Abstract
PTEN is a tumor suppressor gene located on chromosome 10q23 that encodes a protein and phospholipid phosphatase. Somatic mutations of PTEN are found in a number of human malignancies, and loss of expression, or mutational inactivation of PTEN, leads to the constitutive activation of protein kinase B (PKB)/Akt via enhanced phosphorylation of Thr-308 and Ser-473. We recently have demonstrated that the integrin-linked kinase (ILK) can phosphorylate PKB/Akt on Ser-473 in a phosphoinositide phospholipid-dependent manner. We now demonstrate that the activity of ILK is constitutively elevated in a serum- and anchorage-independent manner in PTEN-mutant cells, and transfection of wild-type (WT) PTEN into these cells inhibits ILK activity. Transfection of a kinase-deficient, dominant-negative form of ILK or exposure to a small molecule ILK inhibitor suppresses the constitutive phosphorylation of PKB/Akt on Ser-473, but not on Thr-308, in the PTEN-mutant prostate carcinoma cell lines PC-3 and LNCaP. Transfection of dominant-negative ILK and WT PTEN into these cells also results in the inhibition of PKB/Akt kinase activity. Furthermore, dominant-negative ILK or WT PTEN induces G1 phase cycle arrest and enhanced apoptosis. Together, these data demonstrate a critical role for ILK in PTEN-dependent cell cycle regulation and survival and indicate that inhibition of ILK may be of significant value in PTEN-mutant tumor therapy.
Keywords: tumor suppressor, phospholipid phosphatase, cell adhesion, serum
The PTEN/MMAC/TEP1 tumor suppressor gene located on chromosome 10q23 (1–3) is mutated at high frequency in a wide variety of human cancers, including glioblastoma, melanoma, and carcinomas of the prostate, breast, endometrium, lung, and head and neck (1, 2, 4, 5–11). Germ-line mutations in the PTEN gene are associated with the development of Cowden's disease and Bannayan–Zonana syndrome (12–15). In addition, the phenotype of PTEN-null mice supports the conclusion that PTEN functions as a tumor suppressor gene (16, 17). The homozygous disruption of PTEN results in early embryonic lethality, and heterozygous mice display hyperplastic changes in the prostate, skin, and colon similar to those seen in Cowden's disease. The PTEN gene product shares sequence identity with the protein tyrosine phosphatase family and chicken tensin (18). Recombinant PTEN is capable of dephosphorylating both phosphotyrosine and phosphothreonine, but it also can dephosphorylate phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3], the product of phosphatidylinositol 3-kinase (PI3-kinase) (19, 20). Because many of the cancer-related mutations have been mapped to the phosphatase catalytic domain, it has been suggested that the phosphatase activity of PTEN is required for its tumor suppressor function. Overexpression of PTEN can suppress growth in soft agar and tumor formation in nude mice (21, 22) and also inhibit focal adhesion kinase (FAK), leading to inhibition of cell adhesion and migration (23). However, targeted disruption of PTEN and PTEN mutations in glioblastoma and prostate carcinoma cells result in the serum- and anchorage-independent activation of protein kinase B (PKB)/Akt probably because of increased levels of PI(3,4,5)P3 (16, 24–26). PKB/Akt suppresses apoptosis via several possible downstream effectors, including phosphorylation and inactivation of BAD (27–29), inactivation of caspase-9 (30), and repression of the forkhead transcription factor (31). Consistent with this, the disruption of PTEN leads to the suppression of apoptosis (16) but also to accelerated cell cycle progression (25, 26). Reconstitution with wild-type (WT) PTEN restores apoptotic sensitivity and induces cell cycle arrest (16, 26). These new insights into the tumor-suppressive effects of PTEN recently have been reviewed by Cantley and Neel (32).
The activation of PKB/Akt is regulated in a complex manner via phosphorylation of PKB/Akt on Thr-308 and Ser-473 (33). Although PDK-1 has been shown to phosphorylate Thr-308, it is not clear whether there is a distinct kinase that exclusively phosphorylates Ser-473. It recently has been proposed that PDK-1 acquires Ser-473 phosphorylation activity in the presence of PRK2 peptide (34). However, as yet, there is no evidence that PDK-1 is the only kinase capable of doing this, and the physiological relevance of the conversion of PDK-1 activity toward Ser-473 phosphorylation by the PRK2 peptide has yet to be demonstrated. On the other hand, the integrin-linked kinase (ILK) (35) has been shown to phosphorylate PKB/Akt on Ser-473 in vitro (36). The activity of ILK is sensitive to levels of PI(3,4,5)P3 (36), and, therefore, inactivation of a PI(3,4,5)P3 phosphatase, such as PTEN, would be expected to constitutively activate ILK leading to PKB/Akt activation.
