Abstract
Mammals contain two methionine sulfoxide (MetO) reductases, MsrA and MsrB, that catalyze the thioredoxin-dependent reduction of the S-MetO and R-MetO derivatives, respectively, to methionine. The major mammalian MsrB is a selenoprotein (except in the heart). Here, we show that there is a loss of MsrB activity in the MsrA–/– mouse that correlates with parallel losses in the levels of MsrB mRNA and MsrB protein, suggesting that MsrA might have a role in MsrB transcription. Moreover, mice that were grown on a selenium-deficient (SD) diet showed a substantial decrease in the levels of MsrB-catalytic activity, MsrB protein, and MsrB mRNA in liver and kidney tissues of both WT and MsrA–/– mouse strains. Whereas no significant protein-MetO could be detected in tissue proteins of young mature mice grown on a selenium-adequate diet, growth on the SD diet led to substantial accumulations of MetO in proteins and also of protein carbonyl derivatives in the liver, kidney, cerebrum, and cerebellum, respectively. In addition, accumulation of protein-MetO derivatives increased with age in tissues of mice fed with a selenium-adequate diet. It should be pointed out that even though the total Msr level is at least 2-fold higher in WT than in MsrA–/– mice, SD diet causes an equal elevation of protein-MetO (except in brain cerebellum) and carbonyl levels in both strains, suggesting involvement of other selenoproteins in regulation of the level of cellular protein-MetO accumulation. Furthermore, the development of the ``tip-toe'' walking behavior previously observed in the MsrA–/– mice occurred earlier when they were fed with the SD diet.
Keywords: methionine oxidation, selenoprotein, aging, oxidative stress
Most forms of reactive oxygen species can oxidize protein-bound methionine to a mixture of S and R forms of methionine sulfoxide (MetO). Unlike most other kinds of protein oxidative damage, the oxidation of methionine residues can be repaired by the action of MetO reductases that catalyze the thioredoxin-dependent reduction of the MetO derivatives back to methionine. One of these reductases, MsrA (EC 1.8.4.6), exhibits absolute substrate specificity for S isomer of MetO (1, 2), whereas the other, MsrB, is specific for the reduction of R-MetO (3–5). The importance of these enzymes in metabolism is underscored by the demonstration that null mutations of MsrA lead to an increase in oxidative stress-induced accumulation of damaged proteins, premature death of bacteria and yeast cells, and a shorter life span of mice (6–10). Furthermore, overexpression of MsrA in bacteria, human T cells, and flies protects them from oxidative stress toxicity (6, 8, 11, 12) and leads to an almost doubling of the life span of Drosophila (11). Similar effects of oxidative stress have been observed with null mutants of MsrB in yeast and plants (13). Based on present knowledge, it is evident that the Msr enzymes may have multiple cellular functions, including roles in antioxidant defense (7, 8), protein repair, and enzyme regulation (14–16). It was shown recently that the mammalian form of MsrB is a selenoprotein (3, 17) that utilizes its selenocysteine residue to reduce free and protein-bound R-MetO to methionine (3, 18). In contrast, the MsrA active site utilizes cysteine instead of a selenocysteine residue for the specific reduction of S-MetO to methionine (1). Not yet explained was the further observation that deletion of the MsrA gene in mice led to lower MsrB activity in various tissues, especially in liver, kidney, and brain cerebellum, relative to the parental mouse strain (3). Moreover, MsrA–/– mice exposed to 100% oxygen for 24 h failed to up-regulate thioredoxin reductase (TR) to the level shown in the parental strain, respectively (9). Significantly, the mammalian TR is also a selenoprotein (19), and its level has been shown to be appreciably lower in tissues of rats that had been grown on a selenium-deficient (SD) diet than in those grown on normal diets (20).
The present investigation was designed to determine whether dietary restriction of selenium has any effect on the MsrB and MsrA levels in various tissues of both parental WT and MsrA–/– strains of mice. Both strains also were monitored for changes in more general markers of oxidative stress (protein-MetO and carbonyl content) and for development of an abnormal ``tip-toe'' walking pattern, as was observed previously in the MsrA-knockout mouse (9).
Materials and Methods
Materials. Rabbit antibodies against MsrA and MsrB were prepared and used as described (21). 4-Dimethylaminoazobenzene-4′-sulfonyl (Dabsyl)-R or S-MetO was prepared by first making the R and S isoforms of MetO (1), followed by making the Dabsyl adduct, as described (7).
