Abstract
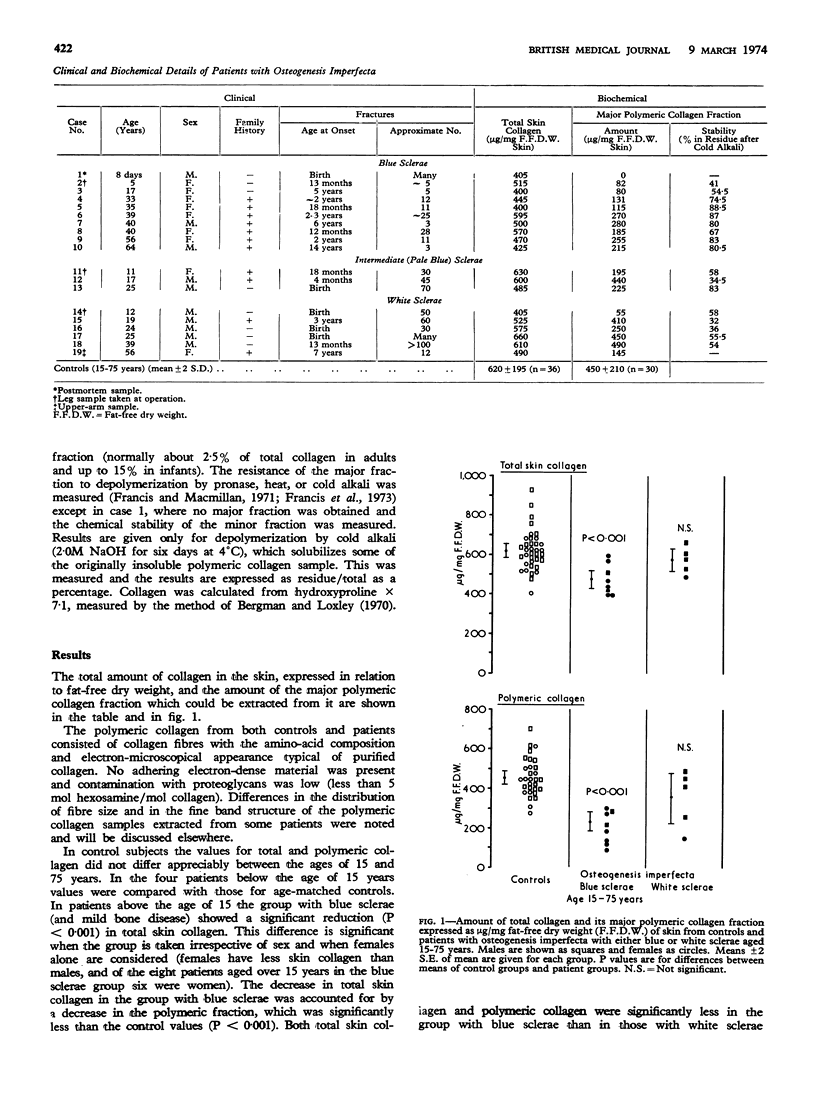
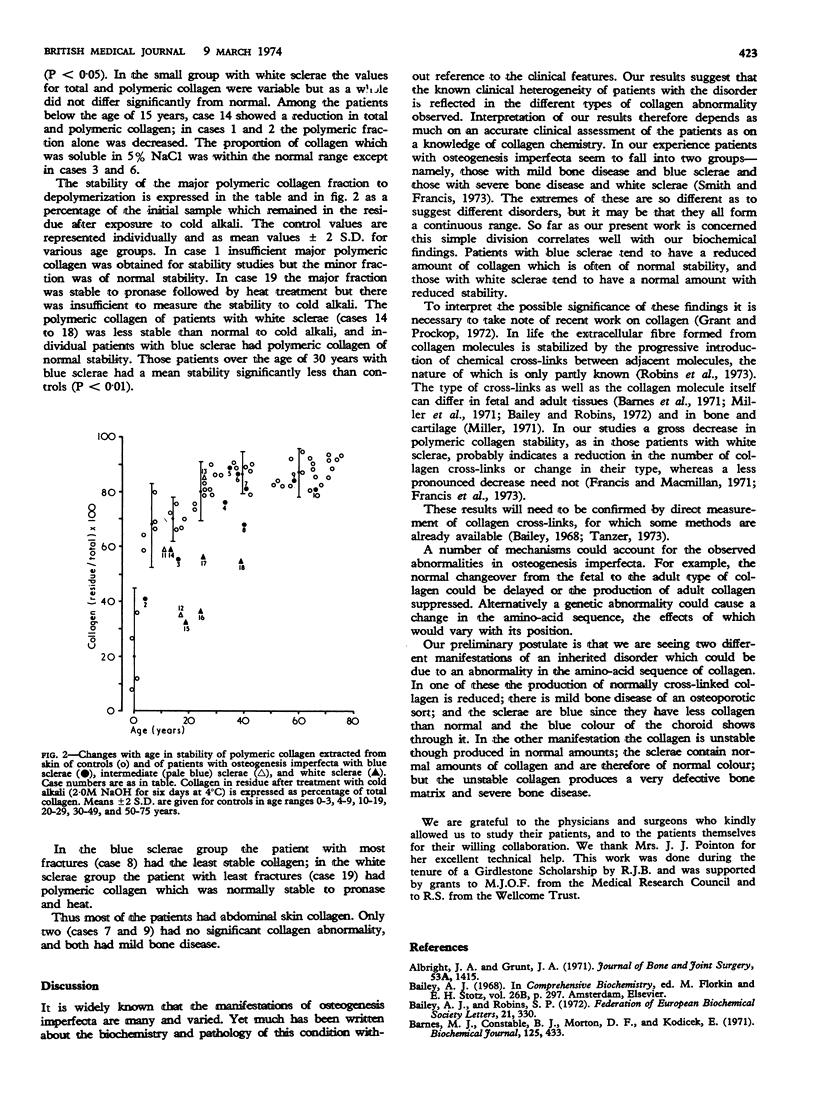
The structural polymeric collagen of the skin of 19 patients with osteogenesis imperfecta has been examined. In those with severe bone disease, who often have white sclerae, this collagen fraction is less resistant to depolymerization than that of age-matched controls, though the total amount is normal. In patients with less severe bone disease, whose sclerae are usually blue, the polymeric collagen may have normal stability but the total amount is reduced. These results suggest defective cross-linking of collagen in severe osteogenesis imperfecta.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P. Embryonic skin collagen. Replacement of the type of aldimine crosslinks during the early growth period. FEBS Lett. 1972 Apr 1;21(3):330–334. doi: 10.1016/0014-5793(72)80195-9. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Hydroxylysine in the N-terminal regions of the 1 - and 2 -chains of various collagens. Biochem J. 1971 Nov;125(2):433–437. doi: 10.1042/bj1250433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman I., Loxley R. The determination of hydroxyproline in urine hydrolysates. Clin Chim Acta. 1970 Feb;27(2):347–349. doi: 10.1016/0009-8981(70)90355-4. [DOI] [PubMed] [Google Scholar]