ILK is a serine and threonine protein kinase containing four ankyrin-like repeats (35) and a putative phosphoinositide phospholipid-binding motif (36). ILK can interact with the cytoplasmic domains of integrin β1- and β3-subunits (35) via its carboxyl terminus, which is required for the localization of ILK in focal adhesion plaques (37). The N-terminal ankyrin repeats recently have been shown to interact with an LIM (cysteine-rich motif first identified in caenorhabditis elegans Lin-11, ISL-1, and mec-3) domain-only protein called PINCH (38). Nck-2, an Src homology (SH) 2 and SH3 domain-containing adapter protein, also interacts with PINCH and is thought to bridge ILK to growth factor receptors (39). In addition, the N-terminal ankyrin repeat also regulates localization of ILK in focal adhesion plaques (37). The activity of ILK is regulated in a PI3-kinase-dependent manner, and ILK can phosphorylate PKB/Akt on Ser-473 in vitro (36). Overexpression of ILK stimulates PKB/Akt-Ser-473 phosphorylation whereas a kinase-deficient form of ILK inhibits this phosphorylation in a dominant-negative manner (36). Overexpression of ILK in some epithelial cells (such as Scp2 mammary epithelial cells and IEC-18 intestinal epithelial cells) results in anchorage-independent cell growth (35), cell cycle progression (40), and tumorigenicity in nude mice (41). Furthermore, ILK down-regulates the expression of E-cadherin and promotes the nuclear translocation and activation of β-catenin (41, 42). In addition to regulating the activity of PKB/Akt, ILK also inhibits the activity of glycogen synthase kinase-3 (GSK-3). Whether this inhibition depends on PKB/Akt remains to be determined, although ILK can phosphorylate GSK-3 in vitro (36). Here we demonstrate that ILK and PKB/Akt are constitutively activated in human prostate carcinoma cells lacking PTEN expression. Furthermore, transfection of kinase-deficient, dominant-negative ILK into these cells dramatically inhibits both serum- and anchorage-independent PKB/Akt-Ser-473 phosphorylation, as well as PKB/Akt kinase activity, and leads to G1 cell cycle arrest and enhanced apoptosis. Inhibition of ILK activity by a small-molecule ILK inhibitor also inhibits serum-independent PKB/Akt-Ser-473 phosphorylation. These data demonstrate that ILK is critical for the PTEN-sensitive regulation of PKB/Akt-dependent cell cycle progression and cell survival.
Materials and Methods
Cell Culture and Transfections.
Three human prostate cancer cell lines were used throughout this study: PC3, LNCaP, and DU145. PC3 and DU145 cells were cultured in DMEM containing 10% FCS. LNCaP cells were cultured in RPMI medium 1640 supplemented with 10% FCS. All cells were passaged in 5% CO2 at 37°C. The cells were transiently transfected with His-V5-tagged ILK WT (ILK-WT) cDNA, His-V5-tagged ILK kinase-deficient (KD) cDNA, or green fluorescent protein (GFP)-tagged PTEN-WT cDNA by using Lipofectin reagent (GIBCO/BRL) according to the manufacturer's guidelines and using 1–4 μg (optimally 3 μg) of cDNA plasmid and 4 μl of Lipofectin reagent. Control cells also were treated overnight with 4 μl of Lipofectin reagent in serum-free medium in the absence or presence of empty vector. The ILK cDNAs were in pcDNA3 vector whereas the PTEN cDNA was in p-EGFP vector. Transfections were carried out overnight and the cells were harvested 24–48 h later. Transfected cells were analyzed for cell viability by using the trypan blue exclusion viability assay.
ILK Kinase Assays.
ILK kinase activity was determined in cell extracts by immunoprecipitation in vitro kinase assays as described (35, 36). Myelin basic protein (MBP) and glutathione S-transferase (GST)-PKB/Akt were used as a substrate for ILK in separate experiments, and phosphorylated proteins were electrophoresed on 12% SDS/PAGE gels. When MBP was used as a substrate, [32P]ATP was used as the phosphate donor in the kinase assay and [32P]MBP was detected by autoradiography or phosphorimage analysis of the gels. When GST-PKB/Akt was used as a substrate, ATP was used as the phosphate donor in the kinase assay, the proteins were electrotransferred from the SDS/PAGE gels to Immobilon poly(vinylidene difluoride) membranes (Millipore), and phosphorylated GST-PKB/Akt was detected by Western blot by using anti-PKB/Akt-phospho-Ser-473 antibody.
PKB/Akt Kinase Assay.
For PKB/Akt kinase assay, cells were lysed in 20 mM Tris (pH 7.5) containing 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF. Equivalent amounts of protein (determined by Bradford assay) were immunoprecipitated with anti-PKB/Akt antibody prebound to protein A-Sepharose beads, and kinase assay was carried out according to the instruction manual of the Akt Kinase Assay Kit (New England Biolabs). GSK-3 fusion protein was used as a substrate for PKB/Akt. Phosphorylated proteins were electrophoresed on 12% SDS/PAGE gels and electrotransferred onto Immobilon poly(vinylidene difluoride) membrane (Millipore) to detect phosphorylated GSK-3 by Western blot using phospho-GSK-3α/β (Ser 21/9) antibody.
Western Blot.
