence for an interaction between LANA and the serine-threonine kinase glycogen synthase kinase 3β (GSK-3β), a negative regulator of β-catenin activity. By modulating GSK-3β activity LANA has the potential to upregulate expression of β-catenin-Tef/Lef-responsive genes, a category that is known to include genes involved in cell proliferative responses, as well as modify the activity of transcription factors that are substrates for GSK-3β.
MATERIALS AND METHODS
Plasmids and antibodies.
LANA and its deletion variants, GSK-3α, and GSK-3β were expressed from SG5 (Stratagene, La Jolla, Calif.)-based vectors: Flag-wtLANA (DY52), Flag-LANA-C (MF10), Flag-LANA-N (MF40), Flag-LANA-dCR (MF24), Flag-LANA-N93 (MF68), Flag-LANA-N155 (MF69), Flag-LANA-N175 (MF71), Flag-LANA-N241 (MF44), Flag-LANA-N275 (MF67), Flag-LANA-C1147 (MF81), Flag-LANA-C1133 (MF82), Flag-LANA-C1108 (MF43), Flag-LANA-C1043 (MF73), and Myc-GSK-3α (MF29). Herpesvirus saimiri ORF73 was ligated as a BamHI fragment into the BamHI site of the modified SG5 vector pJH253 to obtain Flag-ORF73 (MF77). Glutathione S-transferase (GST)-GSK-3β (MF72) was generated by ligating a 1.3-kb BglII GSK-3β DNA fragment into the BamHI site of pGEX-2T (Amersham Pharmacia Biotech, Piscataway, N.J.). The Tcf-luciferase reporter pGL3-OT, mutant reporter pGL3-OF, and β-catenin and mutant β-catenin(S33Y) expression vectors were obtained from K. W. Kinzler (Johns Hopkins School of Medicine). Hemagglutinin (HA)-GSK-3β was obtained from F. McCormick (University of California, San Francisco).
Antibodies used were anti-GSK-3β and β-catenin mouse monoclonal antibodies (BD Transduction Laboratories, San Diego, Calif.), anti-LANA rat monoclonal antibody (Advanced Biotechnologies Inc, Gaithersburg, Md.), anti-HA mouse monoclonal antibody (Roche Applied Science, Indianapolis, Ind.), rabbit polyclonal (Upstate Biotechnology), and anti-Myc, anti-Flag, and anti-β-actin monoclonal antibodies (Sigma, St. Louis, Mo.). Molecular mass standards were purchased from Life Technologies, Grand Island, N.Y.
Immunoprecipitation.
HeLa cells seeded at 106 per 10-cm dish were transfected by the calcium phosphate procedure with Flag-LANA (7 μg), Myc-GSK-3α (7 μg), or HA-GSK-3β (7 μg). Cells were harvested 48 h posttransfection, resuspended in 3 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.8], 0.2% Nonidet P-40, 5% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM EDTA, 50 mM NaCl, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 μg of pepstatin per ml, 5 μg of aprotinin per ml, 0.5 mM NaF, and 1 mM sodium pyrophosphate) and homogenized in a glass Dounce homogenizer. Lysates were precleared by mixing with Sepharose beads (60 μl), followed by centrifugation at 5,000 rpm for 5 min. The supernatant was subjected to a second centrifugation at 15,000 rpm for 15 min. The supernatant was then incubated with anti-HA monoclonal antibody (10 μg) or anti-Myc monoclonal antibody (5 μg) for 2 h at 4°C, followed by incubation with protein G-Sepharose beads (30 μl, swollen volume) for 2 h at 4°C. Beads were washed six times with ice-cold lysis buffer, resuspended in sample buffer (30 μl), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting.
GST affinity assay.
Expression of GST-GSK-3β in transformed Escherichia coli BL21 bacteria was induced by treatment with 0.5 mM isopropylthiogalactopyranoside (IPTG). Bacterial pellets were resuspended in 50 mM Tris-HCl (pH 7.6), 120 mM NaCl, 2 mM DTT, 0.5 mM EDTA, 5% glycerol, 1 μg of lysozyme per ml, 5% Nonidet P-40, 5 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin per ml, and 5 μg of aprotinin per ml and sonicated for 30 s. GST-GSK-3β was purified by binding to glutathione-Sepharose beads (Amersham Pharmacia Biotech, Piscataway, N.J.) for 30 min at 4°C in binding buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 2 mM DTT, 0.5 mM EDTA, 5% glycerol, 1 μg of bovine serum albumin, 0.5% Nonidet P-40, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin per ml, and 5 μg of aprotinin per ml). [35S]methionine- and [35S]cysteine-labeled LANA proteins were synthesized with the TNT transcription-translation system (Promega, Madison, Wis.) and incubated for 1 h at 4°C with the immobilized GST-GSK-3β. Beads were washed six times in binding buffer and resuspended in SDS sample buffer. Bound proteins were analyzed by SDS-PAGE followed by autoradiography.
Preparation of cell extracts and Western blotting.
Tetradecanoyl phorbol acetate (TPA)-treated primary effusion lymphoma (PEL) cells or transfected HeLa cells (2 × 106) were pelleted by centrifugation and solubilized in 500 μl of SDS sample buffer containing 2 mM PMSF, 2 mM N-ethylmaleimide, 5 μg of aprotinin per ml, 0.2 mM Na3VO4, 10 mM NaF, and 10 mM sodium pyrophosphate. The cell lysate was sonicated for 30 s and boiled for 5 min. Lysate (20 μl) was subjected to SDS-PAGE followed by Western blot analysis with peroxidase-conjugated anti-mouse, anti-rat, or anti-rabbit immunoglobulin G as the secondary antibody and the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, N.J.). Bands were visualized by exposure to X-ray film.
Subcellular fractionation.
Nuclei and cytoplasm were fractionated as previously described (73). Briefly, 2 × 106 HeLa or 5 × 106 BC2 or Raji cells were harvested and resuspended in 2 ml of ice-cold hypotonic buffer (20 mM HEPES [pH 7.8], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 5 μg of aprotinin per ml, and 1 μg of pepstatin per ml). After incubation for 10 min on ice, Nonidet P-40 was added to a final concentration of 0.6%, and the sample was vortexed for 10 s. Samples were centrifuged for 20 s at 13,000 rpm. The supernatant was collected and recentrifuged for 2 min at 13,000 rpm. The resulting supernatant formed the cytoplasmic fraction. The pellet containing the nuclei was washed with hypotonic buffer containing 0.6% Nonidet P-40. For direct analysis by SDS-PAGE, the pellet was resuspended in SDS sample buffer. For immunoprecipitation analyses, the pellet was resuspended in 2 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.8], 0.6% Nonidet P-40, 5% glycerol, 1 mM DTT, 0.5 mM EDTA, 50 mM NaCl, 0.5 mM PMSF, 1 μg of pepstatin per ml, and 5 μg of aprotinin per ml) and sonicated for 10 s. Lysates were centrifuged at 13,000 rpm for 2 min. The supernatant formed the nuclear fraction.
Immunofluorescence.
