Abstract
The bacterium Agrobacterium tumefaciens transforms eukaryotic hosts by transferring DNA to the recipient cell where it is integrated and expressed. Bacterial factors involved in this interkingdom gene transfer have been described, but less is known about host-cell factors. Using the yeast Saccharomyces cerevisiae as a model host, we devised a genetic screen to identify yeast mutants with altered transformation sensitivities. Twenty-four adenine auxotrophs were identified that exhibited supersensitivity to A. tumefaciens-mediated transformation when deprived of adenine. We extended these results to plants by showing that purine synthesis inhibitors cause supersensitivity to A. tumefaciens transformation in three plant species. The magnitude of this effect is large and does not depend on prior genetic manipulations of host cells. These data indicate the utility of yeast as a model for the transformation process and identify purine biosynthesis as a key determinant of transformation efficiency. These findings should increase the utility of A. tumefaciens in genetic engineering.
Agrobacterium tumefaciens, a Gram-negative soil bacterium, genetically transforms plants by transferring DNA to the host cell where it is integrated into the host chromosome and expressed. Exogenous DNA sequences introduced into transferred DNA (T-DNA) vectors can be delivered to plants, making A. tumefaciens a cornerstone of plant genetic engineering. Under controlled conditions, A. tumefaciens can also transform mammalian cells and a variety of fungi, including the yeast Saccharomyces cerevisiae (1–6).
Understanding the cellular factors influencing transformation will provide broader insights into the mechanisms underlying interkingdom DNA transfer and should increase the utility of A. tumefaciens in genetic engineering. Bacterial factors that control virulence gene induction as well as processing and delivery of the T-DNA have been studied extensively (7, 8). Recently, a few host-cell factors have been identified that participate in A. tumefaciens-mediated transformation. Studies in Arabidopsis thaliana have implicated histone H2A in chromosomal integration of the T-DNA (9). Studies in S. cerevisiae have implicated a nuclear pore protein in T-DNA nuclear import (10) and nonhomologous end-joining proteins in T-DNA chromosomal integration (11). To date, however, the facile yeast system has not been used to perform a large-scale screen to identify host factors that influence transformation sensitivity. Consequently, we devised a genetic screen to isolate yeast mutants with altered sensitivity to A. tumefaciens-mediated transformation. This approach revealed an unexpected link between transformation efficiency and de novo biosynthesis of adenine, an essential purine precursor of DNA, RNA, and ATP.
Materials and Methods
Strains and Plasmids. The supervirulent A. tumefaciens strain EHA105 harboring pKP506 served as the bacterial donor strain in yeast-transformation experiments (1). The pKP506 plasmid contains the yeast TRP1 marker and the ARS1 replication element, which are flanked by telomere repeat sequences and right border sequences that delineate the T-DNA excised by VirD2 and transferred to the recipient cell. Transformant yeast cells are rendered prototrophic for tryptophan biosynthesis and harbor a 13-kb minichromosome (1).
Standard molecular genetics methods were used for culturing A. tumefaciens (12). Cocultivation medium (CM) was prepared essentially as described (1, 13) with amino acids added as required for yeast strains. CM did not contain tryptophan, and it did not contain adenine except where explicitly noted.
Standard methods were used for culturing and manipulating S. cerevisiae (13). S. cerevisiae strain 10556 2B (W303 genetic background) was subjected to insertional mutagenesis according to established methods (14). Saccharomyces deletion collection S288C strains derived from BY4741 (15) were converted to tr yptophan auxotrophy (trp1::URA3) by using plasmid pNKY1009 (16). The yeast strains used in this study are listed in Table 1.
Table 1. Yeast strains used in this study.
| Yeast strain | Background | Genotype | Source |
|---|---|---|---|
| 10556 2B | W303 | ADE+ ura3-1, his3-11, 15, leu2, trp1-1, MATα | Fink |
| 10556 30D | W303 | ade2-100, ura3-1, leu2, trp1-1, MATa | Fink |
| 10556 3B | W303 | ADE+ ura3-1, leu2, trp1-1, MATα | Fink |
| RRY113 | W303 | ade1::LEU2-LacZ, ura3-1, his3-11, 15, leu2, trp1-1, MATα | This study |
| RRY82 | W303 | ade5,7::LEU2-LacZ, ura3-1, his3-11,15, leu2, trp1-1, MATα | This study |
| RRY86 | W303 | ade6::LEU2-LacZ, ura3-1, his3-11, 15, leu2, trp1-1, MATα | This study |
| RRY817 | S288C | ADE+, trp1::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, MATa | This study |
| RRY826 | S288C | ade1, trp1::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, MATa | This study |
| RRY831 | S288C | ade4, trp1::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, MATa | This study |
| RRY836 | S288C | ade5,7, trp1::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, MATa | This study |
| RRY820 | S288C | ade8, trp1::URA3, his3Δ1, leu2Δ0, met15Δ0, ura3Δ0, MATa | This study |
| MY303 | Σ1278b | ADE+, ura3-52, his3::hisG, trp1::hisG, MATα | Microbia |
| MY1180 | Σ1278b | ade2::hisG, ura3-52, his3::hisG, trp1::hisG, MATα | Microbia |
The strain name, genetic background, relevant genotype, and source of each strain used in this study are indicated.