Western blot analyses using the enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia) were carried out as described (35, 36). The following antibodies were used in this study: anti-PTEN (mouse monoclonal; Oncogene Science); anti-ILK (affinity-purified rabbit polyclonal; Upstate Biotechnology, Lake Placid, NY); anti-P-PKB/Akt-Ser-473 (rabbit polyclonal; New England Biolabs); anti-P-PKB/Akt-Thr-308 (rabbit polyclonal; New England Biolabs); anti-PKB/Akt (rabbit polyclonal; New England Biolabs); anti-P-GSK-3-Ser-21/9 (rabbit polyclonal; New England Biolabs); anti-V5 (mouse monoclonal; Invitrogen); and anti-GFP (mouse monoclonal; Boehringer Mannheim).
Cell Cycle Inhibition by PTEN and ILK KD.
PC3 cells were transfected as above with empty His-V5-tagged vector or vector containing cDNAs encoding PTEN or ILK KD, grown in 10% FBS for 48 h, serum-starved for 18 h, and either refed serum or starved for 3 h. Cells (105) then were harvested, rinsed in cold PBS, and fixed in 4% paraformaldehyde in PBS for 30 min. Samples then were rinsed with PBS and stained with 50 μg/ml propidium iodide (Sigma) in PBS with 10 μg/ml RNase and 1% Triton X-100 for 30 min. Samples were analyzed by using a Coulter EXPO XL-4 flow cytometer. The data shown are representative of four independent experiments.
Induction of Apoptosis by PTEN and ILK KD.
PC3 cells were transfected as above with empty His-V5-tagged vector or vectors containing cDNAs encoding PTEN or ILK KD, maintained in 10% FBS, and serum-starved for 48 h for harvest at 72 h posttransfection. Cells (105) were stained with ApoAlert annexin V-FITC (CLONTECH) according to the manufacturer's instructions and stained with 50 μg/ml propidium iodide in PBS for 15 min. Samples were analyzed by flow cytometry, with the propidium iodide-negative population analyzed for FITC staining.
Results
ILK Activity and PKB/Akt-Ser-473 Phosphorylation in PTEN-Mutant Human Prostate Carcinoma Cells.
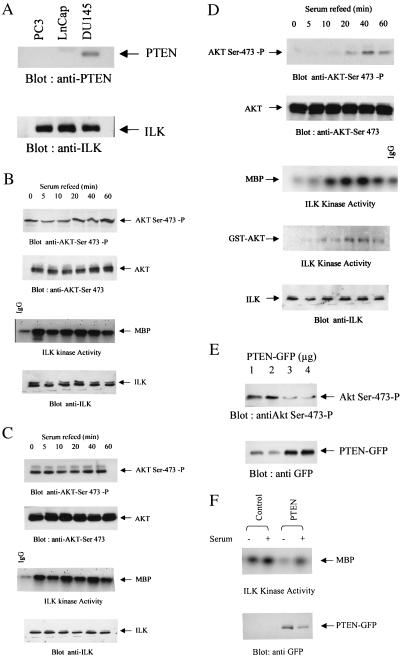
It has been shown recently that mutational inactivation of the tumor suppressor PTEN leads to elevated levels of the PI3-kinase product PI(3,4,5)P3 and increased phosphorylation of PKB/Akt on both Thr-308 and Ser-473 (16, 24). We have demonstrated that ILK is a PI(3,4,5)P3-dependent kinase that can phosphorylate PKB/Akt on Ser-473. We therefore wanted to examine whether inactivation of PTEN also would lead to the constitutive activation of ILK and decided to focus on human prostate cancer cells. PTEN is mutated in about 50% of prostate cancers (7), and of the three human prostate cancer cell lines available, two, PC-3 and LNCaP, are PTEN-negative, and one, DU-145, is PTEN-positive (Fig. 1A). As has been shown previously by others (26), PKB/Akt-Ser-473 phosphorylation levels are high in PC-3 and LNCaP cells in the absence or presence of serum (Fig. 1 B and C), whereas PKB/Akt-Ser-473 is undetectable in the absence of serum and is induced by serum in the PTEN-positive DU-145 cells (Fig. 1D). Consistent with a role of ILK as an upstream regulator of PKB/Akt-Ser-473 phosphorylation, ILK activity is also high in the absence or presence of serum in the PTEN-negative PC-3 and LNCaP cells (Fig. 1 B and C), whereas it is inducible by serum in DU145 cells coordinately with PKB/Akt phosphorylation (Fig. 1D). The detection of a phosphorylated MBP band at 0, 5, and 10 min postserum stimulation, when there is no PKB/Akt-Ser-473 phosphorylation, is a result of nonspecific labeling, as shown by the level of MBP 32P-labeling in IgG control immunoprecipitations. A similar induction of ILK activity by serum in DU145 cells was observed when the GST fusion protein GST-PKB/Akt was used as the substrate in the in vitro ILK kinase assay (Fig. 1D).
Figure 1.
(A) Expression of endogenous PTEN in PC3, LNCaP, and DU145. Stimulation of ILK activity and PKB/Akt-Ser-473 phosphorylation by serum in LNCaP (B), PC3 (C), and DU145 (D) cells. To evaluate stimulation of ILK activity, cells were serum-starved overnight, refed with serum for the indicated time period, and then analyzed for ILK activity by using MBP (B–D), GST-PKB/Akt (D), or PKB/Akt-Ser-473 phosphorylation using anti-PKB/Akt-Ser-473-P antibody (B–D). (E) Dose-dependent inhibition of PKB/Akt-Ser-473 phosphorylation by PTEN. (F) Inhibition of ILK activity in PC3 cells transfected with 3 μg of WT PTEN plasmid.