Animals and Diets. The animals used in this study were the MsrA–/– mice (9) and their parent strain (C57BL/129Svj), which served as the control. Weanling mice were fed a SD torula yeast-based diet or a selenium-adequate (SA) diet containing 0.015 or 0.25 ppm selenium as Na2SeO4, respectively. Diets were prepared by Zeigler based on their SD torula yeast diet (Zeigler Brothers, Gardner, PA). All nutrients, except Se, were provided in the basal diet at levels equal to those recommended by the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies (22, 23). Groups of five mice, fed the diets, were killed at 100 days, and various tissues were removed and frozen at –80°C until assays were performed. Extracts from all tissues were made with PBS as buffer, which contained protease inhibitor mixture (Roche, Gipf-Oberfrick, Switzerland). All mouse experiments were conducted according to guidelines of the National Heart, Lung, and Blood Institute Animal Care and Use Committee.
MsrA and MsrB Activities. Msr activity was measured by using 20 mM DTT, 200 μM Dabsyl-R- or S-MetO (the R or S form of MetO is for monitoring the MsrB or MsrA activity, respectively), and tissue extract supernatant. After 30 min of incubation at 37°C, the separation and quantitation of the product were performed by using an HPLC method, as described (1, 7, 8).
MsrB mRNA Levels. mRNA levels of MsrB were analyzed by isolating total mRNA from livers and kidneys of control and MsrA–/– mice (under each diet condition) by using the FastTrack 2.0 mRNA isolation kit from Invitrogen. Equal amounts of mRNA were taken from each tissue for the first-strand cDNA synthesis by using the SuperScript First-Strand Synthesis System for RT-PCR from Invitrogen. Further amplification of the cDNA transcripts in each case was performed by quantitative PCR using the 5′ forward and 3′ reverse complement primers for MsrB ORF as described (3). The final level of cDNA made for each mRNA sample was evaluated after 1% agar-gel electrophoresis of the PCR mixtures and visualized by ethidium bromide stain.
Determination of Protein-MetO and Carbonyl Groups. Proteins from each extract were subjected to CNBr cleavage to quantify the protein-bound MetO (24). CNBr cleaves peptide bonds on the carboxyl side of Met to yield homoserine but does not attack such bonds involving MetO (25). Peptide hydrolysis with HCl and analysis of the resulting amino acids were carried out on samples before and after treatment with CNBr (26), and the amount of MetO was quantitated by integration of the corresponding peak. Protein carbonyl levels were determined by reacting the proteins from each extract with dinitrophenol followed by spectrometric measurements, as described (27, 28).
Monitoring Mouse Tip-Toe Walking. Walking pattern was documented in both mouse strains by dipping footpads of the hind feet into india ink and allowing the animal to walk on paper strips.
Results
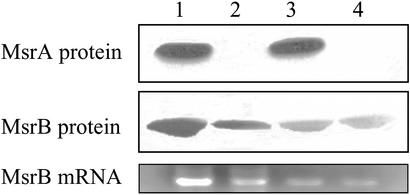
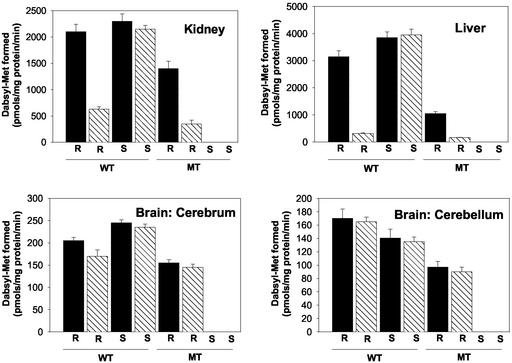
MsrB and MsrA Protein Levels in Various Tissues After SD Diet. Mice were placed on either SD or SA diet for 180 days. Then, various tissue levels of MsrA and MsrB proteins and their abilities to catalyze reduction of S-MetO and R-MetO peptide substrates were measured as described in Materials and Methods. In addition, when changes in protein levels were observed, levels of the respective mRNA were monitored as well. As shown in Fig. 1, the level of MsrA in liver of the parent strain (WT) was unaffected by selenium deficiency, and, as expected, there was no MsrA protein in the MsrA–/– strain (MT). Curiously, the level of MsrB protein in the MT strain fed the SA diet was only 35% of the WT level, as judged by densitometry measurements with the MsrB-specific antibodies (Fig. 1). A more severe decline in MsrB protein was observed in the livers of mice fed the SD diet: MsrB protein levels were only ≈10% or ≈5% in WT and MT liver tissues, respectively (Fig. 1). In addition, there was a reasonable correlation between the MsrB mRNA levels and MsrB protein expression. Patterns similar to those shown in Fig. 1 also were obtained for kidney preparations from these animals (data not shown). Moreover, direct measurements of MsrA and MsrB catalytic activities in livers and kidneys of these WT and MT mice (Fig. 2) were in good agreement with the mRNA and protein patterns summarized in Fig. 1. A very different activity pattern was observed in cerebellum and cerebrum tissues of the brain. In the WT animals, SD diet had little or no effect on the levels of MsrA or MsrB in either of these brain tissues (Fig. 2), and, as expected, no MsrA was detected in the brain of the MT. Interestingly, although unaffected by SD diet, the basal level of MsrB activity in the cerebrum and cerebellum of the MT strain was only ≈75% and ≈50% of that observed in WT, respectively (Fig. 2).