Assays for A. tumefaciens Transformation of S. cerevisiae. S. cerevisiae transformation assays were performed as described (1) with the following modifications. Yeast colonies were grown on yeast extract/peptone/dextrose plates for 72 h and then replica-plated to a fresh yeast extract/peptone/dextrose plate (the master plate) (13). A fresh A. tumefaciens culture was grown on mannitol glutamate/Luria plates for 24 h, transferred to a fresh mannitol glutamate/Luria plate, and incubated at 28°C for an additional 24 h. These bacterial cells then were spread as a lawn on a CM plate and incubated for 24 h at 28°C to induce the virulence program. This temperature was found to maximize bacterial growth while still supporting induction of the virulence program. Induced A. tumefaciens cells were transferred to a fresh CM plate to which the yeast colonies were then replica-plated. Cocultivation of yeast and bacteria was performed at 20°C for 48 h. The cocultivation plate then was replica-plated to yeast synthetic complete medium lacking tryptophan (13). Plates lacking tryptophan were compared with the master plates. This replica-plating transformation assay permits a rapid visual assessment of the transformation sensitivity of individual yeast strains and is amenable to high-throughput analysis.
A quantitative transformation assay was performed essentially as described above except that yeast patches (eight per plate) were used instead of yeast colonies. After cocultivation, the bacteria/yeast mixture was scraped off of the CM plate and resuspended in liquid CM, and serial dilutions were plated on yeast synthetic complete medium lacking or containing tryptophan (13). Transformation efficiency was calculated by dividing the number of yeast transformants (colonies growing on medium lacking tryptophan) by the total number of yeast cells (colonies growing on medium containing tryptophan) after coincubation with A. tumefaciens.
In the ade2 and ADE+ cocultivation assays, strains 10556 2B (ADE+) and 10556 30D (ade2) were mixed in equal amounts on a yeast extract/peptone/dextrose plate and transformation was assayed quantitatively as described above. In subsequent analyses, the 10556 30D and 10556 2B strains were distinguished from one another based on their different adenine and histidine requirements.
Yeast strains were transformed abiotically with 5.0 μg of pKP506 DNA by using standard lithium-acetate methods (17). Transformation efficiencies were determined as described above.
To monitor A. tumefaciens vir gene induction, strain EHA105 containing pVirE-LacZ (18) was incubated with yeast strains under the transformation-assay conditions described above. After cocultivation, bacterial cells were harvested and assayed for β-galactosidase activity (18).
Assays for A. tumefaciens Transformation of Plant Cells. Plant cell cultures were treated with 5 μg/ml 5-fluorouracil, 50 μg/ml mizoribine, 100 μg/ml azaserine, or 100 μg/ml acivicin for 16 h before infection with A. tumefaciens GV3101 bearing pBISN1 at A600 = 0.005 (19). Compounds were not washed out before cocultivation with Agrobacterium cells. Background hydrolysis of β-glucuronidase (GUS) substrate is indicated by uninfected samples, and baseline transformation efficiency is indicated by infected plant cells not treated with any compound. GUS activity was quantitated for Ageratum conyzoides, Nicotiana tabacum BY-2, and A. thaliana Col-0 cells by measuring hydrolysis of the substrate 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid as a function of absorbance at 415 nm in serial dilutions of sample extracts (19–22). Samples were analyzed at 24 h for A. conyzoides and 48 h for more slowly transformed N. tabacum and A. thaliana cell lines. All treatments were performed in parallel. Three independently grown cultures were used to obtain triplicate samples for each treatment to control for possible clonal variation in culture batches. The standard deviation between samples is indicated. Each treatment was found to have similar effects in at least two repetitions. Buffers and GUS substrates were prepared as described (23).
The concentrations of control compounds tested were 0.1–1 μg/ml (3-amino-1,2,4-triazole), 1–10 μg/ml (5-methyl tryptophan), 0.01–1 μg/ml (colchicine), 1–1,000 μg/ml (cycloheximide), and 0.1–100 μg/ml (5-fluorouracil). The highest concentrations of these compounds caused significant plant cell toxicity. For each compound, we tested the highest dose that did not cause major plant cell toxicity in the GUS-activity assays.