To demonstrate a causal relationship between the loss of PTEN and stimulation of PKB/Akt phosphorylation and ILK activity, we transiently transfected WT PTEN into the PTEN-negative PC-3 cells and measured PKB/Akt-Ser-473 phosphorylation (Fig. 1E) and ILK activity (Fig. 1F). As shown in Fig. 1E, PTEN suppresses PKB/Akt-Ser-473 phosphorylation in a dose-dependent manner, and transfection of PC3 cells with an optimal dose of PTEN plasmid (3 μg) also results in the suppression of ILK activity (Fig. 1F), demonstrating that ILK activity can be regulated by PTEN.
Inhibition of ILK Suppresses Constitutive PKB/Akt-Ser-473 Phosphorylation and PKB/Akt Activity in PTEN-Negative Cells.
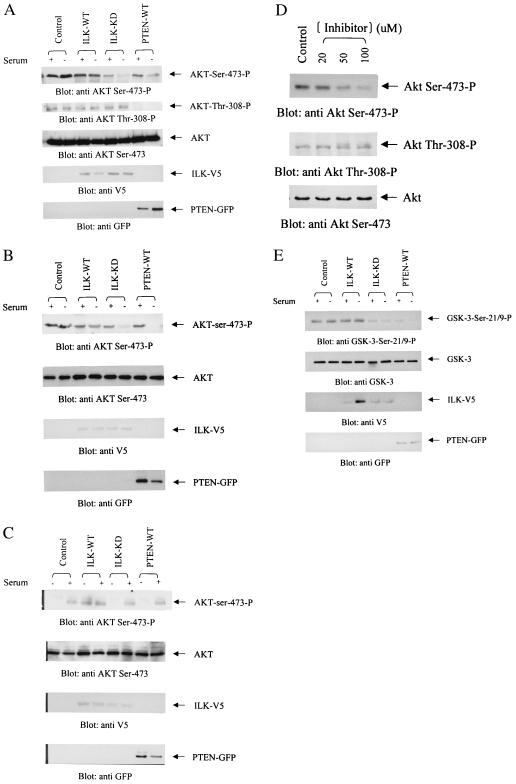
Because PKB/Akt is constitutively phosphorylated on Ser-473 in PTEN-mutant cells (16), we wanted to determine whether this phosphorylation could be suppressed by inhibiting ILK activity. To inhibit endogenous ILK, we transfected cells with a kinase-deficient mutant of ILK (3 μg of cDNA) that behaves as a dominant negative (36). As shown in Fig. 2 A and B, PKB/Akt-Ser-473 phosphorylation is high in PC-3 and LNCaP PTEN-negative cell lines in the absence or presence of serum. Transfection of WT ILK does not further enhance this phosphorylation in these cells. However, transfection of the dominant-negative form of ILK (ILK-KD) results in substantial inhibition of PKB/Akt phosphorylation on Ser-473 but not on Thr-308 (Fig. 2A). As expected, transfection of WT PTEN (3 μg) into these cells results in the inhibition of phosphorylation on both sites. In contrast, transfection of WT ILK into the PTEN-positive DU-145 cells enhances Ser-473 phosphorylation in the absence of serum (Fig. 2C). Transfection of dominant-negative ILK or WT PTEN had little effect on the phosphorylation status of PKB/Akt in these cells. In all three cell lines, PKB/Akt expression levels were unchanged, and all cells expressed the appropriate transgenes as detected by the V5 tag for ILK cDNAs and the GFP tag for the PTEN cDNA (Fig. 2 A–C). These data demonstrate further that PTEN is upstream of ILK and that inhibition of ILK only affects phosphorylation of Ser-473, but not Thr-308.
Figure 2.
Transient overexpression of V5-tagged ILK-WT, ILK-KD, and GFP-tagged PTEN-WT in LNCaP (A), PC3 (B), and DU145 (C) cells. Cells transfected with ILK-WT, ILK-KD, or PTEN-WT were deprived of serum for 18 h commencing 48 h posttransfection. The cells then were refed with serum for 1 h, lysed, and analyzed for PKB/Akt-Ser-473 phosphorylation by using anti-PKB/Akt-Ser-473-P antibody. The second blot in each panel shows equivalent levels of PKB/Akt in each extract as determined by Western blot using anti-PKB/Akt antibody. (D) Treatment of PC3 cells with ILK kinase inhibitor KP-SD-1 abrogates PKB/Akt phosphorylation on Ser-473 but does not inhibit PKB/Akt phosphorylation of Thr-308. PC3 cells were serum-starved and then incubated for 6 h with either 20, 50, or 100 μM of the inhibitor or DMSO (vehicle control) in serum-free DMEM. Equal amounts of proteins from the various treatments were used for immunoblotting with anti-PKB/Akt-Ser-473-P (Top), anti-PKB/Akt-Thr-308-P (Middle), or anti-PKB/Akt (Bottom) antibodies. ILK (WT and KD) and PTEN-WT expression was determined in the transfectants by Western blot with anti-V5 and anti-GFP antibodies, respectively. (E) Determination of PKB/Akt kinase activity in PC3 cells transfected with ILK-WT, ILK-KD, or PTEN-WT. PKB/Akt activity was determined as described in Materials and Methods.