Fig. 1.
Expression of MsrA and MsrB in livers taken from mice fed with SA or SD diet. (Top and Middle) Equal amounts of liver proteins (20 μg) were separated by SDS/PAGE followed by Western blot analysis using specific antibodies against MsrA or MsrB, respectively. Lanes 1 and 3 represent WT samples, and lanes 2 and 4 represent MT samples. Lanes 1 and 2 are liver proteins taken from mice fed the SA diet, and lanes 3 and 4 are liver proteins taken from mice fed the SD diet. Each lane contains pooled proteins collected from five mice at 6 months of age. (Bottom) Quantitation of the MsrB mRNA levels on a 1% agar gel after RT-PCR of mRNA sample from each liver tissue, as described in Materials and Methods. The agar gel is aligned according to lanes 1–4 of the Western blots described above.
Fig. 2.
Msr activities in extracts of tissues taken from mice fed with SA or SD diet. MsrA or MsrB activity was measured by using Dabsyl-MetO as substrate as described in Materials and Methods. Solid bars represent SA diet, and hatched bars represent SD diet. R and S represent MsrB and MsrA activity, respectively. Proteins from five mice at 6 months of age were used for each analysis.
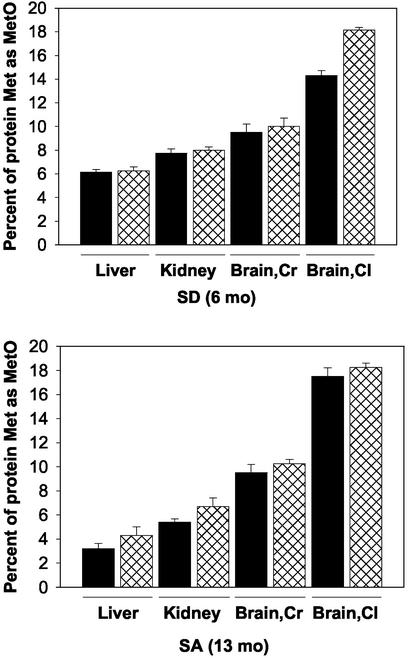
Oxidation of Proteins to MetO and Carbonyl Derivatives. No significant accumulation of protein-bound MetO was detected in any of the tissues examined (liver, kidney, cerebellum, and cerebrum) from both WT and MT strains of mature, young (6-month-old) mice that had been fed on the SA diet (data not shown). However, a substantial fraction (6–18%) of the Met residues were present as the MetO derivative in the tissues of the 6-month-old animals that had been placed on the SD diet (Fig. 3). Moreover, at 13 months of age, a significant accumulation of protein-MetO also occurred in tissues from the SA-fed animals (Fig. 3). The protein-MetO content of the brain fractions (cerebrum and cerebellum) contained the highest levels of MetO, accounting for 10–18% of the total Met in proteins of these tissues. It is noteworthy that the level of MetO in a given tissue of the WT and MT was very similar, except for the cerebellum of the SD mice, where the protein-MetO level in the MT was ≈22% higher than in the WT (Fig. 3).
Fig. 3.