BC2 and BC3 PEL cells and Raji and DG75 B cells were fixed in 50% methanol-acetone (1:1) on glass slides. Cells were incubated with anti-GSK-3β mouse monoclonal antibody (1:100) for 1 h at 25°C. After washing, the cells were incubated with rhodamine-conjugated donkey anti-mouse immunoglobulin G (1:100). To examine the intracellular localization of the Flag-LANA mutants, Flag-LANA plasmids (1.5 μg) were transfected by the calcium phosphate method into HeLa cells seeded at 8 × 105 per well in two-well slide chambers. At 48 h after transfection, cells were fixed in 50% methanol-acetone (1:1) for 15 min at 4°C. Cells were incubated with anti-Flag antibody for 1 h at 25°C. After washing three times in phosphate-buffered saline, the cells were incubated with fluorescein isothiocyanate-conjugated secondary antibody for 1 h at 25°C.
Luciferase assays.
BC2 and BC3 PEL cells and DG75 B cells (2 × 106) were cultured in six-well plates and transfected with Mirus TransIT-LT1 reagent (PanVera Corp., Madison, Wis.). SV-β-gal was used as an internal control for transfection efficiency. Vector (SG5) was used to equalize the amount of DNA in each transfection. HeLa cells were transfected by the calcium phosphate method.
RESULTS
LANA interacts with GSK-3.
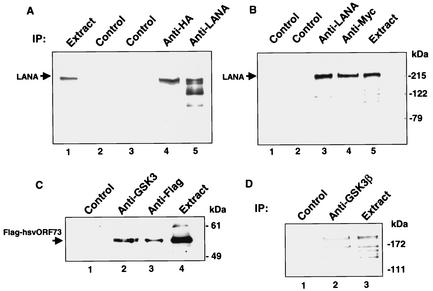
A previously performed yeast two-hybrid screen with LANA as the bait protein identified a number of LANA-interacting proteins, including the corepressor SAP30 and the chromosome binding protein DEK (46, 47). The same screen also identified the serine-threonine kinase GSK-3 as a LANA-interacting protein. There are two closely related isoforms of GSK3, GSK-3α and GSK-3β (86). Interaction with both GSK-3β (Fig. 1A) and GSK-3α (Fig. 1B) was confirmed by coimmunoprecipitation. HeLa cells were cotransfected with LANA and either HA-GSK-3β or Myc-GSK-3α. Immunoprecipitation with anti-HA or anti-Myc antibody followed by Western blotting and probing with anti-LANA monoclonal antibody revealed the presence of LANA in the direct anti-LANA precipitates (Fig. 1A, lane 5; Fig. 1B, lane 3). Coprecipitating LANA was also present in the GSK-3β precipitate (Fig. 1A, lane 4) and in the GSK-3α precipitate (Fig. 1B, lane 4) but not in the precipitates generated with control antibodies (Fig. 1A, lanes 2 and 3; Fig. 1B, lanes 1 and 2). Thus, LANA interacts with GSK-3α and with GSK-3β.
FIG. 1.
LANA interacts with GSK-3β and GSK-3α. Western blots showing coimmunoprecipitation of LANA with HA-GSK-3β (A) and Myc-GSK-3α (B) and coimmunoprecipitation of herpesvirus saimiri (hsv) ORF73 with GSK-3β (C). (A) Extracts of HeLa cells transfected with LANA plus HA-GSK-3β were subjected to immunoprecipitation (IP) with control mouse or rat antibodies (lanes 2 and 3), anti-HA mouse monoclonal antibody (lane 4), or anti-LANA rat monoclonal antibody (lane 5). Lane 1 contained 5% of the extract used for the immunoprecipitation reactions. A Western blot of the precipitated proteins was probed with anti-LANA rat monoclonal antibody. LANA coprecipitated with HA-GSK-3β (lane 4). (B) Extracts of HeLa cells transfected with LANA plus Myc-GSK-3α were immunoprecipitated with the indicated antisera, and a Western blot of the precipitated proteins was probed with anti-LANA rat monoclonal antibody. Lane 1, control rat monoclonal antibody; lane 2, control rabbit polyclonal antibody; lane 3, anti-LANA rat monoclonal antibody; lane 4, anti-Myc rabbit polyclonal antibody; lane 5, extract (5% of the amount used in the immunoprecipitation reactions). LANA coprecipitated with Myc-GSK-3α (lane 4). (C) Extracts of HeLa cells transfected with herpesvirus saimiri Flag-ORF73 plus GSK-3β were subjected to immunoprecipitation with control rabbit polyclonal antibody (lane 1), anti-GSK-3β rabbit polyclonal antibody (lane 2), or anti-Flag rabbit polyclonal antibody (lane 3). The input sample (lane 4) contained 5% of the extract used for the immunoprecipitation reactions. A Western blot of the precipitated proteins was probed with anti-Flag rabbit polyclonal antibody. Flag-ORF73 coprecipitated with GSK-3β (lane 2). (D) Endogenous LANA and GSK-3β coprecipitate. Western blot of BC2 PEL cell nuclear extract immunoprecipitated with control rabbit polyclonal antibody (lane 1) or rabbit anti-GSK-3β polyclonal antibody (lane 2) and probed with anti-LANA rat monoclonal antibody; 5% of the nuclear extract used in the immunoprecipitations was loaded in lane 3.
To determine whether interaction with GSK-3β was a conserved property, the herpesvirus saimiri ORF73 homolog was also tested for GSK-3β interaction. An immunoprecipitation assay was performed on extracts of HeLa cells cotransfected with Flag-ORF73 and GSK-3β followed by Western blot analysis with anti-Flag monoclonal antibody (Fig. 1C). Flag-ORF73 was present in the direct rabbit anti-Flag precipitate (Fig. 1C, lane 3) but not in the precipitate generated with control rabbit antibody (Fig. 1C, lane 1). Flag-ORF73 was also present in the precipitate generated with anti-GSK-3β rabbit antibody (Fig. 1C, lane 2), indicating interaction between herpesvirus saimiri ORF73 and GSK-3β.
To confirm that the interaction between LANA and GSK-3β also occurs in latently infected PEL cells, a coimmunoprecipitation assay was performed with a nuclear extract of BC2 cells (Fig. 1D). Western blotting of the immunoprecipitated proteins with anti-LANA monoclonal antibody revealed the presence of LANA in the anti-GSK-3β precipitate (Fig. 1D, lane 2) but not in the precipitate generated with control rabbit antibody (Fig. 1D, lane 1).
LANA interferes with GSK-3β-mediated degradation of β-catenin.