- FOLLIS R. H., Jr Histochemical studies on cartilage and bone. III. Osteogenesis imperfecta. Bull Johns Hopkins Hosp. 1953 Dec;93(6):386–399. [PubMed] [Google Scholar]
- Falvo K. A., Bullough P. G. Osteogenesis imperfecta: a histometric analysis. J Bone Joint Surg Am. 1973 Mar;55(2):275–286. [PubMed] [Google Scholar]
- Francis M. J., Macmillan D. C. The extraction of polymeric collagen from biopsies of human skin. Biochim Biophys Acta. 1971 Nov 19;251(2):236–245. doi: 10.1016/0005-2795(71)90107-3. [DOI] [PubMed] [Google Scholar]
- Francis M. J., Smith R., Macmillan D. C. Polymeric collagen of skin in normal sunjects and in patients with inherited connective tissue disorders. Clin Sci. 1973 May;44(5):429–438. doi: 10.1042/cs0440429. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Epstein E. H., Jr, Piez K. A. Identification of three genetically distinct collagens by cyanogen bromide cleavage of insoluble human skin and cartilage collagen. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1024–1029. doi: 10.1016/0006-291x(71)90006-4. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L. Cross-linking of collagen. Science. 1973 May 11;180(4086):561–566. doi: 10.1126/science.180.4086.561. [DOI] [PubMed] [Google Scholar]