The control experiment, wherein pretransformed plant cells were exposed to azaserine, was performed as described above with the following modifications. The A. tumefaciens cells were killed 30 h after infection by the addition of timentin (5 mg/ml) and cefotaxime (0.2 mg/ml). Plating experiments confirmed that all A. tumefaciens cells had been killed before the addition of azaserine. Eighteen hours after addition of the antibiotics, azaserine was added at a final concentration of 0.1 mg/ml. Samples were collected immediately and at 8 and 30 h after the addition of the compound and analyzed for GUS expression.
Results
A Genetic Screen for S. cerevisiae Mutants with Altered Sensitivity to A. tumefaciens-Mediated Transformation. We previously described an assay for A. tumefaciens-mediated transformation of S. cerevisiae that measures the collective efficiency of steps in transformation independent of chromosomal integration (1). In this assay, A. tumefaciens strain EHA105 harboring T-DNA donor plasmid pKP506 transfers the TRP1 marker gene to yeast strain recipients, allowing transformed trp1 mutant yeast cells to grow on medium lacking tryptophan. We adapted two variations of this assay for a large-scale genetic screen: (i) a replica-plating assay that provides a rapid visual assessment of the sensitivity of individual yeast colonies to A. tumefaciens transformation; and (ii) a quantitative assay with serial dilutions to determine the fraction of yeast cells transformed. We generated and screened 100,000 yeast transposon insertion mutants and identified yeast strains that exhibited altered transformation sensitivity (see Materials and Methods).
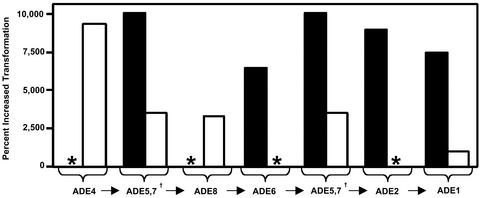
S. cerevisiae Purine Synthesis Mutants Are Supersensitive to A. tumefaciens-Mediated Transformation. Twenty-four mutants supersensitive to A. tumefaciens transformation were isolated, showing transformation efficiencies up to two orders of magnitude higher than isogenic (W303) control strains (Fig. 1). Interestingly, these strains failed to grow on medium lacking the purine adenine. Four complementation groups were defined corresponding to the purine synthesis genes ADE2 (1 allele), ADE6 (6 alleles), ADE1 (5 alleles), and ADE5,7 (12 alleles) (Fig. 1). In all these experiments, yeast and Agrobacterium cells were cocultivated on medium lacking adenine. We next determined whether mutations in other adenine loci also conferred transformation supersensitivity. We obtained similar results for adenine auxotrophs in two other yeast genetic backgrounds. Specifically, S288C yeast strains containing deletions in ADE4, ADE8, ADE1, or ADE5,7 were all supersensitive to A. tumefaciens transformation relative to the isogenic ADE+ control strain (Fig. 1). Further, a Σ1278b yeast strain harboring an ade2 deletion showed a transformation efficiency over three orders of magnitude higher (2.0 × 10–3) than the isogenic ADE+ control strain (1.3 × 10–6). These data show that mutations in six different S. cerevisiae adenine synthesis genes in three different strain backgrounds (W303, S288C, and Σ1278b) caused supersensitivity to A. tumefaciens-mediated transformation. This effect was most pronounced when adenine was absent from the CM plates.
Fig. 1.
Percent increase in transformation sensitivity of S. cerevisiae purine synthesis mutants relative to isogenic ADE+ strains. The first seven steps of yeast de novo purine biosynthesis are depicted below the x axis and are represented by their cognate gene names († denotes that the ADE5,7 gene encodes two enzymatic functions: ADE4 encodes phosphoribosylpyrophosphate amidotransferase; ADE5,7 encodes glycinamide ribotide synthetase and aminoimidazole ribotide synthetase; ADE8 encodes glycinamide ribotide transformylase; ADE6 encodes 5′-phosphoribosylformyl glycinamidine synthetase; ADE2 encodes phosphoribosylaminoimidazole-carboxylase; and ADE1 encodes phosphoribosyl amino imidazolesuccinocarbozamide synthetase). Filled bars represent the transformation sensitivities of W303-derived ade5,7, ade6, and ade1 insertion mutants from the yeast screen (strains RRY113, -82, and -86, respectively) and the W303 ade2 mutant strain (10556 30D) relative to the isogenic ADE+ control strain (10556 2B), which had a transformation sensitivity of 1.0 × 10–3. Open bars represent transformation efficiencies of the S288C deletion mutants ade4, ade5,7, ade8, and ade1 (strains RRY831, -836, -820, and -826, respectively) relative to the isogenic ADE+ control strain (RRY817), which had a transformation sensitivity of 3.8 × 10–4. Yeast strains were incubated with A. tumefaciens strain EHA105 carrying T-DNA donor plasmid pKP506 and transferred to plates with (+TRP) or without (–TRP) tryptophan (1, 12). Transformation sensitivity was defined as the number of transformants (colonies on –TRP plates) divided by the total number of yeast (colonies on +TRP plates). Experiments were performed in triplicate, and the mean is presented. The * indicates yeast mutants not available for testing.