MetO accumulations in various tissues of mice fed with SD or SA diet. Protein-bound MetO levels were analyzed according to the procedure described in Materials and Methods. Solid bars and cross-hatched bars represent WT and MT, respectively. Proteins from five mice were used for each analysis. SD (6 months) or SA (13 months) represents mice at age 6 or 13 months fed the SD or SA diet, respectively. Brain,Cr and Brain,Cl represent cerebrum and cerebellum, respectively.
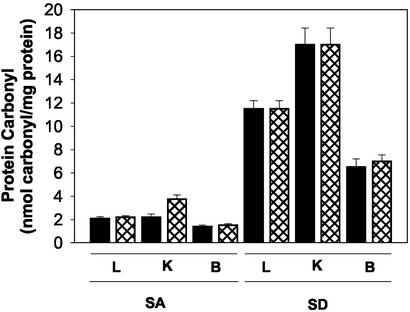
Results, summarized in Fig. 4, demonstrate that selenium deficiency also is associated with large increases in the carbonyl content of proteins in liver, kidney, and brain tissues of both WT and MT mouse strains. The latter observation highlights the possibility that some of the effects of selenium deficiency may reflect increases in the level of oxidative stress (see Discussion).
Fig. 4.
Protein carbonyl accumulations in various mouse tissues fed the SA or SD diet. Protein carbonyl was measured according to the method described previously (24). L, K, and B represent liver, kidney, and brain, respectively. Solid bars and cross-hatched bars represent WT and MT, respectively. Proteins from five mice were used for each analysis.
Tip-Toe Walking Behavior. It was shown earlier that MsrA null mice exhibited a characteristic tip-toe walking behavior after 6 months of age (9), which was interpreted to indicate possible development of a neurological disorder. In the present study, it was found that onset of the tip-toe walking characteristic in the SD mice occurred 3 months earlier than previously observed (9).
Discussion
The basal activity and protein levels of MsrB showed dependency on selenium supply and MsrA expression (Figs. 1 and 2). However, the mechanism by which MsrA regulates MsrB expression is yet to be elucidated. One potential mechanism may involve MsrA-mediated redox state of certain methionine residues of protein(s), which, in turn, may lead to a change in its (their) activity to regulate MsrB expression.
The observation that SD leads to a decrease in the basal activity and protein level of MsrB is not surprising because the mouse MsrB is a selenoprotein (3). That the level of MsrB mRNA is lower in the SD mice indicates that the observed loss of MsrB activity likely reflects a decrease in the expression of normal MsrB protein and is not due to the synthesis of a defective form of the enzyme that is degraded rapidly.
Because selenium deficiency leads to a dramatic loss in MsrB synthesis, it is not surprising that SD diet leads to a substantial increase in the accumulation of MetO-modified protein in various tissues of mature (6-month-old) mice (Fig. 3). However, that SD diet provoked accumulation of nearly identical amounts of protein-MetO in tissues of both WT and the MsrA-knockout mouse strains indicates that factors other than a loss of MsrB also are involved in the generation of protein-bound MetO. Among other possibilities, glutathione peroxidase 1 and TR deserve consideration. In mammals, both are selenoenzymes whose activities and mRNA levels have been shown to decline in some tissues of animals fed with SD diet (29–31). Because glutathione peroxidase 1 is an important antioxidant enzyme, a decrease in its activity could lead to an increase in levels of ROS and, hence, to increased oxidation of Met protein residues. However, a loss of TR could play a more direct role, because it is involved in supplying the reducing equivalents (NADPH) used in the thioredoxin-linked reduction of the S and R forms of MetO by MsrA and MsrB, respectively. Therefore, the selenium deficiency-induced loss of TR might seriously compromise the ability of both MsrA and MsrB to reduce MetO. Moreover, as shown here for MsrB activities in various mouse tissues (Fig. 2), SD diet has been shown earlier to have a pronounced effect on the activities of TR in the rat kidney and liver but had almost no effect on the TR activity in rat brain (29).
The general high protein damage in tissues of mice fed with the SD diet relative to SA diet is manifested by the elevated protein carbonyl levels (4- to 8-fold higher in the SD diet, respectively), in which kidney had the highest level (Fig. 4). The observation that selenium deficiency leads to substantial elevations in the carbonyl content of proteins in various mouse tissues (Fig. 4) is in agreement with results of a recent study showing that there is a marked increase in the protein carbonyl content in livers of SD rabbits (32). Because the activities of TR and MsrB are reduced significantly in selenium-depleted liver and kidney, compared with brain, it is reasonable to suggest that the lowered expression of these proteins in liver and kidney may contribute greatly to the observed enhanced protein carbonylation (Fig. 4).