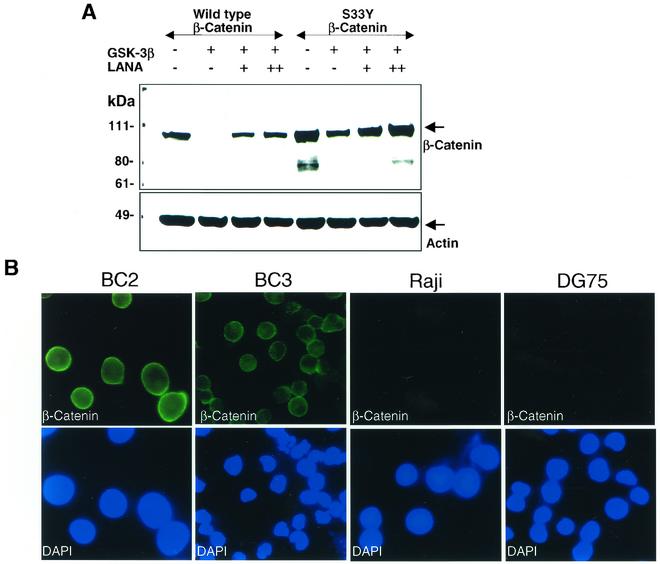
GSK-3β is a key regulatory kinase in the Wnt signaling pathway, where it phosphorylates β-catenin and marks β-catenin for proteasomal degradation (1, 42, 85). We examined the affect of LANA on GSK-3β-mediated turnover of β-catenin (Fig. 2A). In Western blot analyses performed on lysates of transfected HeLa cells, cotransfection of GSK-3β with β-catenin resulted in the loss of detectable β-catenin, as would be expected. However, in the presence of LANA, the ability of GSK-3β to promote β-catenin turnover was greatly reduced, and β-catenin was readily detectable. To further establish that the transfection assay was an appropriate measure of β-catenin turnover, the assay was repeated with β-catenin(S33Y), which carries a mutation in one of the three sites within β-catenin that are phosphorylated by GSK-3β. The S33Y mutant is consequently a less effective target for GSK-3β and has increased stability (59). In contrast to wild-type β-catenin, the S33Y mutant remained detectable in the presence of cotransfected GSK-3β. The addition of LANA resulted in a further small stabilization of β-catenin(S33Y) levels.
FIG. 2.
β-Catenin accumulates in the presence of LANA. (A) Western blot showing rescue of β-catenin from GSK-3β-mediated degradation. Extracts of HeLa cells transfected with 1.5 μg of wild-type or mutant (S33Y) β-catenin, 1 μg of HA-GSK-3β, and either 1 μg (+) or 2.5 μg (++) of Flag-LANA as indicated were analyzed by Western blotting for β-catenin levels with anti-β-catenin monoclonal antibody. Unlike wild-type β-catenin, S33Y mutant β-catenin is partially resistant to GSK-3β-mediated degradation. The membrane was subsequently stripped and reprobed with anti-β-actin monoclonal antibody to demonstrate equal loading of samples. (B) β-Catenin is abundant in PEL cells. Immunofluorescence assay comparing endogenous β-catenin levels in BC2 and BC3 PEL cells with EBV-positive Raji and EBV-negative, KSHV-negative DG75 B cells. Upper panels: Cells were stained with anti-β-catenin monoclonal antibody and fluorescein isothiocyanate-conjugated secondary antibody. Lower panels: Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI).
The level of endogenous β-catenin in KSHV-positive, LANA-expressing BC2 and BC3 PEL cells was compared to that in the EBV-positive Raji and EBV-negative DG75 B-cell lines in an immunofluorescence assay. Strong cytoplasmic staining and faint nuclear β-catenin staining were seen in BC2 and BC3 cells stained with anti-β-catenin primary antibody and fluorescein isothiocyanate-conjugated secondary antibody (Fig. 2B). In contrast, β-catenin was present in much lower levels in Raji cells and DG75 cells (Fig. 2B).
LANA overcomes GSK-3β-mediated repression of a Tcf/Lef reporter.
Accumulation of cytosolic β-catenin allows entry into the nucleus, where β-catenin interacts with Tcf/Lef family transcription factors to stimulate expression of cellular genes whose promoters contain Tcf/Lef binding sites (8, 14, 58). To determine whether antagonism of GSK-3β function by LANA led to increased nuclear β-catenin activity, transient-expression assays were performed in HeLa cells with luciferase reporters carrying three copies of either wild-type Tcf binding sites (GL3-OT) or three copies of a mutated binding site (GL3-OF). Expression from the GL3-OT reporter was activated by cotransfection of β-catenin, and this activation was abolished in the presence of GSK-3β (Fig. 3A, lane 2). Addition of increasing amounts of LANA resulted in a dose-responsive reactivation of the reporter (Fig. 3A, shaded bar, lanes 3 and 4), showing that LANA interaction with GSK-3β led to increased nuclear β-catenin activity. Dose-responsive activation was not seen with the GL3-OF reporter carrying mutated Tcf/Lef binding sites (Fig. 3A, open bar, lanes 3 and 4).
FIG. 3.
LANA reverses GSK-3β-mediated suppression of Tcf/Lef-dependent gene expression. (A) Transient-expression assay performed in HeLa cells transfected with 1 μg of the Tcf-luciferase reporter GL3-OT (shaded bar) or the control reporter GL3-OF (open bar), Tcf4 (0.75 μg), β-catenin (0.75 μg), control SV-β-gal (0.5 μg), HA-GSK-3β (1.5 μg), and Flag-LANA (+, 0.25 μg; ++, 0.5 μg) as indicated. GSK-3β abolished Tcf/Lef reporter expression (lane 2), and this repression was reversed by LANA (lanes 3 and 4). (B) Transient-expression assay performed in KSHV-positive PEL cells (BC2 and BC3) and KSHV-negative DG75 B cells. Cells were transfected with wild-type or mutant Tcf-luciferase reporter (GL3-OT or GL3-OF; 0.9 μg), control SV-β-gal (0.5 μg), and either β-catenin (0.4 μg) (lane 5) or β-catenin plus LANA (0.15 μg) (lane 6). Data are plotted as GL3-OT − GL3-OF. The assay was performed three times, and the standard deviation is shown.
The Tcf-luciferase reporter assay was repeated in BC2 and BC3 PEL cells and virus-negative DG75 B cells to determine whether the observed increase in β-catenin abundance in PEL cells also correlated with increased nuclear β-catenin activity. Reporter activity was ninefold higher in BC2 and BC3 PEL cells than in DG75 cells (Fig. 3B, lanes 1 to 3). Cotransfection of β-catenin into DG75 cells resulted in an increase in reporter expression, indicating that β-catenin levels were limiting in DG75 cells (Fig. 3B, lane 5). Cotransfection of LANA plus β-catenin (Fig. 3B, lane 6) gave a four- to fivefold increase in activity over that seen with β-catenin alone. This is likely to be a result of LANA stabilization of the transfected β-catenin. No response was seen with the mutated GL3-OF reporter. Assays were normalized to control β-galactosidase activity and were performed in triplicate.
Loss of LANA expression correlates with loss of β-catenin accumulation.
LANA is expressed in latently infected PEL cells, but expression is reduced after lytic cycle induction. The effect of lytic induction on β-catenin levels was examined in BC3 PEL cells treated with TPA. Western blot analysis showed strong induction of the lytic cycle K8/RAP protein by 12 h postinduction and continued expression of K8/RAP at 96 h postinduction (Fig. 4). LANA was expressed prior to TPA treatment and remained detectable at 48 h postinduction but not at the later time points of 72 h and 96 h. β-Catenin distribution mirrored that of LANA. β-Catenin was detectable in the extracts prior to induction and at 12, 24, and 48 h, but levels were diminished at 72 h and 96 h. This observation is consistent with the proposed role for LANA in β-catenin stabilization.