Two lines of genetic evidence indicate that the observed supersensitivity to A. tumefaciens-mediated transformation is specific to the disruption of de novo purine biosynthesis in the host. First, no other auxotrophs were identified in our screen. Second, starvation of a his3 mutant for histidine did not significantly increase the transformation efficiency of that strain compared with an isogenic HIS+ strain (data not shown). In this experiment, the his3 mutant (10556 2B) and HIS+ control strain (10556 3C) were transformed with EHA105 harboring pKP506 on CM lacking histidine as described. Both the histidine and adenine mutant cultures contained similar numbers of yeast and bacterial cells at the end of analogous cocultivation experiments, suggesting that the different transformation sensitivities of adenine and histidine mutant strains do not result from differences in growth rates or bacteria/yeast cell ratios (data not shown). These data further support the finding that purine synthesis mutants deprived of adenine are supersensitive to A. tumefaciens-mediated transformation.
S. cerevisiae Purine Synthesis Mutants Are Not Supersensitive to Lithium-Acetate Transformation. As a control, yeast strains were transformed abiotically with 5.0 μg of pKP506 DNA by using standard lithium-acetate methods (17). We found that ade2, ade6, ade1, ade5,7, and ADE+ strains all exhibited similar proportions of transformants (1.8 × 10–5 for ADE+, 1.0 × 10–5 for ade2, 2.2 × 10–5 for ade6,2.8 × 10–5 for ade1, and 4.1 × 10–5 for ade5,7). These data demonstrate that ade mutant supersensitivity to A. tumefaciens transformation was not a function of the TRP1-selectable marker or other features of the pKP506 plasmid.
Adenine Supplementation Reduces Transformation Sensitivity. We further tested the specificity in two chemical supplementation experiments. Yeast cells are able to use exogenously supplied adenine, and we found that its addition decreased transformation sensitivity of an ade2 mutant 88% compared with ade2 cells starved for adenine during cocultivation (Fig. 2). In addition, purine metabolites can be exchanged between yeast cells. To determine whether such exchange would impact transformation efficiency, we mixed equal numbers of ade2 and ADE+ strains and subjected them to A. tumefaciens-mediated transformation. Different selection markers were used to monitor the transformation sensitivity of each strain in the mixture. Consistent with the adenine-supplementation results, ADE+ cells decreased the transformation efficiency of ade2 cells by 98%, presumably by supplying them with adenine or other diffusible purine synthesis intermediates (Fig. 2). Together, these supplementation experiments provide independent evidence that purine deprivation significantly increases the sensitivity of yeast cells to A. tumefaciens-mediated transformation.
Fig. 2.
S. cerevisiae supersensitivity to A. tumefaciens-mediated transformation is rescued by the addition of adenine or ADE+ cells. (A) The transformation sensitivity of ade2 (10556 30D) and ADE+ (10556 2B) yeast strains was determined on medium with (+) or without (–) 400 μM adenine by using A. tumefaciens strain EHA105 carrying T-DNA donor plasmid pKP506 (1, 12). Yeast cells were plated on appropriate medium (–HIS plates to monitor 10556 30D and –ADE plates to monitor 10556 2B) with (+TRP) or without (–TRP) tryptophan. Transformation sensitivity is the number of transformants (colonies on –TRP plates) divided by the total number of yeast (colonies on +TRP plates). The mean and standard deviation are depicted. (B) The same ade2 and ADE+ yeast strains were cultured separately (alone) or together (mixed), and their transformation sensitivities were determined as described.
The A. tumefaciens Virulence Gene-Expression Program Is Not Altered. Within A. tumefaciens cells, virulence (vir) gene transcription is induced during and is essential to the transformation process. Increased vir gene transcription is mediated by a promoter element (Vir box) common to several vir operons including VirE and the reporter construct VirE-LacZ (18). To address the possibility that yeast strains influence A. tumefaciens vir gene induction, we incubated an EHA105 strain containing the VirE-LacZ reporter with ade1, ade5,7,or ADE+ yeast strains and measured β-galactosidase activity at 0, 24, and 48 h. We found similar β-galactosidase activity levels in all samples for all three time points (data not shown). This demonstrates that the transformation supersensitivity of purine synthesis mutants does not result from altered vir gene transcription in A. tumefaciens cells.