The current results provide additional support for the concept that the cyclic oxidation and reduction of methionine residues of proteins may serve an important antioxidant function (24), a thesis also supported by results of earlier studies showing that the loss of MsrA (6–12) or MsrB (13) leads to an increased sensitivity to oxidative stress. However, for reasons discussed above, in addition to the loss of MsrB activity, the SD-induced increase in sensitivity of animals to oxidative stress may reflect losses in any one or more of several other important antioxidant selenoproteins in the cell. The loss of these additional antioxidant enzymes could be the reason for the abolished difference in protein carbonyl content shown between WT and MT kidneys observed under SA diet (Fig. 4). Furthermore, the observation that young, mature (6-month-old) mice did not accumulate significant MetO-modified protein (data not shown), whereas 13-month-old SA mice did (Fig. 3) provides further support for the concept that oxidative stress may contribute to the process of aging. This notion is consistent with the observation that accumulation of MetO-modified proteins occurs earlier in mice fed with the SD diet than with the SA diet.
Of particular interest is the fact that the Msr activities in the brain's cerebrum and cerebellum are considerably lower than those in either the kidney or liver. This difference likely accounts for the fact that the accumulation of protein-MetO in brain tissues is significantly greater than in the other tissues examined (Fig. 3) and calls attention to the possibility that the elevated level of Met oxidation especially in the cerebellum of the MT brain under the SD diet (Fig. 3) underlies the neurological disturbance responsible for the early, age-related onset of tip-toe walking behavior. It is important to note that methionine oxidation occurring under SD diet is primarily the result of selenium depletion, whereas during the aging process many factors could influence the levels of protein-bound MetO. Therefore, the difference in protein-MetO level between WT and MT brain cerebellums (under SD diet) probably is due to selenium depletion-related oxidative stress imposed on MT brains (Fig. 3). Obviously, more research is needed to better understand the physiological events involved in triggering the enhanced protein oxidation in the MT brain.
Acknowledgments
We thank Barbara S. Berlett for excellent technical support.
Abbreviations: MetO, methionine sulfoxide; TR, thioredoxin reductase; SD, selenium-deficient; SA, selenium-adequate; Dabsyl, 4-dimethylaminoazobenzene-4′-sulfonyl; MT, MsrA–/– strain.
References
- 1.Moskovitz, J., Poston, M., Berlett, B. S., Nosworthy, J. N., Szczepanowski, R. & Stadtman, E. R. (2000) J. Biol. Chem. 275, 14167–14172. [DOI] [PubMed] [Google Scholar]
- 2.Sharov, V. S., Ferrington, D. A., Squier, T. C. & Schoneich, C. (1999) FEBS Lett. 455, 247–250. [DOI] [PubMed] [Google Scholar]
- 3.Moskovitz, J., Singh, V. K., Requena, J., Wilkinson, B. J., Jayaswal, R. K. & Stadtman, E. R. (2002) Biochem. Biophys. Res. Commun. 290, 62–65. [DOI] [PubMed] [Google Scholar]
- 4.Grimaud, R., Ezraty, B., Mitchell, J. K., Lafitte, D., Briand, C., Derrick, P. J. & Barras, F. (2001) J. Biol. Chem. 276, 48915–48920. [DOI] [PubMed] [Google Scholar]
- 5.Orly, A., Boschi-Muller, S., Marrand, M., Sanglier-Cianferani, S., Van Dorsselear, A. & Branlant, G. (2002) J. Biol. Chem. 277, 12016–12022. [DOI] [PubMed] [Google Scholar]