FIG. 4.
Loss of LANA expression correlates with loss of β-catenin accumulation. Western blot of extracts of BC3 cells harvested at the indicated times after treatment with TPA to induce KSHV lytic gene expression. One membrane was probed first with mouse anti-β-catenin monoclonal antibody and then stripped and reprobed with mouse anti-β-actin monoclonal antibody. Parallel blots of the same extract were probed with rat anti-LANA monoclonal antibody and anti-K8/RAP rabbit polyclonal antibody. Bands were visualized with the ECL reaction. Loss of LANA at the 72- and 96-h time points after lytic induction correlates with loss of β-catenin accumulation.
LANA alters intracellular distribution of GSK-3β.
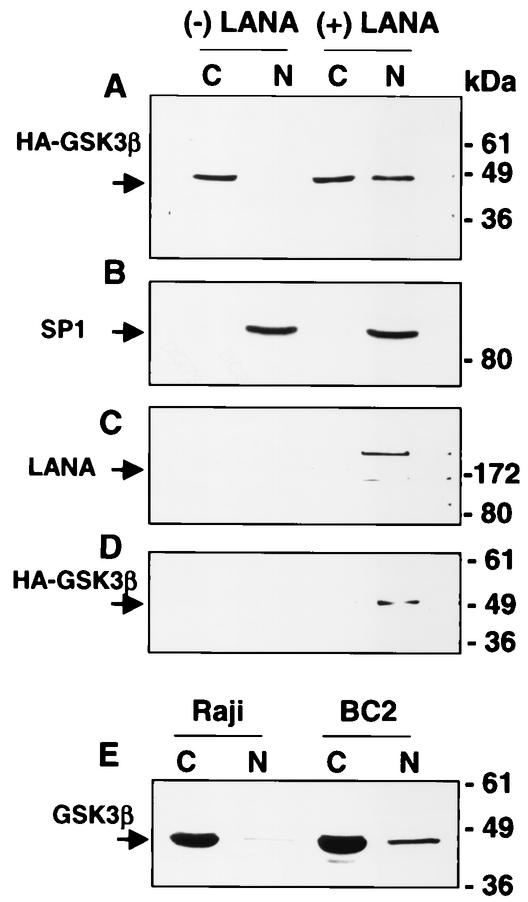
LANA is a nuclear protein, and GSK-3β is predominantly cytoplasmic. However, a small proportion of GSK-3β is known to enter the nucleus during the S phase of the cell cycle (24). We compared the distribution of GSK-3β in HeLa cells transfected with either HA-GSK-3β or HA-GSK-3β plus LANA (Fig. 5). Cells were harvested 2 days after transfection, and nuclear and cytoplasmic fractions were isolated. By Western blot analysis, GSK-3β was almost completely cytoplasmic in cells transfected with HA-GSK-3β alone. In striking contrast, a significant amount of GSK-3β was present in the nucleus of cells cotransfected with LANA (Fig. 5A). The integrity of the nuclear fraction was checked by probing with antibody to the nuclear transcription factor SP1 (Fig. 5B).
FIG. 5.
LANA alters the intracellular localization of GSK-3β. Western blot analyses of fractionated extracts (C, cytoplasmic; N, nuclear) of HeLa cells transfected with 5 μg each of HA-GSK-3β, β-catenin, and either vector (− LANA) or Flag-LANA (+ LANA). (A) Membrane probed with anti-GSK-3β antibody. Nuclear GSK-3β was increased in the presence of LANA. (B) The membrane in A was stripped and reprobed with anti-SP1 rabbit antibody to demonstrate the distribution of a known nuclear protein. (C) The same extracts were immunoprecipitated with anti-GSK-3β monoclonal antibody, and a parallel Western blot was probed with anti-LANA monoclonal antibody. Detection of LANA in the HA precipitate of the nuclear LANA-transfected cells indicates interaction between LANA and GSK-3β in the nucleus. (D) The extracts were immunoprecipitated with anti-LANA monoclonal antibody, and a parallel Western blot was probed with rabbit anti-HA antibody to detect HA-GSK-3β. Detection of nuclear GSK-3β in the LANA precipitate confirmed that interaction between these proteins is occurring in the nucleus. (E) Western blot comparing the distribution of nuclear (N) and cytoplasmic (C) GSK-3β in fractionated Raji B-cell and BC2 PEL cell extracts. The membrane was probed with anti-GSK-3β monoclonal antibody.
As a further demonstration that the interaction between LANA and GSK-3β was taking place in the cell nucleus, the same nuclear and cytoplasmic fractions were immunoprecipitated with anti-HA antibody, and a Western blot of the precipitated proteins was probed with anti-LANA antibody (Fig. 5C). LANA was shown to coprecipitate with HA-GSK-3β in the nuclear fraction of the cotransfected cells. In a reciprocal immunoprecipitation (Fig. 5D), the fractionated extracts were immunoprecipitated with anti-LANA antibody, and a Western blot of the precipitated proteins was probed with anti-HA antibody. HA-GSK-3β was found to coprecipitate with LANA in the nuclear fraction of the cotransfected cells.
To determine whether LANA also led to increased nuclear accumulation of GSK-3β in KSHV-infected cells, EBV-positive Raji B cells and BC2 PEL cells were fractionated into nuclear and cytoplasmic fractions, and the extracts were subjected to Western blotting with anti-GSK-3β monoclonal antibody (Fig. 5E). The amount of GSK-3β detected in the nuclear fraction of BC2 cells was increased relative to that in Raji cells.
Identification of LANA sequences required for GSK-3β interaction.
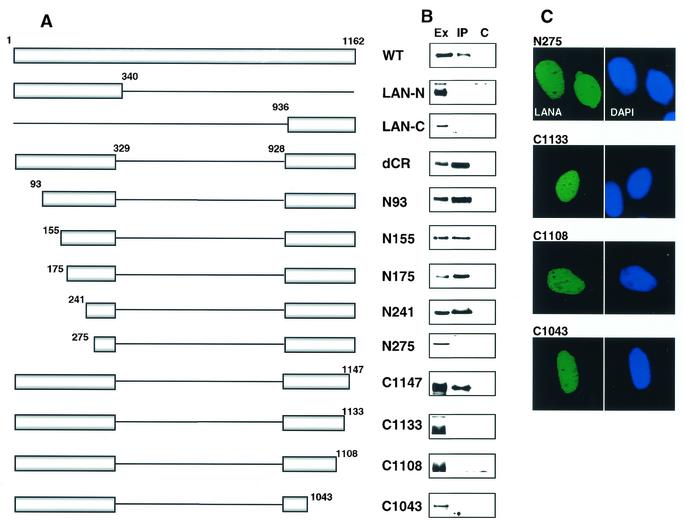
Large deletions of the LANA N terminus, internal repeat region, and C terminus were generated along with a series of smaller N-terminal and C-terminal deletions (Fig. 6A). The different Flag-tagged LANA constructions were cotransfected with HA-GSK-3β into HeLa cells, and the cell extracts were immunoprecipitated with anti-HA antibody followed by Western blotting of the immunoprecipitated proteins. The blots were probed with anti-Flag antibody to detect coprecipitating Flag-LANA and map the regions of LANA required for GSK-3β interaction (Fig. 6B). Examination of the results obtained with the large deletions indicated that the internal repeat region was not required for binding to GSK-3β. However, neither the LANA N terminus nor the LANA C terminus was able to be coprecipitated with HA-GSK-3β, suggesting that more than one region of LANA was necessary for the interaction (Fig. 6B).