Plant Cells Exposed to Purine Synthesis Inhibitors Are Supersensitive to A. tumefaciens-Mediated Transformation. Having shown that disruption of purine synthesis has a profound influence on A. tumefaciens transformation of yeast, we hypothesized that similar disruptions in plant cells might influence their sensitivity to transformation. We investigated the effects of purine and pyrimidine biosynthesis inhibitors on cell culture lines from three plant species: A. conyzoides, N. tabacum, and A. thaliana (22, 21). Plasmid pBISN1, in A. tumefaciens strain GV3101, was used as the T-DNA donor in these transformation assays (19). This plasmid contains GUS coding sequences interrupted by a plant intron, ensuring that GUS expression is confined to transformed plant cells (20). As a measure of transformation efficiency, GUS activity was used to quantitate successful delivery, intracellular targeting, and expression of the T-DNA in recipient plant cells.
Before infection with A. tumefaciens, plant cells were treated with compounds that inhibit key enzymes in the de novo purine biosynthetic pathway (mizoribine), pyrimidine biosynthetic pathway (5-fluorouracil), or both pathways (azaserine and acivicin) (24, 25). All three cell lines exhibited dramatically increased sensitivity to A. tumefaciens transformation after exposure to azaserine and acivicin, showing up to 180-fold increases in GUS activity relative to control Agrobacterium cocultivations not treated with these compounds (Table 2 and Fig. 3).
Table 2. Purine synthesis inhibitors cause supersensitivity to A. tumefaciens transformation in three plant cell cultures: A. conyzoides, N. tabacum BY-2, and A. thaliana Col-0.
| Conditions | A. conyzoides | A. thaliana | N. tabacum |
|---|---|---|---|
| Untransformed | 1.4 ± 0.06 | 1.9 ± 0.5 | 2.4 ± 0.2 |
| Transformed | 20 ± 2.5 | 2.7 ± 0.4 | 5.1 ± 0.6 |
| 5-Fluorouracil | 19 ± 4.4 | 2.7 ± 0.1 | 4.4 ± 0.8 |
| Mizoribine | 1,100 ± 130 | 13 ± 2 | 8.7 ± 3.2 |
| Azaserine | 570 ± 44 | 490 ± 50 | 130 ± 23 |
| Acivicin | 210 ± 17 | 310 ± 15 | 470 ± 240 |
Plant cell cultures were treated with pyrimidine synthesis inhibitor 5-fluorouracil (5 μg/ml), purine synthesis inhibitor mizoribine (50 μg/ml), or purine and pyrimidine synthesis inhibitors azaserine (100 μg/ml) and acivicin (100 μg/ml) for 16 h prior to infection with A. tumefaciens. Two control cultures are shown: the first was not exposed to inhibitors or A. tumefaciens (untransformed); the second was subjected to A. tumefaciens infection (transformed) without exposure to inhibitors. A. tumefaciens strain GV3101 harboring pBISN1 was used for transformations (19). GUS transgene expression in cell extracts was measured by hydrolysis of 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid (OD at 415 nm) of sample extracts (18, 19). All experiments were performed in triplicate. The mean and standard deviation are presented.
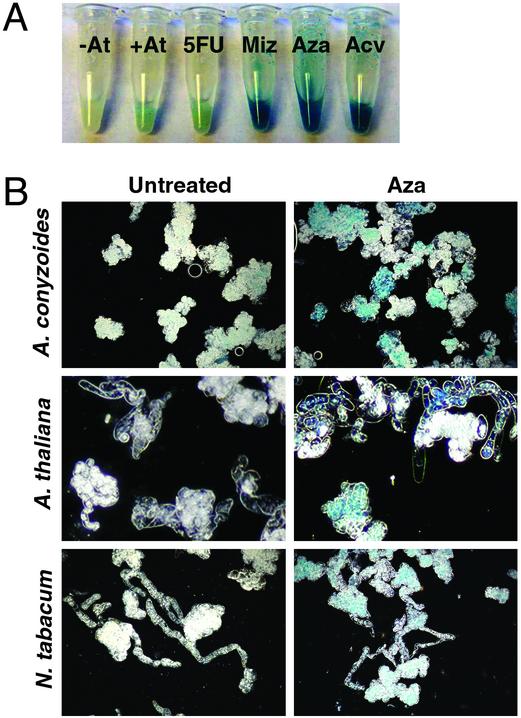
Fig. 3.