Two highly selective inhibitors of ILK activity recently have been identified by us in collaboration with Kinetek Pharmaceuticals, Vancouver, Canada. As shown in Fig. 2D, incubation of one of these inhibitors, KP-SD-1, with serum-starved PC3 cells resulted in a dose-dependent inhibition of PKB/Akt-Ser-473 phosphorylation. This inhibitor does not have any effect on PKB/Akt kinase activity in vitro (data not shown), and, as shown in Fig. 2D, it does not result in the inhibition of PKB/Akt phosphorylation on Thr-308. Full characterization of these inhibitors will be provided elsewhere (J.S., D. Leung, A. Ali, M. Howell, G. Hannigan, J. Woodgett, and S.D., unpublished data). These data support the data on the inhibitory effects of dominant-negative ILK on PKB/Akt-Ser-473 phosphorylation shown in Fig. 2 A and B and show that the inhibitor does not inhibit PDK-1 activity.
Although the inhibition of ILK results in the inhibition of PKB/Akt-Ser-473 phosphorylation (Fig. 2 A, B, and D), we wanted to determine whether this inhibition translated into inhibition of PKB/Akt activity. As shown in Fig. 2E, transfection of kinase-dead, dominant-negative ILK as well as PTEN into PTEN-null PC3 cells does result in the inhibition of PKB/Akt activity.
It is unlikely that the effects of the dominant-negative ILK and WT PTEN on PKB/Akt phosphorylation and ILK kinase activity are caused by effects on cell viability because before serum starvation, i.e., 48 h posttransfection, the viability of the various transfected cells was not significantly different (data not shown).
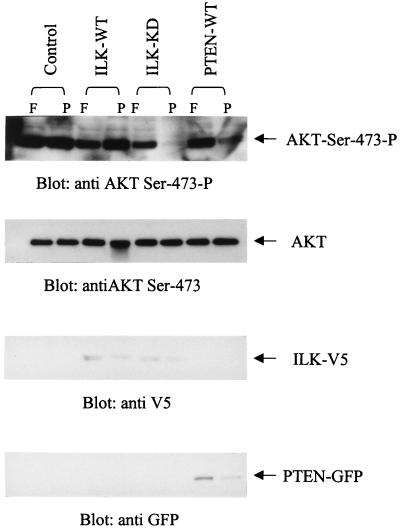
Cell adhesion to extracellular matrices (ECM) stimulates both ILK activity and PKB/Akt-Ser-473 phosphorylation (36, 43, 44). We therefore wanted to determine PKB/Akt-Ser-473 phosphorylation status in PTEN-negative cells that were either anchored or kept in suspension. PC-3 cells were either allowed to adhere to fibronectin or were prevented from adhering by plating on poly-HEMA-coated plates. As shown in Fig. 3, PKB/Akt-Ser-473 phosphorylation is constitutively elevated and is anchorage-independent in the PTEN-negative cells. Transfection of dominant-negative ILK suppressed this phosphorylation in suspended cells, as did transfection of WT PTEN. These data demonstrate that the stimulation of PKB/Akt-Ser-473 phosphorylation by cell–ECM interactions depends on ILK and becomes independent of anchorage in PTEN-negative cells.
Figure 3.
PKB/Akt-Ser 473 phosphorylation in cells plated on fibronectin or kept in suspension (poly-HEMA). ILK-WT-, ILK-KD-, or PTEN-WT-transfected PC3 cells were serum-starved for 18 h, resuspended in serum-free medium, and allowed to adhere on nontissue culture plastic plates coated with either fibronectin (10 μg/ml) or poly-HEMA (15 μg/ml) for 1 h. Cells then were lysed, and the level of PKB/Akt-Ser-473 phosphorylation was determined by Western blotting by using the anti-PKB/Akt-Ser-473-P antibody. ILK (WT and KD) and PTEN-WT expression in the transfectants was determined by Western blotting with anti-V5 and anti-GFP antibodies, respectively.
Collectively, the data presented in Figs. 2 and 3 show that ILK and PTEN are crucial regulators of PKB/Akt activation by serum as well as extracellular matrices and that the constitutive activation of PKB/Akt in PTEN-mutant cells can be reverted to serum and anchorage dependence by either inhibiting ILK or replacing PTEN.