FIG. 6.
Mapping the requirements for GSK-3β interaction. (A) Diagrammatic representation of the Flag-LANA mutants used in the mapping experiments. The boundary amino acids are numbered. (B) Western blot examining coprecipitation of Flag-LANA and HA-GSK-3β. Extracts of HeLa cells cotransfected with HA-GSK-3β (8 μg) and Flag-LANA mutants (8 μg) were immunoprecipitated with anti-HA antibody, and the presence of Flag-LANA in the precipitates was examined by probing with anti-Flag monoclonal antibody. Ex, cell extract; IP, immunoprecipitated with rabbit anti-HA antibody; C, immunoprecipitated with control rabbit antibody; WT, wild type. (C) Immunofluorescence assay showing nuclear localization of the non-GSK-3β-interacting Flag-LANA mutants. HeLa cells were transfected with the Flag-LANA mutants and stained with anti-Flag monoclonal antibody followed by fluorescein isothiocyanate-conjugated secondary antibody (left panel). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (right panel).
Analysis of the N-terminal deletion series revealed that LANA mutants deleted for the N-terminal 93, 155, 175, and 241 amino acids continued to coprecipitate with HA-GSK-3β. However, deletion to amino acid 275 resulted in a loss of interaction. In the case of the C-terminal mutants, deletion to position 1147 did not affect GSK-3β binding, but binding was lost when the deletion was extended to position 1133 or beyond to position 1108 or 1043 (Fig. 6B). LANA contains both an N-terminal nuclear localization signal (amino acids 24 to 30 [63]) and a C-terminal nuclear localization signal (amino acids 863 to 932 [74]) that is unmasked when the C terminus is expressed alone. The length of the central repeat region differs between different LANA isolates, which alters the amino acid numbering in the C terminus, and amino acids 863 to 932 equal amino acids 935 to 1004 in our LANA proteins. All of the LANA constructions used for the mapping experiments therefore contain the region encoding the C-terminal nuclear localization signal. However, these LANA constructions are fusions between the N terminus and the C terminus, and the nuclear localization of LANA proteins of this kind has not been examined experimentally.
To ensure that the lack of interaction between LANA mutants and GSK-3β was not an artifact of improper intracellular localization of LANA, the ability of the LANA mutants N275, C1133, C1108, and C1043 to target to the nucleus was examined in transfected HeLa cells. Immunofluorescence assays showed that these LANA mutants retained the ability to localize to the nucleus (Fig. 6C). Note that LANA exhibits diffuse nuclear staining in the absence of KSHV genomes. The punctate nuclear staining seen in PEL cells reflects colocalization of LANA and KSHV episomal DNA (5). Thus, the coimmunoprecipitation experiments indicated that LANA N-terminal sequences between amino acids 241 and 275 plus LANA C-terminal sequences between amino acids 1133 and 1147 are required for LANA to interact effectively with GSK-3β.
The interaction between LANA and GSK-3β was also examined in GST affinity assays with purified GST-GSK-3β and the in vitro-translated, 35S-labeled Flag-LANA constructions whose expression is shown in Fig. 7B. The results obtained differed slightly from those observed in the coimmunoprecipitation assays but further supported the existence of N-terminal and C-terminal LANA domains for binding to GSK-3β. Consistent with the coimmunoprecipitation assays, LANA deleted for the central repeat region (dCR) interacted with GST-GSK-3β, and the LANA N terminus (LAN-N) did not (Fig. 7A). However, in the GST affinity assay, the LANA C terminus (LAN-C) was capable of binding to GST-GSK-3β in the absence of any N-terminal sequences (Fig. 7A). In addition, LANA truncated at amino acid 1108 (C1108), which is deleted for part of the C-terminal GSK-3β binding domain and did not coimmunoprecipitate with GSK-3β, bound to GST-GSK-3β (Fig. 7A). Further truncation of the C-terminal domain to amino acid 1043 (C1043) severely reduced binding in the GST affinity assay (Fig. 7A).
FIG. 7.
Analysis of LANA interaction with GSK-3β in a GST affinity assay. (A) Autoradiograph showing binding of individual radiolabeled Flag-LANA plasmids to purified bacterially expressed GST-GSK-3β. The LANA C terminus (LAN-C) showed independent binding in this assay, as did the LANA N terminus when expressed in a dimerization-competent construction (C1108) but not when expressed in nondimerizing constructions (C1043 and LAN-N). (B) Autoradiograph showing expression of the different 35S-labeled, in vitro-translated Flag-LANA proteins.
One difference between the C1108 and C1043 constructions is that the former would be capable of dimerization while the latter would not, based on the mapping data of Schwam et al. (74). We interpret these data to suggest that dimerization may be important for GSK-3β interaction. The C terminus of LANA contains both the dimerization domain and a GSK-3β interaction region, while the N terminus also has an interaction domain, but this region requires LANA C-terminal sequences to allow protein dimerization. In this analysis, C1108 would interact with GSK-3β through the N-terminal contact region, while the N-terminal sequences in C1043 and LANA-N would not be capable of effective interaction because of the inability of these proteins to dimerize. Interestingly, the LANA C terminus shows sequence similarity to the GSK-3 binding domain of axin. An alignment between the C-terminal region of LANA required for binding to GSK-3β and the GSK-3β binding domain of axin (90) is presented in Fig. 8.
FIG. 8.
Alignment of the C-terminal region of LANA and herpesvirus saimiri ORF73 with the mapped GSK-3β interaction domains of human axin (hAxin), mouse axin (mAxin), and chicken axin (cAxin) (90). Amino acid similarities between LANA and the herpesvirus saimiri ORF73 and axin proteins are highlighted in bold.
LANA-GSK-3β interaction is necessary for GSK-3β redistribution and for nuclear β-catenin activity.
The ability of the different Flag-LANA mutants to mediate intracellular redistribution of HA-GSK-3β was examined. Cotransfected HeLa cells were harvested and separated into nuclear and cytoplasmic fractions prior to Western blotting. Probing of the blots with anti-HA antibody showed that nuclear accumulation of GSK-3β was dependent on LANA interaction (Fig. 9). Cotransfection with LANA mutants N93, N155, N175, N241, and C1147, which retained GSK-3β binding activity, led to an increased nuclear presence of HA-GSK-3β, while LANA mutants that were impaired for GSK-3β binding (N275, C1133, C1108, and C1043) had minimal impact on GSK-3β relocalization.
FIG. 9.