Transgene-derived GUS expression in plant cells. (A) A. conyzoides cell cultures were treated with various compounds for 16 h before infection with A. tumefaciens strain GV3101 harboring pBISN1. Hydrolysis of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (colorimetric GUS substrate) was observed 24 h after infection. Two control cultures are shown: the first was not exposed to inhibitors or A. tumefaciens (–At); the second was cocultivated with A. tumefaciens (+At) without prior exposure to inhibitors. Four cultures are shown that were pretreated with 5 μg/ml 5-fluorouracil (5FU), 50 μg/ml mizoribine (Miz), 100 μg/ml azaserine (Aza), or 100 μg/ml acivicin (Acv). (B) Dark-field micrographs depict GUS-stained A. conyzoides, N. tabacum BY-2, and A. thaliana Col-0 cells after no treatment (untreated) or treatment with azaserine (Aza) before transformation with A. tumefaciens strain GV3101 harboring pBISN1. All cells were derived from the samples described in Table 2.
To determine whether this effect was specifically attributable to inhibition of purine synthesis, we incubated plant cells with mizoribine or 5-fluorouracil before A. tumefaciens infection. Mizoribine increased the transformation sensitivity cells of A. conyzoides and A. thaliana, but the increase was not as strong as that observed with acivicin and azaserine (Table 2). N. tabacum cells did not show a significant effect after preincubation with mizoribine (Table 2 and Fig. 3). The pyrimidine-specific inhibitor 5-fluorouracil, however, did not elicit a significant increase in sensitivity to transformation in any of the plant cell lines (Table 2 and Fig. 3). These data indicate that purine biosynthesis inhibitors increased transformation sensitivity of two plant species, whereas disruption of pyrimidine synthesis inhibitors did not. However, the strongest effect was obtained by using azaserine or acivicin, which are thought to block both pathways.
Specificity was tested further by applying compounds that disrupt other cellular pathways and processes. In particular, plant cells were pretreated with a wide range of concentrations of colchicine (targets spindle formation) (25), 3-amino-1,2,4-triazole (targets histidine synthesis and catalase) (26, 27), 5-methyl tryptophan (targets tryptophan synthesis) (28), or cycloheximide (targets protein synthesis) (29). None of these treatments increased transformation sensitivity (data not shown). Taken together, these results strongly suggest that supersensitivity to A. tumefaciens-mediated transformation is specifically associated with inhibition of purine biosynthesis.
Azaserine Does Not Cause Increased T-DNA Gene Expression in Pretransformed Plant Cells. We performed two independent experiments to determine whether the high GUS activity detected in cells pretreated with purine synthesis inhibitors resulted from increased transformation efficiency or increased GUS expression per T-DNA delivered. First, we performed microscopic analyses of GUS-stained plant cell cultures after A. tumefaciens-mediated transformation. Pretreatment of A. thaliana and N. tabacum cells with azaserine resulted in increased numbers of cells expressing the GUS transgene relative to untreated controls (Fig. 3). Similar results were obtained with A. conyzoides cells, although the effect was less pronounced because of the high basal level of transformation (Fig. 3). Our second approach was to assess whether azaserine could stimulate GUS expression in pretransformed A. conyzoides cells. In this experiment, plant cells were first transformed by A. tumefaciens cells in the absence of azaserine. Plant cells then were incubated with antibiotics to kill A. tumefaciens cells, which then were removed by several washes. Finally, the plant cells were treated with azaserine and assayed for GUS activity. We found no significant difference between the GUS activity levels of untreated and azaserinetreated A. conyzoides cells in triplicate samples over three time points (data not shown). These results suggest that transformation supersensitivity, caused by purine synthesis inhibitors, reflects an increase in transformation efficiency rather than altered gene expression from preexisting T-DNAs.
Discussion
We developed a genetic screen, using S. cerevisiae, to rapidly identify host factors critical for A. tumefaciens-mediated transformation. We found that yeast adenine auxotrophs were supersensitive to transformation, and the strongest effect was observed when yeast cells were deprived of adenine during cocultivation with Agrobacterium cells. Chemical supplementation and genetic data suggest that this increase in transformation sensitivity was caused by the specific disruption of purine synthesis in yeast host cells. In addition, our finding that purine synthesis inhibitors increased transformation efficiency in three plant species demonstrates the functional equivalence of certain host factors in yeast and plants. These genetic and pharmacological data indicate the utility of yeast as a model for identifying and studying eukaryotic host factors that modulate A. tumefaciens-mediated transformation.