- 6.Moskovitz, J., Rahman, M. A., Strassman, J., Yancey, S. O., Kushner, S. R., Brot, N. & Weissbach, H. (1995) J. Bacteriol. 177, 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskovitz, J., Berlett, S. B., Poston, M. & Stadtman, E. R. (1997) Proc. Natl. Acad. Sci. USA 94, 9585–9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskovitz, J., Flescher, E., Berlett, S. B., Azare, J. A., Poston, M. & Stadtman, E. R. (1998) Proc. Natl. Acad. Sci. USA 95, 14071–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskovitz, J., Bar-Noy, S., Williams, M. W., Requena, J., Berlett, S. B. & Stadtman, E. R. (2001) Proc. Natl. Acad. Sci. USA 98, 12920–12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vineet, K. S., Moskovitz, J., Wilkinson, B. J. & Jayaswal, R. K. (2001) Microbiology 147, 3037–3045. [DOI] [PubMed] [Google Scholar]
- 11.Ruan, H., Tang, X. D., Chen, M. L., Joiner, M. A., Sung, G., Brot, N., Weissbach, H., Heinemann, S. H., Iverson, L., Wu, C. F. & Hoshi, T. (2002) Proc. Natl. Acad. Sci. USA 99, 2748–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St. John, G., Brot, N., Ruan, J., Erdjument-Bromage, H., Tempst, P., Weissbach, H. & Nathan, C. (2001) Proc. Natl. Acad. Sci. USA 98, 9901–9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigo, M. J., Moskovitz, J., Salamini, F. & Bartels, D. (2002) Mol. Genet. Genomics 267, 613–621. [DOI] [PubMed] [Google Scholar]
- 14.Ciorba, M. A., Heinemann, S. H., Weissbach, H., Brot, N. & Hoshi, T. (1997) Proc. Natl. Acad. Sci. USA 94, 9932–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb, F. M., Davis, D. A., Moskovitz, J., Levine, R. L. & Yarchoan, R. (2000) Biochem. J. 346, 305–311. [PMC free article] [PubMed] [Google Scholar]
- 16.Mohri, M. S., Reinach, P. S., Kanayama, A., Shimizu, M., Moskovitz, J., Hisatsune, T. & Miyamoto, Y. (2002) Invest. Ophthalmol. Visual Sci. 43, 3190–3195. [PubMed] [Google Scholar]
- 17.Kryukov, G. V., Kumara, A., Koc, A., Sun, Z. & Gladyshev, V. N. (2002) Proc. Natl. Acad. Sci. USA 99, 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bar-Noy, S. & Moskovitz, J. (2002) Biochem. Biophys. Res. Commun. 297, 956–961. [DOI] [PubMed] [Google Scholar]
- 19.Tamura, T. & Stadtman, T. C. (1996) Proc. Natl. Acad. Sci. USA 93, 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burk, R. F. & Hill, K. F. (1993) Annu. Rev. Nutr. 13, 65–81. [DOI] [PubMed] [Google Scholar]
- 21.Moskovitz, J., Jenkins, N. A., Gilbert, D. J., Copeland, N. G., Frantisek, J., Weissbach, H. & Brot, N. (1996) Proc. Natl. Acad. Sci. USA 93, 3205–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies (1977) J. Nutr. 107, 1340–1348. [DOI] [PubMed] [Google Scholar]
- 23.American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies (1980) J. Nutr. 110, 1726. [DOI] [PubMed] [Google Scholar]
- 24.Levine, R. L., Mosoni, L., Berlett, B. S. & Stadtman, E. R. (1996) Proc. Natl. Acad. Sci. USA 93, 15036–15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fliss, H., Weissbach, H. & Brot, N. (1983) Proc. Natl. Acad. Sci. USA 80, 7160–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy, V. Y., Desrochers, P. E., Pizzo, S. V., Gonias, S. L., Sahakian, J. A., Levine, R. L. & Weiss, S. J. (1994) J. Biol. Chem. 269, 4683–4691. [PubMed] [Google Scholar]
- 27.Starke-Reed, P. E. & Oliver, C. N. (1989) Arch. Biochem. Biophys. 275, 559–567. [DOI] [PubMed] [Google Scholar]
- 28.Requena, J. R., Chao, C. C., Levine, R. L. & Stadtman, E. R. (2001) Proc. Natl. Acad. Sci. USA 98, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill, K. E., McCollum, G. W., Boeglin, M. E. & Burk, R. F. (1997) Biochem. Biophys. Res. Commun. 234, 293–295. [DOI] [PubMed] [Google Scholar]
- 30.Berggren, M. M., Mangin, J. F., Gasdaska, J. R. & Powis, G. (1999) Biochem. Pharmacol. 57, 187–193. [DOI] [PubMed] [Google Scholar]
- 31.Hadley, K. B. & Sunde, R. A. (2001) J. Nutr. Biochem. 12, 693–702. [DOI] [PubMed] [Google Scholar]
- 32.Muller, A. S. & Pallauf, J. (2002) J. Anim. Physiol. Anim. Nutr. (Berlin) 86, 273–287. [DOI] [PubMed] [Google Scholar]