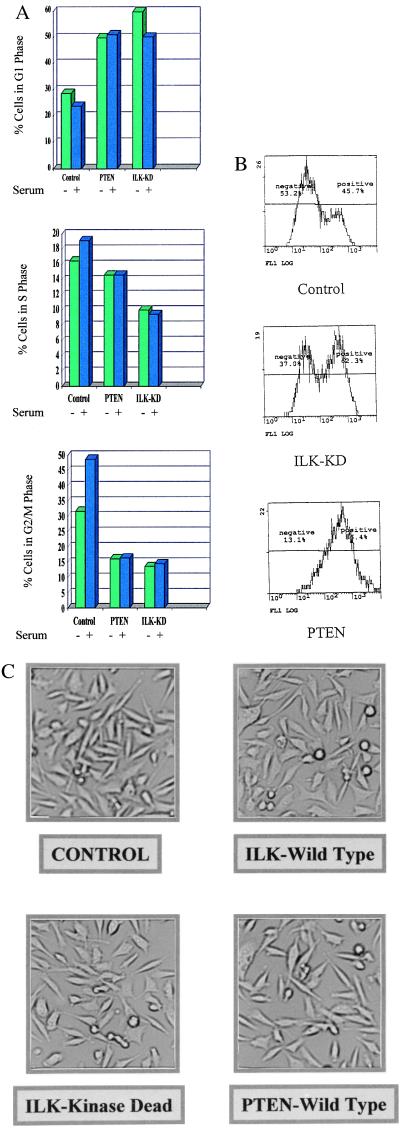
Inhibition of ILK Induces G1 Phase Cell Cycle Arrest and Stimulates Apoptosis in PTEN-Negative Prostate Cancer Cells.
It has been shown previously by others that transfection of WT PTEN into PTEN-mutant cells induces cell cycle arrest as well as apoptosis (25). We therefore wanted to determine whether inhibition of endogenous ILK activity by dominant-negative ILK also would lead to cell cycle arrest and apoptosis. As shown in Fig. 4A, PTEN-transfected PC-3 cells arrest in the G1 phase of the cell cycle compared with control empty vector-transfected PC-3 cells, which continue to cycle in the absence or presence of serum. However, PC-3 cells transfected with the dominant-negative ILK cDNA also arrest in G1 (Fig. 4A). We also noticed a sub-G1 peak in both PTEN and dominant-negative ILK-transfected cells, an indication of the accumulation of apoptotic cells, and wanted to determine whether these cells were indeed undergoing apoptosis. As shown in Fig. 4B, compared with control transfected cells, both PTEN and dominant-negative ILK-transfected cells undergo enhanced apoptosis after 48 h of serum starvation (45). As shown in Fig. 4C, transfection and expression of dominant-negative ILK or WT PTEN did not significantly affect cell adhesion.
Figure 4.
ILK-KD and PTEN-WT induce cell cycle arrest and apoptosis in PC3 cells. (A) Flow cytometric analysis of cell cycle inhibition in PC3 cells by PTEN-WT and ILK-KD. Propidium iodide staining of cells transfected with empty vector (control), PTEN-WT, or ILK-KD and serum-starved for 18 h with or without serum refeed for 3 h. Bar chart shows cell cycle phase distribution percentages determined for each transfection without or with serum refeed for 3 h. (B) Induction of apoptosis in PC3 cells by ILK-KD and PTEN-WT. Cells were transfected with empty vector (control), ILK-KD, or PTEN-WT, serum-starved for 48 h, and stained with annexin-FITC and 50 μg/ml propidium iodide. Histogram shown is FITC staining of propidium iodide-negative cells, which is a measure of the number of cells undergoing apoptosis. (C) Photomicrographs of PC3 cells after transfection with various plasmids. Transfection of PC3 cells with any of these plasmids did not affect cell adhesion.
Our data demonstrate that, as with restoration of PTEN, inhibition of ILK in these cells also induces cell cycle arrest and apoptosis, implying that inhibition of ILK may provide for a novel means of inhibiting the growth and progression of PTEN-mutant tumors.
Discussion
Epithelial cell survival and cell growth are tightly regulated processes that ensure proper tissue homeostasis. Dysregulation of such processes leads to oncogenic transformation and other hyperproliferative disorders. Cell survival and growth are regulated by soluble factors, as well as by components of the extracellular matrix, which interact with their respective receptors and activate signaling cascades, leading to stimulation of cell cycle progression or inhibition of apoptosis. Temporal and spatial inhibition of these signaling cascades is an essential part of the regulation of these processes, and it can be envisaged that loss of such negative regulation would result in increased cell survival and hyperproliferation. The PTEN lipid and protein phosphatase is such a negative regulator of these signaling pathways, and it is now estimated that inactivating mutations of PTEN exist in 60% of all forms of solid tumors.
In this paper we have focused on ILK, which has been shown to phosphorylate PKB/Akt on Ser-473 in vitro (36). Consistent with this property, we have demonstrated that the activity of ILK is serum- and anchorage-independent in PTEN-null prostate carcinoma cells, but is serum- and anchorage-dependent in cells expressing WT PTEN. Furthermore, ILK activity is regulated by PTEN, because re-expression of PTEN by transfection of WT PTEN cDNA in PTEN-null cells inhibits ILK activity. A prediction of our hypothesis is that ILK is intermediate between PTEN and PKB/Akt such that the inhibition of ILK activity in PTEN-null cells would result in the suppression of PKB/Akt-Ser-473 phosphorylation. The activation of ILK in PTEN-null cells presumably is caused by increased PI(3,4,5)P3 levels in these cells, although it is possible that ILK may be activated because of dysregulation of other PTEN targets, such as FAK (46). We have demonstrated here that inhibition of ILK, by either transfection and expression of a dominant-negative form of ILK or by exposure of cells to a small molecule ILK inhibitor, results in a profound inhibition of PKB/Akt-Ser-473, but not of Thr-308 phosphorylation. This inhibition is especially pronounced in cells deprived of serum or adhesion and is accompanied by inhibition of PKB/Akt kinase activity.