This report demonstrates that disruption of purine synthesis in host cells causes supersensitivity to A. tumefaciens transformation. The most striking results were observed with inhibitors that block both purine and pyrimidine synthesis (azaserine and acivicin). The magnitude of this effect is many times greater than similar effects reported in previous studies (25, 30, 31, 32, 33). A less-pronounced effect was observed for the purine synthesis inhibitor mizoribine, whereas the pyrimidine synthesis inhibitor 5-fluorouracil had no effect. These data suggest that disruption of purine synthesis is key. Because these genes are essential for cell viability under most growth conditions, it is not surprising that plant genetic screens failed to identify mutants in the purine biosynthesis pathway. These findings underscore the value of studying transformation mechanisms in the model yeast S. cerevisiae, which has long been used to study biosynthetic pathways and genes essential for cell viability.
Enzymes in the purine biosynthesis pathway have been highly conserved in evolution between yeast and plants and even between prokaryotes and eukaryotes. Purine biosynthesis is central to the biogenesis of many molecules critical for proper cellular metabolism including the DNA and RNA precursors AMP and GMP, the essential coenzymes NAD, NADP, FAD, and CoA, the signaling molecule cAMP, and the ubiquitous energy source ATP (34). In addition, purine pathway intermediates are known to diffuse across cell membranes and might transfer between host cells and A. tumefaciens cells, which raises the possibility that the donor bacteria may also be affected by disruption of purine synthesis in the host cell. Our VirE-lacZ reporter data suggest, however, that Agrobacterium virulence is not altered and that the primary effect of purine synthesis disruption is on the host cell. It has yet to be determined which pathway downstream of purine biosynthesis is most critical in determining host-cell sensitivity to Agrobacterium-mediated transformation. The yeast-transformation system we have developed provides a powerful model system to address these and other questions in future studies.
A previous report suggests a link among bacteria, plants, and de novo purine biosynthesis. In particular, purine biosynthesis in legumes is required for nitrogen assimilation and is regulated by interactions with symbiotic rhizobia partners (34). Rhizobia and A. tumefaciens reside in similar habitats and share extensive homology across their genomes (35). In light of these similarities, it is expected that common mechanisms underlie bacterial–plant interactions, both symbiotic and pathogenic. Thus, insights gained from A. tumefaciens transformation studies may ultimately be more broadly informative of interactions between bacteria and their eukaryotic hosts.
Finally, it will be of particular interest to determine whether purine synthesis inhibitors enhance the production of stable transformants, especially in plants and other eukaryotes recalcitrant to transformation. Such pharmacological treatments would bypass the limitations inherent in using genetic manipulations of the host to improve transformation efficiency and should increase the utility of A. tumefaciens in genetic engineering applications.
Acknowledgments
We thank Cammie Lesser, Paul de Figueiredo, Renata Ditt, Lishan Chen, and Derek Wood for helpful discussions and comments on the manuscript. Yeast strains were kindly provided by Gerald Fink and Microbia, Inc. Plant cell cultures were graciously provided by Delene Oldenburg, Arnold Bendich, Renata Ditt, and Luca Comai. This work was funded by National Institutes of Health Grant RO1 GM32618-30A1 (to E.W.N.). R.L.R. was supported by a Jane Coffin Childs Memorial Fund fellowship. D.E.M. was supported by National Research Service Award Fellowship 1-F32-GM64930.
Abbreviations: T-DNA, transferred DNA; CM, cocultivation medium; GUS, β-glucuronidase.
References
- 1.Piers, K. L., Heath, J. D., Liang, X., Stephens, K. M. & Nester, E. W. (1996) Proc. Natl. Acad. Sci. USA 93, 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groot, M. J., Bundock, P., Hooykaas, P. J. J. & Beijersbergen, A. G. (1998) Nat. Biotechnol. 16, 839–842. [DOI] [PubMed] [Google Scholar]
- 3.Abuodeh, R. O., Orbach, M. J., Mandel, M. A., Das, A. & Galgiani, J. N. (2000) J. Infect. Dis. 181, 2106–2110. [DOI] [PubMed] [Google Scholar]
- 4.Bundock, P., Mroczek, K., Winkler, A. A., Steensma, H. Y. & Hooykaas, P. J. J. (1999) Mol. Gen. Genet. 261, 115–121. [DOI] [PubMed] [Google Scholar]
- 5.Rho, H. S., Kang, S. & Lee, Y. H. (2001) Mol. Cells 12, 407–411. [PubMed] [Google Scholar]
- 6.Kunik, T., Tzfira, T., Kapulnik, Y., Gafni, Y., Dingwall, C. & Citovsky, V. (2001) Proc. Natl. Acad. Sci. USA 98, 1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu, J., Oger, P. M., Schrammeijer, B., Hooykaas, P. J. J., Farrand, S. K. & Winans, S. C. (2000) J. Bacteriol. 182, 3885–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das, A. (1998) Subcell. Biochem. 29, 343–363. [DOI] [PubMed] [Google Scholar]
- 9.Mysore, K. S., Nam, J. & Gelvin, S. B. (2000) Proc. Natl. Acad. Sci. USA 97, 948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballas, N. & Citovsky, V. (1997) Proc. Natl. Acad. Sci. USA 94, 10723–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Attikum, H., Bundock, P. & Hooykaas, P. J. J. (2001) EMBO J. 20, 6550–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cangelosi, G. A., Best, E. A., Martinetti, G. & Nester, E. W. (1991) Methods Enzymol. 204, 384–401. [DOI] [PubMed] [Google Scholar]
- 13.Sherman, F., Fink, G. R. & Hicks, J. (1986) Methods in Yeast Genetics (Cold Spring Harbor Lab. Press, Plainview, NY).