LANA interaction is required for GSK-3β nuclear translocation. Western blot analysis of the intracellular distribution of HA-GSK-3β in HeLa cells cotransfected with the indicated LANA variants. The nuclear (N) and cytoplasmic (C) fractions were probed with anti-HA monoclonal antibody. -, LANA absent. LANA mutants LAN-C, LANA-N, N-275, C1133, C1108, and C1043 were severely impaired for nuclear relocalization of HA-GSK-3β. WT, wild type.
An additional linkage between GSK-3β binding and nuclear β-catenin activity was demonstrated when these LANA mutants were tested for their ability to activate expression from the Tcf-luciferase reporter in transfected HeLa cells. The LANA derivatives that retained GSK-3β binding ability also activated reporter expression (Fig. 10, lanes 3 to 8 and 12). Interestingly, deletion of the N-terminal 93 amino acids in mutants N93, N155, and N175 resulted in increased stimulation of reporter activity over that observed with the equivalent construction retaining these sequences (dCR), although this effect was not observed with the larger N-terminal deletions N204 and N241. The N-terminal 93 amino acids of LANA contain the binding domain for the methyl-CpG binding protein MeCp2 (46). The C-terminal mutants that did not bind GSK-3β in the coimmunoprecipitation assays (C1133, C1108, and C1043) were also inactive in the reporter assay (Fig. 10, lanes 13 to 15), as was the N-terminal non-GSK-binding mutant N275 (Fig. 10, lane 9).
FIG. 10.
LANA interaction with GSK-3β is required for activation of a Tcf-luciferase reporter. Transient-expression assay in which HeLa cells were transfected with the Tcf-luciferase reporter GL3-OT or the mutant reporter GL3-OF (1 μg), Tcf4 (0.75 μg), β-catenin (0.75 μg), HA-GSK-3β (1 μg), control SV-β-gal (0.5 μg), and the indicated Flag-LANA variants (1 μg). Solid bar, GL3-OT; open bar, GL3-OF. The assay was repeated three times, and the standard deviation is indicated.
DISCUSSION
GSK-3β phosphorylates serine and threonine residues in the context of S/TXXXS/Tp where the S/Tp position is phosphorylated by another priming kinase and is unusual for a regulatory kinase in that it is constitutively active and is inhibited as a downstream response to induction. GSK-3β participates in the growth factor, insulin, and Wnt signaling pathways. In the last pathway, GSK-3β is present in a cytoplasmic complex containing axin, the adenomatous polyposis coli tumor suppressor protein (APC), diversin (75), the priming kinases casein kinase Iε and Iα (2, 51), and β-catenin. GSK-3β regulates the stability of β-catenin by phosphorylating residues 33, 37, and 41 in the N terminus of β-catenin, which marks β-catenin for binding by the β-transducin repeat-containing protein (β-Trcp) E3 ubiquitin ligase, ubiquitination, and proteasomal degradation (1, 60).
In the canonical Wnt pathway, secreted Wnt family glycoproteins bind to seven transmembrane receptors of the Frizzled family, which also complex with low-density lipoprotein receptor-related protein (LRP5 and LRP6) coreceptors (61, 82) to mediate hyperphosphorylation of Dishevelled (88). Activated Dishevelled is believed to cause the release of GSK-3β from the complex by promoting the association of Frat/GSK-3-binding protein with GSK-3β, which displaces GSK-3β from axin (34). Loss of GSK-3β leads to hypophosphorylation of β-catenin and β-catenin accumulation and nuclear entry. Nuclear β-catenin does not itself bind DNA but rather complexes with the high mobility group (HMG) domain family of Tcf/Lef transcription factors (37). In the absence of β-catenin, these factors act as repressors through histone deacetylase tethering (12, 71). β-Catenin displaces the repressor complex and recruits coactivators such as CBP/p300, Brg-1, and pygopus (7, 10, 27, 45, 80, 83). Tcf/Lef-responsive genes play a part in cell proliferation, apoptosis, and cell fate decisions and include c-myc, c-myb, c-jun, cyclin D1, c-ETS2 and c-KIT, BMP4, ITF-2, Nr-CAM, Fra, and MDR1 (7, 21, 44, 87).
β-Catenin accumulation occurs in a variety of human cancers (9, 65, 84). Inactivating mutations in APC or mutations in the N terminus of β-catenin that abolish GSK-3β phosphorylation are the most frequently recognized defects and occur in 90% of colorectal cancers (65). Experimentally, fusion of LEF-1 to β-catenin to give constitutive activity results in a protein that transforms chicken embryo fibroblasts (4). Wnt signaling also inhibits apoptosis through β-catenin-mediated transcriptional activity (19). By increasing levels of β-catenin, LANA potentially contributes to growth dysregulation through upregulation of cellular Tcf/Lef-responsive genes, and some of the genes reported to be positively regulated by LANA may also be downstream responders to β-catenin accumulation. For example, cellular interleukin-6 is upregulated in endothelial cells by LANA, and an AP-1 site in the interleukin-6 promoter was identified as being required for this response (3). β-Catenin-Tcf/Lef increases transcription of c-Jun and thus has the potential to increase expression through AP-1 sites.
LANA interacted with both GSK-3α and GSK-3β. These two isoforms of GSK-3 are closely related but may not be identical functionally. In mice, elimination of GSK-3β is lethal, indicating that GSK-3α either is not expressed comparably or does not function comparably to GSK-3β (35). GSK-3α has been subjected to much less scrutiny than the β isoform. However, in GSK-3β knockout mouse cells, there was no defect in Wnt signaling, with GSK-3α substituting effectively for GSK-3β, suggesting that both of these isoforms may participate in β-catenin regulation (85). Two regions of LANA were found to be necessary for efficient binding to GSK-3β, as measured by coimmunoprecipitation, an N-terminal region between amino acids 241 and 275 and a C-terminal region between amino acids 1133 and 1147. These regions appeared to be capable of independent binding to GSK-3β in the GST affinity assay, where the high concentration of bacterially expressed GST fusion protein permits detection of lower-affinity interactions. The GST affinity experiments also suggested that interaction between LANA and GSK-3β may require protein dimerization.
Limited homology was noted between a C-terminal region of LANA (amino acids 1114 to 1146) and the domain of axin (amino acids 373 to 412) that binds to GSK-3β (Fig. 8). There is also considerable amino acid conservation in the C terminus of the primate herpesvirus LANA homologs, and herpesvirus saimiri ORF73 also interacted with GSK-3β in a coimmunoprecipitation assay. A region with a similar level of homology to the GSK-3β binding region of axin can be identified in the ORF73 homolog of herpesvirus saimiri, although the aligning sequences are displaced relative to the equivalent KSHV LANA sequences (Fig. 8). At a minimum, GSK-3β binding appears to be a conserved LANA function, but the consequences of that interaction may not be identical in the different gamma-2 herpesvirus family members. The N terminus of LANA is not well conserved in other primate gamma-2 herpesvirus LANA homologs, and the nature of the requirement for the N-terminal LANA sequences is not clear. Axin is a substrate for GSK-3 as well as containing a GSK-3 binding domain (30). The N terminus of LANA contains multiple potential GSK-3 phosphorylation sites, including sites in the region found to be required for efficient interaction with GSK-3β. It is possible that phosphorylation may be important for optimal GSK-3 interaction with LANA.