It has been demonstrated recently that apart from regulating cell survival and apoptosis, PTEN also regulates cell cycle progression that is accelerated in PTEN-null cells (25, 26). Here, we have shown that WT PTEN as well as dominant-negative ILK induces potent G1 phase cell cycle arrest. In addition, consistent with the inhibition of Ser-473 phosphorylation and kinase activity of PKB/Akt, there is a significant increase in apoptosis of cells transfected with WT PTEN or dominant-negative ILK. These data suggest that dysregulation of ILK activity in the PTEN-null cells plays an important role in the suppression of apoptosis and cell cycle progression in these cells.
At the present time it is not clear whether PKB/Akt is the downstream mediator of the regulation of cell cycle by ILK and PTEN. Preliminary data indicate that suppression of PKB activity in PTEN-null cells by transfection with a powerful dominant-negative PKB/Akt (PKB-AAA) does not induce G1/S cell cycle arrest to the same extent as seen when ILK is inhibited. It is therefore conceivable that ILK may regulate the cell cycle via PKB/Akt-independent pathways involving GSK-3, β-catenin, or AP-1 transcription factor (36, 42, 47).
The findings presented in this paper form the basis of a paradigm that the inactivation of a tumor suppressor results in the dysregulated activation of immediate downstream effectors. The inhibition of such downstream effectors thus may allow for powerful and alternative means of treating tumors harboring mutations in tumor suppressor genes. Here, we have demonstrated that the inhibition of ILK in PTEN-mutant prostate cancer cells induces cell cycle arrest and apoptosis. It is particularly interesting that the effects of inhibiting ILK or PKB/Akt-Ser-473 phosphorylation are much more pronounced under anchorage- and serum-independent conditions. This finding suggests that there is a specificity to the effects of inhibiting ILK activity relating to the properties of malignant transformation, namely, anchorage- and serum-independent growth. These properties are particularly relevant during various stages of metastasis, and, therefore, ILK may be a promising target for the treatment of tumors in which PTEN is dysregulated.
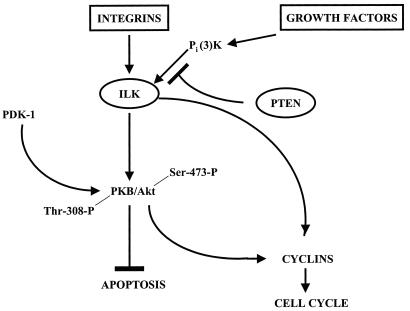
Figure 5.
Schematic representation of the regulation of signal transduction from integrins and growth factor receptors via ILK to downstream kinases regulating cell survival and cell cycle. ILK activity is regulated in a PI3-kinase-dependent manner, and activated ILK phosphorylates PKB/Akt on Ser-473, resulting in its activation and the eventual control of cell proliferation and survival by suppressing apoptosis. ILK also regulates the expression of cyclins, leading to G1 cell cycle progression (40). PTEN encodes a lipid phosphatase with specificity toward PI(3,4,5)P3 and, thus, serves as a critical negative regulator of PI3-kinase-mediated signaling from external stimuli (growth factors, cell adhesion) to cell proliferation and survival largely through its inactivation of ILK and PKB/Akt. Mutational inactivation, or loss, of PTEN thus results in serum- and anchorage-independent activation of ILK and PKB/Akt, leading to constitutive cell survival and cell growth.
Acknowledgments
We thank Dr. Frank Jirik for providing the PTEN cDNA. S.P. is a Research Fellow of the National Cancer Institute of Canada supported with funds provided by the Terry Fox Run. This work was carried out with financial support from a National Cancer Institute of Canada-funded program project on Prostate Cancer Progression.