- 14.Burns, N., Grimwade, B., Ross-Macdonald, P. B., Choi, E. Y., Finberg, K., Roeder, G. S. & Snyder, M. (1994) Genes Dev. 8, 1087–1105. [DOI] [PubMed] [Google Scholar]
- 15.Winzeler, E. A., Shoemaker, D. D., Astromoff, A., Liang, H., Anderson, K., Andre, B., Bangham, R., Benito, R., Boeke, J. D., Bussey, H., et al. (1999) Science 285, 901–906. [DOI] [PubMed] [Google Scholar]
- 16.Alani, E., Cao, L. & Kleckner, N. (1987) Genetics 116, 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiestl, R. H. & Geitz, R. D. (1989) Curr. Genet. 16, 339–345. [DOI] [PubMed] [Google Scholar]
- 18.Stachel, S. E. & Zambryski, P. C. (1986) Cell 46, 325–333. [DOI] [PubMed] [Google Scholar]
- 19.Ditt, R. F., Nester, E. W. & Comai, L. (2001) Proc. Natl. Acad. Sci. USA 98, 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narashimulu, S. B., Deng, X. B., Sarria, R. & Gelvin, S. B. (1996) Plant Cell 8, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagata, T., Nemoto, Y. & Hasezawa, S. (1992) Int. Rev. Cytol. 132, 1–30. [Google Scholar]
- 22.Kanzaki, H., Kagemori, T., Asano, S. & Kawazu, K. (1998) Biosci. Biotechnol. Biochem. 62, 2328–2333. [DOI] [PubMed] [Google Scholar]
- 23.Jefferson, R. A. (1987) Plant Mol. Biol. Rep. 5, 387–405. [Google Scholar]
- 24.Allison, A. C. (2000) Immunopharmacology 47, 63–83. [DOI] [PubMed] [Google Scholar]
- 25.Zubay, G. (1993) Biochemistry (WCB, Oxford), 3rd Ed., pp. 547–584.
- 26.Kanazawa, S., Driscoll, M. & Struhl, K. (1988) Mol. Cell. Biol. 8, 664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda, T., Matsuda, Y., Sugawara, M. & Sagisaka, S. (1992) Biosci. Biotechnol. Biochem. 56, 1911–1915. [DOI] [PubMed] [Google Scholar]
- 28.Bender, J. & Fink, G. R. (1998) Proc. Natl. Acad. Sci. USA 95, 5655–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stryer, L. (1988) Biochemistry (Freeman, New York), pp. 601–626.
- 30.Tzfira, T., Vaidya, M. & Citovsky, V. (2002) Proc. Natl. Acad. Sci. USA 99, 10435–10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amoah, B. K., Wu, H., Sparks, C. & Jones, H. D. (2001) J. Exp. Bot. 52, 1135–1142. [DOI] [PubMed] [Google Scholar]
- 32.Li, X. Q., Liu, C. N., Ritchie, S. W., Peng, J. Y., Gelvin, S. B. & Hodges, T. K. (1992) Plant Mol. Biol. 6, 1037–1048. [DOI] [PubMed] [Google Scholar]
- 33.Mozo, T. & Hooykaas, P. (1992) Plant Mol. Biol. 19, 1019–1030. [DOI] [PubMed] [Google Scholar]
- 34.Smith, P. M. & Atkins, C. A. (2002) Plant Physiol. 3, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood, D. W., Setubal, J. C., Kaul, R., Monks, D. E., Kitajima, J. P., Okura, V. K., Zhou, Y., Chen, L., Wood, G. E., Almeida, N. F., Jr., et al. (2001) Science 294, 2317–2323. [DOI] [PubMed] [Google Scholar]