GSK-3β is predominantly cytoplasmic, but a small proportion of GSK-3β enters the nucleus during S phase and during apoptosis (11, 24). We found that LANA interaction with GSK-3β results in increased nuclear accumulation of GSK-3β. We speculate that nuclear accumulation of GSK-3β may lead to cytoplasmic depletion of the enzyme and escape of β-catenin from phosphorylation and degradation. The increased pool of free cytosolic β-catenin would then be available for nuclear entry and gene activation. This suggestion is supported by the experiments showing that LANA variants that have lost GSK-3β interaction do not relocalize GSK-3β and are defective in their ability to activate a Tcf-responsive reporter.
There is a second aspect to GSK-3β nuclear accumulation that remains to be examined experimentally. Substrates for GSK-3β include nuclear transcription factors such as CREB, c-Jun, c-Myc, C/EBPα, C/EBPβ, and NFATc and nuclear cell cycle regulatory factors such as cyclin D1. In the majority of cases, phosphorylation by GSK-3β interferes with the activity of these proteins. Whether nuclear GSK-3β remains active when complexed with LANA or whether this form of GSK-3β is sequestered in a manner that limits its access to nuclear substrates is an interesting question.
In summary, we have identified relocalization of GSK-3β as one of the mechanisms by which LANA may modify cellular gene expression. Since deregulation of β-catenin has been described in a variety of human cancers, including breast, ovarian, gastric, hepatocellular, thyroid, and renal cell carcinomas and melanoma, it seems likely that the manipulation of GSK-3β by LANA may be a contributing factor in KSHV-associated malignancies.
Acknowledgments
We are grateful to K. Kinzler and B. Vogelstein for Tcf-luciferase wild-type and mutant reporters, β-catenin and mutant β-catenin(S33Y), to F. McCormick for the HA-GSK-3β expression vector, to J. Nicholas for herpesvirus saimiri ORF73, and to G. Hayward for anti-K8/RAP antiserum. We thank F. Chang for manuscript preparation.
This work was funded by National Institutes of Health award RO1 CA85151 to S.D.H.
REFERENCES
- 1.Aberle, H., A. Bauer, J. Stappert, A. Kispert, and R. Kemler. 1997. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 16:3797-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amit, S., A. Hatzubai, Y. Birman, J. S. Andersen, E. Ben-Shushan, M. Mann, Y. Ben-Neriah, and I. Alkalay. 2002. axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 16:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, M., A. Hecht, U. Kruse, R. Kemler, and P. K. Vogt. 1999. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc. Natl. Acad. Sci. USA 96:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 6.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker, N., A. Hurlstone, H. Musisi, A. Miles, M. Bienz, and H. Clevers. 2001. The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J. 20:4935-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker, N., P. J. Morin, and H. Clevers. 2000. The yin-yang of TCF/beta-catenin signaling. Adv. Cancer Res. 77:1-24. [DOI] [PubMed] [Google Scholar]
- 9.Behrens, J. 2000. Control of beta-catenin signaling in tumor development. Ann. N. Y. Acad. Sci. 910:21-35. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ze'ev, A., M. Shtutman, and J. Zhurinsky. 2000. The integration of cell adhesion with gene expression: the role of beta-catenin. Exp. Cell Res. 261:75-82. [DOI] [PubMed] [Google Scholar]
- 11.Bijur, G. N., and R. S. Jope. 2001. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J. Biol. Chem. 276:37436-37442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billin, A. N., H. Thirlwell, and D. E. Ayer. 2000. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol. Cell. Biol. 20:6882-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 14.Brantjes, H., N. Barker, J. van Es, and H. Clevers. 2002. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 383:255-261. [DOI] [PubMed] [Google Scholar]
- 15.Cannon, J. S., J. Nicholas, J. M. Orenstein, R. B. Mann, P. G. Murray, P. J. Browning, J. A. DiGiuseppe, E. Cesarman, G. S. Hayward, and R. F. Ambinder. 1999. Heterogeneity of viral interleukin-6 expression in HHV-8-associated diseases. J. Infect. Dis. 180:824-828. [DOI] [PubMed] [Google Scholar]
- 16.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 17.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 18.Chang, Y., P. S. Moore, S. J. Talbot, C. H. Boshoff, T. Zarkowska, D. Godden-Kent, H. Paterson, R. A. Weiss, and S. Mittnacht. 1996. Cyclin encoded by Kaposi's sarcoma herpesvirus. Nature 382:410. [DOI] [PubMed] [Google Scholar]
- 19.Chen, S., D. C. Guttridge, Z. You, Z. Zhang, A. Fribley, M. W. Mayo, J. Kitajewski, and C. Y. Wang. 2001. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J. Cell Biol. 152:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiou, C. J., L. J. Poole, P. S. Kim, D. M. Ciufo, J. S. Cannon, C. M. apRhys, D. J. Alcendor, J. C. Zong, R. F. Ambinder, and G. S. Hayward. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conacci-Sorrell, M. E., T. Ben-Yedidia, M. Shtutman, E. Feinstein, P. Einat, and A. Ben-Ze'ev. 2002. Nr-CAM is a target gene of the beta-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 16:2058-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotter, M. A., II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 23.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 24.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupin, N., C. Fisher, P. Kellam, S. Arid, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastman, Q., and R. Grosschedl. 1999. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 11:233-240. [DOI] [PubMed] [Google Scholar]
- 28.Ellis, M., Y. P. Chew, L. Fallis, S. Freddersdorf, C. Boshoff, R. A. Weiss, X. Lu, and S. Mittnacht. 1999. Degradation of p27(Kip) cdk inhibitor triggered by Kaposi's sarcoma virus cyclin-cdk6 complex. EMBO J. 18:644-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fakhari, F. D., and D. P. Dittmer. 2002. Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J. Virol. 76:6213-6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frame, S., P. Cohen, and R. M. Biondi. 2001. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7:1321-1327. [DOI] [PubMed] [Google Scholar]
- 31.Friborg, J. J., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 32.Garber, A. C., M. A. Shu, J. Hu, and R. Renne. 2001. DNA binding and modulation of gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:7882-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godden-Kent, D., S. J. Talbot, C. Boshoff, Y. Chang, P. Moore, R. A. Weiss, and S. Mittnacht. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harwood, A. J. 2001. Regulation of GSK-3: a cellular multiprocessor. Cell 105:821-824. [DOI] [PubMed] [Google Scholar]
- 35.Hoeflich, K. P., J. Luo, E. A. Rubie, M. S. Tsao, O. Jin, and J. R. Woodgett. 2000. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406:86-90. [DOI] [PubMed] [Google Scholar]
- 36.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurlstone, A., and H. Clevers. 2002. T-cell factors: turn-ons and turn-offs. EMBO J. 21:2303-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269:335-344. [DOI] [PubMed] [Google Scholar]