Abbreviations
- PKB
protein kinase B
- PI3-kinase
phosphatidylinositol 3-kinase
- PI(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- GSK-3
glycogen synthase kinase-3
- WT
wild type
- KD
kinase deficient
- MBP
myelin basic protein
- GFP
green fluorescent protein
- GST
glutathione S-transferase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.060579697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.060579697
References
- 1.Li D M, Sun H. Cancer Res. 1997;57:2124–2129. [PubMed] [Google Scholar]
- 2.Li J, Yen C, Liaw D, Podyspanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 3.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 4.Rasheed B K, Stenzel T T, McLendon R E, Parsons R, Friedman A H, Friedman H S, Bigner D D, Bigner S H. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- 5.Liu W, James C D, Frederick L, Alderete B E, Jenkins R B. Cancer Res. 1997;57:5254–5257. [PubMed] [Google Scholar]
- 6.Guldberg P, Thor Straten P, Birck A, Ahrenkiel V, Kirkin A F, Zeuthen J. Cancer Res. 1997;57:3660–3663. [PubMed] [Google Scholar]
- 7.Cairns P, Okahami K, Halachmi S, Halachmi N, Esteller M, Herman J G, Jen J, Isaacs W B, Bova G S, Sidransky D. Cancer Res. 1997;57:4997–5000. [PubMed] [Google Scholar]
- 8.Teng D H, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen K L, Vinson V L, Gumpper K L, et al. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 9.Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Cancer Res. 1997;57:3935–3940. [PubMed] [Google Scholar]
- 10.Kohno T, Takahashi M, Manda R, Yokota J. Genes Chromosomes Cancer. 1998;22:152–156. doi: 10.1002/(sici)1098-2264(199806)22:2<152::aid-gcc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Kong D, Suzuki A, Zou T T, Sakurada A, Kemp L W, Wakatsuki S, Yokoyama T, Yamakawa H, Furukawa T, Sato M, et al. Nat Genet. 1997;17:143–144. doi: 10.1038/ng1097-143. [DOI] [PubMed] [Google Scholar]
- 12.Liaw D, Marsh D J, Li J, Dahia P L, Wang S I, Zheng Z, Bose S, Call K M, Tsou H C, Peacocke M, et al. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 13.Nelen M R, van Staveren W C, Peeters E A, Hassel M B, Gorlin R J, Hamm H, Lindboe C F, Fryns J P, Sijmons R H, Woods D G, et al. Hum Mol Genet. 1997;6:1383–1387. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- 14.Tsou H C, Teng D H, Ping X L, Brancolini V, Davis T, Hu R, Xie X X, Gruener A C, Schrager C A, Christiano A M, et al. Am J Hum Genet. 1997;61:1036–1043. doi: 10.1086/301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh D J, Roth S, Lunetta K L, Hemminki A, Dahia P L, Sistonen P, Zheng Z, Caron S, van Orsouw N J, Bodmer W F, et al. Cancer Res. 1997;57:5017–5021. [PubMed] [Google Scholar]
- 16.Stambolic V, Suzuki A, de la Pompa J L, Brothers G M, Mirtsos C, Sasaki T, Ruland J, Penninger J M, Siderovski D P, Mak T W. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 17.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi P P. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 18.Chuang J, Lin D C, Lin S. J Cell Biol. 1995;128:1095–1109. doi: 10.1083/jcb.128.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maehama T, Dixon J E. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 20.Myers M P, Stolarov J P, Eng C, Li J, Wang S I, Wigler M H, Parsons R, Tonks N K. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furnari F B, Lin H, Huang H S, Cavanee W K. Proc Natl Acad Sci USA. 1997;94:12479–12784. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheney I W, Johnson D E, Vaillancourt M T, Avanzini J, Morimoto A, Demers G W, Wills K N, Shabram P W, Bolen J B, Tavtigian S V, et al. Cancer Res. 1998;58:2331–2334. [PubMed] [Google Scholar]
- 23.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada K M. Science. 1998;280:1624–1627. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 24.Haas-Kogan D, Shelev N, Wong M, Mills G, Yount G, Stokoe D. Curr Biol. 1998;8:1195–1198. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 25.Sun H, Lesche R, Li D, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. Proc Natl Acad Sci USA. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy S, Nakamura N, Vasquez F, Batt D B, Perera S, Roberts T M, Sellers W R. Proc Natl Acad Sci USA. 1999;96:2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 28.DelPeso L, Gonzales-Garcia M, Page C, Hemera R, Nunez N. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 29.Kops G J, Ruiter N D, DeVries-Smits A M, Powell D R, Bos J L, Burgering B M. Nature (London) 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 30.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 31.Brunet A, Bonni A, Zigmond M J, Lin M Z, Juo P, Hu L S, Anderson M J, Arden K C, Blenis J, Greenberg M E. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 32.Cantley L C, Neel B G. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downward J. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 34.Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes C P, Alessi D R. Curr Biol. 1999;9:393–404. doi: 10.1016/s0960-9822(99)80186-9. [DOI] [PubMed] [Google Scholar]
- 35.Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M, Radeva G, Filmus J, Bell J C, Dedhar S. Nature (London) 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 36.Delcommenne M, Tan C, Gray V, Ruel L, Woodgett J, Dedhar S. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, Zhang Y, Wu C. J Cell Sci. 1999;112:4589–4599. doi: 10.1242/jcs.112.24.4589. [DOI] [PubMed] [Google Scholar]
- 38.Tu Y, Li F, Goicoechea S, Wu C. Mol Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu Y, Li F, Wu C. Mol Biol Cell. 1999;9:3367–3382. doi: 10.1091/mbc.9.12.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Keightley S Y, Leung-Hagesteijn C-Y, Radeva G, Coppolino M, Goicoechea S, MacDonald J, Dedhar S. J Biol Chem. 1998;273:528–538. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 42.Novak A, Hsu S, Leung-Hagesteijn C Y, Radeva G, PapKoff J, Montesano R, Roskelly C, Grosschedl R, Dedhar S. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Download J. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King W G, Mattaliono M B, Chan T O, Tsichlis P N, Brugge J S. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thiagarajan P, Tait J F. J Biol Chem. 1990;265:17420–17423. [PubMed] [Google Scholar]
- 46.Gu J, Tamura M, Yamada K M. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troussard A A, Tan C, Yoganathan N, Dedhar S. Mol Cell Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]