- 40.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 1999. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am. J. Pathol. 155:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight, J. S., M. A. Cotter II, and E. S. Robertson. 2001. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus transactivates the telomerase reverse transcriptase promoter. J. Biol. Chem. 276:22971-22978. [DOI] [PubMed] [Google Scholar]
- 44.Kolligs, F. T., M. T. Nieman, I. Winer, G. Hu, D. Van Mater, Y. Feng, I. M. Smith, R. Wu, Y. Zhai, K. R. Cho, and E. R. Fearon. 2002. ITF-2, a downstream target of the Wnt/TCF pathway, is activated in human cancers with beta-catenin defects and promotes neoplastic transformation. Cancer Cell 1:145-155. [DOI] [PubMed] [Google Scholar]
- 45.Kramps, T., O. Peter, E. Brunner, D. Nellen, B. Froesch, S. Chatterjee, M. Murone, S. Zullig, and K. Basler. 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109:47-60. [DOI] [PubMed] [Google Scholar]
- 46.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, M. A., M. E. Diamond, and J. L. Yates. 1999. Genetic evidence that EBNA-1 is needed for efficient, stable latent infection by Epstein-Barr virus. J. Virol. 73:2974-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li, M., H. Lee, D. W. Yoon, J.-C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 51.Liu, C., Y. Li, M. Semenov, C. Han, G. H. Baeg, Y. Tan, Z. Zhang, X. Lin, and X. He. 2002. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837-847. [DOI] [PubMed] [Google Scholar]
- 52.Low, W., M. Harries, H. Ye, M. Q. Du, C. Boshoff, and M. Collins. 2001. Internal ribosome entry site regulates translation of Kaposi's sarcoma-associated herpesvirus FLICE inhibitory protein. J. Virol. 75:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann, D. J., E. S. Child, C. Swanton, H. Laman, and N. Jones. 1999. Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J. 18:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattsson, K., C. Kiss, G. M. Platt, G. R. Simpson, E. Kashuba, G. Klein, T. F. Schulz, and L. Szekely. 2002. Latent nuclear antigen of Kaposi's sarcoma herpesvirus/human herpesvirus-8 induces and relocates RING3 to nuclear heterochromatin regions. J. Gen. Virol. 83:179-188. [DOI] [PubMed] [Google Scholar]
- 56.Mittnacht, S., and C. Boshoff. 2000. Viral cyclins. Rev. Med. Virol. 10:175-184. [DOI] [PubMed] [Google Scholar]
- 57.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 58.Morin, P. J. 1999. Beta-catenin signaling and cancer. Bioessays 21:1021-1030. [DOI] [PubMed] [Google Scholar]
- 59.Morin, P. J., A. B. Sparks, V. Korinek, N. Barker, H. Clevers, B. Vogelstein, and K. W. Kinzler. 1997. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787-1790. [DOI] [PubMed] [Google Scholar]
- 60.Orford, K., C. Crockett, J. P. Jensen, A. M. Weissman, and S. W. Byers. 1997. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J. Biol. Chem. 272:24735-24738. [DOI] [PubMed] [Google Scholar]
- 61.Pandur, P., and M. Kuhl. 2001. An arrow for wingless to take off. Bioessays 23:207-210. [DOI] [PubMed] [Google Scholar]
- 62.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piolot, T., M. Tramier, M. Coppey, J. C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Platt, G. M., E. Cannell, M. E. Cuomo, S. Singh, and S. Mittnacht. 2000. Detection of the human herpesvirus 8-encoded cyclin protein in primary effusion lymphoma-derived cell lines. Virology 272:257-266. [DOI] [PubMed] [Google Scholar]
- 65.Polakis, P. 2000. Wnt signaling and cancer. Genes Dev. 14:1837-1851. [PubMed] [Google Scholar]
- 66.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene H-ras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 67.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S.-J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859-868. [DOI] [PubMed] [Google Scholar]
- 69.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roose, J., M. Molenaar, J. Peterson, J. Hurenkamp, H. Brantjes, P. Moerer, M. van de Wetering, O. Destree, and H. Clevers. 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395:608-612. [DOI] [PubMed] [Google Scholar]
- 72.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with mini-extracts prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarz-Romond, T., C. Asbrand, J. Bakkers, M. Kuhl, H. J. Schaeffer, J. Huelsken, J. Behrens, M. Hammerschmidt, and W. Birchmeier. 2002. The ankyrin repeat protein diversin recruits casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16:2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shinohara, H., M. Fukushi, M. Higuchi, M. Oie, O. Hoshi, T. Ushiki, J. Hayashi, and M. Fujii. 2002. Chromosome binding site of latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus is essential for persistent episome maintenance and is functionally replaced by histone H1. J. Virol. 76:12917-12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 78.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun, Q., H. Matta, and P. M. Chaudhary. 2003. The human herpes virus 8-encoded viral FLICE inhibitory protein protects against growth factor withdrawal-induced apoptosis via NF-kappaB activation. Blood 101:1956-1961. [DOI] [PubMed]
- 80.Sun, Y., F. T. Kolligs, M. O. Hottiger, R. Mosavin, E. R. Fearon, and G. J. Nabel. 2000. Regulation of beta-catenin transformation by the p300 transcriptional coactivator. Proc. Natl. Acad. Sci. USA 97:12613-12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swanton, C., D. J. Mann, B. Fleckenstein, F. Neipel, G. Peters, and N. Jones. 1997. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390:184-187. [DOI] [PubMed] [Google Scholar]
- 82.Tamai, K., M. Semenov, Y. Kato, R. Spokony, C. Liu, Y. Katsuyama, F. Hess, J. P. Saint-Jeannet, and X. He. 2000. Low-density lipoprotein-receptor-related proteins in Wnt signal transduction. Nature 407:530-535. [DOI] [PubMed] [Google Scholar]
- 83.Thompson, B., F. Townsley, R. Rosin-Arbesfeld, H. Musisi, and M. Bienz. 2002. A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 4:367-373. [DOI] [PubMed] [Google Scholar]
- 84.Webster, M. T., M. Rozycka, E. Sara, E. Davis, M. Smalley, N. Young, T. C. Dale, and R. Wooster. 2000. Sequence variants of the axin gene in breast, colon, and other cancers: an analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer 28:443-453. [PubMed] [Google Scholar]
- 85.Woodgett, J. R. 2001. Judging a protein by more than its name: GSK-3. Sci. STKE 100:(RE12):1-11. [DOI] [PubMed]
- 86.Woodgett, J. R. 1990. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 9:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada, T., A. S. Takaoka, Y. Naishiro, R. Hayashi, K. Maruyama, C. Maesawa, A. Ochiai, and S. Hirohashi. 2000. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 60:4761-4766. [PubMed] [Google Scholar]
- 88.Yanagawa, S., F. van Leeuwen, A. Wodarz, J. Klingensmith, and R. Nusse. 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9:1087-1097. [DOI] [PubMed] [Google Scholar]
- 89.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in a variety of mammalian cells. Nature (London) 313:812-815. [DOI] [PubMed] [Google Scholar]
- 90.Zeng, L., F. Fagotto, T. Zhang, W. Hsu, T. J. Vasicek, W. L. Perry III, J. J. Lee, S. M. Tilghman, B. M. Gumbiner, and F. Costantini. 1997. The mouse Fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181-192. [DOI] [PubMed] [Google Scholar]
- 91.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]